Abstract
A series of 2-sulfonamidopyridine C-region derivatives of 2-(3-fluoro-4-methylsulfonamidophenyl)propanamide were investigated as hTRPV1 ligands. Systematic modification on the 2-sulfonamido group provided highly potent TRPV1 antagonists. The N-benzyl phenylsulfonamide derivatives 12 and 23 in particular showed higher affinities than that of lead compound 1. Compound 12 exhibited strong analgesic activity in the formalin pain model. Docking analysis of its chiral S-form 12S in our hTRPV1 homology model indicated that its high affinity might arise from additional hydrophobic interactions not present in lead compound 1S.
Keywords: Vanilloid receptor 1, TRPV1 antagonists, Analgesic
1. Introduction
TRPV1 has emerged as an exciting therapeutic target for chronic and inflammatory pain as well as for the numerous other conditions in which C-fiber sensory afferent neurons are involved.1–3 The natural products capsaicin4 and resiniferatoxin5 provided critical initial lead structures guiding the current vigorous efforts by many groups. It is now appreciated that the pharmacophore can be conceptualized as being subdivided into 3 regions, designated A, B, and C. Appropriate substitution in the A region can generate antagonistic activity, with the 3-fluoro-4-sulfonamidophenyl group being an early example.6 The chemical efforts have been significantly aided by structural insights into the TRPV1 binding domain provided by homology modeling,7 cryoEM structural analysis,8,9 and further modeling derived from the cryoEM structure.10 An on-going challenge for the field is to understand the integration of ligand–TRPV1 interactions with endogenous regulatory networks in the context of the whole animal.11 In particular, different antagonists differ in their tendency to induce the side effect of hyperthermia.12 The range of structures with potent TRPV1 antagonism being developed by different groups should provide the tools to address these issues as compounds move forward into clinical testing.13
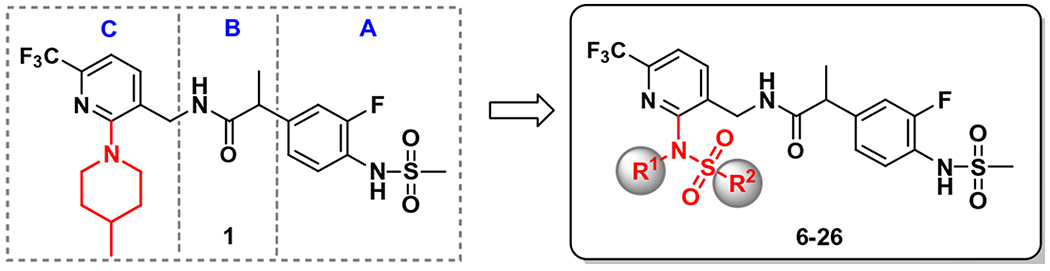
Over the years, we have reported that a series of N-{(6-trifluoromethyl-pyridin-3-yl)methyl}2-(3-fluoro-4-methylsulfonamidophenyl)propanamides were potent hTRPV1 antagonists active against multiple activators.14–20 Initial analyses of the antagonistic template focused on the pyridine C-region where the structure activity relationships for the 2-substituent were extensively explored with a variety of functional groups. A prototype for these series is compound 1, which possesses a 4-methylpiperidinyl group as a 2-substituent. Compound 1 displayed highly potent and (S)-stereospecific antagonism of hTRPV1 activators including capsaicin, low pH, heat (45 °C) and N-arachidonoyl dopamine (NADA) (Fig. 1).14 In addition, in vivo analysis confirmed that compound 1 and congeners blocked capsaicin-induced hypothermia, consistent with their in vitro mechanism of action, and they demonstrated potent antiallodynic activity in a neuropathic pain model. Molecular modeling using our established hTRPV1 homology model indicated that the two principal hydrophobic interactions, between the 6-trifluoromethyl group and the 2-substituents in the C-region and the hydrophobic pockets composed of Leu547/Thr550 and Met514/Leu515, respectively, were critical for the potent activity of the antagonists.14
Figure 1.
Design of 2-sulfonamidopyridine C-region TRPV1 antagonists.
In continuation of our program to discover clinical candidates for antagonism of TRPV1 mediated neuropathic pain, we have sought to further optimize the above template by investigating 2-sulfonamidopyridine C-region derivatives in which hydrophobic R1 and R2 groups were incorporated through a polar sulfonamide linker (Fig. 1). In this study, we synthesized a series of 2-sulfonamido 4-(trifluoromethyl)pyridine C-region derivatives of the antagonistic template and evaluated their binding affinities and antagonism of hTRPV1 activation by capsaicin. With selected potent antagonists in the series, we further characterized their analgesic activities in animal models and performed a docking study with our hTRPV1 homology model to elucidate their binding mode to the receptor.
2. Result and discussion
2.1. Chemistry
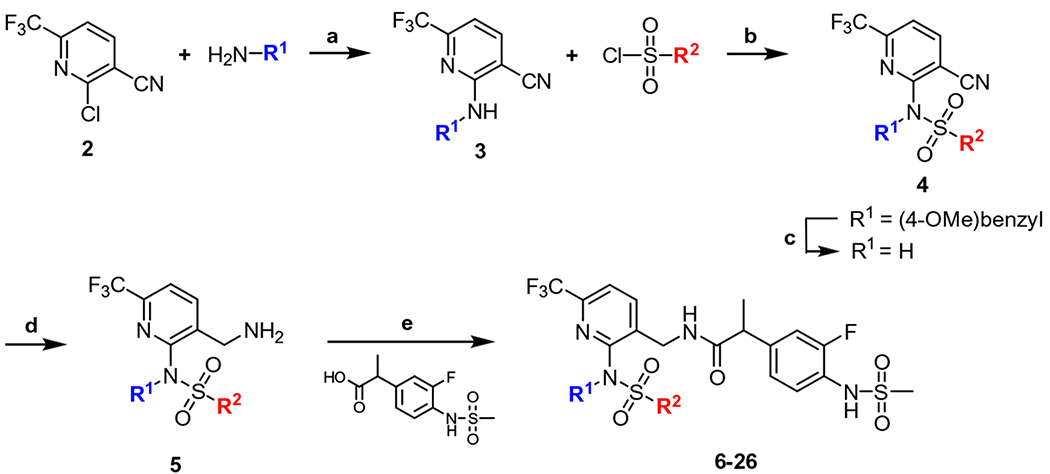
The 2-sulfonamidopyridine derivatives (6–26) were synthesized in 4-steps through a conventional approach starting from the commercially available 2-chloro-6-(trifluoromethyl)nicotinonitrile (2) (Scheme 1). Compound 2 was reacted with various amines (R1NH2) to produce the corresponding 2-amino derivatives (3) by one of two methods. They were then sulfonylated with a series of sulfonyl chlorides (R2SO2Cl) to provide 2-sulfonamido derivatives (4). For the synthesis of the secondary sulfonamide derivative (6), the 4-methoxybenzyl group in 4 was oxidatively deprotected. The nitrile of 4 was reduced to the corresponding amine (5), respectively, which were coupled with the racemic (or chiral S-form) propionic acid as previously reported14 to provide the final compounds (6–26).
Scheme 1.
General synthesis of 2-sulfonamido-6-(trifluoromethyl)pyridine C-region analogs. Reagents and conditions: (a) [Method A] K2CO3, 18-crown-6 ether, CH3CN, reflux, 12 h for 6–8, 11–26; [Method B] Pd(OAc)2, dppf, K2CO3, toluene/THF (1:1 v/v), reflux, 12 h for 9, 10; (b) R2SO2Cl, NaH, DMF, 100 °C, 12 h; (c) CAN, CH3CN/H2O (4:1 v/v), rt, 3 h for 6; (d) 2 M BH3·SMe2 in THF, reflux, 12 h; (e) EDC, HOBt, DMF, rt, 12 h.
2.2. Biological activity
The binding affinities and potencies as agonists/antagonists of the synthesized TRPV1 ligands were assessed in vitro by a binding competition assay with [3H]RTX and by a functional 45Ca2+ uptake assay using human TRPV1 heterologously expressed in Chinese hamster ovary (CHO) cells, as previously described.6,21 For the agonism assay, a saturating concentration of capsaicin (300 nM) was used to define maximal response. For the antagonism assay, the dose-dependent inhibition of the capsaicin (30 nM) stimulated calcium uptake was measured. The Ki values for antagonism take into account the competition between capsaicin and the antagonist. The results are summarized in Tables 1–3, together with the potencies of previous lead compound 1.
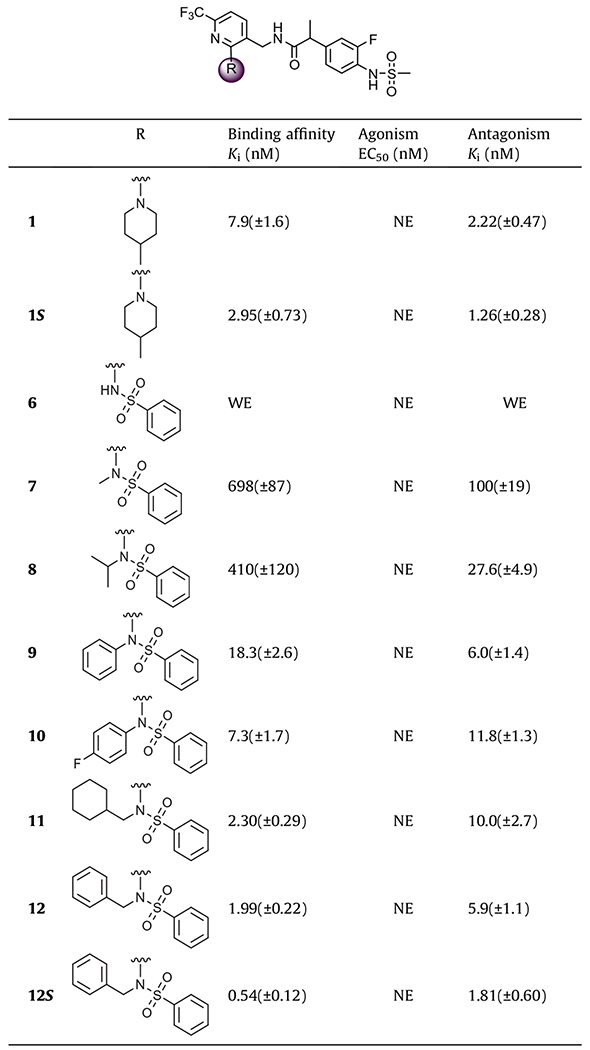
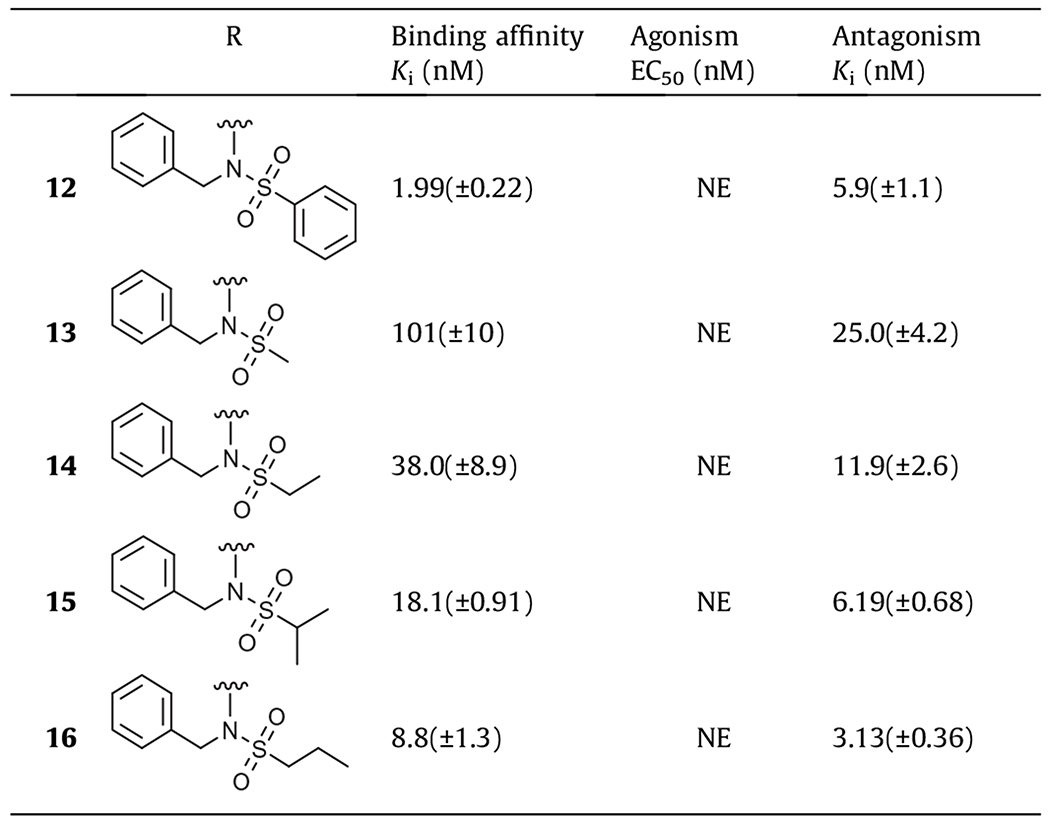
Table 1.
