Abstract
Background:
The circulating immune phenotype was defined in adults and young children with early atopic dermatitis/AD, but chronologic changes in blood of AD infants and children through adolescence have not been explored.
Objective:
To compare immune activation and cytokine polarization in blood of 0–5y/o (n=39), 6–11y/o (n=26), 12–17y/o (n=21) and ≥18y/o (n=43) patients with AD vs. age-matched controls.
Methods:
Flow cytometry was used to measure interferon-γ, interleukin (IL)-9, IL-13, IL-17, and IL-22 cytokines in CD4+/CD8+ T-cells, with ICOS and HLA-DR defining mid- and long-term T-cell activation, respectively, within skin-homing/CLA+ vs. systemic/CLA− T-cells. Unsupervised clustering differentiated patients based on their blood biomarker frequencies.
Results:
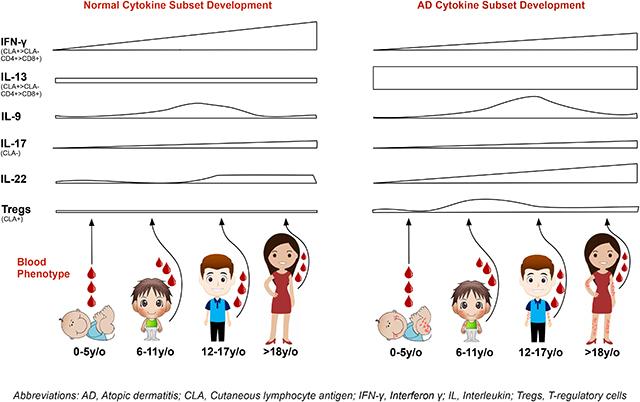
While CLA+ Th1 frequencies were significantly lower in infants with AD vs. all older patients (P<0.01), CLA+ Th2 T-cells were similarly expanded across all AD age groups compared with controls (P<0.05). After infancy, CLA− Th2 frequencies were increased in AD in all age groups, suggesting systemic immune activation with disease chronicity. IL-22 frequencies serially increased from normal in infants to highly significant levels in adolescents and adults compared to respective controls (P<0.01). Unsupervised clustering aligned the AD profiles along an age-related spectrum from infancy to adulthood (ICOS, IL-22 etc.).
Conclusions:
The adult AD phenotype is achieved only in adulthood. Unique cytokine signatures characterizing individual pediatric age endotypes may require age-specific therapies. Future longitudinal studies, comparing the profile of cleared vs. persistent pediatric AD, may define age-specific changes that predict AD clearance.
Keywords: Atopic dermatitis, endotypes, T-cell, CLA, IL-13, IL-22, IFN-γ, ICOS, HLA-DR
Graphical Abstract
Capsule Summary:
Decreased Th1/Th2 ratio characterizes the atopic dermatitis (AD) phenotype across all age groups, while chronic activation, IL-9, IL-22, and Treg changes characterize older patients. Even the adolescent AD profile differs from adult AD.
Introduction
Infancy, childhood and adolescence are critical periods for immune system maturation.1 Early abnormal immune development may cause immune-related disorders. Indeed, 85% of atopic dermatitis (AD) cases present before 5 years of age.2 While young adults have a different AD phenotype from elderly patients,3 immune changes in AD between early childhood and adulthood are unknown.4
Blood studies that dissect developmental changes from infancy through adulthood are limited and primarily focused on T-helper (Th)1/Th2 subsets. Some studies with healthy controls showed expansion of Th1/Th2/T-cytotoxic (Tc)1 subsets with age,5, 6 while others reported no changes in Th2/Tc2 over time.7 Immature interferon (IFN)-γ response8 and low Th1/Tc1 frequencies9–11 were seen in early stages of normal development, and abnormal Th1/Th2 ratios in cord blood and infants who develop AD were described.12–14 However, few studies compared pediatric and adult AD populations6, 15–17 and none directly compared consecutive age groups of AD with age-matched controls, which is critical in understanding normal vs. pathological development of acquired immunity.
The therapeutic arsenal available for 0–12 year old (y/o) AD patients is limited to topical agents and/or broad systemic immune suppressants.4, 18 Active development of targeted therapeutics is ongoing for adults and adolescents with AD and will eventually move to children, further necessitating the elucidation of pediatric endotypes at successive pre-adult age groups to introduce safe, effective, age-tailored targeted approaches.19
We compared T-cell memory subset activation and polarized CD4/CD8 subset frequencies within the skin-homing/cutaneous lymphocyte antigen/CLA+ and systemic/CLA− compartments, in the blood of infants and toddlers (0–5y/o), young children (6–11y/o), adolescents (12–17y/o) and adults (≥18y/o) with moderate-to-severe AD. To differentiate pathologic from physiologic immune maturation, age-matched healthy controls were included. We found that decreased Th1/Th2 ratios were shared across all AD ages, but unique fingerprinting characterizes individual AD age groups, differentiating them from their age-matched peers. Our intracellular and T-cell blood biomarker data grouped the AD cohort, but not the controls, into three unique age phenotypes aligned along a spectrum.
Methods
Patient characteristics and blood samples
Blood was obtained (with signed Institutional Review Board-approved informed consent from parents and patients 12 years and older) from 39 infants and toddlers (0–5y/o; mean 23 months), 26 children (6–11y/o; mean 7.5yrs), 21 adolescents (12–17y/o; mean 14.9yrs) and 43 adults (≥18y/o; mean 43yrs) with moderate-to-severe AD, as well as healthy age-matched controls (24–30 in each group; demographic and laboratory data in Table E1). Sensitivity analyses on patient subsets who were matched for all demographic parameters yielded similar results to analysis including all subjects (Table E2A–B).
Disease severity was scored using SCORing of AD (SCORAD) for adults and SCORAD, Eczema Area and Severity Index (EASI), and Atopic Dermatitis Quickscore (ADQ) in those less than 18y/o. ADQ is a parent-administered tool, which assesses involvement and pruritus of 7 body parts and highly correlates with SCORAD.20 In subjects <18y/o, eosinophil counts and skin-barrier assessment through transepidermal water loss (TEWL) of lesional (LS) and non-lesional (NL) arm skin (AquaFlux Model AF200; Biox, London, England)21 were measured when feasible. No patients received systemic immunosuppressive treatment within 4 weeks prior to this study, thus the data present baseline immune phenotyping of the studied populations. Concomitant allergic manifestations were recorded, and individuals were classified as either having general allergies (allergic rhinitis/asthma/allergic conjunctivitis/environmental), food allergies (FA), both, and neither. Controls had no personal history of AD. Only 1 child and 5 adolescent controls had history of non-cutaneous atopic manifestations, however sensitivity analysis excluding these subjects did not alter the results (Table E2C).
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs were isolated from whole blood by Ficoll-Paque Plus (GE Healthcare, Sweden). Briefly, the blood was laid under Ficoll gradient; after spinning, PBMCs were collected at the interface between the plasma and the Ficoll gradient (see Methods in the Online Repository/OR).
Stimulation of blood cell populations for cytokine responses
Ex-vivo cell activation is required to detect cytokine production, as less than 1% of non-stimulated cells produce cytokines. Whole blood was incubated with PMA (25ng/ml) plus ionomycin (2μg/ml) in the presence of brefeldin A (10μg/ml) for 4 hours at 37°C to induce cytokine responses. After stimulation, red blood cells were lysed with FACS lysing solution to obtain leukocytes (see Methods in the OR).
Cell surface staining and intracellular staining on PBMCs and stimulated and non-stimulated CD4/CD8 T-cells
Peripheral blood mononuclear cells/PBMCs were stained with fluorochrome-labeled Abs to cell surface markers (CD3, CD8, CD4, CD45RO, CCR7, ICOS, HLA-DR, CLA, CCR4, CD25, CD127). Stimulated and non-stimulated blood cells were also stained for cell surface markers [CD3, CD4, and CLA (CD8+ T-cells were gated thorough CD3+CD4− T subpopulation] and permeabilized with FACS/perm to stain for cytokines including Interleukin (IL)-13, IL-22, IL-9, IFN-γ and IL-17 (see Methods in the OR).
Statistical analysis
Statistical analyses were performed with R software (www.R-projets.org). Means and medians were compared using the Welch t-test and Wilcoxon-Mann-Whitney test, respectively. Unsupervised hierarchical clustering of variables (T-cell subset frequencies, age, and clinical scores) was performed using the R package “hclust,” with a McQuitty agglomeration algorithm and Spearman coefficient as a similarity metric and presented as a heat map and a dendrogram. Individual scatter plots were constructed that display Spearman coefficients, 95% confidence intervals, and p-values for AD and healthy samples. We performed k-means unsupervised clustering across principal components of the frequencies of all AD and separately among all healthy control subsets. We found that 3 clusters separated AD patients, but not controls, along a chronologic age spectrum. ANOVA, in conjunction with the Tukey test, was used to find markers that differentiated any two clusters. P-values and fold-changes (FCH) were designated as ***(p<0.001), **(p<0.01), *(p<0.05), and +(p<0.1).
