Abstract
Background
Emergency Department (ED) visits provide an opportunity for hepatitis C virus (HCV) screening for patients who otherwise might be tested. We report on a novel nontargeted, opt-out HCV screening and linkage-to-care program implemented in an urban ED.
Methods
This is a descriptive analysis from 3 months (November 2016-January 2017) of a nontargeted, opt-out ED HCV screening and linkage-to-care program among patients at least 13 years old undergoing phlebotomy for clinical purposes. A multi-purpose Best Practice Advisory (BPA) alerted providers to the program and generated order labels. For patients who authorized testing, specimens were drawn in the ED for HCV antibody (Ab) and reflex confirmatory RNA tests. Public health navigators attempted to contact RNA+ patients and arrange outpatient visits.
Results
HCV Ab tests were performed on 3,808 patients, a 6,950 % increase from preprogram. The proportion of HCV Ab test positivity was 13.2% (504/3808, 95% CI 12.2%, 14.3%) and of those 97.8% (493/504) had a follow up RNA test performed. 292 were confirmed positive for active infection, for an overall RNA positivity rate of 7.7% (95%CI 6.8%, 8.5%) Of those with active infection, 155 (53%) were outside the Centers for Disease Control and Prevention birth cohort for increased risk for HCV including 46 (15.8%, 95% CI 11.8%, 20.4%) who also did not report injection drug use. Linkage attempts were documented on 223 (76.4%) patients and appointments were scheduled for 102 (38% of attempted). 66 patients attended their linkage to care visit (22.5% of all RNA positive patients, 30% of linkage-eligible patients).
Conclusions
Non-targeted opt-out HCV testing can be successfully implemented in an ED setting. A number of patients diagnosed were outside traditional risk groups. Once diagnosed, an ED population may be difficult to engage in care, but a structured interdisciplinary program can successfully link patients to HCV treatment.
Keywords: HCV Linkage to Care, ED HCV Screening Program, HCV Reflex Testing
Introduction
As part of the national strategy to expand Hepatitis C Virus (HCV) diagnosis and treatment, in 2012 the U.S. Centers for Disease Control and Prevention (CDC), and then the U.S. Preventive Services Task Force (USPSTF), endorsed guidance for routine one-time HCV antibody screening for any individual born 1945–1965 (the “birth cohort”), as well as continued targeted testing for drug users and others at high risk for HCV infection.1,2 That guidance increased rates of testing among those born 1945–1965, but it had little impact on those born after 1965.3
The incidence of HCV is rising, however, among those under the age of 40 years.4 Recognizing that stopping HCV transmission requires identifying and curing HCV among people born after 1965 and who are using drugs, CDC and Public Health Departments around the country seek additional venues at which to offer HCV testing and are considering guidance for routine HCV testing among those born after 1965.5
Emergency Departments (EDs) may provide a good venue for HCV testing as well. There are over 136 million ED visits/year in the US and approximately 10% of ambulatory care visits across the country are to an ED.6 The ED has been shown to be an effective and cost-effective venue for HIV testing,7 and best practices exist for public health screening programs in ED settings.8 Additionally, the ED has the potential to reach a segment of the population that does not otherwise access healthcare and may be missed by programs in other healthcare settings.9
Recent data from EDs that have implemented CDC and USPSTF guidance for HCV testing have found satisfactory test uptake, HCV seropositivity rates of up to 10%, and comparable rates of linkage to HCV care and treatment to that observed among patients identified in other healthcare settings.10,11 Those programs, however, employed existing guidance for routine testing among the “birth cohort” and targeted testing for all others. Seroprevalence studies from the ED suggest that targeted testing based on birth cohort and documented IDU history may miss one quarter to one half of all HCV seropositive cases.12
In order to address the concerns that there is a high rate of undiagnosed HCV in our ED patient population, and that some infected patients may be missed using traditional targeted testing, we designed a screening and linkage-to-care (LTC) program that is integrated into routine ED operations. In November, 2016 the Boston Medical Center (BMC) ED implemented a program for nontargeted opt-out HCV using reflex RNA testing among visitors to the ED who were having phlebotomy performed for any reason, without effort to target testing based on birth year or risk behaviors. This manuscript describes the development, implementation, and preliminary screening results of that program.
Methods
OVERVIEW
In November, 2016 BMC, the largest safety net hospital in New England, implemented a program for HCV testing for all patients who presented to the Emergency Department and were having blood drawn for any purpose. Here, we characterize the screening and LTC program design, report on implementation results, including ED program uptake and LTC successes, and report results of the screening program. This project was approved by the Institutional Review Board at Boston University Medical Center.
STUDY SETTING AND POPULATION
BMC is an urban, academic facility that receives approximately 1,150,000 visits per year. The medical center is recognized as the primary “safety net” provider of care for the city’s indigent and most vulnerable population. Among ED patients, 72% are on government-payor insurance, 32% do not speak English as a primary language, and over 70% of the patients are racial or ethnic minorities. Many patients have substance use disorders; 640 patients presented to the ED for opioid overdose in 2013–2014,13 and over 850 patients with opioid related injuries were seen in 2015.
