Abstract
Epidermal expression of adhesion molecules such as desmogleins (Dsg) and cadherins is strongly affected by the differentiation status of keratinocytes. We have previously shown that certain protein kinase C (PKC) isoforms differentially alter the growth and differentiation of human epidermal HaCaT keratinocytes. In this paper, using recombinant overexpression and RNA interference, we define the specific roles of the different PKC isoenzymes in modulation of expression of adhesion molecules in HaCaT keratinocytes. The level of Dsg1, a marker of differentiating keratinocytes, was antagonistically regulated by two Ca-independent ‘novel’ nPKC isoforms; i.e. it increased by the differentiation-promoting nPKCδ and decreased by the growth-promoting nPKCϵ. The expression of Dsg3 (highly expressed in proliferating epidermal layers) was conversely regulated by these isoenzymes, and was also inhibited by the differentiation inducer Ca-dependent ‘conventional’ cPKCα. Finally, the expression of P-cadherin (a marker of proliferating keratinocytes) was regulated by all of the examined PKCs, also in an antagonistic manner (inhibited by cPKCα/nPKCδ and stimulated by cPKCβ/nPKCϵ). Collectively, the presented results strongly argue for the marked, differential, and in some instances antagonistic roles of individual Ca-dependent and Ca-independent PKC isoforms in the regulation of expression of adhesion molecules of desmosomes and adherent junctions in human epidermal keratinocytes.
Keywords: adhesion molecules, differentiation, isoenzymes, protein kinase C
Introduction
Desmosomes are adhesive intercellular junctions of epithelia and consist of desmosomal cadherins, desmogleins (Dsg) and desmocollins (Dsc), linked to the keratin intermediate filament network (1–3). Three isoforms of Dsg and Dsc have been characterized in detail (Dsg1–3, Dsc1–3) and possess distinct patterns of expression within the tissues (4,5). Less is known about a fourth Dsg, Dsg4, which was recently reported in salivary gland, testis, prostate and skin (6). In the epidermis, Dsg1 and 3 show a differentiation-dependent distribution, in which Dsg3 is mostly associated with the basal layers whilst Dsg1 is found in the more differentiated upper layers (7–11). Importantly, Dsg1 and Dsg3 are targets for autoimmune blistering diseases such as pemphigus foliaceus and pemphigus vulgaris, respectively, where the localization of the blistering corresponds to the differentiation-specific expression of the given Dsg isoform (3,12,13).
In addition to desmosomes, the epidermis utilizes another adhesive mechanism, the adherent junction, to maintain the structural integrity of the tissue (14–18). Adherent junctions consist of classical cadherins, including E-cadherins, which are expressed on the cell surfaces of all epidermal layers, and P-cadherins, which can be detected only on the surfaces of the basal and most immediate suprabasal cells of the epidermis (18,19).
Recent intriguing evidence suggests that the desmosomal adhesion molecules may play a second role, contributing to the regulation of epidermal differentiation. Namely, it has been suggested that desmosomal cadherins and the related intracellular mechanisms can regulate the activity of signalling pathways such as that of the protein kinase C (PKC) family of isoforms and can modulate intracellular Ca2+ concentration (20), recognized key regulators in keratinocyte differentiation (21–24). Conversely, these regulatory mechanisms (‘the other way around’) may significantly influence the differentiation-specific expression of the human keratinocyte-specific desmosomal cadherins (25,26). Indeed, suppression of PKC activity resulted in inhibition of formation of desmosomes whereas PKC-activating phorbol esters promoted the development of cell-to-cell contacts via adherent junctions (27). Moreover, recently it has been reported that PKC activation upregulates intercellular adhesion of α-catenin negative human colon cancer cells via induction of desmosomes (28).
PKC possesses multiple isoforms, viz the calcium- and phorbol ester-dependent ‘conventional’ cPKCα, βI, βII, and γ; the calcium-independent ‘novel’ nPKCδ, ϵ, η and θ; the calcium- and phorbol ester-independent ‘atypical’ aPKCζ, and λ/ι; and the unique PKCμ (22,29,30). So far, however, no PKC isoform-specific functions have been documented in the modulation of Dsg and Dsc expression and function; likewise, we also lack a description of the involvement of the PKC system in the regulation of adhesion molecules located in the adherent junctions (e.g. P-cadherin).
In the current study, therefore, we define the roles of specific PKC isoforms in the regulation of expression of various adhesion molecules in human immortalized HaCaT keratinocytes. Using complementary molecular biology techniques [recombinant overexpression, RNA interference (RNAi)], we provide the first evidence that the expression of certain adhesion molecules (Dsgs, cadherins) is not only modulated by the differentiation status of the cells but also by specific PKC isoforms (cPKCα and β, nPKCδ and ϵ) and that these isoforms differentially and antagonistically participate in the process.
Materials and methods
Cell culture
Human immortalized HaCaT keratinocytes were cultured in 25 or 75 cm2 tissue culture flasks (unless otherwise indicated) in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma, St Louis, MO, USA) supplemented with 10% fetal calf serum (Sigma), 2 mm l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 1.25 μg/ml Fungizone (all from TEVA-Biogal, Debrecen, Hungary) at 37°C in a 5% CO2 atmosphere (31,32).
