Abstract
Background
B and T lymphocyte attenuator (BTLA) is a novel immune checkpoint with an unclear role in non–small-cell lung cancer (NSCLC). In contrast, the programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) checkpoint is a potentially curative immunotherapy target in NSCLC. Our study investigated BTLA expression and its relationship with PD-1/PD-L1, tumor-infiltrating lymphocytes (TILs), and clinicopathological features.
Methods
The protein expressions of BTLA, PD-1, and PD-L1 were evaluated by immunohistochemistry (IHC) and TIL abundance was scored in paraffin-embedded tissues from surgically resected specimens from 87 patients with stage I–III NSCLC.
Results
BTLA was expressed in tumor cells in 35 patients with NSCLC (40.2%). In addition, 42 patients (48.3%) were positive for PD-1 in TILs and 31 (35.6%) were positive for PD-L1 in tumor cells. BTLA was overexpressed in patients with lymphatic invasion (P=0.045) and an advanced tumor stage (P=0.034). High expression of BTLA was positively correlated with a high level of PD-L1 (P=0.011). Patients with positive BTLA expression had a shorter relapse-free survival (RFS) than those with negative BTLA expression (P=0.029). Moreover, patients negative for both BTLA and PD-L1 had a longer RFS than patients who were positive for BTLA or PD-L1 or for both checkpoints (P=0.012). The same pattern was shown for overall survival (P=0.031).
Conclusion
High BTLA expression may predict poor prognosis in patients with NSCLC and may represent a new immunotherapy target.
Keywords: B and T lymphocyte attenuator, BTLA, programmed death-1, PD-1, programmed death ligand-1, PD-L1, non-small-cell lung cancer, NSCLC
Introduction
Lung cancer is a common and often fatal malignant tumor, particularly non–small-cell lung cancer (NSCLC), which accounts for 80% of lung cancers, and most are diagnosed at an advanced stage.1 The curative ability of traditional treatment—radiotherapy and chemotherapy—is limited, and it is not easily accepted by patients due to its high toxicity and adverse effects. Molecular targeted therapy has shown promising results in some patients with driver mutations in a short period of remission and the problem of drug resistance is increasingly prominent.2–4 As a novel active immunotherapy, immune checkpoint inhibitors activate cytotoxic T lymphocytes that block programmed death-1 (PD-1), programmed death ligand-1 (PD-L1), and cytotoxic T lymphocyte antigen-4 (CTLA-4), among others, to reverse the immune escape abilities of tumor cells.5,6 Monoclonal antibodies targeting PD-1/PD-L1 have been investigated in clinical trials and approved as second-line treatments for advanced NSCLC.7 Nevertheless, PD-1/PD-L1 pathway inhibitors are only useful in a small number of patients with NSCLC and still show drug resistance after a period of treatment.8 Because this phenomenon suggests the complex role of immune checkpoints, it is imperative to find new therapeutic targets and explore whether different immune checkpoints synergize with each other.
B and T lymphocyte attenuator (BTLA) is a novel member of the CD28 superfamily and potentially an essential target for immunotherapy.9 BTLA is highly expressed in activated T cells but lowly expressed in naïve T cells, B cells, natural killer cells, macrophages, and dendritic cells.10 Herpesvirus entry mediator (HVEM), the ligand of BTLA, is a member of the tumor necrosis factor receptor family. The binding of BTLA to its ligand HVEM can reduce the activation and proliferation of lymphocytes and inhibit the activation and downregulation of T cells. BTLA signaling molecules affect not only Th1 cells, but also Th2 cell differentiation.11 Moreover, the anti-tumor effect of HSP70 vaccine can be improved by downregulation of BTLA expression in a mouse model of cervical cancer.12 Studies have shown that high expression of BTLA in melanoma limits the expression and function of CD8+T cells.13 Finally, studies of single nucleotide polymorphisms suggest that a BTLA polymorphism is connected to the occurrence and prognosis of breast cancer.14
Nonetheless, little is known about the expression and function of BTLA protein in NSCLC. Thus, we determined the expression of BTLA by using immunohistochemistry (IHC), analyzed its relationship with PD-1, PD-L1, tumor-infiltrating lymphocytes (TILs), and clinicopathological features, and explored its effects on progression in NSCLC.
Materials and Methods
Patients and Data
In this study, paraffin-embedded tissue sections were collected from 87 patients undergoing thoracic surgical resection and diagnosed with NSCLC by the Department of Pathology at Shengjing Hospital of China Medical University from February 2013 to June 2014. The Research Ethics Committee of the hospital approved the study (ethics number, 2018ps478k) in accordance with the guidelines of the Helsinki Declaration of 1975, revised in 1983.