IMPLEMENTATION METHODS
I. Stakeholder Engagement
This program is the result of a collaboration between the Emergency Department, the Section of Infectious Diseases, the Department of Laboratory Medicine, the Massachusetts Department of Public Health, with funding from Gilead Sciences, Inc FOCUS program. The objective was to increase diagnosis of HCV and LTC in our institution. Pre-implementation activities included engaging key hospital stakeholders including hospital leadership, ED and outpatient clinicians, laboratory leadership, and representatives from information technology in program design.
The main concern with implementing expanded ED HCV screening brought up by was that the medical record contained written documentation of verbal informed consent for all patients who underwent HCV screening. We addressed this concern by building this documentation into the EMR, so that it appeared automatically in all patients who had an HCV screen ordered. We held an information session with and got approval from our hospital’s Medical Executive Committee, as well as met with the Policies and Procedures Committee to ensure that our processes were in compliance with hospital guidelines.
Structured educational initiatives were performed for residents and faculty (presentation at faculty meeting as well as a one-hour presentation/question period at resident didactics), and nurses (we met with nurse managers and educators as well as attended daily huddles and “ED walk-throughs” with staff nurses for two weeks). There was extensive training of newly hired patient care navigators by infectious disease program managers with experience in this arena.
II. Reflex Laboratory Testing
We developed an onsite pathway to provide Ab screening with reflex testing for HCV RNA and genotype among those specimens identified as being HCV antibody seropositive. This represented a change from prior years when Ab screening and therapeutic monitoring viral load testing was performed on-site, but follow up RNA diagnostic testing and genotyping relied on an off-site reference laboratory – a process that increased time-to-result reporting. Under the new processes, when an HCV test is ordered, the antibody (Ab) screen is performed on the Abbott Architect Anti-HCV/CMIA assay for detection of IgG/IgM antibodies from phlebotomized blood specimens14 and results are reported in the EMR within 2 hours. Any positive Ab screen is automatically reflexed to RNA diagnostic confirmation, viral load, and genotyping if indicated. We check for HCV RNA assay using the Roche COBAS Ampliprep/COBAS Taqman (CAP/CTM) HCV version 2.15 (A single quantitative PCR RNA assay serves as both the diagnostic confirmatory and viral load test). For specimens with detectable HCV RNA, we perform Genotyping on the Abbot M2000.16 The follow-up diagnostic test results are routinely available within 1 week of initial Ab screen.
III. ED Screening Program Design
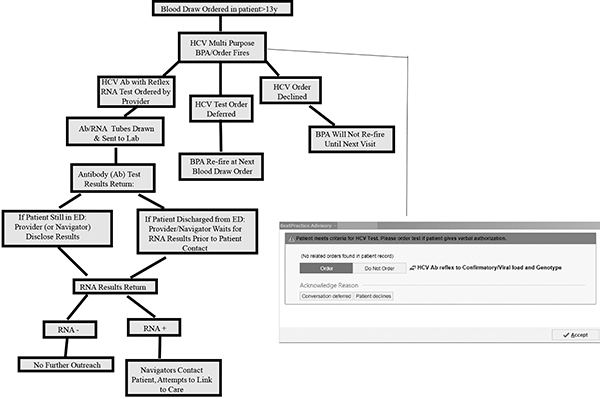
The ultimate strategy we decided on is to offer opt-out HCV screening to all ED patients over 13 years of age who are undergoing phlebotomy for any clinical purpose (Figure 1).
Figure 1:
Operational Model of ED HCV Screening Program
Funding for the actual laboratory tests would be obtained by charging insurance (since 2006 Massachusetts has decreased the number of uninsured due to an individual mandate) and the funding for the development of the laboratory reflex pathway, information technology changes to the Electronic Medical Record (EMR) and support for patient care navigators came from the program grant.
Upon entering any phlebotomy order into the EMR, a multi-functional Best Practice Advisory (BPA) alerts the care provider (nurse, nurse practitioner, physician, physician’s assistant) that the patient is eligible for HCV screening. Patients are considered eligible if they are at least 13 years old and do not have a prior complete HCV panel result in the BMC EMR. A complete HCV panel includes either a negative HCV antibody test or a positive HCV antibody test with follow up RNA testing, and if indicated, a viral load and genotype.
To standardize screening and to comply with institutional leadership mandates, ED providers follow a script in the same language in which they are conducting the medical history, using a medical translator as appropriate: “As a public health measure, we are providing hepatitis C virus testing to all patients getting blood drawn in the ED. This test will be done, unless you decline. Do you have any questions?” Information about the cost of the test is given if a patient asks. This BPA is not only an alert, but functions as a hard stop that the provider must respond to in order to continue with order entry. The provider can, on behalf of the patient, accept, defer, or decline the BPA. If accepted, the BPA generates documentation of verbal patient authorization for testing, triggers an order in the EMR for HCV serum antibody testing, and prints labels for specimen collection. If deferred, the BPA appears again on subsequent phlebotomy orders. If declined, the BPA will not appear for the duration of the current hospitalization, but will reappear on subsequent ED visits.
