Abstract
Background:
Epidemiological evidence remains equivocal on the associations between environmentally relevant levels of per-/polyfluoroalkyl substances (PFASs) and human semen quality.
Objectives:
We aimed to test whether the potential effects on semen quality could be better observed when seminal PFAS levels were used as an exposure marker compared with serum PFAS levels.
Methods:
Matched semen and serum samples from 664 adult men were collected from a cross-sectional population in China from 2015 to 2016. Multiple semen parameters were assessed, along with measurement of 16 target PFASs in semen and serum. Partitioning between semen and serum was evaluated by the ratio of matrix-specific PFAS concentrations. Regression model results were expressed as the difference in each semen parameter associated with the per unit increase in the ln-transformed PFAS level after adjusting for confounders.
Results:
Perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS), and emerging chlorinated polyfluorinated ether sulfonate (6:2 Cl-PFESA) were detected at their highest concentrations in both semen and serum, with median concentrations of 0.23, 0.10, and in semen, respectively, and a semen-to-serum ratio of 1.3:3.1. The between-matrix correlations of these PFAS concentrations were high (). Seminal PFOA, PFOS, and 6:2 Cl-PFESA levels were significantly associated with a lower percentage of progressive sperm and higher percentage of DNA fragmentation (false discovery rate-adjusted ). Associations between serum PFAS levels and semen parameters were generally statistically weaker, except for DNA stainability, which was more strongly associated with serum-based PFASs than with semen-based PFASs.
Conclusions:
Our results suggest the potential for deleterious effects following exposure to 6:2 Cl-PFESA and other PFASs. Compared with serum PFAS levels, the much clearer association of seminal PFAS levels with semen parameters suggests its advantage in hazard assessment on semen quality, although the potential for confounding might be higher. Exposure measurements in target tissue may be critical in clarifying effects related to PFAS exposure. https://doi.org/10.1289/EHP4431
Introduction
Evidence suggests that human semen quality has declined worldwide over the last several decades (Carlsen et al. 1992; Mishra et al. 2018). In addition to adverse lifestyle factors such as cigarette smoking, alcohol and caffeine intake, obesity, stress, and high scrotal temperature (Ilacqua et al. 2018; Sadeu et al. 2010; Sharpe 2000), exposure to environmental contaminants is regarded as another important contributor (Mima et al. 2018; Skakkebaek et al. 2001). Per- and polyfluoroalkyl substances (PFASs) are a group of artificial chemicals now found as global environmental contaminants due to their extensive uses in industrial and consumer products (Lindstrom et al. 2011). With increasing awareness that some common PFASs, such as perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS), are toxic, bioaccumulative, and biopersistent, with long half-lives in humans (e.g., 3.5 and 4.8 y for PFOA and PFOS, respectively) (Olsen et al. 2007), governments and international organizations initiated the phaseout of these compounds in 2000 (Lindstrom et al. 2011). These stricter regulations led to a geographical shift in the production and application of PFOS and PFOA to China (Wang et al. 2016) and the associated development of alternative compounds within Chinese industries, such as chlorinated polyfluorinated ether sulfonate (6:2 Cl-PFESA, trade name F-53B) as a replacement for PFOS (Ruan et al. 2015; Wang et al. 2013). However, growing evidence suggests that these alternatives may be no safer than the legacy compounds they are meant to replace. For example, with an estimated half-life of 15.3 y in humans (longer than that of PFOA and PFOS), 6:2 Cl-PFESA is considered the most biopersistent PFAS reported to date (Shi et al. 2016) and the third most detected PFAS (next to PFOS and PFOA) in the serum of studied populations in China (Pan et al. 2017). Thus, this evidence implies increasing exposure to legacy and novel PFASs in the Chinese population, with the risk to semen quality also of great concern.
Indeed, toxicological studies have shown that exposure to PFASs at high doses (typically at the milligram per kilogram per day level) can impair male fecundity (e.g., decreased serum testosterone and epididymal sperm counts) in rodents (Lau et al. 2007; Wan et al. 2011; Zhang et al. 2014). However, epidemiological evidence remains equivocal regarding the effects of PFASs on male reproductive function at environmentally relevant levels. To date, the associations between PFAS exposure and testicular function have been investigated in a number of different populations, including men from the general population (Joensen et al. 2009, 2013; Buck Louis et al. 2015), male partners of pregnant women (Specht et al. 2012; Toft et al. 2012), subfertile couples attending infertility clinics (Governini et al. 2015; Raymer et al. 2012; Song et al. 2018), and occupational PFOA-exposed workers (Olsen et al. 1998; Sakr et al. 2007). Although such studies have indicated increasingly clear tendencies that higher exposure to PFASs (e.g., PFOS, PFOA) is associated with lower serum testosterone levels (Joensen et al. 2009, 2013; Lopez-Espinosa et al. 2016; Raymer et al. 2012; Zhou et al. 2016), associations with other sex hormones, such as estradiol and luteinizing hormone, remain uncertain (Joensen et al. 2009, 2013; Lopez-Espinosa et al. 2016; Raymer et al. 2012; Sakr et al. 2007; Specht et al. 2012; Zhou et al. 2016). There is a lack of consistent results among the limited investigations concerning PFAS exposure and semen quality parameters (Bach et al. 2016). Although Joensen et al. (2009) observed a significant tendency toward poorer sperm morphology with higher combined serum PFOS and PFOA levels in 105 healthy young men from a Danish population, this finding was not replicated in their later investigation when a larger sample size was included in another sampling year (Joensen et al. 2013). Furthermore, Toft et al. (2012) observed a higher percentage of motile sperm in the highest PFOA-exposed tertile, whereas Song et al. (2018) found negative correlations between sperm motility and PFOA exposure.
The existing body of evidence is largely limited to the assessment of PFASs in serum or plasma. Given their strong binding affinities to serum albumin, PFASs can persist in human serum for years (Olsen et al. 2007), making it a favorable matrix for determining the body burden of PFASs. However, measurement in serum does not necessarily reflect exposure to PFASs in a specific organ or tissue. Compared with PFAS levels in serum, those in semen may more likely represent the actual levels of PFASs entering the male reproductive system. It is possible that the potential effects on testicular function would be more clearly observed if seminal PFASs, rather than serum PFASs, were used as the exposure markers. As such, seminal PFAS levels are worth exploring as potential exposure markers for epidemiological studies. However, one must consider that using a more accurate measure of target organ exposure may introduce confounding that would not occur if a more remote measure of exposure was used (Weisskopf and Webster 2017).
Semen has been used for monitoring various contaminants (Esteban and Castaño 2009) but has been rarely used for monitoring PFASs. Although trace levels of PFASs in semen were detected in earlier studies (Governini et al. 2015; Raymer et al. 2012), the exploration of possible associations with semen quality was hampered by the poor limits of quantitation (LOQs; ), such that even dominant PFASs (e.g., PFOA and PFOS) could not be quantified. However, with the development of modern analytical instruments, the detection sensitivity of PFASs has increased by two to three orders of magnitude, thus allowing the quantification of trace levels of seminal PFASs. In fact, a recent study determined semen PFASs with LOQs reaching parts per trillion (Song et al. 2018). Several PFASs have been detected in semen samples, with results indicating higher correlations (without adjustment for confounders) with semen quality than were detected with serum PFASs. However, this pilot study included only 103 men and two basic semen parameters (i.e., sperm concentration and motility). Research with a larger sample size, more comprehensive outcomes, and further confounders is necessary.
In the present investigation, we collected 664 matched-pair semen and serum samples from men attending an infertility clinic in Nanjing, China. Levels of PFASs, including legacy compounds and the novel alternative 6:2 Cl-PFESA, were quantified in both matrices. With the adjustment of potential covariates, the epidemiological associations with semen quality parameters were analyzed to test whether the effects of PFASs on semen quality could be better evaluated by levels in semen. To the best of our knowledge, this is the first study to investigate the patterns of PFASs between semen and serum. Such information is not only crucial for risk assessment but can also help elucidate the transfer of PFASs to the male reproductive system, which is important for understanding the risks and mechanisms of human reproductive toxicity.
Methods
Study Population and Sample Collection
Male partners of couples were recruited at their first visit to the Reproductive Medical Center, Nanjing Jinling Hospital, Nanjing, China, regardless of their purpose for fertility assessment. The study population was heterogeneous, including men with fecundity issues as well as fertile men who were partners of women with female factor infertility. The criteria for subject selection were minimal; namely, being a) a Chinese citizen years of age and b) able to communicate in Chinese and complete the questionnaire. From June 2015 to July 2016, 1,630 eligible men were invited to join the investigation, with a total of 738 (45.3%) agreeing to participate. With the guidance of a trained nurse, each participant provided signed informed consent and filled out a face-to-face questionnaire; information collected included demographics (e.g., age, race, body weight, height, occupation), health condition [e.g., reproductive (fathering) history and medical history], and lifestyle factors (e.g., smoking and alcohol consumption status). Of the 738 participants, 74 were excluded: 59 reported severe reproductive tract diseases, including testicular cancer, azoospermia, cryptorchidism, grade II and III varicoceles, urogenital infections, testicular hydroceles, hypospadias, or sexually transmitted diseases; 6 were later diagnosed with azoospermia; 2 were taking medication to improve semen quality; 1 worked in a fluorochemical plant, indicating occupational exposure to PFASs; and 6 had inadequate semen volume left for PFAS measurement after routine semen analysis. Thus, the final sample size for this investigation was 664. The study was approved by the Human Subject Committee of Nanjing Jinling Hospital.