In vitro activity of phenylsulfonamide derivatives on hTRPV1a
NE: no effect, WE: weak effect; values are the mean ± SEM of at least three experiments.
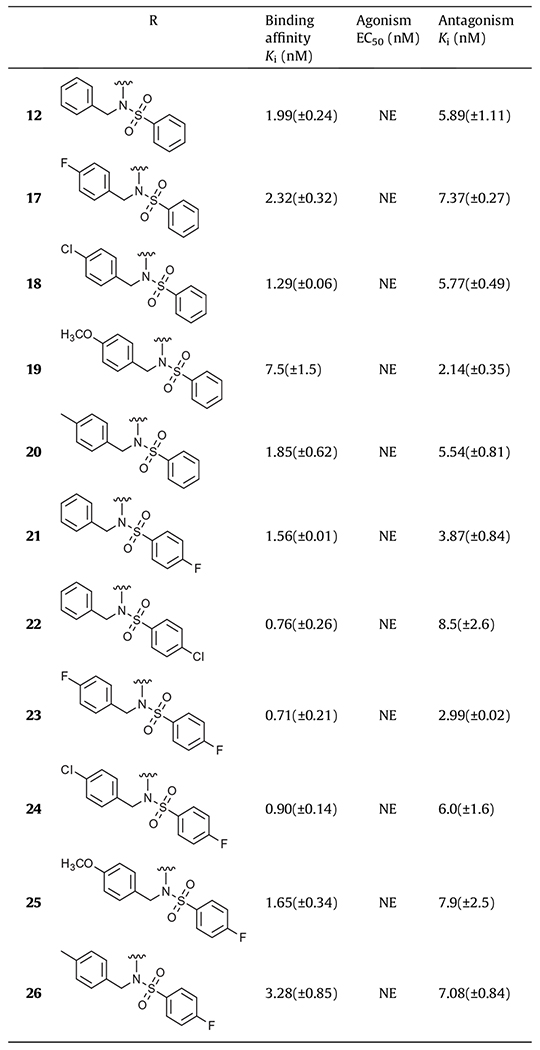
Table 3.
In vitro activity of N-benzyl phenylsulfonamide derivatives on hTRPV1a
NE: no effect; values are the mean ± SEM of at least three experiments.
First, we investigated a series of phenylsulfonamide derivatives (R2 = Ph) in which a variety of N-substituents (R1) including alkyl and aryl groups were explored (Table 1). The secondary phenylsulfonamide derivative (6) was found to have little activity. This result was consistent with previous findings in which secondary amino derivatives14 as 2-substituents in the pyridine C-region showed only weak antagonism. In contrast, incorporation of hydrophobic groups on the nitrogen of the phenylsulfonamide, providing tertiary phenylsulfonamides (7–12), led to potent binding affinity and antagonism. The binding affinities increased with the size of the N-substituent: methyl (7) < isopropyl (8) < phenyl (9) < 4-fluorophenyl (10) < cyclohexylmethyl (11) < benzyl (12). Antagonism by the more potent compounds fell in the range of Ki(ant) = 5–10 nM. Among the compounds, the N-benzyl phenylsulfonamide (12) exhibited high affinity and potent antagonism with Ki = 1.99 nM and Ki(ant) = 5.9 nM, representing a 4-fold enhancement in binding affinity but a 2.5-fold reduction in antagonism compared to lead compound 1. The chiral S-isomer (12S), which has the active S-stereoconfiguration as described previously,14 was prepared and, as expected, showed enhanced potency relative to that of 12 with Ki = 0.54 nM and Ki(ant) = 1.81 nM, which also demonstrated a 5-fold increase in binding affinity but a 1.5-fold decrease in antagonism compared to 1S, S-isomer of lead compound 1.
Next, due to the high potency of 12, we further investigated the structure activity relationship of the phenyl moiety of the phenylsulfonamide in 12 (Table 2). The phenylsulfonamide group of 12 was replaced by the corresponding alkylsulfonamides. The binding affinities and antagonistic potencies were enhanced with the increased lipophilicity of the corresponding alkyl groups: R = Me (13) < R = Et (14) < R = iPr (15) < R = Pr (16). However, none of the derivatives were as potent as compound 12.
Table 2.
In vitro activity of N-benzyl alkylsulfonamide derivatives on hTRPV1a
NE: no effect; values are the mean ± SEM of at least three experiments.
Finally, we investigated 4-substituted N-benzyl and phenylsulfonamide derivatives of 12 for further optimization (Table 3). Four different 4-substituted N-benzyl derivatives with 4-F, 4-Cl, 4-OCH3 and 4-CH3 (17–20) were explored first. The 4-chlorobenzyl derivative (18) showed slightly improved binding affinity compared to that of 12 with Ki = 1.29 nM, and the 4-methoxybenzyl derivative (19) exhibited ca. 3-fold more potent antagonism than that of 12 with Ki(ant) = 2.14 nM. The two 4-substituted phenylsulfonamide derivatives with 4′-F and 4′-Cl (21, 22) were also examined. The 4′-fluorophenyl derivative (21) displayed better binding affinity and antagonism with Ki = 1.56 nM and Ki(cat) = 3.87 nM compared to 12. This promising result prompted us to further investigate 4-substituted benzyl derivatives of 21 (23–26). The N-(4-fluorobenzyl)4′-fluorophenylsulfonamide (23) was found to be the most potent antagonist in this series with Ki = 0.71 nM and Ki(ant) = 2.99 nM, which was 10-fold more potent in binding affinity and similar activity in antagonism compared to compound 1.
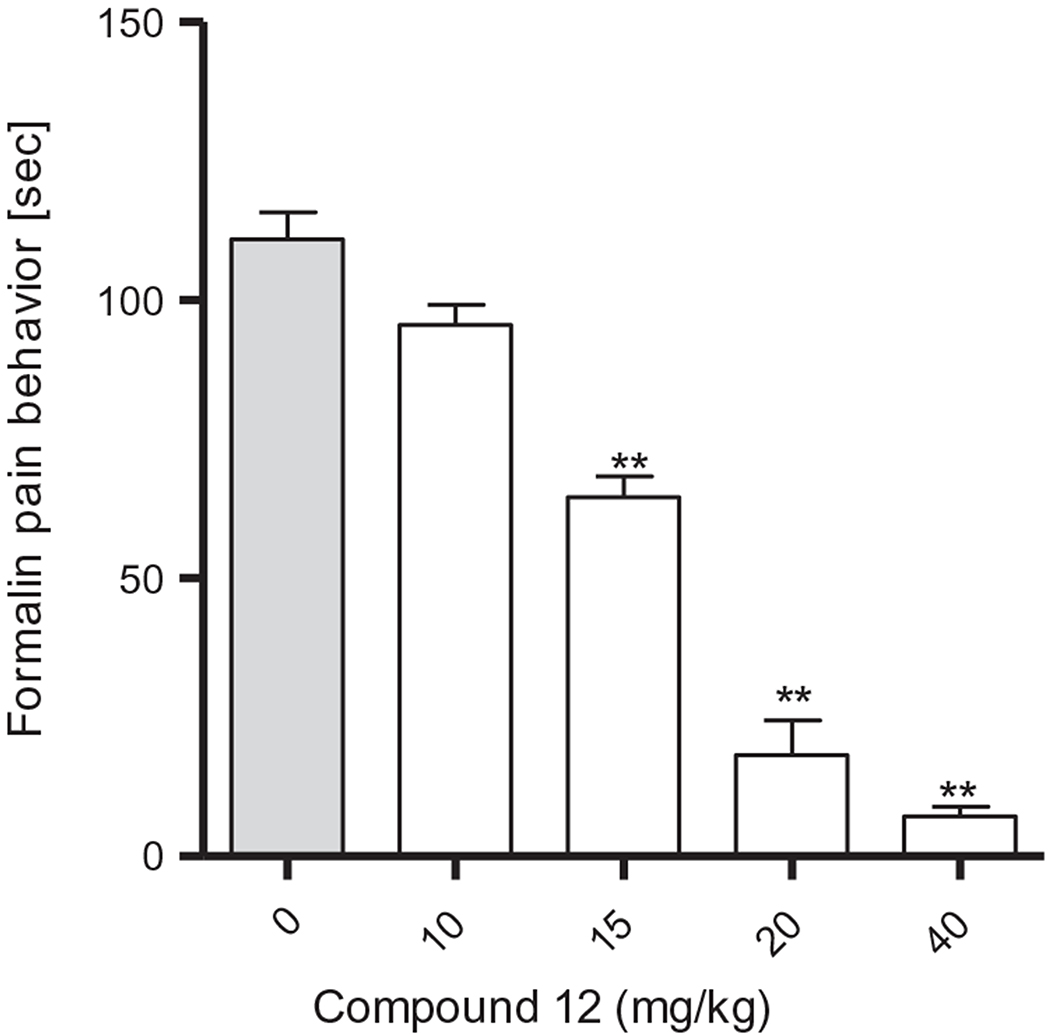
We next evaluated the analgesic activity of compound 12 upon intraperitoneal administration in the formalin mouse pain model (Fig. 2).22,23 Compound 12 demonstrated excellent, dose-dependent analgesic efficacy in the second period (20–30 min after injection). The ED50 was 15.6 mg/kg.
