Abstract
Rspo2 was identified as a novel common integration site (CIS) for the mouse mammary tumor virus (MMTV) in viral induced mouse mammary tumors. Here we show that Rspo2 modulates Wnt signaling in mouse mammary epithelial cells. Co-expression of both genes resulted in an intermediate growth phenotype on plastic and had minor effects on the growth-promoting properties of Wnt1 in soft agar. However, individual Rspo2 and Wnt1 HC11 transfectants as well as the double transfectant were tumorigenic in athymic nude mice, with tumors from each line having distinctive histological characteristics. Rspo2 and Rspo2/Wnt1 tumors contained many spindle cells, consistent with an epithelial–mesenchymal transformation (EMT) phenotype. When Rspo2 and Rspo2/Wnt1 tumor cells were transferred into naïve mice, they exhibited greater metastatic activity than cells derived from Wnt1 tumors. For comparison, C57MG/Wnt1/Rspo2 co-transfectants exhibited invasive properties in three-dimensional (3D) Matrigel cultures that were not seen with cells transfected only with Wnt1 or Rspo2. Use of Dickkopf-1, a specific antagonist of the Wnt/β-catenin pathway, or short hairpin RNA targeting β-catenin expression demonstrated that the invasive activity was not mediated by β-catenin. Our results indicate that Rspo2 and Wnt1 have mutually distinct effects on mammary epithelial cell growth and these effects are context-dependent. While Rspo2 and Wnt1 act synergistically in the β-catenin pathway, other mechanisms are responsible for the invasive properties of stable double transfectants observed in 3D Matrigel cultures.
The Rspo2 gene (designated Int7) was originally identified as a common insertion site (CIS) for the mouse mammary tumor virus (MMTV) in virally induced mouse mammary tumors. Insertion occurred in the 5′ flanking region of the Rspo2 gene (previously designated 2610028F08RIK). MMTV integration results in over-expression of the Rspo2 gene, which is usually silent or expressed only at very low levels during mammary gland development (Lowther et al., 2005). Rspo2 is one of four highly related R-spondin proteins in mammals, sharing ~60% amino acid sequence identity and conserved modular structure (Lowther et al., 2005; Nam et al., 2006). The Rspos contain two furin-like cysteine-rich domains followed by a thrombospondin type I repeat and a positively charged carboxyl-terminal tail. During mouse development, expression of Rspo genes partially overlaps with the pattern of Wnt gene expression (Nam et al., 2007). Rspos have been characterized as being nonconventional secreted activators of the canonical Wnt/β-catenin signaling pathway in that they can stabilize the level of cytosolic β-catenin and dramatically synergize with Wnt ligands to promote β-catenin transcriptional activity (Kazanskaya et al., 2004; Kim et al., 2006, 2008). The Rspo furin-like domains have been shown to be necessary and sufficient for β-catenin activation (Kazanskaya et al., 2004; Kim et al., 2006, 2008; Li et al., 2009). Rspos reportedly antagonize the inhibitory effect of Dickkopf-1 (Dkk1) on the Wnt/β-catenin pathway (Kazanskaya et al., 2004; Nam et al., 2006; Binnerts et al., 2007; Wei et al., 2007). Aside from contributing to binding to heparan proteoglycan (Nam et al., 2006), the functions of the other Rspo domains remain unknown.
The mammalian Wnt gene family plays a critical role in diverse biological processes during development and in the adult, regulating cell proliferation, differentiation, survival, motility, and polarity [reviewed in Gordon and Nusse (2006)]. Wnt ligands control β-catenin turnover through an interaction with their receptors in the Frizzled and low-density lipoprotein receptor-related protein (LRP) families. Upon binding to these receptors, Wnts trigger a series of events that result in the stabilization of cytosolic β-catenin and its accumulation in the nucleus, where it activates the expression of Wnt target genes through its association with members of the T-cell factor (TCF)/lymphocyte enhancing factor (LEF) family (Bejsovec, 2005; Cadigan and Liu, 2006; Gordon and Nusse, 2006). Interestingly, like Rspo2, the Wnt gene family was first discovered in MMTV-induced murine mammary tumors in which proviral insertions turned on the expression of Wnt1 and promoted mammary carcinogenesis (Nusse et al., 1984). Mutation or altered expression of genes that enable the constitutive activation of Wnt/β-catenin signaling has been implicated in malignancy of several organs, including colon, brain, skin, and breast [reviewed in Clevers (2006)].
Little is known about the role of Rspo in mammary gland development and tumorigenesis, although one recent report indicates that Rspo1 is required for normal development (Chadi et al., 2009). Our initial results suggested that Rspo2 is involved in mammary gland tumorigenesis (Lowther et al., 2005). Mammary tumors containing an activated MMTV Rspo2/Int7 were also found to have two or three additional viral insertions that occurred flanking a member of the Wnt (Wnt3a, Wnt10) and/or Fgf (Fgf3, Fgf4) gene families. This implied a possible collaboration between Rspo2 and Wnt/Fgf genes during mammary carcinogenesis. The present work was undertaken to assess the effects of Rspo2 and Wnt1 alone and in combination on mammary epithelial cells in various settings, with a particular emphasis on properties indicative of malignant transformation. We used the C57MG and HC11 mouse cell lines because they retain many characteristics of normal mammary epithelial cells and previously had been utilized in studies of Rspo and Wnt activity (Olson and Papkoff, 1994; Humphreys and Rosen, 1997; Theodorou et al., 2007). Our experiments were conducted in various in vitro and in vivo models and revealed that the interactions between Rspo2 and Wnt1 were context-specific. While Rspo2 had negative or minor effects on Wnt1-associated cell proliferation in monolayer culture, the two factors together enhanced the invasive properties of cells in vitro and Rspo2 promoted metastatic activity in vivo. Consistent with prior reports, Rspo2 enhanced the ability of Wnt1 to stimulate β-catenin transcriptional activity. However, inhibition of this pathway did not block the invasive properties of C57MG transfectants expressing both Rspo2 and Wnt1. These results suggested that another yet to be described mechanism of Rspo/Wnt interaction accounts for the invasive activity of the double transfectants.
Materials and Methods
Antibodies and chemicals
Rabbit anti-V5 (1:5,000, # Ab3792) and rabbit anti-β-catenin (1:1,000, # AB19022) were from Millipore Chemicon (Temecula, CA). Cytokeratin 18 (1:1,000, # 1924-1) was from Epitomics (Burlingame, CA). Cytokeratin 14 (1:5,000, ab53115) and SMA (1:1,000, #ab5694) were from Abcam (Cambridge, MA). Slug (1:500, # H-140), Vimentin (1:1,000, #sc-7557), E-cadherin (1:1,000, # H-108) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-α-tubulin (1:5,000, #T3526) was from Sigma–Aldrich (St. Louis, MO). Alexa Fluor® 594 phalloidin (# A12381) was from Life Technologies (Carlsbad, CA). Recombinant hDkk1 (#1096) was from R&D Systems (Minneapolis, MN), while recombinant human sFRP-1 was prepared as previously described (Uren et al., 2000). In all assays fresh inhibitors were applied every 48 h.
Cloning of mouse Rspo2 and the generation of stable mouse mammary epithelial cell lines expressing Rspo2 and/or Wnt1
Full-length Rspo2 cDNA was amplified using Pfu polymerase (Stratagene, La Jolla, CA) and total cDNA obtained from one of the Rspo2-positive mammary tumors (Lowther et al., 2005). A Cterminal V5-His tag was introduced by PCR onto mouse Rspo2 with the following primers and the resulting DNA product was subcloned into the BamHI and EcoRI sites of pEF6 vector (Invitrogen, Carlsbad, CA): mRspo2F: 5′ CCGGATCCATGCGTTTTTGCCTCTTCT CATTTGCCC 3′, mRspo2R: 5′ CCGAATTCTTGGTTCACTCTGTCTGTAGCGAGG 3′. Two micrograms of Rspo2 vector and/or Wnt1 vector (Invitrogen) was introduced to C57MG and HC11 using FuGENE 6 (Roche, Indianapolis, IN) according to producer’s protocol. After 24 h in culture selection antibiotic was applied on cells, 10 μg/ml blasticidin in case of Rspo2 and 500 μg/ml G418 in case of Wnt1. Cells were kept in presence of selection antibiotic for 14 days. Expression of transgenes was confirmed by RT-PCR and Western blot (WB) analysis.
