Abstract
A series of homologous analogues of prototype antagonist 1 and its urea surrogate were investigated as hTRPV1 ligands. Through one-carbon elongation in the respective pharmacophoric regions, N-(3-fluoro-4-methylsulfonamidomethylphenyl)urea was identified as a novel and potent TRPV1 antagonistic template. Its representative compound 27 showed a potency comparable to that of lead compound 1. Docking analysis of compound 27 in our hTRPV1 homology model indicated that its binding mode was similar with that of 1S.
Keywords: Vanilloid receptor 1, TRPV1 antagonists, Analgesic
TRPV1 has emerged as a promising novel therapeutic target for the management of chronic and inflammatory pain.1–3 Building on initial insights provided by the prototypic ligands for TRPV1, capsaicin4 and resiniferatoxin,5 our understanding of the structure activity requirements for antagonistic ligands is maturing.6,7 Solution of the structure of TRPV1 by cryo electron microscopy8,9 and insights yielded by modeling10,11 provide a powerful complement guiding and validating the development of lead therapeutic structures.
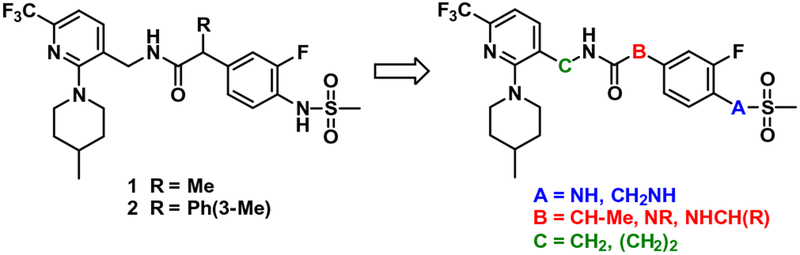
Over the last years, we have demonstrated that a series of 2-(3-fluoro-4-methylsulfonamidophenyl)propanamides were potent hTRPV1 antagonists active against multiple activators.12–19 Their structure activity relationship has been investigated extensively based on the three pharmacophoric regions, designated the A-, B-, and C-regions. In these investigations, compound 1 was identified as a prototype antagonist, possessing 3-fluoro-4-methyl-sulfonamidophenyl in the A-region, propanamide in the B-region, and (6-trifluoromethyl-pyridin-3-yl)methyl in the C-region (Fig. 1).12 Compound 1 exhibited highly potent and (S)-stereospecific antagonism of hTRPV1 activators including capsaicin, low pH, heat (45 °C) and N-arachidonoyl dopamine (NADA). Recently we reported that the α-m-tolyl congener 2 showed high potency and selective antagonism of capsaicin with a 3-fold improvement over prototype 1.18 Molecular modeling using our established hTRPV1 homology model indicated that the enhanced potency of 2 might be attributed to additional specific hydrophobic interactions of the m-tolyl group with the receptor.
Figure 1.
Design of homologated congeners of prototype 1 as a TRPV1 antagonistic template.
In continuation of our program to discover novel antagonistic templates with the goal of developing clinical candidates for TRPV1 mediated neuropathic pain, we have investigated a series of one carbon homologated analogues of prototype antagonist 1 and its urea B-region surrogate 21 (Fig. 1). This homologation approach can provide the optimal orientation of the three pharmacophoric region by varying their positions. Of particular interest, the urea B-region surrogates have an advantage over the corresponding propanamide B-region antagonists previously reported12–19 in terms of synthetic accessibility because the urea is achiral, unlike the chiral propanamide, and the A-region amines, precursors for urea coupling, are more commercially accessible.
In this study, we synthesized one-carbon elongated analogues in the respective A-, B-, C-regions and evaluated their binding affinities and antagonism of hTRPV1 activation by capsaicin. With the optimized potent antagonistic template, we performed a docking study using our hTRPV1 homology model to analyze its binding interactions with the receptor.
