Keywords: cell migration, cell proliferation, exosomes, miR-21, NK-1R, sorting, Substance P
Abstract
Exosomes are cellular vesicles involved in intercellular communication via their specialized molecular cargo, such as miRNAs. Substance P (SP), a neuropeptide/hormone, and its high-affinity receptor, NK-1R, are highly expressed during colonic inflammation. Our previous studies show that SP/NK-1R signaling stimulates differential miRNA expression and promotes colonic epithelial cell proliferation. In this study, we examined whether SP/NK-1R signaling regulates exosome biogenesis and exosome-miRNA cargo sorting. Moreover, we examined the role of SP/NK-1R signaling in exosome-regulated cell proliferation and migration. Exosomes produced by human colonic NCM460 epithelial cells overexpressing NK-1R (NCM460-NK1R) were isolated from culture media. Exosome abundance and uptake were assessed by Western blot analysis (abundance) and Exo-Green fluorescence microscopy (abundance and uptake). Cargo-miRNA levels were assessed by RT-PCR. Cell proliferation and migration were assessed using xCELLigence technology. Colonic epithelial exosomes were isolated from mice pretreated with SP for 3 days. Cell proliferation in vivo was assessed by Ki-67 staining. SP/NK-1R signaling in human colonic epithelial cells (in vitro) and mouse colons (in vivo) increased 1) exosome production, 2) the level of fluorescence in NCM460s treated with Exo-Green-labeled exosomes, and 3) the level of miR-21 in exosome cargo. Moreover, our results showed that SP/NK-1R-induced cell proliferation and migration are at least in part dependent on intercellular communication via exosomal miR-21 in vitro and in vivo. Our results demonstrate that SP/NK-1R signaling regulates exosome biogenesis and induces its miR-21 cargo sorting. Moreover, exosomal miR-21 promotes proliferation and migration of target cells.
NEW & NOTEWORTHY Substance P signaling regulates exosome production in human colonic epithelial cells and colonic crypts in wild-type mice. MiR-21 is selectively sorted into exosomes induced by Substance P stimulation and promotes cell proliferation and migration in human colonocytes and mouse colonic crypts.
INTRODUCTION
Exosomes are small vesicles (30–100 nm) originated from late endosomal compartments, and, upon stimulation such as an infection (1, 8, 18) or activation of G protein-coupled receptor (GPCR) (20, 30), they are released into extracellular spaces by most cell types, including intestinal epithelial cells (18, 32, 33, 50). Intercellular communication conducted by exosomes and their molecular cargo has recently gained increasing attention (38). Studies have shown that exosomes carry molecular cargo, including proteins (9, 29, 49), mRNAs (17, 48), and microRNAs (miRNAs) (14, 48), and transfer this cargo to neighboring or distant cells, leading to regulation of recipient cell function. Moreover, accumulating evidence suggests that exosome-mediated intercellular communication is involved with the development of various diseases, such as cancer (40, 43), infection (18), and inflammation (55, 56).
The neuropeptide/hormone Substance P (SP) and its receptor, NK-1R, are expressed at high levels in the colons of patients with ulcerative colitis (UC) and Crohn’s disease (CD) (13, 22, 24, 34). SP is secreted by neurons, endocrine cells, and macrophages in the intestine, whereas NK-1R, a GPCR, is localized in colonic epithelial cells and immune cells in the intestinal mucosa (45). SP/NK-1R coupling promotes proinflammatory signaling (22, 26, 58) and proliferation (15, 23) through activation of MAPK, AKT, and NF-κB signaling cascades in vitro and colonic inflammation in vivo (4–6, 21, 22, 35, 39, 46). Furthermore, NK-1R deficiency in mice attenuates the development of acute colitis (21), suggesting that SP/NK-1R signaling plays an important role in the pathophysiology of colonic inflammation. On the other hand, SP/NK-1R signaling stimulates differential expression of miRNAs, which form part of the SP/NK-1R signaling-mediated inflammatory network, in human colonic epithelial NCM460 cells overexpressing NK-1R (NCM460-NK1R) (11). Taken together, these data indicate that SP/NK-1R signaling mediates both the activation of signaling cascades and epigenetic regulations in human colonocytes. Interestingly, miR-21, an miRNA upregulated in colonic epithelial cells upon SP stimulation (11), has previously been identified as exosomal cargo of intestinal and nonintestinal cells and has been shown among others to functionally regulate cancer-related responses (2, 31). Thus, in this study, we examined whether SP-induced NK-1R activation regulated exosome biogenesis and exosomal miRNA cargo sorting and its functional significance in human colonic epithelial cells. We showed that SP/NK-1R signaling activation regulated exosome biogenesis and promoted miR-21 sorting into exosomes in human colonocytes. In addition, exosomes carrying miR-21 were readily taken up by naive human colonic epithelial cells in which they promoted cell proliferation and migration in a miR-21-dependent manner.
MATERIALS AND METHODS
Antibodies and reagents.
Protease inhibitors used are as follows: aprotinin (1 μM), benzamidine (5 mM), leupeptin (2 μM), pepstatin (1 μM), phenylmethylsulfonyl-fluoride (1 mM) (Sigma, St. Louis, MO), anti-actin (1:1,000, A2066; Sigma), anti-CD9 (sc-13118; Santa Cruz Biotechnology, Dallas, TX), exosome-depleted FBS (Life Technologies, Carlsbad, CA), anti-miR-21 (Ambion, Austin, TX), and Lipofectamine RNAiMAX (ThermoFisher, Waltham, MA).
Animal studies.
Male (8–12 wk old) C57BL6 wild-type (n = 5/group) mice were injected intraperitoneally and intracolonically with a high (72 μg/mouse, 100 μL) dose of SP (Bachem, King of Prussia, PA) or vehicle (0.1% trifluoroacetic acid), twice a day (8 h apart on days 1 and 2). Mice were euthanized on day 3 by carbon dioxide; colon tissues were collected for harvesting colonic epithelial cells as previously described (42) and immunohistochemistry (IHC). All animal studies were approved by the institutional animal care and use committee.
Cell culture and transfection.
Human colonic epithelial cells (NCM460 and NCM460-NK1R) (58) were maintained in M3D media (Incell, San Antonio, TX) supplemented with 10% (vol/vol) exosome-depleted FBS, 1% l-glutamine, 10 U/mL penicillin, and 100 μg/mL streptomycin at 37°C, 5% CO2. Unless otherwise indicated, NCM460-NK1R cells were treated with SP (100 nM, 6 h, n = 6/group). For miR-21 gene silencing, NCM460-NK1R cells were transfected with antisense miR-21 (anti-miR-21) (Ambion), using Lipofectamine RNAiMAX (Invitrogen). Cells transfected with antisense control miRNA (anticontrol) served as controls (n = 6/group). All transfection was performed 48 h before SP stimulation.
Exosome isolation.
Exosomes were isolated from cell culture media from NCM460-NK1R cells treated as indicated. Isolation was performed by a modified version of previously described protocols (19, 28). Briefly, cell culture media were subjected to differential centrifugation of 1,000 g (5 min, keeping supernatants), 27,000 g (35 min, keeping supernatants), and 33,000 g (16 h, keeping pelleted exosomes). Exosome pellet was resuspended in plain M3D medium for in vitro treatments or TRIzol and RIPA buffer for analysis by RT-PCR and immunoblot, respectively. For exosomes from mouse colonic epithelial cells, mouse colon tissues were homogenized in Buffer A (150 mM NaCl, 10 mM HEPES, pH 7.4, 1 mM EGTA, 0.1 mM MgCl2) supplemented with protease inhibitors using a Teflon homogenizer (Wheaton, Millville, NJ). The lysates were first centrifuged at 1,000 g (5 min) to discard unbroken cells and nuclei. Protein concentration of the supernatant was quantified, and equal amounts of protein from each preparation were used for exosome isolation.