We also performed a power calculation based on our previous flow cytometry study on moderate-to-severe adult AD patients.22 We determined that 39 AD patients would have greater than 90% power (at a significance level of 0.05) to detect differences versus controls in various inflammatory cell subsets (CD4+IL-22+CLA+, CD4+IL-13+CLA−, CD8+IL-17+CLA+, CD8+ Tcm HLA-DR+CLA+).
Results
Flow cytometry was used to measure frequencies of IFN-γ, IL-9, IL-13, IL-17A and IL-22-polarized T-cells, defining Th1/Tc1, Th9/Tc9, Th2/Tc2, Th17/Tc17 and Th22/Tc22 subsets in CD4+/CD8+ T-cells, respectively. Cell surface staining was used to assess expression of mid (inducible co-stimulator molecule/ICOS) and late (HLA-DR) activation markers in central (Tcm/CCR7+CD45RO+) and effector (Tem/CCR7−CD45RO+) memory T-cells in skin homing/cutaneous/CLA+ and systemic/CLA− compartments.
Patients and controls were divided into 4 consecutive age groups (infants and toddlers 0–5y/o, children 6–11y/o, adolescents 12–17y/o and adults ≥18y/o). To display both healthy vs. pathologic developmental changes and immune abnormalities within each age group vs. controls, we present two types of comparison plots; both contain similar data but focus on either AD patients vs. controls for each age group or AD vs. control across all ages. To better represent the effect of values distribution, the comparison plots presented below contain both the mean (in black) and the median (in red) +/− standard error, and their respective p-values. Results discuss the mean values, while median percentages for main comparisons discussed are presented in Table E3.
Skin-homing memory T-cell expansion and ICOS activation feature early AD
Tcm and Tem T-cells are main components of the adaptive immune system, harboring distinct homing capacities.23 While both express the skin homing marker CLA, only Tcm cells retain CCR7 positivity, which enables them to migrate into lymph nodes and function as an immunologic reserve.24 After gating on CD3+ viable T-cells using flow cytometry, CD3+CD4+ and CD3+CD8+ were defined and analyzed separately. CCR7 and CD45RO were used to differentiate memory subsets within the CD4 and CD8 populations. CCR7+CD45RO−defined naive cells, CCR7+CD45RO+ central memory T-cells (Tcm), CCR7−CD45RO+ effector memory T-cells (Tem) and CCR7−CD45RO− Effector/Temra/terminally differentiated T-cells (Fig. E1A–C). We then further defined activated ICOS/HLA-DR activated Tcm/Tem memory subset, using CLA to segregate skin-homing (CLA+) versus systemic subsets (CLA−).
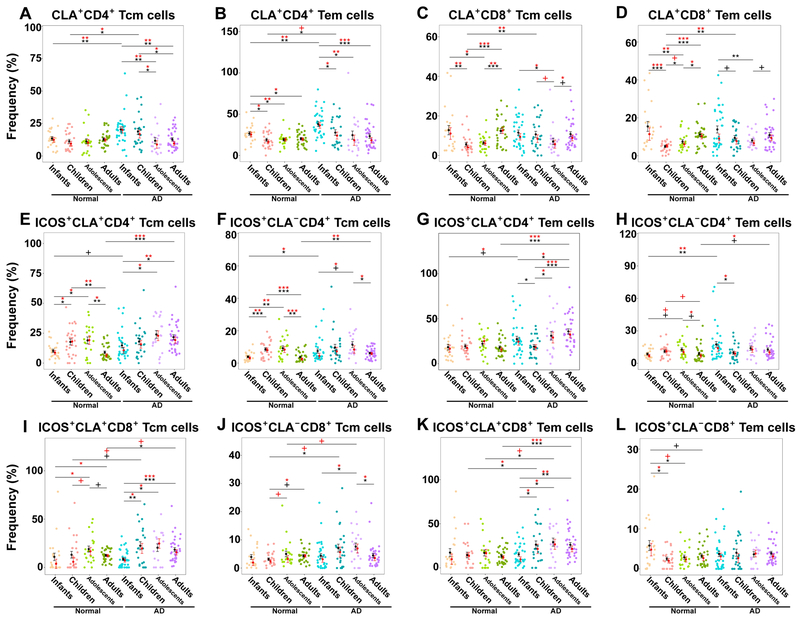
Normal development was characterized by a slight drop of CLA+ CD4+Tem (but not Tcm) cells between infancy and childhood (infants 26.7% vs. children 19%, P=0.01; Figs. 1A–B, E1D–E), but this decrease was significantly more evident in AD (P<0.05; Figs. 1A–B, E1D–E). CD4+CLA+ Tem/Tcm cells were significantly higher in AD infants (Tcm: 20.4% vs. 13.4%, P=0.006; Tem: 38% vs. 26.7%, P=0.002; Fig. E1D–E) and children (Tcm: 18.8% vs. 11.3%, P=0.01; Tem: 27.7% vs. 19%, P=0.03; Fig. E1D–E) than controls, a difference that diminished with increasing age. T-cell memory subset fluctuations with age are presented in Figure E2. Due to increased proportions of effector and naive cells, CD8+ Tcm/Tem subsets dropped between infancy and childhood exclusively in controls (Tcm: infants 12.8% vs. children 5.6%, P=0.008; Tem: infants 15.4% vs. children 5%, P<0.001; Fig. 1C–D), leading to higher frequencies in children with AD (P<0.01), but otherwise frequencies were overall similar between AD and controls over time (Fig. E1F–G). Significant CD4+/CD8+ effector cells increase with age characterized AD vs. controls, who showed decrease of this subset during the years (Fig. E2J, L).
Figure 1.
A-D) The frequency of CLA+ Tem (CD45RO+CCR7−) and Tcm (CD45RO+CCR7+) in CD4+/CD8+ T cells and (E-L) ICOS+ activation in CLA+/− CD4+/CD8+ Tcm/Tem cells in healthy controls and AD patients across ages (ICOS+CLA+CD45RO+CCR7+/−). Bar plots represent means (black)/medians (red) ± SEMs. AD, Atopic dermatitis; CLA, Cutaneous lymphocyte antigen; SEM, Standard error of the mean/median; Tcm, central memory T-cells; Tem, effector memory T-cells. P-values are designated as ***<0.001, **<0.01, *<0.05, and +<0.1.
CD4+ Tcm/Tem ICOS mid-activation continuously increased in both controls and AD, however it decreased significantly between adolescence and adulthood, exclusively in controls (Tcm CLA+: 24% vs. 9%, P=0.003; CLA−: 9.2% vs. 3.7%, P=0.001; Fig. 1E–H). Both skin-homing/CLA+ and systemic/CLA− ICOS-activated CD4+ T-cell frequencies were significantly increased in infants and adults with AD vs. respective controls (P<0.05; Fig. 1E–H). Skin-homing CD8+ Tcm/Tem ICOS activation increased gradually, most notably in AD patients (Fig 1I–L), with frequency differences uniquely seen in adults with AD vs. controls (Tcm: 18.4% vs. 12.5%, P=0.01; Tem: 26% vs. 13.4%, P<0.001; Fig. 1I–L).
The HLA-DR antigen, indicating chronic activation,25 had similar (or even lower) expression in infants with and without AD (P>0.1; Fig. E1H–K), but started to rise in AD children (Tcm+CD4+CLA+ AD: 15.8% vs. 8.4%, P=0.02; CLA−: 7.2% vs. 2.8%, P=0.06; Fig. E1H–I), reaching consistently high levels across CD4+/CD8+/CLA+/CLA−/Tcm/Tem cells in adults with AD compared to healthy individuals (P<0.05; CD8+ Tcm/Tem data not shown; Fig. E1H–K).
Decreased Th1/Th2 ratio characterizes AD across ages
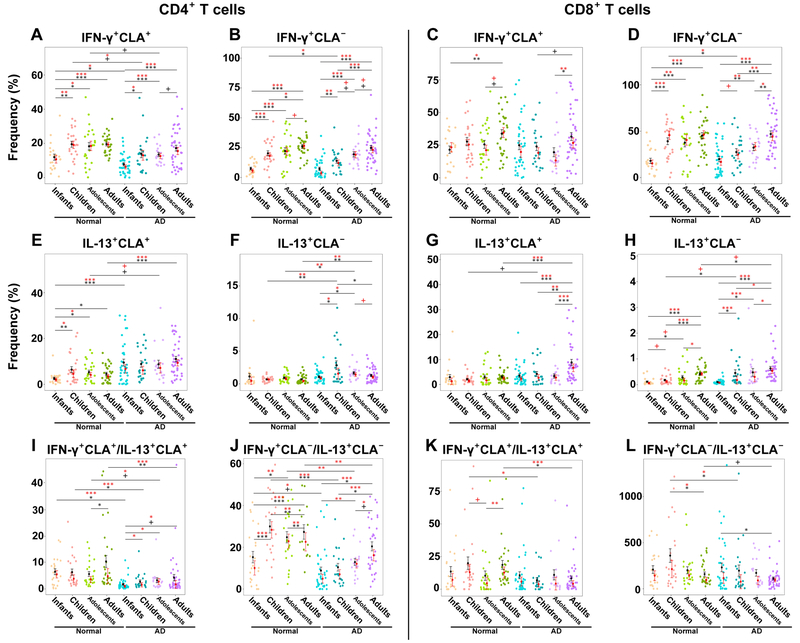
Since T-cell activation leads to cytokine polarization we next studied different polar T-cell subsets. Representative flow cytometry plots and gating strategy are presented in Figure E3. Congruent with past publications,9, 10 IFN-γ levels increased with age in both control and AD subjects, with the lowest frequencies seen in infants (Fig. 2A–D). Nevertheless, in AD IFN-γ did not reach control levels, particularly within the skin homing compartment (CD4+CLA+: infants control 12% vs. AD 7.7%, P=0.04; children control 19.5% vs. AD 13.6%, P=0.05; adolescents control 18% vs. AD 13%, P=0.07) until adulthood (P=0.2; Figs. 2A–D, E4A–D). Even in adulthood, levels trended towards lower frequencies. Interestingly, the only population that showed lower systemic/CLA− CD4+/CD8+ IFN-γ was the 5–12y/o age group (CD4+: control 20% vs. AD 14%, P=0.04; CD8+: control 39% vs. AD 28%, P=0.05; Fig. E4B, D).
Figure 2.
A-D) IFN-γ+, (E-H) IL-13+, and (I-L) IFN-γ+/IL-13+ cytokine frequencies in CLA+, CLA−, CD4+ and CD8+ T-cells in healthy controls and AD patients across ages. The ratio between the % of IFN-γ+CLA+CD4+ (or CD8+) T cells and the % of their IL-13+ counterpart was calculated for each sample but was not multiplied by 100 and is therefore unitless. Bar plots represent means (black)/medians (red) ± SEMs. AD, Atopic dermatitis; CLA, Cutaneous lymphocyte antigen; SEM, Standard error of the mean/median. P-values are designated as ***<0.001, **<0.01, *<0.05, and +<0.1.
In controls, IL-13+CD4+CLA+ was lowest in infants, reaching a plateau in childhood. Conversely, in AD patients, levels were similarly elevated across all ages (P>0.1; Fig. 2E). Systemic/CLA− Th2 was overall low in controls (Fig. 2F). Levels were significantly higher in AD than controls in children (2.8% vs. 0.6%, P=0.005; Fig. E4F), adolescents (1.5% vs. 0.7%, P=0.006; Fig. E4F) and adults (1.3% vs. 0.7%, P=0.005; Fig. E4F). While Tc2 cells were slightly higher in children with vs. without AD, differences in CD8+ subsets were more prominent in adulthood (Figs. 2G–H, E4G–H). Reflecting the Th1 and Th2 imbalances characterizing AD, the Th1/Th2 ratio was decreased in AD than controls in both CLA+/CLA− subsets across the ages. Tc1/Tc2 was significantly lower in children and adults with AD vs. controls (Figs. 2I–L, E4I–L).
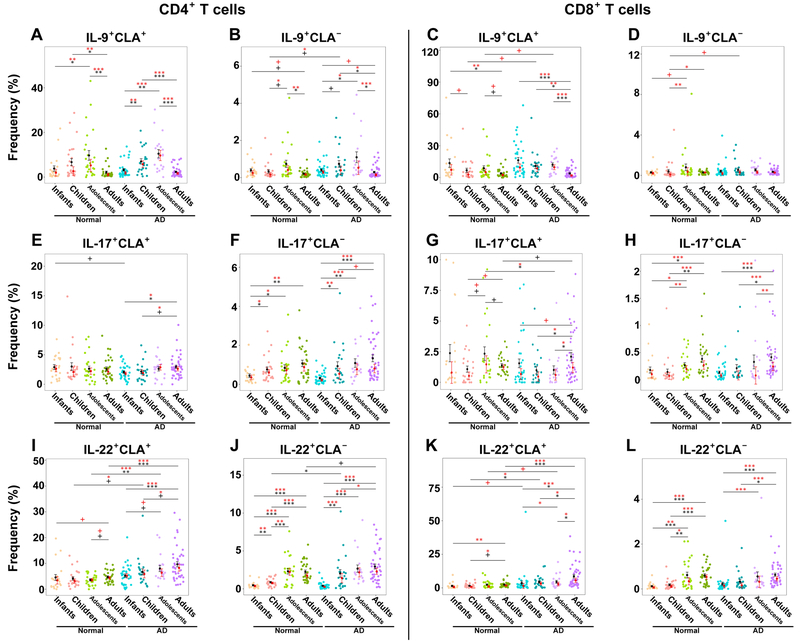
Both healthy controls and AD patients showed Th9 increases over time, peaking in adolescence and dropping in adulthood (Figs. 3A–B, E5A–B). No Th9 differences were observed between AD and controls, besides 5–12y/o children with AD that showed significantly increased CLA− levels (0.76% vs. 0.3%, P=0.04; Fig. E5B). This AD age group also showed increased CLA+/CLA− Tc9 levels (Figs. 3C–D, E5C–D). CLA+ Th17 cells were generally stable and similarly abundant across ages among control and AD (Figs. 3E, E5E), contrary to CLA− Th17 cells which showed developmental expansion in both (Figs. 3F, E5F). Contrary to adolescents with AD that had lower CLA+ Tc17 than controls (1% vs. 4.7%, P=0.04), adults had significantly higher frequencies (2.1% vs. 1.4%, P=0.05; Figs. 3G–H, E5G–H). Systemic/CLA− CD4+/CD8+ IL-22+ similarly increased with age in both controls and AD (Fig. 3J, 3L, E5J, E5L), while skin-homing Th22/Tc22 cells increased with age, primarily in AD (Figs. 3I, 3K, E5I, E5K). Starting in childhood, skin-homing Th22/Tc22 were significantly higher in AD vs. controls, and incrementally increased with age (Th22: children 6.7% vs. 4%, P=0.07; adolescents 7.9% vs. 3.6%, P=0.001; adults 8.2% vs. 4.4%, P<0.0001; Figs. 3I, 3K, E5I, E5K). Polar T-cell subset development in controls and AD is summarized in Table E6 and illustrated in the graphical abstract.
Figure 3.
A-D) IL-9+ and (E-H) IL-17+ and (I-L) IL-22+ frequencies in CLA+, CLA−, CD4+ and CD8+ T-cells in healthy controls and AD patients across ages. Bar plots represent means (black)/medians (red) ± SEMs. AD, Atopic dermatitis; CLA, Cutaneous lymphocyte antigen; SEM, Standard error of the mean/median. P-values are designated as ***<0.001, **<0.01, *<0.05, and +<0.1.
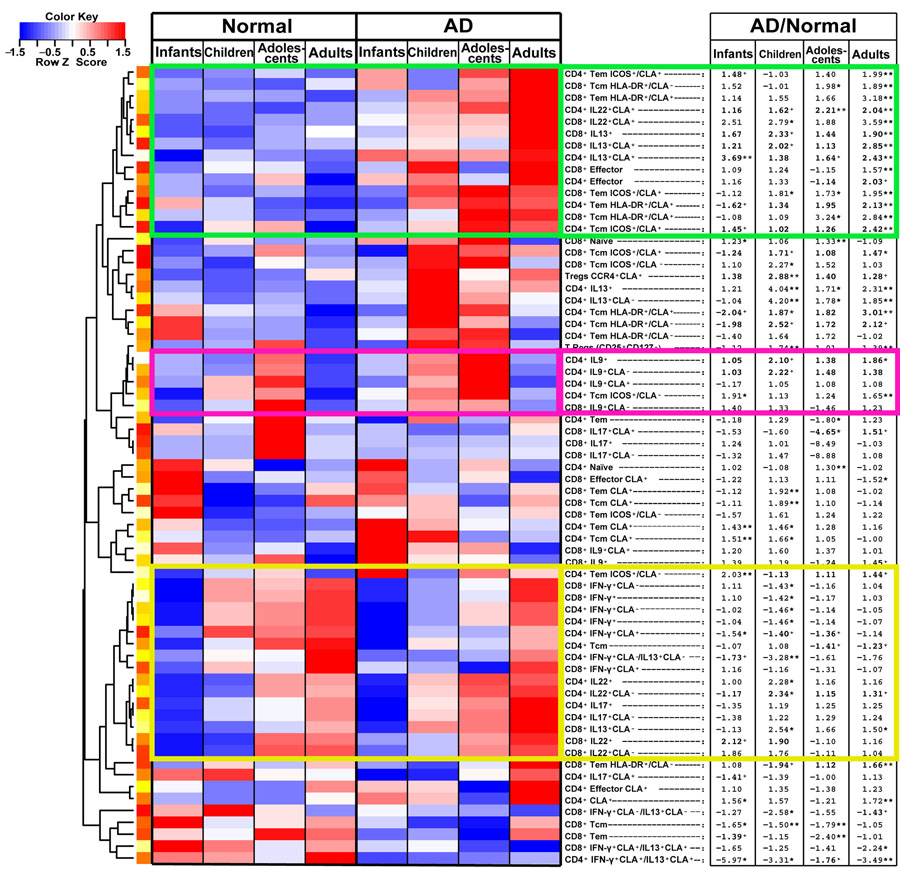
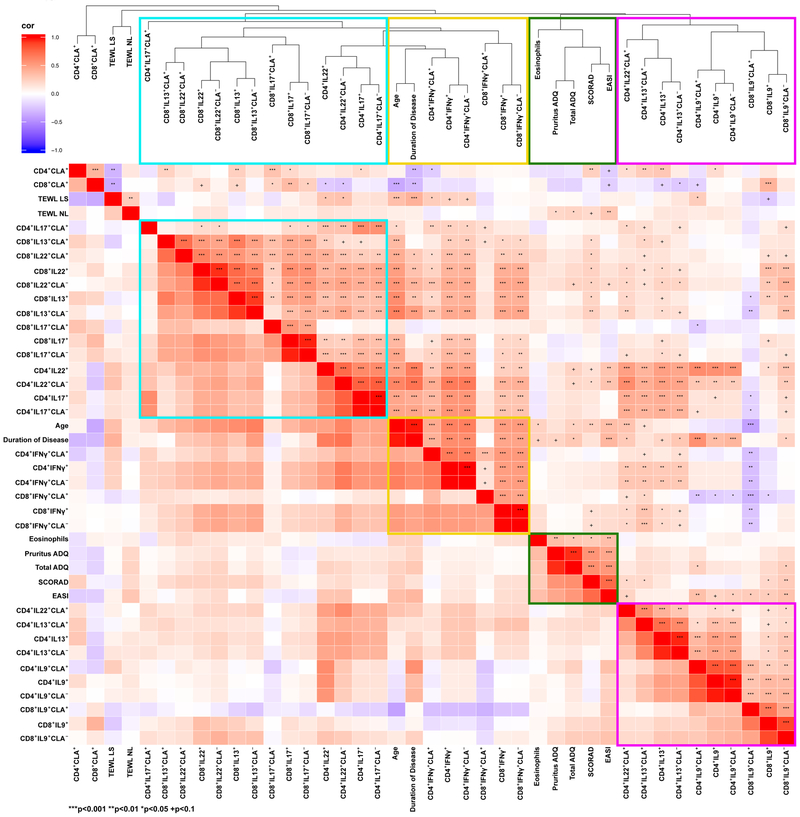
The unsupervised hierarchical clustering heatmap in Figure 4 summarizes the above, displaying all polarized T-cell subsets for control and AD across age groups (red: positive/increase; blue: negative/decrease). Fold-changes (FCHs) of the mean frequencies of AD vs. controls for each age group are presented (stars and plus signs display significance). The green cluster includes subsets that were relatively low and stable among controls, but incrementally increased with age in AD. Most of these subsets were significantly increased in adults with AD vs. controls (FCH>1.57, P<0.05), while younger AD patients showed lower or no significance. The pink box shows increased IL-9 frequencies in childhood, which decline in adulthood, particularly in AD. The yellow cluster shows markers that increased in both controls and AD, therefore minimizing the differences between groups.
Figure 4.
Unsupervised hierarchical clustering heatmap displaying polarized T-cell subsets for control and AD across age groups (red: positive/increase; blue: negative/decrease). Fold-changes (FCHs) of the mean frequencies of AD vs. controls for each age group are listed on the right (stars and plus signs display significance). The green cluster includes subsets that were relatively low and stable among controls, but incrementally increased with age in AD. The pink box shows increased IL-9 frequencies in childhood, which decline in adulthood, particularly in AD. The yellow cluster shows markers that increased in both controls and AD.
T-cell activation, clinical measures and IFN-γ are associated with age and AD chronicity
We also evaluated how clinical characteristics, including AD severity (SCORAD, EASI), patient age, disease duration, eosinophil counts, pruritus and TEWL relate to different polar T-cell subsets. Unsupervised hierarchical clustering of all T-cell subset frequencies, clinical scores, AD duration and age was performed using Spearman correlations as a similarity metric, as displayed in the correlation heatmap and dendrogram in Figure 5 (red: positive correlation; blue: negative correlation, stars and plus signs display significance). Congruent with recent AD data,22, 26 which showed positive correlations between IL-13- and IL-22- and between IL-17- and IL-22-producing T-cells,22, 26, 27 these cytokines largely grouped together (turquoise rectangle). IFN-γ-producing T-cells clustered together with AD duration and patient age (yellow rectangles). A tight cluster gathering multiple clinical measures (green rectangles) was located adjacent to IL-9/IL-13-producing T-cells (purple rectangles). Significant correlations depicted in this heatmap are listed in Table E4, with selected individual scatter plots presented in Figures 6–7 and E6–E8.
Figure 5. Unsupervised hierarchical clustering of polarized cytokine subset frequencies (%) with AD clinical measures.
using Spearman correlation as a similarity metric. CD4+/CD8+ IL-13+, IL-17+ and IL-22+ subsets clustered together (turquoise box). IFN-γ+-producing T-cell subsets grouped together with age and disease duration (yellow box). IL-9+ and some IL-13+ and IL-22+ T-cells (purple box) clustered adjacent to AD clinical measures (green box). The heatmap shows the positive (red) or negative (blue) correlations of all parameters, with color intensity reflecting the strength of the correlation (−1 to +1). Dendrograms are shown as a tree, representing the distance between variables. CLA, Cutaneous lymphocyte antigen. P-values are designated as ***<0.01, **<0.01, *<0.05, and +<0.1.
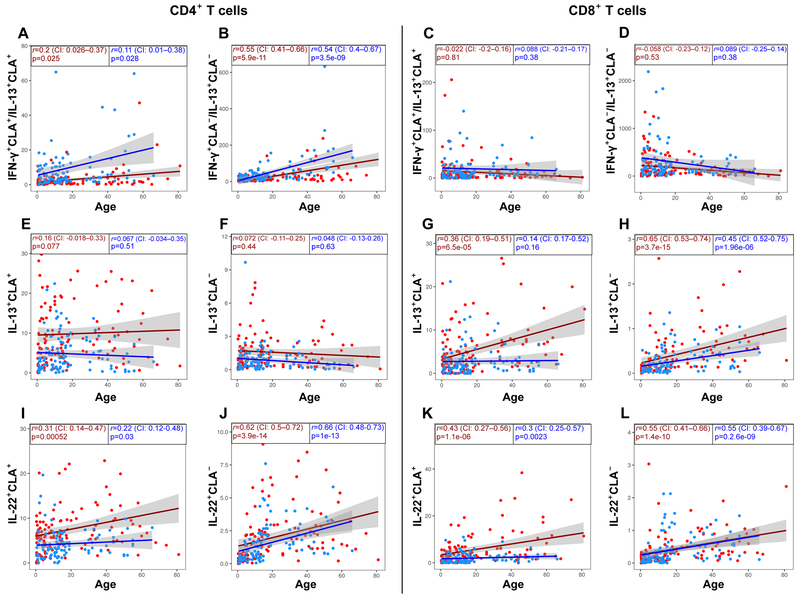
Figure 6.
Spearman correlation scatter plots (linear regression [red=AD/blue=control line] with their CI [in gray]) for (A-E) SCORAD and (F-L) age versus clinical measures and Tem/Tcm subset frequencies (%). Dots colors in plots A-D, F designate different AD patient ages from infancy to adulthood, as shown in Figure 1. CI, Confidence interval; CLA, Cutaneous lymphocyte antigen; EASI, Eczema area and severity index; LS, Lesional; NL, Non-lesional; SCORAD, SCORing of Atopic Dermatitis; Tcm, Central memory T-cells; Tem, Effector memory T-cells; TEWL, Transepidermal water loss.
Figure 7.
Spearman correlation scatter plots (linear regression [red=AD/blue=control line] with their CI [in gray]) for CD4+/CD8+, CLA+/CLA− (A-D) IFN-γ+/IL-13+ producing T-cell ratio, (E-H) IL-13+, and (I-L) IL-22+ producing T-cell frequencies (%) versus age in AD patients (red dots/line) and controls (blue dots/line). CI, Confidence interval; CLA, Cutaneous lymphocyte antigen.
SCORAD and EASI were positively correlated (r=0.74, P<0.001; Fig. 6A). SCORAD also correlated with skin-homing CD4+ cells (r=0.24, P=0.0016; Fig. 6B), pruritus (r=0.54, P<0.0001, Fig. 6C) and eosinophils (r=0.25, P=0.001; Fig. 6D). To evaluate normal vs. pathologic development with age, we comprehensively assessed age correlations in control and AD subjects, presenting both on the same scatter plots for clarity, when applicable. Age correlated positively with severity by SCORAD (r=0.25, P=0.0016; Fig. 6E) and EASI (r=0.34, P<0.001; Fig. 6F), and with all other clinical measures, including pruritus (Fig. 6G), eosinophils (Fig. 6H), and lesional TEWL (Fig. 6I–J).
While skin homing Tcm/Tem cells correlated negatively with age (red=AD, blue=controls; Fig 6K–L), the proportion of ICOS and HLA-DR-activated skin-homing T-cells increased exclusively in AD (Fig. E6A–D). AD duration, which highly correlated with patients’ age (r=0.98, P<0.0001; Fig. E6E), also demonstrated significant positive correlations with EASI and pruritus (Fig. E6F–H). Among the cytokine subsets (Fig. E6I–L), the most significant correlation was noted between AD duration and IFN-γ-producing cells (r=0.58, P<0.0001; Fig. E6L).
