Abstract
NaV1.8 channels play a crucial role in regulating the action potential in nociceptive neurons. A single nucleotide polymorphism in the human NaV1.8 gene SCN10A, A1073V (rs6795970, G>A), has been linked to the diminution of mechanical pain sensation as well as cardiac conduction abnormalities. Furthermore, studies have suggested that this polymorphism may result in a “loss-of-function” phenotype. In the present study, we performed genomic analysis of A1073V polymorphism presence in a cohort of patients undergoing sigmoid colectomy who provided information regarding perioperative pain and analgesic use. Homozygous carriers reported significantly reduced severity in postoperative abdominal pain compared with heterozygous and wild-type carriers. Homozygotes also trended toward using less analgesic/opiates during the postoperative period. We also heterologously expressed the wild-type and A1073V variant in rat superior cervical ganglion neurons. Electrophysiological testing demonstrated that the mutant NaV1.8 channels activated at more depolarized potentials compared with wild-type channels. Our study revealed that postoperative abdominal pain is diminished in homozygous carriers of A1073V and that this is likely due to reduced transmission of action potentials in nociceptive neurons. Our findings reinforce the importance of NaV1.8 and the A1073V polymorphism to pain perception. This information could be used to develop new predictive tools to optimize patient pain experience and analgesic use in the perioperative setting.
NEW & NOTEWORTHY We present evidence that in a cohort of patients undergoing sigmoid colectomy, those homozygous for the NaV1.8 polymorphism (rs6795970) reported significantly lower abdominal pain scores than individuals with the homozygous wild-type or heterozygous genotype. In vitro electrophysiological recordings also suggest that the mutant NaV1.8 channel activates at more depolarizing potentials than the wild-type Na+ channel, characteristic of hypoactivity. This is the first report linking the rs6795970 mutation with postoperative abdominal pain in humans.
Keywords: postoperative pain management, SCN10a polymorphism (rs6795970), whole cell patch clamp for NaV currents
INTRODUCTION
Pain management is a critical component of perioperative care. Provision of appropriate perioperative analgesia has been shown to improve not only patient satisfaction with pain experience (Mann et al. 2000; Pöpping et al. 2008) but also a variety of other patient outcomes, including time to nutritional intake and ambulation (Carli et al. 2002; Major et al. 1996), while reducing healthcare resource utilization (e.g., reducing length of hospital stay) (Major et al. 1996) and a variety of cardiac and pulmonary complications (Kirchhoff et al. 2010; Pöpping et al. 2008). Surgery within the abdominal cavity, including that associated with colorectal procedures, is frequently associated with significant abdominal pain. A variety of analgesic approaches have been developed to specifically manage postoperative abdominal pain in this setting, including a panoply of medications, nonpharmacological therapies, and routes of analgesic administration (Ahmed et al. 2013; Garimella and Cellini 2013). Despite these advances, patient pain experience in the perioperative period following colorectal surgery varies tremendously, even when patient demographics, surgical expertise, surgery type, location, and analgesic modality are considered (Ahmed et al. 2013; Pöpping et al. 2008; Salicath et al. 2018). Thus optimizing pain control frequently remains a challenge in this setting. The reasons for this are multifactorial. One potential explanation relates to the genetic background of each individual patient.
Several studies have demonstrated that particular genetic variants can impart significant risk for increased or decreased perception of pain to human beings. This is especially evident when one considers genes that code for voltage-gated sodium channels (VGSCs). Specifically, NaV1.7, NaV1.8, and NaV1.9 are VGSC subtypes that are preferentially expressed on nociceptive neurons, including those projecting to the gut (Akopian et al. 1996; Beyak et al. 2004; Black et al. 1996; Blum et al. 2002; Gold et al. 2002; Sangameswaran et al. 1997; Zimmermann et al. 2007). Both NaV1.8 and NaV1.9 subtypes are also resistant to tetrodotoxin (TTX), isolated from puffer fish (Akopian et al. 1996; Black et al. 1996). Several polymorphisms, found in the genes encoding NaV1.7 (SCN9A), NaV1.8 (SCN10A), and NaV1.9 (SCN11A), have been associated with significant alterations in pain perception. Some result in “loss-of-function” phenotypes (Bennett and Woods 2014; Duan et al. 2016; Leipold et al. 2013; Nilsen et al. 2009), whereas others have been associated with “gain-of-function” changes (Bennett and Woods 2014; Drenth and Waxman 2007; Huang et al. 2013; Reimann et al. 2010).