β-Catenin silencing by lentiviral transduction of shRNA
Lentiviral vectors were produced by co-transfection of HEK293T cells with the psPax2, pMD2.G, and pLKO.1 constructs (Moffat et al., 2006). The lentiviral helper plasmids, psPax2 and pMD2.G, were purchased from Addgene (Cambridge, MA). We used mouse pLKO.1 lentiviral target gene shRNA sets from Open Biosystems (# RMM4534-NM_007614 for β-catenin), which contained five independent shRNA constructs targeting different regions of β-catenin transcript, respectively. A control vector expressing nontarget scrambled shRNA sequence (CCGGCAACAAGATGAAGAGCACCAACTC GAGTTGGTGCTCTTCATCTTGTTGTTTTT) was purchased from Sigma–Aldrich. Transfections were carried out using FuGENE 6 (Roche). Virus was harvested 48 and 72 h after transfection, and infections of C57MG cells were carried out in the presence of 6 μg/ml of polybrene. Virus-containing supernatant was removed after 24 h. Following transduction, cells were selected with 7 μg/ml puromycin.
Cell culture and proliferation assay
HC11 mammary epithelial cells (Ball et al., 1988) were grown in RPMI 1640 medium containing 10% fetal bovine serum (FBS), L-glutamine (200 nM), 5 μg/ml insulin, and 10 ng/ml epidermal growth factor (EGF). C57MG mammary epithelial cells (Jue et al., 1992) were grown in DMEM supplemented with 10% FBS and 10 μg/ml of insulin. The C57MG/Wnt1 cell line was a gift from Dr. Harold Varmus (Memorial Sloan-Kettering Cancer Center) (Jue et al., 1992). Wnt1 stably expressing Rat2 fibroblasts and the parental Rat2 line were kindly provided by Dr. Anthony Brown (Cornell Medical Center), and maintained as previously reported (Giarre et al., 1998). For growth experiments, parental and transfected HC11 or C57MG cells were plated (Day 0) at a density of 1,000 cells/well in 24-well plates in the media described above. The number of cells was determined with a Coulter Counter either every day or every other day until confluence.
β-Catenin reporter assay
C57MG cells were transfected with 0.75 μg Super8XTOPFlash or Super8XFOPFlash (negative control), both constructs generously provided by Dr. Randall Moon (University of Washington), and 0.25 μg β-galactosidase DNAs using Amaxa system (Lonza Group Ltd., Basel, Switzerland) following manufacturer’s protocol. Twenty-four hours after transfection the medium was changed to conditioned medium from parental (Rat2-CM) or stably expressing Wnt1 Rat2 cells (Wnt1-CM) with or without 500 ng/ml recombinant hRspo2, and cells were incubated for 20 h. Luciferase and β-galactosidase activity were measured using Luciferase Assay System and β-Galactosidase Enzyme Assay System (both from Promega, Madison, WI), according to the manufacturer’s protocols. Luciferase activity was normalized to the β-galactosidase activity, and results were reported as mean ± SD. The reporter assay was also performed in C57MG cells stably expressing Wnt1 or Wnt1/Rspo2 to check the functional consequences of β-catenin knock-down using shRNA. C57MG/Wnt1 and C57/Wnt1/Rspo2 cells were transfected as described above. Cell lysates were harvested 24 h after transfection.
Matrigel penetration assay
C57MG cells were grown in 24-well plates pre-coated with Growth Factor Reduced Matrigel (BD Biosciences, San Jose, CA) and were handled according to manufacturer’s instructions. Singlecell suspensions of C57MG lines were plated (3,500 cells/well) on the 100% Matrigel bed and overlayed with the assay medium and 4% Matrigel. Cells were examined 5 days after plating and visualized with light microscope. Inhibitors were applied as indicated to the top layer every 48 h.
Cell migration and invasion assays
Cell invasion and migration across a basement membrane matrix were evaluated using a commercially available 12-well plate cell invasion/migration assay kit (Chemicon) and following the manufacturer’s instructions. Briefly, ~2 × 105 cells were seeded into individual invasion chambers, and subsequently placed in 12-well plates containing serum (10% FBS) culture medium in the lower chamber and incubated for 24 and 48 h. Non-invading cells were carefully wiped off the upper surface of the filters with a swab. Cells that invaded and migrated through the matrix-containing membrane and reached the lower surface of the invasion chamber were stained with crystal violet and counted in at least three different high power fields (hpf) using a light microscope.
Colony formation in soft agar
The soft agar assay was performed as described previously (Raafat et al., 2007). Briefly, cells in the agar mixture were seeded at 1.5 × 104 or 3 × 104 cells/well in triplicate. The plates were incubated at 37°C with 5% CO2 for 21 days. Colonies measuring 200 mm or larger were counted using the AccuCount 1000 colony counter (Biologics, Inc., Manassas, VA).
Transplantation of HC11 derived mammary epithelial cells in nude mice
The HC11 pools (HC11/parental, HC11/ev (empty vector), HC11/Rspo2, HC11/Wnt1, HC11/Wnt1/Rspo2) were grown to 70% confluence, harvested, and counted.
Two million cells of the indicated cell lines were mixed with 25% Matrigel in 1:1 ratio and injected in the left and right #4 inguinal mammary gland of nude mice. The female nude mice recipients were not bred and were palpated twice weekly. Tumor weight was determined as described previously (Raafat et al., 2007). Tumors were allowed to grow until they reached 500 mg, at which time the tumor-bearing mice were euthanized. Mice were kept under standard laboratory conditions according to the guidelines of the National Cancer Institute.
Metastasis assay
Viably frozen fragments of HC11–Rspo2, –Wnt1, and –Rspo2/Wnt1 tumors were implanted in the left and right #4 inguinal mammary gland of female athymic nude mice. Tumors reaching size of 300–350 mg were removed in survival surgery and mice were observed for any respiratory problems or other symptoms of disease. At any time point if an animal experienced rapid weight loss, debilitating diarrhea, rough coat hair, hunched posture, labored breathing, lethargy, persistent recumbence, jaundice, anemia, significantly abnormal neurological signs, bleeding from any orifice, self-induced trauma, impaired mobility, or had difficulty obtaining food or water it was immediately euthanized. Lungs and spleen were freshly fixed in 4% paraformaldehyde, embedded in paraffin, cut in 5 μm sections.
RNA isolation, RT-PCR, and quantitative real time PCR expression analysis
Total cellular RNAs were obtained from cell lines using TRIzol (Invitrogen). cDNAs were synthesized from 1 μg total RNA with random primers, using the SuperScript III kit according to the manufacturer’s protocol (Invitrogen). Quantitative PCR reactions were performed using primer sets and Brillant II SYBR Green kit (Stratagene). Quantification of GAPDH levels in the same cDNA samples measured in separate PCR reactions served as an internal control. Control conditions like parental cell line or parental cell line transfected with an empty vector or infected with nonsense, non-targeting shRNA were used to define baseline expression. Each cDNA sample was analyzed in triplicate with the Mx3000P (Stratagene) according to the manufacturer’s instructions using primers for Rspo2 R2F5′ CGCAATAAGCG AGCTTAGTTTATGTAT3′, R2R5′ATAGTCTCAATCTAACGGTGCAAAAC3′; Wnt1 W1F5′TGGGTTTCTACTACGTTGCTACTG and W1R5′GTTGTTGTGAAGG TTCATGAGGA3′; β-catenin β-catF5′CTTGGCTGAACCATCACAGA3′, β-catR5′GCTGATGGACCATAACAGCA3′. All primers were designed to span at least one intron in order to exclude amplification from genomic DNA. Primers for other genes were purchased from Origene (Rockville, MD) and used according to the manufacturer’s instructions. Catalog numbers are: E-cadherin MP201510, N-cadherin MP201519, Vimentin MP218224, Snail1 MP215603, Slug MP215604, Twist HP217798, Cytokeratin 18 MP207059, Fibronectin MP204834, Laminin 1 MP207280, SMA MP200230, Tgfβ1 MP217184, and ZO-1 MP216982.
