Abstract
We are investigating the changes in hepatic lipid catabolism that contribute to alcohol-induced fatty liver. Following chronic ethanol (EtOH) exposure, abstinence from alcohol resolves steatosis. Here, we investigated the hepatocellular events that lead to this resolution by quantifying specific catabolic parameters that returned to control levels after EtOH was withdrawn. We hypothesized that, after its chronic consumption, EtOH withdrawal reactivates lipid catabolic processes that restore lipostasis. Male Wistar rats were fed control and EtOH liquid diets for 6 wk. Randomly chosen EtOH-fed rats were then fed control diet for 7 days. Liver triglycerides (TG), lipid peroxides, key markers of fatty acid (FA) metabolism, lipophagy, and autophagy were quantified. Compared with controls, EtOH-fed rats had higher hepatic triglycerides, lipid peroxides, and serum free fatty acids (FFA). The latter findings were associated with higher levels of FA transporters (FATP 2, 4, and 5) but lower quantities of peroxisome proliferator-activated receptor-α (PPAR-α), which governs FA oxidation. EtOH-fed animals also had lower nuclear levels of the autophagy-regulating transcription factor EB (TFEB), associated with lower hepatic lipophagy and autophagy. After EtOH-fed rats were refed control diet for 7 days, their serum FFA levels and those of FATPs fell to control (normal) levels, whereas PPAR-α levels rose to normal. Hepatic TG and malondialdehyde levels in EtOH-withdrawn rats declined to near control levels. EtOH withdrawal restored nuclear TFEB content, hepatic lipophagy, and autophagy activity to control levels. EtOH withdrawal reversed aberrant FA metabolism and restored lysosomal function to promote resolution of alcohol-induced fatty liver.
NEW & NOTEWORTHY Here, using an animal model, we show mechanisms of reversal of fatty liver and injury following EtOH withdrawal. Our data indicate that reactivation of autophagy and lysosome function through the restoration of transcription factor EB contribute to reversal of fatty liver and injury following EtOH withdrawal.
Keywords: ethanol, fatty acid, oxidant stress, steatosis, TFEB
INTRODUCTION
Among the many organs affected by alcohol consumption, the liver sustains the major damage because alcohol is primarily metabolized in the liver (15, 46). Alcohol is metabolized via the cytosolic alcohol dehydrogenase (ADH) and the microsomal cytochrome P-450 2E1 (CYP2E1) (8, 26). Both enzymes oxidize alcohol to generate acetaldehyde, a highly reactive and toxic metabolite that is further oxidized to acetate by the mitochondrial aldehyde dehydrogenase 2 (ALDH2) (8). CYP2E1 metabolism of alcohol also generates excess reactive oxygen species (ROS); this is exacerbated after the enzyme is induced by ethanol (EtOH) (8, 26). ROS produced by alcohol metabolism react with cellular macromolecules, promoting injury due to oxidant stress and alteration of biological function (8, 15, 26).
One of the earliest pathophysiological changes that occur in the liver due to alcohol consumption is the accumulation of fat in hepatocytes (fatty liver or steatosis) (24, 35, 46). This initiates the pathogenesis of alcoholic liver disease (ALD), since fatty liver is considered the “first hit” of alcohol-associated liver disease (AALD), which increases the liver’s vulnerability to “second hit” factors such as gut-derived endotoxins that leak through tight junctions of the gut wall and enter the portal vein to initiate and promote hepatic inflammation and fibrosis (46, 48). Alcoholic fatty liver disease (AFLD) is the first stage in the spectrum of liver injury brought about by alcohol consumption (25), and it is generally reversible with abstinence from alcohol (43). Although accepted from a clinical standpoint that abstinence from alcohol reverses AFLD (18, 43, 45), the molecular mechanisms of recovery from alcoholic fatty liver (AFL) remain unclear. Work from our laboratory showed that EtOH-impaired receptor-mediated endocytosis could be restored to control levels in rats refed control diet for 7 days following 5 wk of EtOH feeding (4). In those studies we did not measure fat content or fat metabolism to see if those parameters returned to normal. In the present study, we took advantage of our EtOH withdrawal and refeeding model to understand mechanisms that contribute to resolution of AFL upon alcohol withdrawal because understanding resolution of fatty liver is not only critical for prognosis of the disease but also for development of targeted therapies to block the progression to more advanced stages of liver disease.
From extensive work carried out in rodent models (1) of alcohol-induced liver injury, it is well established that feeding alcohol in a liquid diet produces fatty liver and mild injury (25, 34). Findings from our laboratory indicate that chronic EtOH exposure disrupts hepatic protein and lipid metabolism, and trafficking, to contribute to AFL and injury in rodents (10, 13, 30, 31, 35, 50). In our more recent work we reported that chronic EtOH exposure disrupts lipophagy, partly by reducing lysosome motility (40) and their numbers to levels that were inadequate to carry out lipid droplet (LD) breakdown (36). Lipophagy is a form of autophagy that selectively degrades LD. Its disruption contributes to hepatic steatosis (36). Here we examined whether refeeding control diet after chronic EtOH administration (to simulate recovery in an animal model) reverses EtOH-induced alterations in lipid metabolism, thereby contributing to recovery from fatty liver. We found that EtOH withdrawal normalizes aberrant fatty acid transport and faulty fatty acid (FA) oxidation and restores cellular degradation machinery, including lysosomal hydrolases and the proteasome, thereby attenuating EtOH-induced fatty liver and injury.
MATERIALS AND METHODS
Reagents.
Antibodies to GAPDH and β-actin were from Millipore Sigma (Burlington, MA). Microtubule-associated protein-1 light chain 3B antibody was from Cell Signaling Technology (Danvers, MA). Anti-P62/sequestosome-1 was purchased from Medical and Biological Laboratories. We obtained peroxisome proliferator-activated receptor-α (PPAR-α) antibody from Santa Cruz (Dallas, TX). Anti-fatty acid-binding protein (FABP4) and anti-cluster of differentiation 36 (CD36) were from Novus Biologicals (Littleton, CO). Anti-fatty acid transporter (FATP) 2 and anti-FATP-5 were from Mybiosource (San Diego, CA) and Biorbyt (San Francisco, CA), respectively. We purchased histone 3 (H3) antibody from Invitrogen (Carlsbard, CA). Anti-ADH was a gift from Dr. Michael Felder, University of South Carolina. Anti-transcription factor EB (TFEB) was purchased from Bethyl Laboratories (Montgomery, TX). Anti-CYP2E1 was from Calbiochem. We purchased protease inhibitor cocktail, the proteasome substrate N-succinyl-l-leucyl-l-leucyl-l-valyl-l-tyrosyl-7-amino-4-methyl-coumarin, and other specialized reagents from Sigma (St. Louis, MO).