The NaV1.8, subtype, expressed primarily in small-diameter dorsal root ganglion (DRG) sensory neurons, is most closely associated with pain perception (Djouhri et al. 2003; Renganathan et al. 2001). This channel subtype appears to be critical for transmission of pain from the viscera. In support of this idea, studies assessing nociceptive response in animal models utilizing chemically induced colitis have demonstrated successful attenuation of visceral pain using either therapeutics (Jarvis et al. 2007) or genetic knockouts that target NaV1.8 (Laird et al. 2002). Of note, one NaV1.8 variant, rs6795970 [a single nucleotide polymorphism (SNP), (c.3218G>A), that results in substitution of alanine to valine at position 1073 (A1073V)], has been associated with diminished mechanical pain sensitivity in humans (Duan et al. 2016). Relatedly, in a cohort of patients with inflammatory bowel disease (IBD), we previously reported that homozygosity for this NaV1.8 polymorphism was significantly more common in patients with hypoalgesic IBD (Gonzalez-Lopez et al. 2018). Electrophysiological characterization of A1073V NaV1.8 heterologously expressed in Neuro-2A cells (Jabbari et al. 2015), ND7/23 cells (Behr et al. 2015), and mouse DRG neurons (Duan et al. 2016) indicated that the variant exhibited different biophysical characteristics, including a hyperpolarizing shift in channel activation. The report by Duan et al. (2016) also indicated that the overall effect of the variant led to a reduction in repetitive firing of DRG neurons due to enhanced channel inactivation. The other studies found that the current density and sustained Na+ currents from A1073V-expressing cells were significantly lower than those of the wild-type channels (Behr et al. 2015; Jabbari et al. 2015). However, little is still known about the impact of this or other NaV1.8 polymorphisms on human visceral or abdominal pain perception, including in the perioperative setting.
The goal of the present study was to determine whether the presence of the NaV1.8 polymorphism A1073V influenced postoperative analgesic requirements in patients undergoing colorectal surgery. Based on our previous observations, we hypothesized that patients homozygous for this variant and undergoing colorectal surgery would exhibit reduced analgesic use in the postoperative period compared with patients carrying one or no copies of this variant (Gonzalez-Lopez et al. 2018). Thus we performed a retrospective analysis of patients who had undergone sigmoid colectomies and genotyped for the A1073V polymorphism. Our primary aim was to evaluate the differences between homozygous carriers of this mutation and other genotypic cohorts in 1) patient reports of abdominal pain severity and 2) analgesic use within a standardized postoperative period. The secondary aim was to assess additional clinical and demographic factors that would influence pain and analgesic use in this setting. Finally, we compared the biophysical properties of the wild-type and variant NaV1.8 heterologously expressed in rat superior cervical ganglion (SCG) neurons.
MATERIALS AND METHODS
Clinical Study Design and Participant Selection
A retrospective analysis was performed using data and blood samples derived from the Intestinal Diseases Natural History Database and Biorepository at Pennsylvania State University Hershey Medical Center between January 1, 2008 and August 31, 2018. This study was performed in accordance with the rules and regulations of the Pennsylvania State University College of Medicine Institutional Review Board (IRB). All study participants consented to enroll in this Biorepository and Database (IRB No. PRAMSHY-98-057).
Inclusion criteria involved the following: 1) adults (>17 yr of age) that 2) underwent an open or laparoscopic sigmoid colectomy, 3) received standard general anesthesia during the operation without intra- or postoperative spinal or epidural anesthesia, and 4) received self-administered (via patient-controlled analgesia devices) parenteral opiate analgesics (i.e., morphine or hydromorphone or fentanyl) as their primary method of abdominal pain control in the postoperative period. Individuals were excluded from the analysis if they underwent any major procedures other than a sigmoid colectomy during the surgery, experienced a significant complication (including acute coronary syndrome, cerebrovascular accident, venous thromboembolism, persistent arrhythmia, aspiration pneumonitis, pneumonia, other infection, anastomotic/intestinal leak or perforation, or major bleeding event resulting in the unexpected drop of >2 units of hemoglobin) during the procedure or in the immediate (24 h) postoperative period. Figure 1 illustrates how study participants were selected, using both the inclusion and exclusion criteria described above.