None of the patients had received any cancer treatment (e.g., chemotherapy, radiotherapy, molecular targeted drugs, or immunotherapy) before the surgery or had any distant metastases. Baseline characteristics and follow-up results (including age, sex, histologic type, smoking status, progression, and overall survival [OS]) were obtained by retrospective analysis of clinical records. Pathologic tumor stage was judged according to the TNM staging system of the 8th UICC/AJCC manual.15
Immunohistochemistry
Paraffin-embedded tissue was sectioned at a thickness of 3.5 μm and baked at 60°C for 2 h. The paraffin slides were dewaxed with xylene and rehydrated in an ethanol series with an autostainer (Leica ST5010, Germany). For antigen retrieval, the tissue sections were placed in a flask filled with citrate buffer (pH 6.0) and heated to boiling for 5 min in a microwave oven, let rest for 5 min, reheated to boiling for 2 min, and let cool to room temperature. After blocking of endogenous peroxidase activity, the sections were incubated with primary antibodies (BTLA, dilution 1:320, catalog number: 21300-1-AP, Proteintech, Chicago, IL; PD-L1 dilution 1:200, catalog number: 66248-1-Ig, Proteintech; and PD-1, dilution 1:200, catalog number: 18106-1-AP, Proteintech) overnight at 4°C. The sections were then incubated with secondary antibodies at room temperature for 30 min, detected with a DAB kit (DAB-1031; MXB Biotechnology, China) under a microscope, and then counterstained with hematoxylin for about 3 min. Finally, they were dehydrated in gradient alcohol and then in a xylene bath by autostainer before being mounted.
Expression of BTLA, PD-1, and PD-L1
All stained sections were evaluated by two independent pathologists who were blinded to the clinical outcomes of the patients, and a final consensus was reached following further review. BTLA immunostaining was scored by multiplying the staining intensity by the positive area. The staining intensity was graded as follows: 0, negative (no staining); 1, weak staining (light yellow); 2, moderate staining (brown yellow); and 3, strong staining (dark brown yellow). The percent positivity was scored as follows: 0, <5%; 1, 5–25%; 2, 26–50%; and 3, >50%. Therefore, the final score of each slide ranged from 0 to 9. The BTLA staining results were classified into two groups according to the final score: (a) negative group = low expression, that is, a total score < 4, giving a total score of 0; (b) positive group = high expression, that is, a total score ≥ 4. The determination and threshold were based on studies of other malignancies.16,17 Patients with ≥ 8% PD-1 staining in TILs18,19 and ≥ 5% PD-1 staining in tumor cells (membrane or cytoplasm) were considered positive according to previous studies.20,21
Assessment of TILs
Under double-blind conditions, two senior pathologists of the Department of Pathology calculated the number of lymphocytes invading the tumor cell nests and stroma according to morphological characteristics in a microscope field of view on the same slides used for BTLA. Lymphocytes at the peripheral edge of the tissue in the sections were not considered to be part of the immune infiltration. A score of 1+ (<30%) indicated low expression of TILs; 2+ (30–60%) indicated moderate expression; and 3+ (>60%) indicated high expression.22,23
Statistical Analysis
The relationship between BTLA expression and clinical baseline characteristics (eg, age, sex, smoking status, histologic type, and stage) was analyzed with the chi-square test. The correlation of the expression levels of BTLA with PD-1, PD-L1, and TILs was analyzed by Spearman’s rank correlation. Relapse-free survival (RFS) was defined as the time from radical surgery to tumor progression or death (mRFS indicates mean RFS). OS was defined as the time from diagnosis (approximately the time of the operation) to death from any cause (mOS indicates mean OS). The survival curves of RFS and OS were estimated by the Kaplan–Meier method, whereas the log rank test was used for comparisons. Multivariate analysis was performed with a Cox regression model for factors with statistical significance after univariate analysis. Statistical analysis was performed using SPSS 19 software, and all statistical tests were two-sided. All statistics were 2-sided and statistical significance was defined as p<0.05.
Results
Patients’ Demographics
Among all patients, 62 (71.3%) were male, and the mean age at diagnosis was 61.83 years old (range, 43–84 years) (Table 1). Fifty-eight patients (66.7%) were smokers. In terms of stage, 29 patients (33.3%) were stage I, 34 (39.1%) were stage II, and 24 (27.5%) were stage III. In addition, 40 patients (46.0%) had adenocarcinoma, 42 (48.2%) had squamous cell carcinoma, 3 (3.5%) had large-cell carcinoma, and 2 (2.3%) had other types.
Table 1.