Overall IFN-γ increased with age in both AD and controls (Fig. E7A–D), however, although the Th1/Th2 (but not the Tc1/Tc2) ratio, and particularly its skin-homing component, increased with age in both groups, it remained significantly decreased in AD across all ages vs. controls (Fig. 7A–D). Conversely, differences between AD and controls were observed in skin-homing Th2/Tc2 throughout development, with controls never reaching AD levels (Fig. 7E–H). CLA+ Th22/Tc22 showed significantly higher developmental increases in AD (Fig. 7I, K), while systemic subsets generally overlapped (Fig. 7J, L). Similar negative trends of IL-9+ (Fig. E7E–H), and expansion of systemic Th17 cells were seen in controls and AD subjects (Fig. E7I–L).
Positive correlations between SCORAD and IL-13, IL-9 and IL-22-producing cells were recorded (P<0.02; Fig. E8A–C). EASI correlated with Th9, Th22 and pruritus (P<0.03; Fig. E8D–F), while pruritus was associated with Th22 and eosinophil counts (P<0.032; Fig. E8G–H). TEWL correlated with CD4+CLA+ cells, only in non-lesional skin (Fig. E8I–J). Th22 positively correlated with TEWL in lesional tissues (Fig. E8K–L), and AD severity correlated with TEWL in non-lesional skin (Fig. E8M–P).
Because Foxp3 staining requires cell permeabilization, surface markers were used for Treg identification. 90% of CD25+CD127−CCR4+ cells co-express Foxp3, therefore CD25+CD127−CCR4+ phenotype defined Tregs.28 In analyzing T-regulatory cells (Tregs), both total Treg (CD25+CD127−) and skin-homing (CCR4+CLA+ fraction) Treg frequencies were captured. The only AD age group that showed significant increases of both total (Fig. E9A) and skin-homing (Fig E9B) Tregs vs. control was 5–12y/o (P<0.01). Developmentally, AD showed similar total Treg trends as controls, whereas discrepancies were more evident in skin-homing subsets (Fig. E9C–D).
Cytokine polarization and T-cell activation differentiate AD patients into separate age clusters along a spectrum
We integrated the T-cell and cytokine biomarkers to differentiate the entire AD cohort based on their blood phenotype. The principal components of all biomarker data for all subjects were analyzed using unsupervised k-means clustering, separately for AD patients and controls. As illustrated in Figure 8, in the AD cohort, the frequencies of different markers defined three meaningful clusters, aligning along a spectrum. While infants (pink ellipse) clustered on the far left, and adults (green ellipse) on the right, children and adolescents (blue ellipse) generally clustered together, between the infants and adults. The markers that best distinguished between each set of clusters appear in the boxes between the two cohorts and are summarized in Table E5. Th1/Th2 ratio, CD8+ activation and IFN-γ-producing T-cells differentiated adults from younger groups. Tregs, T-cell activation, and different cytokine subsets were able to differentiate distinctive AD age groups. Applying the same model to the control population did not distinguish between age groups (Fig. 8).
Figure 8.
Unsupervised clustering of AD patients and healthy controls across all principal components of the blood flow cytometry marker frequencies (%) by k-means. In AD, the frequencies of different markers defined three meaningful age-clusters, aligning along a spectrum. While infants (pink ellipse) clustered on the far left, and adults (green ellipse) on the right, children and adolescents (blue ellipse) clustered together between the other age cohorts. The markers that best distinguished between clusters appear in the boxes between two cohorts (colors of markers parallel the colors of the relative age group). Arrows designate elevated frequencies of a given marker among the specific age group. In healthy controls, clusters did not clearly align patients along an age spectrum. CLA, Cutaneous lymphocyte antigen; ICOS, Inducible co-stimulator molecule; Tregs, T-regulatory cells.
Discussion
This is the first comprehensive study that compares systemic immune profiles of different AD age groups (0–5y/o, 6–11y/o, 12–17y/o and ≥18y/o), with appropriate comparisons to controls. Since circulating CLA+ T-cells have been suggested as peripheral biomarkers in AD,29 we utilized CLA to segregate between skin-homing and systemic immune changes that accompany healthy and AD development. Our data suggest unique endotypes in infants and toddlers, children, adolescents and adults with AD, possibly advocating for personalized, endotype-driven approaches rather than “one-size-fits-all” therapeutic strategies. In addition, the results start to define age-specific differences between pediatric AD patients and age-matched controls as a complement to our recent data showing differences between elderly vs. young AD adult endotypes.3 Our data suggest that even adolescents have a different profile from adults, possibly contributing to the somewhat lower response to the recently approved IL-4Rα antagonist, dupilumab, in adolescents vs. adults.30, 31
Immune responses at birth are immature10 with decreases of naïve T-cells in healthy children with increasing age, paralleled by increases in Tcm/Tem cells.32 Skin-homing Tcm/Tem cells are higher in infants and children with AD compared to controls and decrease with age, exclusively in AD. Elevated skin-homing memory cells cluster AD infants separately from other age groups, suggesting early relevance. Excessive naïve-to-memory switch, which causes an influx of memory cells into the circulation in infancy and early childhood, may play a role in AD initiation, while exclusive decreases in the AD population over time may suggest increased migration of CLA+ Tcm/Tem cells to skin.
ICOS is involved in multiple adaptive immune responses, including induction of various T-cell polarizations,33–35 Treg regulation,36 antibody responses (including IgE switching),37 and innate lymphoid cell (ILC)2-mediated cytokine production.38, 39 ICOS has also been implicated in the pathogenesis of asthma.40, 41 We have previously shown that Tcm/Tem ICOS activation is higher in AD vs. control children.42 Subsequent data showed acclerated systemic/CLA− ICOS T-cell activation in 0–3y/o AD infants.43 Our present data support ICOS expansion in both healthy and AD development, particularly in CD4+ T-cells. However, positive correlations with age were exclusively seen in AD, and specifically in skin-homing memory subsets. Also, infants and adults with AD have higher CD4+CLA+/CLA− ICOS activation than controls, a difference that is also significant for CD8+ subsets in children. The low CD8+ frequencies in infants, which increase with time,10 may explain the earlier activation of CD4+ subsets. Early involvement of ICOS in AD indicates its possible role in disease induction via either stimulation of T-cell subset polarization, Treg modulation, IgE induction, or ILC2s activation. Furthermore, targeting ICOS/ICOS-ligand in an asthma mouse model altered T-cell differentiation and ameliorated asthma.44 Since AD often precedes asthma,45 CLA− ICOS expansion in AD infants may also be associated with non-cutaneous atopic manifestations. These data suggest the potential for ICOS targeting as a preventive or therapeutic approach for AD and atopic associations, though this is speculative and further studies are needed.
The chronic T-cell activation MHC class II antigen, HLA-DR,46 increases with age in healthy individuals.47, 48 HLA-DR activation has been shown in chronic adult AD.42 While it is not elevated in AD infants, its activation increases with age.
At birth, immune responses are Th2-biased, with low Th1/IFN-γ in both healthy newborns8, 9, 49, 50 and those who eventually develop AD.13 Our data shows that Th1 frequencies increase with age in both AD and control infants. However, and congruent with recent blood data,51 AD subjects display significantly lower IFN-γ frequencies than controls, particularly among CD4+CLA+ subsets. Conversely, CLA+Th2 cells are similarly increased across all AD age groups and are significantly higher than controls, even in infants. Systemic/CLA− Th2 cells are significantly higher in AD starting in childhood, which implies systemic Th2 activation with greater chronicity. Consistent with the less dominant role of CD8 suggested in children with early AD42 and low CD8 abundance in infants,10 Tc2 elevations are mostly prominent in adult AD. CLA+ Th1/Th2 ratio correlation with age that is consistently decreased in AD compared to control, and the decreased CLA+/CLA− Th1/Th2 ratio among all AD age groups best reflect the immune imbalance that accompanies AD from birth to adulthood.