Fig. 1.
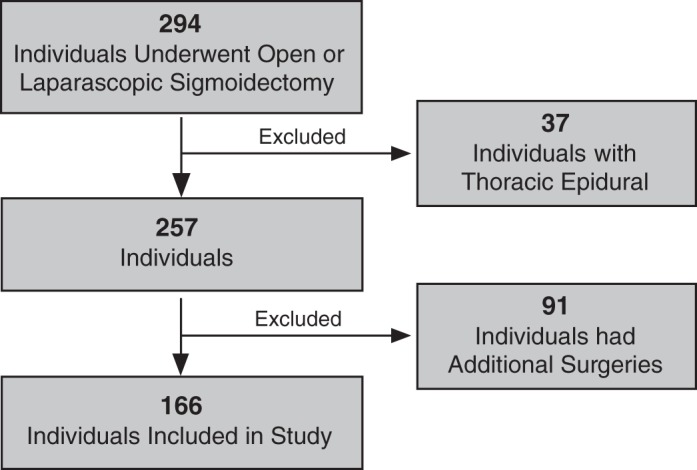
Patient selection algorithm.
The primary outcome of the clinical portion of this study was the highest numeric abdominal pain score (on a Likert-type scale of 0–10, where 0 indicates no pain, and 10, which indicates worst pain ever) self-reported by each patient during the 24-h period immediately following sigmoid colectomy. Patients were asked at least once approximately every 8 h, during the 24-h period immediately following surgery, to rate their maximum abdominal pain. The exact timing of these questions could vary among patients in a given day (due to differences in the completion time of each surgery) by 1–4 h. The secondary outcome was the total postoperative opioid requirement (expressed as mg of opiate equivalents) of each patient during the same period. Morphine equivalents were determined using standard methodology and calculations described by the Centers for Disease Control (https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf) and Centers for Medicine and Medicaid Services (https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf).
Genetic Testing
Patient blood samples were collected in EDTA-containing hematological tubes (BD, Franklin Lakes, NJ) and processed for genomic DNA extraction employing the NucleoSpin blood kit (Macherey-Nagel, Bethlehem, PA). The genomic DNA samples were then prepared for quantitative reverse transcriptase polymerase chain reaction (QRT-PCR) utilizing the Applied Biosystems TaqMan assay reagents (Thermo Fisher Scientific, Grand Island, NY). A probe designed to identify the polymorphism (rs6795970; catalog no. C2926105410, Applied Biosystems) was used for the reaction. The probe was derived from within the amplicon sequence that contained the underlined SNP, AGCTGACATACCTACCTCAGCAGGG[A/G]CCTGAGGAACAGACTCATCTTTCCA. The plate was run on a QuantStudio 12K Flex real-time quantitative PCR system (Applied Biosystems) and analyzed with the QuantStudio 12K Flex software.
SCG Neuron Isolation and cDNA Nuclear Microinjection
The Penn State College of Medicine Institutional Animal Care and Use Committee approved the animal studies. Male Sprague-Dawley rats (5−7 wk) were initially anaesthetized with CO2 and then rapidly decapitated with a laboratory guillotine. SCG neurons were isolated as described previously (Margas et al. 2007). The SCG tissue was quickly cleared of connective tissue in ice-cold Hanks’ balanced salt solution. Thereafter, the SCG tissue was enzymatically dissociated in Earle’s balanced salt solution containing 0.6 mg/mL collagenase D (Roche Applied Science, Mannheim, Germany), 0.4 mg/mL trypsin (Worthington Biochemical, Lakewood, NJ), and 0.1 mg/mL DNase (Sigma-Aldrich, St. Louis, MO) in a shaking water bath at 35°C for 60 min. Thereafter, SCG neurons were dispersed by vigorous shaking, centrifuged twice for 6 min at 44 g, and resuspended in minimum essential medium (MEM; Thermo Fisher Scientific) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% glutamine (Thermo Fisher Scientific). Trophic factors were not added to the culture media to avoid process formation. Finally, the SCG neurons were plated onto 35-mm poly-l-lysine-coated dishes and stored in a humidified incubator supplied with 5% CO2 at 37°C.