Physicians provide preliminary Ab screening results to patients still in the ED when results become available, informing them that they might be infected, and indicating that the linkage navigators will contact them if their confirmatory test results show active disease. They also give patients a CDC fact sheet about HCV. To minimize the impact of the program on ED workflow, patients are not required to wait in the ED for Ab test results. When patients have already left the ED at the time that their HCV Ab test results become available, then program staff contact the patient with results via telephone.
IV. LTC Program
Our HCV LTC program is supported by two dedicated full-time public health navigators and a part-time data analyst. The laboratory generates a daily list of RNA+ patients and provides this to navigators. The navigators attempt to contact each patient, by visiting admitted inpatients or calling discharged patients on phone numbers from the EMR. Navigators attempt to contact all patients to give them positive test results and to link them to a treating provider at BMC. We excluded the following from LTC: 1) patients that receive primary care at an outside medical system that is known to treat HCV, 2) incarcerated persons, 3) pregnant women (until after pregnancy has ended), 4) individuals with viral load<600 IU/mL, 5) those with EMR notes documenting physician opted not to treat at this time, and 6) no contact information in the EMR.
Navigators attempt to reach patients 4 times by telephone, after which they attempt to contact patients via their emergency contact phone number and/or their primary care provider, and simultaneously send a certified letter to the patient’s listed address. The linkage protocol was modified after 2 months to have navigators contact hospitalized patients during their hospital admission. When the navigator successfully contacts a patient (s)he provides test results and schedules a first visit appointment with either a physician or nurse practitioner in general internal medicine, gastroenterology, infectious disease, or addiction services, according to an algorithm developed with outpatient clinicians. When patients miss their scheduled first visit with an HCV treating provider, the navigators repeat their outreach attempts.
IMPLEMENTATION EVALUATION
I. ED Screening Program
The primary outcomes of the ED screening program implementation evaluation were ED testing volume (# tests performed/month), and the change in testing volume pre-intervention to post (mean # tests performed/month in the first 3 months after program implementation – mean #tests performed/month in the year prior to program implementation / mean #tests performed/month in the year prior to program implantation * 100). Secondary outcomes were # of BPA fires, #BPA fires that resulted in HCV testing, and # of providers that ordered HCV screening.
II. LTC Program
We constructed a relational database both for tracking patients in the program and for evaluation purposes (Microsoft Access 2013; Microsoft, Redmond, WA). The navigators and data analyst populated the database using electronic queries of the EMR, manual chart review, and direct communication with patients. We defined a linkage attempt as a phone call to a patient, emergency contact, primary care provider or a visit to the patient while still in the hospital. Patients that attended their first outpatient visit were considered linked to care and those that had no visit 3 months after initial diagnosis were considered unlinked.
We examined the performance of the linkage program including the absolute number and proportion of patients eligible for each step of the testing and linkage cascade to care in this population. We also analyzed the number of attempts that were required to obtain successful LTC.
SCREENING OUTCOMES
Data elements abstracted from the EMR included age, gender, race/ethnicity, previous HCV test results, and co-morbidities. Study staff performed manual chart review to extract histories of substance use based on both problem lists and review of clinical encounter notes. Navigators also communicated directly with patients to ask about drug use history using a formal script.
We report the rates of positive HCV Ab and RNA results (# positive/# tested), percentage of those previously diagnosed with HCV, and number/percent of patients with positive RNA that are outside the birth cohort/identified IDU.
Results
IMPLEMENTATION RESULTS
I. ED Screening Program
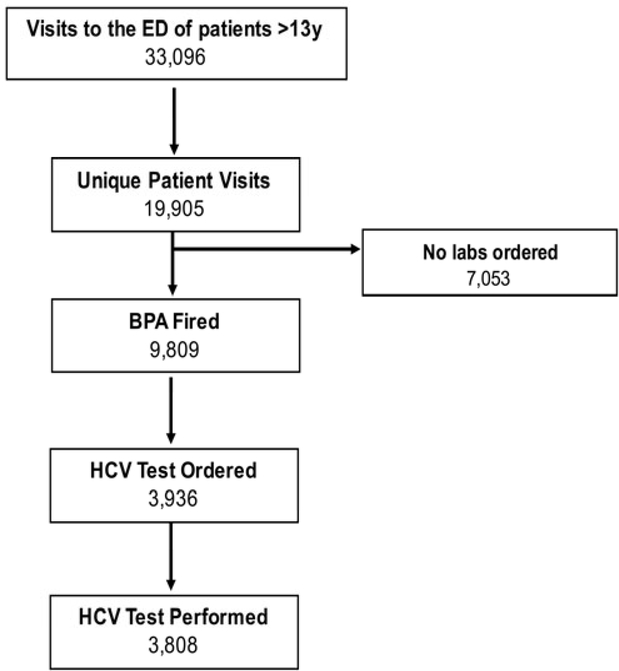
In the year prior to the start of the ED HCV screening program, there were approximately 132,000 ED visits and an average of 18 HCV antibody tests per month were performed in the ED. From November 2, 2016, until January 31, 2017, there were 33,096 visits by 19,905 unique patients age 13 years or older (Figure 2). Of those, 12,852 had labs drawn during at least one of their ED visits. The BPA fired on 9,809 of those patients. Due to information technology data capture limitations, we are unable to determine why the BPA didn’t fire on the remaining 3,043 patients. However, certain providers in the ED are not primarily ED providers (they are mostly off-service residents rotating through the ED) and their orders do not trigger the BPA. Patients seen by these off-service providers likely account for a portion of the non-fires.