Immunoblotting
Cells were harvested in RIPA buffer (150 mM NaCl, 10 mM Tris, pH 7.2, 0.1% SDS, 1.0% Triton X-100, 1% Deoxycholate, 5 mM EDTA) supplemented with the protease inhibitor cocktail complete Mini, EDTA-free and PhosSTOP (Roche). Total protein concentration was determined with BCA Protein Assay Kit (Pierce Biotechnology, Inc., Rockford, IL). Protein (50 μg total), was loaded on 4–20% SDS–PAGE gradient gels and transferred to nitrocellulose membrane using the iBlot Transfer System (Invitrogen). Membranes were blocked with SuperBlock® Dry Blend blocking buffer (Pierce Biotechnology, Inc.) with 0.1% Tween-20 (TBS/T) for 1 h at room temperature (RT) and then incubated with primary antibodies overnight. After washing with TBS/T, membranes were incubated for 1 hat RT with the appropriate secondary antibody conjugated to HRP, diluted in TBS/T. Proteins were visualized using the ECL Western Blotting Detection System (Amersham, GE Healthcare, Piscataway, NJ).
Immunofluorescent (IF) microscopy
Approximately 2 × 104 cells of each analyzed cell line were cultured overnight in Lab-Tek dual chamber slides (Nalge Nunc International, Naperville, IL). Cells were washed three times with PBS and fixed with 4% formaldehyde for 15 min at 4°C. Following a wash with PBS, samples were either incubated with permeabilization buffer (0.1% Triton X-100 in PBS) for 10 min or kept in PBS until the next step (non-permeabilized condition). Further procedures were done as described previously (Raafat et al., 2004, 2007).
Histology and immunohistochemistry
Mammary tissue was fixed in freshly prepared 4% paraformaldehyde, embedded in paraffin, cut in 5 μm sections and stained with hematoxylin and eosin (H&E) for subsequent histological analysis. Further procedures were done as described previously (Raafat et al., 2007). Antigen retrieval was performed by autoclaving slides with Antigen Unmasking Solution (Vector Laboratories, Inc., Burlingame, CA). Immunohistochemical analysis was performed with the ABC method according to the manufacturer’s protocol (Vector Laboratories, Inc.).
Statistics
Quantitative values are represented as the mean ± SD of at least three experiments. The statistical significance of the difference between groups was determined by Student’s t-test. Comparisons resulting in P-values less than 0.05 were considered statistically significant.
Results
Ectopic expression of Rspo2 and Wnt1 in mammary epithelial cell lines
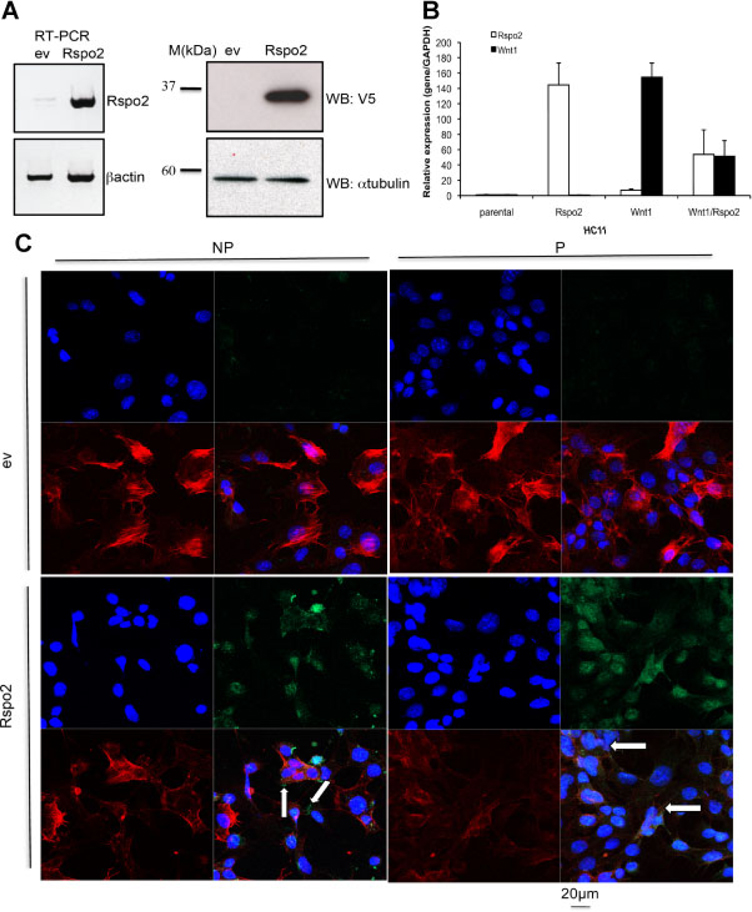
We investigated the effects of Wnt1 and Rspo2 on mouse mammary epithelial cells using stably transfected C57MG and HC11 cells that express untagged mouse Wnt1 and/or mouse Rspo2 tagged with V5-His epitopes at its carboxyl-terminus. Rspo2 expression was confirmed by RT-PCR and WB analysis (Fig. 1A). We monitored levels of expression of Rspo2 and Wnt1 in stable cell lines by performing Real-Time PCR (Fig. 1B). To determine Rspo2 subcellular localization, we stained HC11 monolayer cultures with V5 antibody (green), phalloidin (red) to delineate cell borders, and DAPI (blue) to label nuclei under both permeabilized and non-permeabilized conditions. When cells were not permeabilized, we detected small amounts of Rspo2 outside the cells associated with the membrane or extracellular matrix (Fig. 1C, NP, indicated by arrows), while IF staining of permeabilized cells revealed a strong signal within the cytoplasm and single spots on the membrane (Fig. 1C, P, indicated by arrows). Little or no signal was observed in the parental lines.
Fig. 1.
Rspo2 expression in mouse mammary epithelial cell lines C57MG and HC11. A: Stable expression of Rspo2 in the C57MG mammary epithelial cell line was confirmed by RT-PCR and immunoblotting with anti-V5. β-Actin was an internal control for RT-PCR and anti-α-tubulin blotting was used as a loading control for Western analysis. B: Relative expression of transgenic Rspo2 and Wnt1 in stable lines of HC11 cells. C: Localization of Rspo2 expressed in mouse mammary epithelial cells. HC11 cells stably transfected with an empty vector (ev) or the full-length Rspo2 cDNA construct were fixed and stained under non-permeabilized (NP) or permeabilized (P) conditions to visualize Rspo2 protein localization. Rspo2 proteins were detected with V5 antibody (green, upper and lower right parts), while cell boundaries were indicated by phalloidin (red, lower right and left parts) staining of polymerized actin and DAPI (blue, upper left and lower right parts) stained nuclei.
Rspo2 and Wnt1 have contrasting effects on cell growth in monolayer culture and soft agar
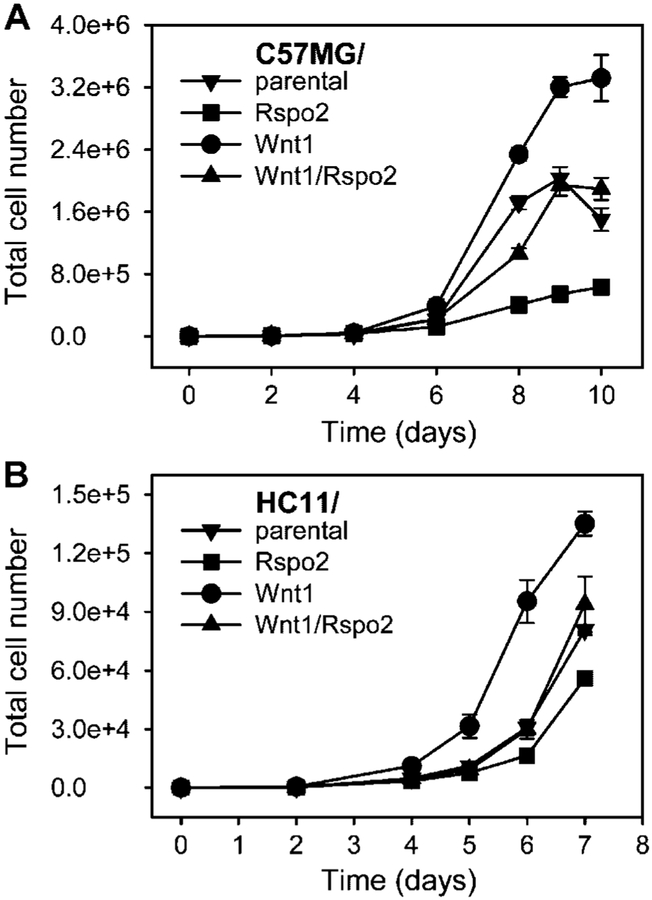
One of the hallmarks of transformed cells is increased proliferation rate. To assess proliferation rate in our model we generated growth curves for each cell line. We plated the same number of cells and subsequently counted the cells at intervals. Stable expression of Rspo2 in C57MG and HC11 cells was associated with a diminished rate of cell proliferation in monolayer culture, whereas Wnt1 expression stimulated cell growth (Fig. 2). For both cell lines, Wnt1/Rspo2 double transfectants had an intermediate growth rate equivalent to that seen with the respective parental cells.