Circulating Th9 cells have skin-homing predilection.52, 53 IL-9 is elevated in both skin and serum of children with AD, and was also correlated with disease severity.54, 55 While the mechanism by which IL-9 contributes to AD is unknown, IL-9 is linked with mast cell, eosinophil and ILC function and has been thought to play a role in AD,56 as well as in other atopic disorders.57–60 Accordingly, IL-9 subsets cluster closest to all AD clinical measures (eosinophils/pruritus/SCORAD/EASI/TEWL). Both normal and AD development are characterized by IL-9 increases with age, which drop in adulthood. We show considerably elevated systemic/CLA− T-cells exclusively among the 5–12y/o AD group vs. controls, consistent with early allergy development in childhood with a subsequent decline in adulthood.61, 62 CLA− systemic activation and cytokine polarization with age support the systemic nature of AD and need for systemic therapeutic approaches in moderate-to-severe disease, as well as the association of comorbidities and increased inflammatory and cardiovascular-associated proteins in more chronic AD.63–66
Black et al.67 showed strong Th17 skewing early in life. While similar CLA+Th17 frequencies are seen in infants and older individuals regardless of AD, systemic/CLA− Th17 cells are relatively low in infants of both groups and mature with time. IL-17 signatures characterize pediatric AD skin,55 but these changes are not mirrored in the circulation. This discrepancy may be due to increased skin residence of Th17 cells, or by reciprocal peripheral regulation of Th17 and Th2 axes.68, 69
Skin-homing Th22 is elevated in children, adolescents and adults with AD vs. controls. Congruent with past studies showing that IL-22 is correlated with AD severity,70 we present positive correlations between IL-22-producing CD4+ cells, SCORAD, EASI and pruritus. CD8+ T-cells are major producers of IL-22 in adult AD skin.70 Lower proportions of CD8+ vs. CD4+ in early childhood47, 71 and the fact that IL-22 marks AD chronicity42 may explain why skin-homing Th22/Tc22 cell frequencies are highest in adolescents and adults. Elevated IL-22 levels, shown to negatively regulate IFN-γ,72 may also contribute to the low Th1 frequencies in AD. Systemic/CLA− Th22/Tc22 subset development was similar in AD and controls, potentially suggesting a greater pathogenic relevance of IL-22 in AD skin73 and the impaired skin barrier.74 Studies have shown positive correlations and cellular co-production of IL-13 and IL-2222, 75 and of IL-17 and IL-22.27, 76 Indeed, these markers cluster together in the correlation heatmap. IFN-γ clusters with patient age and disease duration, differentiating infants from children and adolescents, and adolescents from AD adults. The association of IFN-γ with disease chronicity agrees with previous chronic adult AD data, suggesting that IFN-γ possibly plays a role in inflammatory disorders chronicity, rather than in disease intiation.26, 42, 77 Expansion of CD8+ memory cells, increases in CLA+IL-22-producing cells, and intensification of ICOS+/HLA-DR+ activation with maturity may account for the positive correlations between age, disease duration and severity measures. Increasing severity with age was not seen in a recent study that followed AD infants only between 0–11 months.78 TEWL increases with age in AD and correlates with IL-22 (which also increases with age), likely again reflecting the contribution of IL-22 to the barrier impairment in AD.
Besides maintaining immune tolerance,79 Tregs influence activation of effector cells.80, 81 Only 5–12y/o children with AD show significantly higher total and skin homing Tregs than controls. This age group is characterized by other unique features, including increased skin-homing Tcm/Tem cells, decreased systemic IFN-γ, and increased systemic IL-9 frequencies. Additionally, skin-homing IFN-γ is almost doubled from infancy to childhood AD, although still below age-matched controls. Multiple immunological changes occurring during these years may be involved in AD clearance, or development of non-cutaneous manifestations, commonly occurring during these years.82, 83
Profiling AD across ages is imperative for targeted therapeutic development. Unlike psoriasis, in which targeted treatments lead to remarkable responses in most patients, AD responses to targeted therapeutics are much lower.84 This disparity may be attributed to the multi-cytokine activation in AD, despite the shared Th2 activation, vs. the Th17-centered responses in psoriasis.84 Additionally, AD has a highly varied endotype repertoire, with different immune polarizations.3, 85, 86 Despite common features, particularly elevated Th2 expression, AD is endotypically different across ages, and treatments should rather be tailored to the unique age endotype. While one could hypothesize that the immune changes merely reflect developmental phenomena that are age-related, the lack of clear clustering in controls implicates AD as the driver of the distinct, progressive age-related endotypic characteristics rather than age alone. The clustering model, based on the flow cytometry biomarkers splits the entire cohort into 3 separate age clusters only in AD patients. Expectedly, IFN-γ, IL-22, HLA-DR increased chronologically, distinguishing older subjects from infants with AD. Skin-homing Tcm/Tem cells and systemic ICOS activation, both higher in AD infants, separate infants from other groups. This model demonstrates that a limited group of blood biomarkers can distinguish among various AD endotypes based on patient age, with a spectrum of age-dependent vs. only ‘infantile’ or ‘adult’ phenotypes. Because our data show that the ‘adult’ or ‘stable’ AD phenotype is only achieved in adulthood, it may be possible to intervene before adulthood and prevent the establishment of the adult AD phenotype.
This study has a few limitations. Despite inclusion of different age groups, this study was not longitudinal and thus did not follow the same cohort with time. Additionally, studies in blood do not allow one to measure T-cell subsets in skin, such as tissue-resident memory/Trm T-cells. Furthermore, the study characterized polyclonal T-cell responses, and not antigen-specific responses induced by culprit triggers. Finally, the pathogenicity of immune axes presented here cannot be further dissected without future targeted therapeutic studies to check mechanism.
AD was initially considered an early-onset pediatric disease with 75% “outgrowing” their disease by 10y/o.2, 83, 87, 88 More recent studies have established AD as a disorder that often persists into adulthood.89, 90 Comparing the profile of cleared vs. persistent pediatric AD, ideally through longitudinal studies, will better define age-specific characteristics that predict AD clearance.
Supplementary Material
Clinical Implications:
Diverse immune signatures in different pediatric and adult atopic dermatitis age groups argue for age-specific, rather than uniform, therapeutic interventions.
Funding:
This study was funded by a grant from the LEO Foundation. The study was also supported by the Northwestern Skin Disease Research Center (NIAMS P30 AR057216) and the Northwestern University Clinical And Translational Sciences (NUCATS) Institute (UL1TR001422). JGK has received research support (grants paid to his institution) and/or personal fees from Pfizer, Amgen, Janssen, Lilly, Merck, Novartis, Kadmon, Dermira, Boehringer, Innovaderm, Kyowa, BMS, Serono, BiogenIdec, Delenex, AbbVie, Sanofi, Baxter, Paraxel, Xenoport, and Kineta. ASP is an investigator for Galderma, Incyte, Leo, Pfizer, and Regeneron (grants paid to Northwestern Univ.) and has received honoraria related to AD consulting from AbbVie, Asana, Boehringer, Dermavant, Dermira, Eli Lilly, Forte, Galderma, Leo, Matrisys, Menlo Therapeutics, Morphosys/Galapagos, Novartis, Pfizer, Regeneron, and Sanofi-Genzyme. EGY is an employee of Mount Sinai and has received research funds (grants paid to the institution) from Abbvie, Asana Biosciences, Celgene, Dermavant, DS Biopharma, Galderma, Glenmark, Innovaderm, Janssen Biotech, Leo Pharma, Novan, Novartis, Pfizer, Ralexar, Regeneron, and Union Therapeutics. EGY is also a consultant for Abbvie, Allergan, Amgen, Asana Biosciences, Celgene, Concert, DBV Technologies, Dermira, DS Biopharma, Eli Lilly, EMD Serono, Escalier, Flx Bio, Galderma, Glenmark, Kyowa Kirin, Leo Pharma, Mitsubishi Tanabe, Novartis, Pfizer, Regeneron, Sanofi, and Union Therapeutics.
Abbreviations
- AD
Atopic dermatitis
- ADQ
Atopic dermatitis quickscore
- CD69
Cluster of Differentiation 69
- CLA
Cutaneous Lymphocyte Antigen
- EASI
Eczema area and severity index
- HLA-DR
human leukocyte antigen DR
- IFN-γ
Interferon gamma
- IL
Interleukin
- ICOS
Inducible co-stimulator molecule
- ILC
Innate lymphoid cell
- MHC
Major histocompatibility complex
- PBMC
Peripheral Blood Mononuclear Cell
- SCORAD
SCORing of Atopic Dermatitis
- Tc1
Type 1 Cytotoxic T-cell
- Tc17
Type 17 cytotoxic T-cell
- Tc2
Type 2 cytotoxic T-cell
- Tc22
Type 22 cytotoxic T-cell
- TEWL
Transepidermal water loss
- Tc
T cytotoxic
- Th
T helper
- Y/o
Years old
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Other authors have declared that they have no conflict of interest.
References
- 1.Simon AK, Hollander GA, McMichael A. Evolution of the immunesystem in humans from infancy to old age. Proc Biol Sci 2015; 282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieber T Atopic dermatitis. Ann Dermatol 2010; 22:125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou L, Leonard A, Pavel AB, Malik K, Raja A, Glickman J, et al. Age-specific changes in the molecular phenotype of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2019; 144:144–156. [DOI] [PubMed] [Google Scholar]
- 4.Davidson WF, Leung DYM, Beck LA, Berin CM, Boguniewicz M, Busse WW, et al. Report from the National Institute of Allergy and Infectious Diseases Workshop on “Atopic Dermatitis and the Atopic March: Mechanisms and Interventions”. J Allergy Clin Immunol 2019; 143:894–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandmand M, Bruunsgaard H, Kemp K, Andersen-Ranberg K, Pedersen AN, Skinhoj P, et al. Is ageing associated with a shift in the balance between Type 1 and Type 2 cytokines in humans? Clin Exp Immunol 2002; 127:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamoto N, Kaneko H, Takemura M, Seishima M, Sakurai S, Fukao T, et al. Age-related changes in intracellular cytokine profiles and Th2 dominance in allergic children. Pediatr Allergy Immunol 2006; 17:125–33. [DOI] [PubMed] [Google Scholar]
- 7.Chipeta J, Komada Y, Zhang XL, Deguchi T, Sugiyama K, Azuma E, et al. CD4+ and CD8+ cell cytokine profiles in neonates, older children, and adults: increasing T helper type 1 and T cytotoxic type 1 cell populations with age. Cell Immunol 1998; 183:149–56. [DOI] [PubMed] [Google Scholar]
- 8.Kotiranta-Ainamo A, Rautonen J, Rautonen N. Imbalanced cytokine secretion in newborns. Biol Neonate 2004; 85:55–60. [DOI] [PubMed] [Google Scholar]
- 9.Marodi L Down-regulation of Th1 responses in human neonates. Clin Exp Immunol 2002; 128:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasparoni A, Ciardelli L, Avanzini A, Castellazzi AM, Carini R, Rondini G, et al. Age-related changes in intracellular TH1/TH2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol Neonate 2003; 84:297–303. [DOI] [PubMed] [Google Scholar]
- 11.Debock I, Flamand V. Unbalanced Neonatal CD4(+) T-Cell Immunity. Front Immunol 2014; 5:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Velden VH, Laan MP, Baert MR, de Waal Malefyt R, Neijens HJ, Savelkoul HF. Selective development of a strong Th2 cytokine profile in high-risk children who develop atopy: risk factors and regulatory role of IFN-gamma, IL-4 and IL-10. Clin Exp Allergy 2001; 31:997–1006. [DOI] [PubMed] [Google Scholar]
- 13.Herberth G, Heinrich J, Roder S, Figl A, Weiss M, Diez U, et al. Reduced IFN-gamma- and enhanced IL-4-producing CD4+ cord blood T cells are associated with a higher risk for atopic dermatitis during the first 2 yr of life. Pediatr Allergy Immunol 2010; 21:5–13. [DOI] [PubMed] [Google Scholar]
- 14.Tang ML, Kemp AS, Thorburn J, Hill DJ. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet 1994; 344:983–5. [DOI] [PubMed] [Google Scholar]
- 15.Kaminishi K, Soma Y, Kawa Y, Mizoguchi M. Flow cytometric analysis of IL-4, IL-13 and IFN-gamma expression in peripheral blood mononuclear cells and detection of circulating IL-13 in patients with atopic dermatitis provide evidence for the involvement of type 2 cytokines in the disease. J Dermatol Sci 2002; 29:19–25. [DOI] [PubMed] [Google Scholar]
- 16.Katsunuma T, Kawahara H, Yuki K, Akasawa A, Saito H. Impaired interferon-gamma production in a subset population of severe atopic dermatitis. Int Arch Allergy Immunol 2004; 134:240–7. [DOI] [PubMed] [Google Scholar]
- 17.Jung T, Lack G, Schauer U, Uberuck W, Renz H, Gelfand EW, et al. Decreased frequency of interferon-gamma- and interleukin-2-producing cells in patients with atopic diseases measured at the single cell level. J Allergy Clin Immunol 1995; 96:515–27. [DOI] [PubMed] [Google Scholar]
- 18.Glines KR, Stiff KM, Freeze M, Cline A, Strowd LC, Feldman SR. An update on the topical and oral therapy options for treating pediatric atopic dermatitis. Expert Opin Pharmacother 2019:1–9. [DOI] [PubMed] [Google Scholar]
- 19.Siegfried EC, Igelman S, Jaworsk JC, Antaya RJ, Cordoro KM, Eichenfield LF, et al. Use of dupilimab in pediatric atopic dermatitis: Access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol 2019; 36:172–6. [DOI] [PubMed] [Google Scholar]
- 20.Carel K, Bratton DL, Miyazawa N, Gyorkos E, Kelsay K, Bender B, et al. The Atopic Dermatitis Quickscore (ADQ): validation of a new parent-administered atopic dermatitis scoring tool. Ann Allergy Asthma Immunol 2008; 101:500–7. [DOI] [PubMed] [Google Scholar]
- 21.Laudanska H, Reduta T, Szmitkowska D. Evaluation of skin barrier function in allergic contact dermatitis and atopic dermatitis using method of the continuous TEWL measurement. Rocz Akad Med Bialymst 2003; 48:123–7. [PubMed] [Google Scholar]
- 22.Czarnowicki T, Gonzalez J, Shemer A, Malajian D, Xu H, Zheng X, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol 2015; 136:104–15. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22:745–63. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708–12. [DOI] [PubMed] [Google Scholar]
- 25.Ferenczi K, Burack L, Pope M, Krueger JG, Austin LM. CD69, HLA-DR and the IL-2R identify persistently activated T cells in psoriasis vulgaris lesional skin: Blood and skin comparisons by flow cytometry. J Autoimmun 2000; 14:63–78. [DOI] [PubMed] [Google Scholar]
- 26.Czarnowicki T, He HY, Wen HC, Hashim PW, Nia JK, Malik K, et al. Alopecia areata is characterized by expansion of circulating Th2/Tc2/Th22, within the skin-homing and systemic T-cell populations. Allergy 2018; 73:713–23. [DOI] [PubMed] [Google Scholar]
- 27.Czarnowicki T, He H, Leonard A, Kim HJ, Kameyama N, Pavel AB, et al. Blood endotyping distinguishes the profile of vitiligo from that of other inflammatory and autoimmune skin diseases. J Allergy Clin Immunol 2019; 143:2095–2107. [DOI] [PubMed] [Google Scholar]
- 28.Gorski KTJ, Gavin M, Cottrell S, Cox C, Vincent M, Ferbas J. CCR4 improves phenotypic identification of T-regulatory cells; Validation and implementation of clinical test. Journal Immunol 2010; 184:26.19949081 [Google Scholar]
- 29.Ferran M, Romeu ER, Rincon C, Sagrista M, Arnau AMG, Celada A, et al. Circulating CLA+ T lymphocytes as peripheral cell biomarkers in T-cell-mediated skin diseases. Exp Dermatol 2013; 22:439–42. [DOI] [PubMed] [Google Scholar]
- 30.Simpson EPA, Siegfried EC, Boguniewicz M, Pariser D, Blauvelt A, Hultsch T, Staudinger H, Zhang R, Kamal MA, Davis JD, Ruddy M, Graham NMH, Bansal A. Dupilumab Efficacy and Safety in Adolescents with Moderate-to-Severe Atopic Dermatitis: Results from a Multicenter, Randomized, Placebo-Controlled, Double-Blind, Parallel-Group, Phase 3 Study. Paris, France 2018; 09/15. [Google Scholar]
- 31.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med 2016; 375:2335–48. [DOI] [PubMed] [Google Scholar]
- 32.Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev 2006; 127:274–81. [DOI] [PubMed] [Google Scholar]
- 33.Vieira PL, Wassink L, Smith LM, Nam S, Kingsbury GA, Gutierrez-Ramos JC, et al. ICOS-mediated signaling regulates cytokine production by human T cells and provides a unique signal to selectively control the clonal expansion of Th2 helper cells. Eur J Immunol 2004; 34:1282–90. [DOI] [PubMed] [Google Scholar]
- 34.Paulos CM, Carpenito C, Plesa G, Suhoski MM, Varela-Rohena A, Golovina TN, et al. The Inducible Costimulator (ICOS) Is Critical for the Development of Human T(H)17 Cells. Sci Trans Med 2010; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wikenheiser DJ, Ghosh D, Kennedy B, Stumhofer JS. The Costimulatory Molecule ICOS Regulates Host Th1 and Follicular Th Cell Differentiation in Response to Plasmodium chabaudi chabaudi AS Infection. J Immunol 2016; 196:778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vocanson M, Rozieres A, Hennino A, Poyet G, Gaillard V, Renaudineau S, et al. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J Allergy Clin Immunol 2010; 126:280–9. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi T, Iijima K, Dent AL, Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol 2017; 139:300–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wikenheiser DJ, Stumhofer JS. ICOS Co-Stimulation: Friend or Foe? Front Immunol 2016; 7:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson TR, Quezada SA, Allison JP. Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS). Curr Opin Immunol 2010; 22:326–32. [DOI] [PubMed] [Google Scholar]
- 40.Coyle AJ, Gutierrez-Ramos JC. The role of ICOS and other costimulatory molecules in allergy and asthma. Springer Semin Immunopathol 2004; 25:349–59. [DOI] [PubMed] [Google Scholar]
- 41.Maazi H, Akbari O. ICOS regulates ILC2s in asthma. Oncotarget 2015; 6:24584–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Czarnowicki T, Esaki H, Gonzalez J, Malajian D, Shemer A, Noda S, et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)(+) TH2/TH1 cell imbalance, whereas adults acquire CLA(+) TH22/TC22 cell subsets. J Allergy Clin Immunol 2015; 136:941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esaki H, Czarnowicki T, Gonzalez J, Oliva M, Talasila S, Haugh I, et al. Accelerated T-cell activation and differentiation of polar subsets characterizes early atopic dermatitis development. J Allergy Clin Immunol 2016; 138:1473–7. [DOI] [PubMed] [Google Scholar]
- 44.Uwadiae FI, Pyle CJ, Walker SA, Lloyd CM, Harker JA. Targeting the ICOS/ICOS-L pathway in a mouse model of established allergic asthma disrupts T follicular helper cell responses and ameliorates disease. Allergy 2019; 74:650–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czarnowicki T, Krueger JG, Guttman-Yassky E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J Allergy Clin Immunol 2017; 139:1723–34. [DOI] [PubMed] [Google Scholar]
- 46.Ferenczi K, Burack L, Pope M, Krueger JG, Austin LM. CD69, HLA-DR and the IL-2R identify persistently activated T cells in psoriasis vulgaris lesional skin: blood and skin comparisons by flow cytometry. J Autoimmun 2000; 14:63–78. [DOI] [PubMed] [Google Scholar]
- 47.Hulstaert F, Hannet I, Deneys V, Munhyeshuli V, Reichert T, De Bruyere M, et al. Age-related changes in human blood lymphocyte subpopulations. II. Varying kinetics of percentage and absolute count measurements. Clin Immunol Immunopathol 1994; 70:152–8. [DOI] [PubMed] [Google Scholar]
- 48.Erkeller-Yuksel FM, Deneys V, Yuksel B, Hannet I, Hulstaert F, Hamilton C, et al. Age-related changes in human blood lymphocyte subpopulations. J Pediatr 1992; 120:216–22. [DOI] [PubMed] [Google Scholar]
- 49.Protonotariou E, Malamitsi-Puchner A, Rizos D, Papagianni B, Moira E, Sarandakou A, et al. Age-related differentiations of Th1/Th2 cytokines in newborn infants. Mediators Inflamm 2004; 13:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adkins B Development of neonatal Th1/Th2 function. Int Rev Immunol 2000; 19:157–71. [DOI] [PubMed] [Google Scholar]
- 51.Brunner PM, Israel A, Leonard A, Pavel AB, Kim HJ, Zhang N, et al. Distinct transcriptomic profiles of early-onset atopic dermatitis in blood and skin of pediatric patients. Ann Allergy Asthma Immunol 2019; 122:318–30. [DOI] [PubMed] [Google Scholar]
- 52.Schlapbach C, Gehad A, Yang C, Watanabe R, Guenova E, Teague JE, et al. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med 2014; 6:219ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong MT, Ong DE, Lim FS, Teng KW, McGovern N, Narayanan S, et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity 2016; 45:442–56. [DOI] [PubMed] [Google Scholar]
- 54.Ciprandi G, De Amici M, Giunta V, Marseglia A, Marseglia G. Serum interleukin-9 levels are associated with clinical severity in children with atopic dermatitis. Pediatr Dermatol 2013; 30:222–5. [DOI] [PubMed] [Google Scholar]
- 55.Esaki H, Brunner PM, Renert-Yuval Y, Czarnowicki T, Huynh T, Tran G, et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol 2016; 138:1639–51. [DOI] [PubMed] [Google Scholar]
- 56.Ma L, Xue HB, Guan XH, Shu CM, Zhang JH, Yu J. Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol 2014; 175:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark RA, Schlapbach C. TH9 cells in skin disorders. Semin Immunopathol 2017; 39:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujisawa T, Katsumata H, Kato Y. House dust mite extract induces interleukin-9 expression in human eosinophils. Allergol Int 2008; 57:141–6. [DOI] [PubMed] [Google Scholar]
- 59.Brough HA, Cousins DJ, Munteanu A, Wong YF, Sudra A, Makinson K, et al. IL-9 is a key component of memory TH cell peanut-specific responses from children with peanut allergy. J Allergy Clin Immunol 2014; 134:1329–38. [DOI] [PubMed] [Google Scholar]
- 60.Xie J, Lotoski LC, Chooniedass R, Su RC, Simons FE, Liem J, et al. Elevated antigen-driven IL-9 responses are prominent in peanut allergic humans. PLoS One 2012; 7:e45377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walton S, Nayagam AT, Keczkes K. Age and sex incidence of allergic contact dermatitis. Contact Dermatitis 1986; 15:136–9. [DOI] [PubMed] [Google Scholar]
- 62.Statescu L, Branisteanu D, Dobre C, Solovastru LG, Vasilca A, Petrescu Z, et al. Contact dermatitis - epidemiological study. Maedica (Buchar) 2011; 6:277–81. [PMC free article] [PubMed] [Google Scholar]
- 63.Thijs JL, Strickland I, Bruijnzeel-Koomen C, Nierkens S, Giovannone B, Knol EF, et al. Serum biomarker profiles suggest that atopic dermatitis is a systemic disease. J Allergy Clin Immunol 2018; 141:1523–6. [DOI] [PubMed] [Google Scholar]
- 64.Vekaria AS, Brunner PM, Aleisa AI, Bonomo L, Lebwohl MG, Israel A, et al. Moderate-to-severe atopic dermatitis patients show increases in serum C-reactive protein levels, correlating with skin disease activity. F1000Res 2017; 6:1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brunner PM, Suarez-Farinas M, He H, Malik K, Wen HC, Gonzalez J, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep 2017; 7:8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brunner PM, Silverberg JI, Guttman-Yassky E, Paller AS, Kabashima K, Amagai M, et al. Increasing Comorbidities Suggest that Atopic Dermatitis Is a Systemic Disorder. J Invest Dermatol 2017; 137:18–25. [DOI] [PubMed] [Google Scholar]
- 67.Black A, Bhaumik S, Kirkman RL, Weaver CT, Randolph DA. Developmental regulation of Th17-cell capacity in human neonates. Eur J Immunol 2012; 42:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lynch JP, Ferreira MA, Phipps S. Th2/Th17 reciprocal regulation: twists and turns in the complexity of asthma phenotypes. Ann Transl Med 2016; 4:S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med 2015; 7:301ra129. [DOI] [PubMed] [Google Scholar]
- 70.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 2009; 123:1244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weerkamp F, de Haas EF, Naber BA, Comans-Bitter WM, Bogers AJ, van Dongen JJ, et al. Age-related changes in the cellular composition of the thymus in children. J Allergy Clin Immunol 2005; 115:834–40. [DOI] [PubMed] [Google Scholar]
- 72.Pennino D, Bhavsar PK, Effner R, Avitabile S, Venn P, Quaranta M, et al. IL-22 suppresses IFN-gamma-mediated lung inflammation in asthmatic patients. J Allergy Clin Immunol 2013; 131:562–70. [DOI] [PubMed] [Google Scholar]
- 73.Lou H, Lu J, Choi EB, Oh MH, Jeong M, Barmettler S, et al. Expression of IL-22 in the Skin Causes Th2-Biased Immunity, Epidermal Barrier Dysfunction, and Pruritus via Stimulating Epithelial Th2 Cytokines and the GRP Pathway. J Immunol 2017; 198:2543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fujita H The role of IL-22 and Th22 cells in human skin diseases. J Dermatol Sci 2013; 72:3–8. [DOI] [PubMed] [Google Scholar]
- 75.Teraki Y, Sakurai A, Izaki S. IL-13/IL-22-coproducing T cells, a novel subset, are increased in atopic dermatitis. J Allergy Clin Immunol 2013; 132:971–4. [DOI] [PubMed] [Google Scholar]
- 76.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006; 203:2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012; 130:1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hulshof L, Overbeek SA, Wyllie AL, Chu M, Bogaert D, de Jager W, et al. Exploring Immune Development in Infants With Moderate to Severe Atopic Dermatitis. Front Immunol 2018; 9:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol 2011; 187:2061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McHugh RS, Shevach EM. The role of suppressor T cells in regulation of immune responses. Journal of Allergy and Clinical Immunology 2002; 110:693–702. [DOI] [PubMed] [Google Scholar]
- 81.Hall BM. T Cells: Soldiers and Spies--The Surveillance and Control of Effector T Cells by Regulatory T Cells. Clin J Am Soc Nephrol 2015; 10:2050–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burr ML, Dunstan FD, Hand S, Ingram JR, Jones KP. The natural history of eczema from birth to adult life: a cohort study. Br J Dermatol 2013; 168:1339–42. [DOI] [PubMed] [Google Scholar]
- 83.Carlsten C, Dimich-Ward H, Ferguson A, Watson W, Rousseau R, Dybuncio A, et al. Atopic dermatitis in a high-risk cohort: natural history, associated allergic outcomes, and risk factors. Ann Allergy Asthma Immunol 2013; 110:24–8. [DOI] [PubMed] [Google Scholar]
- 84.Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol 2017; 48:68–73. [DOI] [PubMed] [Google Scholar]
- 85.Czarnowicki T, He H, Krueger JG, Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol 2019; 143:1–11. [DOI] [PubMed] [Google Scholar]
- 86.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol 2014; 134:769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part I: clinical and pathologic concepts. J Allergy Clin Immunol 2011; 127:1110–8. [DOI] [PubMed] [Google Scholar]
- 88.Burr ML, Dunstan FD, Hand S, Ingram JR, Jones KP. The natural history of eczema from birth to adult life: a cohort study. Br J Dermatol 2013; 168:1339–42. [DOI] [PubMed] [Google Scholar]
- 89.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol 2014; 150:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abuabara K, Margolis DJ. Do children really outgrow their eczema, or is there more than one eczema? J Allergy Clin Immunol 2013; 132:1139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.