Abstract
Background:
As a ubiquitous environmental pollutant, methylmercury (MeHg) induces toxic effects in the nervous system, one of its main targets. However, the exact mechanisms of its neurotoxicity have not been fully elucidated. Hypoxia-inducible (), a transcription factor, plays a crucial role in adaptive and cytoprotective responses in cells and is involved in cell survival, proliferation, apoptosis, inflammation, angiogenesis, glucose metabolism, erythropoiesis, and other physiological activities.
Objectives:
The aim of this study was to explore the role of in response to acute MeHg exposure in rat brain and primary cultured astrocytes to improve understanding of the mechanisms of MeHg-induced neurotoxicity and the development of effective neuroprotective strategies.
Methods:
Primary rat astrocytes were treated with MeHg () for . Cell proliferation and cytotoxicity were assessed with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl diphenyltetrazolium bromide (MTT) assay and a lactate dehydrogenase (LDH) release assay, respectively. Reactive oxygen species (ROS) levels were analyzed to assess the level of oxidative stress using 2′,7′-dichlorofluorescin diacetate (DCFH-DA) fluorescence. , and its downstream proteins, glucose transporter 1 (GLUT-1), erythropoietin (EPO), and vascular endothelial growth factor A (VEGF-A) were analyzed by means of Western blotting. Real-time PCR was used to detect the expression of mRNA. Pretreatment with protein synthesis inhibitor (CHX), proteasome inhibitor (MG132), or proline hydroxylase inhibitor (DHB) were applied to explore the possible mechanisms of inhibition by MeHg. To investigate the role of in MeHg-induced neurotoxicity, cobalt chloride (), 2-methoxyestradiol (2-MeOE2), small interfering RNA (siRNA) transfection and adenovirus overexpression were used. Pretreatment with N-acetyl-L-cysteine (NAC) and vitamin E (Trolox) were used to investigate the putative role of oxidative stress in MeHg-induced alterations in levels. The expression of and related downstream proteins was detected in adult rat brain exposed to MeHg () for in vivo.
Results:
MeHg caused lower cell proliferation and higher cytotoxicity in primary rat astrocytes in a time- and concentration-dependent manner. In comparison with the control cells, exposure to MeHg for significantly inhibited the expression of astrocytic , and the downstream genes GLUT-1, EPO, and VEGF-A (), in the absence of a significant decrease in mRNA levels. When protein synthesis was inhibited by CHX, MeHg promoted the degradation rate of . MG132 and DHB significantly blocked the MeHg-induced decrease in expression (). Overexpression of significantly attenuated the decline in MeHg-induced cell proliferation, whereas the inhibition of significantly increased the decline in cell proliferation (). NAC and Trolox, two established antioxidants, reversed the MeHg-induced decline in protein levels and the decrease in cell proliferation (). MeHg suppressed the expression of and related downstream target proteins in adult rat brain.
Discussion:
MeHg induced a significant reduction in protein by activating proline hydroxylase (PHD) and the ubiquitin proteasome system (UPS) in primary rat astrocytes. Additionally, ROS scavenging by antioxidants played a neuroprotective role via increasing expression in response to MeHg toxicity. Moreover, we established that up-regulation of might serve to mitigate the acute toxicity of MeHg in astrocytes, affording a novel therapeutic target for future exploration. https://doi.org/10.1289/EHP5139
Introduction
Methylmercury (MeHg) is a global environmental contaminant targeting the central nervous system (CNS) (Farina and Aschner 2017; Santos et al. 2016). Consumption of MeHg-containing fish products (Canuel et al. 2006; Carrasco et al. 2011; Steuerwald et al. 2000; Stopford and Goldwater 1975) and rice (Rothenberg et al. 2014; Zhang et al. 2010) has been shown to induce neurodegeneration as well as be associated with neurodevelopmental disorders (Belletti et al. 2002; Carocci et al. 2014; Ceccatelli et al. 2010). However, the underlying cellular and molecular mechanisms of MeHg-induced neurotoxicity have yet to be fully elucidated. Meanwhile, there is still a lack of treatment to effectively protect against MeHg-induced brain damage.
Hypoxia-inducible factor-1 (HIF-1), a DNA-binding transcription factor, plays a crucial role in a diverse range of adaptive responses to oxygen tension. HIF-1 is a heterodimer comprising an oxygen-labile () and a constitutively expressed (). Under normal oxygen conditions ( ), prolyl hydroxylase (PHD) has been shown to mediate hydroxylation of (Berra et al. 2003; Ivan et al. 2001). Hydroxylated is rapidly degraded by the ubiquitin proteasome system (UPS), mediated by interaction with the von-Hippel Lindau tumor suppressor gene product (pVHL) (Bruick and McKnight, 2001; Lee et al. 2007). Under hypoxic conditions ( ) or stimulation by other environmental factors, stabilized dimerizes with to transactivate a series of adaptive genes, including vascular endothelial growth factor A (VEGF-A), glucose transporter 1 (GLUT-1), and erythropoietin (EPO) (Semenza and Wang, 1992). Several metals and organic chemicals have been shown to affect expression and activity (Dong et al. 2016; Lee et al. 2009; Liao et al. 2014; Wikenheiser et al. 2013; Wu et al. 2010), whereas increased expression of has been shown to be protective against neurotoxicants (Feng et al. 2014; Lee et al. 2009; Wu et al. 2010). Manganese has also been shown to increase protein levels in Hep2 cells by regulating mitogen-activated protein kinases (MAPKs) (Shin et al. 2010). Clioquinol (a copper and zinc chelator) decreased degradation by inhibiting ubiquitination and hydroxylation in SH-SY5Y cells and HepG2 cells, whereas addition of copper and zinc reversed these effects (Choi et al. 2006). Cadmium was shown to up-regulate expression in human lung epithelial cells via the production of reactive oxygen species (ROS) through the activation of the protein kinase B (Akt), and extracellular signal-regulated protein kinase (ERK) signaling pathways (Jing et al. 2012). Notably, deferoxamine (DFO), an iron chelator, was shown to attenuate neuronal cell death and the generation of ROS caused by MeHg and methylphenyl tetrahydropyridine (MPTP) in in vivo rodent models (Guo et al. 2016; LeBel et al. 1992). Furthermore, it was shown that by inhibiting the activity of proline hydroxylase (PHD) in mouse hippocampal neurons, DFO decreased degradation of and increased intracellular levels of protein (Hamrick et al. 2005). Recently, several studies have further documented that is neuroprotective because it drives the expression of critical genes that diminish neuronal cell death (Chen et al. 2017; Sen and Sen 2016). Increased expression and activity have been shown to promote glycolysis and glucose metabolism, thus countering oxidative stress by producing NADH and NADPH to propagate neuroprotective responses (Soucek et al. 2003). has also been shown to improve cerebral blood flow, which could oppose the toxicity of hypoxia (Iyalomhe et al. 2017). Overexpression of and/or target genes, such as VEGF-A and EPO, may be an early adaptation to the oxidative stressors that characterize MeHg-induced neuropathology. Thus, we speculate that the molecular events that constitute this early adaptation are likely neuroprotective and might mitigate neuronal injury caused by MeHg.
Previous studies have indicated an association between activity and intracellular ROS production. ROS was reported to promote accumulation by the inhibition of PHD catalytic activity via the oxidization of PHD-bound (Koivunen et al. 2007; Pan et al. 2007). However, it has been noted that a paradox exists between them, because the transcriptional program can prevent mitochondrial ROS generation (Jain et al. 2016). It is unclear whether MeHg-induced toxicity is associated with the expression of , whereas the specific relationship between ROS production and also remains unclear. MeHg predominantly accumulates in the CNS, particularly in astrocytes; the latter have been shown to play an important role in mediating MeHg-induced neurotoxicity (Ceccatelli et al. 2010; Shanker et al. 2003), inhibit glutamate uptake, and contribute to secondary excitotoxicity (Aschner et al. 2000; Deng et al. 2014; Liu et al. 2013, 2014). Astrocytes perform diverse important functions in providing support and nutrition (Abbott 2002; Rudge et al. 1994), inducing neuronal differentiation (Barkho et al. 2006) and mediating immune responses (Aschner 1998a, 1998b; Jensen et al. 2013). Therefore, we aimed to investigate the role of in MeHg-induced acute neurotoxicity by using primary rat astrocytes and adult rats, with special emphasis on an ROS mode of toxicity, so as to further identify novel therapeutic targets against MeHg-induced neuronal injury.