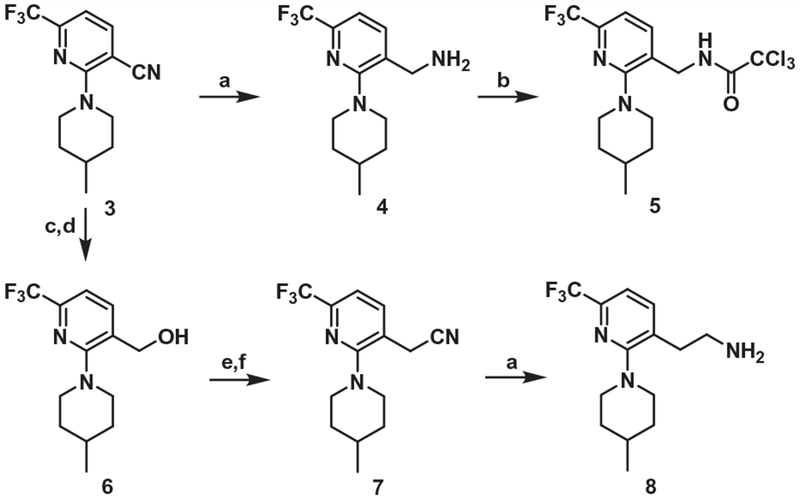
The syntheses of the C-region intermediates for coupling with the A-region are shown in Scheme 1. The C-region amine 4 and its N-trichloroacetyl derivative 5 were prepared from the nitrile 3 as previously reported.12
Scheme 1.
Synthesis of the C-region. Reagents and conditions: (a) BH3·SMe2, THF, reflux, 3 h; (b) Cl3CCOCl, TEA, CH2Cl2, rt, 3 h; (c) KOH, 80% aq EtOH, rt, 12 h (d) LiAlH4, THF, rt, 14 h; (e) PBr3, CH2Cl2, rt, 3 h; (f) KCN, EtOH, reflux, 8 h.
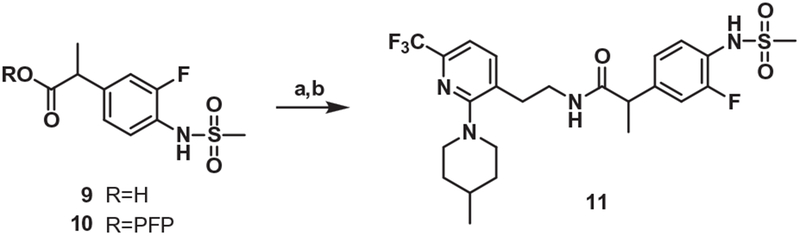
The one-carbon elongated analogue of 4 was also prepared from 3 by the conventional 5-step route. For the synthesis of the one-carbon elongated C-region analogue of 1, the propionic acid 912 was converted to the corresponding pentafluorophenyl ester which reacted with the amine 8 to provide 11 (Scheme 2).
Scheme 2.
Synthesis of the propanamide B-region analog (One-carbon elongated C-region). Reagents and conditions: (a) pentafluorophenol, EDC, DMF, CH2Cl2; (b) compound 8, TEA, CH2Cl2.
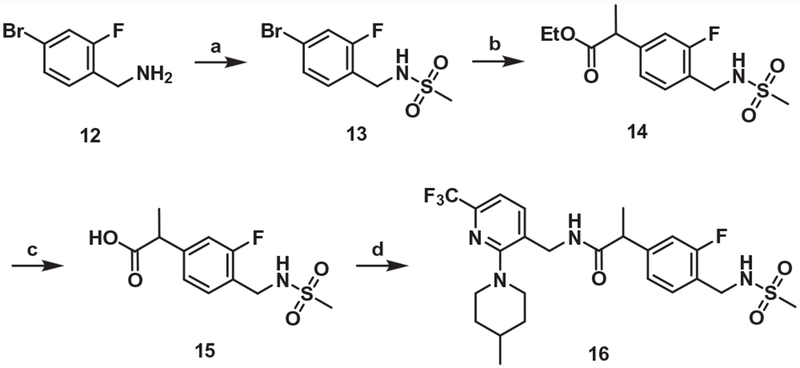
For the synthesis of the one-carbon elongated A-region analogue of 1, the commercially available amine 12 was mesylated and its bromide was substituted with ethyl propionate by a nickel-catalyzed cross-coupling reaction20 to afford 14, which was hydrolyzed and then coupled with the amine 4 to provide 16 (Scheme 3).
Scheme 3.
Synthesis of the propanamide B-region analog (One-carbon elongated A-region). Reagents and conditions: (a) MsCl, pyridine, 0 °C to rt, 2 h; (b) ethyl-2-chloropropionate, NiBr2(bpy)2, Mn, TFA, DMF, 65 °C, 15 h; (c) NaOH, THF/H2O (1:1), 60 °C, 15 h; (d) compound 4, EDC, HOBt, TEA, ACN, rt, 15
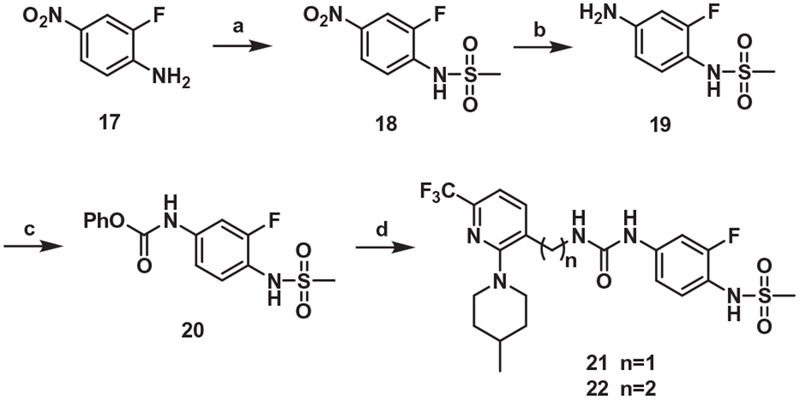
The B-region urea surrogate of 1 and its one-carbon elongated C-region analogue, 21 and 22, respectively, were synthesized by the coupling between the C-region amines and the phenylcarbamate 20, which was prepared from the commercially available amine 17 by the conventional 3 steps (Scheme 4).
Scheme 4.
Synthesis of the urea B-region analog. Reagents and conditions: (a) MsCl, NaH, DMF, 0 °C → rt, 2h; (b) 10% Pd/C, H2, CH2Cl2; (c) phenylchloroformate, pyridine, THF, 0 °C, 1 h; (d) compound 4 for 21, compound 8 for 22, DMAP, MeCN, 50 °C, 6 h.
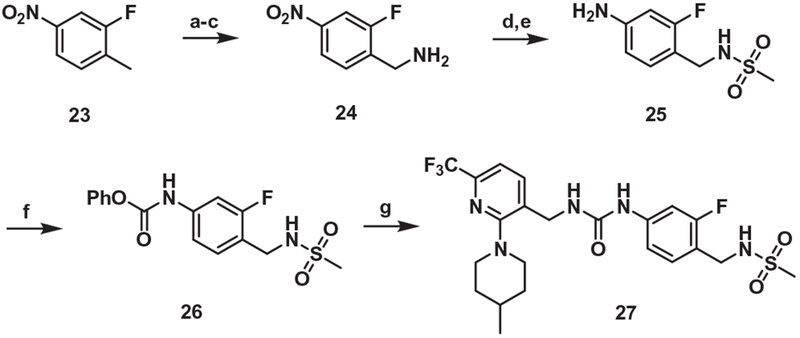
For the synthesis of the one-carbon elongated A-region analogue of 21, the methyl group of commercially available 23 was converted in 3 steps to the corresponding amine 24, which was mesylated and then hydrogenated to give 25. The carbamoylation of 25 followed by coupling with amine 4 produced 27 (Scheme 5).
Scheme 5.
Synthesis of the urea B-region analog (One-carbon elongated A-region). Reagents and conditions: (a) benzoyl peroxide, NBS, CCl4, reflux, 15h; (b) potassium phthalimide, DMF, r.t, 15h; (c) hydrazine monohydrate, PTSA, THF, reflux, 6 h; (d) MsCl, Pyridine, rt, 1 h; (e) 10% Pd/C, H2 gas, THF/EtOH, rt, 15 h; (f) phenylchloroformate, pyridine, THF/CAN; (g) compound 4, DMAP, MeCN, 50 °C, 6 h.
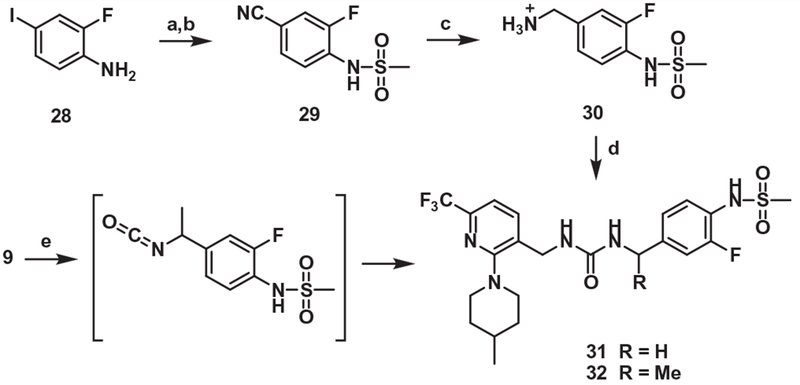
For the synthesis of one-carbon elongated B-region analogues of 21, the commercially available amine 28 was mesylated and its iodo group was converted in 2 steps to the corresponding benzyl amine 30, followed by coupling with the amine 2 to provide 31. On the other hand, the propionic acid 9 underwent Curtius rearrangement and subsequent addition with the amine 4 afforded α-methyl analogue of 31 (Scheme 6).
Scheme 6.
Synthesis of the urea B-region analog (One-carbon elongated B-region). Reagents and conditions: (a) MsCl, Pyridine, rt, 2 h; (b) Zn(CN)2, Pd(PPh3)4, DMF, 150 °C, 15 h; (c) (i) 2 M BH3·SMe2, THF, reflux, 3 h, (ii) 1 M HCl, reflux, 15 h; (d) compound 4, DBU, DMF, 80 °C, 2h; (e) (i) DPPA, TEA, toluene, 110 °C, 1 h, (ii) compound 4, 80 °C, 15h.
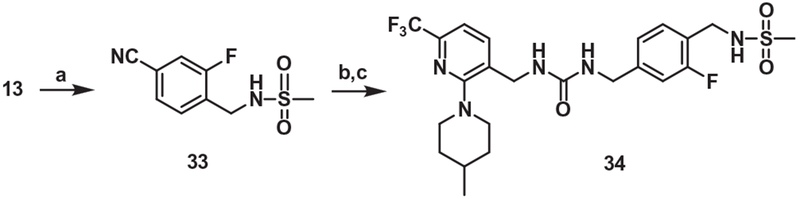
For the synthesis of the one-carbon elongated A and B-region analog of 21, the bromide 13 was converted to the corresponding nitrile 33, which was reduced and then coupled with the trichloroacetamide 5 to provide 34 (Scheme 7).
Scheme 7.
Synthesis of the urea B-region analog (One-carbon elongated A and B-region). Reagents and conditions: (a) Zn(CN)2, Zn, Pd2(dba)3, dppf, DMA, sealed tube, 120 °C, 15 h; (b) 10% Pd/C, MeOH, H2 gas, rt, 15 h; (c) compound 5, DBU, DMF, 80 °C, 2 h.
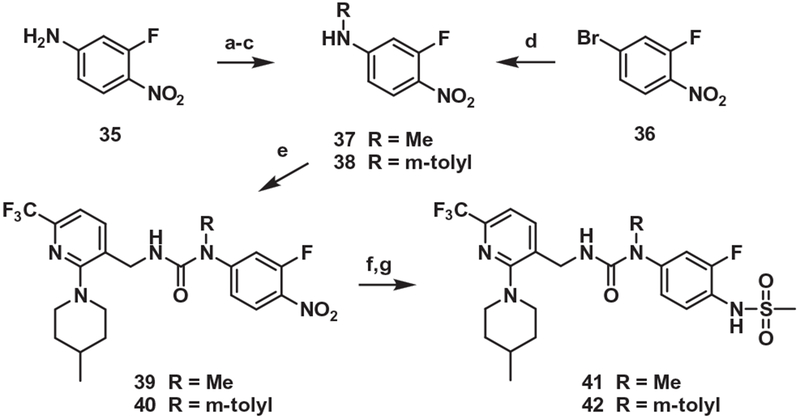
Finally, the commercially available amine 35 was methylated in 3 steps to give 37. Similarly, the bromide 36 was substituted with a m-tolyl group by Buchwald–Hartwig amination to give 38. The carbamoylation of 37 and 38 followed by coupling with the amine 4 provided the adducts 39 and 40, which were reduced and then mesylated to produce 41 and 42, the N-methyl and N-m-tolyl analogues, respectively, of 21 (Scheme 8).
Scheme 8.
Synthesis of the urea B-region analog (N-methyl urea). Reagents and conditions: (a) Boc2O, TEA, CH2Cl2, reflux,15 h; (b) Cs2CO3, CH3I, DMF, 40 °C, 15 h; (c) TFA, CH2Cl2, 0 °C → rt, 2 h; (d) m-toluidine, Pd(OAc)2, Xantphos, Cs2CO3, 1,4-dioxane, reflux,15 h; (e) (i) pyridine, triphosgene, toluene, rt, 2 h, (ii) compound 4, TEA, CH2Cl2, rt, 15 h; (f) 10% Pd/C, H2 gas, MeOH, rt → 40 °C, 30 min; (g) MsCl, pyridine, CH2Cl2, rt, 15 h.
The binding affinities and potencies as agonists/antagonists of the synthesized TRPV1 ligands were assessed in vitro by a binding competition assay with [3H]-resiniferatoxin (RTX) and by a functional 45Ca2+ uptake assay using human TRPV1 heterologously expressed in Chinese hamster ovary (CHO) cells, as previously described.21 For the agonism assay, a saturating concentration of capsaicin (1 μM) was used to define maximal response. For the antagonism assay, the dose-dependent inhibition of the capsaicin (30 nM) stimulated calcium uptake was measured. The Ki values for antagonism take into account the competition between capsaicin and the antagonist. The results are summarized in Table 1, together with the potencies of previous lead compounds 1 and 1S.
Table 1.
In vitro activity of synthesized compounds on hTRPV1a
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | Binding affinity Ki(nM) | Agonism EC50 (nM) | Antagonism Ki(ant) (nM) | |||
| 1 | –NH | CH–Me | CH2 | 7.90 | (±1.6) | NE | 2.22 | (±0.47) |
| 1S | –NH | (S)-CH–Me | CH2 | 2.95 | (±0.73) | NE | 1.26 | (±0.28) |
| 11 | –NH | CH–Me | (CH2)2 | 54 | (±13) | NE | 95 | (±26) |
| 16 | –CH2NH | CH–Me | CH2 | 230 | (±32) | NE | 27.6 | (±5.5) |
| 21 | –NH | NH | CH2 | 83 | (±27) | NE | 149 | (±50) |
| 22 | –NH | NH | (CH2)2 | 210 | (±46) | 29% | 49% | |
| 27 | –CH2NH | NH | CH2 | 6.77 | (±0.48) | NE | 12.5 | (±4.2) |
| 31 | –NH | NHCH2– | CH2 | 59 | (±12) | NE | 18.7 | (±6.1) |
| 32 | –NH | NHCH(Me)– | CH2 | 31.6 | (±3.4) | NE | 28.1 | (±8.5) |
| 34 | –CH2NH | NHCH2– | CH2 | 410 | (±27) | NE | 199 | (±50) |
| 41 | –NH | NH–Me | CH2 | 1160 | (±180) | NE | 59 | (±20) |
| 42 | –NH | NH–Ph(3-Me) | CH2 | 490 | (±120) | NE | 860b | (±290)b |
NE: no effect; values are the mean ± SEM of at least three experiments; where indicated, % represents the % agonism or antagonism at 3–30 μM.
Measured with 30 min pre-incubation before challenge with capsaicin; value with 15 min pre-incubation Ki(ant) = 2200 ± 560 nM.
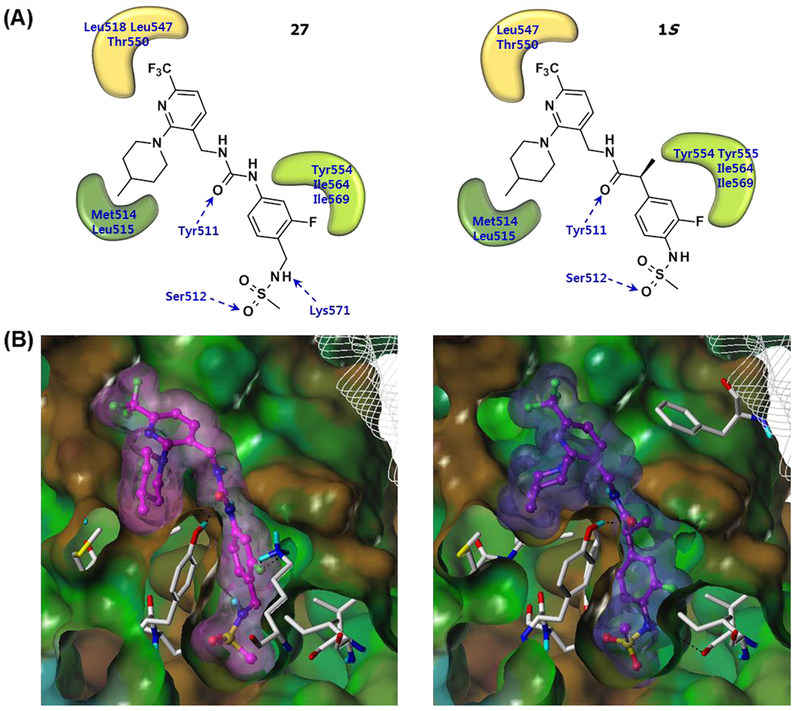
Prototype 1, identified from previous analysis of SAR, possessed its four principal pharmacophores including methylsulfonamide in the A region, propanamide in the B-region, and trifluoromethyl and 4-methylpiperidinyl groups in the C-region. Docking analysis with hTRPV1 revealed that hydrogen bonding and a π-π interaction with Tyr511 and hydrophobic interactions with the two pockets composed of Met514/Leu515 and Leu547/Thr550 were critical for activity (Fig. 2).
Figure 2.
Docking results of 27 and 1S in the hTRPV1 model. (A) 2-D illustration of the binding interactions between 27 (left) and 1S (right) with hTRPV1. Hydrogen bonding interactions are indicated by blue dashed line arrows and hydrophobic interactions are displayed with curved patches. (B) The Fast Connolly surface of hTRPV1 and the van der Waals surface of 27 (left) and 1S (right). The molecular surface of hTRPV1 is created by using MOLCAD and presented with the lipophilic potential property. For clarity, the surface of hTRPV1 is Z-clipped and that of the ligands are colored individually by magenta (27) or purple (1S).
In order to identify whether the pharmacophores in 1 are positioned at optimal distances from one another, we investigated the two one-carbon elongated analogues of 1, viz. 11 and 16. Compound 11, the one-carbon elongated analogue in the C-region, showed 7-fold and 43-fold weaker binding affinity and antagonistic potency, respectively, compared to 1. Compound 16, the one-carbon elongated analogue in the A-region, exhibited 29-fold and 12-fold weaker binding affinity and antagonistic potency, respectively. The results indicated that prototype 1 possessed an optimal interval between the key pharmacophores in the propanamide B-region series.
Next, we explored the corresponding series of urea B-region analogues with one-carbon homologation. The urea surrogate of 1 was examined first. Surprisingly, substitution of propanamide in 1 with the isosteric urea, providing 21, led to a dramatic reduction in activity, with 11-fold and 67-fold decreases in binding affinity and antagonism, respectively, compared to 1. These results suggested that conformational restriction in the urea B-region may affect the positions of the pharmacophores in the A- and C-region, shifting them away from the bioactive conformation. To explore this issue further, we examined one-carbon elongated analogues of 21 in the respective A- and C-regions. Whereas the one-carbon elongated analogue 22 in the C-region proved to be a partial antagonist, the one-carbon elongated analogue 27 in the A-region showed a dramatic improvement in activity with Ki = 6.77 nM and Ki(ant) = 12.5 nM, representing approximately 12-fold increases in binding affinity and antagonistic potency compared to 21. The binding potency of 27 is comparable to that of 1, indicating that compound 27 as a urea B-region antagonist proved to be a novel antagonistic template with high potency. We also examined the homologated analogues in the urea B-region. The one-carbon elongated analogue 31 displayed a moderate increase in potency compared to 21. Its α-methyl surrogate 32 showed activity comparable to that of 31. However, one-carbon elongation in both the A- and B-regions, providing 34, caused a large decline in activity.
Previous SAR study in a series of α-substituted acetamide B-region derivatives demonstrated that the α-m-tolyl derivative 2 showed highly potent and selective antagonism of capsaicin with a 3-fold improvement in potency over the corresponding α-methyl derivative 1, probably due to a specific hydrophobic interaction of the m-tolyl group with hTRPV1.20
Accordingly, we explored the N-methyl urea and N-(m-tolyl) urea B-region analogues, 41 and 42, which were nitrogen congeners of the α-carbon in 1 and 2. Both proved to show only weak binding affinity. Additionally, 42 was unusual in that it failed under our standard assay conditions to show functional activity, either as an antagonist or as an agonist. This failure was traced to a slow unset of action, presumably due to slow penetration into the cells. If the hTRPV1-expressing CHO cells were incubated with 42 for 15 min before challenge with capsaicin, then full antagonism was observed with Ki(ant) = 2200 nM. A further enhancement in antagonistic potency (Ki(ant) = 860 nM) was observed if the pre-incubation time was extended to 30 min.
Using our human TRPV1 (hTRPV1) model12 built based on our rat TRPV1 (rTRPV1) model10, we performed a flexible docking study to investigate the binding interactions of compound 27.22 Compared with the lead compound 1S, compound 27 has a urea group in the B-region and an additional methylene group in the A-region. As shown in Figure 2, the binding mode of 27 was generally similar to that of 1S.12 The urea group in the B-region was able to form a hydrogen bond with Tyr511 and also contributed to the appropriate positioning of A- and C-regions for the hydrophobic interactions. The N-benzylmethanesulfonamide group in the A-region occupied the deep bottom hole and formed hydrophobic interactions with Tyr511, Tyr554, Ile564, and Ile569. The fluorine atom in the A-region made hydrogen bonds with Lys571 and the S═O of the sulfonamide group participated in hydrogen bonding with Ser512. The 3-trifluoromethyl group in the C-region extended toward the hydrophobic area composed of Leu547 and Thr550. Finally, the 4-methylpiperidine ring in the C-region was involved in the hydrophobic interactions with Tyr511, Met514, and Leu515.
In summary, we have investigated a series of homologated analogues of prototype antagonist 1 and its urea B-region surrogate as hTRPV1 ligands. From systematic one-carbon elongation in respective pharmacophoric regions, we identified N-(3-fluoro-4-methylsulfonamidomethylphenyl)urea, a one-carbon elongated analogue of the A-region in the urea B-region series, as a novel and potent TRPV1 antagonistic template. Its representative compound 27 showed high affinity and potent antagonism with Ki = 6.77 nM and Ki(ant) = 12.5 nM which was comparable to that of 1. Since the B-region of compound 27 is achiral urea unlike the chiral propanamide of 1, it has more synthetic accessibility for further optimization and development. Docking analysis of compound 27 in our hTRPV1 homology model indicated that its binding mode was similar with that of 1S previously reported.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (NRF-2014M3A9B5073755), National Leading Research Lab (NLRL) program (2011-0028885), National Natural Science Foundation of China (81502927), Liaoning S&T Project (2014226032, 2015020733 and 2015001002), Hainan S&T Project (KYYS-2014-66), and in part by the Intramural Research Program of the NIH, Center for Cancer Research, NCI (Project Z1A BC 005270) in the USA.
References and notes
- 1.Szallasi A; Blumberg PM Pharmacol. Rev 1999, 51, 159. [PubMed] [Google Scholar]
- 2.Tominaga M; Caterina MJ; Malmberg AB; Rosen TA; Gilbert H; Skinner K; Raumann BE; Basbaum AI; Julius D Neuron 1998, 21, 531. [DOI] [PubMed] [Google Scholar]
- 3.Szallasi A Am. J. Clin. Pathol 2002, 118, 110. [DOI] [PubMed] [Google Scholar]
- 4.(a) Walpole CS; Wrigglesworth R; Bevan S; Campbell EA; Dray A; James IF; Perins MN; Reid DJ; Winter J J. Med. Chem 1993, 36, 2362. [DOI] [PubMed] [Google Scholar]; (b) Walpole CS; Wrigglesworth R; Bevan S; Campbell EA; Dray A; James IF; Masdin KJ; Perkins MN; Winter J J. Med. Chem 1993, 36, 2373. [DOI] [PubMed] [Google Scholar]; (c) Walpole CS; Wrigglesworth R; Bevan S; Campbell EA; Dray A; James IF; Masdin KJ; Perkins MN; Winter J J. Med. Chem 1993, 36, 2381. [DOI] [PubMed] [Google Scholar]
- 5.Appendino G; Szallasi A Life Sci 1997, 60, 681. [DOI] [PubMed] [Google Scholar]
- 6.Min KH; Suh Y-G; Park M-K; Park H-G; Park Y-H; Kim H-D; Oh U; Blumberg PM; Lee J [published erratum appears in Mol. Pharmacol.2003, 63, 958] Mol. Pharmacol 2002, 62, 947.12237342 [Google Scholar]
- 7.(a) Kym PR; Kort ME; Hutchins CW Biochem. Pharmacol 2009, 78, 211. [DOI] [PubMed] [Google Scholar]; (b) Wong GY; Gavva NR Brain Res. Rev 2009, 60, 267. [DOI] [PubMed] [Google Scholar]; (c) Gunthorpe MJ; Chizh BA Drug Discovery Today 2009, 14, 56. [DOI] [PubMed] [Google Scholar]; (d) Lazar J; Gharat L; Khairathkar-Joshi N; Blumberg PM; Szallasi A Expert Opin. Drug Discovery 2009, 4, 159. [DOI] [PubMed] [Google Scholar]; (e) Voight EA; Kort ME Expert Opin. Ther. Pat 2010, 20, 1. [DOI] [PubMed] [Google Scholar]; (f) Szolcsányi J; Sándor Z Trend Pharmacol. Sci 2012, 33, 646. [DOI] [PubMed] [Google Scholar]; (g) Szallasi A; Sheta M Expert Opin. Invest. Drug 2012, 21, 1351. [DOI] [PubMed] [Google Scholar]; (h) De Petrocellis L; Moriello AS Recent Pat. CNS Drug Disc 2013, 8, 180. [DOI] [PubMed] [Google Scholar]; (i) Lee Y; Hong S; Cui M; Sharma PK; Lee J; Choi S Expert Opin. Ther. Pat 2015, 25, 291. [DOI] [PubMed] [Google Scholar]