Exosome uptake.
Exosomes collected from culture media of NCM460-NK1R cells treated with SP (10−7 M, 6 h) or vehicle were labeled with Exo-Green (SBI, Palo Alto, CA) according to manufacturer’s instructions. The labeled exosomes were incubated with naive NCM460 cells maintained in M3D supplemented with exosome-depleted FBS, 1% l-glutamine, 10 U/ml penicillin, and 100 μg/ml streptomycin. Cells were washed 16 h after exosome addition and subjected to AxioVision fluorescent microscopy (Carl Zeiss, Oberkochen, Germany). Fluorescent intensity was quantified by ImageJ version 1.46d (NIH, Bethesda, MD).
Gel electrophoresis and immunoblotting.
Total exosome preparations from conditioned media and mouse colon epithelial cells were normalized using equal donor samples (equal protein in cell lysates and lysates from isolated colonic epithelia). In brief, all samples were subjected to SDS-PAGE and transferred to PVDF membranes in 25 mmol/L Tris, 192 mmol/L glycine. Membranes were blocked (PBS, 10% nonfat dry milk, 0.05% Tween-20) and probed with anti-CD9 and anti-actin antibodies followed by corresponding horseradish peroxidase-labeled secondary antibodies (1:1,000). Blots were developed with enhanced chemiluminescence reagent (ThermoFisher). Western blot bands were quantified using image analyzer LAS-4000 mini (Fujifilm). Data are represented by cropped images from the original membranes.
Quantitative RT-PCR.
Total RNA from all exosome preparations was isolated using standard TRIzol reagent protocol (Life Technologies, Carlsbad, CA). Equal amounts of total RNA (200 ng) from all exosome preparations were used to generate cDNA library using miRCURY LNA Universal RT microRNA PCR cDNA kit (Exiqon). Quantitative RT-PCR (qRT-PCR) for miRNAs was performed using miRNA-specific primers (Exiqon) and miRCURY LNA Universal RT microRNA PCR SYBR Green master mix (Exiqon). qRT-PCR for mRNAs of interest was performed using specific primers (Applied Biosystems), according to the manufacturer's instructions.
Immunohistochemistry and Ki-67 quantification.
Formalin-fixed, paraffin-embedded colon tissues from SP-injected mice and control mice were sectioned at 5 μm. Ki-67 expression was detected by immunohistochemistry. Ki-67 staining was then quantified using a pixel-based quantification performed with an AxioImager.Z1 microscope equipped with AxioVision software version 4.6 (Zeiss, Jena, Germany). Digital images were collected with AxioCam under ×200 magnification, and the densitometric sum of each image was calculated based on the Ki-67 signal intensity and Ki-67-positive area using the AutoMeasure module to avoid selection bias or interobserver effects (16).
Cell proliferation and migration.
Cell proliferation and migration of exosome recipient NCM460 cells was measured using xCELLigence Real-Time Cellular Analysis system (ACEA, San Diego, CA) placed in a humidified incubator (5% CO2, 37°C). Exosomes were collected as described above. For proliferation assays, background impedance of an E-plate 96 (Roche Diagnostics, Indianapolis, IN) was measured following the addition of 100 μL of plain M3D medium to the plates. Cell suspension of NCM460s containing 5 × 103 cells in 50 μL conditioned media [M3D supplemented with 2% (final vol/vol) exosome-depleted FBS, 1% l-glutamine, 10 U/ml penicillin, and 100 μg/ml streptomycin] was seeded into the E-plate 96 wells. Cells were immediately supplemented with equal exosome preparations isolated from exosome donor cells as described above (50 μL, equal protein). Impendence changes were automatically calculated, as live cells interact with electrodes in the E-plates, correlating with exosome recipient NCM460 cell proliferation over time. At 48 h, recipient cells were supplemented with an additional 50 μL of corresponding exosome preparations, and cell proliferation monitoring over time was continued for an additional 72 h. For migration assays, M3D medium as described above was added in the lower chamber of CIM-plate 16 plate (Roche Diagnostics, Indianapolis, IN). Exosome-recipient NCM460 cells were seeded into the upper chamber at 5 × 103 cells/well in serum-free medium mixed with exosome preparations isolated from exosome donor cells as described above (equal amount of protein). The CIM-plate 16 was monitored every 15 min for 72 h. Impedance signal was automatically calculated as live exosome recipient NCM460 cells moved from the upper chamber to the lower chamber, interacting with CIM-plate 16 electrodes correlating with cell migration over time.
Statistical analysis.
Unless otherwise stated, all experiments were performed in triplicate. Student’s t-test was used to compare the statistical significance between control and SP treatment groups. Samples run in triplicate, and data represent means ± SD.
RESULTS
SP regulates exosome biogenesis in human colonic epithelial cells.
Previous studies show that human and mouse intestinal epithelial cells release exosomes (18, 33, 50). On the other hand, exosomes derived from immune cells (52, 56) and mesenchymal stem cells (37, 55) attenuated colonic inflammation during colitis, supporting the notion that molecular cargo carried by exosomes plays an important role in intercellular communications that regulate colitis development. We hypothesized that exosomes secreted by human colonic epithelial cells transfer their molecular cargo to neighboring, naive cells. To show that exosomes are readily taken up by naive NCM460 cells, exosomes isolated from NCM460 cell culture media were labeled with Exo-Green Exo-Glow and were subsequently incubated with naive NCM460 cells for 16 h. Incubated cells were washed to remove excess (non-taken up) exosomes in culture media and were then subjected to fluorescence microscopy. Our results showed that NCM460s exhibited significant green fluorescence, indicating that Exo-Green Exo Glow-labeled exosomes were readily taken up by these cells (Fig. 1, A and B).
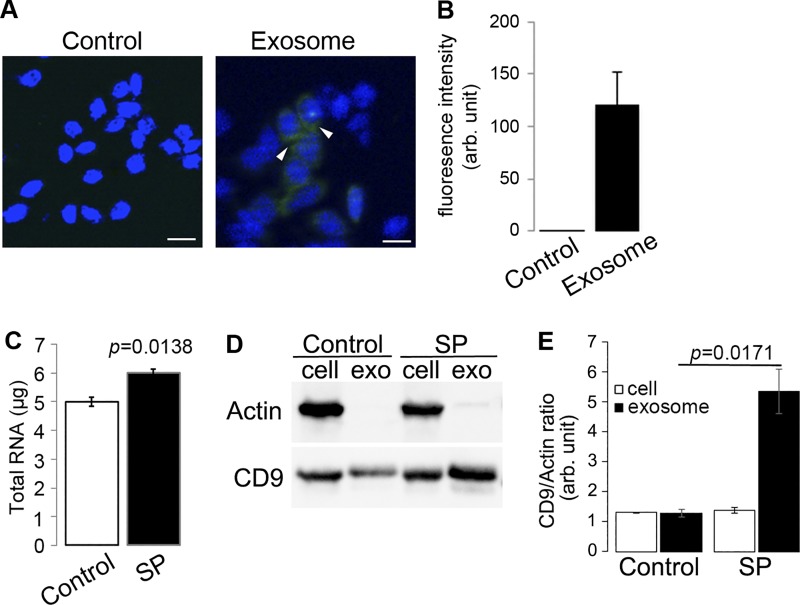
Fig. 1.
Substance P (SP)/NK-1R signaling activation promotes RNA loading to exosomes in human colonocytes and regulates protein expression in colonic epithelial exosomes. A and B: exosomes were collected from culture medium of NCM460 cells and labeled with Exo-Green and incubated with naive NCM460 cells. Fluorescent signals from the exosomes uptaken by NCM460 cells were quantified by ImageJ (scale bar = 10 μm). C: total RNA was harvested from exosomes collected from cell culture media of NCM460-NK1R cells treated with SP (10−7 M, 6 h) or vehicle quantified by spectrometry. D: representative image from Western blot analysis examining CD9 expression in exosomes produced upon SP/NK-1R signaling activation. E: relative amount of exosome production under different treatments was analyzed using densitometry with actin from cell lysates as reference.
Both SP and its receptor, NK-1R, are highly expressed in colonic epithelial cells during colonic inflammation (13, 22, 24, 34). Previously, we have shown that NK-1R signaling activation promotes cell proliferation and proinflammatory responses in human colonic epithelial cells (23, 26, 58) and differentiated miRNA expression (10, 11). To investigate whether SP/NK-1R signaling regulates exosome biogenesis in human colonic epithelial NCM460 cells overexpressing NK-1R (NCM460-NK-1R), cells (1.72 × 106 cells per treatment, n = 3) were incubated with SP (100 nM, 6 h) or control treatment, and culture media were collected. Exosomes were harvested from collected media as described in materials and methods, RNA was isolated from total exosome preparations, and total RNA levels were detected using Nanodrop 2000 spectrophotometer. Our results showed that the amounts of RNA cargo carried by SP-induced exosomes (6.00 ± 0.13 μg) were significantly higher than that by control exosomes (5.00 ± 0.16 μg, P = 0.0138) (Fig. 1C). We further examined SP-stimulated exosome production using immunoblot against CD9, a ubiquitous exosome marker (48). The relative amounts of total exosomes isolated from SP-stimulated NCM460-NK-1R (SP) and vehicle-treated NCM460-NK-1R cells were normalized by the relative amount of cells represented by actin in cell lysates collected from both treatments. Densitometry analysis showed that the amount of exosome was increased in conditioned media upon SP stimulation by 4.1 ± 0.57-fold (P = 0.0171), whereas the amounts of intracellular exosome in SP-stimulated and non-SP-stimulated cells are similar (P = 0.3206, Fig. 1, D and E). Furthermore, SP-exosomes and control exosomes were labeled with Exo-Green Exo-Glow and were subsequently incubated with naive NCM460 cells for 16 h. Fluorescence imaging analysis of NCM460 cells showed a significant increase (P = 0.0002) in fluorescence of NCM460 cells treated with SP-exosomes (Fig. 2), indicating that SP signaling stimulates exosomal biogenesis, which results in an increase of molecular cargo transferred to target cells. Taken together, these results suggest that SP/NK1R signaling activation in colonic epithelial cells increases intestinal epithelial cell-specific exosome biogenesis and enhances intercellular communication between colonic epithelial cells.
Fig. 2.
Substance P (SP)-induced exosomes are more readily taken up by human colonocytes. A: exosomes produced by human colonic epithelial NCM460 cells with or without SP stimulation were labeled with Exo-Green and incubated with naive NCM460 cells. B: uptake of exosomes was analyzed by quantification of fluorescence.
MiR-21 is enriched in exosomes secreted from human colonic epithelial cells in response to SP.
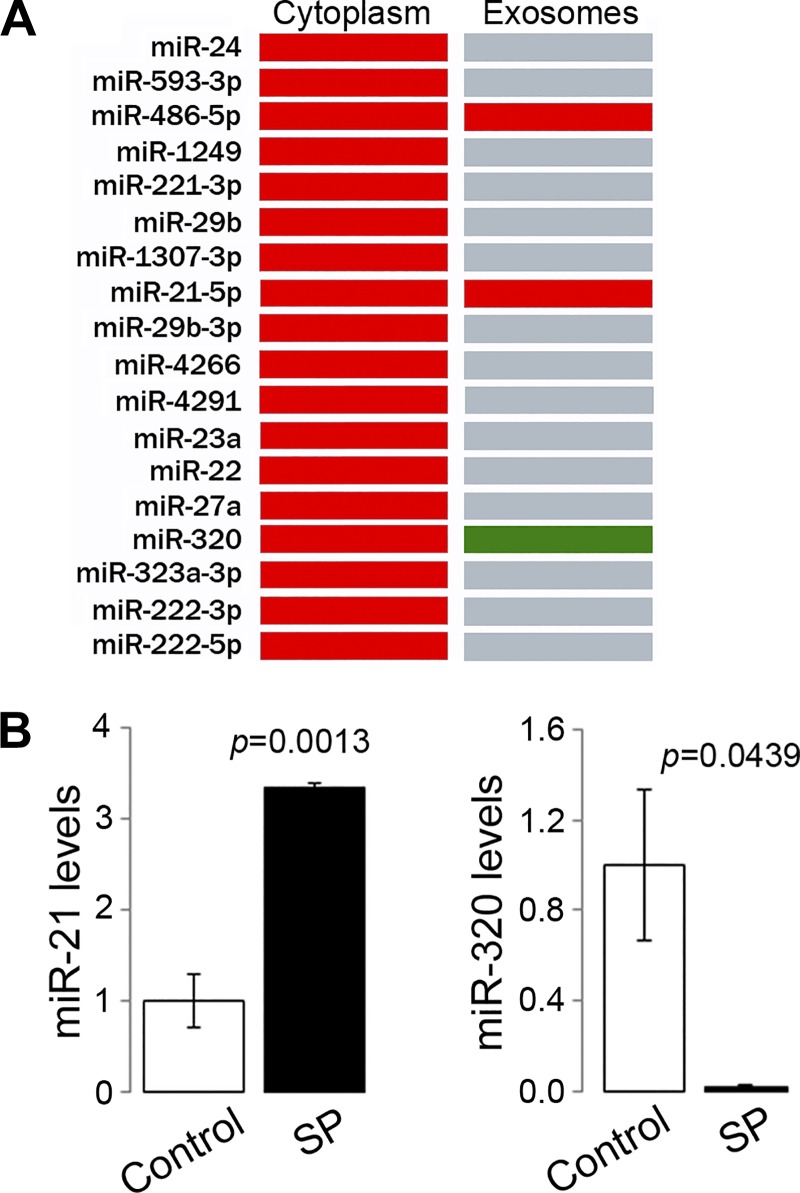
We previously reported that SP/NK-1R signaling regulates the miRNA expression profile of colonic epithelial cells (11). To study whether the differentiated miRNA profile is reflected in the exosomal miRNA cargo, miRNAs were collected from exosomes isolated from NCM460-NK-1R cells stimulated with SP (10−7 M, 6 h) or vehicle and were analyzed for their profile. Our results showed that, of the 18 miRNAs found to be upregulated by SP/NK-1R signaling in the entire cytoplasm of NCM460-NK-1R cells (10), only miR-21, miR-320, and miR-486 were identified in exosomes secreted from NCM460-NK-1R cells with or without SP stimulation (Fig. 3A). Furthermore, RT-PCR analysis revealed that miR-21 was significantly enriched by exosomes released following SP signaling (1.00 ± 0.09 vs. 3.34 ± 0.07, P = 0.0013, Fig. 3B). On the contrary, the level of miR-320b, an miRNA that showed increased expression in whole NCM460-NK1R cells upon SP signaling (11), was significantly decreased (1.00 ± 0.33 vs. 0.02 ± 0.003, P = 0.0439, Fig. 4B) in exosomes isolated from SP-stimulated NCM460-NK1R cells. Therefore, our results suggest that SP/NK-1R signaling in colonic epithelial cells regulates exosomal miRNA sorting, and miR-21 is increased in exosome cargo upon SP stimulation in a selective fashion.
Fig. 3.
MiRNAs are differentially sorted into exosomes upon Substance P (SP)/NK-1R signaling activation. A: miRNAs identified in cytoplasm and exosomes from human colonic epithelial NCM460-NK-1R cells (red: increased expression relative to control; green: reduced expression relative to control; gray: not present). B: levels of miR-21 (left) and miR-320 (right) in exosomes produced by NCM460-NK-1R cells with or without SP treatment were measured using RT-PCR.
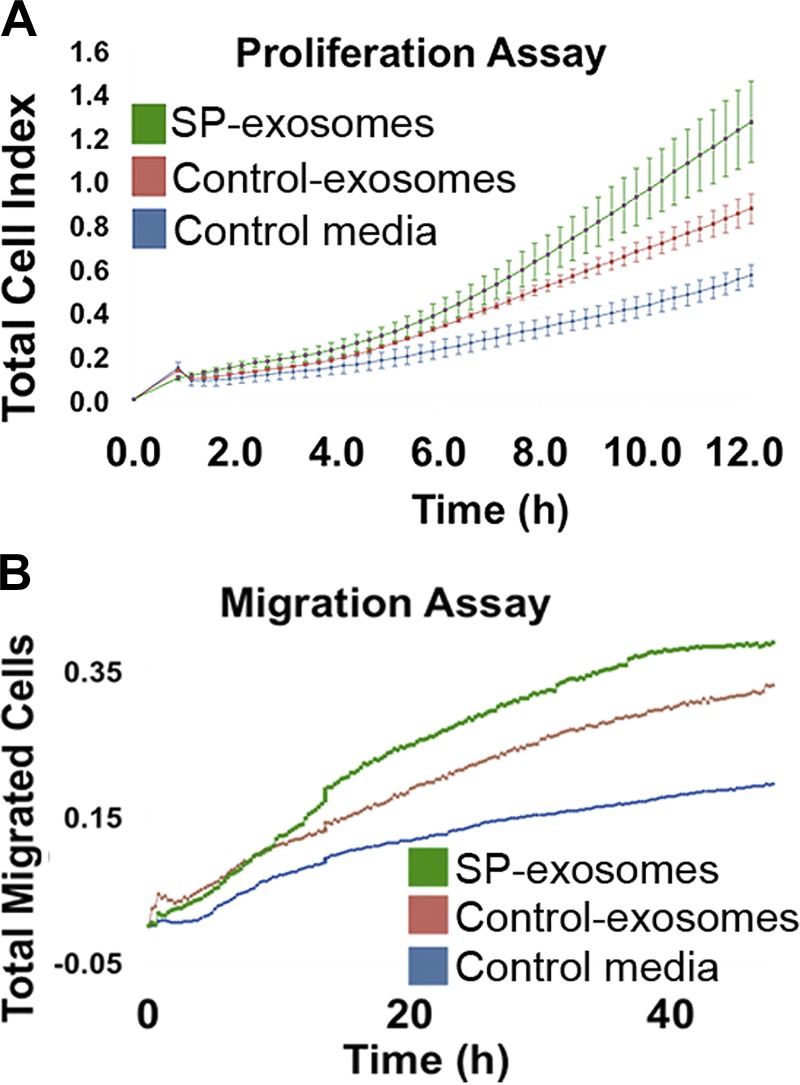
Fig. 4.
Substance P (SP)-exosomes promote cell proliferation and migration in naive NCM460 cells. Cell proliferation (A) and cell migration (B) of naive NCM460 cells treated with SP-exosomes or control exosomes were measured using xCELLigence Real-Time Cellular Analysis system.
MiR-21 in SP-induced colonic epithelial cell exosomes regulates colonic epithelial cell proliferation and migration in vitro.
SP/NK-1R signaling mediates proliferative responses in colonic epithelial cells and preadipocytes (15, 23). To study whether proliferation and migration of colonic epithelial cells can be regulated through exosome-mediated intercellular communication, naive NCM460 cells were incubated with exosomes secreted from SP or vehicle-treated NCM460-NK-1R cells. Naive NCM460 cells incubated with plain culture media were used as controls. As shown in Fig. 4A, although exosomes secreted from NCM460-NK-1R cells treated with vehicle (control exosomes) promoted naive NCM460 cell proliferation, the rate of proliferation was significantly increased in cells treated with exosomes secreted from NCM460-NK-1R cells treated with SP (SP-exosomes, P = 0.0001). Furthermore, SP-exosomes also promoted migration of naive NCM460 cells (P = 0.0015) compared with cells incubated with control exosomes (Fig. 4B). Our results suggested that control exosome cargo was capable of promoting cell proliferation and migration, whereas this effect was more significant for SP-exosome cargo.
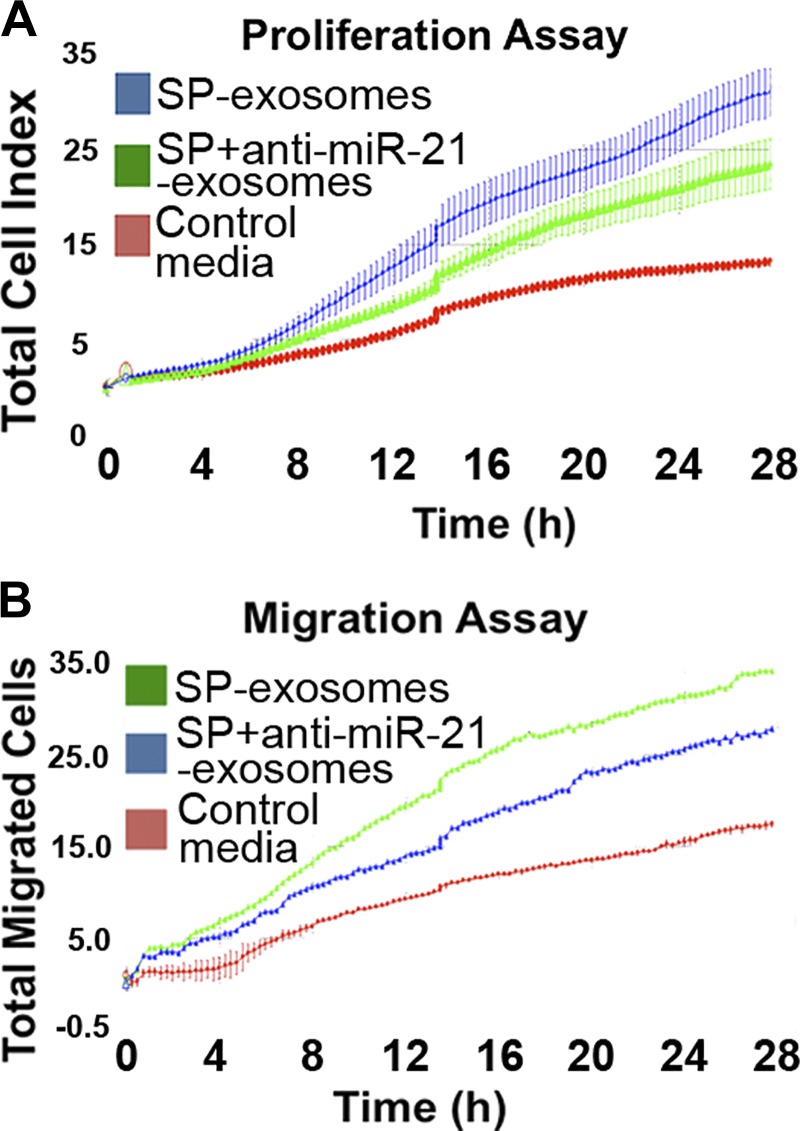
MiRNA cargo in exosomes plays an important role in intercellular communication (48). MiR-21 is one of the miRNAs significantly upregulated during colitis in vivo, as well as following proinflammatory signaling in vitro (3). Furthermore, it has been previously shown that miR-21 promotes cell proliferation and migration in colon cancer cells (3, 44, 54). To examine whether cell proliferation and migration induced by SP-exosomes are mediated by exosomal miR21 cargo, exosomes without miR-21 cargo were produced from SP-stimulated NCM460-NK-1R cells gene-silenced with miR-21 (anti-miR-21); exosomes produced from SP-stimulated NCM460-NK-1R cells transfected with scrambled antisense-miR served as controls. Naive NCM460 cells were incubated with SP-exosomes collected from NCM460-NK-1R cells transfected with or without anti-miR-21, and the rates of proliferation and migration were monitored. Our results showed that exposure of naive NCM460 cells to exosomes prepared from miR-21-silenced NCM460-NK-1R cells shows significantly reduced cell proliferation and migration (P = 0.022 and P = 0.025, respectively, Fig. 5, A and B), suggesting that SP-exosomes promoted cell proliferation and migration, at least partially, through exosomal miR-21 cargo.
Fig. 5.
Exosomal miR-21 promotes cell proliferation and migration in naive NCM460 cells. Exosomes were collected from NCM460-NK-1R cells transfected with antisense-miR-21 or scrambled control and incubated with naive NCM460 cells. Cell proliferation (A) and cell migration (B) of naïve NCM460 cells were measured using xCELLigence Real-Time Cellular Analysis system.
SP treatment promoted exosomal miR-21 export and colonic epithelial cell proliferation in vivo.
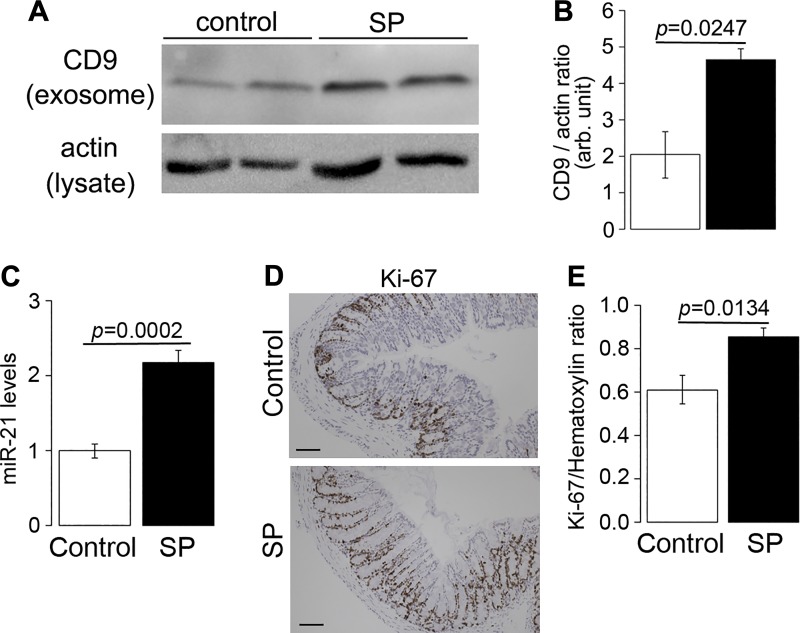
Having shown that SP/NK-1R signaling regulates exosome production and cargo content in vitro, we next investigated whether SP signaling affects colonic epithelial cell exosome biogenesis in vivo. Wild-type mice (8–12 wk old) were intracolonically and intraperitoneally treated with SP or control vehicle as described in materials and methods. Mice were euthanized 3 days posttreatment, colonic epithelia were isolated, and exosomes were collected from equal amounts of protein taken from colonic epithelial lysates. Exosome biogenesis was assessed by Western blot analysis on CD9 expression in exosomal portions collected from colonic epithelia.
As shown in Fig. 6, A and B, exosomal portions collected from mice treated with SP have increased CD9 expression by 2.3 ± 0.26-fold (P = 0.0247), compared with that collected from mice treated with vehicle control, indicating that SP regulates exosomal biogenesis in colonic epithelia in vivo. In addition, results from RT-PCR analysis (Fig. 6C) showed that miR-21 expression was increased in exosomes collected from mice receiving SP treatment (1.0 ± 0.09 vs. 2.18 ± 0.16, P = 0.0002). The above results suggested that SP treatment promoted exosome biogenesis and miR-21 sorting into exosomes in colonic epithelial cells both in vitro and in vivo. Furthermore, the proliferation rates of colonic epithelia in SP-treated mice were analyzed with Ki-67 staining. Results from automated image analysis (Fig. 6, D and E) showed that there was an increase in Ki-67 signals in colonic tissues taken from SP-treated mice, compared with the mice treated with vehicle controls (0.9 ± 0.04 vs. 0.6 ± 0.06, P = 0.0134). Collectively, our results suggest that SP/NK-1R signaling regulates exosomal biogenesis from colonic epithelial cells in vitro and in vivo. Importantly, these exosomes are readily taken up by neighboring colonic epithelial cells, introducing their molecular cargo during the process and possibly altering the intensity of intercellular communication between cells of the colonic epithelium. Taken together, results in this study show that proliferative and migration response induced by SP/NK-1R signaling in colonic epithelial cells can be induced, in part, by transfer of exosomal miR-21 to the cells, in addition to the signaling cascade that is initiated by SP/NK-1R coupling.
Fig. 6.
Substance P (SP) administration promotes exosome production in vivo. Male wild-type C57BL6/J mice (n = 5) were administered with SP (72 μg/dose, 2 doses/day) for 2 days. A and B: representative image from Western blot analysis assessing exosome production. Lysates from colonic epithelial cells with equal amounts of protein were collected for harvesting exosomes. C: miR-21 levels in colonic epithelial cells were measured with RT-PCR. D and E: representative image from Ki-67 immunohistochemistry measuring cell proliferation in colonic epithelial cells in vivo (scale = 50 μm).
DISCUSSION
SP and its receptor, NK1R, have been implicated to play an important role in the pathophysiology of colitis (6, 39, 46) and are expressed in high levels in inflamed colon tissues in patients with inflammatory bowel disease (13, 22, 24, 34). Our previous studies show that SP/NK-1R coupling elicited signaling cascades locally in target human colonocytes, leading to regulation of cellular responses, including inflammation (26, 58), proliferation (23), and apoptosis (25). In addition, SP/NK-1R signaling also mediates angiogenesis in intestinal microvascular endothelial cells by promoting the production of CCN1, an angiogenic factor, in colonocytes (24). However, the role of SP or any other neuropeptide in regulating intercellular communication has not been studied. Herein, we examined whether SP/NK-1R signaling modulated exosome biogenesis and sorting of exosomal cargo. Our results provided evidence that SP/NK-1R signaling activation regulated exosome production in human colonic epithelial cells in vitro (Fig. 1) and in vivo (Fig. 6). Importantly, SP-induced exosomes were more readily taken up by naive human colonic epithelial cells (Fig. 2). Thus the results of our studies show that SP/NK-1R signaling activation promotes exosome-associated intercellular communication.
On the other hand, we previously demonstrated that SP/NK-1R signaling dysregulated the expression of a number of miRNAs in the cytosol of human colonocytes (10, 11), including miR-21 and miR-320. In this study, we also examined whether SP-induced exosomal miRNA expression reflected the dysregulated miRNA expression in cytosol. Interestingly, out of 18 miRNAs that are dysregulated by SP/NK-1R signaling activation, only miR-21, miR-486, and miR-320 were identified in exosomes produced by NCM460-NK-1R cells (Fig. 3). Accumulating evidence shows that miRNAs can act in neighboring or distant tissues through intercellular cross talk, carried in the lumen of exosomes (41), apart from their role in the cell of their origin. Taken together, our results suggest that SP/NK-1R signaling activation increased exosome biogenesis and may promote the transfer of exosomal miRNAs to naïve human colonic epithelial cells.
We previously reported that the total cytoplasmic content of miR-21 is significantly increased in human colonocytes in response to neuropeptide signaling (3, 11). In this study, we showed that increased levels of exosomal miR-21 was produced by human colonocytes in response to SP signaling, compared with that by nonstimulated cells (Fig. 3). Importantly, this differential sorting of miR-21 to exosomes is a regulated process by SP/NK-1R signaling because levels of miR-320, another differentially expressed miRNA that shows increase in cytoplasmic level, were reduced in the exosomal cargo (Fig. 3). Our study is the first to observe that miR-21 is sorted to exosomes in human colonocytes. Differential sorting of miRNAs has been observed in colon cancer cells (7, 47) although its detailed mechanism remains to be examined. Interestingly, other studies have reported that proinflammatory stimulation promoted an increase in miR-21 levels in exosomes in pancreatic beta cells and monocytes in vitro (12, 27).
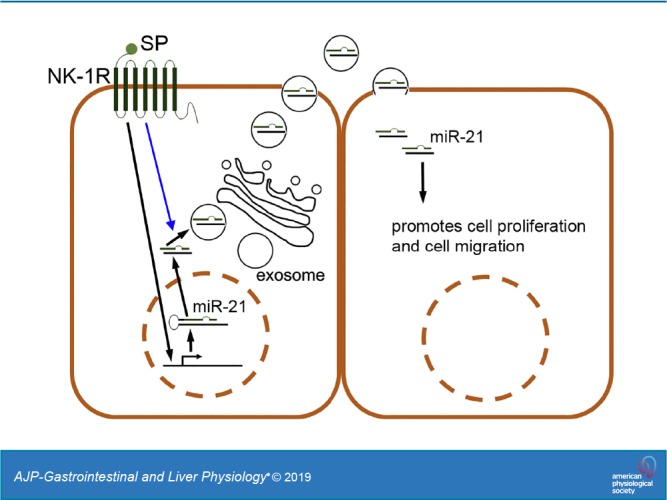
On the other hand, miR-21 is a well-established oncogenic miRNA that promotes cell proliferation and migration (3, 36, 51, 53, 57). Because the development of intestinal inflammation also involves processes such as cell proliferation and migration, herein, the role of SP-induced exosomal miR-21 in colonic epithelial cells was studied. Our results showed that SP-induced exosomes promoted colonic epithelial cell proliferation and migration in naive human colonocytes, in a manner partly dependent on exosomal miR-21 produced by SP-stimulated human colonocytes (Fig. 5). Although the cellular mechanisms and targets of exosomal miR-21 in cell proliferation and migration remain to be studied, it is possible that exosomal miR-21 may inhibit PTEN expression upon uptake of the exosome and, thereby, promote AKT activity in target human colonocytes, as demonstrated in our previous study (3). Overall, our findings suggest that miR-21 is targeted to exosomes following SP/NK1R coupling in human colonocytes and participates in cell proliferation and migration in neighboring human colonocytes that are not initially targeted by SP.
In conclusion, this is the first time that neuropeptide signaling is linked to exosome biogenesis. Furthermore, SP-induced exosomes enable effectors, such as exosomal miR-21 of SP/NK-1R signaling activation, to be transferred to cells that are not initially targeted by SP. Our results implied that exosomes produced by SP-stimulated colonic epithelial cells might amplify SP/NK-1R-associated cellular responses in inflamed colonic tissues by transferring exosomal cargo to neighboring cells that are initially not targeted by SP. Further efforts should focus on the study of sorting the remaining miRNAs present in exosomes in response to SP, focusing on different signaling pathways that may be implicated in the SP-related colitis phenotype.
GRANTS
This study was supported by National Institutes of Health Grants DK60729, DK47373, CURE:DDRC P30 DK 41301, and Animal Core (C. Pothoulakis and D. Iliopoulos); NIH DK110003 (D. Iliopoulos and C. Pothoulakis); CTSI UL1TR000124 (K. Bakirtzi); CCFA (I. K. Law and K. Bakirtzi); the Blinder Research Foundation for Crohn’s Disease and the Eli and Edythe Broad Chair (C. Pothoulakis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.B., D.I., and C.P. conceived and designed research; K.B. and K.F. performed experiments; K.B. and D.I. analyzed data; K.B. and D.I. interpreted results of experiments; K.B. and I.K.L. prepared figures; K.B. and I.K.L. drafted manuscript; C.P. edited and revised manuscript; C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. S. P. Bhat and R. K. Gangalum at Jules Stein Eye Institute UCLA for providing technical support. Integrated Molecular Technologies Core at the University of California at Los Angeles (NIH/NIDDL Center Grant P30 DK 41301) provided us technical support for the xCELLigence Real-Time Cellular Analysis system technology.