Materials and Methods
Primary Rat Astrocyte Isolation and Culture
Sprague-Dawley (SD) rats at the 1–2-d postnatal stage were purchased from the Laboratory Animal Center of Jiangsu University. Primary neonatal rat astrocytes were prepared as previously described (Bai et al. 2015, 2016; Fang et al. 2016; Yang et al. 2018; Yin et al. 2011). The procedures were as follows: Rats were rapidly decapitated, and the cerebral hemispheres were quickly obtained and rinsed with 4°C phosphate buffered solution (PBS). After dissecting away part of the meninges, basal ganglia, midbrain, and blood vessels, the remaining cortical tissues were cut into pieces and dissociated with 0.25% trypsin (Gibco) for 15 min at room temperature in the absence of stirring. Cells were grown in Dulbecco’s Modified Eagle Medium (DMEM; Hyclone) containing 10% fetal bovine serum (Gibco), and 1% penicillin/streptomycin (Gibco). Culture medium was replaced with fresh medium twice a week, and cultures were maintained in a 5% environment at 37°C. Cultured cells were used for experiments when astrocytes reached confluency. Protocols were approved by the Animal Ethics Committee of Jiangsu University of China and were carried out in accordance with the established Guiding Principles for Animal Research.
Reagents and Antibodies
Methylmercuric chloride (MeHgCl; Sigma-Aldrich) was dissolved in PBS to form a stock concentration of ; MG132; 3,4-Dihydroxybenzoic acid (DHB); cobalt chloride (); N-acetyl-cysteine (NAC) and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid); and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl diphenyltetrazolium bromide (MTT)] were obtained from Sigma-Aldrich. Cycloheximide (CHX) was purchased from MedChemExpress; 2-methoxyestradiol (2-MeOE2) was purchased from Selleck Chemicals. For Western blotting analysis, the primary polyclonal antibodies to , VEGF-A, GLUT-1, and EPO were obtained from ImmunoWay, and antibodies to and were obtained from Cell Signaling Technology and CMATAG, respectively. Secondary antibodies used for immunoblotting included horseradish peroxidase (HRP)-conjugated antimouse (Santa Cruz Biotechnology) or anti-rabbit antibodies (Santa Cruz Biotechnology).
Cell Treatment
Astrocytes were treated for with MeHg at concentrations of 1.0, 2.5, 5.0, or in the medium, and the cells treated by culture medium without MeHg were used as the control group. These concentrations were used in previous studies (Allen et al. 2001; Shapiro and Chan 2008). (, ), CHX (, 0.5, 1, or ), MG132 (, ), DHB (, ), (, ), 2-MeOE2 (, ), and NAC (, ) or Trolox (, ) were administered prior to MeHg treatment.
Cell Proliferation Assay
Cell proliferation was measured in astrocytes cultured in 96-well plates () using the MTT assay. At the end of treatment, MTT was added to each well at a final concentration of and incubated at 37°C for . Next, the MTT solution was replaced with dimethyl sulfoxide () for 10 min to dissolve the dark-blue formazan crystals in intact cells, and the absorbance was measured at with a Bio-Rad 680 Microplate Reader (Bio-Rad Laboratories).
Lactate Dehydrogenase (LDH) Release Assay
Cytotoxicity was detected using an LDH release assay kit (Jiancheng Bioengineering Institute of Nanjing) according to the manufacturer’s protocol. Cells were cultured in 96-well plates (), and after treatment supernatants were transferred to clean 96-well plates. Absorbance was analyzed at using a Bio-Rad 680 Microplate Reader.
Measurement of Intracellular ROS Generation
To measure ROS generation, a fluorometric assay was carried out using the cell membrane permeable dye 2′,7′-dichlorofluorescin diacetate (DCFH-DA). In the quantitative experiment of fluorescence intensity, cells cultured in 96-well plate () were treated with different concentrations of MeHg (1, 2.5, 5, and ) for and were then incubated with DCFH-DA in serum-free medium for at 37°C. After washing with PBS, the fluorescence intensity was measured with Cytation™ 5 (Biotek), with an excitation wavelength of and an emission wavelength of . In the imaging experiment, cells cultured in 24-well plates () were treated with MeHg (, ) or (, ). After washing with PBS, the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 15 min at room temperature. Then the cells were incubated with DCFH-DA in serum-free medium for at 37°C. Finally, the resulting fluorescence was detected with a fluorescence microscope (Zeiss Axio Observer). Cells treated with were used as the positive control. The intensity of fluorescence reflects the extent of oxidative stress.
Western Blotting Analysis
To prepare the whole protein lysate, cells were washed three times with cold PBS, lysed in lysis buffer (RIPA lysis buffer containing 1% PMSF, obtained from Beyotime), incubated on ice for 15 min, and collected after centrifugation at 4°C for 15 min. The protein concentration was determined by a BCA protein assay reagent kit (Beyotime). A total of of cell total protein was separated using 10% or 12% SDS-PAGE, then transferred to a PVDF membrane (Millipore). After being blocked with 5% skim milk in TBST ( Tris–HCl, NaCl, 0.1% Tween® 20, pH 7.4) for at room temperature, the membranes were incubated with specific primary antibodies overnight at 4°C (Table 1). On the second day, the membranes were washed three times with TBST buffer and further incubated with HRP-labeled secondary antibodies at room temperature for , and then washed three times in TBST. Finally, signal density of the immunoblots was performed using the MiniChemi Mini Size Chemiluminescent Imaging System (Beijing Sage Creation Science Co., Ltd.) and analyzed with Image J software (National Institutes of Health).
Table 1.
Protein antibodies used for Western blotting.
| Antibodies | Source | Catalog no. | Lot | Dilution |
|---|---|---|---|---|
| ImmunoWay | YT2133 | B3301 | 1:1,000 | |
| Cell Signaling Technology | D28F3 | 2 | 1:1,000 | |
| GLUT-1 | ImmunoWay | YT1928 | B2801 | 1:1,000 |
| EPO | ImmunoWay | YM0237 | B3701 | 1:1,000 |
| VEGF-A | ImmunoWay | YT5108 | B0801 | 1:1,000 |
| CMCTAG | AT0048 | 380436SB | 1:10,000 | |
| Mouse antirabbit IgG-HRP | Santa Cruz Biotechnology | sc-2357 | K0718 | 1:10,000 |
| BP-HRP | Santa Cruz Biotechnology | sc-516102 | E2318 | 1:10,000 |
Adenovirus-Mediated Overexpression
Adenovirus carrying the gene was obtained from Hanbio Biotechnology Co., Ltd. Astrocytes were seeded in 6-well plates at a 60% confluence, and cells were transfected with pHBAd-EF1-MCS-GFP according to later, cells were supplemented with fresh medium and continuously cultured for an additional to observe transfection efficiency by means of Western blotting.
siRNA Transfection
The small interfering RNAs (siRNAs) for were designed and purchased from Guangzhou Ribobio Co., Ltd. The sequences of the three siRNAs were as follows:
#1 (siB07514101244) 5′-TCGACAAGCTTAAGAAAGA dTdT-3′,
#2 (siB07514101312) 5′-GGACAATATAGAAGACATT dTdT-3′ and
#3 (siG081230140326) 5′-CTGATAACGTGAACAAATA dTdT-3′.
Astrocytes were plated in 6-well plates at 60% confluence, transfected with siRNA (), or a negative control (NC, a scrambled sequence) siRNA () with Lipofectamine®2000 in Opti-MEM medium (Invitrogen), according to the manufacturer’s protocol. The transfected cells were incubated at 37°C for an additional and validated the silencing efficacy via Western blotting analysis.
Total RNA Extraction and Quantitative Real-Time PCR (RT-PCR)
Total RNA was extracted from astrocytes using the TRIzol reagent (Invitrogen). Five hundred nanograms of total RNA were reverse-transcribed to cDNA using an RT reagent kit (TaKaRa), which was then amplified with SYBR green dye on a CFX96 Real-Time PCR Detection System (Bio-Rad). The relative quantification of mRNA levels was calculated using the standard relative quantification method.
The primers for rat and were designed and synthesized by Shanghai Generay Biotech Co., Ltd (Shanghai, China), and the sequences were as follows:
:
Forward primer: 5′-TCACAAATCAGCACCAAGCAC-3′
Reverse primer: 5′-AAGGGGAAAGAACAAAACACG-3′
:
Forward primer: 5′-CCTAGACTTCGAGCAAGAGA-3′
Reverse primer: 5′-GGAAGGAAGGCTGGAAGA-3′.
Animal Model
Male SD rats (8 wk old) weighing were obtained from the Laboratory Animal Center of Jiangsu University (Accreditation Number: SCXK [SU] 2018-0012). The rats were housed in a temperature- and humidity-controlled room ( and relative humidity) with a : light:dark cycle (dark phase from 1900 to 0700) and were maintained on standard laboratory chow with ad libitum access to water and food ( per cage). The rats were acclimatized for at least 7 d prior to MeHg exposure.