After a recommended abstinence period of 2 d, each participant provided a semen sample on site by masturbation into a sterile jar. Men with an abstinence period of were asked to return later. Each participant was instructed to collect a complete ejaculate carefully and to record if any spillage occurred. Specimens were immediately analyzed for semen quality parameters, with the remaining aliquot kept at for PFAS analysis.
On the same day as semen collection, each participant provided a venous blood sample. Serum was collected after centrifugation and stored at . Paired serum and semen samples were shipped to the laboratory in Beijing on dry ice and stored at until PFAS analysis.
PFAS Analysis
A total of 16 target PFASs, including perfluorobutanoate (PFBA), perfluoropentanoate (PFPeA), perfluorohexanoate (PFHxA), perfluoroheptanoate (PFHpA), PFOA, perfluorononanoate (PFNA), perfluorodecanoate (PFDA), perfluoroundecanoate (PFUnDA), perfluorododecanoate (PFDoDA), perfluorotridecanoate (PFTriDA), perfluorotetradecanoate (PFTeDA), perfluorobutane sulfonate (PFBS), perfluorohexane sulfonate (PFHxS), PFOS, and 6:2 and 8:2 Cl-PFESAs, were measured following our previously published methods (Pan et al. 2017). For additional information see “Standards and reagents,” “Sample extraction,” and “Instrument analysis” in the Supplemental Material and Table S1. Briefly, serum and semen samples were extracted using ion-pair extraction. The extracts were analyzed using an Acquity UPLC™ coupled to a Xevo™ TQ-S triple quadrupole mass spectrometer (Waters). Procedural blanks, matrix recovery tests, certified reference materials, and additional isotope-labeled injection standards were applied to ensure accurate quantification of PFASs. All labware and solvents were prescreened to reduce possible contamination. Spike recoveries () were validated by spiking mixed native standards (final concentration 0.1 and ) into a blank matrix (commercial fetal bovine serum or pooled semen from three healthy volunteers) and subjected to the same extraction. The recoveries of PFASs in the serum and semen samples ranged from 84% to 112% (see Table S2). The LOQs were established based on three criteria: a) concentration resulting in a signal-to-noise ratio of 10 in different matrices, b) lowest concentration of standard in the calibration curve with measured concentrations within of its theoretical value, and c) concentration factor. The LOQs are shown in Table S2, with values ranging from for serum and for semen. In daily operation, one extraction blank and one quality control sample (SRM1957; NIST) were injected in every 20 samples to ensure accurate quantification of PFASs. An 11-point calibration curve was verified and exhibited excellent linearity in the range of (). Instrumental drift was checked by injecting a standard in every 10 samples. A new calibration curve was reconstructed if the deviation was greater than from its theoretical concentration. No instrumental drift was observed during any batch.
Semen Analysis
Semen samples were analyzed after liquefaction at 37°C. Semen volume was calculated from sample weight, assuming the density of semen to be . Sperm concentration and motility were determined by the CFT-9201 computer-aided sperm analysis system (CASA; Jiangsu Rich Life Science Instrument Co., Ltd.). The analyses were achieved within 60 min of ejaculation to minimize the effect of ejaculation-to-analysis delay on sperm motility. Total sperm count was calculated by multiplying sperm concentration with semen volume. Sperm kinematic parameters were measured along with sperm motility by CASA. Kinematic parameters included curvilinear velocity (VCL), straight-line velocity (VSL), linearity (), average path velocity (VAP), amplitude of lateral head displacement (ALH), and beat cross frequency (BCF). These parameters describe different aspects of sperm movement; VSL, VAP, and LIN are indicators of sperm progression, whereas VCL, ALH, and BCF are indicators of sperm vigor. Because these parameters were strongly correlated with each other, only VSL and VCL were further analyzed as indicators of sperm progression and vigor, respectively. VCL and VSL were selected over other parameters because they are the most well-known kinematic parameters and are not heavily dependent on the type of CASA instrument (WHO 2010). Sperm morphology slides (at least 200 spermatozoa per slide) were fixed and Shorr-stained in duplicate and were evaluated according to the World Health Organization (WHO) criteria. Average percentage of normal morphology was reported if the difference between the two replicates was acceptable; when it was not, the assessment was repeated with two new aliquots of the semen sample. The sperm chromatin stability assay (SCSA) was conducted using a FACSCalibur Flow Cytometer (Becton Dickinson). Parameters, including DNA fragmentation index (DFI; percentage of sperm with damaged DNA) and high DNA stainability (HDS; percentage of sperm with immature chromatin) were then quantified (Evenson et al. 2002). Three trained technicians without any knowledge of the study subjects completed the routine semen analysis, morphological assessment, and SCSA assay, respectively. All three technicians had participated in a continuous quality control program under the supervision of the Quality Control Committee of Nanjing Jinling Hospital. The program was conducted to ensure good agreement in the assessment of sperm concentration using the CASA system against a hemocytometer. In addition, two known concentrations of latex beads (Hamilton-Thorne Inc.) were used to validate the accuracy of the CASA counts. In daily operation, aliquots of preserved semen samples, video recordings, and stained semen slides were analyzed at intervals for internal quality control of sperm concentration, motility, and morphology, respectively.
Statistical Analysis
Basic descriptive statistics were assessed for the distributions of subject demographics, semen parameters, and PFAS concentrations in serum and semen. Several PFASs with extremely low detection frequencies (i.e., PFHpA, PFTeDA, and PFBS, detection rate 2.7–14%) were excluded from further exploration. For all other PFASs, censored likelihood multiple imputation (Boss et al. 2019) was used to impute concentrations below the LOQ. Concentrations lower than the LOQ were imputed according to available data for the subject’s age and linear and quadratic terms of body mass index (BMI and ). These variables were selected over other variables because they significantly predicted PFAS levels with correlations . Pearson’s correlations were used to test the within-matrix and between-matrix correlations of PFAS concentrations. The concentration ratio (semen vs. serum) was calculated to indicate the extent of PFASs entering the male reproductive system from the whole body. It is worth noting that the multiple imputation method makes assumptions about the distribution of the unobserved values, and the potential influence of deviations from these assumptions will increase with the proportion of imputed sample. In that case, relevant results for the PFASs with large proportions below the LOQ, such as PFDoDA, PFTriDA, PFHxS, and 8:2 Cl-PFESA, may be biased, and hence were only exploratory.
The associations of semen quality parameters with each PFAS in serum or semen were evaluated using multivariable linear regression analysis. PFAS concentrations were first modeled as continuous variables (ln-transformed); only target PFASs with detection frequencies greater than 80% were included in the analysis. Sensitivity analysis was then conducted by including both semen and serum PFAS concentrations into the model to describe how estimates vary compared with the serum-only or semen-only estimates. To explore the nonlinear relationships, PFASs were also divided into quartiles and entered into the model as categorical variables using three dichotomous indicator terms, with the lowest quartile used as the reference category. p-Values for linear trends were then derived by fitting the median concentrations of PFASs in each quartile and adding them to the model as continuous variables. To achieve normality of distribution of the residuals, dependent variables were either ln-transformed (semen volume, DFI, and HDS) or cubic-root transformed (sperm concentration and total sperm count). Sperm motility, VCL, VSL, and morphology exhibited close to normal distribution, and hence were included in the regressions untransformed. Residual regression plots were visually assessed to confirm normality assumptions. Subjects reporting spillage during semen collection () were excluded from the models for semen volume, sperm concentration, and total sperm count. In the models of associations, we adjusted a priori for age (years; continuous), BMI and (kilograms per meter squared; continuous), smoking (none, , 1–9, 10–19, and during the last 3 months; categorical), alcohol consumption ( alcoholic beverage/week during the previous 3 months; dichotomous), and abstinence time (days; continuous) because these are known to influence semen quality (Carlsen et al. 2004; Jensen et al. 2004; Kidd et al. 2001; Kucheria et al. 1985; Ramlau-Hansen et al. 2007). Occupational hazards (extreme heat, heavy exertion, chemical exposure, any of these or not; dichotomous) and medical history (self-reported disease vs. not reported; dichotomous) were also evaluated as possible confounders, but neither met the confounder criteria, whose inclusion would cause PFAS estimates to change by (Greenland 1989). To reduce the overall testing error rate, we used false discovery rate (FDR)-adjusted p-values for multiple comparisons. All p-values in models were adjusted using the spreadsheet software developed by Pike (2011). An FDR-adjusted was considered statistically significant in all models. We used R statistical software (version 3.1.1; R Development Core Team) for the censored likelihood multiple imputation [chmi package (Boss et al. 2019)]. All other statistical analyses were performed using IBM PASW statistics (version 25.0; SPSS Inc.).
Results
Population Characteristics
The demographics and semen parameters of the 664 eligible men are displayed in Table 1. The mean (SD) of the age and BMI of participants was and , respectively. Mean abstinence time before semen collection was . Among the participants, 54% were nonsmokers and 55% reported a frequency of alcohol consumption higher than once a week. Many (69%) had never fathered a pregnancy, and few reported potential occupational hazards (25%) or histories of certain diseases (20%).
Table 1.
Subject demographics and semen parameters ().
| Characteristica | Median (5th, 95th) | (%) | |
|---|---|---|---|
| Age (y) | 29.0 (21.0, 39.0) | ||
| BMI () | 23.9 (19.0, 29.4) | ||
| Abstinence (d) | 4.0 (2.0, 10.0) | ||
| Smoking status during last 3 months | |||
| No smoking | 357 (53.8) | ||
| 46 (6.9) | |||
| 1–9 cigarettes/d | 80 (12.0) | ||
| 10–19 cigarettes/d | 121 (18.2) | ||
| 60 (9.0) | |||
| Alcohol consumption during last 3 months | |||
| alcoholic beverage/week | 299 (45.0) | ||
| alcoholic beverage/week | 365 (55.0) | ||
| Occupationb | |||
| Potential occupational hazard | 163 (24.5) | ||
| No hazard | 501 (75.5) | ||
| Medical history | |||
| Self-reported diseasec | 133 (20.0) | ||
| Not reported | 531 (80.0) | ||
| Reproductive history | |||
| Fathered a pregnancy | 203 (30.6) | ||
| Never fathered a pregnancy | 461 (69.4) | ||
| Semen volume (mL) | 3.7 (2.2, 6.9) | ||
| Sperm concentration (million/mL) | 38.1 (4.3, 150) | ||
| Total sperm count (million) | 146 (15.6, 573) | ||
| Progressive motile (%) | 31.8 (10.7, 56.5) | ||
| Curvilinear velocity () | 31.8 (20.1, 45.1) | ||
| Straight-line velocity () | 22.8 (11.6, 33.1) | ||
| Morphologically normal (%) | 4.8 (1.4, 8.1) | ||
| DNA fragmentation index (%) | 15.8 (4.8, 44.8) | ||
| High DNA stainability (%) | 8.9 (4.5, 18.4) | ||
Note: BMI, body mass index; SD, standard deviation.
Data were complete for all variables shown in the table.
Question was “Does your current job involve any of the following? —Heavy exertion, extreme heat, or chemical exposure.” combined into one variable (yes/no). Numbers reporting heavy exertion, extreme heat, and chemical exposure were 134, 30, and 19, respectively.
Included hyperlipidemia, hypertension, hyperglycemia, coronary artery disease, fatty liver disease, renal calculus, hepatitis, psoriasis, hyperthyroidism and/or parotitis, combined into one variable (reported or not reported).
PFAS Profiles in Serum and Semen
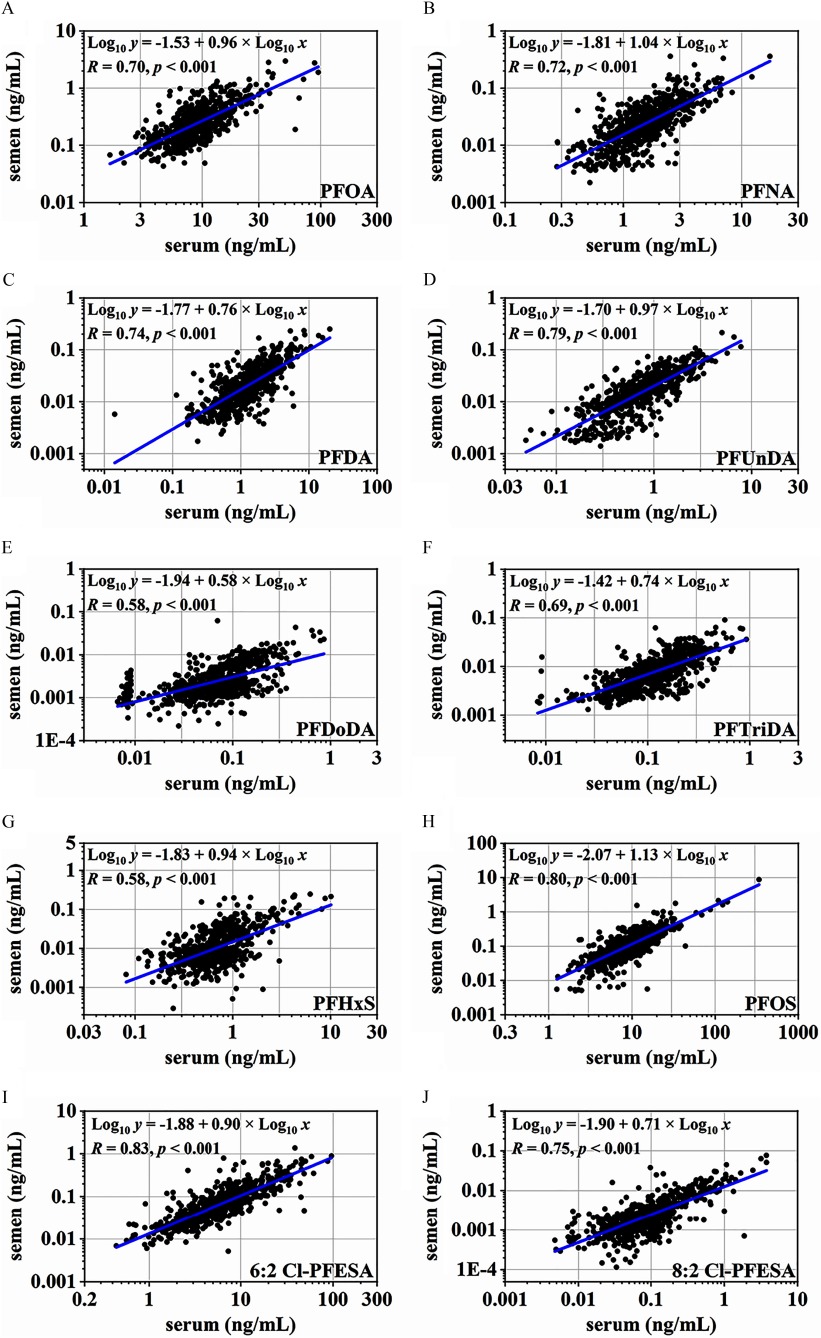
Table 2 shows the distribution of detected PFASs in serum–semen paired samples. PFBA, PFPeA, and PFHxA were not detected in any samples, and hence were not considered any further. Most other target analytes were readily detected in serum (), except for PFHpA, PFTeDA, and PFBS, for which only 24%, 37%, and 80% of tested samples were found to be above the LOQs, respectively. In serum samples, PFOA (median: ), PFOS (), and 6:2 Cl-PFESA () were the three most dominant compounds, accounting for approximately of total PFASs. The scatter plots show moderate-to-high correlations between individual PFAS levels in the two matrices (, ; Figure 1). Significant correlations were also observed among those PFASs in serum and in semen (; see Tables S3 and S4). Compared with those in serum samples, the detection rates and concentrations of PFASs decreased sharply in the corresponding semen samples. The detection rate reached 100% for PFOA and 6:2 Cl-PFESA, and ranged from 77% to 96% for PFOS, PFNA, PFDA, PFUnDA, and PFTriDA. Other PFASs were also found at quantifiable levels in a few samples (2.7–31%) (Table 2). The observed concentration sequence () was identical to that in serum, but at concentrations tens to hundreds of times lower (Wilcoxon rank sum test, for each PFAS). The percentage contribution of PFOA increased significantly from 32% in serum to 50% in semen (Wilcoxon rank sum test, ), whereas the proportion of PFOS and 6:2 Cl-PFESA decreased from 30% and 22% in serum to 20% and 14% in semen, respectively ().
Table 2.
Concentrations (ng/mL) of PFASs in paired serum and semen samples ().
| PFAS | DR (%) | Min | 25th | 50th | 75th | Max | Proportion (%)a |
|---|---|---|---|---|---|---|---|
| Serum | |||||||
| PFHpA | 23.6 | 0.509 | 0.1 | ||||
| PFOA | 100 | 1.660 | 6.804 | 8.567 | 11.04 | 95.69 | 32.1 |
| PFNA | 100 | 0.274 | 1.011 | 1.466 | 2.216 | 17.30 | 5.3 |
| PFDA | 99.8 | 0.014 | 0.706 | 1.240 | 2.031 | 20.24 | 4.6 |
| PFUnDA | 100 | 0.049 | 0.453 | 0.747 | 1.198 | 7.788 | 2.7 |
| PFDoDA | 93.8 | 0.042 | 0.074 | 0.121 | 0.857 | 0.3 | |
| PFTriDA | 99.1 | 0.074 | 0.124 | 0.200 | 0.929 | 0.4 | |
| PFTeDA | 37.0 | 0.021 | 0.115 | 0.0 | |||
| PFBS | 80.3 | 0.017 | 0.033 | 0.053 | 1.054 | 0.2 | |
| PFHxS | 100 | 0.081 | 0.460 | 0.664 | 0.932 | 10.12 | 2.7 |
| PFOS | 100 | 1.256 | 5.568 | 8.378 | 13.09 | 337.0 | 29.7 |
| 6:2 Cl-PFESA | 100 | 0.441 | 3.356 | 6.088 | 9.895 | 96.06 | 21.7 |
| 8:2 Cl-PFESA | 96.5 | 0.043 | 0.081 | 0.153 | 3.769 | 0.4 | |
| Semen | |||||||
| PFHpA | 2.7 | 0.060 | 0.1 | ||||
| PFOA | 100 | 0.043 | 0.153 | 0.229 | 0.362 | 2.966 | 49.6 |
| PFNA | 83.9 | 0.013 | 0.024 | 0.042 | 0.360 | 4.4 | |
| PFDA | 83.4 | 0.012 | 0.020 | 0.034 | 0.250 | 3.8 | |
| PFUnDA | 83.9 | 0.009 | 0.016 | 0.028 | 0.214 | 3.2 | |
| PFDoDA | 29.5 | 0.005 | 0.062 | 0.3 | |||
| PFTriDA | 76.5 | 0.004 | 0.008 | 0.013 | 0.090 | 1.7 | |
| PFTeDA | 14.2 | 0.060 | 0.1 | ||||
| PFBS | 6.2 | 0.094 | 0.1 | ||||
| PFHxS | 30.7 | 0.021 | 0.246 | 1.7 | |||
| PFOS | 96.1 | 0.055 | 0.097 | 0.179 | 8.716 | 20.4 | |
| 6:2 Cl-PFESA | 100 | 0.005 | 0.035 | 0.064 | 0.119 | 1.368 | 14.3 |
| 8:2 Cl-PFESA | 30.6 | 0.004 | 0.077 | 0.3 | |||
Note: Cl-PFESA, chlorinated polyfluorinated ether sulfonate; DR, detection rate; LOQ, limit of quantitation; Max, maximum; Min, minimum; PFAS, per-/polyfluoroalkyl substance; PFBS, perfluorobutane sulfonate; PFDA, perfluorodecanoate; PFDoDA, perfluorododecanoate; PFHpA, perfluoroheptanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFTeDA, perfluorotetradecanoate; PFTriDA, perfluorotridecanoate; PFUnDA, perfluoroundecanoate.
Average proportion among all PFASs in the considered matrix (semen or serum).
Figure 1.
Concentrations of PFASs (ng/mL) in semen versus serum (). All samples were included in the plot. Censored likelihood multiple imputation was used to impute PFAS concentrations below the LOQ. Note: Cl-PFESA, chlorinated polyfluorinated ether sulfonate; LOQ, limit of quantitation; PFAS, per-/polyfluoroalkyl substance; PFDA, perfluorodecanoate; PFDoDA, perfluorododecanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFTriDA, perfluorotridecanoate; PFUnDA, perfluoroundecanoate.
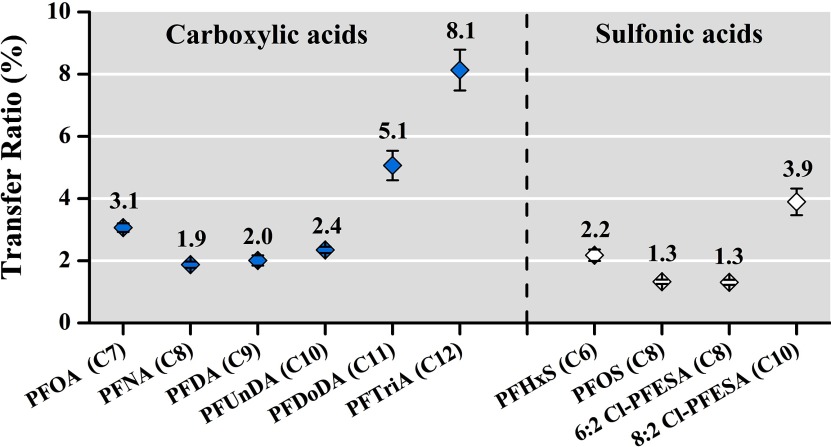
Percentage ratios of individual PFASs in semen versus those in serum were calculated as a hypothetical indicator of PFAS transfer efficiency into the reproductive system from the whole body (Figure 2). The concentration ratios showed a descending trend with increasing perfluorinated carbon number from PFOA (C7) to PFNA (C8); and then increased from PFNA (C8) to PFTriDA (C13), with a U-shaped trend observed for perfluoroalkyl carboxylic acids. A similar U-shaped trend was also observed for per- and polyfluoroalkyl sulfonic acids; the ratio reached the lowest level when sulfonic acids contained eight perfluorinated carbons (i.e., PFOS and 6:2 Cl-PFESA) and increased with increasing or decreasing carbon number (Figure 2). The pattern in concentration ratios was similar after excluding all values with imputation, that is to say, including only paired serum–semen samples with PFAS levels greater than the LOQs in the analysis (see Table S5).
Figure 2.
Percentage ratios of PFAS levels (semen vs. serum) with increasing perfluorinated carbon number (; see Table S5 for corresponding numerical data). Dots correspond to estimated mean values; whiskers indicate 95% confidence intervals. Perfluoroalkyl carboxylic acids and sulfonic acids are separated on the left and right, respectively. Censored likelihood multiple imputation was used to impute PFAS concentrations below the LOQ. Note: Cl-PFESA, chlorinated polyfluorinated ether sulfonate; LOQ, limit of quantitation; PFAS, per-/polyfluoroalkyl substance; PFDA, perfluorodecanoate; PFDoDA, perfluorododecanoate; PFHxS, perfluorohexane sulfonate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFTriDA, perfluorotridecanoate; PFUnDA, perfluoroundecanoate.
PFAS Concentration and Semen Quality
Table 3 shows the associations between semen quality parameters and seminal concentrations of PFASs detected at frequencies above 80% (i.e., PFOA, PFNA, PFDA, PFUnDA, PFOS, and 6:2 Cl-PFESA). The associations in the paired serum samples are provided in Table 4. Concerning seminal PFASs, two semen quality end points were associated with various PFASs: a) reduction in the percentage of progressive sperm (PFOA, PFNA, PFUnDA, PFOS, and 6:2 Cl-PFESA); and b) increase in the percentage of DNA fragmentation (PFOA, PFNA, PFDA, PFOS, and 6:2 Cl-PFESA). Other semen parameters were significantly associated with individual PFASs, most often with PFOA and PFOS. A 1-unit increase in ln-transformed PFOA was associated with cubic-root sperm concentration [; 95% confidence interval (CI): 0.075, 0.311] and cubic-root total sperm count (; 95% CI: 0.061, 0.432); PFOS was associated with lower VSL (; 95% CI: , ). For serum PFASs, significant results were only observed for the percentage of sperm with high DNA stainability. Higher DNA stainability was consistently associated with higher serum PFNA (; 95% CI: 0.063, 0.176), PFDA (; 95% CI: 0.031, 0.115), PFUnDA (; 95% CI: 0.060, 0.152), and 6:2 Cl-PFESA (; 95% CI: 0.037, 0.115). The associations with PFDA, PFUnDA, and 6:2 Cl-PFESA were consistent with those observed for semen PFAS levels, but less so for PFNA. In sensitivity analyses, the magnitudes of the estimates and p-values were essentially unchanged after adjustment for both semen and serum PFASs (see Table S6). Most of the abovementioned associations remained statistically significant, except for the associations with DNA stainability, which became weaker for both semen and serum PFASs (statistical significance was only reached for serum PFNA). Although the p-values increased, a positive association with HDS was still observed for serum PFASs, but basically became null for semen PFASs.
Table 3.
Estimated changes in semen quality parameters associated with a 1-unit increase in ln-transformed PFAS levels in semen ().
| Outcomea | PFOA | p-Value | PFNA | p-Value | PFDA | p-Value | PFUnDA | p-Value | PFOS | p-Value | 6:2 Cl-PFESA | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||||
| Semen volume (mL) b | (, 0.016) | 0.4 | (, 0.013) | 0.4 | (, 0.023) | 0.7 | (, ) | 0.1 | (, 0.024) | 0.8 | (, 0.030) | 1.0 |
| Sperm conc. (million/mL)b | 0.193 (0.075, 0.311) | 0.02 | 0.090 (, 0.188) | 0.2 | 0.046 (, 0.150) | 0.5 | 0.092 (0.002, 0.182) | 0.1 | 0.098 (0.008, 0.188) | 0.1 | 0.069 (, 0.158) | 0.3 |
| Sperm count (million)b | 0.247 (0.061, 0.432) | 0.05 | 0.101 (, 0.254) | 0.4 | 0.048 (, 0.209) | 0.7 | 0.081 (, 0.222) | 0.4 | 0.133 (, 0.274) | 0.2 | 0.104 (, 0.242) | 0.3 |
| Progressive motile (%) | (, ) | 0.03 | (, ) | 0.02 | (, ) | 0.1 | (, ) | 0.04 | (, ) | 0.03 | (, ) | 0.04 |
| VCL () | (, ) | 0.06 | (, ) | 0.1 | (, 0.231) | 0.3 | (, 0.218) | 0.4 | (, ) | 0.1 | (, 0.089) | 0.2 |
| VSL () | (, ) | 0.08 | (, ) | 0.1 | (, ) | 0.1 | (, ) | 0.08 | (, ) | 0.04 | (, ) | 0.1 |
| Morphologically normal (%) | (, 0.127) | 0.5 | (, 0.051) | 0.3 | (, 0.040) | 0.3 | (, 0.057) | 0.3 | 0.004 (, 0.187) | 1.0 | (, 0.159) | 0.9 |
| DFI (%) | 0.136 (0.064, 0.209) | 0.01 | 0.106 (0.047, 0.165) | 0.01 | 0.083 (0.021, 0.145) | 0.05 | 0.048 (, 0.103) | 0.2 | 0.087 (0.033, 0.142) | 0.02 | 0.080 (0.026, 0.135) | 0.03 |
| HDS (%) | 0.035 (, 0.084) | 0.3 | 0.044 (0.002, 0.086) | 0.1 | 0.068 (0.026, 0.110) | 0.02 | 0.069 (0.030, 0.107) | 0.01 | 0.033 (, 0.070) | 0.2 | 0.054 (0.017, 0.090) | 0.03 |
Note: Estimates calculated using linear regression models adjusted for age, BMI, , smoking, alcohol intake, and abstinence time. Censored likelihood multiple imputation was used when PFAS levels were below LOQ. p-Values were false discovery rate (FDR)-adjusted to reduce overall testing error rate. BMI, body mass index; CI, confidence interval; Cl-PFESA, chlorinated polyfluorinated ether sulfonate; conc., concentration; DFI, DNA fragmentation index; HDS, high DNA stainability; LOQ, limit of quantitation; PFAS, per-/polyfluoroalkyl substance; PFDA, perfluorodecanoate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoate; VCL, curvilinear velocity; VSL, straight-line velocity.
Semen volume, DFI, and HDS were ln transformed, sperm concentration and total sperm count were cubic-root transformed, other parameters were not transformed.
Subjects reporting spillage during semen collection () were excluded from these analyses.
Table 4.
Estimated changes in semen quality parameters associated with a 1-unit increase in ln-transformed PFAS levels in serum ().
| Outcomea | PFOA | p-Value | PFNA | p-Value | PFDA | p-Value | PFUnDA | p-Value | PFOS | p-Value | 6:2 Cl-PFESA | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||||
| Semen volume (mL)b | 0.048 (, 0.103) | 0.2 | 0.031 (, 0.078) | 0.4 | 0.033 (, 0.068) | 0.2 | 0.022 (, 0.060) | 0.4 | 0.042 (, 0.085) | 0.2 | 0.044 (0.011, 0.076) | 0.06 |
| Sperm conc. (million/mL)b | 0.083 (, 0.242) | 0.5 | (, 0.077) | 0.5 | (, 0.035) | 0.4 | (, 0.048) | 0.4 | (, 0.124) | 1.0 | (, 0.034) | 0.4 |
| Sperm count (million)b | 0.209 (, 0.457) | 0.2 | (, 0.171) | 0.8 | (, 0.109) | 0.7 | (, 0.118) | 0.7 | 0.061 (, 0.262) | 0.7 | (, 0.134) | 1.0 |
| Progressive motile (%) | (, 1.217) | 0.6 | 0.040 (, 1.862) | 1.0 | 0.861 (, 2.200) | 0.4 | 0.568 (, 2.041) | 0.6 | (, 1.557) | 1.0 | 0.068 (, 1.323) | 1.0 |
| VCL () | 0.280 (, 1.504) | 0.8 | 0.272 (, 1.330) | 0.7 | 0.764 (, 1.540) | 0.2 | 0.492 (, 1.347) | 0.4 | 0.278 (, 1.237) | 0.7 | 0.475 (, 1.203) | 0.4 |
| VSL () | (, 0.963) | 1.0 | (, 0.826) | 1.0 | 0.317 (, 0.963) | 0.5 | 0.062 (, 0.773) | 1.0 | (, 0.762) | 1.0 | 0.190 (, 0.795) | 0.7 |
| Morphologically normal (%) | (, 0.165) | 0.5 | (, 0.020) | 0.2 | (, 0.118) | 0.5 | (, 0.052) | 0.3 | (, 0.189) | 0.7 | (, 0.133) | 0.7 |
| DFI (%) | 0.046 (, 0.144) | 0.5 | (, 0.081) | 1.0 | (, 0.028) | 0.4 | (, 0.035) | 0.5 | 0.040 (, 0.116) | 0.5 | 0.012 (, 0.071) | 0.8 |
| HDS (%) | 0.061 (, 0.127) | 0.2 | 0.119 (0.063, 0.176) | 0.01 | 0.073 (0.031, 0.115) | 0.02 | 0.106 (0.060, 0.152) | 0.01 | 0.059 (0.007, 0.111) | 0.1 | 0.076 (0.037, 0.115) | 0.01 |
Note: Estimates calculated using linear regression models adjusted for age, BMI, , smoking, alcohol intake, and abstinence time. p-Values were false discovery rate (FDR)-adjusted to reduce overall testing error rate. BMI, body mass index; CI, confidence interval; Cl-PFESA, chlorinated polyfluorinated ether sulfonate; conc., concentration; DFI, DNA fragmentation index; HDS, high DNA stainability; PFAS, per-/polyfluoroalkyl substance; PFDA, perfluorodecanoate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoate; VCL, curvilinear velocity; VSL, straight-line velocity.
Semen volume, DFI, and HDS were ln transformed, sperm concentration and total sperm count were cubic-root transformed, other parameters were not transformed.
Subjects reporting spillage during semen collection () were excluded from these analyses.
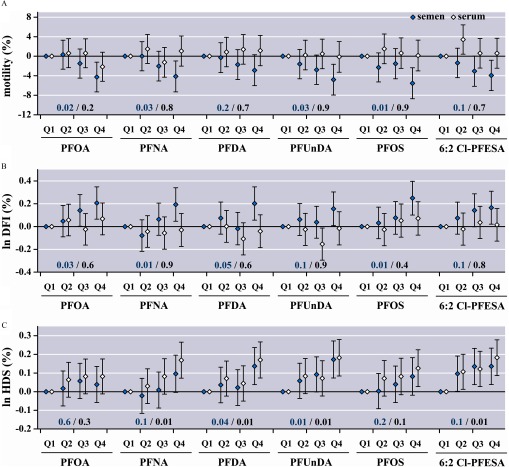
Figure 3 presents the associations of selected outcomes with semen and serum PFAS concentrations across quartiles. The corresponding results for the other outcomes are shown in Figure S1. In accordance with the significant associations from the continuous models, a 2.9–5.5% decline in the percentage of progressive sperm and 0.16–0.25% increase in the per log unit percentage of DNA fragmentation were observed in the highest quartile of seminal PFASs versus the lowest (Figure 3). No significant differences or clear patterns were observed in the serum PFAS quartiles for sperm motility or DNA fragmentation. However, for the percentage of sperm with high DNA stainability, the positive trends across PFAS quartiles in serum tended to be clearer than those in semen. A 0.08–0.18% increase in the per log unit HDS was observed in the highest quartile of serum PFASs versus the lowest and was 1.1–2.0 times greater than the corresponding increment in the highest quartile of semen PFASs compared with the lowest (Figure 3). No clear patterns were observed for other sperm parameters in semen or serum PFAS quartiles (see Figure S1).
Figure 3.
Regression coefficients and 95% confidence intervals for changes in selected semen parameters across PFAS quartiles (; see Tables S7 and S8 for corresponding numerical data). First quartile (Q1) was used as a reference group. Concentration ranges for PFASs across quartiles are listed in Table 2. Models were adjusted for age, BMI, , smoking, alcohol intake, and abstinence time. DNA fragmentation index (DFI) and high DNA stainability (HDS) were ln-transformed. FDR-adjusted p-values were used for trends and are shown in blue (semen) and black (serum PFAS quartiles). Note: BMI, body mass index; Cl-PFESA, chlorinated polyfluorinated ether sulfonate; FDR, false discovery rate; PFAS, per-/polyfluoroalkyl substance; PFDA, perfluorodecanoate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoate; Q, quartile.
Discussion
In the paired semen and serum samples, concentrations of seminal PFASs were two to three orders of magnitude lower but were strongly correlated with those in serum. A U-shaped trend was observed for the concentration ratios (semen vs. serum) of carboxylic and sulfonic acids with increasing chain length, suggesting a possible structure-dependent partitioning of PFASs between the two matrices. Compared with those in serum, seminal PFAS levels were more clearly associated with semen quality parameters in this cross-sectional study of 664 Chinese adults, except for DNA stainability. Sperm motility and DFI were the two outcomes most consistently associated with seminal PFAS levels, with HDS also significantly associated with some PFASs in both semen and serum.
The high detection and abundance of PFASs in serum indicated that PFAS exposure is still widespread and relatively high in Chinese men, even after worldwide regulations on PFOS and PFOA production (Lindstrom et al. 2011). China has banned the production and usage of PFOS (with specific exemptions) since 2014 (MEP 2014a). A grant from the Global Environment Facility was approved in 2017 to support the reduction of PFOS in China (World Bank 2017). PFOA has also been listed as a “product with high pollution and high environmental risk” by the Chinese government (MEP 2014b), although no administrative restriction on its production and usage has yet been promulgated. Serum levels of legacy PFASs, particularly PFOA, were 3- to 11-fold higher in the present population (median ; sampling year: 2015–2016) than in general populations from other countries: for example, the United States (; 2013–2014; CDC 2017), South Korea (; 2009–2010; Lee et al. 2017), Japan (; 2009–2010; Yamaguchi et al. 2013), Spain (; 2007–2009; Bartolomé et al. 2017), Czech Republic (; 2015; Sochorová et al. 2017), Sweden (; 2010–2011; Bjermo et al. 2013), and Norway (; 2010–2011; Averina et al. 2018). Aside from exposure to legacy PFASs, 6:2 Cl-PFESA (commercially F-53B), a replacement chemical for PFOS in the Chinese plating industry (Wang et al. 2013), has become an emerging concern. Similar to previous investigations on pregnant mothers and fetuses in China (Pan et al. 2017), serum concentrations of 6:2 Cl-PFESA (median ) were the third highest among all detected PFASs and accounted for a 2-fold larger proportion (22%) in men than that reported in pregnant women and fetuses (; Pan et al. 2017). To the best of our knowledge, this is the first study to report on baseline levels of 6:2 Cl-PFESA among Chinese male adults and the first to explore its epidemiological association with semen quality. With such comparable concentrations of PFOS in Chinese populations, concerning on toxicity and epidemiological effect of 6:2 Cl-PFESA should not be neglected.
Albeit at levels much lower than those in serum, the widespread presence of the six dominant PFASs (i.e., PFOA, PFOS, 6:2 Cl-PFESA, PFNA, PFDA, and PFUnDA) in semen reflects exposure. Therefore, the next step was to establish correlations between levels in semen and serum to ensure that seminal PFAS levels can be related to actual body burden. Our results demonstrated that concentrations of the six major PFASs among the two matrices were highly correlated (Figure 1). Several studies have reported on the transfer ratios of chemicals from serum (or blood) to semen. Although semen levels can be tens [e.g., lead (Alexander et al. 1998; Telisman et al. 2000)] to thousands [e.g., dioxins (Schecter et al. 1996)] of times lower than levels in serum or blood, they can also be at comparable [e.g., cadmium (Telisman et al. 2000); parabens (Frederiksen et al. 2011)] or higher concentrations [e.g., zinc (Telisman et al. 2000; Xu et al. 1993)]. These discrepancies in concentration ratios indicate different transfer efficiencies and mechanisms for the different chemicals transferring into semen. As for PFASs, we assessed their transfer ratios from serum to semen for the first time and found preliminary evidence that efficiencies for PFASs entering semen may be related to perfluorinated carbon chain length and functional group (Figure 2). However, the observed pattern was driven by PFASs with a large proportion of samples below the LOQ (i.e., PFDoDA, PFTriDA, PFHxS, and 8:2 Cl-PFESA) and thus needs to be interpreted with caution until confirmed in a larger study. Given that PFASs are highly protein-bound in serum (Jones et al. 2003), their binding affinity to serum proteins likely plays a critical role in their transfer efficiency. Those PFASs with greater affinities will bind to serum proteins and consequently limit the bioavailability of the molecule (T Zhang et al. 2013), whereas those PFASs with lower binding affinities to protein will be more easily released from serum and transferred to other matrices, such as semen. The binding capacities to albumin, the most abundant protein in serum, have been shown to increase for C2–C8 PFCAs but to decrease for C9–C12 PFCAs (Bischel et al. 2011). A similar pattern has also been observed for C7–C16 PFCAs binding to fatty acid binding protein (FABP), another major serum protein (LY Zhang et al. 2013). This evidence may partially explain the suggestive U-shaped trend found for PFAS transfer ratios in the present study; however, further investigation is needed to verify the influence from serum proteins and to explore other factors affecting the transmission of PFASs into semen.
Semen consists of a concentrated suspension of spermatozoa stored in epididymides and fluid secretions from accessory sex organs, including seminal vesicles, prostate, bulbourethral glands, and epididymides (WHO 2010). Although it is not a direct measure of levels in sex organs (impractical for human studies), levels in semen can, to some extent, represent the levels of PFASs infiltrating the overall reproductive system, which may be a fraction of the active PFASs affecting reproductive function. Thus, seminal PFAS levels are a more direct marker than serum PFASs, given that individual differences (e.g., serum protein levels) can lead to discrepancies in levels of PFASs entering the reproductive system, despite study subjects experiencing the same serum PFAS exposure. This may explain the clearer association of seminal than serum PFAS levels with semen quality. However, it should be noted that there is greater potential for confounding by physiological conditions when PFAS exposure is assessed in semen than in serum. This is because the closer exposure and outcome are physiologically (e.g., assessed in the same organ), the greater the extraneous factors and hence potential confounders (Weisskopf and Webster 2017). For instance, if a certain condition in the prostate or testis (not influenced by PFAS) can alter both semen volume and other semen parameters, this could induce an indirect association between seminal PFASs (because an effect on semen volume may influence PFAS concentration) and other semen parameters. The potential for this to occur with serum PFAS concentrations is weaker because a condition in the prostate or testis would be less likely to influence serum PFAS levels than semen PFAS levels. The possibility of potential confounding cannot be ruled out in the present study and warrants further exploration.
Our study showed significant associations between higher seminal PFAS levels and decreased progressive sperm (Table 3). The result corroborated earlier findings that PFAS exposure may be correlated with diminished sperm motility, although the PFAS exposure burden in our study population was much lower (e.g., median PFOS level in serum: 8.4 vs. ; Song et al. 2018). The present study also provided positive evidence on previously obscure associations between PFASs and sperm DNA fragmentation. Only a marginally significant increase in DNA fragmentation was reported with increasing serum PFOA levels in one subgroup from the Biopersistent organochlorines in diet and human fertility: Epidemiologic studies of time to pregnancy and semen quality in Inuit and European populations (INUENDO) cohort (Specht et al. 2012), whereas no significant change in DFI with any serum PFAS levels was found in the Longitudinal Investigation of Fertility and the Environment (LIFE) Study (Buck Louis et al. 2015). Here, seminal levels of PFOA, PFNA, PFDA, PFOS, and 6:2 Cl-PFESA were consistently associated with an increased percentage of DNA fragmentation; however, our results did not fully contradict previous studies given that we did not observe any clear patterns between serum PFASs and DFI. Another noteworthy finding was the positive association between PFASs in both serum and semen and the percentage of high DNA stainability, a marker of sperm chromatin immaturity. Only one previous study has focused on this outcome, pointing to a negative association between percentage of DNA stainability and increased perfluorooctane sulfonamide (PFOSA) and 2-(N-methyl-perfluorooctane sulfonamido) acetate (Me-PFOSA-AcOH) levels in serum (Buck Louis et al. 2015). However, neither of these compounds were included in our analysis, which hampers a more complete comparison between the two studies.
Nonetheless, our findings are supported by previous toxicological evidence. It has been shown that PFAS exposure in vitro induces obvious detachment of primary gonocytes from Sertoli cells (ZJ Zhang et al. 2013), which can lead to a higher proportion of immature sperm. This concept supports the higher HDS association we observed in the current study. In addition, animal studies have reported excessive production of reactive oxygen species in the reproductive system induced by PFAS exposure (Guo et al. 2016; Liu et al. 2015). This can, in turn, lead to an increase in oxidative stress and consequent DNA damage (Agarwal et al. 2003) and mitochondrial dysfunction (Burwell and Brookes 2008) during spermatogenesis, which is in accord with the increased DFI and decreased sperm motility observed in the present study. However, some phenomena cannot be explained. In contrast to our hypothesis and finding that semen quality parameters were more significantly associated with PFASs in semen than those in serum, HDS appeared to be more clearly associated with serum PFAS levels. Although PFOA and PFOS were most frequently associated with other semen quality end points, HDS tended to be associated with larger molecule PFASs (i.e., PFNA, PFDA, PFUnDA, and 6:2 Cl-PFESA), and not with PFOA and PFOS. There might be other underlying mechanisms (perhaps not a direct testicular effect) acting upon HDS, and future exploration is still needed. Seminal PFOA levels were unexpectedly associated with higher sperm concentration, although it is unlikely that this suggests a protective effect on spermatogenesis. The linear trend across the PFOA quartile was not statistically significant (see Figure S1); nor were other PFASs significantly associated with sperm concentration. The possibility of a chance finding or uncontrolled confounding effect cannot be ruled out.
The strengths of the present study include the simultaneous collection of paired semen and serum samples and large sample size (664 men). Thus, this study improves our understanding on PFAS partitioning between semen and serum and provides affirmative evidence on the deleterious effects of PFASs on human semen quality. Our results were further strengthened by the correction of multiple comparisons, with FDR-based adjustments conducted to reduce the possibility of chance findings.
However, the following study limitations should also be considered. First, as is the case for most studies focusing on PFASs and semen quality, the temporal relationships between exposure and outcome could not be ascertained based on the cross-sectional design of the research. To corroborate our findings, longitudinal study with repeated measurements of PFAS concentrations and semen quality over time is needed, as well as further exploration on whether any mediators caused or influenced the observed relationships between seminal PFAS levels and semen quality. Second, the study population was recruited from an infertility clinic and, although we excluded those with severe reproductive diseases, the subjects could be considered subfertile and may not be representative of the general population. Third, reproductive hormone measurements are absent from the present study, which hampers exploration on the role of hormones in affecting semen quality. Previous studies have indicated a negative relationship between serum PFASs and testosterone (Joensen et al. 2009, 2013). Based on the fact that testosterone is synthesized in the testes, and our hypothesis that seminal PFAS levels may more directly represent the actual burden to the reproductive system than serum PFAS levels, it would be interesting to determine if there was a clearer association of seminal PFAS levels than serum PFAS levels with serum testosterone.
Conclusions
Based on a cross-sectional study of 664 Chinese men, we found various semen quality parameters to be significantly associated with seminal PFAS levels. The associations with PFAS levels in the matched serum were rarely significant. Our results suggest that PFASs may have deleterious effects on human semen quality at environmentally relevant concentrations. Furthermore, clearer associations of semen quality with seminal PFASs compared with serum PFASs suggest that measuring exposures in the target tissue may be important to clarify effects. Based on the measurement of seminal PFAS levels, this study provides affirmative evidence on previously equivocal results regarding whether PFASs impair human semen quality and highlights that seminal PFASs, albeit at trace levels, may be a more direct exposure marker than serum PFAS levels for epidemiological studies on male reproductive function.
Supplementary Material
Acknowledgments
We thank J. Boss and A. Rix from the University of Michigan, and J. Liao from the Huazhong University of Science and Technology for their kind instruction on censored likelihood multiple imputation. This work was supported by the National Natural Science Foundation of China (21737004 and 21976178), the National Basic Research Program of China (2018YFC1004202), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB14040202).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4431).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Agarwal A, Saleh RA, Bedaiwy MA. 2003. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 79(4):829–843, PMID: 12749418, 10.1016/S0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- Alexander BH, Checkoway H, Faustman EM, van Netten C, Muller CH, Ewers TG. 1998. Contrasting associations of blood and semen lead concentrations with semen quality among lead smelter workers. Am J Ind Med 34(5):464–469, PMID: 9787850, . [DOI] [PubMed] [Google Scholar]
- Averina M, Brox J, Huber S, Furberg AS. 2018. Perfluoroalkyl substances in adolescents in northern Norway: lifestyle and dietary predictors. The Tromsø study, Fit Futures 1. Environ Int 114:123–130, PMID: 29500988, 10.1016/j.envint.2018.02.031. [DOI] [PubMed] [Google Scholar]
- Bach CC, Vested A, Jørgensen KT, Bonde JP, Henriksen TB, Toft G. 2016. Perfluoroalkyl and polyfluoroalkyl substances and measures of human fertility: a systematic review. Crit Rev Toxicol 46(9):735–755, PMID: 27268162, 10.1080/10408444.2016.1182117. [DOI] [PubMed] [Google Scholar]
- Bartolomé M, Gallego-Picó A, Cutanda F, Huetos O, Esteban M, Pérez-Gómez B, et al. 2017. Perfluorinated alkyl substances in Spanish adults: geographical distribution and determinants of exposure. Sci Total Environ 603–604:352–360, PMID: 28633112, 10.1016/j.scitotenv.2017.06.031. [DOI] [PubMed] [Google Scholar]
- Bischel HN, MacManus-Spencer LA, Zhang CJ, Luthy RG. 2011. Strong associations of short-chain perfluoroalkyl acids with serum albumin and investigation of binding mechanisms. Environ Toxicol Chem 30(11):2423–2430, PMID: 21842491, 10.1002/etc.647. [DOI] [PubMed] [Google Scholar]
- Bjermo H, Darnerud PO, Pearson M, Barbieri HE, Lindroos AK, Nälsén C, et al. 2013. Serum concentrations of perfluorinated alkyl acids and their associations with diet and personal characteristics among Swedish adults. Mol Nutr Food Res 57(12):2206–2215, PMID: 23934649, 10.1002/mnfr.201200845. [DOI] [PubMed] [Google Scholar]
- Boss J, Mukherjee B, Ferguson KK, Aker A, Alshawabkeh AN, Cordero JF, et al. 2019. Estimating outcome-exposure associations when exposure biomarker detection limits vary across batches. Epidemiology 30(5):746–755, PMID: 31299670, 10.1097/EDE.0000000000001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Chen Z, Schisterman EF, Kim S, Sweeney AM, Sundaram R, et al. 2015. Perfluorochemicals and human semen quality: the LIFE study. Environ Health Perspect 123(1):57–63, PMID: 25127343, 10.1289/ehp.1307621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell LS, Brookes PS. 2008. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia–reperfusion injury. Antioxid Redox Signal 10(3):579–599, PMID: 18052718, 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. 1992. Evidence for decreasing quality of semen during past 50 years. BMJ 305(6854):609–613, PMID: 1393072, 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E, Petersen JH, Andersson AM, Skakkebaek NE. 2004. Effects of ejaculatory frequency and season on variations in semen quality. Fertil Steril 82(2):358–366, PMID: 15302284, 10.1016/j.fertnstert.2004.01.039. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2017. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2017, Volume One. Atlanta, GA: CDC; https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2017.pdf [accessed 7 May 2019]. [Google Scholar]
- Esteban M, Castaño A. 2009. Non-invasive matrices in human biomonitoring: a review. Environ Int 35(2):438–449, PMID: 18951632, 10.1016/j.envint.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Evenson DP, Larson KL, Jost LK. 2002. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl 23(1):25–43, PMID: 11780920, 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Jørgensen N, Andersson AM. 2011. Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography–tandem mass spectrometry (LC–MS/MS). J Expo Sci Environ Epidemiol 21(3):262–271, PMID: 20216574, 10.1038/jes.2010.6. [DOI] [PubMed] [Google Scholar]
- Governini L, Guerranti C, De Leo V, Boschi L, Luddi A, Gori M, et al. 2015. Chromosomal aneuploidies and DNA fragmentation of human spermatozoa from patients exposed to perfluorinated compounds. Andrologia 47(9):1012–1019, PMID: 25382683, 10.1111/and.12371. [DOI] [PubMed] [Google Scholar]
- Greenland S. 1989. Modeling and variable selection in epidemiologic analysis. Am J Public Health 79(3):340–349, PMID: 2916724, 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Li Q, Shi J, Shi L, Li B, Xu A, et al. 2016. Perfluorooctane sulfonate exposure causes gonadal developmental toxicity in Caenorhabditis elegans through ROS-induced DNA damage. Chemosphere 155:115–126, PMID: 27108369, 10.1016/j.chemosphere.2016.04.046. [DOI] [PubMed] [Google Scholar]
- Ilacqua A, Izzo G, Emerenziani GP, Baldari C, Aversa A. 2018. Lifestyle and fertility: the influence of stress and quality of life on male fertility. Reprod Biol Endocrinol 16(1):115, PMID: 30474562, 10.1186/s12958-018-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Andersson AM, Jørgensen N, Andersen AG, Carlsen E, Petersen JH, et al. 2004. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril 82(4):863–870, PMID: 15482761, 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebæk NE, Jørgensen N. 2009. Do perfluoroalkyl compounds impair human semen quality? Environ Health Perspect 117(6):923–927, PMID: 19590684, 10.1289/ehp.0800517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensen UN, Veyrand B, Antignac JP, Blomberg Jensen M, Petersen JH, Marchand P, et al. 2013. PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Hum Reprod 28(3):599–608, PMID: 23250927, 10.1093/humrep/des425. [DOI] [PubMed] [Google Scholar]
- Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP. 2003. Binding of perfluorinated fatty acids to serum proteins. Environ Toxicol Chem 22(11):2639–2649, PMID: 14587903, 10.1897/02-553. [DOI] [PubMed] [Google Scholar]
- Kidd SA, Eskenazi B, Wyrobek AJ. 2001. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril 75(2):237–248, PMID: 11172821, 10.1016/s0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- Kucheria K, Saxena R, Mohan D. 1985. Semen analysis in alcohol dependence syndrome. Andrologia 17(6):558–563, PMID: 3936380, 10.1111/j.1439-0272.1985.tb01714.x. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99(2):366–394, PMID: 17519394, 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee CK, Suh CH, Kang HS, Hong CP, Choi SN. 2017. Serum concentrations of per- and poly-fluoroalkyl substances and factors associated with exposure in the general adult population in South Korea. Int J Hyg Environ Health 220(6):1046–1054, PMID: 28688604, 10.1016/j.ijheh.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. 2011. Polyfluorinated compounds: past, present, and future. Environ Sci Technol 45(19):7954–7961, PMID: 21866930, 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Liu WW, Yang B, Wu L, Zou WY, Pan XL, Zou T, et al. 2015. Involvement of NRF2 in perfluorooctanoic acid-induced testicular damage in male mice. Biol Reprod 93(2):41, PMID: 26108789, 10.1095/biolreprod.115.128819. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Mondal D, Armstrong B, Eskenazi B, Fletcher T. 2016. Perfluoroalkyl substances, sex hormones, and insulin-like growth factor-1 at 6–9 years of age: a cross-sectional analysis within the C8 Health Project. Environ Health Perspect 124(8):1269–1275, PMID: 26794451, 10.1289/ehp.1509869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEP (Ministry of Environmental Protection of China). 2014a. Announcement on the restriction on the production, use, import and export of nine new persistent organic pollutants. http://www.mee.gov.cn/gkml/hbb/bgg/201404/t20140401_270007.htm [accessed 17 October 2019].
- MEP. 2014b. Comprehensive Directory of Environmental Protection (2013 edition). http://www.gov.cn/gzdt/2014-01/24/content_2574860.htm [accessed 17 October 2019].