Figure 2.
Analgesic activity of compound 12 in the formalin model.
2.3. Molecular modeling
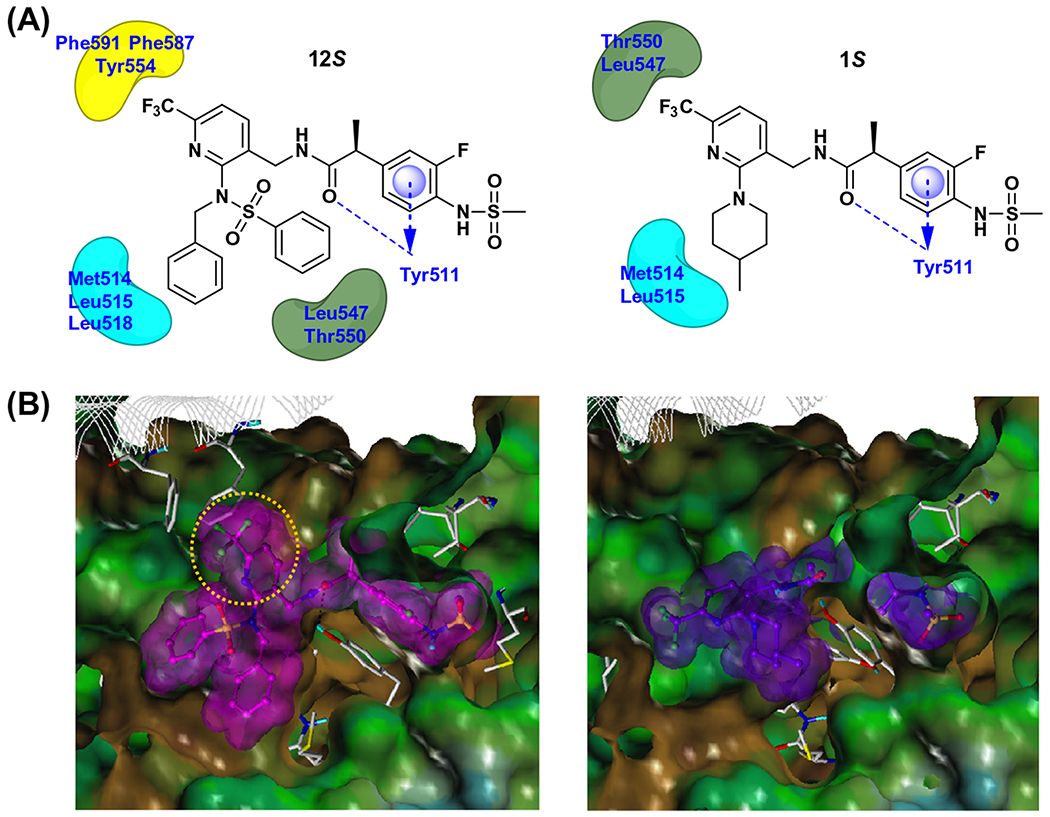
To investigate the binding interactions of compound 12S, we carried out a flexible docking study using our human TRPV1 model14 built based on our rat TRPV1 model7 and compared its behavior to that of lead compound 1S. Previously we had demonstrated that the two principal hydrophobic interactions between the 6-trifluoromethyl group and the 4-methylpiperidinyl ring in the C-region of 1S and the two pockets composed of Leu547/Thr550 and Met514/Leu515, respectively, were critical for its high potency.14 Structurally, compound 12S has the two bulky substituents, including phenylsulfonamide and benzyl groups, at the 2-position of the pyridine C-region, whereas 1S has only a hydrophobic group having a 4-methylpiperidinyl ring.
As shown in Figure 3, the phenylsulfonamide group in the C-region of 12S extended toward the hydrophobic area composed of Leu547 and Thr550 similar to the 6-trifluoromethylpyridine ring in 1. Furthermore, the benzyl group in 12S was involved in the hydrophobic interaction with Met514 and Leu515 as was the 4-methylpiperidinyl group in 1S. However, the 6-trifluoromethylpyridine in 12S made additional hydrophobic interactions with Tyr554 and the residues Phe591, Phe587 from the adjacent monomer. These additional hydrophobic interactions might explain the higher binding affinity of 12S.
Figure 3.
Docking analysis of 12S and 1S in the hTRPV1 homology model. (A) 2-D representation of the interactions between 12S (left) and 1S (right) with hTRPV1. Hydrogen bonding interactions are depicted as blue dashed lines and hydrophobic interactions are shown as arcs. The π–π stacking interaction is marked with a blue disc and arrow. (B) The Fast Connolly surface of hTRPV1 and the van der Waals surface of the docked compounds. MOLCAD was used to create the molecular surface of hTRPV1 and the surface is displayed with the lipophilic potential property. The surface of hTRPV1 is Z-clipped for clarity and that of ligands are colored individually by magenta or purple. The yellow colored circle represents the part of 12S which is involved in the additional hydrophobic interactions.
3. Conclusion
A series of 2-sulfonamidopyridine C-region derivatives of the 2-(3-fluoro-4-methylsulfonamidophenyl)propanamide template have been investigated for their activity on hTRPV1. Systematic modification on the 2-sulfonamido group provided compounds of high affinity and potent antagonism. Compared to lead 1, the N-benzyl phenylsulfonamide derivatives 12 and 23 showed upto a 10-fold increase in binding affinity. Compound 12 was evaluated in the formalin pain model and exhibited strong analgesic activity with ED50 = 15.6 mpk in the second phase. A docking study of compound 12S, the active isomer of 12, in our hTRPV1 homology model revealed three principal hydrophobic interactions of the C-region with the receptor. Among them, the additional hydrophobic interaction with a pocket composed of Phe587/Phe591 might explain the higher affinity of 12S compared to 1S.
4. Experimental
4.1. Chemistry
4.1.1. General
All chemical reagents were commercially available. Melting points were determined on a Büchi Melting Point B-540 apparatus and are uncorrected. Silica gel column chromatography was performed on silica gel 60, 230–400 mesh, Merck. Nuclear magnetic resonance (1H NMR and 13C NMR) spectra were recorded on JEOL JNM-LA 300 [300 MHz (1H), 75 MHz (13C)] and Bruker Avance 400 MHz FT-NMR [400 MHz (1H), 100 MHz (13C)] spectrometers. Chemical shifts are reported in ppm units with Me4Si as a reference standard. Mass spectra were recorded on a VG Trio-2 GC–MS and 6460 Triple Quad LC/MS. All final compounds were purified to >95% purity, as determined by high-performance liquid chromatography (HPLC). HPLC was performed on an Agilent 1120 Compact LC (G4288A) instrument using an Agilent Eclipse Plus C18 column (4.6 × 250 mm, 5 μm) and a Daicel Chiralcel OD-H column (4.6 × 250 mm, 5 μm).
4.1.2. General procedure for amidation
Method A:
To a solution of 2-chloro-4-(trifluoromethyl)-benzonitrile solution (1.00 mmol) in acetonitrile was added potassium carbonate (3.00 mmol) and 18-crown-6 ether (0.10 mmol) and the resulting solution was stirred at room temperature for 30 min. Appropriate amine (NH2R1, 1.5 mmol) was added to the mixture and refluxed for 12 h. After being cooled down to ambient temperature, the reaction was quenched with water and extracted with EtOAc twice. The combined organic extracts were dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc/ hexanes (1:2) as eluant.
Method B:
To a solution of 2-chloro-4-(trifluoromethyl)-benzonitrile solution (1.00 mmol) in toluene/THF (1:1 v/v) was added palladium(II) acetate (0.10 mmol), dppf (0.20 mmol), potassium carbonate (2.00 mmol) and the resulting solution was stirred at room temperature for 30 min. An appropriate amine (NH2R1, 1.50 mmol) was added to the mixture and refluxed for 12 h. After being cooled down to ambient temperature, the reaction was quenched with water and extracted with EtOAc twice. The combined organic extracts were dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc/hexanes (1:2) as eluant.
4.1.2.1. 2-(4-Methoxybenzyl)amino-6-(trifluoromethyl)nicotinonitrile (R1 = (4-OMe)Bn).
Yield 88%, yellow solid, mp = 70–82 °C; 1H NMR (400 MHz, CDCl3) δ 7.78 (d, J = 7.68 Hz, 1H), 7.29 (d, J = 8.32 Hz, 2H), 6.94 (d, J = 7.76 Hz, 1H), 6.86 (d, J = 8.40 Hz, 1H), 5.59 (br s, 1H), 4.62 (d, J = 5.44 Hz, 2H), 3.78 (s, 3H); MS (FAB) m/z 308 [M+H]+.
4.1.2.2. 2-Methylamino-6-(trifluoromethyl)nicotinonitrile (R1 = Me).
Yield 95%, pale yellow solid, mp = 65–73 °C; 1H NMR (300 MHz, CDCl3) δ 7.80 (d, J = 7.86 Hz, 1H), 6.94 (d, J = 7.71 Hz, 1H), 5.41 (s, 1H), 3.11 (d, J = 4.77 Hz, 3H); MS (FAB) m/z 202 [M+H]+.
4.1.2.3. 2-Isopropylamino-6-(trifluoromethyl)nicotinonitrile (R1 = i-Pr).
Yield 70%, yellow solid, mp = 63–65 °C; 1H NMR (300 MHz, CDCl3) δ 7.78 (dd, J = 7.89 Hz, 0.75 Hz, 1H), 6.91 (d, J = 7.86 Hz, 1H), 5.15 (m, 1H), 4.34 (m, 1H), 1.28 (d, J = 6.57 Hz, 6H); MS (FAB) m/z 230 [M+H]+.
4.1.2.4. 2-Phenylamino-6-(trifluoromethyl)nicotinonitrile (R1 = Ph).
Yield 85%, yellow solid, mp = 52–65 °C; 1H NMR (300 MHz, CDCl3) δ 7.96 (dd, J = 7.86, 0.72 Hz, 1H), 7.66 (m, 2H), 7.40 (t, J = 7.53 Hz, 2H), 7.19 (t, J = 1.29 Hz, 1H), 7.15 (d, J = 8.97 Hz, 1H); MS (FAB) m/z 264 [M+H]+.
4.1.2.5. 2-(4-Fluorophenyl)amino-6-(trifluoromethyl)nicotinonitrile (R1 = (4-F)Ph).
Yield 80%, yellow solid, mp = 64–72 °C; 1H NMR (300 MHz, CDCl3) δ 7.96 (d, J = 7.86 Hz, 1H), 7.60 (dd, J = 8.97, 4.59 Hz, 1H), 7.15 (d, J = 7.68 Hz, 2H), 7.09 (t, J = 8.79 Hz, 2H); MS (FAB) m/z 282 [M+H]+.