Figure 2:
Enrollment Flow Chart
HCV Ab screening tests were ordered for 3,936 (40% of BPA fires were accepted) and ultimately sent on 3,808 patients (39%), corresponding to an HCV testing rate of 1,269/month, a 6,950% increase in the rate of testing in the ED compared to the pre-intervention period. During this period 472 ED staff members received the BPA. Among those, 364 providers ordered at least one HCV Ab screening test.
II. Linkage to Care
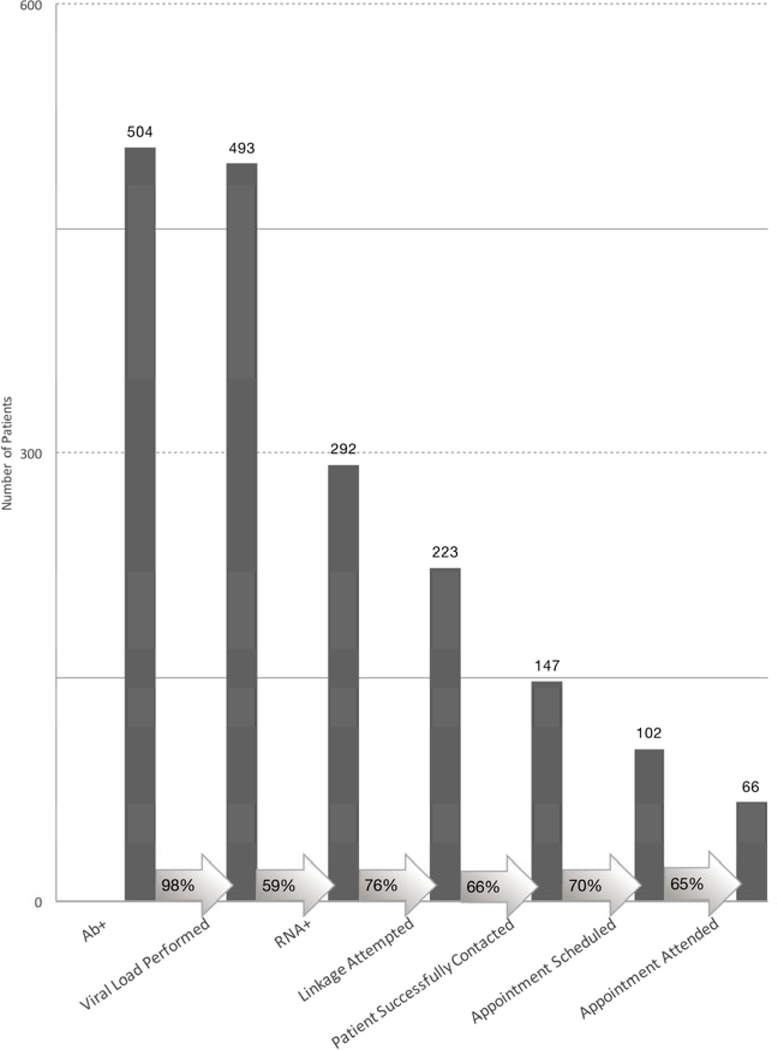
Of 292 RNA+ patients, linkage attempts were documented for 223 (76.4%) patients (Figure 3). Reasons for not attempting linkage were: patients that receive primary care at an outside medical system that is known to treat HCV (25), deceased (1), incarcerated (6), pregnant (5), viral load<600 IU/mL (14), EMR notes documenting physician opted not to treat (15), no reliable contact information in the EMR (3). Among those for whom linkage attempts were made, appointments were scheduled for 102 patients (38% of attempted). Of the 121 patients who ultimately did not have appointments arranged the most common reasons for failure were: unable to reach (76, 63%), in HCV care elsewhere (27, 22%), refused (6, 5%), incarcerated (2, 2%).
Figure 3: Linkage to Care Cascade.
The Linkage to Care Cascade of those identified in the Emergency Department. Column height proportions are based on the initial stage in each part of the cascade.
Proportion of patients progressing to the next stage among eligible patients is shown within the arrows.