Fig. 2.
Rspo2 and Wnt1 over-expression have contrasting effects on cell growth in monolayer culture. Growth curves of parental cell lines and stable transfectants expressing either Rspo2 or Wnt1 alone or in combination were generated as described in the Materials and Methods Section (upper part C57MG, lower part HC11). Cells were seeded in 24-well plates (1,000 cells/well), and were trypsinized and counted at 24 or 48 h intervals. Each counting was done in triplicate and expressed as the mean ± SD.
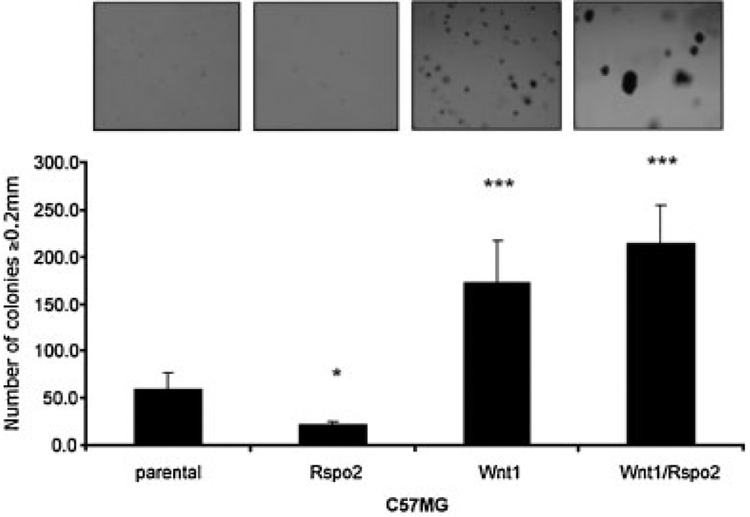
The ability of cells to grow in the absence of a solid substrate generally correlates with malignant transformation (Hanahan and Weinberg, 2000). To determine if Rspo2 expressing cells can grow in an anchorage independent manner we plated each cell line in 0.3% soft agar on top of 0.6% layer of agar and observed them for 21 days. None of the HC11 cells including parental, single, or double Wnt1/Rspo2 transfectants grew in soft agar. However, differential effects of Rspo2 and Wnt1 were evident when C57MG cells were maintained in soft agar (Fig. 3). While C57MG cells expressing Rspo2 grew significantly more slower than parental cells under these conditions (P < 0.05), Wnt1 transfectants formed many relatively small colonies (P < 0.01), as reported for NIH3T3 cells (Bafico et al., 1998). C57MG cells expressing both Rspo2 and Wnt1 yielded a similar number of colonies, but they were appreciably larger than the ones produced by the Wnt1 transfectant, implying a possible cooperation between these proteins in supporting anchorage-independent growth.
Fig. 3.
Growth of Rspo2 and Wnt1 expressing cells in soft agar. Cells from the indicated C57MG lines were placed in 6-well plates in 0.3% soft agar (15,000 cells/well). After 21 days they were visualized by overnight staining with nitrobluetetrazolium and counted for size ≥0.2 mm. Results are from a representative assay performed in triplicate and expressed as the mean ± SD. *P < 0.05, ***P < 0.01 compared to parental line (t-test).
Cells expressing Rspo2 and Wnt1 individually and in combination support tumor growth in nude mice
The HC11 and C57MG cell lines are not tumorigenic in nude mice and therefore are good models to examine the malignant transforming capability of oncogenes in vivo. To determine whether expression of Rspo2 was sufficient to induce mammary tumors in vivo, we injected the left and right #4 inguinal mammary gland of female athymic nude mice with 2 × 106 parental HC11 cells, HC11 cells transfected with empty vector (ev, pEF6), HC11/Rspo2, HC11/Wnt1, and HC11/Wnt1/Rspo2 (cells were mixed with 25% Matrigel at a 1:1 ratio). Mammary tumors were first palpable 3 week after injection. All 10 mammary fat pads inoculated with HC11/Wnt1 cells developed tumors, while only 5 of 10 mammary fat pads inoculated with HC11/Wnt1/Rspo2 cells developed small tumors within 4 weeks (Fig. 4A). Interestingly, although Rspo2 expression did not induce soft agar growth in previous experiments, HC11/Rspo2 cells developed small tumors within 4 weeks (Fig. 4A). This may reflect the collaborative effect of Rspo2 and endogenous Wnt ligands, which are expressed by cells in the mammary fat pad [reviewed by Turashvili et al. (2006)]. We detected Wnt1 and Wnt3a transcripts in mouse mammary tissue (data not shown). We also confirmed Rspo2 and Wnt1 RNA expression in tumors by real time RT-PCR (data not shown). Qualitatively similar tumorigenesis data were obtained with C57MG transfectants, although the onset of tumor formation was substantially longer. This presumably was due to technical factors including the use of a Matrigel suspension when mice were inoculated with HC11 transfectants and the absence of a wild-type Trp53 allele in HC11 cells, as earlier work had shown that Trp53 deficiency accelerates mammary tumorigenesis in Wnt1 transgenic mice (Donehower et al., Genes Dev 1995).
Fig. 4.
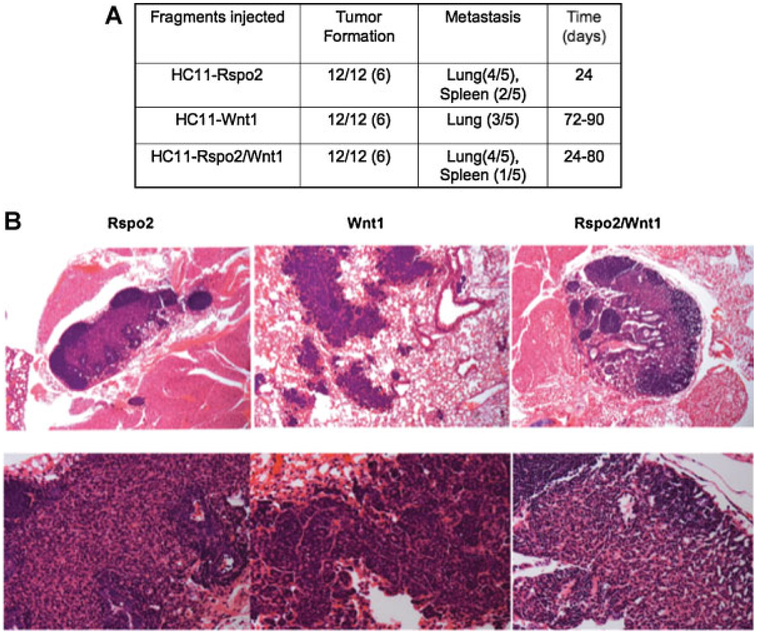
Cells expressing Rspo2 and Wnt1 individually and together are capable of tumor growth in nude mice. A: Pools of two million cells each of HC11/parental, HC11/ev, HC11/Rspo2, HC11/Wnt1, or HC11/Wnt1/Rspo2 were injected in the left and right #4 inguinal mammary gland of female nude mice. Before injection cells were placed in 12.5% Growth Factor Reduced Matrigel suspension to simulate the tumor microenvironment. Tumor growth curves represent the mean tumor size ± SD, determined weekly in two dimensions. HC11 cells did not yield tumors. B: H&E staining of representative tumors. Rspo2 cells gave rise to tumors with swirls of spindle-shaped neoplastic cells. Wnt1 tumors showed cells arranged in several types of structure within the same tumor section (Wnt1 a): Nests with microglandular features (black circle), cord-like organization (red box), spindle-shaped cells arranged in a dense fibrous pattern (green oval) as well as areas of squamous-like metaplasia (black arrows). Higher magnifications showed tumor cells arranged in glandular-like fashion compatible with an adenocarcinoma (Wnt1 b). Wnt1/Rspo2 tumor mass contained cells variable in size and shape that formed swirls containing large spindle-shaped cells. Other areas within the tumor had a more solid and undifferentiated organization characterized by cells with high-grade nuclear pleomorphism. Occasional mitotic figures can be seen (short arrows) (lower part a Wnt1/Rspo2). The original magnification was 100× (a) and 400× (b).