Rats were divided randomly into six groups ():
Group I: Normal saline was injected intraperitoneally for
Group II: MeHg [ body weight (BW)] was injected intraperitoneally for
Group III: MeHg ( BW) was injected intraperitoneally for
Group IV: MeHg ( BW) was injected intraperitoneally for
Group V: MeHg ( BW) was injected intraperitoneally for
Group VI: MeHg ( BW) was injected intraperitoneally for
At the end of the exposure period, the rats were anesthetized by intraperitoneal (IP) injection of pentobarbital to alleviate any potential discomfort and pain. Next, the brain cortices were rapidly frozen in liquid nitrogen and transferred to a freezer. All experimental procedures were conducted following a protocol approved by the Animal Care and Use Committee and Animal Ethics Committee of Jiangsu University (Accreditation Number: SYXK [SU] 2013-0036). All animals used in this study were treated humanely according to the institutional guidelines, with full consideration for the alleviation of distress and discomfort.
Statistical Analysis
All data are presented as the (SD). All assays were repeated at least three times with at least three independently derived astrocyte cultures. Statistical significance was assessed by analysis of variance (ANOVA) with correction for multiple comparisons in post hoc analysis. CHX treatment assay was analyzed by a two-way ANOVA with Dunnett’s post hoc test. Other multiple comparisons were analyzed by one-way ANOVA with Dunnett’s or Tukey’s post hoc test. All statistical analyses were performed using GraphPad Prism software. A p value of was considered to be statistically significant.
Results
Effects of Methylmercury on Astrocyte Proliferation, Cytotoxicity, and Production of ROS
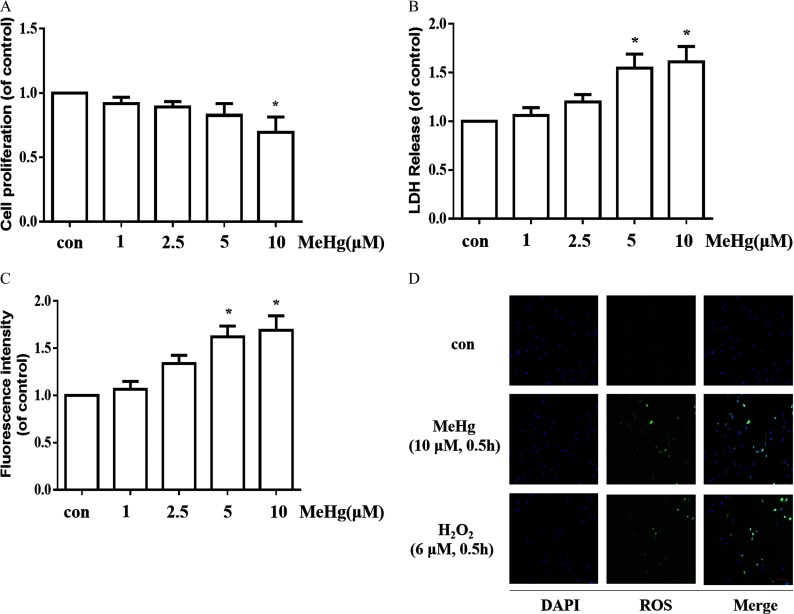
To investigate the effects of MeHg on cell proliferation and cytotoxicity in astrocytes, we incubated astrocytes with this organometal () for . The MTT assay and LDH leakage were subsequently assessed. As shown in Figure 1A, compared with control cells, those treated with MeHg exhibited lower cell proliferation, which appeared to be concentration-dependent. MeHg treatment significantly increased LDH release in astrocytes, especially at concentrations of 5 and (Figure 1B). Exposure to MeHg ( and , ) induced a ∼1.6-fold increase in astrocytic LDH release compared with controls (). Next, we analyzed the effects of MeHg on ROS production by 2′,7′-dichlorofluorescin diacetate (DCFH-DA) fluorescence. As is shown in Figure 1C, MeHg at 5 and caused a significant increase in ROS formation. Additionally, fluorescence images also showed that the fluorescence intensity of ROS was obviously enhanced in MeHg (, ) group (Figure 1D).
Figure 1.
Cell proliferation, cytotoxicity and ROS production after treatment with MeHg for in astrocytes. (A) Cell proliferation in astrocytes was measured using the MTT assay. (B) Cytotoxicity in astrocytes was detected via LDH release. (C) Effects of MeHg treatment on ROS production using the fluorescent probe DCFH-DA. The fluorescence intensity was detected by the microplate reader. (D) Representative images for ROS generation induced by MeHg (, ) treatment. . (, ) was set as a positive control for ROS generation. Green fluorescence indicates ROS and blue (DAPI) indicates the nucleus. Note: Data are presented as from three independent experiments (). con, control (culture medium treatment without MeHg); DAPI, 4′,6-diamidino-2-phenylindole; DCFH-DA, 2′,7′-dichlorofluorescin diacetate; , hydrogen peroxide; LDH, lactate dehydrogenase; MeHg, methylmercury; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl diphenyltetrazolium bromide; ROS, reactive oxygen species. * vs. control by one-way analysis of variance (ANOVA) with Dunnett’s post hoc test.
Effects of Methylmercury on the Expression of Proteins in Astrocytes
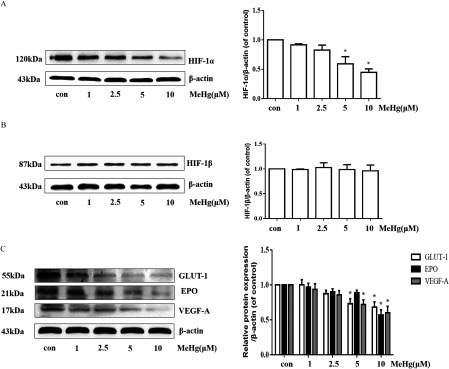
We incubated astrocytes in the absence or presence of MeHg () for and assessed and expression by Western blotting. MeHg-treated astrocytes exhibited a significant reduction in expression in a concentration-dependent manner (Figure 2A). In contrast, remained unaffected irrespective of the MeHg concentration (Figure 2B). Next, we analyzed the expression of targets (GLUT-1, EPO, and VEGF-A) in astrocytes treated with various concentrations of MeHg. A significant decrease in the expression of GLUT-1, EPO, and VEGF-A proteins was observed in astrocytes treated with MeHg for () (Figure 2C). These results are consistent with the ability of MeHg to decrease astrocytic levels, followed by a decrease in, GLUT-1, EPO, and VEGF-A expression.
Figure 2.
Effects of MeHg on the expression of proteins in astrocytes. (A) Western blotting for in astrocytes following MeHg (0, 1, 2.5, 5 or , ) exposure. (B) Western blotting for in astrocytes following MeHg (0, 1, 2.5, 5, or , ) exposure. (C) Effect of MeHg (0, 1, 2.5, 5, or , ) exposure on the expression of the downstream proteins of , including GLUT-1, EPO, and VEGF-A. was used as the internal control. Note: Data are presented as from three independent experiments (). con, control (culture medium treatment without MeHg); EPO, erythropoietin; GLUT-1, glucose transporter 1; , Hypoxia-inducible ; , Hypoxia-inducible ; MeHg, methylmercury; VEGF-A, vascular endothelial growth factor A. * vs. control by one-way ANOVA with Dunnett’s post hoc test.
Effects of Protein Synthesis, Proteasome, and PHD Inhibitors on Protein Expression of and Downstream Effectors in Methylmercury-Treated Astrocytes
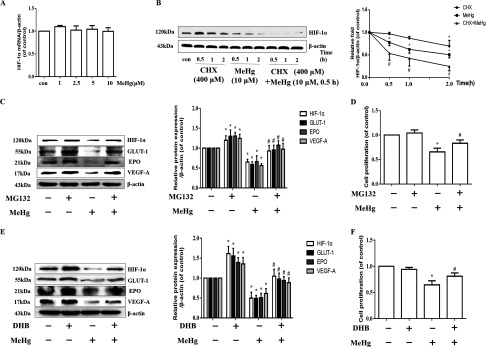
To determine the means by which MeHg regulated expression, we analyzed the expression of the gene with RT-PCR. As shown in Figure 3A, MeHg pretreatment did not affect mRNA levels. Therefore, we further explored the potential causes of the significant reduction in levels caused by MeHg in astrocytes. CHX is a broad-spectrum and nonspecific protein synthesis inhibitor, and astrocytes were harvested after CHX pretreatment () for 0.5, 1, and with and without MeHg treatment (, ). In addition, MeHg treatment alone group ( for 0.5, 1, and ) was also established. Then, protein expression was analyzed (Figure 3B). In comparison with the control group, the protein level of in CHX alone group decreased in a time-dependent manner. Notably, when compared with the corresponding time point of CHX treatment alone group, the level of protein was found to be significantly lower in the group (, Figure 3B). We pretreated astrocytes with or without MG132 (, ), a proteasome inhibitor, followed by MeHg, and incubated the cells for an additional . The cells were harvested at the end of the treatment, and the levels of and its targets were measured via Western blotting. As shown in Figure 3C, MG132 treatment inhibited the significant MeHg-induced reduction in and its target proteins in astrocytes. Previous research has suggested that degradation is attributable to the activation of proline hydroxylase (PHD) (Marxsen et al. 2004). To define the potential contribution of PHD-mediated inhibition of in response to MeHg treatment, we treated astrocytes with DHB [a small-molecule prolyl hydroxylase (PHD) inhibitor] (Siddiq et al. 2005) for and exposed astrocytes to MeHg for . Notably, compared with cells treated with MeHg alone, astrocytic expression in cells treated with MeHg and either the proteasome inhibitor (Figure 3C) or the PHD inhibitor (Figure 3E) was significantly higher. In addition, both the proteasome inhibitor and PHD inhibitor restored MeHg-inhibited cell proliferation (Figure 3D and 3F).
