Abstract
Increased airway smooth muscle (ASM) mass is a key contributor to airway narrowing and airway hyperresponsiveness in asthma. Besides conventional pathways and regulators of ASM proliferation, recent studies suggest that changes in mitochondrial morphology and function play a role in airway remodeling in asthma. In this study, we aimed at determining the role of mitochondrial Bcl-2 adenovirus E1B 19 kDa-interacting protein, Bnip3, in the regulation of ASM proliferation. Bnip3 is a member of the Bcl-2 family of proteins critical for mitochondrial health, mitophagy, and cell survival/death. We found that Bnip3 expression is upregulated in ASM cells from asthmatic donors compared with that in ASM cells from healthy donors and transient downregulation of Bnip3 expression in primary human ASM cells using an siRNA approach decreased cell adhesion, migration, and proliferation. Furthermore, Bnip3 downregulation altered the structure (electron density) and function (cellular ATP levels, membrane potential, and reacitve oxygen species generation) of mitochondria and decreased expression of cytoskeleton proteins vinculin, paxillin, and actinin. These findings suggest that Bnip3 via regulation of mitochondria functions and expression of adhesion proteins regulates ASM adhesion, migration, and proliferation. This study reveals a novel role for Bnip3 in ASM functions and establishes Bnip3 as a potential target in mitigating ASM remodeling in asthma.
Keywords: asthma, Bnip3, growth, remodeling
INTRODUCTION
Airway smooth muscle (ASM), a key determinant of airway pathophysiology, mediates airway contraction, proliferation, and synthetic functions that affect both airway inflammation and remodeling. Across all asthma severities, airway remodeling occurs with an increased thickening of the bronchial wall due to increased ASM mass and other structural changes including enhanced deposition of extracellular matrix proteins (8, 9). Although the precise sequence of events that takes place in the course of remodeling remains unclear, increases in ASM mass contribute to airway narrowing and airflow obstruction. Moreover, the abnormal mass of ASM in the lung caused by airway inflammation correlates with the severity of the disease. At the cellular level, ASM cells derived from subjects with asthma demonstrate increased migration and proliferation in culture compared with that of ASM cells derived from non-asthma donors (4, 22, 27). Despite the clear contribution of airway remodeling to asthma pathobiology, current asthma therapies fail to effectively manage airway remodeling. Therefore, targeting ASM remodeling is one of the important strategies for developing newer drugs in the treatment of asthma (37, 45).
Increasing evidence suggests that changes in mitochondrial morphology and function are intimately associated with many airway pathologies including but not limited to asthma and chronic obstructive pulmonary disease (COPD) (41, 45). Mitochondria are double membrane dynamic organelles that vary in size, shape, and location (48). In mammalian cells, mitochondria are the source of cellular energy produced via oxidative phosphorylation in the form of ATP (36). In addition, mitochondria are key modulators of the redox balance of the cells. During aerobic respiration, mitochondria generate reactive oxygen species (ROS) as the byproduct of electron transfer (36), and in pathological conditions, excessive ROS generation from mitochondria is detrimental. Moreover, mitochondria is a master regulator of calcium homeostasis by taking up and releasing calcium (2, 15, 41). Increased ROS generation and disrupted Ca2+ homeostasis have been implicated in airway diseases (45). Evidence suggests that calcium homeostasis and mitochondrial biogenesis play a role in ASM cell proliferation (51). ASM cells from subjects with asthma are characterized by calcium imbalance that increases mitochondrial biogenesis as indicated by increased mitochondria number and oxygen consumption, which in turn enhance cell proliferation, leading to airway remodeling (34, 51). In addition, oxidative stress via altered mitochondrial function contributes to airway inflammation and ASM remodeling (55). A recent study from our laboratory demonstrated that agonists of bitter taste receptors mitigate mitogen-induced ASM proliferation via their effect on mitochondria (42). Based on these studies, it is possible that therapeutic targeting of mitochondria can potentially mitigate features of airway remodeling. Therefore, establishing the functional role of specific mitochondria proteins is fundamental to developing mitochondria-targeted anti-asthma therapies.
In this study, we aimed at determining the role of Bcl-2 adenovirus E1B 19 kDa-interacting protein 3 (Bnip3), a Bcl-2 family member, in the regulation of ASM cell function. Bnip3 is a regulator not only of autophagy-mediated mitochondrial turnover but also of cell survival/death (26, 57). Our findings demonstrate that downregulation of Bnip3 in primary human ASM cells leads to decreased cell adhesion, migration, and proliferation suggesting an important role of Bnip3 in the regulation of ASM functions. These findings further establish the role of mitochondria in ASM growth.
MATERIALS AND METHODS
Reagents.
Antibodies against Mfn1, Mfn2, and Bnip3 were from Abcam (Burlingame, CA). DLP1 antibody was from BD Bioscience (Franklin Lakes, NJ). Paxillin, vinculin, talin, and tensin 2 antibodies were from Cell Signaling Technology (Beverly, MA). IRDye 680 or 800 secondary antibodies were from Rockland (Gilbertsville, PA). MitoTracker Green and MitoSox red were from Thermo Fisher Scientific (Pittsburg, PA). Anti-β-actin was obtained from Sigma (St. Louis, MO). Hydrogels were from Matrigen (St. Louis, MO).
Cell culture and siRNA transfection.
Human ASM cultures were established using procedures approved by Thomas Jefferson University Institutional Review Board. Human ASM cells were isolated from human tracheae or primary bronchi using enzyme dissociation method and were grown in Ham’s F-12 medium with supplements as described previously (42). Cells in subculture during the second through fifth cell passages were used. A select set of experiments were carried out using ASM cells obtained from asthmatic donors. For experiments requiring serum starvation, the cells were maintained in Ham’s F-12 medium with no serum and supplemented with 1% insulin transferrin selenium (arresting medium) for 24–72 h (42). siRNA transfection was performed in human ASM cells as described previously (42). Briefly, cells were seeded in six-well plates 1 day before transfection. Transfection mixture was prepared by adding 2–5 μl of 5 μM siRNA and 5 μl DharmaFECT1 (GE Healthcare) reagent to serum-free medium. After incubating at room temperature for 20 min, the mixture was transferred to a new tube containing 1 ml of complete medium (final siRNA concentration was 25 nM) and applied to cells. Cells transfected with scrambled or Bnip3 siRNAs were used for all the studies described below. In a selected set of experiments, the cells were cultured on Hydrogel (Matrigen) with varying matrix stiffness.
Cell proliferation assay.
Cell proliferation was determined using CyQuant (Invitrogen, Carlsbad, CA) as described previously (42). Human ASM cells were grown to 50–60% confluency on 96-well cell culture plates with complete F-12 medium and were switched to growth arrest media for 24 h (F-12 + 1% ITS) in the presence or absence of PDGF (10 ng/mL). At 24-, 48-, and 72-h treatment, medium was removed and cells were incubated with CyQuant reagent for 1 h. Fluorescence was determined using Flexstation III (Molecular Devices) at a 530-nm wavelength.
Wound-healing assay.
Scramble or Bnip3 siRNA-transfected human ASM cells were seeded onto a 24-well cell culture plate and grown to confluency. After 24 h of growth arrest, a wound was created on the cell layer using a pipette tip and cells were treated with 10 ng/mL PDGF. Cell migration from the wound edges into the empty space was observed under a bright-field microscope at 24, 48, and 72 h after the initial wound. The data were calculated by determining the percentage of the area of the wound that the cells migrated into from the wound edges.
Immunohistochemistry.
Scramble or Bnip3 siRNA-transfected human ASM cells were plated onto glass coverslips in six-well culture dishes and fixed for 15 min in 4% paraformaldehyde and then permeabilized using 0.3% Triton X-100. Fixed cells were first blocked for 2 h at room temperature in 3% BSA and incubated with rhodamine-phalloidin at room temperature for 1 h or primary antibody at 4°C overnight followed by FITC-conjugated secondary antibody for 1 h. Coverslips were mounted onto glass slides using ProLong antifade medium (Molecular Probes). Fluorescent imaging was performed using a Nikon Confocal Laser Scanning Microscope (Nikon, Inc., Melville, NY).
Cell adhesion assay.
Cell adhesion was measured by using the xCELLigence real-time cell analysis system (ACEA Biosciences) per manufacturer’s protocol. The wells of the E-plate were washed with PBS before cells or plain media were added. Twenty-four hours after the siRNA transfection, human ASM cells were detached by trypsinization and were plated onto E-Plate 16 (ACEA Bioscience) at 12,500 cells/well in a 100-μl volume. The impedance of the plate was then monitored every 3 min for 3–6 h. Fluctuation of the impedance was used to calculate cell index over a period of time as described by the manufacturer using xCELLigence software.
Transmission electron microscopy studies.
The ultrastructural details of the cell were studied using transmission electron microscopy (TEM) to assess mitochondrial morphology and integrity. Cells were grown on glass coverslips in six-well culture plates, grown to confluence, and processed further for transmission electron microscopy as described previously (42). TEM processing and imaging was carried out at the core facility at University of Maryland, Baltimore using FEI Tecnai T12 high-resolution microscope.
Immunoblotting.
Cells were grown to 60–80% confluence in six-well plates and growth arrested for 24–72 h in serum-free F-12/ITS medium. To harvest the protein, cells were washed twice with ice-cold Tris buffer (25 mM Tris and 150 mM NaCl, pH 8.0) and then solubilized in a 25 mM Tris buffer (pH 8.0) containing 150 mM NaCl, 20 mM NaF, 5 mM EGTA, 1 mM EDTA, 10 mM sodium pyrophosphate, 10 mM p-nitrophenyl phosphate, 1 mM benzamidine, 0.1 M phenylmethylsulfonyl fluoride, and 1% (vol/vol) Nonidet P-40 (lysis buffer) for 30 min at 4°C. Following scraping, cell lysates were centrifuged at 13,200 g at 4°C for 10 min. Supernatants were collected, then electrophoresed on SDS-PAGE, transferred to nitrocellulose membranes, and subsequently probed with the indicated primary antibodies and secondary antibodies conjugated with infrared fluorophores (42). Band intensities were visualized and quantified using the Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE). The values were normalized to those of β-actin or tubulin and compared among experimental groups (40, 42).
Microscopy.
Cells were imaged on a Nikon Eclipse Ti2 inverted microscope (Nikon Instruments) controlled by NIS Elements software (Nikon Instruments) using Apo 20 × /0.95WI, DIC N2 and Apo 60 × 1.40 oil, DIC N2 objectives. Live-cell images were acquired in serum-free Ham’s F-12 media at 37°C.
Assessment of mitochondrial morphology, membrane potential, and ROS generation.
Mitochondria morphological and functional studies were performed by live-cell imaging using confocal microscopy (42, 43). For mitochondrial morphology, human ASM cells were incubated with MitoTracker Green following manufacturer’s instructions, and cells were imaged by confocal microscope as previously described (42, 43). For mitochondrial membrane potential measurement, human ASM cells were incubated with 1.5 μM tetramethyl rhodamine ester (TMRE) for 30 min and then washed with PBS to remove the unincorporated dye. TMRE fluorescence was visualized under confocal microscope, and fluorescence intensity was recorded using 545-nm excitation and 580-mm emission. Complete depolarization (maximum de-quench of TMRE fluorescence) was elicited using protonphore 2 μM carbonyl-cyanide-p-trifluoromethoxyphenylhydrazone.
For mitochondrial ROS assay, ASM cells were incubated with MitoSox red (4 μM) for 10 min and washed with PBS to remove the unincorporated dye. MitoSox red fluorescence was recorded using 510-nm excitation and 580-nm emission. Mitochondria images were analyzed using NIH ImageJ 1.44.
Cellular ATP measurement.
Cellular ATP levels were measured in human ASM cells using the ATPlite kit (Perkin Elmer), according to manufacturer’s instructions. Briefly, cells were lysed with mammalian cell lysis buffer, and cell lysates were incubated with substrate reagents following the assay protocol. ATP luciferase was measured in a plate reader as described previously (42). Data were normalized to the luciferase intensity of control cells.
Statistical analysis.
Data are presented as means ± SE values from at least three experiments, in which each experiment was performed using a different ASM culture derived from a unique donor. Individual data points from a single experiment were calculated as the mean value and reported as fold change from control group. Statistically significant differences among groups were assessed by either Student’s t test or one-way ANOVA using Prism Graphpad software 6.0 (Graphpad, La Jolla, CA), with values of P < 0.05 sufficient to reject the null hypothesis.
RESULTS
Bnip3 is upregulated in asthmatic ASM cells and regulates human ASM cell proliferation and migration.
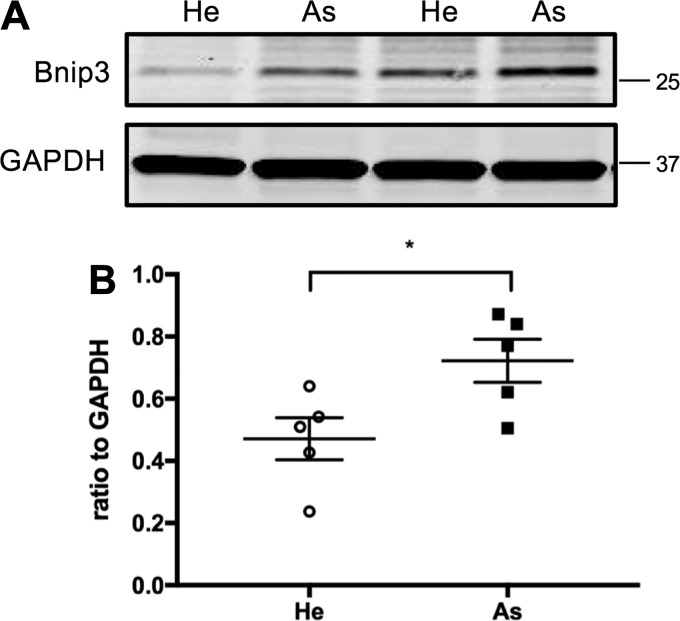
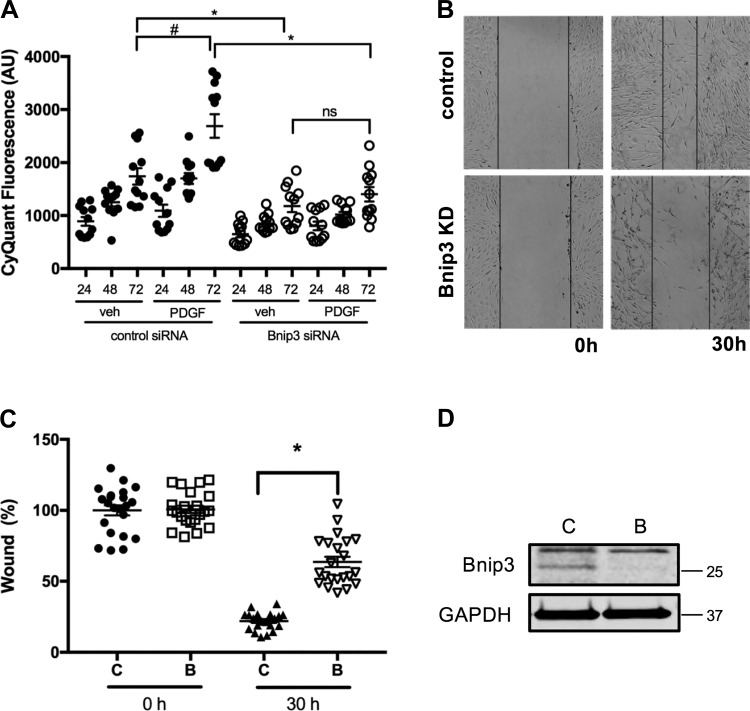
Bnip3 is a member of the Bcl-2 family of proteins, known to modulate cell migration and proliferation in several cell types (33, 47, 56, 58). Excessive proliferation and migration of ASM cells is a hallmark feature of asthma. Therefore, we assessed the level of Bnip3 protein in ASM cells from healthy and asthmatic donors by immunoblotting and found that Bnip3 was significantly (n = 5, P < 0.05) upregulated in asthmatic ASM cells compared with that in ASM cells from healthy donors (Fig. 1). Furthermore, to determine the role for Bnip3 in human ASM proliferation, we examined PDGF-induced human ASM cell growth using scrambled and Bnip3 siRNA-transfected human ASM cells using a CyQuant assay. Human ASM cells transfected with Bnip3 siRNA exhibited significantly decreased proliferation in response to PDGF compared with scrambled siRNA-transfected cells in a time-dependent manner (Fig. 2A). After 72 h of treatment with PDGF, control cells reached a 1.5-fold increase in CyQuant fluorescence compared with 1.2-fold in Bnip3 knockdown cells (n = 12 measurements from 4 different ASM lines, P < 0.05).
Fig. 1.
Upregulation of Bcl-2 adenovirus E1B 19 kDa-interacting protein 3 (Bnip3) protein expression in airway smooth muscle (ASM) cells from asthmatic donors. Proteins were harvested from healthy (He) and asthmatic (As) human ASM cells. A: Bnip3 protein levels were determined by immunoblotting. GAPDH was used as an internal control for protein loading. He, healthy human ASM cells. B: quantification of immunoblots showing Bnip3 protein levels in He and As ASM cells (n = 5; *P < 0.05 He vs. As).
Fig. 2.
Bcl-2 adenovirus E1B 19 kDa-interacting protein 3 (Bnip3) downregulation impaired PDGF-induced human airway smooth muscle (ASM) proliferation and migration. Bnip3 expression was downregulated in human ASM cells by transient transfection of Bnip3 siRNA. Scrambled siRNA-transfected ASM cells were used as control. A: cells were stimulated with either 10 ng/mL of PDGF or vehicle for 24, 48, and 72 h in serum-free F-12 media and cell proliferation was measured by CyQuant assay (n = 12 measurements from 4 different ASM lines, #P < 0.05, significance relative to control siRNA with vehicle treatment condition. *P < 0.05, relative to control siRNA with 72-h PDGF treatment condition; ns: not significant). AU, arbitrary units. B: following transfection of siRNA for 24 h, cells were cultured in serum free F-12 media. Cells were scraped at the center of the dish and were treated with 10 ng/mL of PDGF or vehicle for 30 h. Light microscope images were taken before and after 30 h treatment. Bnip3 KD, Bnip3 knockdown. C: the wound area (between lines) was measured before and after PDGF treatment as percentage of control siRNA area without treatment (n = 24 measurements from 6 different ASM cell lines. *P < 0.05 Bnip3 siRNA vs. scrambled siRNA with 30 h of PDGF treatment). D: immunoblot showing efficiency of Bnip3 knockdown. C, control, scrambled siRNA; B, Bnip3 siRNA.
We determined ASM cell migration using a wound-healing assay in which a wound was created on the cell layer using a pipette tip and cells were allowed to migrate into the wound area from the edges to fill the gap. The scrambled siRNA-transfected human ASM cells closed the gap by 79% over 72 h whereas Bnip3 knockdown cells reached 36.3% recovery during the same time period. These data suggest that PDGF-induced cell migration is significantly decreased by Bnip3 knockdown (Fig. 2, B and C) (n = 24 from 6 different human ASM lines, P < 0.05) in human ASM cells. A representative Western blot image is shown in Fig. 2D to illustrate the Bnip3 knockdown efficiency in human ASM cells.
Bnip3 regulates ASM cell adhesion and spreading.
ASM cell adhesion to substrate via focal adhesion has long been recognized as an essential step in cell migration (20, 39). To further establish the functional role of Bnip3 in human ASM cells, we determined the effect of Bnip3 knockdown on ASM cell adhesion and spreading.
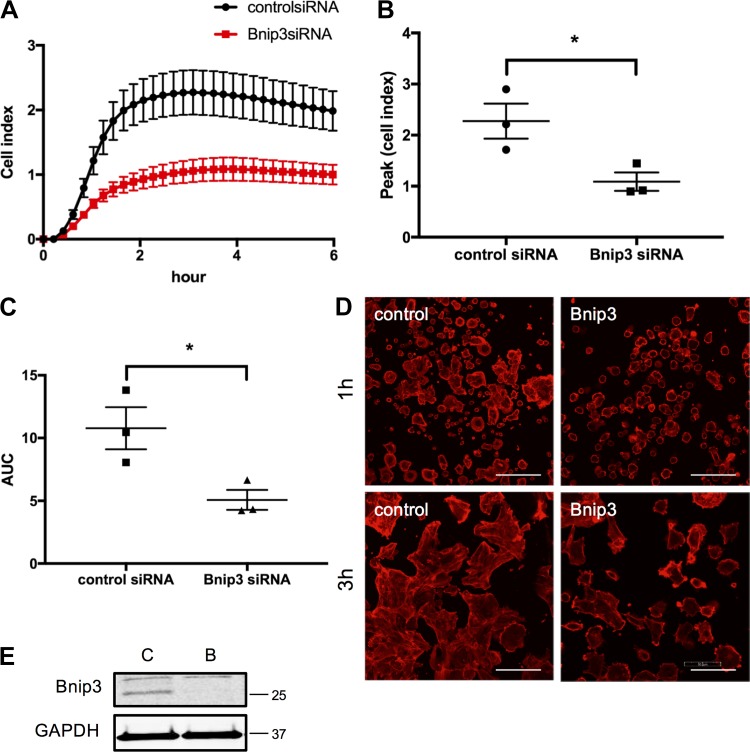
To quantitatively measure the cell adhesion and spreading, we utilized xCELLigence system and monitored cells in real-time after seeding cells on E-plates. Change in impedance at early time points (minutes to few hours) after seeding cells on the culture plates is used as a readout for cell adhesion and cell spreading. Cell adhesion and cell spreading were significantly attenuated in human ASM cells transfected with Bnip3 siRNA compared with scrambled siRNA (Fig. 3), as indicated by the decrease in cell index over time, peak cell index, as well as area under the curve (Fig. 3, A–C). In addition, we performed confocal microscopic analysis of cell attachment and spreading. Similar to what we observed in the xCELLigence assay, all the control cells attached and spread after 3 h (Fig. 3D, left panels) whereas Bnip3 knockdown cells showed attenuated ability to adhere and spread over a period of 3 h (Fig. 3D, right panels). Together these data suggest that Bnip3 knockdown impairs ASM cell adhesion and spreading presumably contributing to the decreased migration and proliferation.
Fig. 3.
Bcl-2 adenovirus E1B 19 kDa-interacting protein 3 (Bnip3) downregulation negatively affects human airway smooth muscle (ASM) cell adhesion and spreading on tissue culture dish. Cell adhesion and spreading were assayed in human ASM cells 72 h after transient transfection of scrambled and Bnip3 siRNA. A–C: cell adhesion and spreading are expressed as cell index and data are represented as real-time (A), peak (B), and integrated (area under the curve, AUC) (C) cell index (*P < 0.05, Bnip3 vs. scrambled siRNA; n = 3 ASM lines from 3 different donors). D: representative confocal images of rhodamine-phalloidin staining showing cell adhesion and spreading 1 and 3 h after seeding cells were seeded on culture dish. Bar = 100 μm. E: immunoblot showing efficiency of Bnip3 knockdown. C, control, scrambled siRNA; B, Bnip3 siRNA.
Bnip3 downregulation impairs cytoskeleton organization and mitochondrial functions.
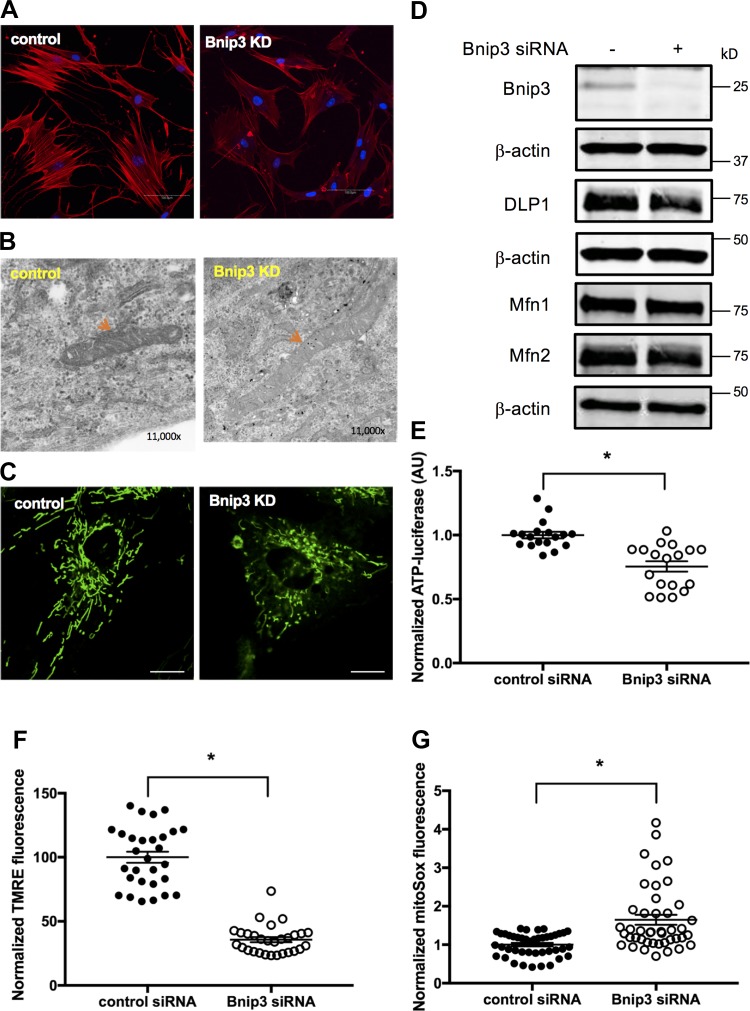
The actin cytoskeleton plays a central role in the process of cell spreading, migration, and proliferation (44, 49). We determined the organization of cytoskeleton (actin filaments) in ASM cells after downregulating Bnip3 expression. In Bnip3 knockdown ASM cells, rhodamine-phalloidin staining exhibited significantly decreased F-actin compared with cells in the control group (Fig. 4A). Furthermore, TEM studies revealed disorganized microfilaments in Bnip3 knockdown cells compared with control cells (data not shown).
Fig. 4.
F-actin and mitochondrial functions were compromised in Bcl-2 adenovirus E1B 19 kDa-interacting protein 3 (Bnip3) siRNA-transfected human airway smooth muscle (ASM) cells. A: human ASM cells were transfected with Bnip3 and scrambled siRNA for 72 h. Cells were stained with rhodamine-phalloidin and DAPI. Representative images of actin stress fiber were acquired by confocal microscopy at excitation of 540 nm. Bnip3 KD, Bnip3 knockdown. Bar = 100 μm. B: representative transmission electron microscopy images (×11,000) showing mitochondrial structure in control and Bnip3 knockdown ASM cells (red arrow). C: representative confocal live-cell image of mitochondria labeled by MitoTracker green. Bar = 20 μm. D: Western blot for determining protein levels of Bnip3, Mfn1, Mfn2, and DLP1 using β-actin as loading control. E–G: mitochondrial functions were assessed by determining cellular ATP levels (E) using a luciferase assay (n = 18 measurements from 6 different ASM cell lines, *P < 0.05, significance relative to scrambled siRNA), mitochondrial membrane potential (F) using tetramethyl rhodamine ester (TMRE) by confocal live-cell imaging (n = 29 cells from 3 separate experiments; *P < 0.05, significance relative to scrambled siRNA), and mitochondrial reactive oxygen species (G) by confocal live-cell imaging using MitoSox red (n = 43 cells from 3 different experiments; *P < 0.05, significance relative to scrambled siRNA). AU, arbitrary units.
Both cell migration and adhesion are energy-dependent processes and Bnip3 is localized in mitochondria, which is the primary source of cellular energy. Therefore, we next assessed the effect of Bnip3 knockdown on the anatomy and physiology of mitochondria. TEM analysis revealed a significant decrease in electron density of mitochondria in Bnip3 knockdown human ASM cells compared with those in scrambled siRNA-transfected cells (Fig. 4B). We further examined mitochondria morphology by live-cell imaging and Western blot analysis (Fig. 4C). There was no significant difference in the mitochondrial aspect ratio and form factor after Bnip3 knockdown in human ASM cells compared with control cells. In addition, there was no significant difference in mitochondrial dynamic protein levels including the levels of dynamin-like protein DLP1 and mitofusin proteins Mfn1 and Mfn2 between control and Bnip3 knockdown ASM cells (Fig. 4D). Collectively, these data suggest that Bnip3 downregulation in ASM cells results in loss of mitochondrial matrix structure without a significant change in mitochondrial fission or fusion.
We determined the functional status of mitochondria in human cells transfected with Bnip3 siRNA by measuring total cellular ATP, mitochondrial membrane potential, and mitochondrial ROS generation. The total intracellular ATP levels were significantly decreased after Bnip3 knockdown (Fig. 4E; n = 18 from 6 different ASM cell lines, P < 0.05) compared with control cells. Confocal live-cell imagining analysis indicated a significant loss in mitochondrial membrane potential in Bnip3 siRNA-transfected ASM cell compared with scrambled siRNA-transfected cells (decreased to 35.69% with control siRNA group set as 100%; n = 29, P < 0.05; Fig. 4F). Furthermore, the mitochondrial ROS generation was significantly increased in Bnip3 knockdown human ASM cells (Fig. 4G; 1.65-fold increase with control set as 1.0; n = 43, P < 0.05) compared with control cells.
Collectively, these findings suggest that Bnip3 knockdown causes mitochondria dysfunction as indicated by a decrease in mitochondrial membrane potential and cellular ATP levels and a significant increase in mitochondrial ROS.
Effect of extracellular matrix composition and stiffness on ASM cell adhesion and spreading.
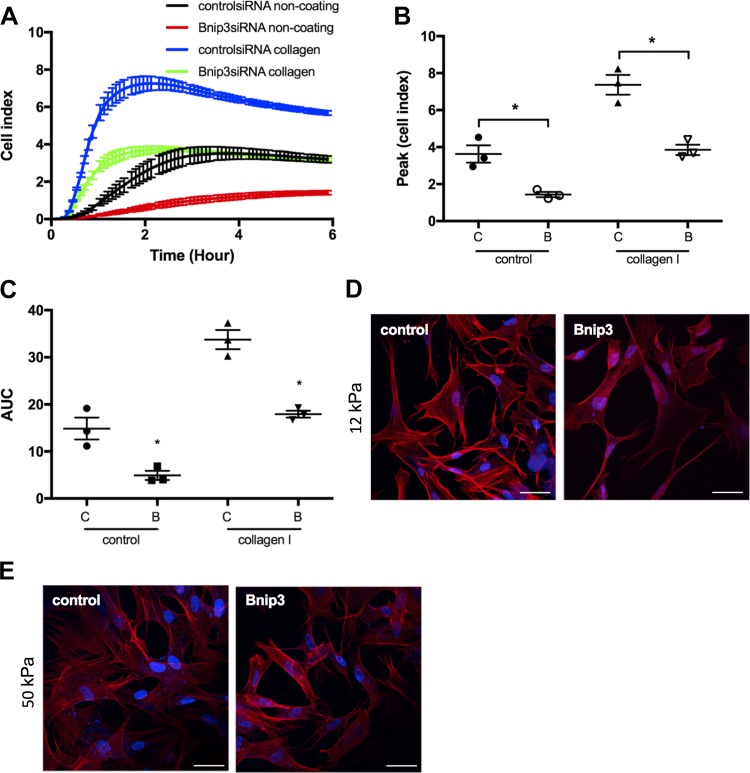
Composition and stiffness of extracellular matrix are known to play pivotal roles in the regulation of adhesion and spreading (biomechanical) properties of ASM cells (30, 54). We next investigated the effect of extracellular matrix composition and stiffness on Bnip3-mediated ASM cell adhesion and spreading using culture plates coated with collagen I and hydrogel plates of different stiffness. As assessed by the xCELLigence system, collagen I increased cell adhesion and spreading in both control siRNA and Bnip3 siRNA groups (Fig. 5, A–C). However, Bnip3 knockdown significantly decreased cell adhesion and spreading as indicated by a lower cell index over time (Fig. 5A), lower peak cell index (Fig. 5B), and decreased area under the curve (Fig. 5C) compared with those of control cells (Fig. 5, A–C).
Fig. 5.
Bcl-2 adenovirus E1B 19 kDa-interacting protein 3 (Bnip3) downregulation decreased human airway smooth muscle (ASM) cell adhesion and spreading on collagen I-coated culture plates and on hydrogels. Cell adhesion and spreading on collagen I and hydrogel matrixes were assessed in human ASM cells 72 h after transient transfection of scrambled and Bnip3 siRNA. A–C: real-time (A), peak (B), and integrated (area under the curve, AUC) (C) measurement of cell adhesion and spreading on collagen were performed using xCELLigence system (n = 3; *P < 0.05 significance relative to scrambled siRNA). D and E: representative confocal images of rhodamine-phalloidin staining showing cell adhesion and spreading 3 h after cells were seeded on hydrogel. DAPI was used as nuclear staining. Bar = 100 μm.
We further seeded control and Bnip3 knockdown ASM cells on hydrogel with increasing stiffness (12 and 50 kPa). Increased extracellular matrix stiffness enhanced the ability of both control and Bnip3 knockdown ASM cells. However, Bnip3 knockdown cells showed decreased ability to attach and spread on hydrogel compared with control cells (Fig. 5, D and E).
Focal adhesion protein levels are decreased by Bnip3 knockdown in human ASM cells.
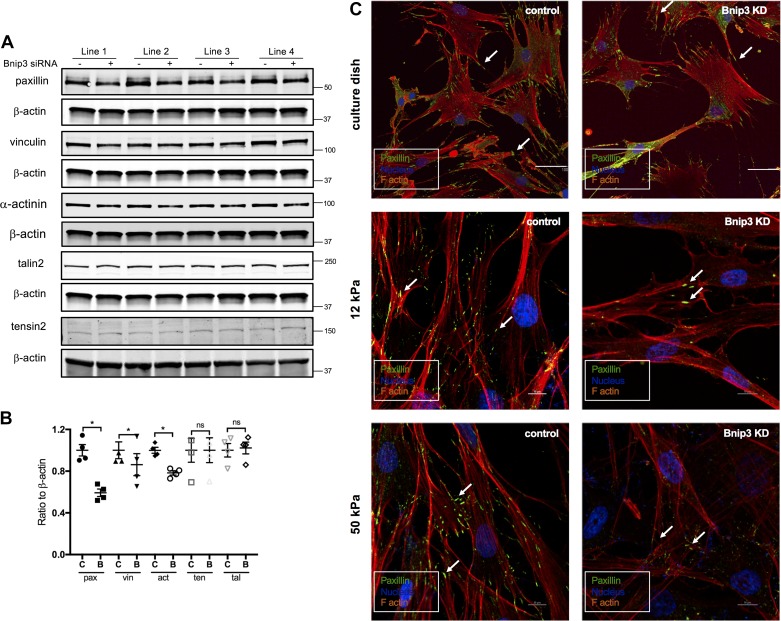
Cell adhesion is determined by the level of expression of focal adhesion proteins or kinase activity of focal adhesion kinase or both. To determine whether the decreased cell adhesion we observed in Bnip3 knockdown cells was due to the changes in focal adhesion protein expression, we examined the protein levels of paxillin, vinculin, talin2, tensin2, and α-actinin. Bnip3 knockdown significantly (P < 0.05, n = 4) decreased paxillin protein levels (Fig. 6, A and B). Similarly, the protein levels of vinculin and α-actinin were also decreased by Bnip3 knockdown, although to a lesser extent (Fig. 6, A and B). There were no significant differences in the expression levels of talin2 and tensin2 between control and Bnip3 knockdown ASM cells (Fig. 6, A and B). There was no significant change in FAK phosphorylation suggesting that downregulation of Bnip3 expression does not modulate focal adhesion kinase activity (data not shown).
Fig. 6.
Bcl-2 adenovirus E1B 19 kDa-interacting protein 3 (Bnip3) downregulation in human airway smooth muscle (ASM) cells decreased paxillin, vinculin, and α-actinin protein levels. Expression of focal adhesion proteins and paxillin puncta in scrambled and Bnip3 siRNA-transfected human ASM cells. A: Western blot showing the protein levels of paxillin (pax), vinculin (vin), α-actinin (act), tensin 2 (ten), talin2 (tal), and Bnip3 using β-actin as loading control. B: quantified data with scrambled siRNA levels set at 1.0. *P < 0.05 significance relative to scrambled siRNA. C, scrambled siRNA; B, Bnip3 siRNA. C: representative confocal images of immunostaining for paxillin (green) in control (left panels) and Bnip3 knockdown (right panels; Bnip3 KD) human ASM cells on culture dish (top two panels; bar = 100 μm) and hydrogel (middle and bottom two panels; bar = 10 μm). Rhodamine (red) and DAPI (blue) were used as counterstaining for F-actin and nucleus, respectively.