Histological examination of Rspo2, Wnt1, and Wnt1/Rspo2 tumors revealed major differences. Wnt1 tumors showed a more varied pattern with characteristics typical of β-catenininduced cancers (Rosner et al., 2002). Within the same tissue section, tumor cells were organized in nests with microglandular features or in cord-like structures surrounded by spindle-shaped cells arranged in a dense fibrous organization. Additionally, Wnt1 tumors had areas compatible with squamous metaplasia (Fig. 4B-Wnt1 a). Areas of tumor with a general arrangement of neoplastic cells around a lumen in a glandular-like pattern were suggestive of adenocarcinoma (Fig. 4B-Wnt1 b). Rspo2-expressing cells, both Rspo2 alone and together with Wnt1 gave rise to tumors containing swirls of spindle-shaped malignant cells similar to tumors containing epithelial–mesenchymal transition (EMT) previously observed in mouse mammary tissue (Fig. 4B-Rspo2 a) (Cardiff et al., 2006). Rspo2 tumors are more homogeneous, comprised of spindle cells arranged in a fasciculated and storiform pattern of growth (Fig. 4B-Rspo2 b); whereas cells in Rspo2/Wnt1 tumors are predominantly arranged in a solid sheet without clear glandular architecture (Fig. 4B-Rspo2/Wnt1 a) and with areas of spindle cells arranged in a fasciculated fashion (Fig. 4B-Rspo2/Wnt1 b). The Rspo2/Wnt1 tumors focally resemble Wnt1 tumors, suggesting an intermediate form.
HC11-Rspo2 tumors exhibit a gene expression profile consistent with EMT
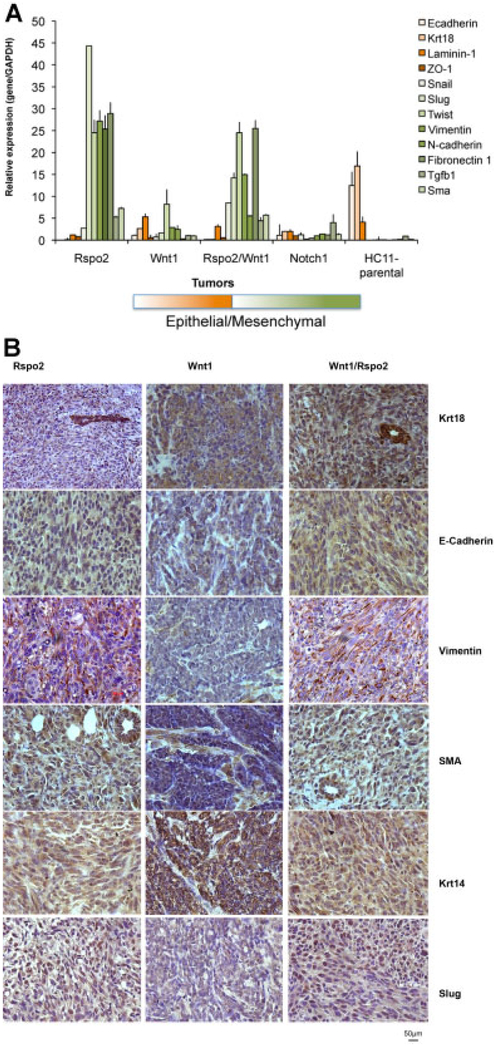
Analysis of gene expression provided additional evidence of an EMT pattern in cells from Rspo2 and Rspo2/Wnt1 tumors. Real-time RT-PCR analysis showed the absence of transcripts for the epithelial markers E-cadherin and cytokeratin 18 (Krt18) in Rspo2 and Rspo2/Wnt1 tumors (Fig. 5A). In contrast, expression of mRNAs encoding the mesenchymal markers Vimentin, N-cadherin, Fibronectin1, myoepithelial marker smooth muscle actin (SMA), Slug, Twist, and Tgfβ1 were highly elevated in Rspo2 and Rspo2/Wnt1 tumors relative to the Wnt1 tumors, parental HC11 cells and normal mammary tissue from nude mice (Fig. 5A). Wnt1 tumors showed moderate expression of a majority of analyzed genes, without a strong EMT signature. The only gene showing an elevated level of expression was Twist. Additionally we performed expression analyzes for Notch1 tumors not having an EMT morphological pattern that were derived from a similar injection of HC11–Notch1 cells into mammary fat pads and the HC11 cell line. In both cases we did not observe an EMT expression pattern signature. Notch1 tumors showed elevated Tgfβ1 mRNA only, while in the parental HC11 cell line the only gene with detectable expression was Krt18.
Fig. 5.
Rspo2 derived tumors show EMT signature. A: Relative expression of the mRNAs encoding EMT-related genes: E-cadherin, Cytokeratin 18 (Krt18), Laminin-1, ZO-1, Snail, Slug, Twist, Vimentin, N-cadherin, Fibronectin1, TGFb1, and SMA in Rspo2, Wnt1, Rspo2/Wnt1, and Notch1 tumors as well as in cultured parental HC11 cells, determined by real-time PCR. GAPDH mRNA was used to normalize the variability in template loading. The data are reported as the means ± SD from one representative tumor from each group analyzed in three repeats. B: Immunohistochemical staining of Rspo2, Wnt1, and Rspo2/Wnt1 tumors for Cytokeratin 18 (Krt18), E-cadherin, Vimentin, Cytokeratin 14 (Krt14), SMA, and Slug. The original magnification was 400×.
IHC analysis of Rspo2 and Rspo2/Wnt1 tumors showed strong staining for cytokeratin 18 (Krt18) in a host duct (indicated by arrow) and much lighter staining in tumor spindle cells (Fig. 5B). The Rspo2 and Rspo2/Wnt1 tumors were almost negative for E-cadherin whereas Wnt1 tumors were intensely stained. The myoepithelial marker cytokeratin 14 (Krt14) and mesenchymal markers, vimentin and SMA, were stained in the epithelial cellular compartment (Fig. 5B) confirming mouse EMT phenotype in Rspo2 and Rspo2/Wnt1 tumors [reviewed in (Damonte et al., 2007)]. In contrast in Wnt1 tumors, the epithelial compartment showed positive staining for Krt18 and Krt14 while the mesenchymal markers were negative in this compartment with light staining in the stroma. In addition IHC analysis of Rspo2 and Rspo2/Wnt1 tumors showed strong nuclear staining for EMT factor Slug (Fig. 5B).
Rspo2-induced tumors showed strong propensity for lung metastasis
To investigate whether expression of the EMT signature correlated with invasiveness, we performed a metastasis assay in which fragments of HC11–Rspo2, –Wnt1, and –Rspo2/Wnt1 tumors were implanted in the left and right #4 inguinal mammary gland of female athymic nude mice. Tumors reaching the size of 300–350 mg were removed in survival surgery and mice were subsequently monitored for any respiratory problems or other symptoms of disease. Most of the tumor bearing mice contained lung metastases. Rspo2 and Rspo2/Wnt1 tumor bearing mice also showed micro-metastases in spleen (Fig. 6A). The first metastatic lesions to appear (24 days after implantation) were those derived from Rspo2 tumors. It took 72–90 days for lung metastases to be detected in Wnt1 tumor bearing mice whereas the time of appearance of Rspo2/Wnt1 metastases varied between 24–80 days. H&E staining showed that the metastases (Fig. 6B, upper row, 100×), especially Rspo2 tumors showed characteristic swirls of spindle cells. Wnt1 and Rspo2/Wnt1 lung metastases showed more epithelial characteristics (Fig. 6B, bottom row, higher magnification 400×).
Fig. 6.
Metastasis of Rspo2, Wnt1, and Rspo2/Wnt1 derived tumors to the lungs and/or spleen. A: Fragments of Rspo2, Wnt1, and Rspo2/Wnt1 tumors were implanted in the left and right#4 inguinal mammary gland of six female athymic nude mice. Tumors reaching size of 300–350 mg were removed in survival surgery and mice were subsequently monitored for evidence of metastases. The numbers in parenthesis reflect the number of mice with mammary tumors or lung/spleen metastasis. B: H&E staining of representative lung metastases. Rspo2 expressing cells gave rise to tumors with swirls of spindle-shaped neoplastic cells. Occasionally areas of densely packed more epithelial-like cells were found. Original magnification 100× upper row, 400× lower row.
Rspo2 and Wnt1 expression together promote cell invasion in Matrigel
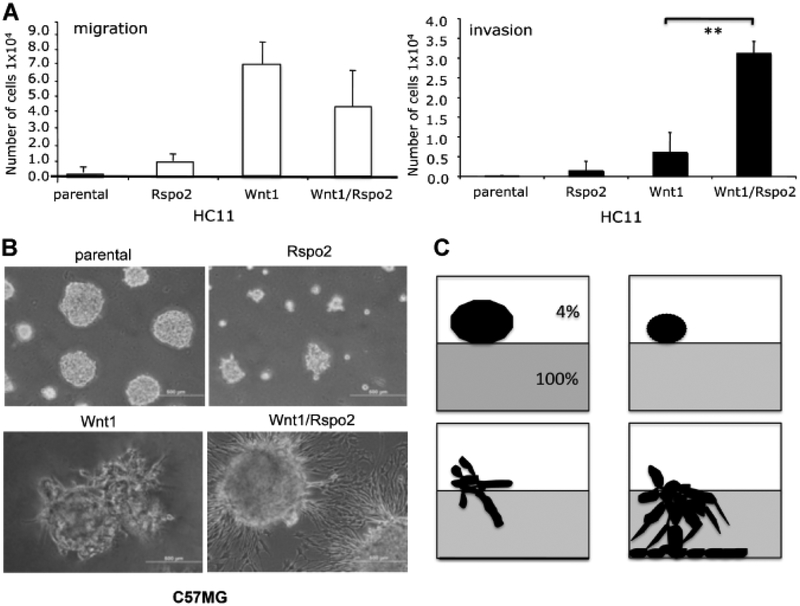
To further evaluate the migration and invasiveness of HC11 parental cells and transfectants we used transwell migration and invasion chambers. Cells were plated on a membrane with 8-μm pores for the migration assay; the membrane was occluded with Matrigel for the invasion assay. A gradient of serum was used as chemoattractant. HC11 cells expressing Wnt1 and Wnt1/Rspo2 showed increased migration, but only cells co-expressing Wnt1 and Rspo2 displayed significantly enhanced invasion (P < 0.02) (Fig. 7A).
Fig. 7.
Rspo2 and Wnt1 activity in migration and invasion assays. A: 3D Matrigel cultures of parental C57MG cells and cells expressing Rspo2 and Wnt1 alone or together. Wells were covered with 100% Matrigel. Cells were plated in 4% Growth Factors Reduced Matrigel at 3,500 cells/well. Colony formation and Matrigel penetration was determined after 5 days. The original magnification was 100×. The focus was set to detect cells that entered the layer of 100%Matrigel. B: Schematic representation of growth pattern observed in 3D Matrigel culture, corresponding to images in (A). C: Transwell migration and invasion assays of parental HC11 cells and transfectants. HC11 cell pools (2.5 × 105/well) were placed in a transwell migration (upper part) or invasion chamber (lower part), with10%FBS in the lower chamber. The number of cells crossing through the pores in the migration assay was determined at 24 h. The number of cells invading through Matrigel-occluded pores was determined at 48 h. The number of migrating or invading cells is expressed as mean ± SD. **P < 0.02 (t-test). Scale bar = 500 μm.
For comparison, the invasiveness of parental and transfected C57MG cells was examined in a 3D Matrigel culture assay. The parental cell line as well as cells expressing Rspo2 failed to invade 100% Matrigel, but formed characteristic spherical colonies in the layer of 4% Matrigel (Fig. 7B,C). Interestingly, C57MG/Rspo2 cells appeared to form smaller acini. While C57MG/Wnt1 cells formed irregularly shaped colonies with protrusions that barely penetrated into the 100% Matrigel, C57MG/Wnt1/Rspo2 cells extensively invaded the matrix, penetrating through it to form monolayers that spread on the plastic surface of the dish (Fig. 7B,C).
Wnt1/Rspo2-dependent penetration of Matrigel and growth in soft agar do not require β-catenin signaling
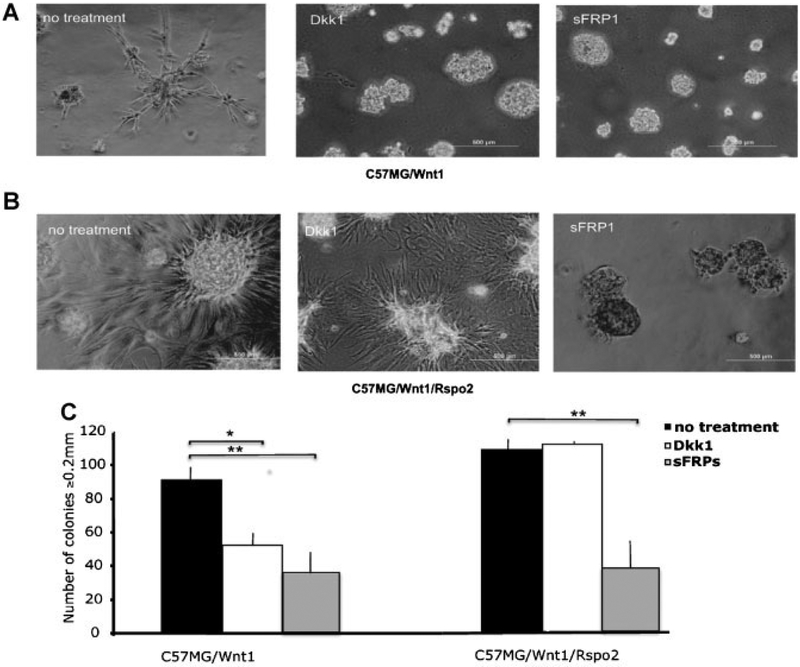
Consistent with prior studies of Rspo/Wnt interaction, we observed a cooperative effect of Rspo2 and Wnt1 on β-catenin signaling in C57MG and HC11 cells (Suppl. Fig. 1A,B). To test the hypothesis that Wnt/β-catenin signaling was necessary for the invasiveness and transformed phenotype exhibited by mammary epithelial cells expressing Wnt1 with or without Rspo2, we performed Matrigel penetration and soft agar assays with C57MG/Wnt1 and C57MG/Wnt1/Rspo2 cells in the presence or absence of various Wnt signaling antagonists. In particular, we used Dkk1 (1 μg/ml) to specifically block the Wnt/β-catenin pathway and sFRP1 (10 μg/ml) to antagonize Wnt signaling in general. The irregular growth pattern of C57MG/Wnt1 cells in Matrigel was inhibited significantly by both reagents (Fig. 8A). In contrast, the penetration of Matrigel by C57MG/Wnt1/Rspo2 cells was abrogated by sFRP1, while the invasive properties of these cells were not affected by Dkk1 (Fig. 8B). Similarly, Dkk1 only decreased the number of soft agar colonies formed by C57MG/Wnt1 cells, not C57MG/Wnt1/Rspo2 cells, while sFRP1 had an inhibitory effect on both cell lines (Fig. 8C).
Fig. 8.
Differential effects of Dkk1 and sFRP1 on C57MG/Wnt1 and C57MG/Wnt1/Rspo2 cells in Matrigel and soft agar assays. C57MG/Wnt1 (A) and C57MG/Wnt1/Rspo2 (B) cells were plated on Matrigel or in soft agar (C) in the presence of growth medium alone (no treatment) or in medium containing 1 μg/ml Dkk1 or 10 μg/ml sFRP1. Fresh inhibitors were applied every 48 h for 5 days in the Matrigel assay or 21days in the soft agar assay. The original magnification was 100×. Experiments were performed in triplicate and results indicate the mean ± SD of a representative experiment. *P < 0.05 (t-test). Scale bar = 500 μm.
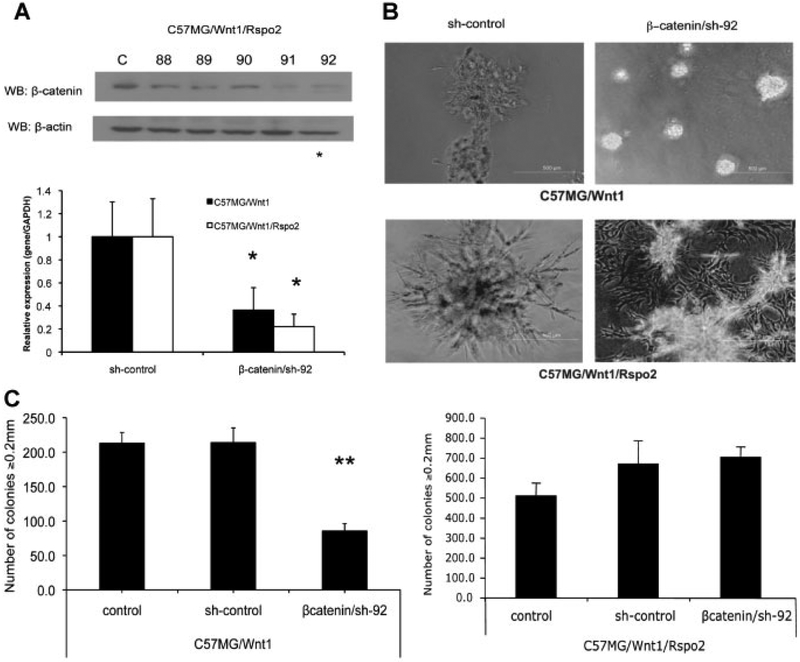
We confirmed these findings by using a lentiviral shRNA system to disrupt expression of β-catenin. Quantitative RT-PCR and WB analysis of lentiviral-infected C57MG/Wnt1 and C57MG/Wnt1/Rspo2 cells demonstrated that the steady state level of β-catenin expression was decreased by approximately 60–80% compared to cells infected with vectors expressing scrambled or nonsense sequences (Fig. 9A). Moreover, knockdown of β-catenin expression by shRNA decreased β-catenin Super8XTOPFlash reporter activity in both Wnt1 and Wnt1/Rspo2 transfectants (Suppl. Fig. 2). Suppression of β-catenin expression in C57MG/Wnt1 cells prevented protrusions and the irregular growth pattern in Matrigel (Fig. 9B) and reduced the number of colonies in soft agar by more than 50%. (Fig. 9C, left part). However, C57MG/Wnt1/Rspo2 cells with diminished β-catenin expression exhibited a more invasive phenotype as cells completely penetrated the Matrigel and formed monolayers on the bottom of the dish (Fig. 9B). Moreover, there was no decline in soft agar growth (Fig. 9C). The scrambled shRNA did not influence cell behavior in either the Matrigel or soft agar assay (Fig. 9B,C). Taken together, these results suggest that, in contrast to Wnt1 alone, the invasiveness and anchorage-independent growth associated with Wnt1 and Rspo2 co-expression are due to mechanisms other than the β-catenin pathway.
Fig. 9.
Differential effects of β-catenin shRNA on C57MG/Wnt1 and C57MG/Wnt1/Rspo2 cells in Matrigel and soft agar assays. A: Silencing of β-catenin expression in C57MG/Wnt1 and C57MG/Wnt1/Rspo2 cells by shRNA lentiviral technology was confirmed by WB (sh-control and 5 b-catenin targets) and real time RT-PCR (β-catenin/sh-92 vs. sh-control). Vector carrying nonsense, non-targeting shRNA was used to define baseline expression (sh-control). B: Representative Matrigel penetration assay for C57MG/Wnt1 and C57MG/Wnt1/Rspo2 cells following β-catenin knockdown (β-catenin/sh-92). Cells were plated in 4% Growth Factors Reduced Matrigel at 3,500 cells/well. Matrigel penetration was determined after 5 days. The original magnification was 100×.Scale bar = 500 μm. C: Colony formation assay for C57MG/Wnt1andC57MG/Wnt1/Rspo2 cells after β-catenin knockdown (β-catenin/sh-92). Cells were placed in 6-well plates in 0.3% soft agar at 15,000 cells/well. After 21 days they were visualized by overnight staining with nitrobluetetrazolium and counted for size ≥0.2 mm. Experiments were performed in triplicate and results indicate the mean ± SD of a representative experiment. **P < 0.02 (t-test).
Discussion
Rspo2 was originally identified as a common integration site (CIS) for MMTV in mouse mammary preneoplastic hyperplastic outgrowth (HOG) lines and in independent mammary tumors. Frequently these HOGs and tumors also had MMTV CIS at members of the Wnt or Fgf gene families raising the possibility that these mutations collaborated in the transition of normal mammary epithelial cells to preneoplastic lesions or malignant tumors. This concept is supported by previous studies showing the biological consequences of cooperation between Wnt1 and Fgf3 in tumorigenesis (Tsukamoto et al., 1988; Muller et al., 1990; Kwan et al., 1992; Stamp et al., 1992). In these studies bitransgenic mice exhibited a pattern of mammary hyperplasia previously observed in Wnt1 transgenic mice and mammary carcinomas arose earlier and more frequently in both males and virgin females than in sibling mice bearing only one of the parental transgenes. Following the example of previous genes altered by MMTV insertions, we tested the hypothesis that Rspo2 is a novel oncogene contributing to mammary gland tumorigenesis.
To evaluate the potential role for Rspo2 in malignant transformation, we investigated properties that are typically dysregulated during cancer progression: proliferation, anchorage independent growth, migration, and invasion (Hanahan and Weinberg, 2000). Surprisingly, the proliferation rate of both C57MG/Rspo2 and HC11/Rspo2 transfectants in monolayer culture was decreased relative to parental lines, in contrast to Wnt1 transfectants, which exhibited an increased growth rate. The Wnt1/Rspo2 double transfectants resembled the parental lines with respect to proliferation on plastic. Moreover, stable expression of Rspo2 did not stimulate the growth of C57MG or HC11 cells in soft agar (Fig. 3B and data not shown), nor did it have a major impact on the anchorage-independent growth of C57MG cells that also over-expressed Wnt1. Taken together, these results provided little evidence to support the idea that Rspo2 participated in mammary neoplasia.
Remarkably different results were obtained when the stable transfectants were implanted into the cleared mammary fat pads of Balb/c nude mice. Consistent with a prior study involving explants of HC11/Rspo3 cells (Theodorou et al., 2007), HC11/Rspo2 cells exhibited oncogenic properties in this model system. Both the HC11/Rspo2 and the HC11/Rspo2/Wnt1 tumors had a sarcomatoid phenotype and an EMT gene signature that distinguished them from the less extensive EMT pattern of HC11/Wnt1 tumors. These tumors only showed upregulation of Twist, a previously reported target of Wnt1 (Howe et al., 2003). Moreover, cells derived from the HC11/Rspo2 and HC11/Rspo2/Wnt1 tumors metastasized more rapidly and widely than cells from HC11/Wnt1 tumors. Rspo2/Wnt1 double transfectants also displayed more invasive behavior than Wnt1 transfectants in 3D Matrigel assays, although invasive activity was not characteristic of Rspo2 transfectants in the in vitro assays. Our findings documented the transforming potential of Rspo2 and demonstrated that it could synergize with Wnt1 in certain contexts to promote aggressive tumor behavior. However, the variation in cellular responses to Rspo2 expression in different settings emphasized the importance of environment in determining Rspo2 activity and its interaction with Wnt1. We suspect that local factors such as matrix components and surfaces as well as selective pressures in vivo contributed to the distinctive cellular behavior associated with Rspo2 and Wnt expression in different contexts.
Our data challenge conventional views about the signaling mechanism of Rspos. While we confirmed that Rspo2 synergizes with Wnt1 to stimulate the β-catenin pathway, this was not required for the invasive properties of C57MG/Rspo2/Wnt1 cells in 3D Matrigel cultures or their growth in soft agar. In contrast, β-catenin signaling was critical for C57MG/Wnt1 cells in these assays. Presumably the co-expression of Rspo2 and Wnt1, combined with the influences of the environment, elicits other mechanisms that warrant further investigation. In this regard, it is worth noting that Rspo3 was recently reported to stimulate activation of c-Jun N-terminal Kinase (JNK) (Ohkawara et al., 2011). It is possible that some effects of Rspo2 described in our study occur independent of Wnts.