- 8.Liao M; Cao E; Julius D; Cheng Y Nature 2013, 504, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao E; Liao M; Cheng Y; Julius D Nature 2013, 504, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH; Lee Y; Ryu H; Kang DW; Lee J; Lazar J; Pearce LV; Pavlyukovets VA; Blumberg PM; Choi S J. Comput. Aided Mol. Des 2011, 25, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z; Pearce LV; Xu X; Yang X; Yang P; Blumberg PM; Xie XQ J. Chem. Inf. Model 2015, 55, 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MS; Ryu H; Kang DW; Cho S-H; Seo S; Park YS; Kim M-Y; Kwak EJ; Kim YS; Bhondwe RS; Kim HS; Park S-G; Son K; Choi S; DeAndrea-Lazarus I; Pearce LV; Blumberg PM; Frank R; Bahrenberg G; Stockhausen H; Kögel BY; Schiene K; Christoph T; Lee J J. Med. Chem 2012, 55, 8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorat SA; Kang DW; Ryu H; Kim MS; Kim HS; Ann J; Ha T-H; Kim SE; Son K; Choi S; Blumberg PM; Frank R; Bahrenberg G; Schiene K; Christoph T; Lee J Eur. J. Med. Chem 2013, 64, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ha T-H; Ryu H; Kim S-E; Kim HS; Ann J; Tran P-T; Hoang V-H; Son K; Cui M; Choi S; Blumberg PM; Frank R; Bahrenberg G; Schiene K; Christoph T; Frormann S; Lee J Bioorg. Med. Chem 2013, 21, 6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu H; Seo S; Cho S-H; Kim HS; Jung A; Kang DW; Son K; Cui M; Hong S-H; Sharma PK; Choi S; Blumberg PM; Frank-Foltyn R; Bahrenberg G; Stockhausen H; Schiene K; Christoph T; Frormann S; Lee J Bioorg. Med. Chem. Lett 2014, 24, 4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu H; Seo S; Cho S-H; Kim MS; Kim M-Y; Kim HS; Ann J; Tran P-T; Hoang V-H; Byun J; Cui M; Son K; Sharma PK; Choi S; Blumberg PM; Frank-Foltyn R; Bahrenberg G; Koegel B-Y; Christoph T; Frormann S; Lee J Bioorg. Med. Chem. Lett 2014, 24, 4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryu H; Seo S; Lee J-Y; Ha T-H; Lee S; Jung A; Ann J; Kim S-E; Yoon S; Hong M; Blumberg PM; Frank-Foltyn R; Bahrenberg G; Schiene K; Stockhausen H; Christoph T; Frormann S; Lee J Eur. J. Med. Chem 2015, 93, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran P-T; Kim HS; Ann J; Kim S-E; Kim C; Hong M; Hoang V-H; Ngo VTH; Hong S; Cui M; Choi S; Blumberg PM; Frank-Foltyn R; Bahrenberg G; Stockhausen H; Christoph T; Lee J Bioorg. Med. Chem. Lett 2015, 25, 2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ann J;Jung A; Kim M-Y; Kim H-M; Ryu H; Kim S; Kang DW; Hong S; Cui M; Choi S; Blumberg PM; Frank-Foltyn R; Bahrenberg G; Stockhausen H; Christoph T; Lee J Bioorg. Med. Chem 2015, 23, 6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durandetti M; Nédélec J-Y; Périchon J J. Org. Chem 1996, 61, 1748. [DOI] [PubMed] [Google Scholar]
- 21.Veghel DV; Cleynhens J; Pearce LV; Blumberg PM; Laere KV; Verbruggen A; Bormans G Nucl. Med. Biol 2013, 40, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The 3D structures of the ligands were generated with Concord and energy minimized with MMFF94s force field and MMFF94 charge until the rms of Powell gradient was 0.05 kcal mol−1 A−1 in SYBYL-X 2.0 (Tripos Int., St. Louis, MO, USA). The flexible docking study on our hTRPV1 model was performed using GOLD v.5.2 (Cambridge Crystallographic Data Centre, Cambridge, UK), which employees a genetic algorithm (GA) and allows for full ligand flexibility and partial protein flexibility. The binding site was defined as 8 Å around the capsaicin complexed in the hTRPV1 model. The side chains of the nine residues which are important for ligand binding, (i.e., Tyr511, Ser512, Met514, Leu515, Leu518, Phe543, Leu547, Thr550, and Asn551) were allowed to be flexible with ‘crystal mode’ in GOLD. Compound 27 was docked using the GoldScore scoring function, and the other parameters remained as default. All the computation calculations were undertaken on an Intel® Xeon™ Quad-core 2.5 GHz workstation with Linux Cent OS release 5.5.