Generation of PKC-overexpressing HaCaT cells
Stable overexpression of various PKC isoforms in HaCaT keratinocytes was performed as described previously (33–35). Experiments were routinely carried out on pools of transfected cells, but the results were confirmed on at least three individual clones for each isoform. As described before (33–35), the efficacy of recombinant overexpression was monitored by Western blotting and kinase assays (not shown).
Suppression of levels of endogenous PKC isoforms by RNA interference
Cells were seeded in six-well tissue culture plates in DMEM containing serum but lacking antibiotics. At 40–50% confluence, cells were transfected with RNAi probes against cPKCα and β and nPKCδ and ϵ, with scrambled RNAi probes (used as control), or with fluorescein-labelled control siRNA (all from Santa Cruz Biotech, Santa Cruz, CA, USA) previously mixed (and incubated at room temperature for 25 min) with transfection medium also containing the transfection reagent (both provided by Santa Cruz for use in siRNA transfection protocols). The efficacy of siRNA-driven ‘knock-down’ of the PKCs was daily evaluated by Western blot and quantitative ‘real-time’ Q-PCR techniques (see below) for 4 days (35). The transfection procedure resulted in >80% cell survival rate and >70% transfection efficiency (data not shown).
Antibodies
All primary antibodies against PKC isoenzymes were developed in rabbits and were shown to react specifically with the given PKC isoforms in HaCaT keratinocytes (32,33,35–37). Anti-PKCα, β and ϵ were from Sigma, whereas anti-PKCδ was from Santa Cruz. In addition, monoclonal mouse antibodies against certain adhesion molecules, such as Dsg1 (Progen, Heidelberg, Germany), Dsg3 (Serotec, Düsseldorf, Germany) and P-Cadherin (P-cad, BD Biosciences Pharmingen, San Diego, CA, USA) were employed. For an endogenous control, the expression of β-actin (antibody from Santa Cruz) was determined. Specificities of all antibodies were also tested by applying isoform-specific neutralizing peptides, which blocked the immunostaining in all cases (data not shown).
Western blot analysis
The Western blot technique was employed to assess expression of adhesion molecules and to verify the efficacy of RNAi suppression of the PKC isoforms at the protein level (32,33,35–38). In brief, cells were washed with ice-cold phosphate-buffered saline (PBS), harvested in homogenization buffer (20 mm Tris–HCl, 5 mm EGTA, 1 mm 4-(2-aminoethyl)benzenesulphonyl fluoride, 20 μm leupeptin, pH 7.4; all from Sigma) and disrupted by sonication on ice. Protein content of samples was measured by a modified bicinchonic acid protein assay (Pierce, Rockford, IL, USA). Total cell lysates were mixed with SDS-PAGE sample buffer and boiled for 10 min at 100 C. The samples were subjected to SDS-PAGE (8% gels were loaded with 20–30 μg protein per lane) and transferred to nitrocellulose membranes (BioRad, Vienna, Austria). Membranes were then blocked with 5% dry milk in PBS and probed with the appropriate primary antibodies against the given adhesion molecules or PKC isoforms. Peroxidase-conjugated goat anti-rabbit or anti-mouse IgG antibodies (BioRad) were used as secondary antibodies, and the immunoreactive bands were visualized by an ECL Western blotting detection kit (Amersham, Little Chalfont, UK). Immunoblots were subjected to densitometric analysis using an Intelligent Dark Box (Fuji, Tokyo, Japan) and the Image Pro Plus 4.5.0 software (Media Cybernetics, Silver Spring, MD, USA) (34,35,39), and then normalized densitometric values of the individual lanes of several independent experiments were determined and expressed as mean ± SEM. To assess equal loading, membranes were stripped in 200 ml of 50 m Tris–HCl buffer (pH 7.5) containing 2% SDS and 0.1 β-mercaptoethanol (all from Sigma) at 65 C for 1 h and were re-probed with a mouse β-actin antibody (Sigma) followed by a similar visualization procedure as described above.
Quantitative ‘real-time’ Q-PCR
To assess the efficacy of RNAi of the PKC isoforms at the gene level, Q-PCR was carried out on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) by using the 5¢ nuclease assay (35,39). Total RNA was isolated using TRIzol (Invitrogen, Paisley, UK) and then 2–3 μg of total RNA were reverse transcribed into cDNA by using 15 units of AMV reverse transcriptase (Promega, Madison, WI, USA) and 0.025 μg/μl random primers (Promega). PCR amplification was carried out by using the TaqMan primers and probes (Assay ID: Hs00176973_m1 for PKCα; Assay ID: Hs00176998_m1 for PKCβ; Assay ID: Hs00178914_m1 for PKCδ; Assay ID: Hs00178455_m1 for PKCϵ) using the TaqMan Universal PCR Master Mix Protocol (Applied Biosystems). As internal controls, transcripts of β-actin were determined (Assay ID: Hs_99999903_m1).
