Abstract
BACKGROUND
Pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT) is associated with improved overall survival (OS) in breast-cancer patients, but it is unclear how post-NACT response influences radiotherapy administration in patients presenting with node-positive disease. We sought to determine whether nodal pCR is associated with likelihood of receiving nodal radiation and whether radiotherapy among patients experiencing nodal pCR is associated with improved OS.
METHODS
cN1 female breast cancer patients diagnosed 2010–2015 who were ypN0 (i.e., nodal pCR, n=12,341) or ypN1 (i.e., residual disease, n=13,668) post-NACT were identified in the National Cancer Database. Multivariate logistic regression was used to identify factors associated with receiving radiotherapy. Cox proportional hazards modeling was used to estimate the association between radiotherapy and adjusted OS.
RESULTS
26,009 patients were included. 43.9% (n=5,423) of ypN0 and 55.3% (n=7,556) of ypN1 patients received nodal radiation. Rates of nodal radiation remained the same over time among ypN0 patients (trend test p=0.29) but increased among ypN1 patients from 49% in 2010 to 59% in 2015 (trend test p<0.001). After adjusting for covariates, nodal pCR (vs no stage change) was associated with decreased likelihood of nodal radiation after mastectomy (~20% decrease) and lumpectomy (~30% decrease, both p<0.01). After mastectomy, nodal (vs no) radiation conferred no significant survival benefit in ypN0 patients but approached significance for ypN1 patients (hazard ratio [HR] 0.83, 95% CI 0.69–0.99, p=0.04, overall p-value=0.11). After lumpectomy, nodal radiation was associated with improved adjusted OS for ypN0 (HR 0.38, 95% CI 0.22–0.66) and ypN1 patients (HR 0.44, 95% CI 0.30–0.66, both p<0.001), but this improvement was not significantly greater than that associated with breast-only radiation.
CONCLUSIONS
ypN0 patients were less likely to receive nodal radiation than ypN1 patients, suggesting that selective omission already occurs and, in the context of limited survival data, could potentially be appropriate for select patients.
Keywords: axilla, breast cancer, lymph nodes, neoadjuvant chemotherapy, pathologic complete response, radiation
Summary
Among breast-cancer patients presenting with cN1 disease, nodal pathologic complete response (ypN0) after neoadjuvant chemotherapy (NACT) was associated with decreased likelihood of post-mastectomy and post-lumpectomy nodal radiation (NRT). There was no survival benefit associated with nodal (vs no) radiation in ypN0 patients post-mastectomy, but benefit approached significance among ypN1 patients. NRT was associated with improved survival for ypN0 and ypN1 patients post-lumpectomy but this benefit was not significantly greater than that conferred by breast-only radiation.
Introduction
Pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT) is associated with improved survival in breast cancer patients, an association that persists even when pCR is limited to the breast or nodal compartments.1,2 But while pCR provides important prognostic information for those who experience it, it is unclear how response to NACT should be incorporated into decisions regarding adjuvant locoregional therapy.
The ongoing multi-institutional clinical trial NSABP B-51/RTOG 1304 was designed for the purpose of assessing whether adjuvant nodal radiation following lumpectomy or mastectomy might safely be omitted in patients with breast cancer who present with cN1 disease and achieve pCR in the axillary nodes (i.e., ypN0).3 The primary endpoint for this trial is locoregional recurrence, but overall survival is an important secondary outcome.
In routine clinical care, the National Comprehensive Cancer Network (NCCN) guidelines for adjuvant radiation in breast cancer patients recommend radiotherapy for all patients with any residual nodal disease after NACT and for radiation to be considered in cN1 patients who experience nodal pCR. But with increasing utilization of and improved response to NACT,4,5 it is unclear how improvements in post- NACT response might be influencing treatment decisions about radiation and what, if any, additional survival benefit might be conferred by administering radiation to patients who experience a significant response to preoperative systemic therapy.
Given the morbidity of locoregional axillary therapy (e.g., lymphedema following axillary radiation and lymph node dissection) and increased efforts to avoid overtreatment of the axilla, we sought to determine whether nodal pCR, i.e., eradication of disease in the axillary nodes following NACT, is associated with differential administration of adjuvant radiation. Specifically, we asked whether rates of post-NACT nodal radiation have changed over time with increasingly effective systemic therapy and whether differences in radiation receipt are associated with differences in overall survival among women with nodal pCR. We hypothesized that rates of nodal radiation will have declined over time with increasing rates of nodal pCR. We also hypothesized that patients who experienced nodal pCR (i.e., ypN0) would be less likely to receive adjuvant nodal radiation, even if it otherwise might have been indicated based on pre-NACT clinical stage. Finally, we hypothesized that non-receipt of radiation in patients achieving nodal pCR would be associated with no significant difference in short-term overall survival when compared to patients who achieved nodal pCR and did receive radiation.
Methods
Patient Cohort
Female patients≥ 18 years old diagnosed with cT1–3, cN1 invasive breast cancer between 2010 and 2015 and who received surgery after NACT were identified from the 2004–2015 National Cancer Data Base (NCDB) Participant User File. 2010 was selected as the beginning of our cohort date to reflect when HER2 coding became standardized in the NCDB, an important consideration given the high rates of pCR among HER2+ patients receiving preoperative systemic therapy.5,6 Our intent was to identify patients for whom adjuvant radiation would typically be recommended based on their clinical stage of disease at presentation but for whom there might be variable levels of receipt based on pathologic response to neoadjuvant systemic therapy. Accordingly, we limited our analysis of node-positive patients to those with cN1 disease who became either node-negative (ypN0) or continued to have a small amount of persistent nodal disease (ypN1) after NACT. Our rationale was that radiotherapy would typically be recommended for nearly all patients with significant nodal disease (cN2–3) at presentation regardless of response to chemotherapy,7 pathologic response to systemic therapy is anatomically more challenging to confirm in cN2–3 disease, and some discretion is still used in the administration of radiotherapy for pN1 disease regardless of systemic therapy sequence.8
Clinical node (cN) classification is defined in the NCDB according to pathologic diagnosis obtained via needle biopsy, imaging studies (excluding lymphoscintigraphy), and/or clinical examination demonstrating characteristics highly suspicious for malignancy. Patients with noninvasive disease (i.e., Stage 0 or ductal carcinoma in situ [DCIS]) at diagnosis or on post-NACT pathological review (i.e., ypTis); patients with inflammatory breast cancer on post-NACT pathological review (i.e., ypT4d); clinical or pathological stage M1 disease; no or unknown number of examined lymph nodes (LNs); a surgical procedure coded as “none,” “local tumor destruction only,” “not otherwise specified,” or “unknown”; and/or missing or incomplete radiation, clinical or pathologic stage, or surgical information were excluded. We also excluded patients who received neoadjuvant radiation or who had radiation administered to a site other than the ipsilateral breast, chest wall, and/or regional LNs. Although some definitions of pCR allow for the presence of residual DCIS, we chose to exclude patients with pathological stage ypTis. This strict definition of complete pCR allowed us to minimize heterogeneity in our primary endpoint, given emerging evidence that ypTis may be associated with worse long-term outcomes than ypT0.9
With regards to tumor biomarkers, hormone receptor-positive (HR+) was defined as estrogen receptor-positive (ER+) and/or progesterone receptor-positive (PR+) while hormone receptor-negative (HR-) was defined as estrogen receptor-negative (ER-) and progesterone receptor-negative (PR-). The cohort was divided into 3 subtypes based on combinations of HR and HER2 status: (1) HR+/HER2-, (2) HER2+ (including both HR+ and HR-), and (3) HR-/HER2-(i.e., triple-negative). pCR was defined as the absence of any residual invasive carcinoma or DCIS on pathologic review of a surgical specimen following NACT. Response to NACT was categorized as follows:
no stage change (i.e., cTN = ypTN);
breast downstage/node-positive (i.e., cT>ypT, ypN1)
breast-only pCR (i.e., ypT0, ypN1/N1mic);
discordant/nodal pCR (i.e., change from lower T to higher T classification [cT<ypT], ypN0);
no breast change/nodal pCR (i.e., no change in T classification [cT=ypT], ypN0);
breast downstage/nodal pCR (i.e., change from higher T to lower T classification [cT>ypT], ypN0);
overall pCR (i.e., ypT0N0);
upstage (i.e., a change from lower cT stage to higher ypT [cTN<ypTN], ypN1).