Figure 3.
Effects of protein expression of and its downstream effectors and effects on cell proliferation induced by CHX, MG132, or DHB in MeHg-treated astrocytes. (A) Astrocytes were treated with MeHg (0, 1, 2.5, 5, or , ) and mRNA was evaluated. mRNA was normalized to and plotted relative to the control. * vs. the control by one-way ANOVA with Dunnett’s post hoc test. (B) Cells were pretreated with CHX for 0.5, 1 and and then exposed to MeHg for . MeHg treatment alone group ( for 0.5, 1 and ) was also established. protein levels were analyzed by western blotting. was used to normalize the protein level and the intensities were presented as fold changes relative to the control. * vs. the control by one-way ANOVA with Dunnett’s post hoc test. # the group vs. the corresponding time point of CHX alone group by two-way ANOVA with Dunnett’s post hoc test. (C) Cells were pretreated with MG132 (, ) and then treated with MeHg for . Western blotting was used to evaluate protein levels of , GLUT-1, EPO, and VEGF-A. (D) Effect of MG132 (, ) on the cell proliferation was evaluated using an MTT assay. (E) Effect of DHB pretreatment (, ) on the expression of , GLUT-1, EPO, and VEGF-A was detected by western blotting. (F) Effect of DHB pretreatment (, ) on the cell proliferation was determined by an MTT assay. For Western blotting analyses, was used to normalize the protein level. Note: Statistical analysis was performed by one-way ANOVA with Tukey’s post hoc test. Data are presented as from three independent experiments (). CHX, cycloheximide; con, control (culture medium treatment without MeHg); DHB, 3,4-Dihydroxybenzoic acid; EPO, erythropoietin; GLUT-1, glucose transporter 1; , Hypoxia-inducible ; MeHg, methylmercury; MG132, proteasome inhibitor; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl diphenyltetrazolium bromide; VEGF-A, vascular endothelial growth factor A. * vs. control, # vs. MeHg only-group.
Effects of Pharmacologic and Genetic Manipulation of on Cell Proliferation
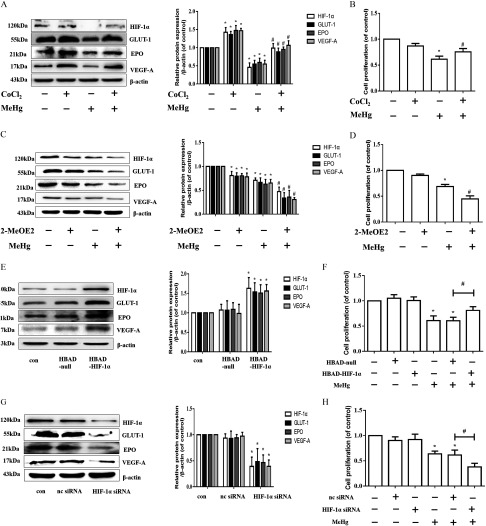
We hypothesized that decreased protein contributes to astrocytic cytotoxicity. To further confirm this hypothesis, we treated astrocytes with (, ) or 2-MeOE2 (, ) in the absence or presence of MeHg ( exposure). We used , an inducer of expression (Triantafyllou et al. 2006), and 2-MeOE2, a inhibitor (Chen et al. 2008; Parada-Bustamante et al. 2015; Pribluda et al. 2000), to regulate expression in MeHg-treated astrocytes (Figure 4A and 4C). Analogous to the data shown in Figure 3D and 3F, overexpression of protein mitigated the MeHg-reduced cell proliferation in astrocytes () (Figure 4B), whereas down-regulation of protein significantly aggravated the decline in astrocytic proliferation caused by MeHg () (Figure 4D). To confirm this observation, we further used siRNA and adenovirus overexpression, which target the gene (Figure 4E to 4H). Consistent with the results of and 2-MeOE2 treatment, overexpression of significantly attenuated the decline in MeHg-induced cell proliferation (Figure 4F), whereas inhibition of significantly increased the decline in cell proliferation (Figure 4H; ).
Figure 4.
Effects of pharmacologic and genetic manipulation of on cell proliferation. (A) Astrocytes were pretreated with (, ) and then treated with MeHg for . Western blotting was used to evaluate protein levels of , GLUT-1, EPO, and VEGF-A. (B) Effect of pretreatment (, ) on the cell proliferation was evaluated using an MTT assay. (C) Effect of 2-MeOE2 pretreatment (, ) on the expression of , GLUT-1, EPO, and VEGF-A was detected by Western blotting. (D) Effect of 2-MeOE2 pretreatment (, ) on the cell proliferation was evaluated using an MTT assay. For Western blotting analyses, representative blots are shown, and the intensities are presented as fold changes relative to the control group ( as the internal control). * vs. control, # vs. MeHg-only group. (E) The protein expression levels of , GLUT-1, EPO, and VEGF-A after overexpression of . * vs. HBAD-null group. (F) Effect of adenovirus-induced overexpression on the decrease in MeHg-induced cell proliferation as determined by an MTT assay. * vs. control group, # vs. group. (G) Effect of siRNA on the protein expression of , GLUT-1, EPO, and VEGF-A. (H) Effect of siRNA on the MeHg-induced decrease in cell proliferation. Note: Statistical analysis was performed by one-way ANOVA with Tukey’s post hoc test. Data are presented as the from three independent experiments (). , cobalt chloride; con, control (culture medium treatment without MeHg); EPO, erythropoietin; GLUT-1, glucose transporter 1; HBAD, pHBAd-EF1-MCS-GFP; , Hypoxia-inducible ; 2-MeOE2, 2-methoxyestradiol; MeHg, methylmercury; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl diphenyltetrazolium bromide; nc siRNA, negative control (scrambled sequence) siRNA; siRNA, small interfering RNA; VEGF-A, vascular endothelial growth factor A. * vs. nc siRNA group. * vs. control group, # vs. group.
Protein Expression and Cell Proliferation after Pretreatment with Antioxidants in Methylmercury-Treated Astrocytes
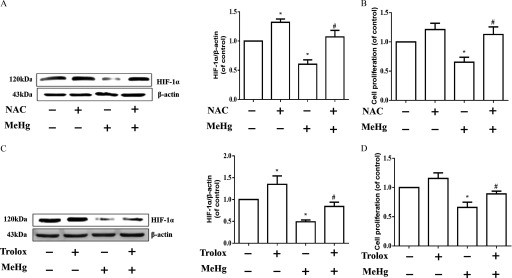
We investigated whether ROS production were associated with decreased expression in MeHg-exposed astrocytes. Astrocytes were pretreated with NAC (, ) and Trolox (, ) followed by MeHg for . NAC and Trolox are antioxidants that are commonly used to mitigate ROS (Aremu et al. 2008; Bai et al. 2015; Kaur et al. 2010). As shown in Figures 5A and 5C, cells pretreated with either NAC or Trolox and then treated with MeHg exhibited higher protein levels, similar to those of control cells, than those treated with MeHg alone. Pretreated cells exhibited higher levels (as much as ) of cell proliferation than those treated with MeHg alone () (Figure 5B and 5D).
Figure 5.
protein expression and cell proliferation after pre-treatment with antioxidants in MeHg-treated astrocytes. (A) Effect of NAC pretreatment (, ) on protein expression. (B) Effect of NAC pretreatment on the decreased cell proliferation induced by MeHg. (C) Effect of Trolox pretreatment (, ) on protein expression. (D) Effect of Trolox pretreatment on the MeHg-induced decrease in cell proliferation. Note: Statistical analysis was performed by one-way ANOVA with Tukey’s post hoc test. Data are presented as the from three independent experiments (). con, control (culture medium treatment without MeHg); , Hypoxia-inducible ; MeHg, methylmercury; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl diphenyltetrazolium bromide; NAC, N-acetyl-cysteine, Trolox, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. * vs. control, # vs. MeHg-only group.