Results
Expression of adhesion molecules changes during the high cell density-induced differentiation of HaCaT keratinocytes
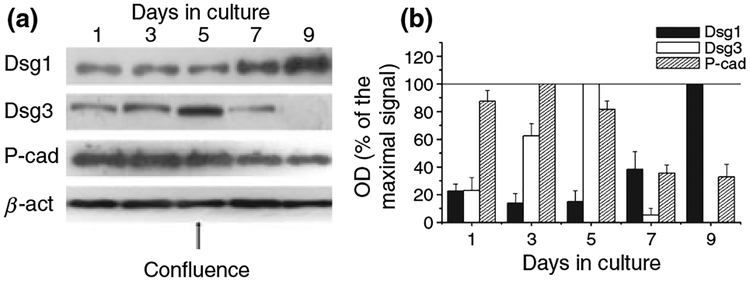
We have previously shown (37) that HaCaT cell cultures start to differentiate upon reaching confluence (high cell density-induced differentiation). Hence, we first defined the alterations in the expression patterns of the adhesion molecules Dsg1, Dsg3 and P-cad during culture of HaCaT keratinocytes. As revealed by Western blotting (Fig. 1), the expression of the molecules differentially altered with days in culture. The level of Dsg1 (which is mostly expressed in the suprabasal layers of the human epidermis in vivo) (7) was very low in the subconfluent, proliferating cultures. However, as expected, the expression of the molecule markedly increased in parallel with the onset of differentiation.
Figure 1.
Alteration of expression of adhesion molecules during the high cell density-induced differentiation of HaCaT keratinocytes. (a) HaCaT cells were harvested at various culturing days (confluence was reached at day 5), similar amounts of proteins were subjected to SDS-PAGE, and Western immunoblotting was performed using antibodies against the adhesion molecules Dsg1, Dsg3 and P-cad. To assess equal loading, nitrocellulose membranes were stripped and re-probed with an anti-β-actin antibody (β-act). The figure is a representative of four to five experiments yielding similar results. (b) The amounts of the adhesion molecules were quantitated by densitometry (optical density; OD), the values were normalized to the OD values of β-actin, and the results were expressed as the percentage of the maximal amount of the given molecule within the experiment. Values represent the mean ± SEM of four to five independent experiments.
In contrast, the expression of Dsg3 first increased with time in the highly proliferating cultures and then dropped to practically undetectable levels in the postconfluent (differentiating) cells (Fig. 1). Partly similar to these data, the level of P-cad was highest in the preconfluent cultures and significantly decreased with the onset of the high cell density-induced differentiation programme. These findings were in good accord with previous results demonstrating that Dsg3 and P-cad are chiefly expressed in vivo in the highly proliferation stratum basale of the human epidermis (7). It appears, therefore, that the proliferating and differentiating HaCaT cell culture provides a model for the in vivo like differentiation-dependent alterations in the expression of certain adhesion molecules.
Expression of adhesion molecules differentially alters in HaCaT keratinocytes overexpressing certain PKC isoforms
We have previously also shown (37) that recombinant overexpression of certain PKC isoforms significantly affected the in vitro and in vivo proliferation and differentiation of HaCaT keratinocytes. Comparison of various PKC overexpresser HaCaT keratinocytes at similar proliferation and differentiation stages revealed that the overexpression of cPKCα or nPKCδ decreased cellular proliferation rate but, of great importance, markedly increased the expression of differentiation markers (e.g. involucrin, filaggrin, transglutaminase-1). In contrast, overexpression of cPKCβ or nPKCϵ resulted in hyperproliferative transformation of the cells and the suppression of differentiation.
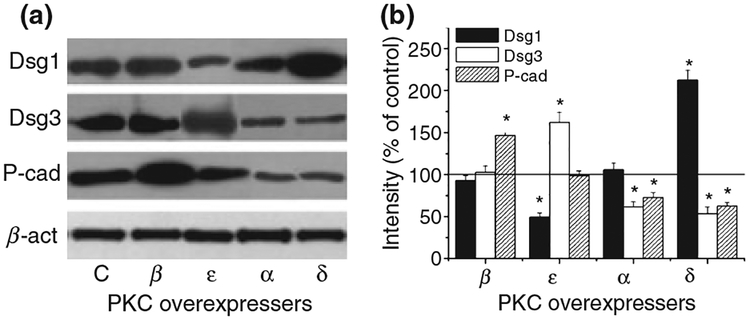
In the next phase of our study, we therefore investigated the expression of the above adhesion molecules in the PKC overexpresser HaCaT keratinocytes. In the course of these experiments, it was also of importance to compare the levels of the adhesion molecules on cells with similar proliferation/differentation stages. Since the growth rates of the PKC overexpresser cells were markedly different (37), the expression of the adhesion molecules was determined on cells harvested at 80–85% confluence, as described before (37). Western blot analysis revealed that the expression pattern of the adhesion molecules depends not simply on the differentiation status of the cells but also on the specific PKC isoform whose overexpression induced the altered differentiation of the cells. In the cPKCβ-HaCaT cells, which were hyperproliferative, the level of P-cad markedly and significantly (P < 0.05, n = 5) increased compared with control (empty vector transfected) keratinocytes (Fig. 2). However, we observed no alteration in the expression of Dsg1 or Dsg3. In contrast to these finding, in the nPKCϵ overexpresser (also characterized by decreased differentiation and increased proliferation), the expression of Dsg3 increased (P < 0.05, n = 6), that of Dsg1 decreased (P < 0.05, n = 5), whereas the level of P-cad did not change (when compared with control).
Figure 2.
Expression pattern of adhesion molecules in HaCaT keratinocytes overexpressing individual PKC isoforms. (a) Stable transfectants of HaCaT cells overexpressing the different PKC isoforms or the control empty vector (C) were harvested at similar proliferation and differentiation stages (i.e. 80–85% confluence) and similar amounts of proteins were subjected to Western immunoblotting to detect the adhesion molecules Dsg1, Dsg3 and P-cad. To assess equal loading, nitrocellulose membranes were stripped and re-probed with an anti-β-actin antibody (β-act). The figure is a representative of four to five experiments yielding similar results. (b) The amounts of the adhesion molecules were quantitated by densitometry (optical density; OD), then normalized to those of β-actin, and finally expressed as the percentage of the control (empty vector transfected) regarded as 100%. Values represent the mean ± SEM of five to six independent experiments. Asterisks mark significant (P < 0.05) differences, defined by Student’s t-test, compared with control.
Although to a lesser extent, a differentially modified expression pattern of the adhesion molecules was also observed in those PKC overexpresser HaCaT cells which were characterized by accelerated differentiation and suppressed proliferation (Fig. 2). In cPKCα-HaCaT cells, the expression of Dsg3 and P-cad (‘markers’ of the proliferating keratinocytes of stratum basale) significantly decreased (in both cases, P < 0.05, n = 5) whereas that of Dsg1 was negligibly altered compared with control. In the nPKCδ overexpresser keratinocytes, levels of Dsg3 and P-cad were likewise suppressed (in both cases, P < 0.05, n = 6). A difference, however, was that Dsg1 (a ‘marker’ of the suprabasal, differentiating epidermal layers) was appreciably elevated (P < 0.05, n = 5).
Expression of adhesion molecules also differentially alters in HaCaT keratinocytesin which the levels of certain PKC isoformswere suppressed by RNAi
To complement the above studies measuring the effect of recombinantly overexpressed PKC isoenzymes, in the next phase of our experiments we selectively ‘knocked-down’ individual PKC isoforms using the RNAi technique (35) and then investigated the effect of such interventions on the expression of the adhesion molecules.
First, we assessed the efficacy of RNAi treatment for suppression of the expression of individual PKC isoforms. Using Western blot (Fig. 3a), followed by densitometry analysis (Fig. 3b), as well as Q-PCR techniques at various time-points after RNAi transfection, we found that the levels of all PKC isoforms investigated were markedly (approximately 20% of the scrambled RNAi probe-transfected control, Fig. 3b,c) and, of great importance, selectively suppressed by the RNAi probes 48 h after transfection. The selectivity of the intervention was supported by the fact that in no case did an RNAi probe affect the levels of the other (i.e. ‘non-targeted’) PKC isoforms or of the endogenous control β-actin; further, the scrambled RNAi probes did not modify the expression of the ‘targeted’ PKC isoenzyme (Fig. 3a).
Figure 3.
Effect of RNAi-driven ‘knock-down’ of individual PKC isoforms on the expression of adhesion molecules in HaCaT keratinocytes. (a) Various specific RNAi probes (Sp) against the PKC isoforms as well as the scrambled (control) RNAi probes (Sc) were introduced to the cells as described under ‘Materials and methods’ section. Forty-eight hours after transfection, cells were harvested and subjected to Western blot analysis (WB) to define the expression of the ‘targeted’ PKC isoform; the other PKC isoforms; the various adhesion molecules (Dsg1, Dsg3 and P-cad); and the endogenous control β-actin (β-act). In addition, to monitor the lack of effect of transfection, the expression of PKC isoforms was compared in non-transfected (NT) and scrambled RNAi probes (Sc) transfected cells. The figure is a representative of five to six experiments yielding similar results. (b) Time-course of efficacy of inhibition of PKC isoform expression as assessed by Western blotting at various time-points after transfection with RNAi. The amounts of the individual PKC isoforms were quantitated by densitometry (optical density; OD), then normalized to those of β-actin, and finally expressed as the percentage of the scrambled RNAi probes transfected (control) samples regarded as 100%. (c) Time-course of efficacy of inhibition of PKC isoform mRNA levels as assessed by Q-PCR at various time-points after transfection. PKC-specific transcripts were determined as described under ‘Materials and methods’ section; then, values were normalized to those of β-actin and finally expressed as the percentage of the scrambled RNAi probe transfected (control) samples regarded as 100%. (d) Western blot analysis of adhesion molecules 48 h after transfection. The amounts of the adhesion molecules were quantitated by densitometry (optical density; OD), then normalized to those of β-actin, and finally expressed as the percentage of the scrambled RNAi probes transfected (control) samples regarded as 100%. In b–d, points represent the mean ± SEM of five to six independent experiments and asterisks mark significant (P < 0.05) differences, defined by Student’s t-test, compared with the respective controls.
We therefore measured the expression of the adhesion molecules 48 h after transfection. As seen in Fig. 3a,d, Western blot analysis of the RNAi-mediated ‘knock-down’ cells revealed that suppression of individual PKC isoforms had effects opposite of those when the same individual isoforms were overexpressed, with only minor differences. Thus, suppression of cPKCα resulted in elevation of Dsg3 and P-cad (in both cases, P < 0.05, n = 5) whereas suppression of nPKCδ resulted in elevation of Dsg3 and P-cad (in both cases, P < 0.05, n = 6), together with a decrease in Dsg1 (P < 0.05, n = 5). In cPKCβ ‘knock-down’ cells, the expression of P-cad decreased (P < 0.05, n = 5), with no change in Dsg1 and Dsg3 whilst in nPKCϵ ‘knock-down’ keratinocytes, Dsg1 levels were elevated and Dsg3 and P-cad levels were reduced (in all cases, P < 0.05, n = 5–6). Other than for the reduction in P-cad in the nPKCϵ ‘knock-down’ keratinocytes, whereas P-cad was unaffected in the nPKCϵ overexpressers, the effects of the suppression of the individual PKC isoforms was opposite of those for their overexpression (Fig. 2).