Animal treatments.
All protocols were approved by the Institutional Animal Care and Use Committee at the Veterans’ Affairs Nebraska, Western Iowa Health Care System Research Service. We followed the eighth edition of the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health. Male Wistar rats weighing 175–200 g, purchased from Charles River Laboratories (Portage, MI), were weight matched and fed control or EtOH-containing Lieber-DeCarli diets for 6 wk (36). Next, randomly chosen EtOH-fed rats were gradually weaned from the EtOH diet to avoid withdrawal symptoms. On day 1 of weaning, rats were given two parts of EtOH diet plus one part of control diet. On day 2, we fed rats with equal parts of EtOH and control diets. From days 3 to 7, we fed EtOH-free control diet as described previously (4). At euthanize rats, we collected blood from the axillary artery. Following exsanguination and pneumothorax, the liver of each animal was removed, a portion was homogenized, and the homogenate was subjected to subcellular fractionation, as described (13). Hepatic nuclear fractions were prepared as detailed (29), and the relative purity of nuclear and cytosolic fractions was determined by the amounts of marker proteins, histone 3 for nuclear fractions and GAPDH for cytosolic fractions.
Serum analyses.
The clinical laboratory at the Omaha Veterans Affairs Medical Center performed automated measurements of alanine transaminase and aspartate transaminase activities in rat sera. We measured the levels of nonesterified fatty acids (NEFA) in sera, using a colorimetric assay from Cell Biolabs (San Diego, CA).
Proteasome, lysosomal cathepsins, and lysosomal acid lipase assays.
The chymotrypsin-like activity of the proteasome and activities of cathepsins B and L were assayed fluorometrically, as previously published (47). Lysosomal acid lipase (LAL) activity was measured after incubating tissue homogenates with the fluorogenic substrate 4-methylumbelliferone as described earlier.
RNA isolation and real-time PCR.
Total RNA, isolated from liver pieces using the Pure Link RNA mini kit (Invitrogen, Frederick, MD), was reverse transcribed using Taqman reverse transcription reagents (Applied Biosystems) to generate cDNAs. mRNA levels were quantified from amplified cDNA samples using PCR master mix, specific primers, and SYBR green as the fluorescent marker. Primers used for PCR are listed in Table 1. Relative mRNA levels were normalized to RNA encoding β-actin.
Table 1.
Primer sets used for detection of mRNAs encoding proteins that regulate fatty acid metabolism
| Gene | Forward Primer 5′–3′ | Reverse Primer 5′–3′ |
|---|---|---|
| CD36 | AACCCAGAGGAAGTGGCAAAG | GACAGTGAAGGCTCAAAGATGG |
| FABP-4 | GTAGAA GGGGAC TTGGTCGTC | GCC TTTCATGACACA TTCCAC |
| FATP-2 | TGACGATCACACA GGAA GGA | CCA GAAA TCTCTCGGACA GC |
| FATP-5 | TTACCC TCGACAACAACAACC | CTTCCC GTCC TTCA GTTTTCT |
| PPAR-α | GTCCTCTGGTTGTCCCCTTG | GTCAGTTCACAGGGAAGGCA |
CD36, cluster of differentiation 36; FABP-4, fatty acid-binding protein-4; FATP, fatty acid transporter; PPAR-α, peroxisome proliferator-activated receptor-α.
Hepatic triglycerides.
Preweighed frozen liver pieces were subjected to total lipid extraction (17). The filtered lipid extracts were saponified to quantify triglycerides using Thermo DMA reagent (Thermo Electron, Middletown, VA). Results were calculated as milligram triglyceride using a triolein standard and normalized per gram of liver.
Lipid peroxidation.
Crude liver homogenates were used to measure lipid peroxides as thiobarbituric acid reactive substances (TBARS), using a previously published procedure (3a). Malondialdehyde (MDA) was used as the standard, and results are reported as MDA equivalents per gram liver.
LD isolation.
LDs from crude liver extracts were purified by gradient centrifugation as described earlier (21). Briefly, postnuclear supernatant (PNS) fractions of liver homogenates were subjected to discontinuous sucrose gradient to collect the white band (LD fraction) at the top of the gradient. The latter band corresponding to LDs was further purified by additional centrifugation and washing steps as described (37). LD-enriched fractions were suspended in TE buffer for Western blot analysis.
Detection of proteins on Western blots.
Protein samples were separated under denaturing conditions on SDS-polyacrylamide minigels and transferred to nitrocellulose membranes. The membranes were incubated overnight with primary antibodies at 4°C. After being washed, membranes were incubated with secondary antibodies conjugated to green or red infrared dye for 1 h. Proteins were detected using the Odyssey infrared imaging system. We quantified protein band densities with Li-Cor analysis software.
Statistical analysis.
Data are expressed as means ± SE. We determined statistical significance between groups by one-way analysis of variance (ANOVA), using a Newman-Keuls post hoc analysis. A P value ≤ 0.05 was considered statistically significant.
RESULTS
EtOH withdrawal alleviated EtOH-induced oxidant stress and injury.
We sought to identify liver parameters that would return to normal after EtOH withdrawal in rats chronically fed EtOH for 6 wk. We observed initial signs of resolution of liver injury, including reductions in serum alanine transaminase (ALT) and aspartate transaminase (AST) activities and liver triglycerides as early as 2 and 3 days of feeding control diet following EtOH withdrawal (data not shown). However, we found that 7 days of feeding control diet following EtOH withdrawal (hereafter referred to as “7-day refed”) is necessary to achieve significant reversal of the parameters associated with liver pathology. Here, we describe all parameters that partially or completely returned to control levels after 7 days of EtOH withdrawal. Feeding the control or EtOH diet or refeeding the control diet following EtOH withdrawal did not alter body weights among the three groups (control = 380 ± 8 g; EtOH fed = 355 ± 11 g; 7-day refed = 372 ± 43 g). The relative liver weight (expressed as g/100 g body wt) was significantly higher in EtOH-fed animals than controls. EtOH withdrawal for 7 days did not significantly affect the relative liver weight of rats previously fed EtOH (control = 3.1 ± 0.08; EtOH fed = 4.0 ± 0.11; 7-day refed = 3.9 ± 0.1, P ≤ 0.0004). Hepatic protein content per 100 g body weight in EtOH-fed rats was significantly higher than that of pair-fed controls. EtOH withdrawal caused a partial decline in liver protein content (expressed as mg protein/100 g body wt) that had been elevated by EtOH feeding (control = 637 ± 30; EtOH fed = 829 ± 24; 7-day refed = 743 ± 33, P = 0.0002, ANOVA). However, liver protein content in EtOH-withdrawn animals was still significantly higher than pair-fed controls after 7 days of refeeding.
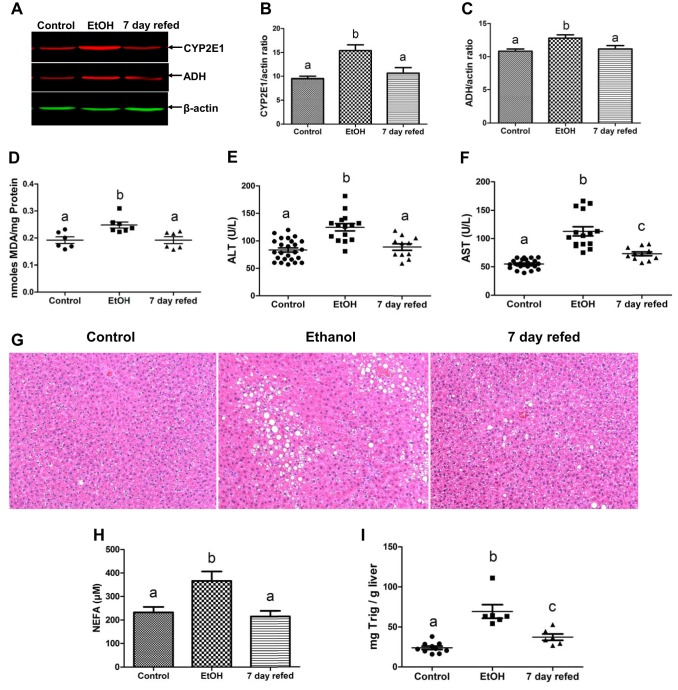
Compared with pair-fed controls, EtOH-fed rats exhibited mild liver injury as judged by higher activities of ALT and AST in their sera (Fig. 1). Both enzyme activities returned to control (ALT) or near control levels (AST) after EtOH withdrawal and refeeding control diet for 7 days. Compared with control animals, the contents of the major EtOH-metabolizing enzymes CYP2E1 and ADH were induced by 50 and 18%, respectively, in livers of EtOH-fed rats (Fig. 1, A–C), suggesting an acceleration of EtOH oxidation. Indeed, the latter inductions were associated with a 30% elevation in the levels of lipid peroxides, measured as MDA, an index of oxidant stress (Fig. 1D). EtOH withdrawal and refeeding returned ADH and CYP2E1 to control levels, with a concomitant decline in hepatic lipid peroxides (Fig. 1D).
Fig. 1.
Ethanol (EtOH) withdrawal alleviated EtOH-induced liver pathologies. Representative Western blot (A) and densitometric quantification of cytochrome P-450 2E1 (CYP2E1, B) and alcohol dehydrogenase (ADH, C) in liver homogenates. Hepatic lipid peroxides (D), serum alanine transaminase (ALT, E) and aspartate transaminase (AST, F), and hematoxylin and eosin (H&E)-stained paraffin section images obtained by light microscopy (G), serum nonesterified fatty acids (NEFA) levels (H), and liver triglycerides (I) in the livers of rats treated as indicated in the abscissa. Data are means ± SE of 6–25 animals/group. Bars with different letters are significantly different. Bars with the same letter are not significantly different, P ≤ 0.05.
EtOH withdrawal attenuated hepatic fat accumulation.
Hepatic fat accumulation, indicated by triglyceride levels, was 2.6-fold higher in livers of EtOH-fed rats than in pair-fed controls (Fig 1I). This was verified histologically in liver sections of EtOH-fed rats, clearly showing accumulation of LDs (Fig. 1G). We further detected 1.8-fold higher levels of NEFA in the sera of EtOH-fed rats. NEFAs are known to exacerbate EtOH-induced fatty liver, by their accelerated transport in liver cells and reesterification into triglycerides. EtOH withdrawal and refeeding control diet completely normalized serum NEFA levels to those of control animals (Fig. 1H), whereas in 7-day refed rats it only partially reversed liver triglycerides, which remained significantly higher than in pair-fed controls (Fig. 1I).
EtOH withdrawal normalizes the levels of signaling proteins that regulate hepatic fatty acid trafficking and oxidation.
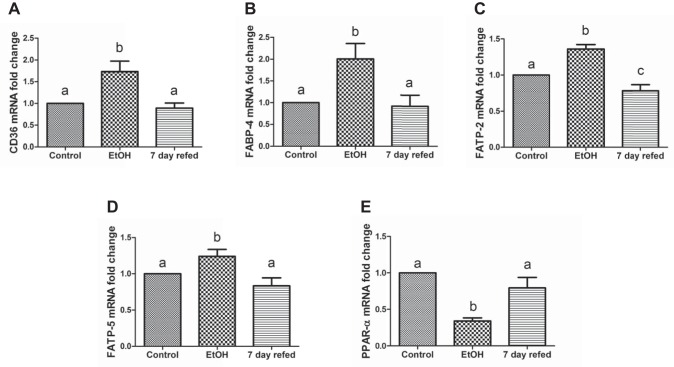
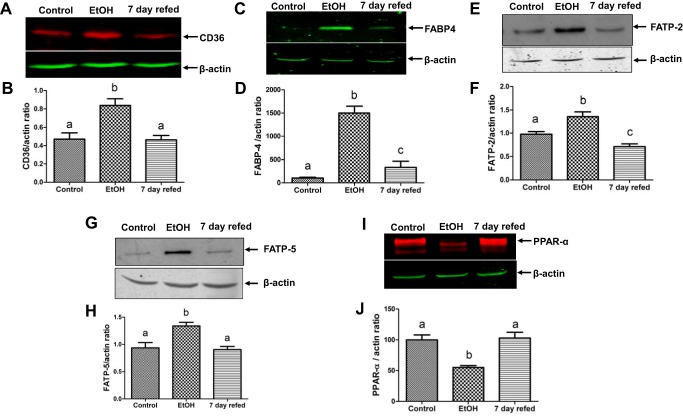
Serum free fatty acids taken up by the liver contribute to hepatic fat accumulation (55). Here we determined whether there is a corresponding increase in the levels of proteins involved in hepatocellular fatty acid uptake and trafficking in response to elevated serum NEFA levels induced by EtOH feeding (Fig. 1H). To this end, we measured the mRNA and protein levels of CD36, FABP-4, and fatty acid transport proteins FATP-2 and FATP-5. Each of these participates in fat metabolism by facilitating cellular fatty acid uptake and esterification with glycerol, thereby playing key roles in the development of fatty liver disease. EtOH feeding simultaneously elevated both the mRNA and the contents of all the aforementioned FA uptake and transport proteins (Figs. 2 and 3). However, when EtOH was withdrawn and replaced with control diet, the aforementioned mRNAs and the proteins they encode all returned to control levels (Figs. 2 and 3). The mRNA and protein levels of the transcription factor PPAR-α, which regulates genes encoding enzymes of fatty acid oxidation, were downregulated more than twofold in livers of EtOH-fed rats. EtOH withdrawal and refeeding control diet restored the mRNA and protein levels of PPAR-α to control levels (Figs. 2E and 3J).
Fig. 2.
Ethanol (EtOH) withdrawal normalized mRNA levels of proteins involved in fatty acid uptake, trafficking, and oxidation. mRNA levels of cluster of differentiation 36 (CD36, A) fatty acid-binding protein (FABP)-4 (B), fatty acid transporter (FATP)-2 (C), FATP-5 (D), and peroxisome proliferator-activated receptor-α (PPAR-α, E) in livers of rats treated as indicated in the abscissa. Data are means ± SE of 6–12 animals/group. Bars with different letters are significantly different. Bars with the same letter are not significantly different, P ≤ 0.05.
Fig. 3.
Ethanol (EtOH) withdrawal normalized proteins involved in fatty acid uptake, trafficking, and oxidation. Representative Western blot and densitometric quantification of cluster of differentiation 36 (CD36) in total homogenates (A and B), fatty acid-binding protein (FABP)-4 (C and D), fatty acid transporter (FATP)-2 (E and F), FATP-5 (G and H), and peroxisome proliferator-activated receptor-α (PPAR-α, I and J) in postnuclear supernatants made from liver homogenates of rats treated as indicated in the abscissa. Data are means ± SE of 6–8 animals/group. Bars with different letters are significantly different. Bars with the same letter are not significantly different, P ≤ 0.05.
EtOH withdrawal normalizes hepatic autophagy and proteasome activities.
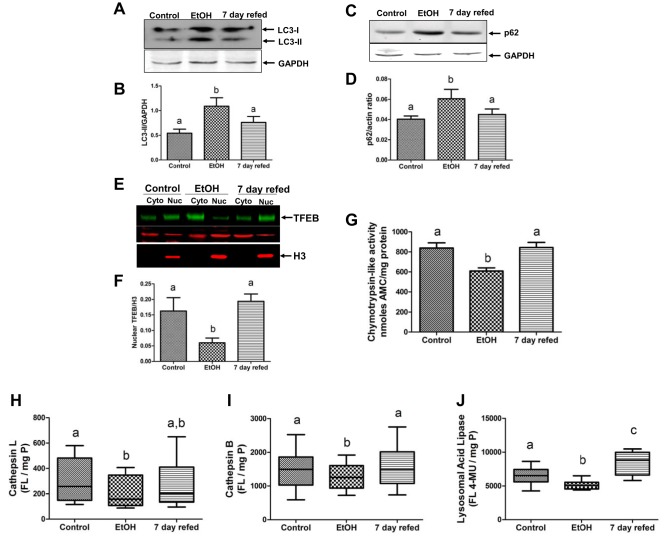
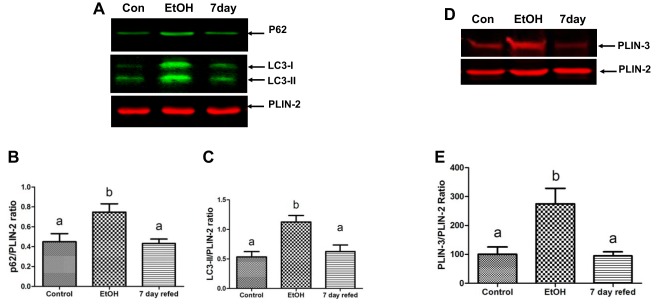
Autophagy and the ubiquitin proteasome system (UPS) are cellular macromolecule, organelle, and protein degradative pathways both capable of removing damaged organelles, excess LDs, damaged proteins, and protein aggregates (12, 33). Compared with livers of pair-fed controls, chronic EtOH feeding increased the hepatic levels of microtubule-associated protein 1 light chain 3 II (LC3-II), a marker of autophagosomes, which sequester and deliver cytoplasmic cargo to lysosomes for degradation (Fig. 4, A and B). The increase in hepatic LC3-II levels occurred simultaneously with an EtOH-induced rise in P62 (sequestosome-1) protein, an autophagic adapter protein that is degraded along with autophagic vacuole cargo during autophagy activation (Fig. 4, C and D) (22, 47, 50). The nuclear levels of TFEB, which activates genes involved in autophagy, lysosome, and mitochondrial biogenesis, were downregulated by EtOH exposure. Compared with controls, the mRNA encoding TFEB was also twofold lower in livers of EtOH-fed rats (see below). The protein levels of TFEB in the cytoplasm, which is the inactive form of TFEB, were significantly higher in the livers of EtOH-fed rats (see below). The transcriptionally active form of TFEB, measured in the nuclear compartment, revealed that, compared with pair-fed controls, chronic EtOH feeding reduced nuclear TFEB by threefold (Fig. 4, E and F). However, 7-day refeeding restored TFEB mRNA to near control levels (relative quantification: pair-fed control = 1 ± 0; EtOH fed =0.5 ± 0.04; 7-day refed = 0.86 ± 0.1, P = 0.001). Nuclear TFEB protein levels were elevated by threefold (Fig. 4 E and F), and the levels of cytosolic (inactive) TFEB protein fell by 1.8-fold (pair fed = 1.48 ± 0.08; EtOH = 2.7 ± 0.77; 7-day refed = 1.5 ± 0.13, P = 0.01). When we calculated the nuclear-to-cytosolic ratio of TFEB, which we found to be a reliable indicator of autophagy activity in a mouse model (50), we found that 7-day refeeding restored this ratio to control (normal) levels (pair fed = 0.8 ± 0.07; EtOH = 0.25 ± 0.05; 7-day refed = 0.9 ± 0.1, P = 0.01). The restoration of TFEB was accompanied by normalization of the activities of selected lysosomal proteinases, cathepsins B and L, and the lysosomal acid lipase, a LD hydrolase. All three enzymes declined significantly in activity after chronic EtOH feeding (Fig. 4, H–J). After 7-day refeeding, the activities of all three enzymes were restored to control levels (Fig. 4, H–J).
Fig. 4.
Nuclear transcription factor EB (TFEB) levels and hepatic autophagy are restored following ethanol withdrawal and 7-day refeeding. Representative Western blot and densitometric quantification of microtubule-associated protein 1 light chain 3 II (LC3-II, A and B) and P62 (C and D) in postnuclear supernatants. E and F: TFEB levels in cytosol and nuclear fractions. Chymotrypsin-like proteasome activity (G), specific activities of lysosomal hydrolases cathepsin L (H), cathepsin B (I), and lysosomal acid lipase (J) in postnuclear supernatants made from liver homogenates of rats treated as indicated in the abscissa. Data are means ± SE of 6–8 animals/group. Bars with different letters are significantly different. Bars with the same letter are not significantly different, P ≤ 0.05.
Furthermore, as we have reported before (50, 51), chronic EtOH feeding decreased the chymotrypsin-like activity of the proteasome by 40% in livers of EtOH-fed rats compared with controls. This EtOH-induced decline was completely reversed by 7-day refeeding (Fig. 4G).
EtOH withdrawal alleviates the accumulation of autophagy machinery on LDs.
To determine autophagic targeting of LDs, we quantified markers of autophagy in LDs isolated from the livers of control, EtOH, and 7-day refed animals. We quantified the levels of autophagosomes (LC3-II) and P62, and LD protein perilipin-3 (PLIN-3), that tend to accumulate on LDs due to a slowdown in autophagic degradation of LDs (21). Compared with pair-fed controls, LDs isolated from EtOH-fed rats exhibited a 2.2- and a 1.4-fold increase in LC3-II and P62 levels, respectively. However, EtOH withdrawal and refeeding the control diet for 7 days completely reversed this effect of EtOH on autophagy markers. Similarly, PLIN-3 levels that were induced 2.8-fold following EtOH exposure were also reversed to control levels upon 7-day refeeding (Fig. 5).
Fig. 5.
Ethanol (EtOH) withdrawal normalizes autophagic association with lipid droplets. Representative Western blot (A and D) and densitometric quantification of P62 (B), microtubule-associated protein 1 light chain 3 II (LC3-II, C), and perilipin-3 (PLIN-3, E) in lipid droplet-enriched fractions prepared from the livers of rats treated as indicated in the abscissa. Data are means ± SE of 6–8 animals/group. Bars with different letters are significantly different. Bars with the same letter are not significantly different, P ≤ 0.05.
DISCUSSION
About 90% of people with alcohol use disorders (AUD) develop fatty liver (28), which is a risk factor for progression to advanced stages of ALD and occurs early in the onset of ALD (19). Currently, there are no established or effective Food and Drug Administration-approved drugs to treat ALD (2, 53). Abstinence from alcohol is the only option that reliably reverses alcohol-induced steatosis (43). The cessation of alcohol consumption also ceases EtOH metabolism. This is important because, to date, most, if not all, EtOH-induced disruptions reported in liver, including steatosis, require EtOH oxidation (5, 6, 26). Here, 7 days after EtOH administration (and its metabolism) ceased upon refeeding control diet, circulating free fatty acids (FFAs) and their liver transporters were reduced to normal. Both were accompanied by a restoration of hepatoprotective autophagy to mitigate EtOH-induced fatty liver and subsequent injury.
Fat accumulation and mild liver injury are now predictable consequences of feeding rodents the Lieber-DeCarli EtOH liquid diet (1). We and others reported that EtOH metabolism plays a crucial role in inducing the aforementioned hallmark features of ALD in EtOH-fed rodents (5, 14, 49, 50). Here, EtOH withdrawal and refeeding the control diet for 7 days normalized the induced levels of ADH and CYP2E1, thereby decreasing the generation of lipid peroxides (Fig. 1D). The latter reactive oxygen species are generated by secondary reactions of unsaturated fatty acids with oxygen radicals generated by induced levels of CYP2E1, causing hepatocyte damage (14) and release of hepatocellular ALT and AST in plasma (14).
One of the major mechanisms that contributes to fatty liver development is the uptake by the liver of circulating FFA derived from EtOH-induced lipolysis in adipose tissue (55). FFA uptake by hepatocytes is facilitated and regulated by a set of transporter proteins (3, 20, 55). Here, EtOH feeding caused a rise in circulating FFA levels (Fig. 1H) and a corresponding adaptive increase in the contents of CD36, FABP4, FATP-2, and FATP-5 (Fig. 3), all of which participate in FA uptake and their subsequent condensation with glycerol, producing triglycerides that are incorporated into LDs (3, 11, 20). The EtOH-induced elevation in circulating FFAs was exacerbated by a concomitant downregulation of PPAR-α (Fig. 3, I and J), which regulates genes involved in fatty acid oxidation (16). After EtOH was withdrawn, 7-day refeeding likely normalized adipocyte lipolysis, thereby decreasing circulating FFAs (Fig. 1H). This was further associated with a corresponding reduction in hepatic FA uptake proteins (Fig. 3, A–H) and a restoration to normal of PPAR-α (Fig. 3, I and J), allowing FA oxidation to resume normally. Both of these partially alleviated EtOH-induced fat accumulation (Fig. 1, G and I). Interestingly, although EtOH withdrawal promoted fat utilization, it did not completely reverse EtOH-induced fatty liver (Fig. 1I), probably because the amount of residual fat (and protein) in livers of EtOH-fed rats overwhelmed the degradation and subsequent oxidation systems, requiring a longer recovery period to completely reverse fatty liver.