BTLA, PD-1 and PD-L1 Expression and Clinical Pathological Factors
| Variables | Total, n (%) | BTLA | PD-1 | PD-L1 | |||
|---|---|---|---|---|---|---|---|
| Positive n (%) | P value | Positive n (%) | P value | Positive n (%) | P value | ||
| Gender | |||||||
| Male | 62(71.3%) | 25(40.3%) | 0.978 | 33(53.2%) | 0.146 | 23(37.1%) | 0.653 |
| Female | 25(28.7%) | 10(40.0%) | 9(36.0%) | 8(32.0%) | |||
| Age, mean | 61.83 | ||||||
| <60 | 32(36.8%) | 14(43.8%) | 0.610 | 13(40.6%) | 0.276 | 14(43.8%) | 0.228 |
| ≥60 | 55(63.2%) | 21(38.2%) | 29(52.7%) | 17(30.9%) | |||
| Smoking | |||||||
| Non-smoker | 29(33.3%) | 9 (31.0%) | 0.216 | 10(34.5%) | 0.069 | 5(17.2%) | 0.011* |
| Smoker | 58(66.7%) | 26(44.8%) | 32(55.2%) | 26(44.8%) | |||
| Sublobe | |||||||
| Left lobe | 41(47.1%) | 18(43.9%) | 0.510 | 19(46.3%) | 0.733 | 13(31.7%) | 0.470 |
| Right lobe | 46(52.9%) | 17(37.0%) | 23(50.0%) | 18(39.1%) | |||
| Surgery | |||||||
| Standard | 83(95.4%) | 33(39.8%) | 0.683 | 39(47.0%) | 0.273 | 27(32.5%) | 0.006* |
| Limited | 4 (4.8%) | 2(50.0%) | 3(75.0%) | 4(100%) | |||
| Pathology | |||||||
| Adenocarcinoma | 40(46.0%) | 18(45.0%) | 0.403 | 22(55.0%) | 0.247 | 17(42.5%) | 0.217 |
| Non-adenocarcinoma | 47(54.0%) | 17(36.2%) | 20(42.6%) | 14(29.8%) | |||
| Histological grade | |||||||
| G1 | 20(23.0%) | 9(45.0%) | 0.754 | 8 (40.0%) | 0.660 | 6(30.0%) | 0.815 |
| G2 | 44(50.6%) | 16(36.4%) | 23(52.3%) | 16(36.4%) | |||
| G3 | 23(26.4%) | 10(43.5%) | 11(47.8%) | 9(39.1%) | |||
| T stage | |||||||
| ≤5cm | 65(74.7%) | 24(36.9%) | 0.280 | 29(44.6%) | 0.240 | 22(33.8%) | 0.550 |
| >5cm | 22(25.3%) | 11(50.0%) | 13(59.1%) | 9(40.9%) | |||
| Lymph node metastasis | |||||||
| No | 51(58.6%) | 16(31.4%) | 0.045* | 22(43.1%) | 0.254 | 13(25.5%) | 0.019* |
| Yes | 36(41.4%) | 19(52.8%) | 20(55.6%) | 18(50.0%) | |||
| Vessel invasion | |||||||
| No | 83(95.4%) | 32(38.6%) | 0.147 | 39(47.0%) | 0.273 | 29(34.9%) | 0.614 |
| Yes | 4 (4.6%) | 3 (75.0%) | 3 (75.0%) | 2(50.0%) | |||
| Stage | |||||||
| I-II | 63(72.4%) | 21(33.3%) | 0.034* | 28(44.4%) | 0.247 | 19(30.2%) | 0.084 |
| III | 24(27.6%) | 14(58.3%) | 14(58.3%) | 12(50.0%) | |||
| TILs percentage | |||||||
| High | 23(26.4%) | 12(52.2%) | 0.393 | 10(43.5%) | 0.524 | 6(26.1%) | 0.501 |
| Medium | 30(34.5%) | 11(36.7%) | 17(56.7%) | 11(36.7%) | |||
| Low | 34(39.1%) | 12(35.3%) | 15(44.1%) | 14 (41.2%) | |||
Note: *Stands for the value of p < 0.05.
Abbreviations: BTLA, B and T lymphocyte attenuator; PD-1, programmed death-1; PD-L1, programmed death ligand-1.
Characterization of BTLA, PD-1, and PD-L1 Expression in NSCLC and Their Association with Clinicopathological Factors
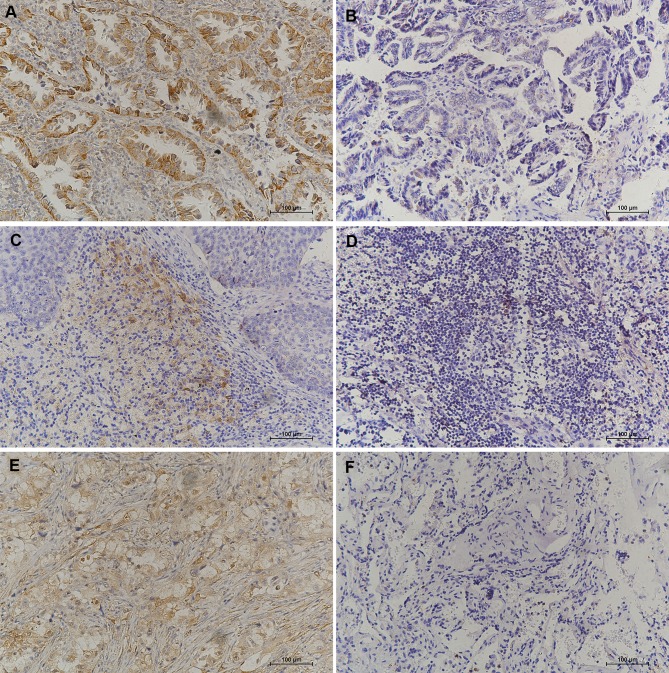
The IHC results showed that BTLA protein was mainly expressed in the membrane and cytoplasm of tumor cells but could occasionally be seen scattered in TILs. Statistical analysis showed that BTLA expression was detected in 35 patients (40.2%). We also found that 42 patients (48.3%) were positive for PD-1 in TILs and 31 (35.6%) were positive for PD-L1 in tumor cells (Figure 1). BTLA was overexpressed in patients with lymph node metastasis (P=0.045) and an advanced pathologic stage (P=0.034). In addition, the expression of PD-L1 was higher in smokers (P=0.011) and in patients with standard surgery (P=0.006) or lymph node metastasis (P=0.019). Expression of PD-1 in TILs was not significantly correlated with clinicopathological factors (Table 1).
Figure 1.
Representative IHC staining for BTLA, PD-1, and PD-L1 (200×) in NSCLC. (A) Positive BTLA expression. (B) Negative BTLA expression. (C) Positive PD-1 expression. (D) Negative PD-1 expression. (E) Positive PD-L1 expression. (F) Negative PD-L1 expression.
Abbreviations: IHC, immunohistochemical; NSCLC, non-small cell lung cancer; BTLA, B and T lymphocyte attenuator; PD-1, programmed death-1; PD-L1, programmed death ligand-1.
Correlations of BTLA Expression with PD-1, PD-L1, and TIL Abundance
Assessment of TILs found that 34 patients (39.1%) had a score of 1+, 30 (34.5%) had 2+, and 23 (26.4%) had 3+. There was a positive correlation between the expression of BTLA and PD-L1 in Spearman correlation analysis (r=0.271, P=0.011) (Table 2).
Table 2.
The Correlation of BLTA Expression with PD-1, PD-L1 and TILs
| Characteristics | BTLA Expression | |||
|---|---|---|---|---|
| Positive | Negative | r-value | P-value | |
| PD-1 expression | ||||
| Positive | 19 (45.2%) | 23 | 0.099 | 0.363 |
| Negative | 16 (35.6%) | 29 | ||
| PD-L1 expression | ||||
| Positive | 18 (58.1%) | 13 | 0.271 | 0.011* |
| Negative | 17 (30.4%) | 39 | ||
| TILs percentage | ||||
| High | 12 (52.2%) | 11 | 0.126 | 0.246 |
| Medium | 11 (36.7%) | 19 | ||
| Low | 12 (35.3%) | 22 | ||
Note: *Stands for the value of p < 0.05.
Abbreviations: BTLA, B and T lymphocyte attenuator; PD-1, programmed death-1; PD-L1, programmed death ligand-1; TILs, tumor-infiltrating lymphocytes.
Clinical Impact of BTLA on Prognosis
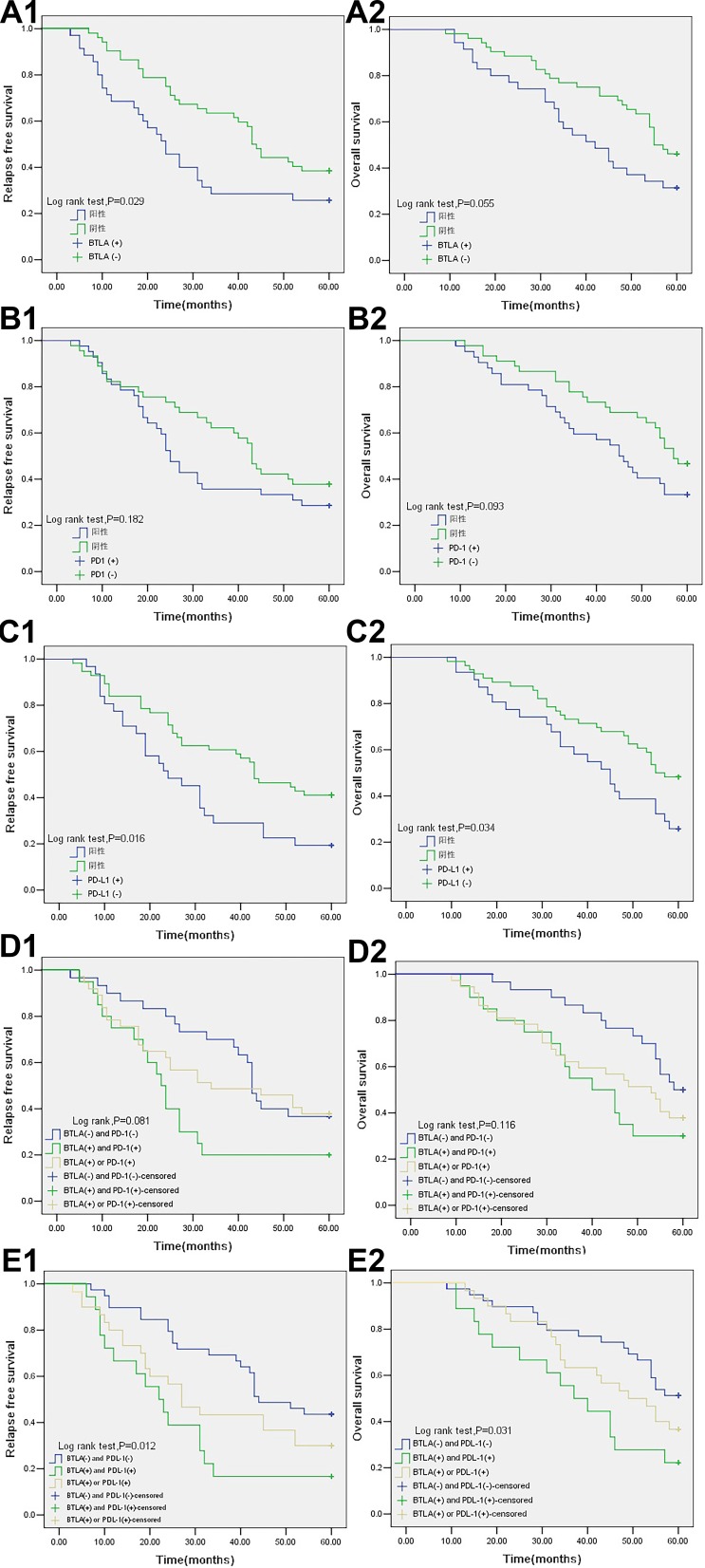
The median follow-up time was 54 months, with a range of 9 to 60 months. Kaplan–Meier analysis revealed that patients with positive BTLA or PD-L1 expression had a shorter mRFS than those with negative expression (29.40 months [95% CI 22.60–36.20] vs 41.33 months [95% CI 36.30–46.35], P=0.029; 29.74 months [95% CI 23.21–36.27] vs 40.29 months [95% CI 35.02–45.55], P=0.016) (Figure 2A1 and C1). The patients with positive PD-L1 expression also had a shorter mOS than those with negative expression (41.16 months [95% CI 35.18–47.14] vs 47.89 months [95% CI 43.74–52.05], P=0.034) (Figure 2C2). The positive expression of BTLA also showed a tendency for a negative impact on OS, because the P-value approached 0.05 (P=0.055) (Figure 2A2). We also found that patients who were both BTLA negative and PD-L1 negative had a longer RFS than patients who were either BTLA or PD-L1 positive or positive for both checkpoints (43.44 months [95% CI 37.80–49.07] vs 33.90 months [95% CI 26.36–41.44] vs 25.94 months [95% CI 17.85–34.04], P=0.012) (Figure 2E1). The same pattern was shown for OS (49.59 months [95% CI 44.80–54.38] vs 45.07 months [95% CI 39.43–50.71] vs 37.33 months [95% CI 29.24–45.43], P=0.031) (Figure 2E2). PD-1 expression and co-expression with BTLA were not prognostic factors for RFS or OS (P>0.05) (Figure 2B and D).
Figure 2.
Kaplan-Meier survival curves in patients with NSCLC. (A1) RFS of BTLA expression (P=0.029). (A2) OS of BTLA expression (P=0.055). (B1) RFS of PD-1 expression (P=0.182). (B2) OS of PD-1 expression (P=0.093). (C1) RFS of PD-L1 expression (P=0.016). (C2) OS of PD-L1 expression (P=0.034). (D1) RFS of BTLA and PD-1 expression (P=0.081). (D2) OS of BTLA and PD-1 expression (P=0.116). (E1) RFS of BTLA and PD-L1 expression (P=0.012). (E2) OS of BTLA and PD-L1 expression (P=0.031).
Abbreviations: NSCLC, non-small cell lung cancer; BTLA, B and T lymphocyte attenuator; PD-1, programmed death-1; PD-L1, programmed death ligand-1.
Univariate and Multivariate Analysis of RFS and OS
Univariate analysis indicated that lymphatic metastasis, tumor stage, BTLA expression, and PD-L1 expression were significant risk factors for RFS. In addition, lymphatic metastasis, tumor stage, and PD-L1 expression were significant prognostic factors for OS (Table 3). In multivariate analysis, the independent risk factors were tumor stage (HR 3.617, 95% CI 2.072–6.312, P<0.001) and PD-L1 expression (HR 1.774, 95% CI 1.044–3.014, P=0.034) for RFS and lymphatic metastasis (HR 2.359, 95% CI 1.268–4.388, P=0.007) and tumor stage (HR 3.023, 95% CI 1.601–5.709, P=0.001) for OS (Table 4).
Table 3.
Univariate Analysis of the Prognostic Factors of Survival in Patients with NSCLC
| Variable | Relapse-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gender | ||||
| (Male vs female) | 0.964(0.548–1.698) | 0.900 | 0.671(0.358–1.258) | 0.213 |
| Age | ||||
| (≥60 vs <60) | 0.690(0.410–1.162) | 0.163 | 0.755(0.437–1.308) | 0.316 |
| Smoke status | ||||
| (Yes vs No) | 1.642(0.931–2.899) | 0.087 | 1.555(0.852–2.836) | 0.150 |
| Pathology | ||||
| (Non-adenocarcinoma vs adenocarcinoma) | 0.885(0.528–1.484) | 0.644 | 0.968(0.561–1.670) | 0.906 |
| Grade | ||||
| (G3 vs G1-G2) | 0.990(0.692–1.417) | 0.957 | 1.096(0.757–1.586) | 0.629 |
| T stage | ||||
| (>5cm vs ≤5cm) | 1.480(0.841–2.607) | 0.174 | 1.465(0.804–2.671) | 0.212 |
| Lymphatic metastasis | ||||
| (Yes vs No) | 2.362(1.405–3.970) | 0.001* | 3.379(1.933–5.907) | <0.001* |
| Vessel invasion | ||||
| (Yes vs No) | 0.840(0.205–3.448) | 0.809 | 1.038(0.252–4.272) | 0.959 |
| Tumor stage | ||||
| (III vs I-II) | 3.710(2.137–6.440) | <0.001* | 4.386(2.468–7.792) | <0.001* |
| BTLA expression | ||||
| (Positive vs negative) | 1.763(1.048–2.965) | 0.033* | 1.693(0.979–2.929) | 0.060 |
| PD-1 expression | ||||
| (Positive vs Negative) | 1.414(0.843–2.373) | 0.189 | 1.585(0.917–2.739) | 0.099 |
| PD-L1 expression | ||||
| (Positive vs negative) | 1.868(1.107–3.153) | 0.019* | 1.788(1.033–3.097) | 0.038* |
| TILs | ||||
| (High vs low-medium) | 0.753(0.537–1.056) | 0.101 | 0.759(0.531–1.083) | 0.129 |
Note: *Stands for the value of p < 0.05.
Abbreviations: BTLA, B and T lymphocyte attenuator; PD-1, programmed death-1; PD-L1, programmed death ligand-1; TILs, tumor-infiltrating lymphocytes; HR, hazard ratio; CI, confidence interval.
Table 4.
Multivariate Analysis of the Prognostic Factors of Survival in Patients with NSCLC
| Variable | Relapse-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Lymphatic metastasis | ||||
| (Yes vs No) | 1.477(0.809–2.696) | 0.204 | 2.359(1.268–4.388) | 0.007* |
| Tumor stage | ||||
| (III vs I-II) | 3.617(2.072–6.312) | <0.001* | 3.023(1.601–5.709) | 0.001* |
| BTLA expression | ||||
| (Positive vs negative) | 1.219(0.695–2.140) | 0.490 | – | – |
| PD-L1 expression | ||||
| (Positive vs negative) | 1.774(1.044–3.014) | 0.034* | 1.545(0.870–2.744) | 0.138 |
Note: *Stands for the value of p < 0.05.
Abbreviations: BTLA, B and T lymphocyte attenuator; HR, hazard ratio; CI, confidence interval.
Discussion
BTLA is another recently discovered immunosuppressive receptor of the CD28 superfamily, in addition to CTLA-4 and PD-L1/PD-1. It is a type 1 transmembrane glycoprotein, and the cytoplasmic domain contains an immunoreceptor tyrosine-based inhibitory motif (ITIM), an immunoreceptor tyrosine-based switch motif (ITSM), and a GRB-2 recognition consensus sequence.13,24 The interaction of BTLA with its ligand HVEM can downregulate the immune response by suppressing lymphocyte proliferation and inhibiting the secretion of cytokines (IL-2, IFN-γ, IL-4, and IL-10).25–28 Phosphorylation of the critical functional molecules, such as Syk, in the BCR signaling pathway is restrained after BTLA activation, which inhibits the proliferation of B lymphocytes.29 Follicular helper T cells (Tfh) also have high expression of BTLA and may help to regulate the IgG response.30 Therefore, BTLA may be a significant therapeutic target that plays a role in not only cellular immunity, but also humoral immunity.
As far as we know, this is the first study to demonstrate that BTLA can be constitutively detected in tumor cells by 40.2% of patients of NSCLC (I–III stage) using IHC and to analyze the correlations of BTLA with PD-1, PD-L1, and TILs. Compared with a previous study that found BTLA expression on intratumoral CD8+ T cells from patients with non–small cell lung cancer,31 we found that BTLA was mainly present in pulmonary carcinoma cells and a small amount was expressed in TILs. This phenomenon, which can be expressed on both tumor cells and TILs, indicates the complexity of BTLA mediated immunosuppression and needs further study. In our study, BTLA levels were significantly higher in NSCLC patients with traditional risk factors such as lymphatic metastasis and high tumor pathological stage.32–34 Furthermore, we determined that patients with positive BTLA expression had a shorter RFS than those with negative BTLA expression, and the same pattern appeared to be shown for OS (P=0.055). These results indicate that BTLA expression is related to the progression and poor prognosis of NSCLC, which is consistent with the results of previous studies of gastric cancer.16,17 Feng et al16 even revealed that the expression of BTLA in cancer tissue was an independent prognostic factor for gastric cancer, although BTLA expression was not an independent factor for RFS and OS in multivariate analysis of our patients with NSCLC. This difference may be related to tumoral heterogeneity or because the correlation of BTLA expression with lymphatic invasion and tumor stage weakened its effect on the statistical significance in the Cox regression model.
In addition, we determined that BTLA expression was positively correlated with PD-L1 in tumor cells. Meanwhile, we found that BTLA-positive status alone or both BTLA and PD-L1 positivity were correlated with early postoperative recurrence and short OS, further indicating that the group expressing both proteins showed an even worse prognosis. It may be that BTLA overexpression reduces immune surveillance ability and has a synergistic effect with PD-L1. In some previous studies, PD-L1 was linked to worse survival outcomes not only in NSCLC, but also in other malignancies.35–38 Analogously, we also observed that PD-L1 expression was higher in smokers and those with lymph node metastasis and was an independent prognostic factor for RFS.
Although a checkpoint blockade antibody targeting PD-L1 has achieved impressive clinical efficacy, only a few patients—about 20%—benefit from monoclonal therapy.39 Co-expression of multiple immune checkpoints has frequently been found in exhausted T cells in tumors.40 Therefore, the complexity and variability of the neoplastic immune microenvironment may be one of the reasons for the low clinical efficacy. Thus, simultaneous blockade of BTLA and PD-L1 could be a strategy for cancer treatment.
There are some limitations to our study. First, the study population was not large and might have resulted in a selection bias. Second, there is currently no uniform standard for the evaluation of BTLA in NSCLC, and we adopted the cutoff determination used by previous studies of other malignant tumors. Third, this is a retrospective study that lacked some data, such as driver mutation status, that should be examined in further work.
Conclusion
To the best of our knowledge, our research demonstrated that BTLA was expressed in tumor tissue of NSCLC for the first time. BTLA was statistically highly expressed in patients with clinical risk factors. Meanwhile, BTLA expression was positively correlated with PD-L1 expression. BTLA expression or both BTLA and PD-L1 positivity was related to a shorter RFS and OS, implying a synergistic action between the two immune checkpoint molecules. Accordingly, we speculate that a high level of BTLA expression is a risk factor for the prognosis of NSCLC and that BTLA might be a novel therapeutic target for immunotherapy.
Funding Statement
This study was supported by the provincial Natural Science Foundation of Liaoning, China (No. 2014021032).
Compliance with Ethical Standards
The study received the approval of the clinical research ethics committee of Shengjing Hospital of China Medical University (approval number, 2018PS478K).
Disclosure
The authors declare that they have no conflict of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.He Y, Li S, Ren S, et al. Impact of family history of cancer on the incidence of mutation in epidermal growth factor receptor gene in non-small cell lung cancer patients. Lung Cancer. 2013;81(2):162–166. doi: 10.1016/j.lungcan.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 3.He Y, Wang Y, Boyle T, et al. Hepatic metastases is associated with poor efficacy of erlotinib as 2nd/3rd line therapy in patients with lung adenocarcinoma. Med Sci Monit. 2016;22:276–283. doi: 10.12659/MSM.896607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohashi K, Maruvka YE, Michor F, Pao W. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol. 2013;31(8):1070–1080. doi: 10.1200/JCO.2012.43.3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504–535. doi: 10.6004/jnccn.2017.0050 [DOI] [PubMed] [Google Scholar]
- 8.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv324. doi: 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karakatsanis S, Bertsias G, Roussou P, Boumpas D. Programmed death 1 and B and T lymphocyte attenuator immunoreceptors and their association with malignant T-lymphoproliferative disorders: brief review. Hematol Oncol. 2014;32(3):113–119. doi: 10.1002/hon.v32.3 [DOI] [PubMed] [Google Scholar]
- 10.Sedy JR, Gavrieli M, Potter KG, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6(1):90–98. doi: 10.1038/ni1144 [DOI] [PubMed] [Google Scholar]
- 11.Pasero C, Olive D. Interfering with coinhibitory molecules: BTLA/HVEM as new targets to enhance anti-tumor immunity. Immunol Lett. 2013;151(1–2):71–75. doi: 10.1016/j.imlet.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 12.Han L, Wang W, Fang Y, et al. Soluble B and T lymphocyte attenuator possesses antitumor effects and facilitates heat shock protein 70 vaccine-triggered antitumor immunity against a murine TC-1 cervical cancer model in vivo. J Immunol. 2009;183(12):7842–7850. doi: 10.4049/jimmunol.0804379 [DOI] [PubMed] [Google Scholar]
- 13.Haymaker CL, Wu RC, Ritthipichai K, et al. BTLA marks a less-differentiated tumor-infiltrating lymphocyte subset in melanoma with enhanced survival properties. Oncoimmunology. 2015;4(8):e1014246. doi: 10.1080/2162402X.2015.1014246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Z, Li D, Jiang W, et al. Association of BTLA gene polymorphisms with the risk of malignant breast cancer in Chinese women of Heilongjiang Province. Breast Cancer Res Treat. 2010;120(1):195–202. doi: 10.1007/s10549-009-0462-6 [DOI] [PubMed] [Google Scholar]
- 15.Kay FU, Kandathil A, Batra K, Saboo SS, Abbara S, Rajiah P. Revisions to the tumor, node, metastasis staging of lung cancer (8(th) edition): rationale, radiologic findings and clinical implications. World J Radiol. 2017;9(6):269–279. doi: 10.4329/wjr.v9.i6.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng XY, Wen XZ, Tan XJ, et al. Ectopic expression of B and T lymphocyte attenuator in gastric cancer: a potential independent prognostic factor in patients with gastric cancer. Mol Med Rep. 2015;11(1):658–664. doi: 10.3892/mmr.2014.2699 [DOI] [PubMed] [Google Scholar]
- 17.Lan X, Li S, Gao H, et al. Increased BTLA and HVEM in gastric cancer are associated with progression and poor prognosis. Onco Targets Ther. 2017;10:919–926. doi: 10.2147/OTT.S128825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–5100. doi: 10.1158/1078-0432.CCR-04-0428 [DOI] [PubMed] [Google Scholar]
- 19.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra137. doi: 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. doi: 10.1158/0008-5472.CAN-05-4303 [DOI] [PubMed] [Google Scholar]
- 22.Schalper KA, Brown J, Carvajal-Hausdorf D, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015;107:3. doi: 10.1093/jnci/dju435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y, Rozeboom L, Rivard CJ, et al. PD-1, PD-L1 protein expression in non-small cell lung cancer and their relationship with tumor-infiltrating lymphocytes. Med Sci Monit. 2017;23:1208–1216. doi: 10.12659/MSM.899909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavrieli M, Watanabe N, Loftin SK, Murphy TL, Murphy KM. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem Biophys Res Commun. 2003;312(4):1236–1243. doi: 10.1016/j.bbrc.2003.11.070 [DOI] [PubMed] [Google Scholar]
- 25.Watanabe N, Gavrieli M, Sedy JR, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–679. doi: 10.1038/ni944 [DOI] [PubMed] [Google Scholar]
- 26.M’Hidi H, Thibult ML, Chetaille B, et al. High expression of the inhibitory receptor BTLA in T-follicular helper cells and in B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Am J Clin Pathol. 2009;132(4):589–596. doi: 10.1309/AJCPPHKGYYGGL39C [DOI] [PubMed] [Google Scholar]
- 27.Derre L, Rivals JP, Jandus C, et al. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120(1):157–167. doi: 10.1172/JCI40070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang XF, Chen YJ, Wang Q, et al. Distinct expression and inhibitory function of B and T lymphocyte attenuator on human T cells. Tissue Antigens. 2007;69(2):145–153. doi: 10.1111/j.1399-0039.2006.00710.x [DOI] [PubMed] [Google Scholar]
- 29.Vendel AC, Calemine-Fenaux J, Izrael-Tomasevic A, Chauhan V, Arnott D, Eaton DL. B and T lymphocyte attenuator regulates B cell receptor signaling by targeting Syk and BLNK. J Immunol. 2009;182(3):1509–1517. doi: 10.4049/jimmunol.182.3.1509 [DOI] [PubMed] [Google Scholar]
- 30.Kashiwakuma D, Suto A, Hiramatsu Y, et al. B and T lymphocyte attenuator suppresses IL-21 production from follicular Th cells and subsequent humoral immune responses. J Immunol. 2010;185(5):2730–2736. doi: 10.4049/jimmunol.0903839 [DOI] [PubMed] [Google Scholar]
- 31.Thommen DS, Schreiner J, Muller P, et al. Progression of lung cancer is associated with increased Dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res. 2015;3(12):1344–1355. doi: 10.1158/2326-6066.CIR-15-0097 [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Yue CF, Zhou WH, et al. Aurora-A contributes to cisplatin resistance and lymphatic metastasis in non-small cell lung cancer and predicts poor prognosis. J Transl Med. 2014;12:200. doi: 10.1186/1479-5876-12-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin H, Ni Y, Tong J, et al. Elevated expression of CRYAB predicts unfavorable prognosis in non-small cell lung cancer. Med Oncol. 2014;31(8):142. doi: 10.1007/s12032-014-0142-1 [DOI] [PubMed] [Google Scholar]
- 34.Schneider F, Derrick V, Davison JM, Strollo D, Incharoen P, Dacic S. Morphological and molecular approach to synchronous non-small cell lung carcinomas: impact on staging. Mod Pathol. 2016;29(7):735–742. doi: 10.1038/modpathol.2016.66 [DOI] [PubMed] [Google Scholar]
- 35.Sun JM, Zhou W, Choi YL, et al. Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol. 2016;11(7):1003–1011. doi: 10.1016/j.jtho.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 36.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia (New York, NY). 2006;8(3):190–198. doi: 10.1593/neo.05733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151–2157. doi: 10.1158/1078-0432.CCR-06-2746 [DOI] [PubMed] [Google Scholar]
- 39.Ma W, Gilligan BM, Yuan J, Li T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9(1):47. doi: 10.1186/s13045-016-0277-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19(18):4917–4924. doi: 10.1158/1078-0432.CCR-12-1972 [DOI] [PMC free article] [PubMed] [Google Scholar]