- Mima M, Greenwald D, Ohlander S. 2018. Environmental toxins and male fertility. Curr Urol Rep 19(7):50, PMID: 29774504, 10.1007/s11934-018-0804-1. [DOI] [PubMed] [Google Scholar]
- Mishra P, Negi MPS, Srivastava M, Singh K, Rajender S. 2018. Decline in seminal quality in Indian men over the last 37 years. Reprod Biol Endocrinol 16(1):103, PMID: 30352581, 10.1186/s12958-018-0425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Gilliland FD, Burlew MM, Burris JM, Mandel JS, Mandel JH. 1998. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J Occup Environ Med 40(7):614–622, PMID: 9675720, 10.1097/00043764-199807000-00006. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhu Y, Zheng T, Cui Q, Buka SL, Zhang B, et al. 2017. Novel chlorinated polyfluorinated ether sulfonates and legacy per-/polyfluoroalkyl substances: placental transfer and relationship with serum albumin and glomerular filtration rate. Environ Sci Technol 51(1):634–644, PMID: 27931097, 10.1021/acs.est.6b04590. [DOI] [PubMed] [Google Scholar]
- Pike N. 2011. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol 2(3):278–282, 10.1111/j.2041-210X.2010.00061.x. [DOI] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Aggerholm AS, Jensen MS, Toft G, Bonde JP. 2007. Is smoking a risk factor for decreased semen quality? A cross-sectional analysis. Hum Reprod 22(1):188–196, PMID: 16966350, 10.1093/humrep/del364. [DOI] [PubMed] [Google Scholar]
- Raymer JH, Michael LC, Studabaker WB, Olsen GW, Sloan CS, Wilcosky T, et al. 2012. Concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) and their associations with human semen quality measurements. Reprod Toxicol 33(4):419–427, PMID: 21736937, 10.1016/j.reprotox.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan T, Lin YF, Wang T, Liu RZ, Jiang GB. 2015. Identification of novel polyfluorinated ether sulfonates as PFOS alternatives in municipal sewage sludge in China. Environ Sci Technol 49(11):6519–6527, PMID: 25961764, 10.1021/acs.est.5b01010. [DOI] [PubMed] [Google Scholar]
- Sadeu JC, Hughes CL, Agarwal S, Foster WG. 2010. Alcohol, drugs, caffeine, tobacco, and environmental contaminant exposure: reproductive health consequences and clinical implications. Crit Rev Toxicol 40(7):633–652, PMID: 20662712, 10.3109/10408444.2010.493552. [DOI] [PubMed] [Google Scholar]
- Sakr CJ, Kreckmann KH, Green JW, Gillies PJ, Reynolds JL, Leonard RC. 2007. Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers. J Occup Environ Med 49(10):1086–1096, PMID: 18000414, 10.1097/JOM.0b013e318156eca3. [DOI] [PubMed] [Google Scholar]
- Schecter A, McGee H, Stanley JS, Boggess K, Brandt-Rauf P. 1996. Dioxins and dioxin-like chemicals in blood and semen of American Vietnam veterans from the state of Michigan. Am J Ind Med 30(6):647–654, PMID: 8914711, . [DOI] [PubMed] [Google Scholar]
- Sharpe RM. 2000. Lifestyle and environmental contribution to male infertility. Br Med Bull 56(3):630–642, PMID: 11255550, 10.1258/0007142001903436. [DOI] [PubMed] [Google Scholar]
- Shi YL, Vestergren R, Xu L, Zhou Z, Li CX, Liang Y, et al. 2016. Human exposure and elimination kinetics of chlorinated polyfluoroalkyl ether sulfonic acids (Cl-PFESAs). Environ Sci Technol 50(5):2396–2404, PMID: 26866980, 10.1021/acs.est.5b05849. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. 2001. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16(5):972–978, PMID: 11331648, 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Sochorová L, Hanzlíková L, Černá M, Drgáčová A, Fialová A, Švarcová A, et al. 2017. Perfluorinated alkylated substances and brominated flame retardants in serum of the Czech adult population. Int J Hyg Environ Health 220(2 Pt A):235–243, PMID: 27743851, 10.1016/j.ijheh.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Song XF, Tang SY, Zhu HM, Chen ZY, Zang ZJ, Zhang YA, et al. 2018. Biomonitoring PFAAs in blood and semen samples: investigation of a potential link between PFAAs exposure and semen mobility in China. Environ Int 113:50–54, PMID: 29421407, 10.1016/j.envint.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Specht IO, Hougaard KS, Spanó M, Bizzaro D, Manicardi GC, Lindh CH, et al. 2012. Sperm DNA integrity in relation to exposure to environmental perfluoroalkyl substances—a study of spouses of pregnant women in three geographical regions. Reprod Toxicol 33(4):577–583, PMID: 22449571, 10.1016/j.reprotox.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Telisman S, Cvitković P, Jurasović J, Pizent A, Gavella M, Rocić B. 2000. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ Health Perspect 108(1):45–53, PMID: 10620523, 10.2307/3454294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft G, Jönsson BAG, Lindh CH, Giwercman A, Spano M, Heederik D, et al. 2012. Exposure to perfluorinated compounds and human semen quality in Arctic and European populations. Hum Reprod 27(8):2532–2540, PMID: 22647447, 10.1093/humrep/des185. [DOI] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Wong MH, Lee KF, Yeung WSB, Giesy JP, et al. 2011. Testicular signaling is the potential target of perfluorooctanesulfonate-mediated subfertility in male mice. Biol Reprod 84(5):1016–1023, PMID: 21209418, 10.1095/biolreprod.110.089219. [DOI] [PubMed] [Google Scholar]
- Wang SW, Huang J, Yang Y, Hui YM, Ge YX, Larssen T, et al. 2013. First report of a Chinese PFOS alternative overlooked for 30 years: its toxicity, persistence, and presence in the environment. Environ Sci Technol 47(18):10163–10170, PMID: 23952109, 10.1021/es401525n. [DOI] [PubMed] [Google Scholar]
- Wang T, Vestergren R, Herzke D, Yu JC, Cousins IT. 2016. Levels, isomer profiles, and estimated riverine mass discharges of perfluoroalkyl acids and fluorinated alternatives at the mouths of Chinese rivers. Environ Sci Technol 50(21):11584–11592, PMID: 27689437, 10.1021/acs.est.6b03752. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Webster TF. 2017. Trade-offs of personal versus more proxy exposure measures in environmental epidemiology. Epidemiology 28(5):635–643, PMID: 28520644, 10.1097/EDE.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2010. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed Geneva, Switzerland: WHO; http://apps.who.int/iris/bitstream/handle/10665/44261/9789241547789_eng.pdf [accessed 7 May 2019]. [Google Scholar]
- World Bank. 2017. China—Reduction and Phase-out of PFOS in Priority Sectors Project. https://www.worldbank.org/en/news/loans-credits/2017/04/07/china-reduction-and-phase-out-of-pfos-in-priority-sectors-project [accessed 17 October 2019].
- Xu B, Chia SE, Tsakok M, Ong CN. 1993. Trace-elements in blood and seminal plasma and their relationship to sperm quality. Reprod Toxicol 7(6):613–618, PMID: 8118112, 10.1016/0890-6238(93)90038-9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Arisawa K, Uemura H, Katsuura-Kamano S, Takami H, Sawachika F, et al. 2013. Consumption of seafood, serum liver enzymes, and blood levels of PFOS and PFOA in the Japanese population. J Occup Health 55(3):184–194, PMID: 23574777, 10.1539/joh.12-0264-oa. [DOI] [PubMed] [Google Scholar]
- Zhang HX, Lu Y, Luo B, Yan SM, Guo XJ, Dai JY. 2014. Proteomic analysis of mouse testis reveals perfluorooctanoic acid-induced reproductive dysfunction via direct disturbance of testicular steroidogenic machinery. J Proteome Res 13(7):3370–3385, PMID: 24940614, 10.1021/pr500228d. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liang JR, Zhu HY, Li CZ, Wu Q. 2013. PFOS and PCB 153 have direct adverse effects on neonatal testis modeled using a coculture of primary gonocyte and Sertoli cells. Environ Toxicol 28(6):322–331, PMID: 21544924, 10.1002/tox.20723. [DOI] [PubMed] [Google Scholar]
- Zhang LY, Ren XM, Guo LH. 2013. Structure-based investigation on the interaction of perfluorinated compounds with human liver fatty acid binding protein. Environ Sci Technol 47(19):11293–11301, PMID: 24006842, 10.1021/es4026722. [DOI] [PubMed] [Google Scholar]
- Zhang T, Sun HW, Lin Y, Qin XL, Zhang YF, Geng X, et al. 2013. Distribution of poly- and perfluoroalkyl substances in matched samples from pregnant women and carbon chain length related maternal transfer. Environ Sci Technol 47(14):7974–7981, PMID: 23777259, 10.1021/es400937y. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Hu LW, Qian ZM, Chang JJ, King C, Paul G, et al. 2016. Association of perfluoroalkyl substances exposure with reproductive hormone levels in adolescents: by sex status. Environ Int 94:189–195, PMID: 27258660, 10.1016/j.envint.2016.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.