The lysosome-dependent autophagy pathway has received considerable attention in recent years due to its importance in maintaining liver homeostasis (9, 38, 52). It is likely that autophagic breakdown of LDs was partly responsible for the attenuation of fatty liver following EtOH withdrawal. We and others showed that chronic EtOH feeding to mice inhibits hepatic lipophagy (36, 39, 40) and macroautophagy (i.e., autophagy) (7, 23, 32, 47, 50), causing lipid and protein accumulation, and cellular stress, while restoration of hepatic autophagy/lipophagy alleviates EtOH-induced liver pathologies (27). In other recent work on EtOH regulation of autophagy and lipophagy, we have shown that chronic EtOH feeding impedes the nuclear localization of TFEB (50) to downregulate de novo lysosome biogenesis and simultaneously cause accumulation of the autophagic adapter protein P62 on LDs (36), indicating a block in lipophagy of LDs marked by P62 for degradation, owing in part to inadequate levels of lysosomes for LD breakdown (36, 50). Here, we show that chronic EtOH feeding to rats similarly disrupted the nuclear localization of TFEB and enriched its content in the cytoplasm (Fig. 4, E and F). We propose that the EtOH-induced downregulation of proteasome activity reported here (Fig. 4G) results in a failure to degrade the inactive cytosolic (and phosphorylated) form of TFEB (44), leading to its cytosolic accumulation in that compartment (50). Disruption of TFEB nuclear localization was further accompanied by a downregulation of lysosomal proteolytic and lipolytic activity (Fig. 4, H–J), with a simultaneous accumulation of autophagosomes (LC3-II) and of the autophagic adapter protein P62 both in whole liver and on isolated LDs (Fig. 4, A–D and Fig. 5, A–C), all indicating a downregulation of autophagy and its participation in lipid metabolism (36, 50) in livers of EtOH-fed rats. In contrast, EtOH withdrawal restored TFEB nuclear localization (Fig. 4, E and F), normalized proteasome activity (Fig. 4G), and reversed EtOH-induced accumulation of autophagosomes and P62 protein content in the whole liver (Fig. 4, A–D) and on LD fractions (Fig. 5, A–C), indicating normalization of hepatic autophagy. Importantly, restoration of TFEB in the nuclear compartment restored lysosomal proteolytic and acid lipase activities (Fig. 4, H–J), which was further associated with a partial reversal of EtOH-induced hepatic protein and triglyceride accumulation to suggest that restoration of autophagy and lysosome hydrolase activity likely allowed normal resumption of proteolysis and lipid metabolism after EtOH withdrawal. Although all of our data indicate that EtOH withdrawal restored lysosomal function to alleviate steatosis, it is plausible that EtOH withdrawal also restored lipolysis, which partially contributed to attenuation of steatosis because LD catabolism occurs through the coordinated cross talk between lipolysis and lipophagy (21, 41, 54). The involvement of lysosomal participation in LD catabolism is further confirmed by our finding that 7-day refeeding reversed EtOH-induced levels of LD membrane protein PLIN-3 (Fig. 5, D and E), which upon its stabilization protects the LDs from degradation through lipolysis and lipophagy (21, 42). PLIN-3 is a substrate of chaperone-mediated autophagy (21, 42), which is the uptake and degradation directly by lysosomes of selected subsets of proteins like PLIN-3, which contains a KFERQ-like pentapeptide sequence (21, 42). Such degradation is executed by chaperone-mediated autophagy-positive lysosomes, which express both heat shock cognate 70 and lysosome-associated membrane protein 2A on the lysosomal membrane, that facilitate substrate entry and their degradation (21, 42). Collectively these findings suggest that EtOH withdrawal promoted LD catabolism through the lysosomal pathway.
In summary, by quantifying specific catabolic parameters that returned to normal (control) levels following EtOH withdrawal, we found that EtOH withdrawal attenuated fatty liver predominantly by reactivating autophagy and lysosomal function. Our findings suggest that these mechanisms that alleviated EtOH-induced fatty liver (Fig. 6) are hepatoprotective and that targeting these pathways may benefit the management and/or prevention of advanced stages of liver injury, including steatohepatitis and fibrosis. Importantly, treatment with pharmacological agents that promote hepatocyte autophagy may accelerate reversal of liver injury following EtOH withdrawal.
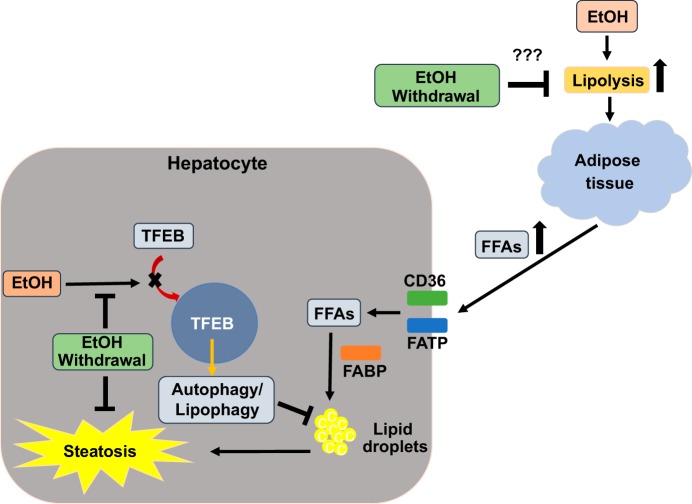
Fig. 6.
Proposed mechanism by which ethanol (EtOH) withdrawal alleviates EtOH-induced liver injury. Chronic EtOH feeding induces adipose tissue lipolysis, which generates elevated levels of circulating free fatty acids (FFAs). The latter are taken up by the liver through fatty acid (FA) uptake proteins, including cluster of differentiation 36 (CD36) and fatty acid transporters (FATPs) and incorporated into lipid droplets with the help of fatty acid-binding proteins (FABPs) to induce fat accumulation (steatosis). EtOH exposure also impedes the nuclear localization of transcription factor EB (TFEB), thereby disrupting hepatocyte autophagy. Such disruption causes accumulation of undegraded proteins and lipids. We hypothesize that EtOH withdrawal halts adipose tissue lipolysis (“???”), thereby normalizing circulating FFA levels, and their uptake into hepatocytes. This reversal of FA accumulation accompanies restoration of nuclear TFEB to normal, which in turn restores lysosomal functions, allowing normal degradation of accumulated hepatic proteins and lipids to restore proteostasis and lipostasis. Short thick arrows, increase due to EtOH exposure; black arrows, activation or a subsequent pathway step; cross bars, inhibition; question mark, “further investigations required.”