4.1.2.6. 2-(Cyclohexylmethyl)amino-6-(trifluoromethyl)nicotinonitrile (R1 = (Cy)Me).
Yield 81%, yellow solid, mp = 65–78 °C; 1H NMR (300 MHz, CDCl3) δ 7.78 (d, J = 7.68 Hz, 1H), 6.91 (d, J = 7.71 Hz, 1H), 5.40 (s, 1H), 3.40 (t, J = 6.24 Hz, 2H), 1.76–1.61 (m, 4H), 1.26–1.21 (m, 4H), 1.05–0.98 (m, 2H); MS (FAB) m/z 284 [M+H]+.
4.1.2.7. 2-(Benzylamino-6-(trifluoromethyl)nicotinonitrile (R1 = Bn).
Yield 83%, pale yellow solid, mp = 78–85 °C; 1H NMR (300 MHz, CDCl3) δ 7.81 (d, J = 7.86 Hz, 1H), 7.38–7.25 (m, 5H), 6.97 (t, J = 7.89 Hz, 1H), 5.70 (br s, 1H), 4.71 (d, J = 5.67 Hz, 2H); MS (FAB) m/z 278 [M+H]+.
4.1.2.8. 2-(4-Fluorobenzyl)amino-6-(trifluoromethyl)nicotinonitrile (R1 = (4-F)Bn).
Yield 70%, dark yellow solid, mp = 87–92 °C; 1H NMR (300 MHz, CDCl3) δ 7.82 (d, J = 7.7 Hz, 1H), 7.38–7.34 (m, 2H), 7.06–6.97 (m, 3H), 4.68 (d, J = 5.7 Hz, 2H); MS (FAB) m/z 296 [M+H]+.
4.1.2.9. 2-(4-Chlorobenzyl)amino-6-(trifluoromethyl)nicotinonitrile (R1 = (4-Cl)Bn).
Yield 70%, yellow solid, mp = 82–95 °C; 1H NMR (300 MHz, CDCl3) δ 7.85 (d, J = 7.71 Hz, 1H), 7.33–7.32 (m, 4H), 7.00 (d, J = 7.71 Hz, 1H), 5.73 (s, 1H), 4.69 (d, J = 5.67 Hz, 2H); MS (FAB) m/z 312 [M+H]+.
4.1.2.10. 2-(4-Methylbenzyl)amino-6-(trifluoromethyl)nicotinonitrile (R1 = (4-Me)Bn).
Yield 88%, yellow solid, mp = 91–97 °C; 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 7.80 Hz, 1H), 7.26 (d, J = 7.72 Hz, 2H), 7.19–7.12 (m, 3H), 6.94 (d, J = 7.76 Hz, 1H), 5.72 (br s, 1H), 4.65 (d, J = 5.52 Hz, 2H), 2.33 (s, 3H); MS (FAB) m/z 292 [M+H]+.
4.1.3. General procedure for sulfonamidation
A solution of 2-(alkyl/aryl amino)-5-(trifluoromethyl)nicotinonitrile (1.00 mmol) in DMF was cooled to 0 °C and sodium hydride (60% dispersion in oil; 2.50 mmol) was added. The resulting solution was stirred at 0 °C for 30 min and the appropriate sulfonyl chloride (R2SO2Cl) was added dropwised to the mixture and then stirred at 100 °C for 12 h. The reaction was quenched with water and extracted with DCM twice. The combined organic extracts were dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc/hexanes (1:4) as eluant.
4.1.3.1. N-(3-Cyano-6-(trifluoromethyl)pyridin-2-yl)-N-(4-methoxybenzyl)benzenesulfonamide (R1 = (4-OMe)Bn, R2 = Ph).
Yield 78%, yellow solid, mp = 92–100 °C; 1H NMR (300 MHz, CDCl3) δ 8.10 (d, J = 8.07 Hz, 1H), 7.74–7.53 (m, 6H), 7.15–7.12 (m, 2H), 6.71–6.68 (m, 2H), 4.67 (s, 2H), 3.70 (s, 3H); MS (FAB) m/z 448 [M+H]+.
4.1.3.2. N-(3-Cyano-6-(trifluoromethyl)pyridin-2-yl)-N-methylbenzenesulfonamide (R1 = Me, R2 = Ph).
Yield 85%, orange solid, mp = 95 °C; 1H NMR (300 MHz, CDCl3) δ 8.29 (d, J = 7.86 Hz, 1H), 7.65–7.53 (m, 4H), 7.54 (t, J = 7.89 Hz, 2H), 3.22 (s, 3H); MS (FAB) m/z 342 [M+H]+
4.1.3.3. N-(3-Cyano-6-(trifluoromethyl)pyridin-2-yl)-N-isopropylbenzenesulfonamide (R1 = i-Pr, R2 = Ph).
Yield 35%, yellow solid, mp = 96 °C; 1H NMR (300 MHz, CDCl3) δ 8.33 (d, J = 7.86 Hz, 1H) 7.86–7.77 (m, 4H), 7.63–7.50 (m, 5H) 2.71 (s, 3H), 1.08 (d, J = 6.78 Hz, 6H); MS (FAB) m/z 370 [M+H]+.
4.1.3.4. N-(3-Cyano-6-(trifluoromethyl)pyridin-2-yl)-N-phenylbenzenesulfonamide (R1 = Ph, R2 = Ph).
Yield 83%, red yellow, mp = 95–103 °C; 1H NMR (300 MHz, CDCl3) δ 8.15 (d, J = 8.07 Hz, 1H), 7.63 (d, J = 8.04 Hz, 2H), 7.67 (d, J = 8.07 Hz 1H), 7.62 (t, J = 7.35 Hz, 1H), 7.46 (t, J = 7.68 Hz, 2H), 7.41–7.31 (m, 5H); MS (FAB) m/z 404 [M+H]+.
4.1.3.5. N-(3-Cyano-6-(trifluoromethyl)pyridin-2-yl)-N-(4-fluorophenyl)benzenesulfonamide (R1 = (4-F)Ph, R2 = Ph).
Yield 77%, red yellow solid, mp = 95–103 °C; 1H NMR (300 MHz, CDCl3) δ 8.17 (d, J = 7.89 Hz, 1H), 7.74 (d, J = 7.53 Hz, 1H), 7.71 (d, J = 8.07 Hz, 2H), 7.64 (t, J = 7.32 Hz, 1H), 7.48 (t, J = 7.86 Hz, 2H), 7.31 (m, 2H), 7.05 (t, J = 8.43 Hz, 2H); MS (FAB) m/z 422 [M+H]+.
4.1.3.6. N-(3-Cyano-6-(trifluoromethyl)pyridin-2-yl)-N-(cyclohexylmethyl)benzenesulfon amide (R1 = (Cy)Me, R2 = Ph).
Yield 50%, yellow solid, mp = 83–94 °C; 1H NMR (300 MHz, CDCl3) δ 8.31 (d, J = 7.89 Hz, 1H), 7.77 (d, J = 8.04 Hz, 1H), 7.67–7.48 (m, 5H), 3.44 (d, J = 6.96 Hz, 2H), 1.76–1.65 (m, 4H), 1.28–1.20 (m, 4H), 0.88–0.86 (m, 2H); MS (FAB) m/z 424 [M+H]+.
4.1.3.7. N-Benzyl-N-(3-cyano-6-(trifluoromethyl)pyridin-2-yl)benzenesulfonamide (R1 = Bn, R2 = Ph).
Yield 80%, yellow solid, mp = 80–95 °C; 1H NMR (300 MHz, CDCl3) δ 8.11 (d, J = 8.04 Hz, 1H), 7.66 (d, J = 8.07 Hz, 1H), 7.75–7.67 (m, 2H), 7.60–7.50 (m, 3H), 7.24–7.15 (m, 5H), 4.74 (s, 2H); MS (FAB) m/z 418 [M+H]+.
4.1.3.8. N-Benzyl-N-(3-cyano-6-(trifluoromethyl)pyridin-2-yl)methanesulfonamide (R1 = Bn, R2 = Me).
Yield 74%, pale yellow solid, mp = 102–110 °C; 1H NMR (300 MHz, CDCl3) δ 8.11 (d, J = 8.04 Hz, 1H), 7.66 (d, J = 8.07 Hz, 1H), 7.20–7.33 (m, 5H), 5.03 (s, 2H), 3.16 (s, 3H); MS (FAB) m/z 356 [M+H]+.
4.1.3.9. N-Benzyl-N-(3-cyano-6-(trifluoromethyl)pyridin-2-yl)ethanesulfonamide (R1 = Bn, R2 = Et).
Yield 55%, red yellow solid, mp = 82–98 °C; 1H NMR (300 MHz, CDCl3) δ 8.07 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 7.9 Hz, 1H), 7.34–7.19 (m, 5H), 5.06 (s, 2H), 3.40–3.33 (q, J = 7.5 Hz, 2H), 1.48 (t, J = 7.5 Hz, 3H); MS (FAB) m/z 370 [M+H]+.
4.1.3.10. N-Benzyl-N-(3-cyano-6-(trifluoromethyl)pyridin-2-yl)propane-1-sulfonamide (R1 = Bn, R2 = Pr).
Yield 74%, yellow solid, mp = 95–103 °C; 1H NMR (300 MHz, CDCl3) δ 8.07 (d, J = 8.10 Hz, 1H), 7.62 (d, J = 7.90 Hz, 1H), 7.34–7.21 (m, 5H), 5.05 (s, 2H), 3.30 (t, J = 7.70 Hz, 2H), 2.06–1.94 (m, 2H), 1.12 (t, J = 7.50 Hz, 3H); MS (FAB) m/z 384 [M+H]+.
4.1.3.11. N-Benzyl-N-(3-cyano-6-(trifluoromethyl)pyridin-2-yl)propane-2-sulfonamide (R1 = Bn, R2 = i-Pr).
Yield 22%, yellow solid, mp = 79–86 °C; 1H NMR (300 MHz, CDCl3) δ 8.05 (d, J = 7.90 Hz, 1H), 7.60 (d, J = 8.10 Hz, 1H), 7.32–7.21 (m, 5H), 5.09 (s, 2H), 3.63 (m, 1H), 1.48 (d, J = 6.80 Hz, 6H); MS (FAB) m/z 384 [M+H]+.
4.1.3.12. N-(3-Cyano-6-(trifluoromethyl)pyridin-2-yl)-N-(4-fluorobenzyl)benzenesulfonamide (R1 = (4-F)Bn, R2 = Ph).
Yield 98%, red yellow solid, mp = 92–98 °C; 1H NMR (300 MHz, CDCl3) δ 8.14 (d, J = 8.1 Hz, 1H), 7.73–7.54 (m, 6H), 7.24–7.19 (m, 2H), 6.90–6.85 (m, 2H), 4.70 (s, 2H); MS (FAB) m/z 436 [M+H]+.
4.1.3.13. N-Benzyl-4-chloro-N-(3-cyano-6-(trifluoromethyl)pyridin-2-yl)benzenesulfonamide (R1 = Bn, R2 = (4-Cl)Ph).
Yield 87%, yellow solid, mp = 84–96 °C; 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 7.96 Hz, 1H), 7.85 (d, J = 8.52 Hz, 2H), 7.48 (m, 3H), 7.19–7.15 (m, 5H), 4.70 (s, 2H); MS (FAB) m/z 452 [M+H]+.
4.1.3.14. N-(3-Cyano-6-(trifluoromethyl)pyridin-2-yl)-4-fluoro-N-(4-fluorobenzyl)benzenesul-fonamide (R1 = (4-F)Bn, R2 = (4-F)Ph).
Yield 89%, yellow solid, mp = 87–96 °C; 1H NMR (300 MHz, CDCl3) δ 8.16 (d, J = 7.86 Hz, 1H), 7.75 (m, 3H), 7.19–7.15 (m, 5H), 6.85 (m, 2H), 4.70 (s, 2H); MS (FAB) m/z 454 [M+H]+.
4.1.3.15. N-(4-Chlorobenzyl)-N-(3-cyano-6-(trifluoromethyl)pyridin-2-yl)benzenesulfonamide (R1 = (4-Cl)Bn, R2 = Ph).
Yield 68%, yellow solid, mp = 78–87 °C; 1H NMR (300 MHz, CDCl3) δ 8.16 (d, J = 7.86 Hz, 1H), 7.79–7.64 (m, 4H), 7.59–7.50 (m, 2H), 7.23–7.15 (m, 4H), 4.70 (s, 2H); MS (FAB) m/z 452 [M+H]+.
4.1.3.16. N-(4-Chlorobenzyl)-N-(3-cyano-6-(trifluoromethyl)pyridin-2-yl)-4-fluorobenzenesul-fonamide (R1 = (4-Cl)Bn, R2 = (4-F)Ph).
Yield 85%, yellow solid, mp = 86–92 °C; 1H NMR (300 MHz, CDCl3) δ 8.16 (d, J = 7.86 Hz, 1H), 7.75 (m, 3H), 7.19–7.15 (m, 5H), 6.85 (m, 2H), 4.70 (s, 2H); MS (FAB) m/z 470 [M+H]+.
4.1.3.17. N-(3-Cyano-6-(trifluoromethyl)pyridin-2-yl)-4-fluoro-N-(4-methoxybenzyl)benzene-sulfonamide (R1 = (4-OCH3)Bn, R2 = (4-F)Ph).
Yield 82%, yellow solid, mp = 78–93 °C; 1H NMR (300 MHz, CDCl3) δ 8.10 (d, J = 8.07 Hz, 1H) 7.98 (d, J = 8.07 Hz, 2H), 7.40 (m, 2H), 7.12 (m, 2H), 6.89 (m, 3H), 4.67 (s, 2H), 3.80 (s, 3H); MS (FAB) m/z 466 [M+H]+.
4.1.3.18. N-(3-Cyano-6-(trifluoromethyl)pyridin-2-yl)-N-(4-methylbenzyl)benzenesulfonamide (R1 = (4-Me)Bn, R2 = Ph).
Yield 72%, yellow solid, mp = 84–94 °C; 1H NMR (300 MHz, CDCl3) δ 8.16 (d, J = 7.86 Hz, 1H), 7.79–7.64 (m, 5H), 7.59–7.50 (m, 2H), 7.23–7.15 (m, 3H), 4.70 (s, 2H), 2.19 (s, 3H); MS (FAB) m/z 432 [M+H]+.
4.1.3.19. N-Benzyl-N-(3-cyano-6-(trifluoromethyl)pyridin-2-yl)-4-fluorobenzenesulfonamide (R1 = Bn, R2 = (4-F)Ph).
Yield 75%, yellow solid, mp = 73–85 °C; 1H NMR (400 MHz, CDCl3) δ 8.09 (d, J = 7.96 Hz, 1H), 7.85 (d, J = 8.52 Hz, 2H), 7.48 (m, 3H), 7.19–7.15 (m, 5H), 4.70 (s, 2H); MS (FAB) m/z 436 [M+H]+.
4.1.3.20. N-(3-Cyano-6-(trifluoromethyl)pyridin-2-yl)-4-fluoro-N-(4-methylbenzyl)-benzene-sulfonamide (R1 = (4-Me)Bn, R2 = (4-F)Ph).
Yield 72%, yellow solid, mp = 75–87 °C; 1H NMR (300 MHz, CDCl3) δ 8.16 (d, J = 7.86 Hz, 1H), 7.79–7.64 (m, 4H), 7.59–7.50 (m, 2H), 7.23–7.15 (m, 3H), 4.70 (s, 2H), 2.19 (s, 3H); MS (FAB) m/z 450 [M+H]+.
4.1.4. Procedure for N-PMB deprotection by ceric ammonium nitrate oxidation
Ceric ammonium nitrate (2.10 mmol) was added to a solution of N-(3-cyano-6-(trifluoromethyl)py-ridin-2-yl)-N-(4-methoxybenzyl)benzenesulfonamide (1.00 mmol) in acetonitrile/H2O (4:1 v/v) at 0 °C. The resulting orange solution was stirred at 0 °C for 30 min, and then at ambient temperature for 1 h. The reaction mixture was diluted with EtOAc and saturated NaHCO3 was added. The resulting suspension was stirred for 30 min at room temperature and then filtered through a pad of celite. After the two layers were separated, the organic layer was washed with brine. The combined organic extracts were dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc/hexanes (1:4) as eluant.
4.1.4.1. N-(3-Cyano-6-(trifluoromethyl)pyridin-2-yl)benzenesulfonamide (R1 = H, R2 = Ph).
Yield 97%, yellow solid, mp = 75–87 °C; 1H NMR (300 MHz, CDCl3) δ 8.22–8.20 (m, 2H), 8.02 (d, J = 8.04 Hz, 1H), 7.64–7.52 (m, 3H), 7.36 (d, J = 7.86 Hz, 1H); MS (FAB) m/z 328 [M+H]+.
4.1.5. General procedure for nitrile reduction
To a stirred solution of nitrile (1.00 mmol) in anhydrous THF (10 ml) was added 2 M BH3·SMe2 in THF (1.10 mmol) at room temperature. After being refluxed for 8 h, the mixture was cooled to ambient temperature, 2 N HCl was added dropwise, and the solution was then refluxed for 30 min. After cooling to ambient temperature, the mixture was neutralized with 2 N NaOH and extracted with EtOAc several times. The combined organic layers were washed with brine, dried over MgSO4, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using MeOH/DCM (1:10) as eluant.
4.1.5.1. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)benzenesulfonamide (R1=H, R2 = Ph).
Yield 32%, clear oil; 1H NMR (300 MHz, DMSO) δ 7.94–7.91 (m, 2H), 7.45 (d, J = 7.32 Hz, 1H), 7.32–7.30 (m, 3H), 6.81 (d, J = 7.50 Hz, 1H), 3.88 (s, 2H); MS (FAB) m/z 332 [M+H]+.
4.1.5.2. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-methylbenzenesulfonamide (R1 = Me, R2 = Ph).
Yield 69%, clear oil; 1H NMR (300 MHz, CDCl3) δ 8.19 (d, J = 7.86 Hz, 1H), 7.67–7.61 (m, 4H), 7.50 (t, J = 7.68 Hz, 2H), 4.26 (s, 2H), 3.09 (s, 3H); MS (FAB) m/z 346 [M+H]+.
4.1.5.3. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-isopropylbenzenesulfonamide (R1 = i-Pr, R2 = Ph).
Yield 44%, yellow oil; 1H NMR (300 MHz, CDCl3) δ 8.25 (d, J = 8.07 Hz, 1H), 7.80–7.83 (m, 2H), 7.72 (d, J = 8.07 Hz, 1H), 7.59 (m, 1H), 7.46 (m, 2H), 4.32 (m, 2H), 2.49 (s, 2H), 1.25 (m, 1H), 1.07 (d, J = 6.57 Hz, 1H), 0.86 (d, J = 6.42 Hz, 1H); MS (FAB) m/z 374 [M+H]+.
4.1.5.4. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-phenylbenzenesulfonamide (R1 = Ph, R2 = Ph).
Yield 57%, yellow oil; 1H NMR (300 MHz, CDCl3) δ 8.11 (d, J = 7.86 Hz, 1H), 7.68 (m, 2H), 7.67 (m, 1H), 7.58 (t, J = 7.32 Hz, 1H), 7.42 (t, J = 7.53 Hz, 2H), 7.37–7.28 (m, 5H), 4.05 (s, 2H); MS (FAB) m/z 408 [M+H]+.
4.1.5.5. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-(4-fluorophenyl)benzenesul-fonamide (R1 = (4-F)Ph, R2 = Ph).
Yield 52%, dark yellow oil; 1H NMR (300 MHz, CDCl3) δ 8.14 (d, J = 7.89 Hz, 1H), 7.69 (d, J = 8.04 Hz, 1H), 7.67–7.57 (m, 4H), 7.54 (d, J = 8.04 Hz, 2H), 7.41–7.34 (m, 2H), 7.01–6.91 (m, 2H), 4.79 (s, 2H); MS (FAB) m/z 426 [M+H]+.
4.1.5.6. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-(cyclohexylmethyl)benzene-sulfonamide (R1 = (Cy)Me, R2 = Ph).
Yield 72%, yellow oil; 1H NMR (300 MHz, CDCl3) δ 8.25 (d, J = 7.86 Hz, 1H), 7.66 (d, J = 7.89 Hz, 1H), 7.63–7.44 (m, 5H), 4.34 (s, 2H), 3.36 (d, J = 6.39 Hz, 2H), 1.67–1.59 (m, 4H), 1.12–1.06 (m, 4H), 0.86–0.88 (m, 2H); MS (FAB) m/z 428 [M+H]+.
4.1.5.7. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-benzylbenzenesulfonamide (R1 = Bn, R2 = Ph).
Yield 37%, yellow oil; 1H NMR (300 MHz, CDCl3) δ 7.98 (d, J = 7.86 Hz, 1H), 7.69–7.62 (m, 3H), 7.57–7.49 (m, 3H), 7.18–7.11 (m, 5H), 4.64 (s, 1H), 3.90 (s, 1H); MS (FAB) m/z 422 [M+H]+.
4.1.5.8. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-benzylmethanesulfonamide (R1 = Bn, R2 = Me).
Yield 81%, clear oil; 1H NMR (300 MHz, CDCl3) δ 7.98 (d, J = 8.04 Hz, 1H), 7.63 (d, J = 7.89 Hz, 1H), 7.22–7.19 (m, 3H), 7.17–7.13 (m, 2H), 4,85 (s, 2H), 3.71 (s, 2H), 3.11 (s, 3H); MS (FAB) m/z 360 [M+H]+.
4.1.5.9. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-benzylethanesulfonamide (R1 = Bn, R2 = Et).
Yield 32%, yellow oil; 1H NMR (300 MHz, CDCl3) δ 7.98 (d, J = 7.9 Hz, 1H), 7.61 (d, J = 7.9 Hz, 1H), 7.22–7.13 (m, 5H), 4.89 (s, 2H), 3.69 (s, 2H), 3.29 (q, J = 7.50 Hz, 2H), 1.49 (t, J = 7.50 Hz, 3H); MS (FAB) m/z 374 [M+H]+.
