Abstract
This review summarizes a presentation given during the “Countermeasures to Cardiovascular Aging Symposium” that was part of the American Physiological Society Conference on Cardiovascular Aging: New Frontiers and Old Friends held in Westminster, CO, in August 2017. Endothelial dysfunction, a characteristic of vascular aging, is a major risk factor for age-associated cardiovascular diseases. In women, the decline in endothelial function is attenuated until menopause, whereafter the rate of decline accelerates to match that seen in men. Sex differences in the decline in endothelial function have been attributed to changes in sex hormones with aging. Women have a progressive impairment in endothelial function across the stages of the menopause transition, related in part to declining estradiol levels. In contrast to women, little is known about the impact of declining testosterone levels on endothelial function in men. Some evidence suggests greater endothelial dysfunction in men with low testosterone compared with men with higher testosterone. The underlying causes of endothelial dysfunction with sex hormone deficiency are unknown but may be related to endothelial nitric oxide synthase dysfunction and oxidative stress. Lifestyle behaviors, including habitual endurance exercise, attenuates and reverses the age-associated decline in endothelial function in older men. However, in older women, these exercise adaptations are diminished or absent, possibly related to estrogen deficiency. Understanding how declines in sex hormones contribute to the vascular aging process in both women and men will inform effective sex-specific intervention strategies to preserve vascular health and prevent cardiovascular diseases.
Keywords: aging, endothelial biology, exercise, women
INTRODUCTION
Cardiovascular disease (CVD) is the number one cause of death in adults in the United States, being responsible for ~800,000 deaths annually (5). The prevalence of CVD increases with advancing age in both women and men. Women have a lower prevalence of CVD until midlife, but by the sixth and seventh decades, the prevalence rates are similar between the sexes (5). The lower prevalence of CVD in women in their premenopausal years has been attributed to the female sex hormone estradiol, which is diminished during the menopause transition. The impact of sex hormones on vascular aging in adults may help to explain some of the observed sex differences in age-associated CVD. Endothelial dysfunction is a biomarker of vascular aging (27), and both estradiol and testosterone modulate endothelial function (16, 36). Regular aerobic exercise is associated with a reduced risk of CVD and also influences the vascular aging process. However, evidence suggests that the benefits of regular exercise on endothelial function may be sex specific and may be dependent on the sex hormone environment (39).
This brief review provides a summary of the presentation on the modulatory role of sex hormones on vascular aging that was given during the “Countermeasures to Cardiovascular Aging Symposium” as part of the 2017 American Physiological Society Conference on Cardiovascular Aging: New Frontiers and Old Friends. In this review, the effects of sex hormones on age-associated endothelial dysfunction in healthy adult women and men, as well as the modulatory influence of sex hormones on endothelial adaptations to regular exercise training are discussed.
SEX DIFFERENCES IN AGE-ASSOCIATED ENDOTHELIAL DYSFUNCTION
The vascular endothelium is a single layer of cells that provides a protective barrier to preserve the integrity of the vascular wall. One of the key features of age-associated endothelial dysfunction is a decrease in endothelium-dependent vasodilation. Sex differences in the rate of decline in endothelium-dependent vasodilation have been reported (7, 44). Endothelium-dependent vasodilation, measured via brachial artery flow-mediated dilation (FMD), was preserved in men until the fourth decade of life, whereas in women, FMD was preserved until the fifth decade but subsequently then declined at a faster rate compared with men (7). There was no effect of age on the brachial artery response to nitroglycerine, indicating that vascular smooth muscle cell function was not impaired. Similar observations have been reported in the coronary circulation; coronary endothelial function was greater in cycling women under the age of 50 yr compared with age-matched men but comparable between postmenopausal women and men over the age of 50 yr (32). Because the age at which endothelial dysfunction observed in women coincided with the age typical of menopause, estrogen was suggested to provide endothelial protection in premenopausal women that is lost as women become postmenopausal (7, 32).
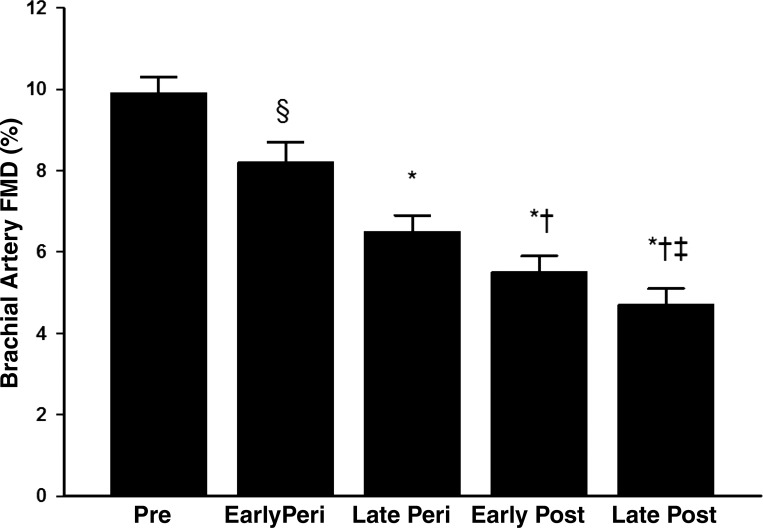
The menopause transition, or perimenopause, is a period of time of profound changes in the hormonal environment that can take several years and ends after 1 yr of amenorrhea (41). Because previous aging studies only classified women as premenopausal or postmenopausal (7, 44), we investigated whether the onset of endothelial dysfunction occurred during the perimenopausal years in healthy women categorized into stages of the menopausal transition. Endothelial function was progressively reduced across the stages of the menopause transition, even after statistical adjustment for age and CVD risk factors (Fig. 1). In late perimenopausal women, the reduction in brachial artery FMD (6.5%) was more pronounced than early perimenopausal women despite being similar age (34). These data support the idea that declining ovarian function across the stages of the menopause transition contributes to vascular aging in women.
Fig. 1.
Endothelial function measured via brachial artery flow-mediated dilation (FMD) is progressively reduced across the stages of the menopause transition. *P < 0.001 and §P = 0.03 vs. premenopausal women; †P < 0.001 vs. early perimenopausal women; ‡P < 0.001 vs. late perimenopausal women. Premenopausal comprises regular menstrual cycles with no change in usual cycle length (21–35 days); early perimenopausal (peri) comprises >2 cycles with cycle length changes of ≥7 days; late perimenopausal comprises ≥2 but <12 mo of amenorrhea; early postmenopausal (post) comprises ≤5 yr postmenopause; and late postmenopausal comprises >5 yr postmenopause. [From Moreau et al. (34).]
Little is known about how declines in gonadal hormones contribute to vascular aging in men. Unlike women, whose endogenous estradiol levels undergo an abrupt decrease with menopause, a parallel change in testosterone is not observed in men. Although total and bioavailable (free) testosterone levels decline with age, only 20% of 60-yr-old men and 50% of 80-yr-old men having serum total testosterone below the normal range for young men (18). In population-based studies including men with CVD risk factors, low serum testosterone is associated with reduced endothelial function (2, 12). However, the role of testosterone deficiency in modulating the age-associated decline in endothelial function in the absence of disease is less clear. What is also less clear is whether changes in estradiol levels contribute to the decline in endothelial dysfunction with aging in men. In young healthy men, endothelial function decreased after 6 wk of treatment with the aromatase inhibitor anastrozole, suggesting a possible modulatory role of estrogens on endothelial function in men (29).
BIOLOGICAL MECHANISMS MEDIATING ENDOTHELIAL DYSFUNCTION WITH SEX HORMONE DEFICIENCY
To determine the mechanisms underlying endothelial dysfunction with sex hormone deficiency, it is important to understand the independent actions of estradiol and testosterone in women and men. Both estradiol and testosterone have acute vasorelaxing properties, producing dilation in coronary and peripheral large arteries via nongenomic endothelium-dependent and -independent (i.e., direct effects on the vascular smooth muscle cells) mechanisms (33, 37, 38, 43, 49). The endothelium-dependent effects of both hormones are related, in part, to an increase in nitric oxide (NO) bioavailablity (9, 33) via estrogen and androgen receptor-mediated activation of endothelial NO synthase (eNOS), the enzyme that synthesizes NO from the substrate l-arginine (9, 33, 51). The activation of eNOS is related, in part, to phosphorylation of eNOS via the activation by phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway. The endothelium-independent effects of both sex hormones involve the PI3K-Akt/NO/cGMP/PKG signaling pathway, resulting in stimulation of large-conductance Ca2+-activated K+ (BKCa) channels, vascular smooth muscle cell repolarization, and vasodilation (10, 38).
In postmenopausal women, we (35, 36) and others (43, 46) have shown improvements in endothelial function with estradiol treatment; however, endothelial function is not restored to levels seen in premenopausal women. The FMD response with estradiol treatment in postmenopausal women is similar to that observed in perimenopausal women (34–36), suggesting that aging is also a contributing factor to endothelial dysfunction in women. In this regard, Sherwood et al. (43) reported that postmenopausal women aged 50–59 yr had a marked improvement in endothelial function 18 h after estradiol treatment, whereas women aged 60–79 yr showed no evidence of improvement.
Similar to women, testosterone treatment has been shown to improve endothelial function in men. Brachial artery FMD and nitroglycerine-mediated (i.e., endothelium-independent) vasodilation improved after 12 wk of oral testosterone in men with coronary artery disease (21). We recently showed an increase in endothelial function from after 6 and 12 mo treatment with testosterone in older men who had borderline to low testosterone levels (19). In contrast, other studies have demonstrated no beneficial effect and even potential harm with testosterone administration (20, 30). These findings may be due to administering testosterone to men who are not sufficiently androgen deficient or, alternatively, the inability of the exogenous testosterone to adequately increase testosterone to levels needed to improve vasodilation (30).
The regulatory role of estradiol in men, and testosterone in women, on endothelial function is not completely understood. Resistance vessel endothelial function improved after 8 wk of low-dose estradiol in hypogonadal older men (26), and 6 wk of parenteral testosterone improved endothelial function in postmenopausal women who were chronically using estradiol therapy (50).
The decline in endothelial function with aging, is attributed in part, to reduced bioavailability of NO, secondary to NO scavenging by reactive oxygen species (ROS; Fig. 2) (25). We (36) and others (13) have shown that infusion of the antioxidant vitamin C, a common experimental model that temporarily and reversibly reduces ROS and removes the tonic oxidative stress-related suppression of endothelial function, increased brachial artery FMD in both older sedentary men and estrogen-deficient postmenopausal women.
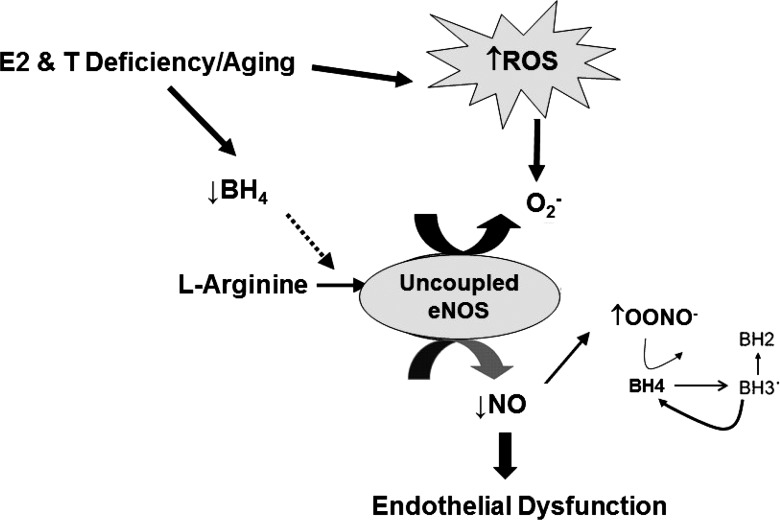
Fig. 2.
Working model of two of the key underlying mechanisms by which declines in sex hormones with the menopause transition in women, and andropause in men, contributes to age-associated endothelial dysfunction. Estrogen and testosterone deficiency, coupled with aging, results in an overproduction of reactive oxygen species (ROS) due to the loss of the antioxidant properties of estrogen and testosterone. Sex hormone deficiency may also reduce tetrahydropbiopterin (BH4) synthesis, causing endothelial nitric oxide (NO) synthase (eNOS) to uncouple and produce superoxide () instead of NO. Collectively, the increase in ROS would inactivate NO and increase the production of peroxynitrite (ONOO−), which would oxidize BH4 and cause further uncoupling of eNOS and increased production, resulting in reduced NO bioavailability and impaired endothelial function.
Evidence suggests that sex hormone deficiency likely contributes to age-associated oxidative stress suppression of endothelial function. Both estradiol and testosterone modulate ROS generation within multiple cellular compartments, including the plasma membrane (e.g., NADPH oxidase), peroxisomes (e.g., lipid oxidation), mitochondria (e.g., oxidative phosphorylation), and cytoplasm (e.g., xanthine oxidase) (1, 4, 22, 23, 47). Virdis et al. (45) demonstrated that a local infusion of vitamin C reversed resistance vessel endothelial dysfunction induced by oophorectomy in premenopausal women. Moreover, vitamin C infusion did not affect resistance vessel endothelial function in the women before oophorectomy, in healthy controls, or in oophorectomized women treated with estradiol for 3 mo (45). Collectively, these data support the idea that the impairment in endothelial function with estrogen deficiency is mediated, at least in part, by oxidative stress (Fig. 2). Whether low testosterone contributes to oxidative stress-induced vascular aging in men is unclear and warrants further investigation.
A second key mechanism underlying age-associated reductions in NO bioavailability is decreased NO biosynthesis related to eNOS dysfunction (Fig. 2). Under physiological conditions, eNOS produces NO from the interaction with l-arginine and tetrahydrobiopterin (BH4), an essential cofactor for normal eNOS function. However, if BH4 is limited, eNOS can uncouple, resulting in increased production of superoxide instead of NO (3). Peroxynitrite and other oxidases (i.e., NADPH oxidase) can oxidize BH4 to BH2, reducing its bioavailability for eNOS (3, 48). In older men, BH4 supplementation restored endothelial function to levels observed in young men (14).
Although no data are available with regards to testosterone, there is evidence to suggest that estradiol may regulate BH4 bioavailability and eNOS function. Aortic BH4 content, NO, and endothelial vasodilatory function were higher in estradiol-treated animals compared with ovariectomized animals (28). Additionally, we showed that brachial artery FMD increased 3 h after oral BH4 administration in estrogen-deficient postmenopausal women but, as expected, had no effect in premenopausal controls or in postmenopausal women treated with estradiol (35).
SEX HORMONE REGULATION OF ENDOTHELIAL ADAPTATIONS TO EXERCISE TRAINING
Regular exercise is promoted as a therapeutic strategy for delaying and improving vascular aging. However, evidence suggests sex specificity to endothelial adaptions to endurance exercise training in older adults. Although endurance exercise has been shown to prevent and even restore the age-related endothelial dysfunction in older men (11, 14, 39), older women have minimal to no endothelial adaptations to endurance exercise (6, 36, 39, 42). We recently demonstrated that endothelial function is improved with endurance exercise training in postmenopausal women treated with estradiol but not in those treated with placebo, suggesting an essential role of estradiol in vascular adaptations to endurance exercise in women (36).
The mechanisms by which estradiol permits endothelial adaptations in postmenopausal women are unclear but could be related to an increased resistance to oxidative damage due to enhanced antioxidant defenses by estradiol. We previously demonstrated that vitamin C infusion increased brachial artery FMD in both sedentary and endurance-trained estrogen-deficient postmenopausal women and after 12 wk of endurance exercise training in placebo-treated postmenopausal women. In contrast, there was no effect of vitamin C after 12 wk of endurance exercise training in estradiol-treated postmenopausal women (36). In a swim training study conducted in female rats, oxidative stress markers increased and endogenous antioxidants levels decreased in ovariectomized female rats after 90 days of training but were decreased and increased, respectively, in intact female rats (31).
Estradiol and exercise may also work in synergy to modulate intracellular signaling and gene expression in endothelial cells. Exercise and estradiol share common intracellular signaling pathways to activate and phosphorylate eNOS to release NO: exercise via mechanosensors (i.e., integrins and G protein-coupled receptors) (52) and estradiol via estrogen receptors (8). Future studies should examine whether prescribing endurance exercise training with pharmacological/nonpharmacological therapies that can act via estrogen receptors (e.g., resveratrol and phytoestrogens) can enhance exercise training responses in estrogen-deficient postmenopausal women (24, 40).
Whether testosterone plays an essential role in vascular adaptations to exercise training in older men is unknown. A lifestyle intervention prescribing a healthy diet plus exercise training showed improvements in endothelial function after 6 and 12 wk in older men undergoing androgen deprivation therapy for prostate cancer compared with a nonlifestyle control intervention, but endothelial function was no longer different between the two interventions by 24 wk (17). In contrast, in a parallel-arm, open-label observation study of diet plus exercise with or without testosterone in severely obese hypogonadal men, endothelial function improved in the diet plus exercise plus testosterone condition but not in the diet plus exercise alone condition (15). Finally, we showed improvements in brachial artery FMD with progressive resistance training in older men with borderline to low testosterone levels who were randomized to receive either low-dose or usual-dose testosterone but not in men treated with placebo (19).
SUMMARY
Vascular aging, featuring endothelial dysfunction, is the major risk factor for age-associated CVD. As the population ages, increasing numbers of adults will be living with CVD, and thus effective prevention strategies are needed to mitigate the negative impact on quality of life and substantial societal and economic burdens of CVD. Regular exercise is promoted as a firstline strategy for the primary prevention of CVD in older adults. Because declines in gonadal function may contribute to the vascular aging process in both women and men and may also diminish beneficial endothelial adaptations to exercise training, a better understanding of the impact of declines in sex hormones on the vascular endothelium at the cellular and systemic levels is needed to inform future sex-specific therapies for the prevention of CVD.
GRANTS
This work was supported by the National Institutes of Health Grants AG-027678, AG-049762, R56-HL-114073, AG-20683, P30-DK-048520, and UL1-TR-001082, the University of Colorado Center for Women’s Health Research, and the Eastern Colorado Veterans Affairs Geriatric Research Education and Clinical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
K.L.M. conceived and designed research; K.L.M. prepared figures; K.L.M. drafted manuscript; K.L.M. edited and revised manuscript; K.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The author thanks the collaborators and colleagues within the Investigators in Metabolism, Aging, Gender, and Exercise Research Group for support.
REFERENCES
- 1.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res 892: 255–262, 2001. doi: 10.1016/S0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 2.Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M, Ouchi Y. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res 30: 1029–1034, 2007. doi: 10.1291/hypres.30.1029. [DOI] [PubMed] [Google Scholar]
- 3.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413–420, 2004. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 4.Barud W, Palusiński R, Bełtowski J, Wójcicka G. Inverse relationship between total testosterone and anti-oxidized low density lipoprotein antibody levels in ageing males. Atherosclerosis 164: 283–288, 2002. doi: 10.1016/S0021-9150(02)00069-2. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2017 update: a report from the American Heart Association. Circulation 135: e146–e603, 2017. [Erratum in Circulation 135: e646, 2017]. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey DP, Pierce GL, Howe KS, Mering MC, Braith RW. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol 100: 403–408, 2007. doi: 10.1007/s00421-007-0447-2. [DOI] [PubMed] [Google Scholar]
- 7.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 8.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev 23: 665–686, 2002. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 9.Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation 94: 2614–2619, 1996. doi: 10.1161/01.CIR.94.10.2614. [DOI] [PubMed] [Google Scholar]
- 10.Deenadayalu V, Puttabyatappa Y, Liu AT, Stallone JN, White RE. Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase. Am J Physiol Heart Circ Physiol 302: H115–H123, 2012. doi: 10.1152/ajpheart.00046.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. doi: 10.1161/01.CIR.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 12.Empen K, Lorbeer R, Dörr M, Haring R, Nauck M, Glaser S, Krebs A, Reffelmann T, Ewert R, Völzke H, Wallaschofski H, Felix SB. Association of testosterone levels with endothelial function in men: results from a population-based study. Arterioscler Thromb Vasc Biol 32: 481–486, 2011. doi: 10.1161/ATVBAHA.111.232876. [DOI] [PubMed] [Google Scholar]
- 13.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francomano D, Bruzziches R, Barbaro G, Lenzi A, Aversa A. Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: a pilot study. J Endocrinol Invest 37: 401–411, 2014. doi: 10.1007/s40618-014-0066-9. [DOI] [PubMed] [Google Scholar]
- 16.Francomano D, Fattorini G, Gianfrilli D, Paoli D, Sgrò P, Radicioni A, Romanelli F, Di Luigi L, Gandini L, Lenzi A, Aversa A. Acute endothelial response to testosterone gel administration in men with severe hypogonadism and its relationship to androgen receptor polymorphism: a pilot study. J Endocrinol Invest 39: 265–271, 2016. doi: 10.1007/s40618-015-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert SE, Tew GA, Fairhurst C, Bourke L, Saxton JM, Winter EM, Rosario DJ. Effects of a lifestyle intervention on endothelial function in men on long-term androgen deprivation therapy for prostate cancer. Br J Cancer 114: 401–408, 2016. doi: 10.1038/bjc.2015.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging . Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 86: 724–731, 2001. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 19.Hildreth KL, Schwartz RS, Vande Griend J, Kohrt WM, Blatchford PJ, Moreau KL. Effects of testosterone and progressive resistance exercise on vascular function in older men. J Appl Physiol 125: 1693–1701, 2018. doi: 10.1152/japplphysiol.00165.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones RD, Hugh Jones T, Channer KS. The influence of testosterone upon vascular reactivity. Eur J Endocrinol 151: 29–37, 2004. doi: 10.1530/eje.0.1510029. [DOI] [PubMed] [Google Scholar]
- 21.Kang SM, Jang Y, Kim J, Chung N, Cho SY, Chae JS, Lee JH. Effect of oral administration of testosterone on brachial arterial vasoreactivity in men with coronary artery disease. Am J Cardiol 89: 862–864, 2002. doi: 10.1016/S0002-9149(02)02202-6. [DOI] [PubMed] [Google Scholar]
- 22.Keaney JF Jr, Shwaery GT, Xu A, Nicolosi RJ, Loscalzo J, Foxall TL, Vita JA. 17 beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation 89: 2251–2259, 1994. doi: 10.1161/01.CIR.89.5.2251. [DOI] [PubMed] [Google Scholar]
- 23.Kemper MF, Stirone C, Krause DN, Duckles SP, Procaccio V. Genomic and non-genomic regulation of PGC1 isoforms by estrogen to increase cerebral vascular mitochondrial biogenesis and reactive oxygen species protection. Eur J Pharmacol 723: 322–329, 2014. doi: 10.1016/j.ejphar.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem 280: 7460–7468, 2005. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 25.Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 43: 562–571, 1999. doi: 10.1016/S0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 26.Komesaroff PA, Fullerton M, Esler MD, Dart A, Jennings G, Sudhir K. Low-dose estrogen supplementation improves vascular function in hypogonadal men. Hypertension 38: 1011–1016, 2001. doi: 10.1161/hy1101.095006. [DOI] [PubMed] [Google Scholar]
- 27.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 28.Lam KK, Lee YM, Hsiao G, Chen SY, Yen MH. Estrogen therapy replenishes vascular tetrahydrobiopterin and reduces oxidative stress in ovariectomized rats. Menopause 13: 294–302, 2006. doi: 10.1097/01.gme.0000182806.99137.5e. [DOI] [PubMed] [Google Scholar]
- 29.Lew R, Komesaroff P, Williams M, Dawood T, Sudhir K. Endogenous estrogens influence endothelial function in young men. Circ Res 93: 1127–1133, 2003. doi: 10.1161/01.RES.0000103633.57225.BC. [DOI] [PubMed] [Google Scholar]
- 30.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev 24: 313–340, 2003. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 31.Macedo UB, Martins RR, Freire Neto FP, Oliveira YM, Medeiros AC, Brandão-Neto J, Rezende AA, Almeida MD. Oophorectomy hinders antioxidant adaptation promoted by swimming in Wistar rats. Appl Physiol Nutr Metab 38: 148–153, 2013. doi: 10.1139/apnm-2012-0121. [DOI] [PubMed] [Google Scholar]
- 32.Mathews L, Iantorno M, Schär M, Bonanno G, Gerstenblith G, Weiss RG, Hays AG. Coronary endothelial function is better in healthy premenopausal women than in healthy older postmenopausal women and men. PLoS One 12: e0186448, 2017. doi: 10.1371/journal.pone.0186448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendelsohn ME. Mechanisms of estrogen action in the cardiovascular system. J Steroid Biochem Mol Biol 74: 337–343, 2000. doi: 10.1016/S0960-0760(00)00110-2. [DOI] [PubMed] [Google Scholar]
- 34.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97: 4692–4700, 2012. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 302: H1211–H1218, 2012. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong PJ, Patrizi G, Chong WC, Webb CM, Hayward CS, Collins P. Testosterone enhances flow-mediated brachial artery reactivity in men with coronary artery disease. Am J Cardiol 85: 269–272, 2000. doi: 10.1016/S0002-9149(99)00630-X. [DOI] [PubMed] [Google Scholar]
- 38.Perusquía M, Stallone JN. Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am J Physiol Heart Circ Physiol 298: H1301–H1307, 2010. doi: 10.1152/ajpheart.00753.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 120: 13–23, 2011. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riesco E, Aubertin-Leheudre M, Maltais ML, Audet M, Dionne IJ. Synergic effect of phytoestrogens and exercise training on cardiovascular risk profile in exercise-responder postmenopausal women: a pilot study. Menopause 17: 1035–1039, 2010. doi: 10.1097/gme.0b013e3181da7915. [DOI] [PubMed] [Google Scholar]
- 41.Santoro N. Perimenopause: from research to practice. J Womens Health (Larchmt) 25: 332–339, 2016. doi: 10.1089/jwh.2015.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos-Parker JR, Strahler TR, Vorwald VM, Pierce GL, Seals DR. Habitual aerobic exercise does not protect against micro- or macrovascular endothelial dysfunction in healthy estrogen-deficient postmenopausal women. J Appl Physiol 122: 11–19, 2017. doi: 10.1152/japplphysiol.00732.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol 27: 1782–1787, 2007. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- 44.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582, 1996. doi: 10.1161/01.HYP.28.4.576. [DOI] [PubMed] [Google Scholar]
- 45.Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation 101: 2258–2263, 2000. doi: 10.1161/01.CIR.101.19.2258. [DOI] [PubMed] [Google Scholar]
- 46.Vitale C, Mercuro G, Cerquetani E, Marazzi G, Patrizi R, Pelliccia F, Volterrani M, Fini M, Collins P, Rosano GM. Time since menopause influences the acute and chronic effect of estrogens on endothelial function. Arterioscler Thromb Vasc Biol 28: 348–352, 2008. doi: 10.1161/ATVBAHA.107.158634. [DOI] [PubMed] [Google Scholar]
- 47.Wagner AH, Schroeter MR, Hecker M. 17β-Estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J 15: 2121–2130, 2001. doi: 10.1096/fj.01-0123com. [DOI] [PubMed] [Google Scholar]
- 48.Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 44: 381–386, 2004. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- 49.Webb CM, McNeill JG, Hayward CS, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation 100: 1690–1696, 1999. doi: 10.1161/01.CIR.100.16.1690. [DOI] [PubMed] [Google Scholar]
- 50.Worboys S, Kotsopoulos D, Teede H, McGrath B, Davis SR. Evidence that parenteral testosterone therapy may improve endothelium-dependent and -independent vasodilation in postmenopausal women already receiving estrogen. J Clin Endocrinol Metab 86: 158–161, 2001. doi: 10.1210/jcem.86.1.7103. [DOI] [PubMed] [Google Scholar]
- 51.Yu J, Akishita M, Eto M, Ogawa S, Son BK, Kato S, Ouchi Y, Okabe T. Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology 151: 1822–1828, 2010. doi: 10.1210/en.2009-1048. [DOI] [PubMed] [Google Scholar]
- 52.Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol 587: 3911–3920, 2009. doi: 10.1113/jphysiol.2009.172916. [DOI] [PMC free article] [PubMed] [Google Scholar]