GRANTS
This study was supported by National Institute on Alcohol Abuse and Alcoholism Grants 5RC1-AA-019032 and 1R01-AA-020735-01 (C. A. Casey and M. A. McNiven, Multiple PI awards) and the Department of Veterans Affairs (C. A. Casey).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.G.T. and C.A.C. conceived and designed research; P.G.T., K.R., L.Y., J.L.K., and C.A.C. performed experiments; P.G.T., J.L.K., and C.A.C. analyzed data; P.G.T., K.R., T.M.D., M.A.M., and C.A.C. interpreted results of experiments; P.G.T. prepared figures; P.G.T. and C.A.C. drafted manuscript; P.G.T., T.M.D., M.A.M., and C.A.C. edited and revised manuscript; P.G.T., K.R., L.Y., T.M.D., J.L.K., M.A.M., and C.A.C. approved final version of manuscript.
REFERENCES
- 1.Arteel GE. Animal models of alcoholic liver disease. Dig Dis 28: 729–736, 2010. doi: 10.1159/000324280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergheim I, McClain CJ, Arteel GE. Treatment of alcoholic liver disease. Dig Dis 23: 275–284, 2005. doi: 10.1159/000090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonen A, Luiken JJ, Glatz JF. Regulation of fatty acid transport and membrane transporters in health and disease. Mol Cell Biochem 239: 181–192, 2002. doi: 10.1023/A:1020511125085. [DOI] [PubMed] [Google Scholar]
- 3a.Buttkus H, Rose RJ. Amine-malonaldehyde condensation products and their relative color contribution in the thiobarbituric acid test. J Am Oil Chem Soc 49: 440–443, 1972. [Google Scholar]
- 4.Casey CA, Kragskow SL, Sorrell MF, Tuma DJ. Ethanol-induced impairments in receptor-mediated endocytosis of asialoorosomucoid in isolated rat hepatocytes: time course of impairments and recovery after ethanol withdrawal. Alcohol Clin Exp Res 13: 258–263, 1989. doi: 10.1111/j.1530-0277.1989.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 5.Cederbaum AI. Regulation of pathways of alcohol metabolism by the liver. Mt Sinai J Med 47: 317–328, 1980. [PubMed] [Google Scholar]
- 6.Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol 20: 17756–17772, 2014. doi: 10.3748/wjg.v20.i47.17756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao X, Wang S, Zhao K, Li Y, Williams JA, Li T, Chavan H, Krishnamurthy P, He XC, Li L, Ballabio A, Ni HM, Ding WX. Impaired TFEB-mediated lysosome biogenesis and autophagy promote chronic ethanol-induced liver injury and steatosis in mice. Gastroenterology 155: 865–879 e812, 2018. doi: 10.1053/j.gastro.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crabb DW. Ethanol oxidizing enzymes: roles in alcohol metabolism and alcoholic liver disease. Prog Liver Dis 13: 151–172, 1995. [PubMed] [Google Scholar]
- 9.Czaja MJ, Ding WX, Donohue TM Jr, Friedman SL, Kim JS, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JH, Perlmutter DH, Randall G, Ray RB, Tsung A, Yin XM. Functions of autophagy in normal and diseased liver. Autophagy 9: 1131–1158, 2013. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalton SR, Wiegert RL, Baldwin CR, Kassel KM, Casey CA. Impaired receptor-mediated endocytosis by the asialoglycoprotein receptor in ethanol-fed mice: implications for studying the role of this receptor in alcoholic apoptosis. Biochem Pharmacol 65: 535–543, 2003. doi: 10.1016/S0006-2952(02)01555-1. [DOI] [PubMed] [Google Scholar]
- 11.Doege H, Grimm D, Falcon A, Tsang B, Storm TA, Xu H, Ortegon AM, Kazantzis M, Kay MA, Stahl A. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J Biol Chem 283: 22186–22192, 2008. doi: 10.1074/jbc.M803510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolganiuc A, Thomes PG, Ding WX, Lemasters JJ, Donohue TM Jr. Autophagy in alcohol-induced liver diseases. Alcohol Clin Exp Res 36: 1301–1308, 2012. doi: 10.1111/j.1530-0277.2012.01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donohue TM Jr, McVicker DL, Kharbanda KK, Chaisson ML, Zetterman RK. Ethanol administration alters the proteolytic activity of hepatic lysosomes. Alcohol Clin Exp Res 18: 536–541, 1994. doi: 10.1111/j.1530-0277.1994.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 14.Donohue TM Jr, Osna NA, Trambly CS, Whitaker NP, Thomes PG, Todero SL, Davis JS. Early growth response-1 contributes to steatosis development after acute ethanol administration. Alcohol Clin Exp Res 36: 759–767, 2012. doi: 10.1111/j.1530-0277.2011.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donohue TM Jr, Thomes PG. Ethanol-induced oxidant stress modulates hepatic autophagy and proteasome activity. Redox Biol 3: 29–39, 2014. doi: 10.1016/j.redox.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem 278: 27997–28004, 2003. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 18.Forrest EH, Atkinson SR, Richardson P, Masson S, Ryder S, Thursz MR, Allison M, Group STM. ACG clinical guideline for alcoholic liver disease: the MELD threshold for corticosteroid treatment has yet to be established. Am J Gastroenterol, 114: 175–176, 2019. doi: 10.1038/s41395-018-0076-x. [DOI] [PubMed] [Google Scholar]
- 19.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572–1585, 2011. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev 90: 367–417, 2010. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 21.Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol 17: 759–770, 2015. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Zughaier SM. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12: 1–222, 2016. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li SY, Gilbert SA, Li Q, Ren J. Aldehyde dehydrogenase-2 (ALDH2) ameliorates chronic alcohol ingestion-induced myocardial insulin resistance and endoplasmic reticulum stress. J Mol Cell Cardiol 47: 247–255, 2009. doi: 10.1016/j.yjmcc.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieber CS. Alcohol and the liver: metabolism of alcohol and its role in hepatic and extrahepatic diseases. Mt Sinai J Med 67: 84–94, 2000. [PubMed] [Google Scholar]
- 25.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 34: 9–19, 2004. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta 257: 59–84, 1997. doi: 10.1016/S0009-8981(96)06434-0. [DOI] [PubMed] [Google Scholar]
