Endothelium-derived nitric oxide (NO) and endothelial cell (EC) function
One of the mechanisms ECs employ to maintain EC vascular homeostasis is releasing vasodilators such as NO1. Exposure of ECs to pathological insults disrupts NO production and NO bioavailability2 that lead to EC dysfunction; setting the ground for atherosclerotic plaque formation and other cardiovascular diseases3. In ECs, NO is mainly catalyzed by eNOS4. Reduced NO production can be attributed to impaired eNOS activity, as eNOS expression increases rather than decreases during EC dysfunction, presumably due to elevated levels of hydrogen peroxide, a dismutation product of O2.− 5. Reduced NO bioavailability can be due to decreased NO production and/or increased NOS degradation6.
Pathology of miR-199a dysregulation
miR-199a-5p and miR-199a-3p are produced from two arms of a single molecule of miR-199a precursor7. In ECs, miR-199a-5p acts as a potent regulator of angiogenesis8, 9. Inhibition of miR-199a-5p in human dermal microvascular ECs results in angiogenesis whereas induction of miR-199a-5p inhibits angiogenic response in Matrigel® culture. miR-199a-5p negatively regulates angiogenic responses by directly targeting v-ets erythroblastosis virus E26 oncogene homolog 1 (Ets-1), thereby regulating the Ets-1-MMP1 pathway9. Zeng et al. described the role of miR-199a-5p in pulmonary microvascular EC proliferation, as miR-199a-5p delivery suppresses cell proliferation whereas miR-199a-5p knockdown has an opposite effect10. In ECs infected with human cytomegalovirus, miR-199a-5p expression is upregulated, leading to the enhanced cell migration and tube formation via downregulation of the SIRT1/eNOS pathway11. miR-199a is also involved in Fabry disease development and EC dysfunction12, 13.
Role of miR-199a in NO production in ECs and NO bioavailability
In this issue of the Journal of ATVB, Joris et al nicely demonstrate that both miR-199a-3p and miR-199a-5p are abundantly expressed in human and bovine aortic ECs and that human and bovine miR sequences are identical. The authors used bio-informatics tools to identify VEGFA, Calcineurin, and SOD1 as potential miR-199a-3p/−5p targets. In BAECs treated with a miR inhibitor LNA, NO production increased by two-fold whereas expression of eNOS, DDAH1, PRMT1, and the endogenous competitive substrate for eNOS remained unchanged. In contrast, treatment of BAECs with non-selective NOS inhibitor L-NAME decreased NO production to the basal level while expression of eNOS regulators were unaffected. The authors reasoned that LNA-mediated miR-199a-3p/−5p inhibition promotes eNOS activity via Ser1177/Thr495 phosphorylation through upregulating the PI3K/Akt and calcineurin pathways and increases NO bioavailability by reducing O2-° levels via increasing SOD1 expression. LNA-mediated miR-199a-3p/−5p inhibition also promotes VEGF-mediated tube formation and improves vascular tone. To validate in vitro findings, they treated mice with antagomiRs, and noted increased NO-dependent relaxation and eNOS-S1177 phosphorylation in aortas, as well as a decreased O2-. level in aortic rings, that correlated with higher circulating Hb-NO in the venous blood along with greater expression of calcineurin and SOD1. These results suggest that inhibition of miR-199a-3p/−5p improves EC function. In the mouse model of angiotensin-mediated hypertension and cardiac hypertrophy, expression of both miR-199a-3p/−5p is induced in heart and aorta whereas only miR-199a-5p is induced in plasma14.
miR-199a in ECs: how much do we not know?
What has been known about miR biogenesis is that between the two RNA strands from the two arms of the DNA region, one strand is preferentially selected for entry into a silencing complex (mature, miR) whereas the other is degraded (immature, miR*). Lately, emerging evidence has revealed that some miRs* are functional and expressed abundantly15. Both mature forms derived from the precursor miR-199-a were identified in mice in 200316, and their expression was found in humans later7, 17. They were then renamed miR-199a-5p and miR-199a-3p7, 16, 18–21. The miR-199a precursor is highly conserved across species, suggesting that miR-199a-5p and miR-199a-3p possess important biological functions7. Whereas the role of miR-199a in cancer as well as in cardiomyocyte apoptosis have been studied extensively, its function in EC biology remains to be characterized. In the present study, Joris et al found that miR-199a-5p and miR-199a-3p are highly expressed in both human and bovine ECs. Their studies have revealed that miR-199a-5p and miR-199a-3p play independent roles in EC dysfunction via the regulation of NO production and bioavailability. Inhibition of miR-199a-3p and miR-199a-5p independently increases NO bioavailability via inducing eNOS enzymatic activity and reducing NO degradation. Consequently, VEGF-mediated tube formation by ECs is promoted, and contractile tone is improved.
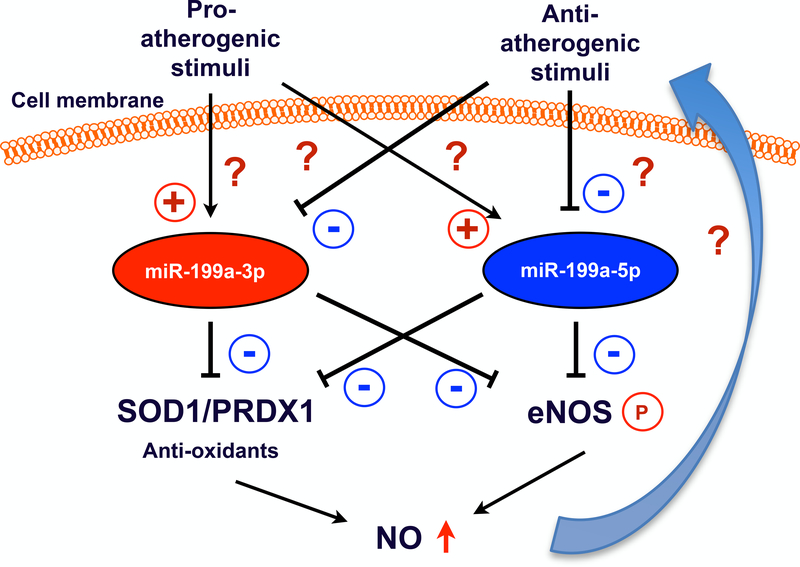
The present study has broadened the biological spectrum of miR-199a functions from cancer biology, cardiomyocyte apoptosis and heart failure to EC dysfunction and vascular homeostasis. Why and how important both mature forms derived from the same miR-199a precursor are required in the regulation of NO production and bioavailability is unknown. With the current knowledge on miR-199a function in ECs, further studies are necessary to gain deeper insights into pathological implications of miR-199a (Figure). Firstly, NO is produced when ECs are exposed to mechanical forces including hemodynamic shear stress and intraluminal pressure that trigger biochemical signaling involving multiple enzymes and mechanosensors, ultimately leading to eNOS activation22. Several mechanosensors are identified to initiate signal transduction in ECs such as mechanosensing ion channels, G-protein-coupled receptors, integrins-transmembrane receptors23–26. Therefore, it is plausible to examine potential roles for miR-199a-5p and miR-199a-3p in shear-induced NO production, and whether they are regulated by mechanosensors. Secondly, although miR-199a is encoded by two loci within two different introns on dynamin 2 and 3: miR-199a-1 is on chromosome 19 of dynamin 2 (intron 15) whereas miR-199a-2 is on chromosome 1 of Dynamin 3 (intron 14) but there is no reported data showing that the expression of miR-199a precursors is regulated by dynamin genes, indicating that miR-199a precursors can be regulated by their own promoters7. Additionally, it is well known that miR-targeting LNA has high affinity to the target sequence, but miR-targeting LNA treatment might also result in off-target effects including altering gene expression in neighbor tissues27. Hence, to determine molecular mechanisms by which the expression of miR-199a in ECs is regulated, as well as how miR-199a-5p and miR-199a-3p are differently regulated should provide us with a better approach in the prevention of their pathology. Thirdly, because circulating miRs can be detected in different fluid compartments such as blood, saliva, and urine, it is possible that miR-199a-5p and miR-199a-3p expression can be a harbinger of various coronary artery disease stages from subclinical atherosclerotic disease28. Thus, linking miR-199a-5p and miR-199a-3p to atherosclerotic disease burden as primarily diagnostic markers could help us to define their prognostic significance in coronary artery disease. Another limitation in the current study is the use of bovine aortic ECs, which lack miR-199a-3p, and therefore significantly limits our understanding of the functional difference between miR-199a-5p and miR-199a-3p.
Figure. miR-199a-3p and miR-199a-5p coordinately regulate NO bioavailability via inhibiting anti-oxidants expression and eNOS phosphorylation.
It remains unclear how miR-199a-3p and miR-199a-5p expression are modulated by pro-atherogenic and anti-atherogenic stimuli (including NO itself).
Acknowledgements
We thank Dr. Keigi Fujiwara for discussions and critical reading of the manuscript.
Sources of Funding
The research activities of us are supported by grant from the National Institute of Health (NIH) to Drs. Le (HL-134740) and Abe (HL-130193, HL-123346, and HL-118462).
Footnotes
Conflict of Interest Disclosures
None
References
- 1.Sandoo A, van Zanten JJ, Metsios GS, Carroll D and Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010;4:302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N and Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10:4–18. [DOI] [PubMed] [Google Scholar]
- 3.Mudau M, Genis A, Lochner A and Strijdom H. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr. 2012;23:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forstermann U and Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37, 837a-837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Z and Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin Med Res. 2006;4:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Vanhoutte PM and Leung SW. Vascular nitric oxide: Beyond eNOS. J Pharmacol Sci. 2015;129:83–94. [DOI] [PubMed] [Google Scholar]
- 7.Gu S and Chan WY. Flexible and versatile as a chameleon-sophisticated functions of microRNA-199a. Int J Mol Sci. 2012;13:8449–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai L, Lou W, Zhu J, Zhou X and Di W. MiR-199a inhibits the angiogenic potential of endometrial stromal cells under hypoxia by targeting HIF-1alpha/VEGF pathway. Int J Clin Exp Pathol. 2015;8:4735–44. [PMC free article] [PubMed] [Google Scholar]
- 9.Chan YC, Roy S, Huang Y, Khanna S and Sen CK. The microRNA miR-199a-5p down-regulation switches on wound angiogenesis by derepressing the v-ets erythroblastosis virus E26 oncogene homolog 1-matrix metalloproteinase-1 pathway. J Biol Chem. 2012;287:41032–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng L, Xing L, Zeng C, Wu T, Gui Y, Li W, Lan T, Yang Y, Gu Q, Qi C, Zhang Q, Tang F, He X and Wang L. Inactivation of PI3Kdelta induces vascular injury and promotes aneurysm development by upregulating the AP-1/MMP-12 pathway in macrophages. Arterioscler Thromb Vasc Biol. 2015;35:368–77. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Liu L, Wang R, Tuo H, Guo Y, Yi L, Wang J and Wang D. MiR-199a-5p promotes migration and tube formation of human cytomegalovirus-infected endothelial cells through downregulation of SIRT1 and eNOS. Arch Virol. 2013;158:2443–52. [DOI] [PubMed] [Google Scholar]
- 12.Cammarata G, Scalia S, Colomba P, Zizzo C, Pisani A, Riccio E, Montalbano M, Alessandro R, Giordano A and Duro G. A pilot study of circulating microRNAs as potential biomarkers of Fabry disease. Oncotarget. 2018;9:27333–27345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler J, Rot S, Bache M, Kappler M, Wurl P, Vordermark D, Taubert H and Greither T. miR-199a-5p regulates HIF-1alpha and OSGIN2 and its expression is correlated to soft-tissue sarcoma patients’ outcome. Oncol Lett. 2016;12:5281–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joris V, León-Gómez E, Menchi L, Lobysheva I, Di Mauro V, Esfahani H, Condorelli G, Balligand J-L, Catalucci D and Dessy C. MiR-199a-3p and miR-199a-5p take part to a redundant network of regulation of the NOS/NO pathway in the endothelium. Arterioscler Thromb Vasc Biol. 2018;in press. [DOI] [PubMed] [Google Scholar]
- 15.Guo L and Lu Z. The fate of miRNA* strand through evolutionary analysis: implication for degradation as merely carrier strand or potential regulatory molecule? PLoS One. 2010;5:e11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagos-Quintana M, Rauhut R, Meyer J, Borkhardt A and Tuschl T. New microRNAs from mouse and human. RNA. 2003;9:175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozomara A and Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozomara A and Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krichevsky AM and Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim LP, Glasner ME, Yekta S, Burge CB and Bartel DP. Vertebrate microRNA genes. Science. 2003;299:1540. [DOI] [PubMed] [Google Scholar]
- 21.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foa R, Schliwka J, Fuchs U, Novosel A, Muller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De Vita G, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M and Tuschl T. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sriram K, Laughlin JG, Rangamani P and Tartakovsky DM. Shear-Induced Nitric Oxide Production by Endothelial Cells. Biophys J. 2016;111:208–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker CF, Strop P, Bass RB, Hansen KC, Locher KP, Ren G, Yeager M, Rees DC and Kochendoerfer GG. Conversion of a mechanosensitive channel protein from a membrane-embedded to a water-soluble form by covalent modification with amphiphiles. J Mol Biol. 2004;343:747–58. [DOI] [PubMed] [Google Scholar]
- 24.Chachisvilis M, Zhang YL and Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci U S A. 2006;103:15463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shyy JY and Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–75. [DOI] [PubMed] [Google Scholar]
- 26.Johnson BD, Mather KJ and Wallace JP. Mechanotransduction of shear in the endothelium: basic studies and clinical implications. Vasc Med. 2011;16:365–77. [DOI] [PubMed] [Google Scholar]
- 27.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M and Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–9. [DOI] [PubMed] [Google Scholar]
- 28.Feinberg MW and Moore KJ. MicroRNA Regulation of Atherosclerosis. Circ Res. 2016;118:703–20. [DOI] [PMC free article] [PubMed] [Google Scholar]