Focal adhesions are large, dynamic multiprotein complex structures that form mechanical links between intracellular actin bundles and the extracellular substrate. We further performed immunofluorescence studies using paxillin as a marker of focal adhesion complex. Paxillin localized at focal adhesion was visualized as a puncta-like structure. The paxillin “puncta” at the site of focal adhesion per cell adhesion were less in Bnip3 knockdown cells than in control cells (Fig. 6C, right three panels). Decrease in adhesion puncta formation was more obvious on hydrogels of higher stiffness showing significantly decreased paxillin “puncta” in Bnip3 knockdown cells compared with control cells (Fig. 6C, right middle and bottom panels).
Collectively, these findings demonstrate that Bnip3 regulates ASM cell adhesion, spreading, migration, and proliferation by affecting expression of adhesion proteins and formation of cytoskeletal and focal adhesion complex.
DISCUSSION
In this study, we have identified a novel mechanism in regulating ASM cell proliferation via the mitochondrial protein Bnip3. Our studies demonstrate for the first time a role for Bnip3 in mitogen-induced ASM proliferation. Bnip3 is not only associated with mitochondrial autophagy but is also involved in the regulation of various cellular functions including proliferation, and migration (33, 47, 56, 58). Knocking down Bnip3 significantly decreased PDGF-induced ASM cell proliferation suggesting a novel mechanism that can be targeted for mitigation of airway remodeling in asthma and COPD. Even though the role of Bnip3 in the pathogenesis of neurodegenerative disorders, cancer, and cardiovascular disease has been reported (14, 19, 46), the precise mechanism by which Bnip3 contributes to human pathologies remains poorly understood. Several animal model and in vitro studies have provided evidence for the role of Bnip3 in the regulation of cell proliferation and migration (33, 47, 56, 58). Consistent with the role of Bnip3 in disease pathogenesis, studies have shown that cellular stress, hypoxia, and starvation upregulate Bnip3 expression in multiple cell types (13, 58). Our findings demonstrate, for the first time, that Bnip3 is upregulated in asthmatic ASM cells compared with that in healthy ASM cells and presumably contributes to asthmatic phenotype of ASM cells.
Bnip3 has been reported as either promoting cell survival or cell death dependent on the levels of Bnip3 expression and cellular localization (47). Overexpression of Bnip3 leads to increased growth of tumors and downregulation of Bnip3-inhibited tumor growth (52). Similarly, tail vein injection of Bnip3 shRNA targeted to cardiac myocytes inhibited ischemia-induced cardiac remodeling (10). These studies suggest the function of Bnip3 in cell death regulation is context dependent. Bnip3 is an atypical proapoptotic molecule, and it is likely that Bnip3-mediated cell death depends on the concomitant expression of other proapoptotic Bcl-2 family proteins needed for induction of apoptosis-induced cell death. Therefore, it is important to establish what functional effect, if any, Bnip3 has in the regulation cell behavior, which is first critical step in exploring Bnip3 in the pathogenesis of obstructive airway diseases. In this context, our data suggest that Bnip3 downregulation may be beneficial in mitigating airway remodeling.
Bnip3 knockdown in ASM cells resulted in structural (decreased electron density of mitochondria) and functional (decreased ATP levels and mitochondrial membrane potential and increased ROS levels) changes in mitochondria. Interestingly, we did not find an increase in mitochondrial fragmentation or any change in expression of the mitochondrial dynamic proteins DLP1, Mfn1, Mfn2, and Opa1 (42). Consistent with our findings, loss of Bnip3 in hepatocytes reduced mitochondrial functions (23). However, it is unclear if cell survival or death function of Bnip3 is directly related to its regulation of mitochondrial structure and function. Bnip3 also plays a critical role in mitophagy thereby initiating the process of eliminating dysfunctional mitochondria (38). Failing to eliminate damaged mitochondria may cause the accumulation of damaged mitochondria and increased ROS generation (53). Mitochondrial ROS could either enhance or inhibit ASM cell proliferation in a dosage- and duration-dependent manner. At physiological conditions, ROS act as the second messenger for ASM cell proliferation (3). For example, ASM proliferation induced by fetal bovine serum or PDGF was significantly reduced by utilizing antioxidants such as N-acetylcysteine, catalase, and probucol (3). However, excessive ROS could inhibit cell growth via redox-dependent oxidation of signaling proteins (5, 7, 17, 35). ROS can interact with biomolecules resulting in oxidation of proteins, mutation in DNA, and lipid leading to cell cycle arrest (1, 5, 32). Further studies are needed to understand whether Bnip3 knockdown causes oxidative damage of ASM cell proteins.
Bnip3 knockdown decreased the ability of ASM cells to adhere and spread with a concomitant decrease in the expression level of focal adhesion proteins paxillin, vinculin, and α-actinin. The mechanism by which Bnip3 regulates expression of focal adhesion proteins is not clear. Recent studies suggest that Bnip3 can localize to the nucleus and act as a transcriptional repressor to decrease gene expression (6). In fact, Bnip3 knockdown resulted in decrease in expression of adhesion proteins at mRNA level (data not shown) suggesting transcriptional regulation of expression of focal adhesion proteins by Bnip3. However, we cannot exclude the possibility that decreased focal adhesion proteins are the secondary effect of changes in the mitochondrial function. Decreased mitochondrial membrane potential and increased ROS both indicate mitochondrial dysfunction in Bnip3 knockdown cells. Particularly, increased mitochondrial ROS can regulate cytoskeleton dynamics and cell adhesion via direct or indirect mechanisms. We did not observe significant changes in FAK phosphorylation (data not shown), but other studies have demonstrated that proteins such as FAK and Src are redox-sensitive molecules and ROS can regulate kinase activity of FAK and cytoskeletal rearrangement (12, 21). ROS can also directly act on actin via oxidative modification of reactive cysteine (16). In fact, ROS produced at the cell-matrix contact are essential mediators for actin stress fibers formation during cell spreading via oxidative modification of thiol groups (16). By doing so, ROS-mediated redox modification seems to affect cell-cell and cell-matrix adhesion and cell spreading (16, 28).
Decreased cell adhesion is associated with impaired actin stress fiber formation in cells lacking Bnip3, which may also result in decreased cell migration and cell division/proliferation (33). Actin stress fibers consist of the polymers of filamentous actin and drive a large number of cellular process such as changes in cell shape, cell mobility, cytokinesis, and intracellular transport via exerting or resisting forces. Recent studies support a role for actin stress fiber organization in promoting cell proliferation and migration/metastasis (50, 59). Recent studies demonstrated that paxillin mutations affecting focal adhesions cause altered mitochondrial dynamics (31). Furthermore, focal adhesion and cell shape change are associated with cell proliferation likely via cell cycle regulation (11, 18, 29). It appears that focal adhesion, mitochondria function, and ASM proliferation are interconnected, but the detailed mechanisms involved are yet to be explored.
In summary, our studies suggest that manipulation of mitochondria-associated proteins such as Bnip3 may prove to be a novel antiproliferative strategy in mitigating ASM remodeling. Although we did not assess the effect of Bnip3 downregulation on ASM cell stiffness or contractile response, it is reasonable to hypothesize that Bnip3 downregulation decreases ASM cell stiffness and contraction based on the rationale that actin filaments and cell adhesion plays a major role in force generation in ASM cells (24, 25, 60). This should further favor therapeutic targeting of Bnip3 in ASM cells as this would overcome both airway hyperresponsiveness and airway remodeling.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-137030 and HL-146645 (D. A. Deshpande).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.P., R.A.P., and D.A.D. conceived and designed research; S.P. and S.D.S. performed experiments; S.P., S.D.S., and D.A.D. analyzed data; S.P. and D.A.D. interpreted results of experiments; S.P., S.D.S., and D.A.D. prepared figures; S.P., R.A.P., and D.A.D. drafted manuscript; S.P., R.A.P., and D.A.D. edited and revised manuscript; S.P., S.D.S., R.A.P., and D.A.D. approved final version of manuscript.
REFERENCES
- 1.Alic N, Higgins VJ, Dawes IW. Identification of a Saccharomyces cerevisiae gene that is required for G1 arrest in response to the lipid oxidation product linoleic acid hydroperoxide. Mol Biol Cell 12: 1801–1810, 2001. doi: 10.1091/mbc.12.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345, 2011. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brar SS, Kennedy TP, Whorton AR, Murphy TM, Chitano P, Hoidal JR. Requirement for reactive oxygen species in serum-induced and platelet-derived growth factor-induced growth of airway smooth muscle. J Biol Chem 274: 20017–20026, 1999. doi: 10.1074/jbc.274.28.20017. [DOI] [PubMed] [Google Scholar]
- 4.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 3: 507–511, 1990. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- 5.Burhans WC, Heintz NH. The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radic Biol Med 47: 1282–1293, 2009. doi: 10.1016/j.freeradbiomed.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Burton TR, Henson ES, Azad MB, Brown M, Eisenstat DD, Gibson SB. BNIP3 acts as transcriptional repressor of death receptor-5 expression and prevents TRAIL-induced cell death in gliomas. Cell Death Dis 4: e587, 2013. doi: 10.1038/cddis.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carreras MC, Converso DP, Lorenti AS, Barbich M, Levisman DM, Jaitovich A, Antico Arciuch VG, Galli S, Poderoso JJ. Mitochondrial nitric oxide synthase drives redox signals for proliferation and quiescence in rat liver development. Hepatology 40: 157–166, 2004. doi: 10.1002/hep.20255. [DOI] [PubMed] [Google Scholar]
- 8.Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis 147: 405–410, 1993. doi: 10.1164/ajrccm/147.2.405. [DOI] [PubMed] [Google Scholar]
- 9.Carroll N, Lehmann E, Barret J, Morton A, Cooke C, James A. Variability of airway structure and inflammation in normal subjects and in cases of nonfatal and fatal asthma. Pathol Res Pract 192: 238–248, 1996. doi: 10.1016/S0344-0338(96)80227-5. [DOI] [PubMed] [Google Scholar]
- 10.Chaanine AH, Gordon RE, Kohlbrenner E, Benard L, Jeong D, Hajjar RJ. Potential role of BNIP3 in cardiac remodeling, myocardial stiffness, and endoplasmic reticulum: mitochondrial calcium homeostasis in diastolic and systolic heart failure. Circ Heart Fail 6: 572–583, 2013. doi: 10.1161/CIRCHEARTFAILURE.112.000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science 276: 1425–1428, 1997. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 12.Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol 161: 933–944, 2003. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JW, Jo A, Kim M, Park HS, Chung SS, Kang S, Park KS. BNIP3 is essential for mitochondrial bioenergetics during adipocyte remodelling in mice. Diabetologia 59: 571–581, 2016. doi: 10.1007/s00125-015-3836-9. [DOI] [PubMed] [Google Scholar]
- 14.Chourasia AH, Macleod KF. Tumor suppressor functions of BNIP3 and mitophagy. Autophagy 11: 1937–1938, 2015. doi: 10.1080/15548627.2015.1085136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340, 2011. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiaschi T, Cozzi G, Raugei G, Formigli L, Ramponi G, Chiarugi P. Redox regulation of beta-actin during integrin-mediated cell adhesion. J Biol Chem 281: 22983–22991, 2006. doi: 10.1074/jbc.M603040200. [DOI] [PubMed] [Google Scholar]
- 17.Flattery-O’Brien JA, Dawes IW. Hydrogen peroxide causes RAD9-dependent cell cycle arrest in G2 in Saccharomyces cerevisiae whereas menadione causes G1 arrest independent of RAD9 function. J Biol Chem 273: 8564–8571, 1998. doi: 10.1074/jbc.273.15.8564. [DOI] [PubMed] [Google Scholar]
- 18.Folkman J, Moscona A. Role of cell shape in growth control. Nature 273: 345–349, 1978. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 19.Fordjour PA, Wang L, Gao H, Li L, Wang Y, Nyagblordzro M, Agyemang K, Fan G. Targeting BNIP3 in inflammation-mediated heart failure: a novel concept in heart failure therapy. Heart Fail Rev 21: 489–497, 2016. doi: 10.1007/s10741-016-9557-4. [DOI] [PubMed] [Google Scholar]
- 20.Gerlach BD, Tubbesing K, Liao G, Rezey AC, Wang R, Barroso M, Tang DD. Phosphorylation of GMFγ by c-Abl coordinates lamellipodial and focal adhesion dynamics to regulate airway smooth muscle cell migration. Am J Respir Cell Mol Biol 61: 219–231, 2019. doi: 10.1165/rcmb.2018-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol 25: 6391–6403, 2005. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girodet PO, Ozier A, Bara I, Tunon de Lara JM, Marthan R, Berger P. Airway remodeling in asthma: new mechanisms and potential for pharmacological intervention. Pharmacol Ther 130: 325–337, 2011. doi: 10.1016/j.pharmthera.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Glick D, Zhang W, Beaton M, Marsboom G, Gruber M, Simon MC, Hart J, Dorn GW II, Brady MJ, Macleod KF. BNip3 regulates mitochondrial function and lipid metabolism in the liver. Mol Cell Biol 32: 2570–2584, 2012. doi: 10.1128/MCB.00167-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunst SJ, Tang DD. The contractile apparatus and mechanical properties of airway smooth muscle. Eur Respir J 15: 600–616, 2000. doi: 10.1034/j.1399-3003.2000.15.29.x. [DOI] [PubMed] [Google Scholar]
- 25.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 295: C576–C587, 2008. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gustafsson AB. Bnip3 as a dual regulator of mitochondrial turnover and cell death in the myocardium. Pediatr Cardiol 32: 267–274, 2011. doi: 10.1007/s00246-010-9876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, Gerthoffer WT. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem 274: 24211–24219, 1999. doi: 10.1074/jbc.274.34.24211. [DOI] [PubMed] [Google Scholar]
- 28.Hurd TR, DeGennaro M, Lehmann R. Redox regulation of cell migration and adhesion. Trends Cell Biol 22: 107–115, 2012. doi: 10.1016/j.tcb.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol 109: 317–330, 1989. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irons L, Owen MR, O’Dea RD, Brook BS. Effect of loading history on airway smooth muscle cell-matrix adhesions. Biophys J 114: 2679–2690, 2018. doi: 10.1016/j.bpj.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawada I, Hasina R, Lennon FE, Bindokas VP, Usatyuk P, Tan YH, Krishnaswamy S, Arif Q, Carey G, Hseu RD, Robinson M, Tretiakova M, Brand TM, Iida M, Ferguson MK, Wheeler DL, Husain AN, Natarajan V, Vokes EE, Singleton PA, Salgia R. Paxillin mutations affect focal adhesions and lead to altered mitochondrial dynamics: relevance to lung cancer. Cancer Biol Ther 14: 679–691, 2013. doi: 10.4161/cbt.25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klatt P, Molina EP, De Lacoba MG, Padilla CA, Martinez-Galesteo E, Barcena JA, Lamas S. Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. FASEB J 13: 1481–1490, 1999. doi: 10.1096/fasebj.13.12.1481. [DOI] [PubMed] [Google Scholar]