Twenty-two appointments were made after one telephone attempt by the navigator, 12 after 2 attempts, 14 after 3 attempts, 16 after 4 attempts, 18 after certified letter, 3 after in-person visits, and 17 after physician outreach. Ultimately, sixty-six patients attended their linkage to care visit (22.6% of all RNA positive patients (95% CI 17.8%, 27.4%), 29.6% of linkage-attempted patients (95% CI 23.6%, 35.6%).
SCREENING RESULTS
Among the 3808 who were tested, the rate of HCV Ab test positivity was 13.2% (504/3808, 95% CI 12.2%, 14.3%, Table 1). Of those 97.8% (493/504) had a follow up RNA test performed. 292 were HCV RNA positive, corresponding to a rate of active HCV infection of 7.7% (95%CI 6.8%, 8.5%). Of those who were RNA positive, 187 (64.0%) were confirmed in the EMR as having previously tested positive for HCV Ab, 42 (14.4%) self-reported prior HCV positive test, and 63 (21.6%, 95% CI 17.0%, 26.7%) were considered to be newly diagnosed cases.
Table 1:
Demographics of Patients Screened for HCV
| Antibody Positive | Antibody Negative | Unadjusted Odds Ratio (95% CI) | |
|---|---|---|---|
| Characteristic | N = 504 (%) | N = 3303 (%) | |
| Age, mean (SD), y | 49.7 (14.3) | 48.9 (18.2) | - |
| Sex | |||
| Female | 182 (36.1) | 1819 (55.1) | Reference Group |
| Male | 322 (63.9) | 1484 (44.9) | 2.17 (1.79 – 2.63) |
| Race/Ethnicity | |||
| Black | 175 (34.7) | 1555 (47.1) | Reference Group |
| Hispanic | 82 (16.3) | 794 (24.0) | 0.92 (0.70 – 1.21) |
| Asian | 5 (1.0) | 73 (2.2) | 0.61 (0.24 – 1.53) |
| American Indian or Alaska Native | 3 (0.6) | 6 (0.2) | 4.44 (1.10 – 17.92) |
| White | 221 (43.8) | 660 (20.0) | 2.98 (2.39 – 3.70) |
| Declined to answer | 18 (3.6) | 215 (6.5) | 0.74 (0.45 – 1.23) |
| Documented IDU (current or previous) | |||
| No | 150 (29.8) | 2857 (86.5) | Reference Group |
| Yes | 318 (63.1) | 96 (2.9) | 63.09 (47.63 – 83.58) |
| Unknown | 36 (7.1) | 350 (10.6) | 1.96 (1.34 – 2.87) |
| Birth Cohort | |||
| Born before 1945 | 19 (3.8) | 427 (12.9) | Reference Group |
| Born 1945–1965 | 236 (46.8) | 1096 (33.2) | 4.84 (2.99 – 7.83) |
| Born after 1965 | 249 (49.4) | 1780 (53.9) | 3.14 (1.95 – 5.07) |
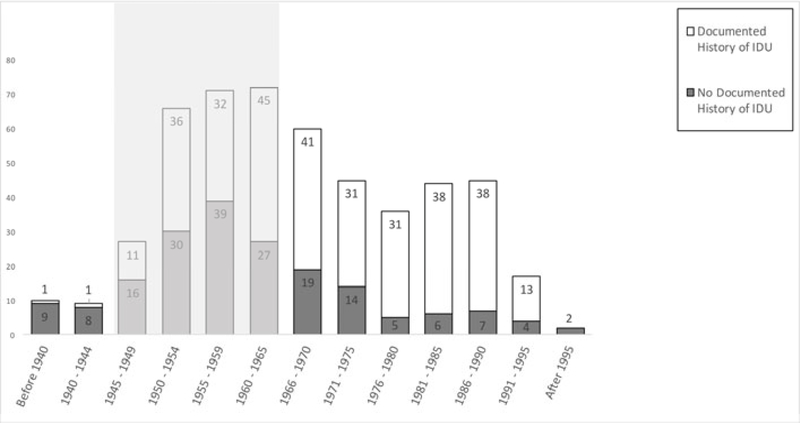
Overall 318 (63.1%) Ab+ patients had past or current injection drug use (IDU) documented in their record (Table 1). There were 236 (46.8%) Ab+ members of the baby boomer birth cohort and 249 (49.4%) Ab+ patients younger than the birth cohort and over half the patients with active HCV infection (53%) were diagnosed outside of the birth cohort. Seventy-four (14.7%, 95%CI 11.7%, 18.1%) patients that were antibody positive and 46 (15.8%, 95% CI 11.8%, 20.4%) patients that were RNA positive were neither in the birth cohort nor had a history of IDU (Figure 4).
Figure 4: Antibody+ Results by Birth Year.