The human wild-type NaV1.8 insert (NCBI No. NM_006514.3) was cloned in the pcDNA3.1 vector (Genscript, Piscataway, NJ) via the Xho1 and EcoR1 sites. The mutant A1073V NaV1.8 plasmid was generated via site-directed mutagenesis (Agilent Technologies, Santa Clara, CA). Both constructs were sequence verified before injection. Three to six hours postdissociation, the SCG neurons were injected with the cDNA plasmid encoding either human wild-type NaV1.8 or A1073V NaV1.8 at a final concentration of 0.3−0.5 μg/μL with an microinjector 5246 and micromanipulator 5171 (both from Eppendorf, Hauppague, NY). In addition, all neurons were coinjected with the enhanced green fluorescent protein (pEGFP) at a final concentration of 0.005 μg/μL to facilitate later identification of neurons receiving successful nuclear injection. The pEGFP-expressing neurons were visualized with a filter set containing an excitation filter at 480 nm, a dichroic beam splitter of 505 nm, and an emission filter at 535 nm. All electrophysiological recordings were performed 16–24 h post-cDNA nuclear injection.
Electrophysiology
The whole cell patch-clamp technique was used to record Na+ currents with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) from acutely isolated SCG neurons. The recordings were carried out at room temperature. The recording pipet electrodes, made from borosilicate glass capillaries (no. 8250; King Precision Glass, Claremont, CA), were also coated with Sylgard (Dow Corning, Midland, MI). Custom-designed software (F6), developed by Stephen R. Ikeda (National Institute on Alcohol Abuse and Alcoholism), was used for voltage protocol generation and data acquisition on a Mac Mini (Apple Computer, Cupertino, CA) equipped with an ITC-18 data acquisition interface (HEKA Instruments, Holliston, MA). Current traces were filtered at 2 kHz (−3 dB) using a four-pole low-pass Bessel filter and digitized at 10 kHz. Furthermore, the cell membrane capacitance and series resistance (80–85%) were electronically compensated, and the lag was set to 7 μs. The liquid junction potentials were not corrected.
For recording Na+ currents, the external solution consisted of (in mM) 70 NaCl, 80 tetraethylammonium (TEA)-Cl, 5 MnCl2, 10 HEPES, 10 glucose, and 0.0003 TTX, pH 7.4. The pipette solution contained (in mM) 120 N-methyl-d-glucamine, 20 TEA-OH, 10 sucrose, 11 EGTA, 10 HEPES, 1 CaCl2, 4 Mg-ATP, 0.3 Na2GTP, and 14 Tris-creatine phosphate, pH 7.2 with methanesulfonic acid. The current density (pA/pF) was determined by dividing the peak Na+ current amplitude by the membrane capacitance. The cell membrane capacitance was obtained from the uncompensated capacitative currents that were elicited by a 5-ms step pulse from −80 to −70 mV. The cell membrane capacitance was obtained employing the equation Cm = Q/V, where Cm is the capacitance, Q is the charge stored in the capacitor/cell membrane, and V is the voltage-step amplitude. The current traces were corrected for linear leakage current, which was determined from hyperpolarizing command pulses.
Statistical Analysis
Univariate analysis.
Comparisons among continuous variables were made using the Student t test for two variables or one-way ANOVA (with Bonferroni posttest) for more than two variables. Categorical variables were compared using chi-square or Fisher’s exact testing. In most cases, we chose to evaluate the homozygous carrier cohort to a combined heterozygous and wild-type carrier cohort due to previous findings demonstrating that it is only the homozygous genotype that is associated with phenotypic changes in human pain perception (Duan et al. 2016; Gonzalez-Lopez et al. 2018). All values are means ± SE or percentages with 95% confidence intervals unless described otherwise. P values <0.05 were considered significant.
CART analysis.
We employed classification and regression tree (CART) analysis to estimate the likelihood of severe postoperative pain among individuals undergoing colectomy under general anesthesia and enrolled in the study. Several candidate predictors (i.e., sex, pre- and perioperative opioid use, smoking status, etc.) were used to build the classification trees using XLSTAT (Addinsoft, