Summary
Interactions between melanocytes and neighboring cells in the skin (keratinocytes and fibroblasts) play important roles in regulating human skin color. We recently reported that neuregulin-1 (NRG1) is highly expressed in fibroblasts from Fitzpatrick type VI skin (the darkest) and at least in part determines the constitutive color of human skin. We have now characterized the bioactive motif of NRG1 that is involved in modulating melanin production in human melanocytes. We found that 8-mer motifs (PSRYLCKC and LCKCPNEF) increased melanin production but did not increase the proliferation of melanocytes; the minimum fragment that could elicit that effect was the tetrapeptide LCKC. This smaller bioactive peptide might have an advantage in clinical applications in which it modulates only pigmentation and does not stimulate melanocyte proliferation.
Interactions between melanocytes and neighboring cells in the skin (keratinocytes and fibroblasts) play important roles in the regulation of skin color in humans (Imokawa, 2004; Yamaguchi and Hearing, 2009; Yamaguchi et al., 2007). A large number of secreted paracrine factors have been shown to be involved in regulating pigment production in response to environmental cues and also to control topological differences in skin color. We recently reported that neuregulin-1 (NRG1) is highly expressed and secreted by fibroblasts derived from Fitzpatrick type VI skin (the darkest) and plays an important role in regulating the constitutive pigmentation of human skin (Choi et al., 2010). NRG1 has been characterized as an essential protein that is involved in regulating the development of the nervous system, heart, breast, and other organ systems (Esper et al., 2006; Falls, 2003). We showed that treatment of melanocyte cultures and of 3D skin reconstructs with recombinant human NRG1 (rhNRG1) increased the proliferation and the pigmentation of melanocytes. We also found that NRG1 increases levels of phospho-Akt and completely abrogates the expression of ErbB2, one of the receptors activated by NRG1.
Type I β1 NRGs are initially synthesized as transmembrane precursors called proNRGs, which have a COOH-terminal cytoplasmic domain and a single membrane spanning transmembrane domain. proNRG is cleaved just outside the transmembrane domain, and the soluble polypeptide, which contains the immunoglobulin-like domain (residues 50–114, gray box in Figure 1A) and the calcium-binding EGF-like domain (residues 190–221, open box in Figure 1A), is released (Li and Loeb, 2001). It is known that the EGF domain of NRG1 is sufficient to activate the downstream signaling via ErbB receptors (Yarden and Sliwkowski, 2001). In this study, we characterized the bioactive motif of NRG1 that is involved in the modulation of melanin production in human melanocytes. For that purpose, we synthesized overlapping peptides of various sizes that encompass the EGF domain of NRG1 and measured changes in melanin production and melanocyte growth elicited by those peptides.
Figure 1.
Sequences of human NRG1 and 16-mer peptides studied. (A) The full sequence of human NRG1 is shown, with the Heparinbinding IG-like domain (residues 50–114, gray box) and the calcium-binding EGF-like domain (residues 190–221, unfilled box) identified. Note that there are two sequences reported for the EGF domain that differ at 4 residues (underlined). (B) Sequence of the EGF domain of NRG1 and overlapping 16-mer peptides (WC1–WC4). (C) Changes in melanin production elicited by 16-mer peptides. Darkly pigmented human melanocytes (HEM-DP) were treated with rhNRG1 (6.25 nM) or 16-mer peptides (100 nM) every 2 days for 8 days. Control samples were treated with vehicle only (0.1% BSA in PBS). N = 4. Melanocytes were dissolved in 200 ll 1N NaOH, and melanin concentrations were measured by absorbance at 405 nm using a spectrophotometer, as previously detailed (Virador et al., 1999). Melanin content is expressed as lg melanin per ml NaOH. Student’s paired t-test was used for all statistical analyses. N = 4. *P < 0.05; **P < 0.01.
The commercially available rhNRG1 (R&D Systems, Minneapolis, MN, USA) used in our previous study that activates pigment production by melanocytes contains the EGF domain plus several residues on either side of that. To identify the bioactive motif of NRG1 within that EGF domain, we first designed four 16-mer peptides that each overlapped by 8 residues within the EGF domain (Figure 1B). Two different sequences for the EGF domain of NRG1 have been reported (NP_039250 [Sequence I] and Q02297 [Sequence II]), which differ at residues 213–215 (PNE versus QPG) and residue 219 (D versus A). Therefore, we designed two different peptides for residues 206–221; all peptides were synthesized at >95% purity by Biosynthesis (Lewisdale, TX, USA) and were dissolved in water prior to use. None of the peptides was further modified, including CYS. We treated darkly pigmented human melanocytes (HEM-DP; Cascade Biologics, Portland, OR, USA) grown in culture (Choi et al., 2010) with those peptides (at 100 nM) every other day for 8 days (n = 4) and then measured the melanin content to check the bioactivity of each peptide, as previously described (Virador et al., 1999). rhNRG1 (residues 176–246) was used at 50 ng ⁄ ml [= 6.25 nM, the same concentration as our previous study (Choi et al., 2010)], as a positive control. Treatment with rhNRG1 or with WC3 (residues 197–212) resulted in significant increases in melanin content (Figure 1C).
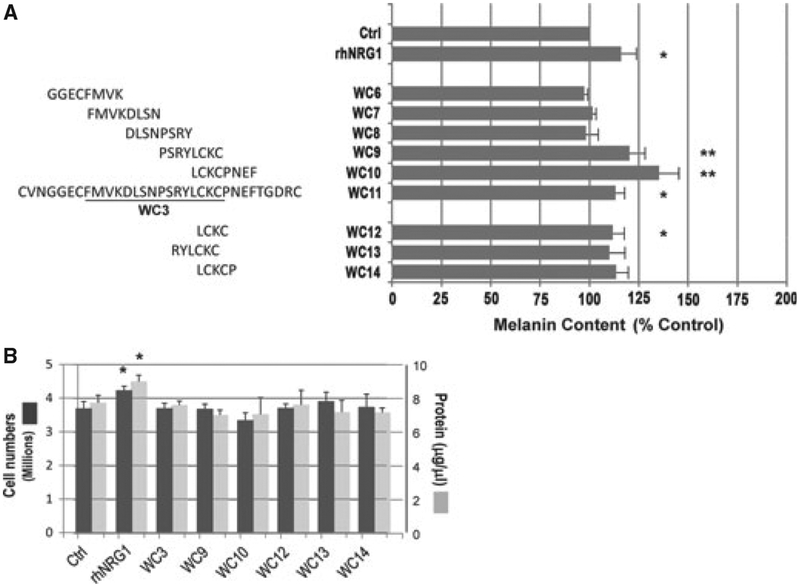
We then synthesized five 8-mer peptides around the WC3 region to further narrow down the bioactive motif. WC6 includes 4 residues preceding WC3 and the initial 4 residues of WC3, while WC10 includes the last 4 residues of WC3 and the following 4 residues (Figure 2A). Those peptides were also used at 100 nM, and again, darkly pigmented human melanocytes were treated every day for 8 days (n = 3). As positive controls, we used WC11, which is the EGF domain of NRG1, at 100 nM, as well as rhNRG1 at 6.25 nM. The results reveal that WC9 and WC10, as well as the full EGF domain (WC11), and rhNRG1, significantly increased the melanin content.
Figure 2.
Effect of NRG1 smaller peptides on pigmentation. (A) Upper, sequences of overlapping 8-mer peptides (WC6–WC10) and WC11, which cover entire EGF domain of NRG1. Right, melanin production elicited by treatment with 8-mer peptides; treatments and assay conditions are as described for Figure 1. Data are reported as % control. WC9, N = 6, all others, N = 3. *P < 0.05; **P < 0.01. Lower, effects of peptides WC12, WC13 and WC14, presented as reported previously. WC12, N = 6, all others, N = 3. *P < 0.05; (B) Cell and total protein concentrations after 8 days of treatment with various peptides, rhNRG1 or control. After cell number counting, melanocytes were solubilized in lysis buffer (M-per by Thermo Fisher Scientific, Rockford, IL, USA) and, after centrifugation, the supernatants were used to measure protein concentration. N = 3. *P < 0.05.