4.1.5.10. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-benzylpropane-1-sulfonami-de (R1 = Bn, R2 = Pr).
Yield 55%, yellow oil; 1H NMR (300 MHz, CDCl3) δ 7.97 (d, J = 8.0 Hz, 1H), 7.61 (d, J = 7.5 Hz, 1H), 7.22–7.13 (m, 5H), 4.88 (s, 2H), 3.68 (s, 2H), 3.24 (t, J = 8.1 Hz, 2H), 2.04–1.90 (m, 2H), 1.08 (t, J = 7.4 Hz, 3H); MS (FAB) m/z 388 [M+H]+.
4.1.5.11. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-benzylpropane-2-sulfonami-de (R1 = Bn, R2 = i-Pr).
Yield 30%, yellow oil; 1H NMR (300 MHz, CDCl3) δ 8.00 (d, J = 7.9 Hz, 1H), 7.60 (d, J = 8.1 Hz, 1H), 7.23–7.20 (m, 3H), 7.15–7.10 (m, 2H), 4.93 (s, 2H), 3.68–3.65 (m, 1H), 3.60 (s, 2H), 1.49 (d, J = 6.8 Hz, 6H); MS (FAB) m/z 388 [M+H]+.
4.1.5.12. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-(4-fluorobenzyl)benzenesul-fonamide (R1 = (4-F)Bn, R2 = Ph).
Yield 39%, yellow oil; 1H NMR (300 MHz, CDCl3) δ 8.04 (d, J = 7.30 Hz, 1H), 7.68–7.49 (m, 6H), 7.13–7.08 (m, 2H), 6.89–6.82 (m, 2H), 4.62 (br s, 2H). 3.92 (s, 2H); MS (FAB) m/z 440 [M+H]+.
4.1.5.13. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-benzyl-4-chlorobenzenesul fonamide (R1 = Bn, R2 = (4-Cl)Ph).
Yield 75%, yellow oil; 1H NMR (300 MHz, CDCl3) δ 8.01 (d, J = 7.86 Hz, 1H), 7.57–7.63 (m, 3H), 7.48–7.51 (m, 2H), 7.16 (m, 3H), 7.11 (m, 2H), 4.62 (br s, 2H), 3.70 (s, 2H); MS (FAB) m/z 456 [M+H]+.
4.1.5.14. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-4-fluoro-N-(4-fluorobenzyl)be-nzenesulfonamide (R1 = (4-F)Bn, R2 = (4-F)Ph).
Yield 64%, yellow oil; 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 8.00 Hz, 1H), 7.64 (m, 2H), 7.62 (d, J = 7.96 Hz, 1H), 7.23 (m, 4H), 6.98–7.03 (t, J = 8.68 Hz, 2H), 4.62 (s, 2H). 3.92 (s, 2H); MS (FAB) m/z 458 [M+H]+.
4.1.5.15. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-(4-chlorobenzyl)benzenesul-fonamide (R1 = (4-Cl)Bn, R2 = Ph).
Yield 67%, pale yellow oil; 1H NMR (300 MHz, CDCl3) δ 8.05 (d, J = 8.04 Hz, 1H), 7.69–7.50 (m, 6H), 7.16–7.07 (m, 4H), 4.61 (s, 2H), 3.96 (s, 2H); MS (FAB) m/z 456 [M+H]+.
4.1.5.16. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-(4-chlorobenzyl)-4-fluorobe-nzenesulfonamide (R1 = (4-Cl)Bn, R2 = (4-F)Ph).
Yield 60%, yellow oil; 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 8.00 Hz, 1H), 7.69–7.50 (m, 3H), 7.14–7.24 (m, 6H), 4.61 (s, 2H), 3.96 (s, 2H); MS (FAB) m/z 474 [M+H]+.
4.1.5.17. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-(4-methoxybenzyl)benzene-sulfonamide (R1 = (4-OCH3)Bn, R2 = Ph).
Yield 73%, yellow oil; 1H NMR (300 MHz, CDCl3) δ 8.00 (d, J = 7.9 Hz, 1H), 7.68–7.62 (m, 3H), 7.57–7.49 (m, 3H), 7.03 (d, J = 8.6 Hz, 2H), 6.68 (d, J = 8.8 Hz, 2H), 4.58 (s, 2H), 3.91 (s, 2H), 3.70 (s, 3H); MS (FAB) m/z 452 [M+H]+.
4.1.5.18. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-4-fluoro-N-(4-methoxybenzyl)-benzenesulfonamide (R1 = (4-OCH3)Bn, R2 = (4-F)Ph).
Yield 85%, yellow oil; 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 8.00 Hz, 1H), 7.69 (m, 3H), 7.62 (d, J = 7.92 Hz, 1H), 7.23 (d, J = 11.6 Hz, 1H), 7.14 (d, J = 8.12 Hz, 1H), 6.99 (m, 3H), 4.61 (s, 2H), 3.96 (s, 2H), 3.05 (s, 3H); MS (FAB) m/z 470 [M+H]+.
4.1.5.19. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-(4-methylbenzyl)benzenesul-fonamide (R1 = (4-Me)Bn, R2 = Ph).
Yield 84%, yellow oil; 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 8.00 Hz, 2H), 7.75 (m, 5H), 6.97 (m, 4H), 4.61 (s, 2H), 3.96 (s, 2H), 2.35 (s, 3H); MS (FAB) m/z 436 [M+H]+.
4.1.5.20. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-N-benzyl-4-fluorobenzenesulfo-namide (R1 = Bn, R2 = (4-F)Ph).
Yield 82%, yellow oil; 1H NMR (300 MHz, CDCl3) δ 8.01 (d, J = 7.86 Hz, 1H), 7.57–7.63 (m, 2H), 7.50 (m, 3H), 7.16 (m, 3H), 7.11 (m, 2H), 4.62 (br s, 2H), 3.70 (s, 2H); MS (FAB) m/z 440 [M+H]+.
4.1.5.21. N-(3-(Aminomethyl)-6-(trifluoromethyl)pyridin-2-yl)-4-fluoro-N-(4-methylbenzyl)be-nzenesulfonamide (R1 = (4-Me)Bn, R2 = (4-F)Ph).
Yield 84%, yellow oil; 1H NMR (300 MHz, CDCl3) δ 8.00 (d, J = 8.00 Hz, 1H), 7.65 (m, 2H), 7.52 (m, 3H), 7.09 (d, J = 11.37 Hz, 2H), 6.77 (m, 2H), 4.61 (s, 2H), 3.96 (s, 2H), 2.24 (s, 3H); MS (FAB) m/z 454 [M+H]+.
4.1.6. General procedure for amide coupling
A mixture of 2-(3-fluoro-4-(methylsulfonamido)phenyl) propanoic acid (1.00 mmol), amine (1.10 mmol), 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (1.10 mmol) and 1-hydroxybenzotriazole hydrate (1.50 mmol) in DMF (5 ml) was stirred for 12 h at room temperature. The reaction mixture was extracted with EtOAc (10 ml). The aqueous phase was saturated with aq NaCl and extracted again with EtOAc (15 ml). The combined organic extracts were washed with 1 N HCl (5 ml) and brine (5 ml), dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel using EtOAc/hexanes (1:2) as eluant.
4.1.6.1. 2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(phenylsulfonamido)-6-(triflu-oromethyl)pyridin-3-yl)methyl)propanamide (6).
Yield 56%, white solid, mp = 102–109 °C; 1H NMR (300 MHz, CDCl3) δ 8.14 (d, 2H, J = 7.68 Hz, 2H), 7.56–7.38 (m, 5H), 7.16 (t, 2H), 7.04 (d, J = 7.86 Hz, 1H), 6.59 (br t, 2H), 4.33 (m, 2H), 3.55 (q, J = 7.5 Hz, 1H), 3.03 (s, 3H), 1.50 (d, J = 7.14 Hz, 3H), 1.26 (m, 1H); MS (FAB) m/z 575 [M+H]+.
4.1.6.2. 2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(N-methylphenylsulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (7).
Yield 48%, white solid, mp = 77–88 °C; 1H NMR (300 MHz, CDCl3) δ 8.06 (d, J = 7.86 Hz, 1H), 7.65 (m, 1H), 7.59 (d, J = 7.86 Hz, 1H), 7.52–7.48 (m, 5H), 7.13 (d, J = 11.37 Hz, 1H), 7.09 (d, J = 8.43 Hz, 1H), 6.71 (brt, 1H), 6.5 (br s, 1H), 4.69 (m, 2H), 3.57 (q, J = 7.32 Hz, 1H), 3.06 (s, 3H), 2.94 (s, 3H), 1.52 (d, J = 6.96 Hz, 3H); MS (FAB) m/z 589 [M+H]+.
4.1.6.3. 2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(N-isopropylphenylsulfonamid-o)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (8).
Yield 54%, white solid, mp = 83–92 °C; 1H NMR (300 MHz, DMSO) δ 9.57 (s, 1H), 8.7 (br s, 1H), 7.9 (br s, 1H), 7.70 (m, 3H), 7.59 (d, J = 7.86 Hz, 2H), 7.45 (m, 1H), 7.25 (m, 1H), 7.19 (d, J = 11.37 Hz, 1H), 4.55 (m, 2H), 4.30 (m, 1H), 3.78 (q, J = 7.32 Hz, 1H), 3.02 (s, 1H), 1.40 (d, J = 6.96 Hz, 3H), 1.07 (s, 3H), 0.83 (s, 3H); MS (FAB) m/z 617 [M+H]+.
4.1.6.4. 2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(N-phenylphenylsulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (9).
Yield 95%, white solid, mp = 98–102 °C; 1H NMR (300 MHz, CDCl3) δ7.96 (d, J = 7.86 Hz, 1H), 7.60 (m, 5H), 7.52 (d, J = 8.07 Hz, 1H), 7.44 (t, J = 8.43 Hz, 3H), 7.31 (m, 7H), 7.13 (dd, J = 12.7 Hz, 2H), 6.40 (t, 1H), 4.56 (m, 2H), 3.55 (dd, J = 6.09 Hz, 1H), 3.03 (s, 3H), 1.51 (d, J = 7.14 Hz, 3H); MS (FAB) m/z 651 [M+H]+.
4.1.6.5. 2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(N-(4-fluorophenyl)phenylsulfonam-ido)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (10).
Yield 65%, white solid, mp = 90–98 °C; 1H NMR (300 MHz, CDCl3) δ 7.96 (d, J = 7.89 Hz, 1H), 7.63 (m, 3H), 7.53 (d, J = 7.71 Hz, 3H), 7.47 (dd, J = 14.2 Hz, 3H), 7.34 (m, 3H), 7.15 (m, 2H), 6.97 (m, 2H), 4.60 (m, 2H), 3.59 (dd, J = 4.30 Hz, 1H), 2.99 (s, 3H), 1.53 (d, J = 6.33 Hz, 3H); MS (FAB) m/z 669 [M+H]+.
4.1.6.6. N-((2-(N-(Cyclohexylmethyl)phenylsulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (11).
Yield 44%, white solid, mp = 85–91 °C; 1H NMR (300 MHz, CDCl3) δ 8.15 (m, 1H), 8.05 (m, 1H), 7.56 (m, 7H), 7.15 (d, J = 15.8 Hz, 2H), 6.72 (m, 1H), (s, 1H), 5.06 (br t, 1H), 4.39 (m, 1H), 3.57 (s, 1H), 3.36 (d, J = 6.06 Hz, 2H), 2.92 (s, 3H), 2.05 (m, 1H), 1.46 (m, 10H); MS (FAB) m/z 671 [M+H]+.
4.1.6.7. N-((2-(N-Benzylphenylsulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (12).
Yield 59%, white solid, mp = 75–88 °C; 1H NMR (300 MHz, CD3OD) δ 7.71 (m, 1H), 7.63–7.53 (m, 6H), 7.43 (t, J = 8.22 Hz, 1H), 7.22–7.13 (m, 7H), 4.66 (s, 2H), 4.52 (s, 2H), 3.67 (q, J = 7.14 Hz, 1H), 2.96 (s, 3H), 1.45 (d, J = 7.14 Hz, 3H); MS (FAB) m/z 665 [M+H]+.
4.1.6.8. (S)-N-((2-(N-Benzylphenylsulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (12S).
Yield 62%, white solid, mp = 85–90 °C, (c = 0.01, CHCl3); 1H NMR (400 MHz, DMSO) δ 9.53 (br s, 1H), 8.41 (t, J = 5.44 Hz, 1H), 7.76 (d, J = 7.80 Hz, 2H), 7.63–7.53 (m, 6H), 7.43 (t, J = 8.22 Hz, 1H), 7.22–7.13 (m, 7H), 4.62 (s, 2H), 4.42 (s, 2H), 3.70 (q, J = 6.72 Hz, 1H), 3.00 (s, 3H), 1.36 (d, J = 6.92 Hz, 3H); 13C NMR (300 MHz, CDCl3) δ 173.40, 171.15, 155.72, 152.48, 150.76, 146.57, 146.09, 141.71, 140.09, 140.00, 138.93, 136.32, 134.28, 133.62, 133.23, 129.49, 128.96, 128.57, 128.38, 124.30, 123.75, 120.61, 60.36, 54.13, 46.30, 39.55, 38.13, 21..02, 18.39, 14.16; MS (FAB) m/z 665 [M+H]+.
4.1.6.9. N-((2-(N-Benzylmethylsulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (13).
Yield 38%, white solid, mp = 65–72 °C; 1H NMR (300 MHz, CD3OD) δ 7.67 (d, J = 8.04 Hz, 1H), 7.57 (d, J = 7.86 Hz, 1H), 7.42 (t, J = 8.25 Hz, 1H), 7.23–7.10 (m, 7H), 4.86 (s, 2H), 4.30 (s, 2H), 3.64 (q, J = 7.14 Hz, 1H), 3.11 (s, 3H), 2.97 (s, 3H), 1.42 (d, J = 7.14 Hz, 3H); MS (FAB) m/z 603 [M+H]+.
4.1.6.10. N-[2-(Benzyl-ethanesulfonyl-amino)-6-trifluoromethylpyridin-3-ylmethyl]-2-(3-fluo-ro-4-methanesulfonylamino-phenyl)-propionamide (14).
Yield 59%, white solid, mp = 66–73 °C; 1H NMR (300 MHz, CD3OD) δ 7.65 (d, J = 7.89 Hz, 1H), 7.57 (d, J = 7.68 Hz, 1H), 7.42 (t, J = 8.43 Hz, 1H), 7.22–7.10 (m, 7H), 4.29 (d, 2H), 3.63 (q, J = 7.14 Hz, 1H), 3.34 (m, 2H), 2.97 (s, 3H), 1.42 (d, J = 7.14 Hz, 3H), 1.37 (d, J = 7.32 Hz, 3H); MS (FAB) m/z 617 [M+H]+.
4.1.6.11. N-((2-(N-Benzylpropan-2-ylsulfonamido)-6-(trifluoromethyl)pyridin-3-yl)-methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (15).
Yield 49%, white solid, mp = 67–80 °C; 1H NMR (300 MHz, CDCl3) δ 7.91 (d, J = 7.68 Hz, 1H), 7.55 (d, 1H), 7.48 (d, 1H), 7.22 (m, 3H), 7.08–7.02 (m, 4H), 6.2 (br, 1H), 5.9 (br, 1H), 5.33 (d, 1H), 4.89 (d, 1H), 4.8 (m, 1H), (m, 1H), 3.01 (s, 3H), 2.01 (d, J = 7.14 Hz, 3H), 1.98 (d, J = 7.14 Hz, 3H), 1.40 (d, J = 7.14 Hz, 3H), 0.86 (m, 2H); MS (FAB) m/z 631 [M+H]+.
4.1.6.12. N-((2-(N-Benzylpropylsulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (16).
Yield 56%, white solid, mp = 62–70 °C; 1H NMR (300 MHz, CD3OD) δ 7.66 (d, J = 8.04 Hz, 1H), 7.57 (d, J = 5.04 Hz, 1H), 7.42 (t, J = 8.25 Hz, 1H), 7.22–7.10 (m, 7H), 4.89 (s, 2H), 4.28 (s, 2H), 3.63 (q, J = 7.14 Hz, 1H), 3.29 (m, 2H), 2.97 (s, 3H), 1.87 (sextet, J = 7.89 Hz, 2H), 1.42 (d, J = 7.14 Hz, 3H), 1.04 (t, J = 7.50 Hz, 3H); MS (FAB) m/z 631 [M+H]+.
4.1.6.13. 2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(N-(4-fluorobenzyl)phenyl-sulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (17).
Yield 46%, white solid, mp = 71–81 °C; 1H NMR (300 MHz, CDCl3) δ 7.92 (br, 1H), 7.67 (m, 1H), 7.56–7.47 (m, 7H), 7.13–7.03 (m, 4H), 6.84 (t, J = 7.35 Hz, 2H), 6.45 (br t, 1H), 4.90 (br s, 1H), 4.60 (br s, 1H), 4.40 (br s, 1H), 4.10 (br s, 1H), 3.46 (q, J = 7.14 Hz, 1H), 2.93 (s, 3H), 1.49 (d, J = 7.14 Hz, 3H); MS (FAB) m/z 683 [M+H]+.
4.1.6.14. N-((2-(N-(4-Chlorobenzyl)phenylsulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (18).
Yield 36%, white solid, mp = 75–83 °C; 1H NMR (300 MHz, CDCl3) δ 7.92 (d, J = 5.01 Hz, 1H), 7.64 (m, 1H), 7.51 (m, 7H), 7.11 (t, J = 6.33 Hz, 3H), 7.00 (t, J = 7.35 Hz, 3H), 6.38 (s, 1H), 4.82 (m, 1H), 4.57 (m, 1H), 4.37 (s, 1H), 4.11 (m, 1H), 3.44 (dd, J = 10.1 Hz, 3H), 2.92 (s, 3H), 1.46 (d, J = 5.34 Hz, 3H); MS (FAB) m/z 700 [M+H]+.
4.1.6.15. 2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(N-(4-methoxybenzyl)phenyl-sulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (19).
Yield 89%, white solid, mp = 67–76 °C; 1H NMR (300 MHz, CDCl3) δ 7.90 (s, 1H), 7.59 (m, 7H), 7.11 (d, J = 11.6 Hz, 1H), 7.05 (d, J = 8.07 Hz, 1H), 6.98 (d, J = 8.40 Hz, 2H), 6.68 (d, J = 8.25 Hz, 2H), 6.35 (br s, 2H), 4.49 (m, 4H), 3.71 (s, 3H), 3.42 (s, 1H), 2.94 (s, 3H), 1.46 (d, J = 6.96 Hz, 3H); MS (FAB) m/z 695 [M+H]+.
4.1.6.16. 2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-(N-(4-methylbenzyl)phenyl-sulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (20).
Yield 82%, white solid, mp = 85–90 °C; 1H NMR (400 MHz, DMSO) δ 9.53 (br s, 1H), 8.41 (br t, 1H), 7.76 (d, J = 8.12 Hz, 2H), 7.59–7.62 (m, 6H), 7.32–7.36 (t, J = 8.24 Hz, 1H), 7.22 (d, J = 11.48 Hz, 1H), 7.15 (d, J = 8.16 Hz, 1H), 6.98 (br t, 3H), 4.57 (s, 2H), 4.44 (s, 2H), 3.70 (q, J = 6.96 Hz, 1H), 3.00 (s, 3H), 2.17 (s, 3H), 1.36 (d, 3H, J = 6.92 Hz); MS (FAB) m/z 679 [M+H]+.
4.1.6.17. N-((2-((N-Benzyl-4-fluorophenyl)sulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (21).
Yield 54%, white solid, mp = 74–84 °C; 1H NMR (300 MHz, CD3OD) δ 8.47 (m, J = 5.67 Hz, 2H), 7.78 (d, J = 8.04 Hz, 1H), 7.69 (dd, J = 8.52 Hz, 2H), 7.61 (d, J = 8.07 Hz, 1H), 7.46 (t, J = 8.76 Hz, 2H), 7.34 (t, J = 8.22 Hz, 1H), 7.19 (m, 7H), 4.63 (s, 2H), 4.42 (s, 2H), 3.01 (s, 3H), 1.36 (d, J = 6.96 Hz, 3H); MS (FAB) m/z 684 [M+H]+.
4.1.6.18. N-((2-((N-Benzyl-4-chlorophenyl)sulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (22).
Yield 76%, white solid, mp = 85–90 °C; 1H NMR (300 MHz, CDCl3) δ 8.47 (t, J = 5.67 Hz, 1H), 7.46–7.58 (m, 7H), 7.19 (m, 3H), 7.02–7.07 (m, 4H), 4.63 (s, 2H), 4.42 (s, 2H), 3.01 (s, 3H), 1.36 (d, J = 6.96 Hz, 3H); MS (FAB) m/z 670 [M+H]+.
4.1.6.19. 2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-((4-fluoro-N-(4-fluorobenzyl)-phenyl) sulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (23).
Yield 85%, white solid, mp = 92–98 °C; 1H NMR (400 MHz, DMSO) δ 9.53 (br s, 1H), 8.45 (t, J = 5.32 Hz, 1H), 7.78 (d, J = 8.00 Hz, 1H), 7.66–7.69 (m, 1H), 7.62 (d, J = 8.00 Hz, 1H), 7.44 (t, J = 8.60 Hz, 2H), 7.33 (t, J = 8.28 Hz, 1H), 7.22 (d, J = 11.64 Hz, 1H), 7.13–7.18 (m, 3H), 6.98 (t, J = 8.60 Hz, 3H), 4.62 (s, 2H), 4.39 (s, 2H), 3.70 (q, J = 6.92 Hz, 1H), 3.00 (s, 3H), 1.36 (d, J = 6.92 Hz, 3H); MS (FAB) m/z 701 [M+H]+.