- 33.Maes H, Van Eygen S, Krysko DV, Vandenabeele P, Nys K, Rillaerts K, Garg AD, Verfaillie T, Agostinis P. BNIP3 supports melanoma cell migration and vasculogenic mimicry by orchestrating the actin cytoskeleton. Cell Death Dis 5: e1127, 2014. doi: 10.1038/cddis.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, Siew L, Simcock DE, McVicker CG, Kanabar V, Snetkov VA, O’Connor BJ, Karner C, Cousins DJ, Macedo P, Chung KF, Corrigan CJ, Ward JP, Lee TH. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc Natl Acad Sci USA 106: 10775–10780, 2009. doi: 10.1073/pnas.0902295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Márton M, Tihanyi N, Gyulavári P, Bánhegyi G, Kapuy O. NRF2-regulated cell cycle arrest at early stage of oxidative stress response mechanism. PLoS One 13: e0207949, 2018. doi: 10.1371/journal.pone.0207949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol 16: R551–R560, 2006. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 37.Nayak AP, Deshpande DA, Penn RB. New targets for resolution of airway remodeling in obstructive lung diseases. F1000 Res 7: 680, 2018. doi: 10.12688/f1000research.14581.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ney PA. Mitochondrial autophagy: origins, significance, and role of BNIP3 and NIX. Biochim Biophys Acta 1853: 2775–2783, 2015. doi: 10.1016/j.bbamcr.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 39.Paluch EK, Aspalter IM, Sixt M. Focal adhesion-independent cell migration. Annu Rev Cell Dev Biol 32: 469–490, 2016. doi: 10.1146/annurev-cellbio-111315-125341. [DOI] [PubMed] [Google Scholar]
- 40.Pan S, Berk BC. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ Res 100: 213–219, 2007. doi: 10.1161/01.RES.0000256089.30318.20. [DOI] [PubMed] [Google Scholar]
- 41.Pan S, Conaway S Jr, Deshpande DA. Mitochondrial regulation of airway smooth muscle functions in health and pulmonary diseases. Arch Biochem Biophys 663: 109–119, 2019. doi: 10.1016/j.abb.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan S, Sharma P, Shah SD, Deshpande DA. Bitter taste receptor agonists alter mitochondrial function and induce autophagy in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 313: L154–L165, 2017. doi: 10.1152/ajplung.00106.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan S, Wang N, Bisetto S, Yi B, Sheu SS. Downregulation of adenine nucleotide translocator 1 exacerbates tumor necrosis factor-α-mediated cardiac inflammatory responses. Am J Physiol Heart Circ Physiol 308: H39–H48, 2015. doi: 10.1152/ajpheart.00330.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pocaterra A, Santinon G, Romani P, Brian I, Dimitracopoulos A, Ghisleni A, Carnicer-Lombarte A, Forcato M, Braghetta P, Montagner M, Galuppini F, Aragona M, Pennelli G, Bicciato S, Gauthier N, Franze K, Dupont S. F-actin dynamics regulates mammalian organ growth and cell fate maintenance. J Hepatol 71: 130–142, 2019. doi: 10.1016/j.jhep.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 45.Prakash YS, Halayko AJ, Gosens R, Panettieri RA Jr, Camoretti-Mercado B, Penn RB; ATS Assembly on Respiratory Structure and Function . An official American Thoracic Society Research Statement: current challenges facing research and therapeutic advances in airway remodeling. Am J Respir Crit Care Med 195: e4–e19, 2017. doi: 10.1164/rccm.201611-2248ST. [DOI] [PubMed] [Google Scholar]
- 46.Rogers RS, Tungtur S, Tanaka T, Nadeau LL, Badawi Y, Wang H, Ni HM, Ding WX, Nishimune H. Impaired mitophagy plays a role in denervation of neuromuscular junctions in ALS mice. Front Neurosci 11: 473, 2017. doi: 10.3389/fnins.2017.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh A, Azad M, Shymko MD, Henson ES, Katyal S, Eisenstat DD, Gibson SB. The BH3 only Bcl-2 family member BNIP3 regulates cellular proliferation. PLoS One 13: e0204792, 2018. doi: 10.1371/journal.pone.0204792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soubannier V, McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta 1793: 154–170, 2009. doi: 10.1016/j.bbamcr.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Tang DD, Gerlach BD. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir Res 18: 54, 2017. doi: 10.1186/s12931-017-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tavares S, Vieira AF, Taubenberger AV, Araújo M, Martins NP, Brás-Pereira C, Polónia A, Herbig M, Barreto C, Otto O, Cardoso J, Pereira-Leal JB, Guck J, Paredes J, Janody F. Actin stress fiber organization promotes cell stiffening and proliferation of pre-invasive breast cancer cells. Nat Commun 8: 15237, 2017. doi: 10.1038/ncomms15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trian T, Benard G, Begueret H, Rossignol R, Girodet PO, Ghosh D, Ousova O, Vernejoux JM, Marthan R, Tunon-de-Lara JM, Berger P. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med 204: 3173–3181, 2007. doi: 10.1084/jem.20070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vara-Perez M, Maes H, Van Dingenen S, Agostinis P. BNIP3 contributes to the glutamine-driven aggressive behavior of melanoma cells. Biol Chem 400: 187–193, 2019. doi: 10.1515/hsz-2018-0208. [DOI] [PubMed] [Google Scholar]
- 53.Wang CH, Wu SB, Wu YT, Wei YH. Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp Biol Med (Maywood) 238: 450–460, 2013. doi: 10.1177/1535370213493069. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Li R, Zhong R. Extracellular matrix promotes proliferation, migration and adhesion of airway smooth muscle cells in a rat model of chronic obstructive pulmonary disease via upregulation of the PI3K/AKT signaling pathway. Mol Med Rep 18: 3143–3152, 2018. doi: 10.3892/mmr.2018.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiegman CH, Michaeloudes C, Haji G, Narang P, Clarke CJ, Russell KE, Bao W, Pavlidis S, Barnes PJ, Kanerva J, Bittner A, Rao N, Murphy MP, Kirkham PA, Chung KF, Adcock IM, Brightling CE, Davies DE, Finch DK, Fisher AJ, Gaw A, Knox AJ, Mayer RJ, Polkey M, Salmon M, Singh D; COPDMAP . Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 136: 769–780, 2015. doi: 10.1016/j.jaci.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu P, Cao Y, Zhao R, Wang Y. miR-96-5p regulates wound healing by targeting BNIP3/FAK pathway. J Cell Biochem 120: 12904–12911, 2019. doi: 10.1002/jcb.28561. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ 16: 939–946, 2009. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Zhang D, Yan T, Jiang X, Zhang C, Zhao L, Li L, Tang D, Zhang Q, Jia J, Zhang J, Huang Y. BNIP3 promotes the motility and migration of keratinocyte under hypoxia. Exp Dermatol 26: 416–422, 2017. doi: 10.1111/exd.13248. [DOI] [PubMed] [Google Scholar]
- 59.Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci 33: 122–128, 2012. doi: 10.1016/j.tips.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W, Gunst SJ. Interactions of airway smooth muscle cells with their tissue matrix: implications for contraction. Proc Am Thorac Soc 5: 32–39, 2008. doi: 10.1513/pats.200704-048VS. [DOI] [PMC free article] [PubMed] [Google Scholar]