- 27.Lin CW, Zhang H, Li M, Xiong X, Chen X, Chen X, Dong XC, Yin XM. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol 58: 993–999, 2013. doi: 10.1016/j.jhep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol Suppl 62: S38–S46, 2015. doi: 10.1016/j.jhep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology 128: 2066–2076, 2005. doi: 10.1053/j.gastro.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McVicker BL, Casey CA. Effects of ethanol on receptor-mediated endocytosis in the liver. Alcohol 19: 255–260, 1999. doi: 10.1016/S0741-8329(99)00043-9. [DOI] [PubMed] [Google Scholar]
- 31.McVicker BL, Rasineni K, Tuma DJ, McNiven MA, Casey CA. Lipid droplet accumulation and impaired fat efflux in polarized hepatic cells: consequences of ethanol metabolism. Int J Hepatol 2012: 978136, 2012. doi: 10.1155/2012/978136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menk M, Graw JA, Poyraz D, Möbius N, Spies CD, von Haefen C. Chronic alcohol consumption inhibits autophagy and promotes apoptosis in the liver. Int J Med Sci 15: 682–688, 2018. doi: 10.7150/ijms.25393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osna NA, Thomes PG, Donohue TM Jr. Involvement of autophagy in alcoholic liver injury and hepatitis C pathogenesis. World J Gastroenterol 17: 2507–2514, 2011. doi: 10.3748/wjg.v17.i20.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res 33: 191–205, 2009. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasineni K, Casey CA. Molecular mechanism of alcoholic fatty liver. Indian J Pharmacol 44: 299–303, 2012. doi: 10.4103/0253-7613.96297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasineni K, Donohue TM Jr, Thomes PG, Li Yang, Tuma DL, McNiven MA, Casey CA. Ethanol-induced steatosis involves impairment of lipophagy, associatedwith reduced dynamin2 activity. Hepatol Commun 1: 501–512, 2017. doi: 10.1002/hep4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasineni K, McVicker BL, Tuma DJ, McNiven MA, Casey CA. Rab GTPases associate with isolated lipid droplets (LDs) and show altered content after ethanol administration: potential role in alcohol-impaired LD metabolism. Alcohol Clin Exp Res 38: 327–335, 2014. doi: 10.1111/acer.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R. Autophagy in liver diseases. J Hepatol 53: 1123–1134, 2010. doi: 10.1016/j.jhep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Schott MB, Rasineni K, Weller SG, Schulze RJ, Sletten AC, Casey CA, McNiven MA. β-Adrenergic induction of lipolysis in hepatocytes is inhibited by ethanol exposure. J Biol Chem 292: 11815–11828, 2017. doi: 10.1074/jbc.M117.777748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulze RJ, Rasineni K, Weller SG, Schott MB, Schroeder B, Casey CA, McNiven MA. Ethanol exposure inhibits hepatocyte lipophagy by inactivating the small guanosine triphosphatase Rab7. Hepatol Commun 1: 140–152, 2017. doi: 10.1002/hep4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulze RJ, Sathyanarayan A, Mashek DG. Breaking fat: the regulation and mechanisms of lipophagy. Biochim Biophys Acta Mol Cell Biol Lipids 1862, 10 Pt B: 1178–1187, 2017. doi: 10.1016/j.bbalip.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schweiger M, Zechner R. Breaking the barrier--chaperone-mediated autophagy of perilipins regulates the lipolytic degradation of fat. Cell Metab 22: 60–61, 2015. doi: 10.1016/j.cmet.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 43.Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, Tsukamoto H. Alcoholic liver disease. Nat Rev Dis Primers 4: 16, 2018. doi: 10.1038/s41572-018-0014-7. [DOI] [PubMed] [Google Scholar]
- 44.Sha Y, Rao L, Settembre C, Ballabio A, Eissa NT. STUB1 regulates TFEB-induced autophagy-lysosome pathway. EMBO J 36: 2544–2552, 2017. doi: 10.15252/embj.201796699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol 113: 175–194, 2018. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomes PG, Donohue TM. Role of early growth response-1 in the development of alcohol-induced steatosis. Curr Mol Pharmacol 10: 179–185, 2017. doi: 10.2174/1874467208666150817112529. [DOI] [PubMed] [Google Scholar]
- 47.Thomes PG, Ehlers RA, Trambly CS, Clemens DL, Fox HS, Tuma DJ, Donohue TM Jr. Multilevel regulation of autophagosome content by ethanol oxidation in HepG2 cells. Autophagy 9: 63–73, 2013. doi: 10.4161/auto.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomes PG, Osna NA, Bligh SM, Tuma DJ, Kharbanda KK. Role of defective methylation reactions in ethanol-induced dysregulation of intestinal barrier integrity. Biochem Pharmacol 96: 30–38, 2015. doi: 10.1016/j.bcp.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Thomes PG, Osna NA, Davis JS, Donohue TM Jr. Cellular steatosis in ethanol oxidizing-HepG2 cells is partially controlled by the transcription factor, early growth response-1. Int J Biochem Cell Biol 45: 454–463, 2013. doi: 10.1016/j.biocel.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomes PG, Trambly CS, Fox HS, Tuma DJ, Donohue TM Jr. Acute and chronic ethanol administration differentially modulate hepatic autophagy and transcription factor EB. Alcohol Clin Exp Res 39: 2354–2363, 2015. doi: 10.1111/acer.12904. [DOI] [PubMed] [Google Scholar]
- 51.Thomes PG, Trambly CS, Thiele GM, Duryee MJ, Fox HS, Haorah J, Donohue TM Jr. Proteasome activity and autophagosome content in liver are reciprocally regulated by ethanol treatment. Biochem Biophys Res Commun 417: 262–267, 2012. doi: 10.1016/j.bbrc.2011.11.097. [DOI] [PubMed] [Google Scholar]
- 52.Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol 14: 170–184, 2017. doi: 10.1038/nrgastro.2016.185. [DOI] [PubMed] [Google Scholar]
- 53.Vuittonet CL, Halse M, Leggio L, Fricchione SB, Brickley M, Haass-Koffler CL, Tavares T, Swift RM, Kenna GA. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm 71: 1265–1276, 2014. doi: 10.2146/ajhp140028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zechner R, Madeo F, Kratky D. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat Rev Mol Cell Biol 18: 671–684, 2017. doi: 10.1038/nrm.2017.76. [DOI] [PubMed] [Google Scholar]
- 55.Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, Sun X, Yin X, Sun X, Kim S, McClain CJ, Zhang X, Zhou Z. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol 180: 998–1007, 2012. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]