Selective inhibition of nPKCδ significantly alters the expression of adhesion molecules in HaCaT keratinocytes
Finally, we examined the effect of rottlerin, a compound often used as a selective nPKCδ inhibitor (40). Consistent with the role of PKCδ as an inhibitor of HaCaT proliferation, previous studies have shown that the treatment of HaCaT cells with rottlerin caused enhanced proliferation (41). Here, we determined its effect on adhesion molecules.
As was expected, treatment of (control, non-transfected) HaCaT keratinocytes with rottlerin altered the expression pattern of the adhesion molecules in a dose-dependent fashion (Fig. 4). In full agreement with the results from overexpression and RNAi treatment, the inhibition of nPKCδ with rottlerin significantly suppressed the level of Dsg1 (a ‘marker’ of differentiating keratinocytes) whilst it elevated the expression of Dsg3 and P-cad (‘markers’ of proliferating epidermal cells) (in all cases, P < 0.05, n = 5–6). Although confidence in the interpretation is limited because of possible effects of rottlerin (especially at higher concentrations) in systems other than PKC (42), these findings further support the concept that the endogenous nPKCδ activity plays a key role in the regulation of the adhesion molecules Dsg1, Dsg3 and P-cad.
Figure 4.
Effect of the nPKCδ inhibitor rottlerin on the expression of adhesion molecules in HaCaT keratinocytes. (a) Cells were treated with various concentrations of rottlerin for 48 h, then harvested and similar amounts of proteins were subjected to Western immunoblotting to detect the adhesion molecules Dsg1, Dsg3 and P-cad. To assess equal loading, nitrocellulose membranes were stripped and re-probed with an anti-β-actin antibody (β-act). The figure is a representative of four to five experiments yielding similar results. (b) The amounts of the adhesion molecules were quantitated by densitometry (optical density; OD), then normalized to those of β-actin, and finally expressed as the percentage of the vehicle-treated (control, C) cells regarded as 100%. Points represent the mean ± SEM of four to five independent experiments. Asterisks mark significant (P < 0.05) differences, defined by Student’s t-test, compared with control.
Discussion
Previous studies have clearly shown that the epidermal expression of adhesion molecules in the human skin is regulated by numerous factors (e.g. serum content, calcium concentration, signalling pathways) which all modify the differentiation status of the cells (14,21,24,26). In the current study, we used molecular approaches to present that the differentiation-dependent expression of certain adhesion molecules (Dsg1, Dsg3 and P-cad) is specifically regulated by members of the PKC family which, as we have shown before, are also involved in the regulation of epidermal growth and differentiation. Furthermore, individual PKC isoforms have differential effects, with some being opposite in action to others.
We and others have previously reported that the expression level of nPKCδ was appreciably elevated in differentiating keratinocytes and that the activation and/or overexpression of nPKCδ resulted in the initiation of the differentiation programme and, in parallel, mediated the apoptotic effect of several inducers (33,37,43–45). In good accord with these findings, in the current study we found that nPKCδ stimulated the expression of the ‘differentiation marker’ Dsg1 whereas it inhibited the expression of Dsg3 and P-cad, ‘markers’ of the basal epidermal layers. Interestingly, cPKCα – which was also shown to play a central role in the terminal differentiation programme of epidermal keratinocytes (23,33,46) – also decreased the expression of Dsg3 and P-cad. However, cPKCα did not affect the level of Dsg1, suggesting that, although both isoforms promote epidermal differentiation, their roles in the regulation of expression of adhesion molecules are (slightly) different.
The expression of the adhesion molecules was conversely regulated by another ‘pair’ of PKCs. nPKCϵ – which was previously implicated as an effective promoter of in vivo and in vitro keratinocyte growth (33,46,47) – inhibited the expression of Dsg1 whilst it increased the level of Dsg3 and P-cad. However, the role of another growth-promoting isoform (33) cPKCβ – whose expression was shown to be altered in the hyperproliferative skin disorder psoriasis (48) – was only minor since it was found to only affect P-cad, which it increased. These data likewise demonstrate the differential role of certain PKC in the regulation of adhesion molecules.
Interestingly, our findings indicate that the level of Dsg1 is regulated only by the two Ca-independent nPKC isoforms, which have antagonistic effects (i.e. Dsg1 is increased by the differentiation-promoting nPKCδ and is decreased by the growth-promoting nPKCϵ). It appears, therefore, that although calcium was previously shown to markedly increase the expression of Dsg1 (and to stimulate differentiation) (13,26), this process does not depend directly on the activation of Ca-dependent cPKCs.
The expression of Dsg3 (highly expressed in the proliferating basal layers of the epidermis) was also markedly regulated by the Ca-independent nPKC isoforms, although in the direction opposite that of Dsg1 (i.e. Dsg3 was decreased by nPKCδ and increased by nPKCϵ). However, the Ca-dependent cPKCα was also involved in its regulation, leading to inhibition of its expression. Intriguingly, Osada et al. (49) have previously shown that Dsg3-targeting auto-antibodies from patients with pemphigus vulgaris induced a marked translocation of cPKCα and nPKCδ, reflecting their activation (they did not investigate nPKCϵ). These results suggest that, in addition to the regulation of Dsg3 expression by PKC isoforms which we describe here, the initiation of the adhesion molecule-dependent intracellular signalling may also involve certain PKCs.
Finally, as revealed in the RNAi experiments (and almost identically in the overexpression studies), the expression of P-cad was regulated by all of the examined PKCs. The two ‘pairs’ of Ca-dependent and Ca-independent isoforms (cPKCα/nPKCδ and cPKCβ/nPKCϵ) antagonistically modified the level of the adherent junction protein, suggesting that P-cad may function as a key adhesion molecular ‘target’ of the complex PKC-dependent regulatory ‘pathways’ in human keratinocytes.
In conclusion, although there are evidences in the literature, that desmosomal assembly is influenced by extra-cellular Ca2+ concentration, by PKC signalling and that classical cadherin-mediated adhesion may also facilitate desmosome formation (20–24), our present study is the first to define the roles of specific PKC isoforms in the regulation of expression of various adhesion molecules. Besides regulation of expression, some data also suggest that PKC generates a switch between the Ca-dependent and Ca-independent, hyper-adhesive states of desmosomes in HaCaT cells (50). On the other hand, the PKC system possibly takes part not only in regulation of adhesive activity of desmosomes, but also in pemphigus vulgaris IgG (PV IgG) induced cell–cell detachment, since the PKC inhibitor bisindolylmaleimide prevented blistering caused by PV IgG after passive transfer to neonatal mice (51). Besides the PKC system, the p38MAPK, Rho family GTPases, C-myc and phospholipase C (and the related complex signalling events) are also implicated as potential signalling pathways important for pemphigus vulgaris pathogenesis (52), but their direct role in the regulation of expression of desmosomal cadherins is unclear. Although further studies are needed to determine the in vivo relevance of our findings and to identify the downstream elements of the PKC-dependent intracellular signalling mechanisms, the presented results strongly argue for the marked, differential and in some instances antagonistic roles of individual Ca-dependent and Ca-independent PKC isoforms in the regulation of expression of adhesion molecules of desmosomes and adherent junctions in human epidermal keratinocytes.
Acknowledgements
The technical assistance of Ms Ibolya Varga is gratefully appreciated. This work was supported by the following Hungarian research grants: OTKA T49231, K63153 and K60262. Tamás Bíró is the recipient of the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.
Abbreviations:
- cad
cadherin
- Dsg
desmoglein
- PKC
protein kinase
References
- 1.Koch PJ, Franke WW. Desmosomal cadherins: another growing multigene family of adhesin molecules. Curr Opin Cell Biol 1994: 6: 682–687. [DOI] [PubMed] [Google Scholar]
- 2.North AJ, Bardsley WG, Hyam J et al. Molecular map of the desmosomal plague. J Cell Sci 1999: 112: 4325–4336. [DOI] [PubMed] [Google Scholar]
- 3.Amagai M, Ahmed AR, Kitajima Y et al. Are desmoglein auto-antibodies essential for the immunopathogenesis of pemphigus vulgaris, or just ‘witnesses of disease’? Exp Dermatol 2006: 15: 815–831. [DOI] [PubMed] [Google Scholar]
- 4.Garrod DR, Merritt AJ, Nie Z. Desmosomal cadherins. Curr Opin Cell Biol 2002: 14: 537–545. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa H, Li K, Tamai K, Sawamura D, Uitto J. Cloning of the mouse desmoglein 3 gene (Dsg3): interspecies conservation within the cadherin superfamily. Exp Dermatol 2000: 9: 229–239. [DOI] [PubMed] [Google Scholar]
- 6.Whittock NV, Bower C. Genetic evidence for a novel human desmosomal cadherin, Desmoglein 4. J Invest Dermatol 2003: 120: 523–530. [DOI] [PubMed] [Google Scholar]
- 7.Arnemann J, Sullivan KH, Magee AI, King IA, Buxton RS. Stratification-related expression of isoforms of desmosomal cadherins in human epidermis. J Cell Sci 1993: 104: 741–750. [DOI] [PubMed] [Google Scholar]
- 8.Garrod DR Desmosomes and hemidesmosomes. Curr Opin Cell Biol 1993: 5: 30–40. [DOI] [PubMed] [Google Scholar]
- 9.Legan PK, Yue KKM, Chidgey MAJ, Holton RW, Wilkinson RW, Garrod DR. The bovine desmocollin family: a new gene and expression patterns reflecting epithelial cell proliferation and differentiation. J Cell Biol 1994: 126: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrod DR, Chidgey MAJ, North AJ. Desmosomes: differentiation, development, dynamics and disease. Curr Opin Cell Biol 1996: 8: 670–678. [DOI] [PubMed] [Google Scholar]
- 11.Garrod DR, Merritt AJ, Nie Z. Desmosomal adhesion: structural basis, molecular mechanism and regulation. Mol Membr Biol 2002: 19: 81–94. [DOI] [PubMed] [Google Scholar]
- 12.Amagai M Pemphigus: autoimmunity to epidermal cell adhesion molecules. Adv Dermatol 1996: 11: 319–352. [PubMed] [Google Scholar]
- 13.Amagai M, Koch P, Nishikawa T, Stanley JR. Pemphigus vulgaris antigen (desmoglein 3) is localizes in the lower epidermis, the site of blister formation in patients. J Invest Dermatol 1996: 106: 351–355. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JE, Jensen PJ, Wheelock MJ. Cadherin function is required for human keratinocytes to assemble desmosomes and stratify in response to calcium. J Invest Dermatol 1994: 102: 870–877. [DOI] [PubMed] [Google Scholar]
- 15.Ide A, Hashimoto T, Amagai M, Tanaka M, Nishikawa T. Detection of autoantibodies against bullous pemphigoid and pemphigus antigens by an enzyme-linked immunosorbent assay using the bacterial recombinant proteins. Exp Dermatol 1995: 4: 112–116. [DOI] [PubMed] [Google Scholar]
- 16.Kowalczyk AP, Borgwardt JE, Green KJ. Analysis of desmosomal cadherin-adhesive function and stoichiometry of desmosomal cadherin–plakoglobin complexes. J Invest Dermatol 1996: 107: 293–300. [DOI] [PubMed] [Google Scholar]
- 17.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol 1997: 113: 119–146. [DOI] [PubMed] [Google Scholar]
- 18.Mahoney MG, Hu Y, Brennan D, Bazzi H, Christiano AM, Wahl JK III. Delineation of diversified desmoglein distribution in stratified squamous epithelia: implications in diseases. Exp Dermatol 2006: 15: 101–109. [DOI] [PubMed] [Google Scholar]
- 19.Mége R-M, Gavard J, Lambert M. Regulation of cell–cell junctions by cytoskeleton. Curr Opin Cell Biol 2006: 18: 541–548. [DOI] [PubMed] [Google Scholar]
- 20.Kitajima Y, Aoyama Y, Seishima M. Transmembrane signaling for adhesive regulation of desmosomes and hemidesmosomes, and for cell–cell detachment induced by pemphigus IgG in cultured keratinocytes: involvement of protein kinase C. J Investig Dermatol Symp Proc 1999: 4: 137–144. [DOI] [PubMed] [Google Scholar]
- 21.Hennings H, Michel D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation or growth and differentiation of mouse epidermal cells in culture. Cell 1980: 19: 245–254. [DOI] [PubMed] [Google Scholar]
- 22.Mitev V, Miteva L. Signal transduction in keratinocytes. Exp Dermatol 1999: 8: 96–108. [DOI] [PubMed] [Google Scholar]
- 23.Lee YS, Yuspa SH, Dlugosz AA. Differentiation of cultured human epidermal keratinocytes at high cell densities is mediated by endogenous activation of the protein kinase C pathway. J Invest Dermatol 1998: 111: 762–766. [DOI] [PubMed] [Google Scholar]
- 24.Denning MF, Dlugosz AA, Cheng C et al. Cross-talk between epidermal growth factor receptor and protein kinase C during calcium-induced differentiation of keratinocytes. Exp Dermatol 2000: 9: 192–199. [DOI] [PubMed] [Google Scholar]
- 25.Sheu HM, Kitajima Y, Yaoita H. Involvement of protein kinase C in translocation of desmoplakins from cytosol to plasma membrane during desmosomes formation in human squamous cell carcinoma cells grown in low to normal calcium concentration. Exp Cell Res 1989: 185: 176–190. [DOI] [PubMed] [Google Scholar]
- 26.Denning MF, Guy SG, Ellerbroek SM, Norvell SM, Kowalczyk AP, Green KJ. The expression of desmoglein isoforms in cultured human keratinocytes is regulated by calcium, serum, and protein kinase C. Exp Cell Res 1998: 239: 50–59. [DOI] [PubMed] [Google Scholar]
- 27.Kitajima Y, Inoue S, Nagao S, Nagata K, Yaoita H, Nozawa Y. Biphasic effects of 12-O-tetradecanoylphorbol-13-acetate on the cell morphology of low calcium-grown human epidermal carcinoma cells: involvement of translocation and down regulation of protein kinase C. Cancer Res 1988: 48: 964–970. [PubMed] [Google Scholar]
- 28.van Hengel J, Gohon L, Bruyneel E et al. Protein kinase C activation upregulates intercellular adhesion of alpha-catenin-negative human colon cancer cell variants via induction of desmosomes. J Cell Biol 2007: 137: 1103–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishizuka Y The molecular heterogeneity of protein kinase C and its implication for cellular regulation. Nature 1988: 334: 661–665. [DOI] [PubMed] [Google Scholar]
- 30.Gutcher I, Webb PR, Anderson NG. The isoform-specific regulation of apoptosis by protein kinase C. Cell Mol Life Sci 2003: 60: 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bíró T, Szabó I, Kovács L, Hunyadi J, Csernoch L. Distinct sub-populations in HaCaT cells as revealed by the characteristics of intracellular calcium release induced by phosphoinositide-coupled agonists. Arch Dermatol Res 1998: 290: 270–276. [DOI] [PubMed] [Google Scholar]
- 32.Gönczi M, Papp H, Bíró T, Kovács L, Csernoch L. Effect of protein kinase C on transmembrane calcium fluxes in HaCaT keratinocytes. Exp Dermatol 2002: 11: 25–33. [DOI] [PubMed] [Google Scholar]
- 33.Papp H, Czifra G, Bodó E et al. Opposite roles of protein kinase C isoforms in proliferation, differentiation, apoptosis, and tumorigenicity of human HaCaT keratinocytes. Cell Mol Life Sci 2004: 61: 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ács P, Bögi K, Marquez AM et al. The catalytic domain of protein kinase C chimeras modulates the affinity and targeting of phorbol ester induced translocation. J Biol Chem 1997: 272: 22148–22153. [DOI] [PubMed] [Google Scholar]
- 35.Griger Z, Páyer E, Kovács I et al. Protein kinase Cβ and δ isoenzymes promote arachidonic acid production and proliferation of MonoMac-6 cells. J Mol Med 2007: 85: 1031–1042. [DOI] [PubMed] [Google Scholar]
- 36.Gönczi M, Szentadrássy N, Fü löp L et al. Stretch-activated channels influence the membrane potential and alter the proliferation of keratinocytes in vitro. Exp Dermatol 2007: 16: 302–310. [DOI] [PubMed] [Google Scholar]
- 37.Papp H, Czifra G, Lázár J et al. Protein kinase C isozymes regulate proliferation and high cell density-mediated differentiation of HaCaT keratinocytes. Exp Dermatol 2003: 12: 811–824. [DOI] [PubMed] [Google Scholar]
- 38.Kiss B, Bíró T, Czifra G et al. Investigation of micronized titanium-dioxide penetration in human skin xenografts and its effect on cellular functions of human skin-derived cells. Exp Dermatol doi: 10.1111/j/1600-0625.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- 39.Bodó E, Bíró T, Telek A et al. A ‘hot’ new twist to hair biology – involvement of vanilloid receptor-1 (VR1/TRPV1) signaling in human hair growth control. Am J Pathol 2005: 166: 985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gschwendt M, Müller H-J, Kialbassa K et al. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun 1994: 199: 93–98. [DOI] [PubMed] [Google Scholar]
- 41.Dietrich C, Gumpert N, Heit I, Borchert-Stuchltrager M, Oesch F, Wieser R. Rottlerin induces a transformed phenotype in human keratinocytes. Biochem Biophys Res Commun 2001: 282: 575–579. [DOI] [PubMed] [Google Scholar]
- 42.Alessi DR The protein kinase C inhibitors Ro 318220 and GF 109203X are equally potent inhibitors of MAPKAP kinase-1β and p70 S6 kinase. FEBS Lett 1997: 402: 121–123. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Lorenzo PS, Bogi K, Blumberg PM, Yuspa SH. Protein kinase Cδ targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by and adenoviral vector. Mol Cell Biol 1999: 19: 8547–8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denning MF, Wang Y, Nickoloff BJ, Wrone-Smith T. Protein kinase C delta is activated by caspase-dependent proteolysis during ultraviolet radiation-induced apoptosis of human keratinocytes. J Biol Chem 1998: 273: 29995–30002. [DOI] [PubMed] [Google Scholar]
- 45.Denning MF, Wang Y, Tibudan S, Alkan S, Nickoloff BJ, Qin JZ. Caspase activation and disruption of mitochondrial membrane potential during UV radiation-induced apoptosis of human keratinocytes requires activation of protein kinase C. Cell Death Differ 2002: 9: 40–52. [DOI] [PubMed] [Google Scholar]
- 46.Zang LC, Ng DC, Bikle DD. Role of protein kinase α in calcium induced keratinocyte differentiation: defective regulation in squamous cell carcinoma. J Cell Physiol 2003: 195: 249–259. [DOI] [PubMed] [Google Scholar]
- 47.Jansen AP, Dreckschmidt NE, Verwiebe ED, Wheeler DL, Oberley TD, Verma AK. Regulation of the induction of epidermal ornithine decarboxylase and hyperplasia to the different skin tumor-promotion susceptibilities of protein kinase C alpha, -delta, and -epsilon transgenic mice. Int J Cancer 2001: 93: 635–643. [DOI] [PubMed] [Google Scholar]
- 48.Fisher GJ, Tavakkol A, Leach K et al. Differential expression of protein kinase C isoenzymes in normal and psoriatic adult human skin: reduced expression of protein kinase C-betaII in psoriasis. J Invest Dermatol 1993: 101: 553–559. [DOI] [PubMed] [Google Scholar]
- 49.Osada K, Sheisima M, Kitajima Y. Pemphigus IgG activates and translocates protein kinase C from the cytosol to the particulate/cytoskeleton fractions in human keratinocytes. J Invest Dermatol 1997: 108: 482–487. [DOI] [PubMed] [Google Scholar]
- 50.Kimura TE, Merrit AJ, Garrod DR. Calcium-independent desmosomes of keratinocytes are hyper-adhesive. J Invest Dermatol 2007: 127: 775–781. [DOI] [PubMed] [Google Scholar]
- 51.Sánchez-Carpintero I, España A, Pelacho B et al. In vivo blockade of pemphigus vulgaris acantholysis by inhibition of intracellular signal transduction cascades. Br J Dermatol 2004: 151: 565–570. [DOI] [PubMed] [Google Scholar]
- 52.Sharma P, Mao X, Payne AS. Beyond steric hindrance: the role of adhesion signaling pathways in the pathogenesis of pemphigus. J Dermatol Sci 2007: 48: 1–14. [DOI] [PubMed] [Google Scholar]