In conclusion we have shown that Rspo2 expression contributes to mouse mammary gland tumorigenesis. HC11 cells expressing Wnt1 and Rspo2 independently or together can develop into tumors in nude mice. Rspo2-derived tumors exhibit a strong EMT phenotype and are highly metastatic to the lung as well as the spleen. C57MG cells co-expressing Rspo2 and Wnt1 also display a transformed and invasive phenotype in vitro that is not mediated by β-catenin signaling.
Supplementary Material
Acknowledgments
We thank Harold Varmus for providing the C57MG/Wnt1 cell line, Anthony Brown for the Rat2/Wnt1 and parental Rat2 lines and Randall Moon for the Super8XTOPFlash and Super8XFOPFlash constructs. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, and Center for Cancer Research.
Contract grant sponsor: NIH.
Contract grant sponsor: National Cancer Institute.
Contract grant sponsor: Center for Cancer Research.
Literature Cited
- Bafico A, Gazit A, Wu-Morgan SS, Yaniv A, Aaronson SA. 1998. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene 16:2819–2825. [DOI] [PubMed] [Google Scholar]
- Ball RK, Friis RR, Schoenenberger CA, Doppler W, Groner B. 1988. Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J 7:2089–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejsovec A 2005. Wnt pathway activation: New relations and locations. Cell 120:11–14. [DOI] [PubMed] [Google Scholar]
- Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas WE III, Dixon M, Hazell SA, Wagle M, Nie WS, Tomasevic N, Williams J, Zhan X, Levy MD, Funk WD, Abo A. 2007. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci USA 104:14700–14705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. 2006. Wnt signaling: Complexity at the surface. J Cell Sci 119:395–402. [DOI] [PubMed] [Google Scholar]
- Cardiff RD, Gregg JP, Miller JW, Axelrod DE, Borowsky AD. 2006. Histopathology as a predictive biomarker: strengths and limitations. J Nutr 136:2673S–2675S. [DOI] [PubMed] [Google Scholar]
- Chadi S, Buscara L, Pechoux C, Costa J, Laubier J, Chaboissier MC, Pailhoux E, Vilotte JL, Chanat E, Le Provost F. 2009. R-spondin1 is required for normal epithelial morphogenesis during mammary gland development. Biochem Biophys Res Commun 390:1040–1043. [DOI] [PubMed] [Google Scholar]
- Clevers H 2006. Wnt/beta-catenin signaling in development and disease. Cell 127:469–480. [DOI] [PubMed] [Google Scholar]
- Damonte P, Gregg JP, Borowsky AD, Keister BA, Cardiff RD. 2007. EMT tumorigenesis in the mouse mammary gland. Lab Invest 87:1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Vogel H, McArthur MJ, Montgomery CA, Park SH, Thompson CA, Ford RJ, Bradley A 1995. Effects of genetic background on tumorigenesis in p53-deficient mice. Mol. Carcinog 14:16–22. [DOI] [PubMed] [Google Scholar]
- Giarre M, Semenov MV, Brown AM. 1998. Wnt signaling stabilizes the dual-function protein beta-catenin in diverse cell types. Ann N Y Acad Sci 857:43–55. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. 2006. Wnt signaling: Multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281:22429–22433. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2000. The hallmarks of cancer. Cell 100:57–70. [DOI] [PubMed] [Google Scholar]
- Howe LR, Watanabe O, Leonard J, Brown AM. 2003. Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res 63:1906–1913. [PubMed] [Google Scholar]
- Humphreys RC, Rosen JM. 1997. Stably transfected HC11 cells provide an in vitro and in vivo model system for studying Wnt gene function. Cell Growth Differ 8:839–849. [PubMed] [Google Scholar]
- Jue SF, Bradley RS, Rudnicki JA, Varmus HE, Brown AM. 1992. The mouse Wnt-1 gene can act via a paracrine mechanism in transformation of mammary epithelial cells. Mol Cell Biol 12:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. 2004. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell 7:525–534. [DOI] [PubMed] [Google Scholar]
- Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, Abo A, Tomizuka K, Funk WD. 2006. R-Spondin proteins: A novel link to beta-catenin activation. Cell Cycle 5:23–26. [DOI] [PubMed] [Google Scholar]
- Kim KA, Wagle M, Tran K, Zhan X, Dixon MA, Liu S, Gros D, Korver W, Yonkovich S, Tomasevic N, Binnerts M, Abo A. 2008. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell 19:2588–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan H, Pecenka V, Tsukamoto A, Parslow TG, Guzman R, Lin TP, Muller WJ, Lee FS, Leder P, Varmus HE. 1992. Transgenes expressing the Wnt-1 and int-2 proto-oncogenes cooperate during mammary carcinogenesis in doubly transgenic mice. Mol Cell Biol 12:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Yen TY, Endo Y, Klauzinska M, Baljinnyam B, Macher B, Callahan R, Rubin JS. 2009. Lossof-function point mutations and two-furin domain derivatives provide insights about R-spondin2 structure and function. Cell Signal 21:916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther W, Wiley K, Smith GH, Callahan R. 2005. A new common integration site, Int7, for the mouse mammary tumor virus in mouse mammary tumors identifies a gene whose product has furin-like and thrombospondin-like sequences. J Virol 79:10093–10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, Carpenter AE, Foo SY, Stewart SA, Stockwell BR, Hacohen N, Hahn WC, Lander ES, Sabatini DM, Root DE. 2006. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124:1283–1298. [DOI] [PubMed] [Google Scholar]
- Muller WJ, Lee FS, Dickson C, Peters G, Pattengale P, Leder P. 1990. The int-2 gene product acts as an epithelial growth factor in transgenic mice. EMBO J 9:907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. 2006. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenindependent gene expression. J Biol Chem 281:13247–13257. [DOI] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Yoon JK. 2007. Dynamic expression of R-spondin family genes in mouse development. Gene Expr Patterns 7:306–312. [DOI] [PubMed] [Google Scholar]
- Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. 1984. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature 307:131–136. [DOI] [PubMed] [Google Scholar]
- Ohkawara B, Glinka A, Niehrs C. 2011. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev Cell 20:303–314. [DOI] [PubMed] [Google Scholar]
- Olson DJ, Papkoff J. 1994. Regulated expression of Wnt family members during proliferation of C57mg mammary cells. Cell Growth Differ 5:197–206. [PubMed] [Google Scholar]
- Raafat A, Bargo S, Anver MR, Callahan R. 2004. Mammary development and tumorigenesis in mice expressing a truncated human Notch4/Int3 intracellular domain (h-Int3sh). Oncogene 23:9401–9407. [DOI] [PubMed] [Google Scholar]
- Raafat A, Zoltan-Jones A, Strizzi L, Bargo S, Kimura K, Salomon D, Callahan R. 2007. Kit and PDGFR-alpha activities are necessary for Notch4/Int3-induced tumorigenesis. Oncogene 26:662–672. [DOI] [PubMed] [Google Scholar]
- Rosner A, Miyoshi K, Landesman-Bollag E, Xu X, Seldin DC, Moser AR, MacLeod CL, Shyamala G, Gillgrass AE, Cardiff RD. 2002. Pathway pathology: Histological differences between ErbB/Ras and Wnt pathway transgenic mammary tumors. Am J Pathol 161:1087–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp G, Fantl V, Poulsom R, Jamieson S, Smith R, Peters G, Dickson C. 1992. Nonuniform expression of a mouse mammary tumor virus-driven int-2/Fgf-3 transgene in pregnancyresponsive breast tumors. Cell Growth Differ 3:929–938. [PubMed] [Google Scholar]
- Theodorou V, Kimm MA, Boer M, Wessels L, Theelen W, Jonkers J, Hilkens J. 2007. MMTV insertional mutagenesis identifies genes, gene families and pathways involved in mammary cancer. Nat Genet 39:759–769. [DOI] [PubMed] [Google Scholar]
- Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. 1988. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 55:619–625. [DOI] [PubMed] [Google Scholar]
- Turashvili G, Bouchal J, Burkadze G, Kolar Z. 2006. Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology 73:213–223. [DOI] [PubMed] [Google Scholar]
- Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS. 2000. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem 275:4374–4382. [DOI] [PubMed] [Google Scholar]
- Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X. 2007. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J Biol Chem 282:15903–15911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.