Protein Expression of and Downstream Effectors following MeHg Administration in in Vivo Rat Models
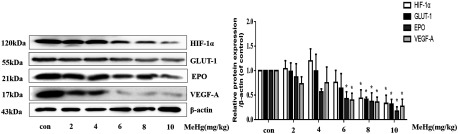
Finally, to evaluate the in vivo effects of MeHg on protein expression, we established a SD rat model. Animals were administered with MeHg (0, 2, 4, 6, 8, and , each group with 6 rats) by intraperitoneal injection and sacrificed later. The expression of and its target proteins in brain tissues was measured by Western blotting. As shown in Figure 6, MeHg treatment significantly decreased the expression of and its targets, especially in rats exposed to and MeHg ().
Figure 6.
Protein expression of and downstream effectors following MeHg administration in in vivo rat models. Adult Sprague-Dawley rats were administered with MeHg (0, 2, 4, 6, 8, ) by intraperitoneal injection for . Whole brain lysates were analyzed by Western blotting for the indicated proteins. Note: Data are presented as (). con, control (intraperitoneal injection of saline); EPO, erythropoietin; GLUT-1, glucose transporter 1; , Hypoxia-inducible ; MeHg, methylmercury; VEGF-A, vascular endothelial growth factor A. * vs. control group by one-way ANOVA with Dunnett’s post hoc test.
Discussion
In the present study, we report that, compared with the vehicle control, treatment with 5 or MeHg for resulted in lower cell proliferation and higher ROS generation in primary rat astrocytes, and these results were associated with lower expression of . To define the mechanism(s) of the MeHg-induced alteration in expression, first we detected the mRNA level of after MeHg treatment. The results showed that MeHg exposure resulted in a significant reduction in levels without affecting transcription. Then, we speculated that whether MeHg affected the degradation of protein. The results showed that treating cells with results in lower protein expression of than does treating cells with CHX alone in three different time points. Because CHX blocks protein synthesis, it would stand to reason that the effect of MeHg in these cells is associated with protein degradation. Additionally, we also demonstrated that the proteasome inhibitor (MG132) and the PHD inhibitor (DHB), both of which block protein degradation of , partially restored the inhibitory effect of MeHg on protein, further suggesting that the MeHg-induced decrease in protein is due to the promoted degradation. Furthermore, taking advantage of genetic and pharmacological methods to alter inherent levels of the protein, we provide evidence directly linking MeHg toxicity with inhibition. It is noteworthy that lower protein expression was related to the production of ROS, as demonstrated by NAC and Trolox. Taken together, our novel observations suggest that ROS act as an important regulator of inhibition in MeHg-treated astrocytes and that overexpression of may afford a novel neuroprotective effect in the treatment of MeHg-induced neurotoxicity. Moreover, we also found that MeHg inhibited the expression of in the SD rat brain, which better revealed the relationship between MeHg and the signaling pathway found in the in vitro and in vivo experiments.
MeHg is an established neurotoxicant (Aschner and Syversen 2005; Clarkson and Magos 2006; Culbreth and Aschner, 2016; Farina et al. 2011a, 2011b), whose mechanism(s) of toxicity have yet to be fully understood; however, oxidative stress seems to play a role (Aschner et al. 2007; Santos et al. 2018; Ishihara et al. 2016). Oxidative stress is one of the known mechanisms for the induction of . Previous studies have consistently shown that metals, such as lead (Das et al. 2015), aluminum (Mailloux and Appanna 2007), manganese (Han et al. 2009), and zinc (Pan et al. 2013; Park et al. 2018), as well as other xenobiotics, such as isoflurane (Cao et al. 2018; Sun et al. 2013), can increase expression. In contrast, concentration-dependent inhibition of in Hep3B cells has been reported in response to cadmium and inorganic mercury (Hg) treatment (Horiguchi et al. 2000).
We speculated that two factors contribute to the decreased level of protein in response to MeHg treatment. First, MeHg-induced mitochondrial damage might cause a reduction in oxygen consumption and increase relative oxygen content, leading to redistribution of oxygen and binding to PHD and an increase in PHD activity (Wu et al. 2010), whereas other metals, including inorganic mercury, mimicked a hypoxic state and induced pseudohypoxia to promote the translocation of (Mailloux and Appanna 2007). MeHg did not change the level of in brain microvascular pericytes (Hirooka et al. 2013). Second, MeHg might increase 2-oxoglutarate (2-OG) levels, a cofactor of PHD. 2-OG has been shown to increase PHD activity, resulting in increased degradation of and a net decrease in protein (Dehne et al. 2010; Mailloux et al. 2009; Matsumoto et al. 2006). As shown in Figure 3, MeHg appeared to have activated PHD and UPS, thus promoting degradation.
regulates the expression of a large number of genes that are involved in glucose metabolism, energy production, angiogenesis, migration, and other cellular processes (Liu et al. 2012; Semenza 2002). Thus, reduced availability of and the subsequent reduction in expression of its target genes may cause cellular dysfunction. VEGF-A, GLUT-1, and EPO are typical downstream target proteins of (Ashok et al. 2017; Cramer et al. 2003; Mense et al. 2006; Semenza 2002). VEGF-induced angiogenesis has previously been shown to improve the blood supply to neurons and slow the clinical deterioration in Alzheimer’s disease (Bogaert et al. 2006). VEGF and EPO have been shown to improve tissue oxygen content by promoting erythropoiesis and angiogenesis, which may stimulate genes involved in glucose transport and metabolism (Ashok et al. 2017; Semenza 2002). It is noteworthy that complexes can regulate the biological functions of various genes, such as those involved in vascular growth, oxygen transport, and energy metabolism (Czibik 2010). Thus, we suggest that the mechanism of MeHg neurotoxicity involves, at least in part, a reduction in and its downstream proteins (GLUT-1, EPO, and VEGF-A), leading to secondary alterations in blood flow, tissue oxygenation, and ATP production. In addition, studies on the regulation of PHD have generated interest as investigational drugs for anemia and neurodegenerative diseases. The use of a small molecule of PHD inhibitor (FG-4592), has been promoted to clinical trials for treating anemia by increasing erythropoiesis through mediated transcription (Besarab et al. 2016; Chen et al. 2017; Haase 2017), as well as for treating Parkinson’s disease by positive regulation of redox biology and mitochondrial function (Li et al. 2018). In the present study, the suppression of MeHg toxicity by both and adenovirus-targeted overexpression suggested that activation of the pathway can reverse inhibition of cell proliferation by MeHg (Figure 4). Thus, we suggest that can be pursued as a novel target for MeHg poisoning.
MeHg-induced toxicity is mediated, at least in part, by oxidative stress (Polunas et al. 2011; Yee and Choi 1996). In addition, rapid degradation of may result from ROS generation (Beattie et al. 2005; Cairo et al. 1996; Henkel and Krebs 2004; Jaeckel et al. 2005; Li et al. 2006; Sato and Kondoh 2002). We initially considered that ROS generation could play a role in the altered protein expression to mediate MeHg toxicity. Treatment of rat astrocytes with oxygen radical scavengers (NAC and Trolox) resulted in higher protein expression and increased cell proliferation (Figure 5), suggesting that detoxification of ROS by is associated with its neuroprotective effects. Reductions in expression in MeHg-treated astrocytes and rats suggest that the up-regulation of affords neuroprotective effects. Taking into account the limitations of the pharmacological and genetic regulations of clinical toxicity and the ability of antioxidants to promote expression, the use of antioxidants should be considered for clinical detoxification. However, MeHg can affect many cellular and molecular functions if the MeHg exposure concentrations and duration are sufficient. Thus, additional research on the toxicological significance of suppression by acute exposure to MeHg is warranted, in addition to a more precise delineation of its protective mechanisms.
In conclusion, we demonstrated that MeHg led to astrocytic neurotoxicity in vitro by reducing levels of and its downstream proteins, which may result from increasing PHD activity, which in turn promoted degradation. Furthermore, the up-regulation of imparted protection against MeHg neurotoxicity. Taken together, these findings—from both in vivo and in vitro experiments—suggest that may be as of yet an unrecognized and important mediator or regulator of MeHg-induced neurotoxicity, and this means of increasing activation in astrocytes may afford a therapeutic strategy for mitigating MeHg poisoning.
Acknowledgments
The authors are grateful to P. Spencer at Oregon Health & Science University, M. Ghert at McMaster University, and J. Cai at University of Oklahoma Health Science Center for their critical comments. This work was supported in part by the Natural Science Foundation of China (Nos. 30872139, 81273124, 31100964) and R01 ES07331, R01 ES10563, and ES020852 from the National Institute of Environmental Health Sciences (NIEHS).
Footnotes
These authors contributed equally to this work as first authors.