Statistical Analysis
Likelihood of receiving nodal radiation was our primary outcome. Because the NCDB does not capture information on recurrence or cause-specific mortality, overall survival was our secondary outcome.
Chi-square, analysis of variance (ANOVA), and Kruskal-Wallis tests, as appropriate, were used to assess differences in categorical and continuous variables, for which we report proportions and median values with interquartile ranges (IQRs). A two-sided Cochran-Armitage trend test was used to assess whether there were changes over time in rates of radiation receipt and rates of complete nodal response (i.e., ypN0). Logistic regression was used to estimate the association between post-NACT response and likelihood of receiving nodal radiation after adjustment for known, relevant patient, disease, and treatment characteristics including extent of axillary surgery (0–9 LNs removed vs ≥10 LNs removed, with the latter serving as a proxy for axillary lymph node dissection [ALND] in the absence of ALND data in the NCDB)10 and tumor subtype (HR+/HER2-, HER2+, and triple-negative). This model was conducted separately for mastectomy and lumpectomy recipients, was built in the generalized estimating equations framework, and accounted for the correlation of patients treated at the same facility by incorporating an exchangeable correlation structure. Odds ratios (ORs), 95% confidence intervals (CIs), and two-sided p-values are reported.
Overall survival (OS) was defined as the time from diagnosis to death or last follow-up. As required by NCDB guidelines, patients diagnosed in 2015 were excluded from survival analyses due to insufficient length of follow-up. Cox proportional hazards modeling was used to estimate the association between radiation receipt (no radiation, breast/chest-wall radiation only, breast/chest wall and nodal radiation), nodal response to NACT (ypN0, ypN1), and OS after adjustment for clinically relevant patient, disease, and treatment characteristics. Survival analyses were also conducted separately for ypN0 and ypN1 patients. A robust sandwich covariance estimator was included in all Cox models to account for the correlation of patients treated at the same facility. We report hazard ratios (HRs) and 95% CIs with two-tailed p-values.
P-value<0.05 was deemed significant for all analyses, and no adjustments were made for multiple comparisons. Only patients with complete data were included in each model. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) and R 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria). Our institutional review board granted this study exempt status due to use of de-identified patient data.
Results
Demographic, clinical, and treatment characteristics
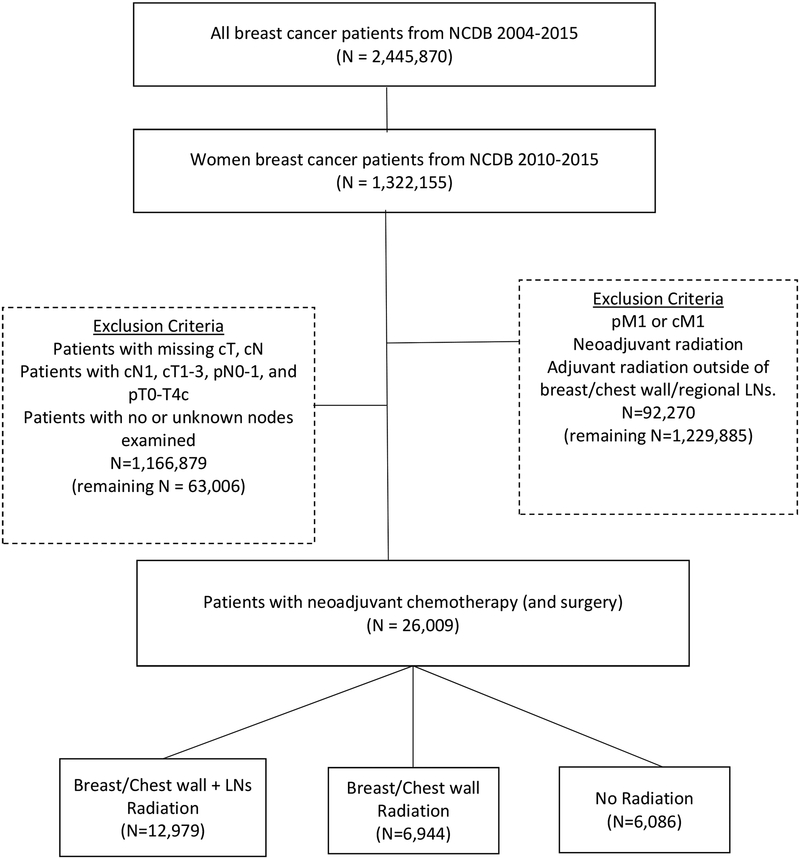
We identified 26,009 women who met inclusion criteria (median age 51, IQR 43–60, Table 1, Figure 1): 23.4% (n=6,086) received no radiation, 26.7% (n=6,944) received radiation to the breast/chest wall [BCWRT], and 49.9% [n=12,979]) received radiation to both the breast/chest wall and regional LNs [BCW+NRT]. Median length of follow-up was 40.1 months. There was no difference in race/ethnicity between patients receiving no radiation, BCWRT, or BCW+NRT. Patients living ≤50 miles from their treatment facility had higher rates of radiation than patients living >50 miles away (≤50 miles: 77.2% vs >50 miles: 70.4%, p<0.001).
Table 1.
Demographic Characteristics, cN1 Breast Cancer Patients who Underwent Surgery following Neoadjuvant Chemotherapy (NACT), National Cancer Data Base (NCDB), 2010–2015 (n=26,009)a,b
| All Patients | Breast/Chest Wall + Lymph Node Radiation | Breast/Chest Wall Radiation | No Radiation | ||
|---|---|---|---|---|---|
| N=26,009 (100%) | N=12,979 (49.9%) | N=6,944 (26.7%) | N=6,086 (23.4%) | P-Value | |
| Age Group | |||||
| 40–70 | 20,378 (78.3%) | 10,237 (78.9%) | 5,565 (80.1%) | 4,576 (75.2%) | <0.001 |
| <40 | 4,194 (16.1%) | 2,127 (16.4%) | 1,022 (14.7%) | 1,045 (17.2%) | |
| >70 | 1,437 (5.5%) | 615 (4.7%) | 357 (5.1%) | 465 (7.6%) | |
| Race/Ethnicity | |||||
| Hispanic | 2,151 (8.3%) | 1,065 (8.2%) | 549 (7.9%) | 537 (8.8%) | 0.07 |
| Non-Hispanic Black | 4,598 (17.7%) | 2,224 (17.1%) | 1,297 (18.7%) | 1,077 (17.7%) | |
| Non-Hispanic White | 17,153 (66%) | 8,635 (66.5%) | 4,545 (65.5%) | 3,973 (65.3%) | |
| Non-Hispanic Other | 1,368 (5.3%) | 694 (5.3%) | 350 (5%) | 324 (5.3%) | |
| Charlson/Deyo Comorbidity Score | |||||
| 0 | 22,920 (88.1%) | 11,457 (88.3%) | 6,172 (88.9%) | 5,291 (86.9%) | 0.01 |
| 1 | 2,603 (10%) | 1,284 (9.9%) | 658 (9.5%) | 661 (10.9%) | |
| ≥ 2 | 486 (1.9%) | 238 (1.8%) | 114 (1.6%) | 134 (2.2%) | |
| Local Income Level | |||||
| < 35,000 | 7,658 (29.4%) | 3,693 (28.5%) | 2,105 (30.3%) | 1,860 (30.6%) | 0.002 |
| ≥ 35,000 | 18,351 (70.6%) | 9,286 (71.5%) | 4,839 (69.7%) | 4,226 (69.4%) | |
| Insurance Status | |||||
| Private | 17,274 (66.4%) | 8,833 (68.1%) | 4,603 (66.3%) | 3,838 (63.1%) | <0.001 |
| Medicaid/Government | 3,491 (13.4%) | 1,692 (13%) | 940 (13.5%) | 859 (14.1%) | |
| Medicare | 3,971 (15.3%) | 1,820 (14%) | 1,067 (15.4%) | 1,084 (17.8%) | |
| Not Insured | 963 (3.7%) | 490 (3.8%) | 247 (3.6%) | 226 (3.7%) | |
| Local Education Level | |||||
| < 80% High School Graduation Rate | 10,815 (41.6%) | 5,544 (42.7%) | 2,874 (41.4%) | 2,397 (39.4%) | <0.001 |
| ≥ 80% High School Graduation Rate | 9,735 (37.4%) | 4,710 (36.3%) | 2,593 (37.3%) | 2,432 (40%) | |
| Facility Type | |||||
| Academic | 9,858 (37.9%) | 4,804 (37%) | 2,732 (39.3%) | 2,322 (38.2%) | <0.001 |
| Community | 1,871 (7.2%) | 917 (7.1%) | 506 (7.3%) | 448 (7.4%) | |
| Comprehensive | 10,744 (41.3%) | 5,345 (41.2%) | 2,840 (40.9%) | 2,559 (42%) | |
| Integrated Network | 3,515 (13.5%) | 1,902 (14.7%) | 862 (12.4%) | 751 (12.3%) | |
| Facility Location | |||||
| Midwest | 6,743 (25.9%) | 3,563 (27.5%) | 1,825 (26.3%) | 1,355 (22.3%) | <0.001 |
| Northeast | 4,661 (17.9%) | 2,432 (18.7%) | 1,254 (18.1%) | 975 (16%) | |
| South | 10,109 (38.9%) | 4,773 (36.8%) | 2,702 (38.9%) | 2,634 (43.3%) | |
| West | 4,132 (15.9%) | 2,063 (15.9%) | 1,070 (15.4%) | 999 (16.4%) | |
| Great circle distance (miles)c | |||||
| <10 | 13,090 (50.3%) | 6,734 (51.9%) | 3,504 (50.5%) | 2,852 (46.9%) | <0.001 |
| 10–50 | 10,643 (40.9%) | 5,292 (40.8%) | 2,786 (40.1%) | 2,565 (42.1%) | |
| >50 | 2,195 (8.4%) | 914 (7%) | 632 (9.1%) | 649 (10.7%) | |
Limited to patients with post-neoadjuvant chemotherapy nodal classification of ypN0 or ypN1.
Percentages represent column proportions for each variable except for the header row subgroups.
Distance between the patient’s residence (defined as the center of the patient’s zip code or the patient’s city if the zip code is not available) and the street address of the reporting/treating facility.
Figure 1.
cN1 Breast Cancer Patients who Underwent Surgery following Neoadjuvant Chemotherapy, National Cancer Data Base (NCDB), 2010–2015 (n=26,009)a
A majority of patients underwent mastectomy (63.6%), presented with cT2 primary tumors (56.6%), and had high-grade (57.1%) disease (Table 2). Patients receiving nodal radiation had a higher number of positive LNs (median number of positive nodes was 2 for BCW+NRT and 1 for BCWRT and no radiation) and a higher proportion of patients with extensive axillary surgery (≥10 LNs removed) vs patients who only received breast/chest wall radiation or no radiation at all (BCW+NRT: 52.6%, BCWRT: 48.9%, no radiation: 47.9%, both p<0.001). Over ¾ of patients received radiation of some kind (76.6%, n=19,923). Notably, 5.2% (n=1,355) of patients were recommended to undergo radiation treatment but never received it.
Table 2.
Clinical and Treatment Characteristics, cN1 Breast Cancer Patients who Underwent Surgery following Neoadjuvant Chemotherapy (NACT), National Cancer Data Base (NCDB), 2010–2015 (n=26,009)a,b
| All Patients | Breast/Chest Wall + Lymph Node Radiation | Breast/Chest Wall Radiation | No Radiation | ||
|---|---|---|---|---|---|
| N=26,009 (100%) | N=12,979 (49.9%) | N=6,944 (26.7%) | N=6,086 (23.4%) | P-Value | |
| Grade | |||||
| 1 | 1,075 (4.1%) | 537 (4.1%) | 275 (4%) | 263 (4.3%) | 0.01 |
| 2 | 8,180 (31.5%) | 4,206 (32.4%) | 2,166 (31.2%) | 1,808 (29.7%) | |
| 3 | 14,845 (57.1%) | 7,337 (56.5%) | 3,970 (57.2%) | 3,538 (58.1%) | |
| Unknown | 1,909 (7.3%) | 899 (6.9%) | 533 (7.7%) | 477 (7.8%) | |
| Histology | |||||
| Ductal | 22,715 (87.3%) | 11,226 (86.5%) | 6,155 (88.6%) | 5,334 (87.6%) | 0.01 |
| Lobular | 1,067 (4.1%) | 574 (4.4%) | 247 (3.6%) | 246 (4%) | |
| Other | 218 (0.8%) | 99 (0.8%) | 56 (0.8%) | 63 (1%) | |
| Tumor Subtype/Receptor Group | |||||
| HR+/HER2− | 10,411 (40%) | 5,576 (43%) | 2,743 (39.5%) | 2,092 (34.4%) | <0.001 |
| HER2+ | 8,946 (34.4%) | 4,274 (32.9%) | 2,372 (34.2%) | 2,300 (37.8%) | |
| TNBC | 6,281 (24.1%) | 3,002 (23.1%) | 1,718 (24.7%) | 1,561 (25.6%) | |
| Clinical T Classification | |||||
| cT1 | 4,794 (18.4%) | 2,151 (16.6%) | 1,330 (19.2%) | 1,313 (21.6%) | <0.001 |
| cT2 | 14,730 (56.6%) | 7,193 (55.4%) | 4,039 (58.2%) | 3,498 (57.5%) | |
| cT3 | 6,485 (24.9%) | 3,635 (28%) | 1,575 (22.7%) | 1,275 (20.9%) | |
| Pathological T Classification | |||||
| ypT0 | 6,641 (25.5%) | 3,048 (23.5%) | 1,793 (25.8%) | 1,800 (29.6%) | <0.001 |
| ypT1 | 12,122 (46.6%) | 6,084 (46.9%) | 3,335 (48%) | 2,703 (44.4%) | |
| ypT2 | 5,673 (21.8%) | 2,980 (23%) | 1,437 (20.7%) | 1,256 (20.6%) | |
| ypT3 | 1,471 (5.7%) | 821 (6.3%) | 353 (5.1%) | 297 (4.9%) | |
| ypT4 | 102 (0.4%) | 46 (0.4%) | 26 (0.4%) | 30 (0.5%) | |
| Pathological N Classification | |||||
| ypN0 | 12,341 (47.4%) | 5,423 (41.8%) | 3,531 (50.8%) | 3,387 (55.7%) | <0.001 |
| ypN1 | 13,668 (52.6%) | 7,556 (58.2%) | 3,413 (49.2%) | 2,699 (44.3%) | |
| Response to NACT | |||||
| Breast-only pCR | 1,070 (4.1%) | 584 (4.5%) | 261 (3.8%) | 225 (3.7%) | <0.001 |
| Discordant/Nodal pCR | 150 (0.6%) | 65 (0.5%) | 51 (0.7%) | 34 (0.6%) | |
| Breast Downstage/Nodal pCR | 4,735 (18.2%) | 2,153 (16.6%) | 1,391 (20%) | 1,191 (19.6%) | |
| Breast Downstage/Node-positive | 6,009 (23.1%) | 3,547 (27.3%) | 1,466 (21.1%) | 996 (16.4%) | |
| No stage change | 5,864 (22.5%) | 3,031 (23.4%) | 1,516 (21.8%) | 1,317 (21.6%) | |
| No Breast Change/Nodal pCR | 1,885 (7.2%) | 741 (5.7%) | 557 (8%) | 587 (9.6%) | |
| Overall pCR | 5,571 (21.4%) | 2,464 (19%) | 1,532 (22.1%) | 1,575 (25.9%) | |
| Upstage | 725 (2.8%) | 394 (3%) | 170 (2.4%) | 161 (2.6%) | |
| Type of Breast Surgery | |||||
| Lumpectomy | 9,474 (36.4%) | 4,829 (37.2%) | 3,930 (56.6%) | 715 (11.7%) | <0.001 |
| Mastectomy | 16,535 (63.6%) | 8,150 (62.8%) | 3,014 (43.4%) | 5,371 (88.3%) | |
| Breast Surgery Type*ypN classification | |||||
| Lumpectomy-ypN0 | 4,842 (18.6%) | 2,246 (17.3%) | 2,215 (31.9%) | 381 (6.3%) | <0.001 |
| Lumpectomy-ypN 1 | 4,632 (17.8%) | 2,583 (19.9%) | 1,715 (24.7%) | 334 (5.5%) | |
| Mastectomy-ypN0 | 7,499 (28.8%) | 3,177 (24.5%) | 1,316 (18.9%) | 3,006 (49.4%) | |
| Mastectomy-ypN 1 | 9,036 (34.7%) | 4,973 (38.3%) | 1,698 (24.4%) | 2,365 (38.9%) | |
| Extent of Axillary Surgery (i.e., number of LNs removed and examined) | |||||
| 1–9 LNs | 12,874 (49.5%) | 6,154 (47.4%) | 3,549 (51.1%) | 3,171 (52.1%) | <0.001 |
| ≥ 10 LNs | 13,135 (50.5%) | 6,825 (52.6%) | 3,395 (48.9%) | 2,915 (47.9%) | |
| Positive Lymph Nodes [LNs] | |||||
| 1 (1 – 3) | 2 (1 – 3) | 1 (1 – 2) | 1 (1 – 2) | <0.001 | |
| Endocrine Therapy | |||||
| Adjuvant Endocrine Therapy Only | 12,813 (49.3%) | 7,093 (54.6%) | 3,551 (51.1%) | 2,169 (35.6%) | <0.001 |
| Neoadjuvant Endocrine Therapy | 1,525 (5.9%) | 649 (5%) | 385 (5.5%) | 491 (8.1%) | |
| No Endocrine | 10,671 (41%) | 4,826 (37.2%) | 2,766 (39.8%) | 3,079 (50.6%) | |
Limited to patients with post-neoadjuvant chemotherapy nodal classification of ypN0 or ypN1.
Percentages represent column proportions for each variable except for the header row subgroups.
Response to NACT and radiation receipt over time
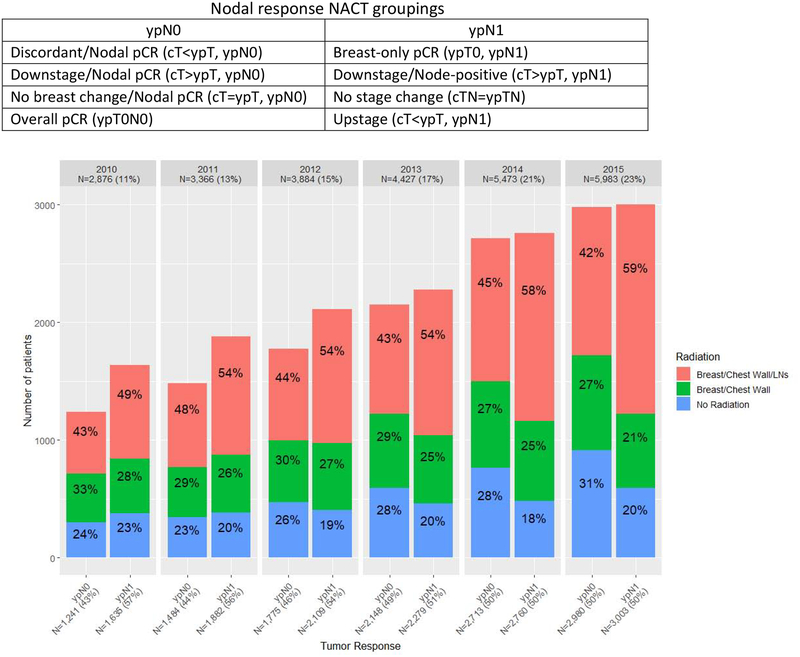
Between 2010 and 2015, rates of nodal pCR increased significantly from 43% in 2010 to 50% in 2015 (trend test p<0.001, Figure 2), but over the entire period, more than half of patients in our cohort still had residual nodal disease after NACT (i.e., ypN1, 52.6%, n=13,668, Table 2). Among patients who had nodal pCR (i.e., ypN0, 47.4%, n=12,341), 45.1% (n=5,571) had overall pCR (ypTN0), 15.2% (n=1,885) had no change in the breast, 34.6% (n=4,735) had downstage in the breast, and 1.2% (n=150) had upstage in the breast (i.e., a discordant response).
Figure 2.
Radiation Receipt by Response to Neoadjuvant Chemotherapy (NACT), cN1 Breast Cancer Patients who Underwent Surgery post‐NACT, National Cancer Data Base (NCDB), 2010‐2015 (n=26,009)a
a Limited to patients with post‐neoadjuvant chemotherapy nodal classification of ypN0 or ypN1. Two‐sided Cochran‐Armitage trend tests: (1) rates of nodal pCR (ypN0) after NACT, p<0.001, (2) rates of nodal radiation for ypN0 patients, p=0.29, (3) rates of nodal radiation for ypN1 patients, p<0.001.
Over time, rates of BCW+NRT did not change in ypN0 patients (trend test p=0.29), while among patients with residual nodal disease, rates of BCW+NRT increased significantly, from 49% in 2010 to 59% in 2015 (trend test p<0.001). Nevertheless, only 55.2% (n=7,556) of all ypN1 patients received BCW+NRT and nearly 1/5 of ypN1 patients (19.7%, n=2,699) received no radiation of any kind.
Effect of post-NACT response on receipt of nodal radiation
After mastectomy
After adjusting for known covariates and compared with having no stage change in the breast or LNs, having nodal pCR with no change in the breast (cT=ypT OR 0.76, 95% CI 0.62–0.93) or with breast downstage (cT>ypT OR 0.83, 95% CI 0.71–0.97) was associated with decreased likelihood of nodal radiation among patients who underwent mastectomy (p=0.003, Table 3). Living >50 miles away from treatment (vs <10 miles) was also associated with lower likelihood of receiving nodal radiation (OR 0.75, 95% CI 0.63–0.89, p=0.007). Having higher grade disease, receiving care within an Integrated Network Cancer Center (vs an Academic Cancer Center), and being diagnosed in 2015 (vs 2010) were all independently associated with an increased likelihood of receiving nodal radiation (all p<0.05).
Table 3.
Likelihood of Receiving Nodal Radiation after Neoadjuvant Chemotherapy (NACT) and Surgery, cN1 Breast Cancer Patients, National Cancer Data Base (NCDB), 2010–2015
| Mastectomy (N=8,793) | Lumpectomy (N=7,201) | |||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-Value | Overall P-Value | OR (95% CI) | P-Value | Overall P-Value | |
| Age group | ||||||
| <40 | -REF- | 0.76 | -REF- | 0.69 | ||
| 40–70 | 1.03 (0.92 – 1.16) | 0.57 | 1.07 (0.9 – 1.28) | 0.42 | ||
| >70 | 1.09 (0.84 – 1.41) | 0.52 | 1.04 (0.78 – 1.38) | 0.79 | ||
| Response | ||||||
| No stage change | -REF- | 0.003 | -REF- | <0.001 | ||
| Breast-only pCR | 1.19 (0.92 – 1.53) | 0.18 | 1.05 (0.83 – 1.35) | 0.67 | ||
| Discordant/Nodal pCR | 0.72 (0.41 – 1.25) | 0.24 | 0.38 (0.15 – 0.95) | 0.04 | ||
| Breast Downstage/Nodal pCR | 0.83 (0.71 – 0.97) | 0.02 | 0.69 (0.58 – 0.81) | <0.001 | ||
| Breast Downstage/Node-positive | 1.06 (0.92 – 1.22) | 0.43 | 1.23 (1.05 – 1.43) | 0.01 | ||
| No Breast Change/Nodal pCR | 0.76 (0.62 – 0.93) | 0.008 | 0.66 (0.54 – 0.8) | <0.001 | ||
| Overall pCR | 0.91 (0.78 – 1.07) | 0.26 | 0.8 (0.68 – 0.93) | 0.005 | ||
| Upstage | 1.1 (0.84 – 1.45) | 0.50 | 1.32 (0.92 – 1.9) | 0.13 | ||
| Race/Ethnicity | ||||||
| Non-Hispanic White | -REF- | 0.74 | -REF- | 0.57 | ||
| Hispanic | 1 (0.84 – 1.18) | 0.99 | 1.11 (0.93 – 1.33) | 0.25 | ||
| Non-Hispanic Black | 1.06 (0.93 – 1.21) | 0.40 | 1.04 (0.91 – 1.19) | 0.53 | ||
| Non-Hispanic Other | 1.08 (0.89 – 1.3) | 0.43 | 1.09 (0.89 – 1.34) | 0.38 | ||
| Charlson/Deyo Comorbkity Score | ||||||
| 0 | -REF- | 0.36 | -REF- | 0.58 | ||
| 1 | 0.96 (0.81 – 1.12) | 0.59 | 1.08 (0.94 – 1.24) | 0.30 | ||
| ≥ 2 | 1.29 (0.86 – 1.93) | 0.23 | 1.04 (0.74 – 1.45) | 0.83 | ||
| Insurance Status | ||||||
| Private | -REF- | 0.73 | -REF- | 0.63 | ||
| Medicaid/Government | 0.97 (0.84 – 1.12) | 0.69 | 0.92 (0.8 – 1.06) | 0.25 | ||
| Medicare | 0.96 (0.82 – 1.12) | 0.60 | 0.94 (0.82 – 1.08) | 0.37 | ||
| Not Insured | 1.13 (0.85 – 1.51) | 0.40 | 0.99 (0.77 – 1.27) | 0.93 | ||
| Grade | ||||||
| 1 | -REF- | 0.02 | -REF- | 0.67 | ||
| 2 | 1.26 (1.01 – 1.58) | 0.04 | 1.08 (0.84 – 1.39) | 0.55 | ||
| 3 | 1.38 (1.1 – 1.73) | 0.006 | 1.11 (0.86 – 1.43) | 0.41 | ||
| Facility Type | ||||||
| Academic | -REF- | 0.003 | -REF- | 0.43 | ||
| Community | 1.01 (0.78 – 1.31) | 0.95 | 1.17 (0.91 – 1.49) | 0.22 | ||
| Comprehensive | 1.14 (0.95 – 1.38) | 0.16 | 1.08 (0.91 – 1.29) | 0.35 | ||
| Integrated Network | 1.55 (1.22 – 1.98) | <0.001 | 1.17 (0.95 – 1.44) | 0.14 | ||
| Facility Location | ||||||
| West | -REF- | 0.03 | -REF- | 0.87 | ||
| Midwest | 1.18 (0.92 – 1.51) | 0.19 | 1 (0.8 – 1.25) | 0.99 | ||
| Northeast | 1.17 (0.9 – 1.54) | 0.24 | 0.98 (0.77 – 1.25) | 0.89 | ||
| South | 0.9 (0.71 – 1.14) | 0.39 | 0.93 (0.75 – 1.16) | 0.53 | ||
| Great circle distance (miles) | ||||||
| <10 | -REF- | 0.007 | -REF- | 0.001 | ||
| 10–50 | 0.95 (0.86 – 1.05) | 0.31 | 1.01 (0.9 – 1.12) | 0.92 | ||
| >50 | 0.75 (0.63 – 0.89) | 0.001 | 0.71 (0.6 – 0.85) | <0.001 | ||
| Extent of Axillary Surgery (i.e., number of LNs removed and examined) | ||||||
| ≥ 10 LNs | -REF- | 0.41 | -REF- | 0.61 | ||
| 1–9 LNs | 0.96 (0.87 – 1.06) | 0.40 | 0.97 (0.88 – 1.08) | 0.61 | ||
| Histology | ||||||
| Ductal | -REF- | 0.28 | -REF- | 0.84 | ||
| Lobular | 1.17 (0.96 – 1.44) | 0.12 | 0.93 (0.69 – 1.24) | 0.61 | ||
| Other | 0.94 (0.6 – 1.48) | 0.79 | 1.09 (0.61 – 1.95) | 0.78 | ||
| cT | ||||||
| cT1 | -REF- | 0.51 | -REF- | 0.007 | ||
| cT2 | 1.08 (0.95 – 1.23) | 0.24 | 0.93 (0.82 – 1.07) | 0.31 | ||
| cT3 | 1.06 (0.91 – 1.25) | 0.45 | 1.17 (0.97 – 1.4) | 0.10 | ||
| Tumor Subtype/Receptor Group | ||||||
| HR+/HER2− | -REF- | 0.13 | -REF- | 0.004 | ||
| HER2+ | 1.04 (0.93 – 1.17) | 0.49 | 0.84 (0.75 – 0.94) | 0.002 | ||
| TNBC | 0.91 (0.8 – 1.04) | 0.17 | 0.97 (0.86 – 1.09) | 0.59 | ||
| Year of Diagnosis | ||||||
| 2010 | -REF- | 0.004 | -REF- | <0.001 | ||
| 2011 | 1.26 (1.04 – 1.51) | 0.01 | 1.15 (0.94 – 1.4) | 0.17 | ||
| 2012 | 1.08 (0.9 – 1.3) | 0.42 | 1.2 (0.97 – 1.48) | 0.09 | ||
| 2013 | 1.16 (0.95 – 1.41) | 0.15 | 1.15 (0.94 – 1.41) | 0.17 | ||
| 2014 | 1.31 (1.09 – 1.57) | 0.004 | 1.41 (1.16 – 1.72) | <0.001 | ||
| 2015 | 1.41 (1.16 – 1.72) | <0.001 | 1.55 (1.27 – 1.89) | <0.001 | ||
The model has accounted for the correlation of patients treated at the same hospital.
BCWRT, breast/chest-wall radiation. BCWN+RT, breast/chest-wall + nodal radiation.
After lumpectomy
After adjusting for covariates, having any form of nodal pCR – even when paired with upstage in the breast – was associated with a decreased likelihood of receiving post-lumpectomy nodal radiation (all p<0.001, Table 3) when compared with having no stage change. Having downstage in the breast but persistent nodal disease was associated with an increased likelihood of receiving nodal radiation (BCW+NRT OR 1.23, 95% CI 1.05–1.43, p<0.001, Table 3). As with mastectomy patients, living >50 miles away from treatment (vs <10 miles) was associated with lower likelihood of receiving nodal radiation (OR 0.71, 95% CI 0.60–0.85) as was being HER2+ (vs HR+/HER2-, OR 0.84, 95% CI 0.75–0.94, both p<0.01). Given high rates of nodal pCR in HER2+ patients,11 we examined the interaction of Response to NACT*Tumor Subtype and found it was not significant. Higher cT classification and later year of diagnosis were both associated with increased likelihood of receiving BCW+NRT (both p<0.01).
Overall survival
After mastectomy
After adjusting for known covariates, there was no survival benefit conferred by receipt of either BCWRT or BCW+NRT (vs no radiation, p=0.20, Supplemental Table 1). Experiencing nodal pCR was associated with improved OS as compared to having residual nodal disease (HR 0.43, 95% CI 0.37 – 0.49, p<0.001) but when we tested for an interaction between receipt of radiation and nodal response to NACT, it was insignificant, indicating that the effect of radiation on adjusted OS did not vary by nodal response.
When we stratified the adjusted analysis by post-NACT nodal status, there continued to be no survival benefit associated with receipt of any form of post-mastectomy radiation (vs no radiation) in ypN0 patients, but benefit approached significance among ypN1 patients receiving BCW+NRT (vs no radiation: HR 0.83, 95% CI 0.69–0.99, p=0.04, Type III/overall p-value=0.11, Table 4a). For both ypN0 and ypN1 patients, having fewer LNs removed and examined, triple-negative tumor subtype (TNBC vs HR+/HER2-), and higher cT stage were all associated with worse adjusted OS (all p<0.05).
Table 4.
Adjusted Overall Survival, cN1 Breast Cancer Patients following Neoadjuvant Chemotherapy, Stratified by Nodal Response, National Cancer Data Base (NCDB), 2010–2014
| a. Mastectomy recipients | ||||||
|---|---|---|---|---|---|---|
| ypN0 (N=4,577, Events=312) | ypN1 (N=5,561, Events=747) | |||||
| HR (95% CI) | P-Value | Overall P-Value | HR (95% CI) | P-Value | Overall P-Value | |
| Age group | ||||||
| <40 | -REF- | 0.01 | -REF- | 0.40 | ||
| 40–70 | 1.47 (1.07 – 2.02) | 0.02 | 1.04 (0.85 – 1.27) | 0.71 | ||
| >70 | 2.36 (1.29 – 4.32) | 0.005 | 1.28 (0.89 – 1.84) | 0.19 | ||
| Radiation | ||||||
| No Radiation | -REF- | 0.41 | -REF- | 0.11 | ||
| BCW+NRT | 1.01 (0.79 – 1.29) | 0.94 | 0.83 (0.69 – 0.99) | 0.04 | ||
| BCWRT | 0.82 (0.59 – 1.14) | 0.24 | 0.92 (0.73 – 1.15) | 0.46 | ||
| Race/Ethnicity | ||||||
| Non-Hispanic White | -REF- | 0.13 | -REF- | 0.07 | ||
| Hispanic | 0.65 (0.39 – 1.08) | 0.10 | 0.78 (0.58 – 1.06) | 0.12 | ||
| Non-Hispanic Black | 1.09 (0.81 – 1.48) | 0.57 | 1.20 (0.99 – 1.46) | 0.06 | ||
| Non-Hispanic Other | 0.61 (0.32 – 1.17) | 0.14 | 0.88 (0.58 – 1.32) | 0.53 | ||
| Charlson/Deyo Comorbkity Score | ||||||
| 0 | -REF- | 0.33 | -REF- | 0.41 | ||
| 1 | 1.13 (0.8 – 1.6) | 0.50 | 0.88 (0.69 – 1.12) | 0.31 | ||
| ≥ 2 | 1.67 (0.8 – 3.46) | 0.17 | 1.23 (0.77 – 1.97) | 0.39 | ||
| Insurance Status | ||||||
| Private | -REF- | 0.25 | -REF- | 0.008 | ||
| Medicaid/Government | 1.28 (0.91 – 1.79) | 0.16 | 1.27 (1.04 – 1.56) | 0.02 | ||
| Medicare | 1.35 (0.94 – 1.94) | 0.11 | 1.43 (1.13 – 1.80) | 0.003 | ||
| Not Insured | 1.26 (0.75 – 2.13) | 0.38 | 1.27 (0.84 – 1.92) | 0.25 | ||
| Grade | ||||||
| 1 | -REF- | 0.07 | -REF- | <0.001 | ||
| 2 | 2.97 (0.94 – 9.34) | 0.06 | 1.54 (0.99 – 2.39) | 0.055 | ||
| 3 | 3.44 (1.12 – 10.51) | 0.03 | 2.82 (1.79 – 4.42) | <0.001 | ||
| Facility Type | ||||||
| Academic | -REF- | 0.74 | -REF- | 0.13 | ||
| Community | 1.3 (0.8 – 2.11) | 0.29 | 0.76 (0.54 – 1.06) | 0.10 | ||
| Comprehensive | 1.08 (0.83 – 1.41) | 0.58 | 1.08 (0.91 – 1.29) | 0.36 | ||
| Integrated Network | 1.01 (0.72 – 1.43) | 0.94 | 0.96 (0.75 – 1.23) | 0.76 | ||
| Facility Location | ||||||
| West | -REF- | 0.40 | -REF- | 0.23 | ||
| Midwest | 1 (0.69 – 1.44) | 0.98 | 1.2 (0.95 – 1.52) | 0.13 | ||
| Northeast | 1.1 (0.72 – 1.68) | 0.67 | 1.26 (0.99 – 1.61) | 0.06 | ||
| South | 0.84 (0.6 – 1.18) | 0.32 | 1.09 (0.88 – 1.35) | 0.41 | ||
| Extent of Axillary Surgery (i.e., number of LNs removed and examined) | ||||||
| ≥ 10 LNs | -REF- | 0.003 | -REF- | 0.046 | ||
| 1–9 LNs | 1.44 (1.14 – 1.82) | 0.003 | 1.16 (1 – 1.35) | 0.046 | ||
| Histology | ||||||
| Ductal | -REF- | 0.03 | -REF- | 0.27 | ||
| Lobular | 1.55 (0.87 – 2.73) | 0.13 | 1.13 (0.82 – 1.56) | 0.46 | ||
| Other | 2.18 (1.07 – 4.46) | 0.03 | 1.53 (0.84 – 2.77) | 0.16 | ||
| cT | ||||||
| cT1 | -REF- | <0.001 | -REF- | <0.001 | ||
| cT2 | 0.8 (0.56 – 1.15) | 0.23 | 0.75 (0.60 – 0.93) | 0.01 | ||
| cT3 | 1.32 (0.92 – 1.88) | 0.13 | 2.59 (2.14 – 3.13) | <0.001 | ||
| Tumor Subtype/Receptor Group | ||||||
| HR+/HER2− | -REF- | <0.001 | -REF- | <0.001 | ||
| HER2+ | 0.58 (0.43 – 0.78) | <0.001 | 1.24 (0.98 – 1.58) | 0.07 | ||
| TNBC | 1.45 (1.1 – 1.91) | 0.008 | 1.73 (1.36 – 2.20) | <0.001 | ||
| b. Lumpectomy recipients | ||||||
| ypN0 (N=2,906, Events=158) | ypN1 (N=2,858, Events=295) | |||||
| HR (95% CI) | P-Value | Overall P-Value | HR (95% CI) | P-Value | Overall P-Value | |
| Age group | ||||||
| <40 | -REF- | 0.77 | -REF- | 0.27 | ||
| 40–70 | 1.13 (0.65 – 1.98) | 0.67 | 0.83 (0.55 – 1.25) | 0.38 | ||
| >70 | 0.89 (0.33 – 2.39) | 0.81 | 1.13 (0.61 – 2.1) | 0.70 | ||
| Radiation | ||||||
| No Radiation | -REF- | <0.001 | -REF- | <0.001 | ||
| BCW+NRT | 0.38 (0.22 – 0.66) | <0.001 | 0.44 (0.3 – 0.66) | <0.001 | ||
| BCWRT | 0.32 (0.18 – 0.57) | <0.001 | 0.35 (0.23 – 0.54) | <0.001 | ||
| Race/Ethnicity | ||||||
| Non-Hispanic White | * | -REF- | 0.05 | |||
| Hispanic | 0.72 (0.42 – 1.24) | 0.24 | ||||
| Non-Hispanic Black | 1.32 (1 – 1.73) | 0.05 | ||||
| Non-Hispanic Other | 0.66 (0.33 – 1.3) | 0.22 | ||||
| Charlson/Deyo Comorbkity Score | ||||||
| 0 | -REF- | 0.67 | -REF- | 0.003 | ||
| 1 | 1.23 (0.74 – 2.04) | 0.42 | 1.52 (1.1 – 2.09) | 0.01 | ||
| ≥ 2 | 0.79 (0.23 – 2.79) | 0.72 | 2.22 (1.2 – 4.13) | 0.01 | ||
| Insurance Status | ||||||
| Private | -REF- | 0.26 | -REF- | <0.001 | ||
| Medicaid/Government | 1.21 (0.76 – 1.91) | 0.43 | 1.65 (1.18 – 2.31) | 0.004 | ||
| Medicare | 1.28 (0.79 – 2.08) | 0.31 | 1.87 (1.37 – 2.55) | <0.001 | ||
| Not Insured | 1.78 (0.93 – 3.42) | 0.08 | 1.42 (0.79 – 2.57) | 0.24 | ||
| Grade | ||||||
| 1 | -REF- | 0.38 | -REF- | <0.001 | ||
| 2 | 3.87 (0.5 – 29.84) | 0.19 | 1.1 (0.52 – 2.34) | 0.80 | ||
| 3 | 3.4 (0.44 – 26.06) | 0.24 | 2.28 (1.06 – 4.91) | 0.03 | ||
| Facility Type | ||||||
| Academic | * | -REF- | 0.87 | |||
| Community | 0.87 (0.53 – 1.45) | 0.60 | ||||
| Comprehensive | 0.89 (0.68 – 1.19) | 0.44 | ||||
| Integrated Network | 0.95 (0.62 – 1.44) | 0.81 | ||||
| Facility Location | ||||||
| West | * | -REF- | 0.19 | |||
| Midwest | 0.98 (0.63 – 1.52) | 0.93 | ||||
| Northeast | 1.22 (0.83 – 1.81) | 0.31 | ||||
| South | 1.37 (0.93 – 2.03) | 0.11 | ||||
| Extent of Axillary Surgery (i.e., number of LNs removed and examined) | ||||||
| ≥ 10 LNs | * | -REF- | 0.64 | |||
| 1–9 LNs | 0.94 (0.74 – 1.20) | 0.64 | ||||
| Histology | ||||||
| Ductal | -REF- | 0.80 | -REF- | 0.35 | ||
| Lobular | 1.19 (0.26 – 5.48) | 0.82 | 0.96 (0.38 – 2.47) | 0.94 | ||
| Other | 0.54 (0.08 – 3.75) | 0.53 | 2.01 (0.78 – 5.17) | 0.15 | ||
| cT | ||||||
| cT1 | -REF- | 0.22 | -REF- | 0.007 | ||
| cT2 | 1.37 (0.86 – 2.17) | 0.19 | 1.59 (1.15 – 2.19) | 0.005 | ||
| cT3 | 1.61 (0.94 – 2.78) | 0.08 | 1.80 (1.21 – 2.67) | 0.003 | ||
| Tumor Subtype/Receptor Group | ||||||
| HR+/HER2− | -REF- | <0.001 | -REF- | <0.001 | ||
| HER2+ | 0.68 (0.44 – 1.04) | 0.07 | 0.59 (0.41 – 0.84) | 0.004 | ||
| TNBC | 1.5 (0.99 – 2.28) | 0.06 | 1.82 (1.40 – 2.37) | <0.001 | ||
BCWRT, breast/chest-wall radiation. BCWN+RT, breast/chest-wall + nodal radiation.
Not adjusted for in lumpectomy group due to potential overfitting caused by insufficient number of events.
As required by NCDB guidelines, patients diagnosed in 2015 were excluded from survival analyses due to insufficient length of follow-up. The models have accounted for the correlation of patients treated at the same hospital.
After lumpectomy
As with mastectomy, nodal pCR was associated with improved adjusted OS (HR 0.45, 95% CI 0.36–0.56, p<0.001). Compared with receiving no radiation, both BCWRT and BCW+NRT were associated with improved OS in both ypN0 and ypN1 lumpectomy recipients (all p<0.001, Supplemental Table 1).
After stratification by post-NACT nodal response, these findings remained essentially the same for both ypN0 (BCWRT HR 0.32, 95% CI 0.18–0.57; BCW+NRT HR 0.38, 95% CI 0.22–0.66) and ypN1 patients (BCWRT HR 0.35, 95% CI 0.23–0.54; BCW+NRT HR 0.44, 95% CI 0.3–0.66, all p<0.001, Table 4b). Tumor subtype was the only other factor found to be associated with improved survival in this cohort (p<0.001).
Discussion
Rates of nodal pCR have increased over time among breast cancer patients presenting with limited node-positive disease (cN1), likely due to increasingly effective preoperative systemic therapy. Among cN1 patients who achieved nodal pCR, rates of nodal radiotherapy receipt have remained the same over time. But among cN1 patients with persistent nodal involvement, rates of nodal irradiation have increased, suggesting that radiation oncologists are using response to NACT to guide treatment decisions even in the absence of prospectively acquired data of the kind we hope to obtain from the companion trials Alliance A1120212 and NSABP B-51/RT 1304.3
Post-mastectomy radiation (PMRT) with or without nodal coverage was not associated with improved adjusted OS in ypN0 patients beyond that already associated with a patient’s response to NACT, although among ypN1 patients who received nodal radiation there may be some survival benefit. These findings demonstrate that radiation oncologists are not only already omitting PMRT in select patients that present with limited nodal disease, but also that this omission may not compromise OS, even in patients with low-volume, residual nodal disease after NACT.
Among patients in our cohort who received lumpectomy, the vast majority (92.5%) received some form of radiation. Post-lumpectomy radiation with or without nodal coverage was associated with improved survival after lumpectomy regardless of nodal response to NACT, reflecting the appropriate inclusion of radiation as part of guideline-concordant care in patients undergoing post-NACT lumpectomy. Notably, as evidenced by significant overlap in the confidence intervals for the adjusted survival analyses, there was no significant difference in survival between BCWRT and BCW+NRT among lumpectomy patients. This finding suggests that the main benefit of radiation in this cohort of patients is largely derived from its role in treating unresected disease in the breast.
However, the results of our survival analysis should be interpreted with caution. We recognize that our failure to detect a survival benefit from nodal radiation may be due to several reasons unrelated to actual treatment efficacy including (1) the relative paucity of events, especially in light of the significant association between node-specific radiation and survival observed in previous clinical trials,13,14 (2) the often-imprecise distinction between BCWRT and BCW+NRT both in actual treatment delivery and with regards to clinician and coder documentation,15 and (3) the short period of follow-up in our cohort (40.1 months). Indeed, given that the survival benefit from post-lumpectomy radiation is typically derived 10 to 15 years after treatment, any survival difference observed within only a few years of treatment may, in part and even with adjustment, be due to baseline differences between those who did and did not receive the different forms of treatment being compared.
We are, however, reassured by the robustness of our Cox proportional hazards model with regards to the inclusion of many potential confounders including patient data on race/ethnicity; biomarker subtype; and proxies for access to care including insurance status, type of facility at which treatment was received, and distance from treatment facility.
Our study had several additional limitations. Radiation is primarily administered for the purpose of preventing locoregional recurrence, but the NCDB does not report breast cancer-specific survival or recurrence rates, thus overall survival was the only long-term outcome studied. Although there is significant evidence that receipt of radiotherapy can improve survival when indicated for patients with breast cancer, longer follow-up will be required to confirm whether reductions in locoregional recurrence secondary to radiation receipt translate into improved survival.16
Pre- and postoperative staging, radiation dosage, and treatment sequence are reported by the NCDB’s member institutions, but these assessments are not subjected to central review that might otherwise identify and reclassify cases for which this data were incorrect. In particular, the use of regional nodal irradiation may not have been accurately captured. Moreover, not all initial nodal staging was biopsy-proven, so our analysis may include patients with pathologically benign but radiographically or anatomically concerning lymphadenopathy that were inappropriately considered cN1; these patients would be expected to do better than true cN1 patients and could potentially influence survival results. Finally, we limited our analysis to cN1 patients, thus, caution should be used before extending our findings to patients with cN2–3 and/or non-axillary nodal disease.
Conclusion
Our findings indicate that practice patterns have evolved in parallel with improvements in the effectiveness of preoperative systemic therapy. We found that nodal response to NACT is strongly associated with likelihood of radiation receipt in patients presenting with cN1 disease and for whom radiation would historically have been indicated, an indication that practice patterns are changing in advance of clinical trial findings. Rates of nodal pCR have increased over time in the context of increasingly effective NACT, and patients who fail to experience nodal pCR have become more likely to receive nodal radiation.
In patients undergoing mastectomy, there was no additional survival benefit conferred by nodal radiation, though our findings suggest that there may be some benefit from nodal radiation in ypN1 patients, i.e., those with small-volume residual disease. Among patients receiving lumpectomy, breast radiation with or without nodal radiation was strongly associated with improved OS for both ypN0 and ypN1 patients, but the addition of nodal radiation did not significantly add to the survival benefit already conferred by breast radiation. These findings require validation in prospective randomized trials such as NSABP B-51/RTOG 1304,3 which should help us collectively establish whether, in the context of increasingly effective systemic therapy, we can continue to safely de-escalate locoregional treatment to the axilla and potentially omit nodal radiation in select clinically node-positive patients following NACT.
Supplementary Material
Acknowledgements
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and its participating hospitals are the source of the de-identified data used herein; the CoC has not verified these data and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Funding
Dr. Fayanju is currently supported by the National Institutes of Health (NIH) under Award Number 1K08CA241390 (PI: Fayanju) and was previously supported by the National Center for Advancing Translational Sciences (NCATS) of the NIH under Award Number 1KL2TR002554 (PI: Svetkey). Dr. Suneja is supported by grants K08CA228631 (PI: Suneja) and P30AI064518 (PI: Weinhold) from the NIH. Dr. Greenup is supported by the NIH BIRCWH K12HD043446 (PI: Andrews). This work is also supported by the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest to Disclose: Dr. Force reports receipt of personal fees from Genomic Health, NanoString, and Pfizer that are unrelated to the current project. Other than government-issued grant funding (see below), all other authors have no relevant conflicts of interest to disclose.
References
- 1.Fayanju OM, Ren Y, Thomas SM, et al. The Clinical Significance of Breast-only and Node-only Pathologic Complete Response (pCR) After Neoadjuvant Chemotherapy (NACT): A Review of 20,000 Breast Cancer Patients in the National Cancer Data Base (NCDB). Annals of surgery. 2018;268(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. [DOI] [PubMed] [Google Scholar]
- 3.Mamounas EP, White JR, Bandos H, et al. NSABP B-51/RTOG 1304: Randomized phase III clinical trial evaluating the role of postmastectomy chest wall and regional nodal XRT (CWRNRT) and post-lumpectomy RNRT in patients (pts) with documented positive axillary (Ax) nodes before neoadjuvant chemotherapy (NC) who convert to pathologically negative Ax nodes after NC. Journal of Clinical Oncology. 2014;32(15_suppl):TPS1141–TPS1141. [Google Scholar]
- 4.Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(12):2694–2702. [DOI] [PubMed] [Google Scholar]
- 5.Gianni L, Pienkowski T, Im Y-H, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. The Lancet Oncology. 2016;17(6):791–800. [DOI] [PubMed] [Google Scholar]
- 6.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384. [DOI] [PubMed] [Google Scholar]
- 7.McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. Breast Cancer (Version 1.2019). 2019; http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 10 May 2019.
- 9.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. [DOI] [PubMed] [Google Scholar]
- 10.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(18):2946–2953. [DOI] [PubMed] [Google Scholar]
- 11.Mamtani A, Barrio AV, King TA, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Annals of surgical oncology. 2016;23(11):3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boughey JC. Comparison of Axillary Lymph Node Dissection With Axillary Radiation for Patients With Node-Positive Breast Cancer Treated With Chemotherapy. https://clinicaltrials.gov/ct2/show/NCT01901094.
- 13.Poortmans PM, Collette S, Kirkove C, et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. The New England journal of medicine. 2015;373(4):317–327. [DOI] [PubMed] [Google Scholar]
- 14.Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. The New England journal of medicine. 2015;373(4):307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jagsi R, Chadha M, Moni J, et al. Radiation field design in the ACOSOG Z0011 (Alliance) Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(32):3600–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whelan TJ, Julian J, Wright J, Jadad AR, Levine ML. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol. 2000;18(6):1220–1229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.