Dark gray bars represent those patients with a reactive test would have been missed with combined IDU-based and birth cohort testing compared with opt-out testing
Discussion
This report characterizes one of the earliest ED screening programs to employ nontargeted opt-out testing for HCV. We found that this model, aided by a decision support tool in the form of a BPA with a hard-stop, results in a high volume of screening in a population with high HCV seroprevalence. We identified a large number of cases, over 20% of which were new diagnoses. Further, we demonstrate that current guidance for testing only among those born 1945–1965 and those with known risk factors such as IDU would likely miss many cases of HCV in the ED.
We also identified challenges to ED testing in this site. Though screening rates were high in our program, we did not achieve universal screening, even among those undergoing phlebotomy, as the BPA didn’t fire on all patients undergoing phlebotomy, and only 40% of patients for whom the BPA fired underwent testing. When patients were identified as HCV-infected, it was difficult to contact them, despite having dedicated staff and resources for follow-up. Finally, even when staff successfully contacted a patient, loss to follow-up remained high.
Several findings of this study advance prior work done in Emergency Departments, and merit further discussion. First is the streamlined nature of the opt-out testing process that contributed to the high number of patients screened. In designing the program, we made key implementation decisions that impacted work flow: we employed information technology (IT) to incorporate the multi-purpose BPA into the EMR. This has been reported in the literature for HIV testing, but has not been a prominent feature in reported HCV programs.17 The specific BPA we employed functioned not only as a flag to alert the provider that the patient was eligible, but was actually a hard stop that the provider was forced to respond to, and we believe that impacted uptake in a positive way.
Additionally, we decided to screen only patients who were otherwise getting phlebotomy. That choice reflects a compromise between the desire for “near-universal” screening and the need to mitigate additional ED workflow burden. A large number of ED patients never had phlebotomy performed, and were unable to benefit from the program. However, patient throughput is of utmost priority to ED practitioners; utilizing IT capabilities to mitigate provider workload and piggybacking testing onto care that is already being provided, independent of screening, are essential to ensuring stakeholder support as well as ensuring uptake upon program implementation.
Third is the high percentage of Ab+ tests (97.8%) that had confirmatory RNA follow-up testing. This is due to reflex RNA test workflow that was instituted at the hospital laboratory prior to starting the ED HCV Screening program. This operational model is unusual but is becoming a best-practice.18 We believe this is the first manuscript reporting on an ED HCV screening program that employs reflex RNA testing. Nationwide, lack of RNA follow up is a point of significant drop off in the HCV treatment cascade, both in ED as well as ambulatory care settings, where rates range from 52%−82%.10,11,19–20. When it is possible to “reflex” Ab results to RNA and genotype, doing so will likely improve HCV follow-up and outcomes.
Fourth, this study adds substantial value to the HCV literature by reporting outcomes from screening outside targeted high-risk groups in an ED setting. This is the first ED program that we are aware of to screen in this manner. Had we employed only “birth cohort” testing, our program would have missed 268 cases (53%) of HCV. This finding mirrors the state and national trend, and previous ED seroprevalence studies, with increased HCV infection among persons aged 15–24.4,12,21 This increase was driven by the injection opiate epidemic in MA,22 which foreshadowed a trend nationally. IDU continues to be the most common risk factor identified by the CDC.23 Active persons who inject drugs are a particularly important target group for diagnostic and treatment interventions. Frequently new diagnoses of HCV in the birth cohort population represents patients infected in the distant past. However, people who inject drugs are actively transmitting the virus, making this group the primary driver of the increasing HCV epidemic.24
However, even if we had targeted the birth cohort and reported IDU history, we still would have missed a considerable percentage of Ab+ and RNA+ patients (14.7% and 15.8% respectively). White10 found 2.6% and Merchant25 found that half of newly diagnosed HCV would have been missed had they tested only patients currently targeted in national screening recommendations. These findings suggest that patients may not be comfortable disclosing risk factors, or may not be aware that prior risky behavior still puts them at risk for disease26. Future programs should consider implementing a screening model that tests patients outside of the disclosed high risk group.
Also worthy of further discussion are the challenges that our work reveals. First, despite effort to ensure that program workflow was integrated into general ED care, provider and patient uptake of this screening program was modest; the BPA did not fire on all patients undergoing phlebotomy, though the reasons it didn’t fire are impossible to determine given constraints in the information technology system. Additionally, only 38% of the BPA firing resulted in an HCV test. Screening program limitations precluded collecting data for patients who declined the test. However, the fact that almost 23% of the providers did not order any HCV screening test suggests that at least part of the failure was due to the providers’ failure to offer testing, rather than the patient failing to accept the test. Significant effort was spent prior to and during program initiation to familiarize staff with the program, and this remains an area we hope to continue to improve. Since reviewing this data, we have implanted a system in which the navigators go to the ED once/month to remind staff of and answer questions about the program. Even given these limitations of complete provider uptake, however, HCV screening rates still increased from 18 antibody tests to 1,269 antibody tests per month.
Another challenge that we faced was linking patients with HCV infection to HCV care. Ultimately, we successfully linked 30% of HCV RNA+ patients that were eligible for linkage at our institution to an outpatient HCV visit, which represents 22% of all HCV RNA+ patients. The person-hours required to achieve this modest success was high: 47% of patients that had appointments scheduled did so after more than 2 linkage attempts, and a significant portion of patients (34%) were never successfully contacted. These numbers are similar to other ED screening programs reported in the literature. An ED program at University of Alabama, Birmingham linked 30.8% and one at Highland Hospital reported 34.1% linkage.27 The ED population is notoriously difficult to access, and the subpopulation infected with HCV frequently have additional risk factors of drug-abuse that make engagement more difficult. We are continuing to improve processes to try to capture these patients. We recently created an EMR flag that automatically pages the patient care navigators when patients lost-to-follow-up re-present to the ED, but we do not yet have data on its efficacy in linking patients.
And though a 22% linkage rate may sound discouraging, the 66 patients that were ultimately linked through this program represent a segment of the population that had not been diagnosed/linked to care through other HCV testing venues. And though we don’t have data on treatment, other ED testing sites have demonstrated treatment rates comparable to rates for patients identified in other settings.27 There may be a rate of linkage to care below which it is not beneficial to implement HCV screening, however there is not acceptance in the scientific community at what this rate is.
Fourth, it is notable that almost 80% of the patients that were found to be HCV infected in our program had already been diagnosed with HCV, according to laboratory data at our institution or self-report. There are many possible reasons for this, including that prior to reflex RNA testing, many patients failed to get confirmatory testing after positive Ab screens. Additionally, prior to the development of direct acting anti-viral agents, the medications for HCV were not well tolerated, and many patients declined treatment. Finally, many HCV infected patients have other co-morbidities or life stressors that prohibit them from being able to engage with HCV care. Those patients have not been cured, and re-identifying those cases by our program provided a new opportunity to initiate navigational support to link these patients to care.
Limitations
Our program and its evaluation has limitations. First, this represents data from a single center in an inner city with a high underlying disease burden, and findings may not be similar at other institutions. In addition, the pre/post design for intervention evaluation does not include a control group and it is therefore not possible to formally test the causality of the ED testing program on testing rates. It is notable, however, that we appreciated a 6,950% increase in HCV testing in the ED from the pre-intervention to post-intervention periods. While secular trends and confounding remain a theoretical possibility, it is difficult to imagine a feasible secular trend or sampling bias that could result in such a large effect size. Because of IT limitations we don’t have complete information about why the BPA did not always fire, and we have not collected qualitative data about why the test was not ordered sometimes when the BPA did fire. The ED work-flow precluded informed consent for collecting research data that were not already be collected as part of routine care, we could not interview patients who declined HCV screening.
Conclusions
Our data demonstrate that it is possible to implement a successful ED HCV screening and linkage-to-care program in an inner city hospital. Doing so greatly increases the volume of testing and identifies many cases among patients outside of classic risk groups. Our data support implementing HCV testing in an Emergency Department for patients undergoing phlebotomy for clinical purposes, using reflex RNA testing, without attempt to target by risk factors. Doing so, however, will require inter-disciplinary collaboration, financial support, and a dedicated infrastructure focused on ensuring linkage to HCV care.
Acknowledgments
We wish to thank the navigation staff, including Katy Scrudder, Michael Silver, and Lindsey White for their assistance in the development and implementation of the ED HCV Screening Program. We also wish to thank the staff of the clinical laboratory, Laboratory Information Systems, and Information Technology Services for their efforts in support of this program
Funding Sources/Disclosures: Support for the ED HCV testing program was provided by a grant from Gilead Sciences, Inc.’s FOCUS Program
Benjamin Linas reports additional grant funding by Providence/Boston Center for AIDS Research and Center for Health Economics
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
References
- 1.CDC DVH - Hepatitis C Information For the Health Professional [Internet]. [cited 2012 Jul 10];Available from: http://www.cdc.gov/hepatitis/HCV/index.htm
- 2.Moyer VA. Screening for Hepatitis C Virus Infection in Adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2013;159(5):349–57. [DOI] [PubMed] [Google Scholar]
- 3.Barocas JA, Wang J, White LF, et al. Hepatitis C Testing Increased Among Baby Boomers Following The 2012 Change To CDC Testing Recommendations. Health Aff (Millwood) 2017;36(12):2142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Commentary | U.S. 2015 Surveillance Data for Viral Hepatitis | Statistics & Surveillance | Division of Viral Hepatitis | CDC [Internet]. 2017. [cited 2017 Nov 24];Available from: https://www.cdc.gov/hepatitis/statistics/2015surveillance/commentary.htm
- 5.Ward J Hepatitis/Liver Hepatitis C Virus: Gone by 2030? [Internet]. 2017;Available from: http://www.croiwebcasts.org/console/player/33550?mediaType=slideVideo&
- 6.National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables - 3c2185e6fd3bbba3a2a20a598687902eaf55.pdf [Internet]. [cited 2016 Nov 29];Available from: https://pdfs.semanticscholar.org/fe7b/3c2185e6fd3bbba3a2a20a598687902eaf55.pdf
- 7.Haukoos JS, Campbell JD, Conroy AA, et al. Programmatic Cost Evaluation of Nontargeted Opt-Out Rapid HIV Screening in the Emergency Department. PLOS ONE 2013;8(12):e81565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Policy Statement. Ann Emerg Med 2007;50(2):209. [DOI] [PubMed] [Google Scholar]
- 9.Liaw W, Petterson S, Rabin DL, Bazemore A. The Impact of Insurance and a Usual Source of Care on Emergency Department Use in the United States. Int J Fam Med [Internet] 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White DAE, Anderson ES, Pfeil SK, Trivedi TK, Alter HJ. Results of a Rapid Hepatitis C Virus Screening and Diagnostic Testing Program in an Urban Emergency Department. Ann Emerg Med 2016;67(1):119–28. [DOI] [PubMed] [Google Scholar]
- 11.Galbraith JW, Franco RA, Donnelly JP, et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology 2015;61(3):776–782 [DOI] [PubMed] [Google Scholar]
- 12.Hsieh Y-H, Rothman RE, Laeyendecker OB, et al. Evaluation of the Centers for Disease Control and Prevention Recommendations for Hepatitis C Virus Testing in an Urban Emergency Department. Clin Infect Dis 2016;62(9):1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison J, Walley AY, Feldman JA, et al. Identifying Patients for Overdose Prevention With ICD-9 Classification in the Emergency Department, Massachusetts, 2013–2014. Public Health Rep 2016;131(5):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger A, Rabenau H, Allwinn R, Doerr HW. Evaluation of the new ARCHITECT anti-HCV screening test under routine laboratory conditions. J Clin Virol 2008;43(2):158–61. [DOI] [PubMed] [Google Scholar]
- 15.Pas S, Molenkamp R, Schinkel J, et al. Performance Evaluation of the New Roche cobas AmpliPrep/cobas TaqMan HCV Test, Version 2.0, for Detection and Quantification of Hepatitis C Virus RNA. J Clin Microbiol 2013;51(1):238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciotti M, Marcuccilli F, Guenci T, et al. A multicenter evaluation of the Abbott RealTime HCV Genotype II assay. J Virol Methods 2010;167(2):205–7. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Mauntel-Medici C, Heinert S, Baghikar S. Harnessing the Power of the Electronic Medical Record to Facilitate an Opt-Out HIV Screening Program in an Urban Academic Emergency Department: J Public Health Manag Pract 2017;23(3):264–8. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Testing for HCV Infection: An Update of Guidance for Clinicians and Laboratorians. Morb Mortal Wkly Rep 2013;62:1–4. [PMC free article] [PubMed] [Google Scholar]
- 19.Blackburn NA, Patel RC, Zibbell JE. Improving Screening Methods for Hepatitis C Among People Who Inject Drugs: Findings from the HepTLC Initiative, 2012–2014. Public Health Rep 2016;131(Suppl 2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yehia BR, Schranz AJ, Umscheid CA, Lo Re V. The Treatment Cascade for Chronic Hepatitis C Virus Infection in the United States: A Systematic Review and Meta-Analysis. PLoS ONE 2014;9(7):e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyons MS, Kunnathur VA, Rouster SD, et al. Prevalence of Diagnosed and Undiagnosed Hepatitis C in a Midwestern Urban Emergency Department. Clin Infect Dis 2016;62(9):1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Hepatitis C Virus Infection Among Adolescents and Young Adults --- Massachusetts, 2002−-2009. Morb Mortal Wkly Rep 60(17):537–41. [PubMed] [Google Scholar]
- 23.Surveillance for Viral Hepatitis – United States, 2015. [Internet]. [cited 2017 Jul 24];Available from: https://www.cdc.gov/hepatitis/statistics/2015surveillance/commentary.htm
- 24.Williams IT, Bell BP, Kuhnert W, Alter MJ. Incidence and Transmission Patterns of Acute Hepatitis C in the United States, 1982–2006. Arch Intern Med 2011;171(3):242–8. [DOI] [PubMed] [Google Scholar]
- 25.Merchant RC, Baird JR, Liu T, Taylor LE. HCV among The Miriam Hospital and Rhode Island Hospital Adult ED Patients. R I Med J 2013 2014;97(7):35–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Galbraith JW. Hepatitis C Virus Screening: An Important Public Health Opportunity for United States Emergency Departments. Ann Emerg Med 2016;67(1):129–30. [DOI] [PubMed] [Google Scholar]
- 27.Anderson ES, Galbraith JW, Deering LJ, et al. Continuum of Care for Hepatitis C Virus Among Patients Diagnosed in the Emergency Department Setting. Clin Infect Dis 2017;64(11):1540–6. [DOI] [PubMed] [Google Scholar]