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Abbott NJ. 2002. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat 200(6):629–638, PMID: 12162730, 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JW, El-Oqayli H, Aschner M, Syversen T, Sonnewald U. 2001. Methylmercury has a selective effect on mitochondria in cultured astrocytes in the presence of [U-13C] glutamate. Brain Res 908(2):149–154, PMID: 11454325, 10.1016/S0006-8993(01)02628-2. [DOI] [PubMed] [Google Scholar]
- Aremu DA, Madejczyk MS, Ballatori N. 2008. N-acetylcysteine as a potential antidote and biomonitoring agent of methylmercury exposure. Environ Health Perspect 116(1):26–31, PMID: 18197295, 10.1289/ehp.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M. 1998a. Immune and inflammatory responses in the CNS: modulation by astrocytes. Toxicol Lett 102-103(3):283–287, PMID: 10022267, 10.1016/s0378-4274(98)00324-5. [DOI] [PubMed] [Google Scholar]
- Aschner M. 1998b. Astrocytes as mediators of immune and inflammatory responses in the CNS. Neurotoxicology 19(2):269–281, PMID: 9553964, 10.1016/s1383-5742(97)00039-2. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T. 2005. Methylmercury: recent advances in the understanding of its neurotoxicity. Ther Drug Monit 27(3):278–283, PMID: 15905795, 10.1097/01.ftd.0000160275.85450.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. 2007. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res 40(3):285–291, PMID: 17334523, 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- Aschner M, Yao CP, Allen JW, Tan KH. 2000. Methylmercury alters glutamate transport in astrocytes. Neurochem Int 37(2–3):199–206, PMID: 10812205, 10.1016/S0197-0186(00)00023-1. [DOI] [PubMed] [Google Scholar]
- Ashok BS, Ajith TA, Sivanesan S. 2017. Hypoxia-inducible factors as neuroprotective agent in Alzheimer’s disease. Clin Exp Pharmacol Physiol 44(3):327–334, PMID: 28004401, 10.1111/1440-1681.12717. [DOI] [PubMed] [Google Scholar]
- Bai Y, Yin C, Zhao W, Li B, Qian H, Ma J, et al. . 2015. Differential protection of pre- versus post-treatment with curcumin, Trolox, and N-acetylcysteine against acrylonitrile-induced cytotoxicity in primary rat astrocytes. Neurotoxicology 51:58–66, PMID: 26409646, 10.1016/j.neuro.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Bai Y, Zhao W, Yin C, Fang Y, Ma J, Li B, et al. . 2016. Preconditioning of endoplasmic reticulum stress protects against acrylonitrile-induced cytotoxicity in primary rat astrocytes: the role of autophagy. Neurotoxicology 55:112–121, PMID: 27260289, 10.1016/j.neuro.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, et al. . 2006. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev 15(3):407–421, PMID: 16846377, 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie JH, Owen HL, Wallace SM, Arthur JR, Kwun IS, Hawksworth GM, et al. . 2005. Metallothionein overexpression and resistance to toxic stress. Toxicol Lett 157(1):69–78, PMID: 15795095, 10.1016/j.toxlet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Belletti S, Orlandini G, Vettori MV, Mutti A, Uggeri J, Scandroglio R, et al. . 2002. Time course assessment of methylmercury effects on C6 glioma cells: submicromolar concentrations induce oxidative DNA damage and apoptosis. J Neurosci Res 70(5):703–711, PMID: 12424738, 10.1002/jnr.10419. [DOI] [PubMed] [Google Scholar]
- Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J. 2003. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1α in normoxia. EMBO J 22(16):4082–4090, PMID: 12912907, 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besarab A, Chernyavskaya E, Motylev I, Shutov E, Kumbar LM, Gurevich K, et al. . 2016. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol 27(4):1225–1233, PMID: 26494833, 10.1681/ASN.2015030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert E, Damme PV, Bosch LVD, Robberecht W. 2006. Vascular endothelial growth factor in amyotrophic lateral sclerosis and other neurodegenerative diseases. Muscle Nerve 34(4):391–405, PMID: 16856151, 10.1002/mus.20609. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. 2001. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294(5545):1337–1340, PMID: 11598268, 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Cairo G, Castrusini E, Minotti G, Bernelli-Zazzera A. 1996. Superoxide and hydrogen peroxide-dependent inhibition of iron regulatory protein activity: a protective stratagem against oxidative injury. FASEB J 10(11):1326–1335, PMID: 8836047, 10.1096/fasebj.10.11.8836047. [DOI] [PubMed] [Google Scholar]
- Canuel R, de Grosbois SB, Atikessé L, Lucotte M, Arp P, Ritchie C, et al. . 2006. New evidence on variations of human body burden of methylmercury from fish consumption. Environ Health Perspect 114(2):302–306, PMID: 16451872, 10.1289/ehp.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Li Z, Ma L, Ni C, Li L, Yang N, et al. . 2018. Isoflurane-induced postoperative cognitive dysfunction is mediated by hypoxia-inducible factor-1α-dependent neuroinflammation in aged rats. Mol Med Rep 17(6):7730–7736, PMID: 29620198, 10.3892/mmr.2018.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carocci A, Rovito N, Sinicropi S, Genchi G. 2014. Mercury toxicity and neurodegenerative effects. In: Reviews of Environmental Contamination and Toxicology. Reviews of Environmental Contamination and Toxicology (Continuation of Residue Reviews), vol 229. Whitacre D, eds. Cham, Switzerland: Springer, 1–18, PMID: 24515807, 10.1007/978-3-319-03777-6_1. [DOI] [PubMed] [Google Scholar]
- Carrasco L, Benejam L, Benito J, Bayona JM, Díez S. 2011. Methylmercury levels and bioaccumulation in the aquatic food web of a highly mercury-contaminated reservoir. Environ Int 37(7):1213–1218, PMID: 21658770, 10.1016/j.envint.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Daré E, Moors M. 2010. Methylmercury-induced neurotoxicity and apoptosis. Chem Biol Interact 188(2):301–308, PMID: 20399200, 10.1016/j.cbi.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Chen W, Jadhav V, Tang J, Zhang JH. 2008. HIF-1 alpha inhibition ameliorates neonatal brain damage after hypoxic-ischemic injury. Acta Neurochir Suppl 102:395–399, PMID: 19388354, 10.1007/978-3-211-85578-2_77. [DOI] [PubMed] [Google Scholar]
- Chen N, Qian J, Chen J, Yu X, Mei C, Hao C, et al. . 2017. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant 32(8):1373–1386, PMID: 28371815, 10.1093/ndt/gfx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SM, Choi KO, Park YK, Cho H, Yang EG, Park H. 2006. Clioquinol, a Cu (II)/Zn (II) chelator, inhibits both ubiquitination and asparagine hydroxylation of hypoxia-inducible factor-1alpha, leading to expression of vascular endothelial growth factor and erythropoietin in normoxic cells. J Biol Chem 281(45):34056–34063, PMID: 16973622, 10.1074/jbc.M603913200. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. 2006. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36(8):609–662, PMID: 16973445, 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, et al. . 2003. HIF-1 alpha is essential for myeloid cell-mediated inflammation. Cell 112(5):645–657, PMID: 12628185, 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbreth M, Aschner M. 2016. Dysregulation of glutamate cycling mediates methylmercury-induced neurotoxicity. Adv Neurobiol 13:295–305, PMID: 27885634, 10.1007/978-3-319-45096-4_11. [DOI] [PubMed] [Google Scholar]
- Czibik G. 2010. Complex role of the HIF system in cardiovascular biology. J Mol Med 88(11):1101–1111, PMID: 20574807, 10.1007/s00109-010-0646-x. [DOI] [PubMed] [Google Scholar]
- Das KK, Jargar JG, Saha S, Yendigeri SM, Singh SB. 2015. α-tocopherol supplementation prevents lead acetate and hypoxia-induced hepatic dysfunction. Indian J Pharmacol 47(3):285–291, PMID: 26069366, 10.4103/0253-7613.157126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehne N, Hintereder G, Brüne B. 2010. High glucose concentrations attenuate hypoxia-inducible factor-1α expression and signaling in non-tumor cells. Exp Cell Res 316(7):1179–1189, PMID: 20184881, 10.1016/j.yexcr.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Deng Y, Xu Z, Xu B, Liu W, Wei Y, Li Y, et al. . 2014. Exploring cross-talk between oxidative damage and excitotoxicity and the effects of riluzole in the rat cortex after exposure to methylmercury. Neurotox Res 26(1):40–51, PMID: 24519665, 10.1007/s12640-013-9448-6. [DOI] [PubMed] [Google Scholar]