4.1.6.20. N-((2-((N-(4-Chlorobenzyl)-4-fluorophenyl)sulfonamido)-6-(trifluoromethyl)-pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (24).
Yield 78%, white solid, mp = 90–95 °C; 1H NMR (400 MHz, DMSO) δ 9.53 (br s, 1H), 8.48 (t, J = 5.36 Hz, 1H), 7.78 (d, J = 8.00 Hz, 1H), 7.64–7.69 (m, 2H), 7.44 (t, J = 8.60 Hz, 2H), 7.33 (t, J = 8.28 Hz, 1H), 7.22 (d, J = 11.64 Hz, 1H), 7.13–7.18 (m, 3H), 6.98 (t, J = 8.60 Hz, 3H), 4.63 (s, 2H), 4.43 (s, 2H), 3.70 (q, J = 6.92 Hz, 1H), 3.00 (s, 3H), 1.36 (d, J = 6.92 Hz, 3H); MS (FAB) m/z 718 [M+H]+.
4.1.6.21. 2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-((4-fluoro-N-(4-methoxy-benzyl)phenyl)sulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (25).
Yield 78%, white solid, mp = 85–90 °C; 1H NMR (400 MHz, DMSO) δ 9.51 (br s, 1H), 8.42 (t, J = 5.28 Hz, 1H), 7.84 (d, J = 7.24 Hz, 1H), 7.75 (m, 3H), 7.61 (d, J = 7.92 Hz, 1H), 7.42–7.46 (t, J = 8.64 Hz, 2H), 7.33 (t, J = 8.28 Hz, 1H), 7.22 (d, J = 11.60 Hz, 1H), 7.13 (d, J = 8.12 Hz, 1H), 6.99 (t, J = 8.60 Hz, 3H), 4.58 (s, 2H), 4.43 (s, 2H), 3.70 (q, J = 6.92 Hz, 1H), 3.00 (s, 3H), 2.18 (s, 3H), 1.36 (d, J = 6.92 Hz, 3H); MS (FAB) m/z 713 [M+H]+.
4.1.6.22. 2-(3-Fluoro-4-(methylsulfonamido)phenyl)-N-((2-((4-fluoro-N-(4-methylbenzyl)-phenyl)-sulfonamido)-6-(trifluoromethyl)pyridin-3-yl)methyl)propanamide (26).
Yield 83%, white solid, mp = 85–90 °C; 1H NMR (400 MHz, DMSO) δ 8.42 (t, J = 5.28 Hz, 1H), 7.77 (d, J = 7.96 Hz, 1H), 7.66–7.69 (m, 2H), 7.59 (d, J = 7.92 Hz, 1H), 7.42–7.46 (t, J = 8.64 Hz, 2H), 7.33 (t, J = 8.28 Hz, 1H), 7.22 (d, J = 11.60 Hz, 2H), 7.13 (d, J = 8.12 Hz, 1H), 6.96 (d, J = 8.44 Hz, 2H), 6.73 (d, J = 8.36 Hz, 2H), 4.56 (s, 2H), 4.38 (s, 2H), 3.70 (q, J = 6.92 Hz, 1H), 3.64 (s, 3H), 3.00 (s, 3H), 1.36 (d, J = 6.92 Hz, 3H); MS (FAB) m/z 697 [M+H]+.
4.2. Molecular modeling
The 3D structures of the molecules were generated with Concord and energy minimized with MMFF94s force field and MMFF94 charge until the rms of Powell gradient was 0.05 kcal mol−1 A−1 in SYBYL-X 2.0 (Tripos Int., St. Louis, MO, USA). The flexible docking study on our hTRPV1 model14 was performed using GOLD v.5.2 (Cambridge Crystallographic Data Centre, Cambridge, UK), which employees a genetic algorithm (GA) and allows for full ligand flexibility and partial protein flexibility. The binding site was defined as 8 Å around the capsaicin complexed in the hTRPV1 model. The side chains of the nine residues which are important for ligand binding, (i.e., Tyr511, Ser512, Met514, Leu515, Leu518, Phe543, Leu547, Thr550, and Asn551) were allowed to be flexible with ‘crystal mode’ in GOLD. Compound 12S was docked using the GoldScore scoring function, and the other parameters remained as default. All the computation calculations were undertaken on an Intel® Xeon™ Quad-core 2.5 GHz workstation with Linux Cent OS release 5.5.
Acknowledgments
This research was supported by research grants from the Korea Science and Engineering Foundation (KOSEF) (NRF-2007-0056817) and the National Leading Research Lab (NLRL) program (2011-0028885) in South Korea, and in part by the Intramural Research Program of the NIH, Center for Cancer Research, NCI (Project Z1A BC 005270) in the USA.
References and notes
- 1.Szallasi A; Blumberg PM Pharmacol. Rev 1999, 51, 159. [PubMed] [Google Scholar]
- 2.Tominaga M; Caterina MJ; Malmberg AB; Rosen TA; Gilbert H; Skinner K; Raumann BE; Basbaum AI; Julius D Neuron 1998, 21, 531. [DOI] [PubMed] [Google Scholar]
- 3.Szallasi A Am. J. Clin. Pathol 2002, 118, 110. [DOI] [PubMed] [Google Scholar]
- 4.(a) Walpole CS; Wrigglesworth R; Bevan S; Campbell EA; Dray A; James IF; Perins MN; Reid DJ; Winter JJ Med. Chem 1993, 36, 2362. [DOI] [PubMed] [Google Scholar]; (b) Walpole CS; Wrigglesworth R; Bevan S; Campbell EA; Dray A; James IF; Masdin KJ; Perkins MN; Winter J J. Med. Chem 1993, 36, 2373. [DOI] [PubMed] [Google Scholar]; (c) Walpole CS; Wrigglesworth R; Bevan S; Campbell EA; Dray A; James IF; Masdin KJ; Perkins MN;Winter J J. Med. Chem 1993, 36, 2381. [DOI] [PubMed] [Google Scholar]
- 5.Appendino G; Szallasi A Life Sci. 1997, 60, 681. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH; Lee Y; Ryu H; Kang DW; Lee J; Lazar J; Pearce LV; Pavlyukovets VA; Blumberg PM; Choi S J. Comput. Aided Mol. Des 2011, 25, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao M; Cao E; Julius D; Cheng Y Nature 2013, 504, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao E; Liao M; Cheng Y; Julius D Nature 2013, 504, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Z; Pearce LV; Xu X; Yang X; Yang P; Blumberg PM; Xie XQ J. Chem. Inf. Model 2015, 55, 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y; Toth A; Tran R; Szabo T; Welter JD; Blumberg PM; Lee J; Kang SU; Lim JO; Lee J Mol. Pharmacol 2003, 64, 325. [DOI] [PubMed] [Google Scholar]
- 12.Garami A; Shimansky YP; Pakai E; Oliveira DL; Gavva NR; Romanovsky AA J. Neurosci 2010, 30, 1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Kym PR; Kort ME; Hutchins CW Biochem. Pharmacol 2009, 78, 211. [DOI] [PubMed] [Google Scholar]; (b) Wong GY; Gavva NR Brain Res. Rev 2009, 60, 267. [DOI] [PubMed] [Google Scholar]; (c) Gunthorpe MJ; Chizh BA Drug Discovery Today 2009, 14, 56. [DOI] [PubMed] [Google Scholar]; (d) Lazar J; Gharat L; Khairathkar-Joshi N; Blumberg PM; Szallasi A Exp. Opin. Drug Disc 2009, 4, 159. [DOI] [PubMed] [Google Scholar]; (e) Voight EA; Kort ME Exp. Opin. Ther. Pat 2010, 20, 1. [DOI] [PubMed] [Google Scholar]; (f) Szolcsányi J; Sándor Z Trend Pharmacol. Sci 2012, 33, 646. [DOI] [PubMed] [Google Scholar]; (g) Szallasi A; Sheta M Exp. Opin. Investig. Drug 2012, 21, 1351. [DOI] [PubMed] [Google Scholar]; (h) De Petrocellis L; Moriello AS Recent Patents CNS Drug Disc. 2013, 8, 180. [DOI] [PubMed] [Google Scholar]; (i) Lee Y; Hong S; Cui M; Sharma PK; Lee J; Choi S Exp. Opin. Ther. Pat 2015, 25, 291. [DOI] [PubMed] [Google Scholar]
- 14.Kim MS; Ryu H; Kang DW; Cho S-H; Seo S; Park YS; Kim M-Y; Kwak EJ; Kim YS; Bhondwe RS; Kim HS; Park S-g.; Son K; Choi S; DeAndrea-Lazarus I; Pearce LV; Blumberg PM; Frank R; Bahrenberg G; Stockhausen H; Kögel BY; Schiene K; Christoph T; Lee J J. Med. Chem 2012, 55, 8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorat SA; Kang DW; Ryu H; Kim MS; Kim HS; Ann J; Ha T-H; Kim SE; Son K; Choi S; Blumberg PM; Frank R; Bahrenberg G; Schiene K; Christoph T; Lee J Eur. J. Med. Chem 2013, 64, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha T-H; Ryu H; Kim S-E; Kim HS; Ann J; Tran P-T; Hoang V-H; Son K; Cui M; Choi S; Blumberg PM; Frank R; Bahrenberg G; Schiene K; Christoph T; Frormann S; Lee J Bioorg. Med. Chem 2013, 21, 6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryu H; Seo S; Cho S-H; Kim HS; Jung A; Kang DW; Son K; Cui M; Hong S-h.; Sharma PK; Choi S; Blumberg PM; Frank-Foltyn R; Bahrenberg G; Stockhausen H; Schiene K; Christoph T; Frormann S; Lee J Bioorg. Med. Chem. Lett 2014, 24, 4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryu H; Seo S; Cho S-H; Kim MS; Kim M-Y; Kim HS; Ann J; Tran P-T; Hoang V-H; Byun J; Cui M; Son K; Sharma PK; Choi S; Blumberg PM; Frank-Foltyn R; Bahrenberg G; Koegel B-Y; Christoph T; Frormann S; Lee J Bioorg. Med. Chem. Lett 2014, 24, 4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu H; Seo S; Lee J-Y; Ha T-H; Lee S; Jung A; Ann J; Kim S-E; Yoon S; Hong M; Blumberg PM; Frank-Foltyn R; Bahrenberg G; Schiene K; Stockhausen H; Christoph T; Frormann S;Lee J Eur. J. Med. Chem 2015, 93, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ann J; Jung A; Kim M-Y; Kim H-M; Ryu H; Kim S; Kang DW; Hong S; Cui M; Choi S; Blumberg PM; Frank-Foltyn R; Bahrenberg G; Stockhausen H; Christoph T; Lee J Bioorg. Med. Chem 2015, 23, 6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veghel DV; Cleynhens J; Pearce LV; Blumberg PM; Laere KV; Verbruggen A; Bormans G Nucl. Med. Biol 2013, 40, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubuisson D; Dennis SG Pain 1977, 4, 161. [DOI] [PubMed] [Google Scholar]
- 23.Tjolsen A; Berge OG; Hunskaar S; Rosland JH; Hole K Pain 1992, 51, 5. [DOI] [PubMed] [Google Scholar]