REFERENCES
- 1.Anderson MR, Pleet ML, Enose-Akahata Y, Erickson J, Monaco MC, Akpamagbo Y, Velluci A, Tanaka Y, Azodi S, Lepene B, Jones J, Kashanchi F, Jacobson S. Viral antigens detectable in CSF exosomes from patients with retrovirus associated neurologic disease: functional role of exosomes. Clin Transl Med 7: 24, 2018. doi: 10.1186/s40169-018-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS, Li Y, Lu ES, Kwan K, Wong KK, Schmandt R, Lu KH, Mok SC. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun 7: 11150, 2016. doi: 10.1038/ncomms11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakirtzi K, Hatziapostolou M, Karagiannides I, Polytarchou C, Jaeger S, Iliopoulos D, Pothoulakis C. Neurotensin signaling activates microRNAs-21 and −155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors. Gastroenterology 141: 1749–1761, 2011. doi: 10.1053/j.gastro.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castagliuolo I, Keates AC, Qiu B, Kelly CP, Nikulasson S, Leeman SE, Pothoulakis C. Increased substance P responses in dorsal root ganglia and intestinal macrophages during Clostridium difficile toxin A enteritis in rats. Proc Natl Acad Sci USA 94: 4788–4793, 1997. doi: 10.1073/pnas.94.9.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castagliuolo I, Morteau O, Keates AC, Valenick L, Wang CC, Zacks J, Lu B, Gerard NP, Pothoulakis C. Protective effects of neurokinin-1 receptor during colitis in mice: role of the epidermal growth factor receptor. Br J Pharmacol 136: 271–279, 2002. doi: 10.1038/sj.bjp.0704697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castagliuolo I, Riegler M, Pasha A, Nikulasson S, Lu B, Gerard C, Gerard NP, Pothoulakis C. Neurokinin-1 (NK-1) receptor is required in Clostridium difficile-induced enteritis. J Clin Invest 101: 1547–1550, 1998. doi: 10.1172/JCI2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, Demory Beckler M, Weaver AM, Vickers K, Prasad N, Levy S, Zhang B, Coffey RJ, Patton JG. KRAS-dependent sorting of miRNA to exosomes. eLife 4: e07197, 2015. doi: 10.7554/eLife.07197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Wang X, Yu Y, Xiao Y, Huang J, Yao Z, Chen X, Zhou T, Li P, Xu C. Serum exosomes of chronic gastritis patients infected with Helicobacter pylori mediate IL-1α expression via IL-6 trans-signalling in gastric epithelial cells. Clin Exp Immunol 194: 339-349, 2018. doi: 10.1111/cei.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng ZB, Zhuang X, Ju S, Xiang X, Mu J, Liu Y, Jiang H, Zhang L, Mobley J, McClain C, Feng W, Grizzle W, Yan J, Miller D, Kronenberg M, Zhang HG. Exosome-like nanoparticles from intestinal mucosal cells carry prostaglandin E2 and suppress activation of liver NKT cells. J Immunol 190: 3579–3589, 2013. doi: 10.4049/jimmunol.1203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang K, Law IKM, Padua D, Sideri A, Huang V, Kevil CG, Iliopoulos D, Pothoulakis C. MicroRNA-31–3p is involved in Substance P (SP)-associated inflammation in human colonic epithelial cells and experimental colitis. Am J Pathol 188: 586-599, 2018. doi: 10.1016/j.ajpath.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang K, Sideri A, Law IK, Bakirtzi K, Polytarchou C, Iliopoulos D, Pothoulakis C. Identification of a novel substance P (SP)-neurokinin-1 receptor (NK-1R) microRNA-221-5p inflammatory network in human colonic epithelial cells. Cell Mol Gastroenterol Hepatol 1: 503–515, 2015. doi: 10.1016/j.jcmgh.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukushima Y, Okamoto M, Ishikawa K, Kouwaki T, Tsukamoto H, Oshiumi H. Activation of TLR3 and its adaptor TICAM-1 increases miR-21 levels in extracellular vesicles released from human cells. Biochem Biophys Res Commun 500: 744–750, 2018. doi: 10.1016/j.bbrc.2018.04.146. [DOI] [PubMed] [Google Scholar]
- 13.Goldin E, Karmeli F, Selinger Z, Rachmilewitz D. Colonic substance P levels are increased in ulcerative colitis and decreased in chronic severe constipation. Dig Dis Sci 34: 754–757, 1989. doi: 10.1007/BF01540348. [DOI] [PubMed] [Google Scholar]
- 14.Gotanda K, Hirota T, Saito J, Fukae M, Egashira Y, Izumi N, Deguchi M, Kimura M, Matsuki S, Irie S, Ieiri I. Circulating intestine-derived exosomal miR-328 in plasma, a possible biomarker for estimating BCRP function in the human intestines. Sci Rep 6: 32299, 2016. doi: 10.1038/srep32299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross K, Karagiannides I, Thomou T, Koon HW, Bowe C, Kim H, Giorgadze N, Tchkonia T, Pirtskhalava T, Kirkland JL, Pothoulakis C. Substance P promotes expansion of human mesenteric preadipocytes through proliferative and antiapoptotic pathways. Am J Physiol Gastrointest Liver Physiol 296: G1012–G1019, 2009. doi: 10.1152/ajpgi.90351.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman JM, Baritaki S, Ruiz JJ, Sideri A, Pothoulakis C. Corticotropin-releasing hormone receptor 2 signaling promotes mucosal repair responses after colitis. Am J Pathol 186: 134–144, 2016. doi: 10.1016/j.ajpath.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosseini M, Khatamianfar S, Hassanian SM, Nedaeinia R, Shafiee M, Maftouh M, Ghayour-Mobarhan M, ShahidSales S, Avan A. Exosome-encapsulated microRNAs as potential circulating biomarkers in colon cancer. Curr Pharm Des 23: 1705–1709, 2017. doi: 10.2174/1381612822666161201144634. [DOI] [PubMed] [Google Scholar]
- 18.Hu G, Gong A-Y, Roth AL, Huang BQ, Ward HD, Zhu G, Larusso NF, Hanson ND, Chen X-M. Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog 9: e1003261, 2013. doi: 10.1371/journal.ppat.1003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeppesen DK, Hvam ML, Primdahl-Bengtson B, Boysen AT, Whitehead B, Dyrskjøt L, Orntoft TF, Howard KA, Ostenfeld MS. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J Extracell Vesicles 3: 25011, 2014. doi: 10.3402/jev.v3.25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajimoto T, Mohamed NNI, Badawy SMM, Matovelo SA, Hirase M, Nakamura S, Yoshida D, Okada T, Ijuin T, Nakamura SI. Involvement of Gβγ subunits of Gi protein coupled with S1P receptor on multivesicular endosomes in F-actin formation and cargo sorting into exosomes. J Biol Chem 293: 245–253, 2018. doi: 10.1074/jbc.M117.808733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koon HW, Shih D, Karagiannides I, Zhao D, Fazelbhoy Z, Hing T, Xu H, Lu B, Gerard N, Pothoulakis C. Substance P modulates colitis-associated fibrosis. Am J Pathol 177: 2300–2309, 2010. doi: 10.2353/ajpath.2010.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koon HW, Shih DQ, Hing TC, Chen J, Ho S, Zhao D, Targan SR, Pothoulakis C. Substance P induces CCN1 expression via histone deacetylase activity in human colonic epithelial cells. Am J Pathol 179: 2315–2326, 2011. doi: 10.1016/j.ajpath.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koon HW, Zhao D, Na X, Moyer MP, Pothoulakis C. Metalloproteinases and transforming growth factor-alpha mediate substance P-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. J Biol Chem 279: 45519–45527, 2004. doi: 10.1074/jbc.M408523200. [DOI] [PubMed] [Google Scholar]
- 24.Koon HW, Zhao D, Xu H, Bowe C, Moss A, Moyer MP, Pothoulakis C. Substance P-mediated expression of the pro-angiogenic factor CCN1 modulates the course of colitis. Am J Pathol 173: 400–410, 2008. doi: 10.2353/ajpath.2008.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koon HW, Zhao D, Zhan Y, Moyer MP, Pothoulakis C. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc Natl Acad Sci USA 104: 2013–2018, 2007. doi: 10.1073/pnas.0610664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koon HW, Zhao D, Zhan Y, Simeonidis S, Moyer MP, Pothoulakis C. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves PKCdelta activation. J Pharmacol Exp Ther 314: 1393–1400, 2005. doi: 10.1124/jpet.105.088013. [DOI] [PubMed] [Google Scholar]
- 27.Lakhter AJ, Pratt RE, Moore RE, Doucette KK, Maier BF, DiMeglio LA, Sims EK. Beta cell extracellular vesicle miR-21-5p cargo is increased in response to inflammatory cytokines and serves as a biomarker of type 1 diabetes. Diabetologia 61: 1124–1134, 2018. doi: 10.1007/s00125-018-4559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebowitz J, Lewis MS, Schuck P. Modern analytical ultracentrifugation in protein science: a tutorial review. Protein Sci 11: 2067–2079, 2002. doi: 10.1110/ps.0207702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, Jones HR, Sumagin R, Hilgarth RS, Alam A, Fredman G, Argyris I, Rijcken E, Kusters D, Reutelingsperger C, Perretti M, Parkos CA, Farokhzad OC, Neish AS, Nusrat A. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest 125: 1215–1227, 2015. doi: 10.1172/JCI76693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin M-C, Chen S-Y, Tsai H-M, He P-L, Lin Y-C, Herschman H, Li H-J. PGE2 /EP4 signaling controls the transfer of the mammary stem cell state by lipid rafts in extracellular vesicles. Stem Cells 35: 425–444, 2017. doi: 10.1002/stem.2476. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Sun H, Wang X, Yu Q, Li S, Yu X, Gong W. Increased exosomal microRNA-21 and microRNA-146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int J Mol Sci 15: 758–773, 2014. doi: 10.3390/ijms15010758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallegol J, van Niel G, Heyman M. Phenotypic and functional characterization of intestinal epithelial exosomes. Blood Cells Mol Dis 35: 11–16, 2005. doi: 10.1016/j.bcmd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Mallegol J, Van Niel G, Lebreton C, Lepelletier Y, Candalh C, Dugave C, Heath JK, Raposo G, Cerf-Bensussan N, Heyman M. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology 132: 1866–1876, 2007. doi: 10.1053/j.gastro.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 34.Mantyh CR, Gates TS, Zimmerman RP, Welton ML, Passaro EP Jr, Vigna SR, Maggio JE, Kruger L, Mantyh PW. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci USA 85: 3235–3239, 1988. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantyh CR, Maggio JE, Mantyh PW, Vigna SR, Pappas TN. Increased substance P receptor expression by blood vessels and lymphoid aggregates in Clostridium difficile-induced pseudomembranous colitis. Dig Dis Sci 41: 614–620, 1996. doi: 10.1007/BF02282350. [DOI] [PubMed] [Google Scholar]
- 36.Mao B, Xiao H, Zhang Z, Wang D, Wang G. MicroRNA-21 regulates the expression of BTG2 in HepG2 liver cancer cells. Mol Med Rep 12: 4917–4924, 2015. doi: 10.3892/mmr.2015.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao F, Wu Y, Tang X, Kang J, Zhang B, Yan Y, Qian H, Zhang X, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. BioMed Res Int 2017: 5356760, 2017. doi: 10.1155/2017/5356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics 73: 1907–1920, 2010. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Pothoulakis C, Castagliuolo I, LaMont JT, Jaffer A, O’Keane JC, Snider RM, Leeman SE. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci USA 91: 947–951, 1994. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preet R, Dixon DA. Mutant KRAS exosomes influence the metabolic state of the colon microenvironment. Cell Mol Gastroenterol Hepatol 5: 647–648, 2018. doi: 10.1016/j.jcmgh.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato-Kuwabara Y, Melo SA, Soares FA, Calin GA. The fusion of two worlds: non-coding RNAs and extracellular vesicles–diagnostic and therapeutic implications. Int J Oncol 46: 17–27, 2015. doi: 10.3892/ijo.2014.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141: 1762–1772, 2011. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 43.Schillaci O, Fontana S, Monteleone F, Taverna S, Di Bella MA, Di Vizio D, Alessandro R. Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: their emerging role in tumor heterogeneity. Sci Rep 7: 4711, 2017. doi: 10.1038/s41598-017-05002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi C, Yang Y, Xia Y, Okugawa Y, Yang J, Liang Y, Chen H, Zhang P, Wang F, Han H, Wu W, Gao R, Gasche C, Qin H, Ma Y, Goel A. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut 65: 1470–1481, 2016. doi: 10.1136/gutjnl-2014-308455. [DOI] [PubMed] [Google Scholar]
- 45.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev 94: 265–301, 2014. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stucchi AF, Shebani KO, Leeman SE, Wang CC, Reed KL, Fruin AB, Gower AC, McClung JP, Andry CD, O’Brien MJ, Pothoulakis C, Becker JM. A neurokinin 1 receptor antagonist reduces an ongoing ileal pouch inflammation and the response to a subsequent inflammatory stimulus. Am J Physiol Gastrointest Liver Physiol 285: G1259–G1267, 2003. doi: 10.1152/ajpgi.00063.2003. [DOI] [PubMed] [Google Scholar]
- 47.Teng Y, Ren Y, Hu X, Mu J, Samykutty A, Zhuang X, Deng Z, Kumar A, Zhang L, Merchant ML, Yan J, Miller DM, Zhang HG. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat Commun 8: 14448, 2017. doi: 10.1038/ncomms14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 49.Van Niel G, Mallegol J, Bevilacqua C, Candalh C, Brugière S, Tomaskovic-Crook E, Heath JK, Cerf-Bensussan N, Heyman M. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut 52: 1690–1697, 2003. doi: 10.1136/gut.52.12.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 121: 337–349, 2001. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 51.Venturutti L, Romero LV, Urtreger AJ, Chervo MF, Cordo Russo RI, Mercogliano MF, Inurrigarro G, Pereyra MG, Proietti CJ, Izzo F, Díaz Flaqué MC, Sundblad V, Roa JC, Guzmán P, Bal de Kier Joffé ED, Charreau EH, Schillaci R, Elizalde PV. Stat3 regulates ErbB-2 expression and co-opts ErbB-2 nuclear function to induce miR-21 expression, PDCD4 downregulation and breast cancer metastasis. Oncogene 35: 2208–2222, 2016. doi: 10.1038/onc.2015.281. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Tian J, Tang X, Rui K, Tian X, Ma J, Ma B, Xu H, Lu L, Wang S. Exosomes released by granulocytic myeloid-derived suppressor cells attenuate DSS-induced colitis in mice. Oncotarget 7: 15356–15368, 2016. doi: 10.18632/oncotarget.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie J, Chen M, Zhou J, Mo MS, Zhu LH, Liu YP, Gui QJ, Zhang L, Li GQ. miR-7 inhibits the invasion and metastasis of gastric cancer cells by suppressing epidermal growth factor receptor expression. Oncol Rep 31: 1715–1722, 2014. doi: 10.3892/or.2014.3052. [DOI] [PubMed] [Google Scholar]
- 54.Xiong B, Cheng Y, Ma L, Zhang C. MiR-21 regulates biological behavior through the PTEN/PI-3 K/Akt signaling pathway in human colorectal cancer cells. Int J Oncol 42: 219–228, 2013. doi: 10.3892/ijo.2012.1707. [DOI] [PubMed] [Google Scholar]
- 55.Yang J, Liu X-X, Fan H, Tang Q, Shou Z-X, Zuo D-M, Zou Z, Xu M, Chen Q-Y, Peng Y, Deng S-J, Liu Y-J. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One 10: e0140551, 2015. doi: 10.1371/journal.pone.0140551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang X, Meng S, Jiang H, Chen T, Wu W. Exosomes derived from interleukin-10-treated dendritic cells can inhibit trinitrobenzene sulfonic acid-induced rat colitis. Scand J Gastroenterol 45: 1168–1177, 2010. doi: 10.3109/00365521.2010.490596. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Zhang C, Hu L, He Y, Shi Z, Tang S, Chen Y. Abnormal expression of miR-21 and miR-95 in cancer stem-like cells is associated with radioresistance of lung cancer. Cancer Invest 33: 165–171, 2015. doi: 10.3109/07357907.2015.1019676. [DOI] [PubMed] [Google Scholar]
- 58.Zhao D, Kuhnt-Moore S, Zeng H, Pan A, Wu JS, Simeonidis S, Moyer MP, Pothoulakis C. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves Rho family small GTPases. Biochem J 368: 665–672, 2002. doi: 10.1042/bj20020950. [DOI] [PMC free article] [PubMed] [Google Scholar]