- Dong SY, Guo YJ, Feng Y, Cui XX, Kuo SH, Liu T, et al. . 2016. The epigenetic regulation of HIF-1α by SIRT1 in MPP(+) treated SH-SY5Y cells. Biochem Biophys Res Commun 470(2):453–459, PMID: 26768367, 10.1016/j.bbrc.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang YT, Guo CJ, Zhang PP, Zhao W, Xing G, Wang S, et al. . 2016. Role of autophagy in methylmercury-induced neurotoxicity in rat primary astrocytes. Arch Toxicol 90(2):333–345, PMID: 25488884, 10.1007/s00204-014-1425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Aschner M. 2017. Methylmercury-induced neurotoxicity: focus on pro-oxidative events and related consequences. Adv Neurobiol 18:267–286, PMID: 28889272, 10.1007/978-3-319-60189-2_13. [DOI] [PubMed] [Google Scholar]
- Farina M, Aschner M, Rocha JB. 2011a. Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol 256(3):405–417, PMID: 21601588, 10.1016/j.taap.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Rocha JB, Aschner M. 2011b. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci 89(15–16):555–563, PMID: 21683713, 10.1016/j.lfs.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Liu T, Li XQ, Liu Y, Zhu XY, Jankovic J, et al. . 2014. Neuroprotection by Orexin-A via HIF-1α induction in a cellular model of Parkinson’s disease. Neurosci Lett 579:35–40, PMID: 25038418, 10.1016/j.neulet.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Guo C, Hao LJ, Yang ZH, Chai R, Zhang S, Gu Y, et al. . 2016. Deferoxamine-mediated up-regulation of HIF-1α prevents dopaminergic neuronal death via the activation of MAPK family proteins in MPTP-treated mice. Exp Neurol 280:13–23, PMID: 26996132, 10.1016/j.expneurol.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Haase VH. 2017. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial Int 21:S110–S124, PMID: 28449418, 10.1111/hdi.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick SE, McQuillen PS, Jiang X, Mu D, Madan A, Ferriero DM. 2005. A role for hypoxia-inducible factor-1alpha in desferoxamine neuroprotection. Neurosci Lett 379(2):96–100, PMID: 15823423, 10.1016/j.neulet.2004.12.080. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JS, Choi D, Lee Y, Hong S, Choi J, et al. . 2009. Manganese (II) induces chemical hypoxia by inhibiting HIF-prolyl hydroxylase: implication in manganese-induced pulmonary inflammation. Toxicol Appl Pharmacol 235(3):261–267, PMID: 19263519, 10.1016/j.taap.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Henkel G, Krebs B. 2004. Metallothioneins: zinc, cadmium, mercury, and copper thiolates and selenolates mimicking protein active site features-structural aspects and biological implications. Chem Rev 104(2):801–824, PMID: 14871142, 10.1021/cr020620d. [DOI] [PubMed] [Google Scholar]
- Hirooka T, Yamamoto C, Yasutake A, Eto K, Kaji T. 2013. Expression of VEGF-related proteins in cultured human brain microvascular endothelial cells and pericytes after exposure to methylmercury. J Toxicol Sci 38(6):837–845, PMID: 24213003, 10.2131/jts.38.837. [DOI] [PubMed] [Google Scholar]
- Horiguchi H, Kayama F, Oguma E, Willmore WG, Hradecky P, Bunn HF. 2000. Cadmium and platinum suppression of erythropoietin production in cell culture: clinical implications. Blood 96(12):3743–3747, PMID: 11090055, 10.1182/blood.V96.12.3743.h8003743_3743_3747. [DOI] [PubMed] [Google Scholar]
- Ishihara Y, Tsuji M, Kawamoto T, Yamazaki T. 2016. Involvement of reactive oxygen species derived from mitochondrial in neuronal injury elicited by methylmercury. J Clin Biochem Nutr 59(3):182–190, PMID: 27895385, 10.3164/jcbn.16-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. . 2001. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292(5516):464–468, PMID: 11292862, 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Iyalomhe O, Swierczek S, Enwerem N, Chen Y, Adedeji MO, Allard J, et al. . 2017. The role of hypoxia-inducible factor 1 in mild cognitive impairment. Cell Mol Neurobiol 37(6):969–977, PMID: 27858285, 10.1007/s10571-016-0440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeckel P, Krauss G, Menge S, Schierhorn A, Rücknagel P, Krauss GJ. 2005. Cadmium induces a novel metallothionein and phytochelatin 2 in an aquatic fungus. Biochem Biophys Res Commun 333(1):150–155, PMID: 15939401, 10.1016/j.bbrc.2005.05.083. [DOI] [PubMed] [Google Scholar]
- Jain IH, Zazzeron L, Goli R, Alexa K, Schatzman-Bone S, Dhillon H, et al. . 2016. Hypoxia as a therapy for mitochondrial disease. Science 352(6281):54–61, PMID: 26917594, 10.1126/science.aad9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CJ, Massie A, De Keyser J. 2013. Immune players in the CNS: the astrocyte. J Neuroimmune Pharmacol 8(4):824–839, PMID: 23821340, 10.1007/s11481-013-9480-6. [DOI] [PubMed] [Google Scholar]
- Jing Y, Liu LZ, Jiang Y, Zhu Y, Guo NL, Barnett J, et al. . 2012. Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells. Toxicol Sci 125(1):10–19, PMID: 21984483, 10.1093/toxsci/kfr256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Evje L, Aschner M, Syversen T. 2010. The in vitro effects of Trolox on methymercury-induced neurotoxicity. Toxicology 276 (1):73–78, PMID: 20637824, 10.1016/j.tox.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Koivunen P, Hirsilä M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J. 2007. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem 282(7):4524–4532, PMID: 17182618, 10.1074/jbc.M610415200. [DOI] [PubMed] [Google Scholar]
- LeBel CP, Ali SF, Bondy SC. 1992. Deferoxamine inhibits methyl mercury-induced increases in reactive oxygen species formation in rat brain. Toxicol Appl Pharmacol 112(1):161–165, PMID: 1310167, 10.1016/0041-008X(92)90292-Z. [DOI] [PubMed] [Google Scholar]
- Lee DW, Rajagopalan S, Siddiq A, Gwiazda R, Yang L, Beal MF, et al. . 2009. Inhibition of prolyl hydroxylase protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity: model for the potential involvement of the hypoxia-inducible factor pathway in Parkinson disease. J Biol Chem 284(42):29065–29076, PMID: 19679656, 10.1074/jbc.M109.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA, Roth RA, LaPres JJ. 2007. Hypoxia, drug therapy and toxicity. Pharmacol Ther 113(2):229–246, PMID: 17046066, 10.1016/j.pharmthera.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Li Q, Chen H, Huang X, Costa M. 2006. Effects of 12 metal ions on iron regulatory protein 1 (IRP-1) and hypoxia-inducible factor-1 alpha (HIF-1α) and HIF-regulated genes. Toxicol Appl Pharmacol 213(3):245–255, PMID: 16386771, 10.1016/j.taap.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Cui XX, Chen YJ, Wu TT, Xu H, Yin H, et al. . 2018. Therapeutic potential of a prolyl hydroxylase inhibitor FG-4592 for Parkinson’s diseases in vitro and in vivo: regulation of redox biology and mitochondrial function. Front Aging Neurosci 10:121, PMID: 29755339, 10.3389/fnagi.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao TL, Chen SC, Tzeng CR, Kao SH. 2014. TCDD induces the hypoxia-inducible factor (HIF)-1α regulatory pathway in human trophoblastic JAR cells. Int J Mol Sci 15(10):17733–17750, PMID: 25272228, 10.3390/ijms151017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Shen SM, Zhao XY, Chen GQ. 2012. Targeted genes and interacting proteins of hypoxia inducible factor-1. Int J Biochem Mo Biol 3(2):165–178, PMID: 22773957. [PMC free article] [PubMed] [Google Scholar]
- Liu W, Xu Z, Deng Y, Xu B, Wei Y, Yang T. 2013. Protective effects of memantine against methylmercury-induced glutamate dyshomeostasis and oxidative stress in rat cerebral cortex. Neurotox Res 24(3):320–337, PMID: 23504438, 10.1007/s12640-013-9386-3. [DOI] [PubMed] [Google Scholar]
- Liu W, Xu Z, Deng Y, Xu B, Yang H, Wei Y, et al. . 2014. Excitotoxicity and oxidative damages induced by methylmercury in rat cerebral cortex and the protective effects of tea polyphenols. Environ Toxicol 29(3):269–283, PMID: 22223486, 10.1002/tox.21755. [DOI] [PubMed] [Google Scholar]