Finally, we designed 4- to 6-mer peptides around the 4 residues (LCKC) that are common in WC9 and WC10 (Figure 2A, bottom). We treated darkly pigmented human melanocytes with those peptides at 100 nM every day for 8 days (n = 3). After multiple experiments (in triplicate each time), we found that each of those 4- to 6-mer peptides increased melanin content to comparable levels (10–15% above controls), although only WC12 (LCKC) did so at a statistically significant level. We suspect that the variability in effects of WC10,WC11, and WC12, and their reduced efficacy compared with the longer peptides (WC9 and WC10) reflect problems with stability and ⁄ or decreased binding affinity to the receptor.
We then examined whether any of these peptides affected the proliferation of human melanocytes. Interestingly, none of the smaller peptides increased the proliferation of melanocytes although rhNRG1 significantly increased the proliferation compared with the control (Figure 2B, dark bars), as we reported before (Choi et al., 2010). Similarly, the total protein content of each sample did not change after treatment with the smaller peptides while treatment with rhNRG1 significantly increased the total protein content (Figure 2B, light bars). Overall, our results show that the WC3, WC9 and WC10 peptides increase only the pigmentation while the full-length rhNRG1 increases both the proliferation and the pigmentation of melanocytes.
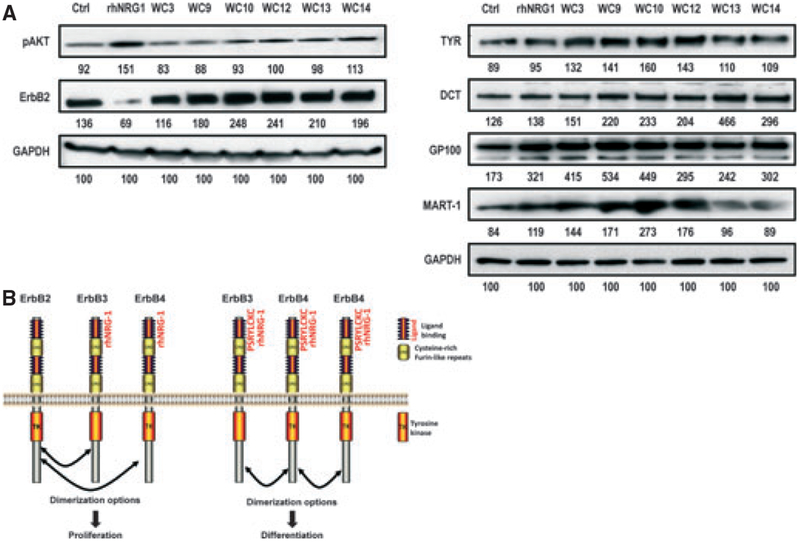
We then examined levels of pAkt and ErbB2 to see whether they were changed by the smaller NRG1 peptides compared with rhNRG1. In our previous study (Choi et al., 2010), we showed that the rhNRG1-treated melanocytes maintained higher levels of pAkt compared with controls, even after the cells became confluent. Interestingly, treatment with WC3 or WC9 showed the same level of pAkt as the control, while treatment with rhNRG1 showed the highest increase in pAkt (as we reported before) and the other smaller peptides moderately increased the pAkt level (Figure 3A, left). The change in ErbB2 level elicited by rhNRG1 was not elicited by the smaller peptides. These results confirm that treatment with rhNRG1 completely abrogates ErbB2 levels; however, none of the smaller peptides had such an effect on ErbB2 protein levels. The peptides that are 8-mers or smaller (WC9, WC10, WC12, WC13, and WC14) actually increased the level of ErbB2 (about 2fold). The significance and mechanism of the increase in ErbB2 level by peptides need to be further investigated.
Figure 3.
Effect of NRG1 peptides on expression levels of pAkt, ErbB2, and various melanocyte-specific proteins. (A) Left, after 8 days of treatment of darkly pigmented melanocytes with peptides, rhNRG1 or control (vehicle only), proteins were extracted and western blotting was used to detect levels of pAkt, ErbB2 and GAPDH (used as a loading control) as previously detailed (Choi et al., 2010). Numbers below each band reflect the quantitation of that band corrected for loading against GAPDH. Antibodies used were pAkt (Ser473) (1:1000; Cell Signaling Technology, Danvers, MA, USA), ErbB2 rabbit monoclonal (1:1000; Cell Signaling Technology) and GAPDH (FL-335) (1:10 000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Right, levels of tyrosinase, DCT, GP100, MART1, and GAPDH (used as a loading control). Numbers below each band reflect the quantitation of that band corrected for loading against GAPDH. Antibodies used were aPEP7h (1:5000) for TYR, aPEP8h (1:3000) for TRP2 and aPEP13h (1:3000) for GP100, as previously described (Virador et al., 2001), as well as MART1 (Ab-3) (1:1000; Thermo Fisher Scientific, Kalamazoo, MI, USA) and GAPDH (FL-335) as noted previously. (B) Proposed scheme of ErbB receptor and ligand binding in regulating growth and differentiation of melanocytes.
We also examined the expression of melanocyte-specific proteins elicited by treatment with rhNRG1 and the smaller peptides. Treatment with rhNRG1 dramatically increased the level of GP100 (~85% compared with the control) and to a lesser extent, MART1 (~40%), tyrosinase (7%), and DCT (10%) (Figure 3A, right). Interestingly, the levels of all 4 of those melanogenic proteins increased more dramatically after treatment with the shorter peptides WC3, WC9, WC10, or WC12 (ranging from 20 to >300%).
Identification of the smallest bioactive motif of a given protein is an important issue to generate more potent and efficient bioactive molecules and to facilitate their delivery through the skin barrier. We had already shown that the EGF domain of rhNRG1 is sufficient to activate downstream signaling via ErbB receptors (Choi et al., 2010). The goal of this study was to identify the smallest bioactive motif within the EGF domain that could modulate melanin production by human melanocytes. We found that two 8-mer motifs (WC9 and WC10) significantly increased melanin production in human melanocytes. That 8-mer motif increased pigmentation about 20%, which is the same level of increase elicited by rhNRG1. As we showed in our previous study (Choi et al., 2010), this level of change can quite significantly impact visible color as relatively small increases in pigment production cause large visible changes in skin color over extended periods of time. Furthermore, WC9 and WC10 do not increase the proliferation of melanocytes, unlike rhNRG1, which is a desirable effect for in vivo applications. We also found that the changes in levels of pAkt (increased) or ErbB2 (decreased) that were elicited by treatment with rhNRG1 did not occur in response to treatment with the smaller peptides.
NRG1canbindtoErbB3orErbB4, which canformheterodimers with ErbB2, or they can form homodimers by themselves (Esper et al., 2006; Falls, 2003). Although ErbB2-ErbB3and ⁄ orErbB2-ErbB4heterodimersarethemostcommonly found, other receptor combinations also have been reported (Tao and Maruyama, 2008). Tao and Maruyama reported that ErbB3-ErbB4 heterodimers form in NIH3T3 cells and that ErbB3-ErbB4 heterodimers show even higher interactions than ErbB3-ErbB3 or ErbB4-ErbB4 homodimers. It is worth noting that the ErbB3 homodimer may not be an effective option as ErbB3 has an impaired tyrosine kinasedomain(Bergeret al.,2004). It is well established that upon ligand binding, EGF receptors are internalized and degraded in lysosomes ⁄ proteasomes (Di Fiore and Gill, 1999) and we also confirmed the proteasomal degradation of ErbB2 in our previous study (Choi et al., 2010). Some receptors may be recycled, but usually not all of them are recycled (Masui et al., 1993). Considering the level of ErB2 receptors expressed after the binding of smaller NRG1 peptides, itseemsunlikelythatErbB2isinvolvedinthesignaling cascade of those smaller peptides. Another group reported that NRG1 significantly enhances cell migration but not proliferation in normal melanocytes (Gordon-Thomson et al., 2005). Thediscrepancybetweentheirstudyandoursprobablyreflectsdifferencesinthemethodsoftreatment(asingle 3-day treatment versus every other day or every day for 8 days treatment). Therefore, the smaller NRG1 peptides mayonlytriggertheformationofErbB4-ErbB4homodimers and ⁄ or ErB3-ErbB4 heterodimers, which may be related to the differentiation of human melanocytes, whereasrhNRG1 might induce the formation of ErbB4-ErbB4 homodimers or ErbB3-Erb4heterodimers, which leads to the differentiation pathway, and ErbB2-ErbB3 or ErbB2-ErbB4 heterodimers, which leads to the proliferation pathway, as shown schematically in Figure 3B. Further study should be followed to test this hypothesis.
We also found that the smaller peptides that included the LCKC motif significantly increased the levels of tyrosinase, GP100 and MART1, more dramatically than the full-length rhNRG1. Therefore, it seems that this LCKC bioactive motif specifically regulates pigmentation without affecting proliferation via the increase in the melanocyte-specific melanogenic proteins.
Significance.
Paracrine factors secreted from keratinocytes and fibroblasts play important roles in the regulation of human skin pigmentation. Previously, we showed that neuregulin-1 (NRG1) is highly expressed in fibroblasts derived from the dark skin (type VI), and treatment with NRG1 increases melanocyte proliferation and pigmentation. In this study, we identified a minimal bioactive motif of NRG1 that only increases pigmentation and not proliferation. This small tetrapeptide may have an advantageous clinical application in terms of more efficient delivery through the skin barrier as well as the specific control of pigmentation without affecting the proliferation of melanocytes.
Acknowledgements
This research was supported in part by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health.
References
- Berger MB, Mendrola JM, and Lemmon MA (2004). ErbB3 ⁄ - HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett. 569, 332–336. [DOI] [PubMed] [Google Scholar]
- Choi W, Wolber R, Gerwat W, Mann T, Batzer J, Smuda C, Liu H, Kolbe L, and Hearing VJ (2010). The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J. Cell Sci 123, 3102–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore PP, and Gill GN (1999). Endocytosis and mitogenic signailing. Curr. Opin. Cell Biol 11, 483–488. [DOI] [PubMed] [Google Scholar]
- Esper RM, Pankonin MS, and Loeb JA (2006). Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res. Rev 51, 161–175. [DOI] [PubMed] [Google Scholar]
- Falls DL (2003). Neuregulins: functions, forms, and signaling strategies. Exp. Cell Res 284, 14–30. [DOI] [PubMed] [Google Scholar]
- Gordon-Thomson C, Jones J, Mason RS, and Moore GP (2005). ErbB receptors mediate both migratory and proliferative activities in human melanocytes and melanoma cells. Melanoma Res. 15, 21–28. [DOI] [PubMed] [Google Scholar]
- Imokawa G (2004). Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 17, 96–110. [DOI] [PubMed] [Google Scholar]
- Li Q, and Loeb JA (2001). Neuregulin-heparan-sulfate proteoglycan interactions produce sustained erbB receptor activation required for the induction of acetylcholine receptors in muscle.J. Biol. Chem 276, 38068–38075. [DOI] [PubMed] [Google Scholar]
- Masui H, Castro L, and Mendelsohn J (1993). Consumption of EGF by A431 cells: evidence for receptor recycling. J. Cell Biol 120, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao RH, and Maruyama IN (2008). All EGF(ErbB) receptors have preformed homoand heterodimeric structures in living cells. J. Cell Sci 121, 3207–3217. [DOI] [PubMed] [Google Scholar]
- Virador V, Kobayashi N, Matsunaga J, and Hearing VJ (1999). A standardized protocol for assessing regulators of pigmentation. Anal. Biochem 270, 207–219. [DOI] [PubMed] [Google Scholar]
- Virador V, Matsunaga N, Matsunaga J et al. (2001). Production of melanocyte-specific antibodies to human melanosomal proteins: expression patterns in normal human skin and in cutaneous pigmented lesions. Pigment Cell Res. 14, 289–297. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, and Hearing VJ (2009). Physiological factors that regulate skin pigmentation. BioFactors 35, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Brenner M, and Hearing VJ (2007). The regulation of skin pigmentation. J. Biol. Chem 282, 27557–27561. [DOI] [PubMed] [Google Scholar]
- Yarden Y, and Sliwkowski MX (2001). Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol 2, 127–137. [DOI] [PubMed] [Google Scholar]