- Mailloux RJ, Appanna VD. 2007. Aluminum toxicity triggers the nuclear translocation of HIF-1α and promotes anaerobiosis in hepatocytes. Toxicol In Vitro 21(1):16–24, PMID: 16979867, 10.1016/j.tiv.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Mailloux RJ, Puiseux-Dao S, Appanna VD. 2009. Alpha-ketoglutarate abrogates the nuclear localization of HIF-1α in aluminum-exposed hepatocytes. Biochimie 91(3):408–415, PMID: 19028544, 10.1016/j.biochi.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Marxsen JH, Stengel P, Doege K, Heikkinen P, Jokilehto T, Wagner T, et al. . 2004. Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases. Biochem J 381(Pt 3):761–767, PMID: 15104534, 10.1042/BJ20040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Imagawa S, Obara N, Suzuki N, Takahashi S, Nagasawa T, et al. . 2006. 2-Oxoglutarate downregulates expression of vascular endothelial growth factor and erythropoietin through decreasing hypoxia-inducible factor-1alpha and inhibits angiogenesis. J Cell Physiol 209(2):333–340, PMID: 16883594, 10.1002/jcp.20733. [DOI] [PubMed] [Google Scholar]
- Mense SM, Sengupta A, Zhou M, Lan C, Bentsman G, Volsky DJ, et al. . 2006. Gene expression profiling reveals the profound upregulation of hypoxia-responsive genes in primary human astrocytes. Physiol Genomics 25(3):435–449, PMID: 16507782, 10.1152/physiolgenomics.00315.2005. [DOI] [PubMed] [Google Scholar]
- Pan R, Chen C, Liu WL, Liu KJ. 2013. Zinc promotes the death of hypoxic astrocytes by upregulating hypoxia-induced hypoxia-inducible factor-1alpha expression via poly (ADP-ribose) polymerase-1. CNS Neurosci Ther 19(7):511–520, PMID: 23582235, 10.1111/cns.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Mansfield KD, Bertozzi CC, Rudenko V, Chan DA, Giaccia AJ, et al. . 2007. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol Cell Biol 27(3):912–925, PMID: 17101781, 10.1128/MCB.01223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada-Bustamante A, Valencia C, Reuquen P, Diaz P, Rincion-Rodriguez R, Orihuela PA. 2015. Role of 2-methoxyestradiol, an endogenous estrogen metabolite, in health and disease. Mini Rev Med Chem 15(5):427–438, PMID: 25723461, 10.2174/1389557515666150226121052. [DOI] [PubMed] [Google Scholar]
- Park YH, Bae HC, Kim J, Jeong SH, Yang SI, Son SW. 2018. Zinc oxide nanoparticles induce HIF-1α protein stabilization through increased reactive oxygen species generation from electron transfer chain complex III of mitochondria. J Dermatol Sci 91(1):104–107, PMID: 29622477, 10.1016/j.jdermsci.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Polunas M, Halladay A, Tjalkens RB, Philbert MA, Lowndes H, Reuhl K. 2011. Role of oxidative stress and the mitochondrial permeability transition in methylmercury cytotoxicity. Neurotoxicology 32(5):526–534, PMID: 21871920, 10.1016/j.neuro.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribluda VS, Gubish ER Jr, Lavallee TM, Treston A, Swartz GM, Green SJ. 2000. 2-Methoxyestradiol: an endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev 19(1–2):173–179, PMID: 11191057, 10.1023/a:1026543018478. [DOI] [PubMed] [Google Scholar]
- Rothenberg SE, Windham-Myers L, Creswell JE. 2014. Rice methylmercury exposure and mitigation: A comprehensive review. Environ Res 133:407–423, PMID: 24972509, 10.1016/j.envres.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge JS, Li Y, Pasnikowski EM, Mattsson K, Pan L, Yancopoulos GD, et al. . 1994. Neurotrophic factor receptors and their signal transduction capabilities in rat astrocytes. Eur J Neurosci 6(5):693–705, PMID: 8075814, 10.1111/j.1460-9568.1994.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Santos AAD, Hort MA, Culbreth M, López-Granero C, Farina M, Rocha JB, et al. . 2016. Methylmercury and brain development: a review of recent literature. J Trace Elem Med Biol 38:99–107, PMID: 26987277, 10.1016/j.jtemb.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AAD, López-Granero C, Farina M, Rocha JB, Bowman AB, Aschner M. 2018. Oxidative stress, caspase-3 activation and cleavage of ROCK-1 play an essential role in MeHg-induced cell death in primary astroglial cells. Food Chem Toxicol 113:328–336, PMID: 29428217, 10.1016/j.fct.2018.01.057. [DOI] [PubMed] [Google Scholar]
- Sato M, Kondoh M. 2002. Recent studies on metallothionein: protection against toxicity of heavy metals and oxygen free radicals. Tohoku J Exp Med 196(1):9–22, PMID: 12498322, 10.1620/tjem.196.9. [DOI] [PubMed] [Google Scholar]
- Semenza G. 2002. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol 64(5–6):993–998, PMID: 12213597, 10.1016/S0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Wang GL. 1992. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12(12):5447–5454, PMID: 1448077, 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen T, Sen N. 2016. Treatment with an activator of hypoxia-inducible factor 1, DMOG provides neuroprotection after traumatic brain injury. Neuropharmacology 107:79–88, PMID: 26970014, 10.1016/j.neuropharm.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker G, Syversen T, Aschner M. 2003. Astrocyte-mediated methylmercury neurotoxicity. BTER 95(1):1–10, PMID: 14555794, 10.1385/BTER:95:1:1. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Chan HM. 2008. Characterization of demethylation of methylmercury in cultured astrocytes. Chemosphere 74(1):112–118, PMID: 18950830, 10.1016/j.chemosphere.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Shin HJ, Choi MS, Ryoo NH, Nam KY, Park GY, Bae JH, et al. . 2010. Manganese-mediated up-regulation of HIF-1alpha protein in Hep2 human laryngeal epithelial cells via activation of the family of MAPKs. Toxicol In Vitro 24(4):1208–1214, PMID: 20152896, 10.1016/j.tiv.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Siddiq A, Ayoub IA, Chavez JC, Aminova L, Shah S, LaManna JC, et al. . 2005. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem 280(50):41732–41743, PMID: 16227210, 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek T, Cumming R, Dargusch R, Maher P, Schubert D. 2003. The regulation of glucose metabolism by HIF-1 mediates a neuroprotective response to amyloid beta peptide. Neuron 39(1):43–56, PMID: 12848931, 10.1016/S0896-6273(03)00367-2. [DOI] [PubMed] [Google Scholar]
- Steuerwald U, Weihe P, Jørgensen PJ, Bjerve K, Brock J, Heinzow B, et al. . 2000. Maternal seafood diet, methylmercury exposure and neonatal neurologic function. J Pediatr 136(5):599–605, PMID: 10802490, 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- Stopford W, Goldwater LJ. 1975. Methylmercury in the environment: a review of current understanding. Environ Health Perspect 12:115–118, PMID: 1227851, 10.1289/ehp.7512115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li QF, Zhang Y, Hu R, Jiang H. 2013. Isoflurane preconditioning increases survival of rat skin random-pattern flaps by induction of HIF-1α expression. Cell Physiol Biochem 31(4–5):579–591, PMID: 23635649, 10.1159/000350078. [DOI] [PubMed] [Google Scholar]
- Triantafyllou A, Liakos P, Tsakalof A, Georgatsou E, Simos G, Bonanou S. 2006. Cobalt induces hypoxia-inducible factor-1alpha (HIF-1alpha) in HeLa cells by an iron-independent, but ROS-, PI-3K and MAPK-dependent mechanism. Free Radic Res 40(8):847–856, PMID: 17015263, 10.1080/10715760600730810. [DOI] [PubMed] [Google Scholar]
- Wikenheiser J, Karunamuni G, Sloter E, Walker MK, Roy D, Wilson DL, et al. . 2013. Altering HIF-1α through 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure affects coronary vessel development. Cardiovasc Toxicol 13(2):161–167, PMID: 23264063, 10.1007/s12012-012-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li X, Xie W, Jankovic J, Le W, Pan T. 2010. Neuroprotection of deferoxamine on rotenone-induced injury via accumulation of HIF-1 alpha and induction of autophagy in SH-SY5Y cells. Neurochem Int 57(3):198–205, PMID: 20546814, 10.1016/j.neuint.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Yang B, Bai Y, Yin C, Qian H, Xing G, Wang S, et al. . 2018. Activation of autophagic flux and the Nrf2/ARE signaling pathway by hydrogen sulfide protects against acrylonitrile-induced neurotoxicity in primary rat astrocytes. Arch Toxicol 92(6):2093–2108, PMID: 29725710, 10.1007/s00204-018-2208-x. [DOI] [PubMed] [Google Scholar]
- Yee S, Choi BH. 1996. Oxidative stress in neurotoxic effects of methylmercury poisoning. Neurotoxicology 17(1):17–26, PMID: 8784815. [PubMed] [Google Scholar]
- Yin Z, Lee E, Ni M, Jiang H, Milatovic D, Rongzhu L, et al. . 2011. Methylmercury-induced alterations in astrocyte functions are attenuated by ebselen. Neurotoxicology 32(3):291–299, PMID: 21300091, 10.1016/j.neuro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Feng XB, Larssen T, Qiu GL, Vogt RD. 2010. In inland China, rice, rather than fish, is the major pathway for methylmercury exposure. Environ Health Perspect 118(9):1183–1188, PMID: 20378486, 10.1289/ehp.1001915. [DOI] [PMC free article] [PubMed] [Google Scholar]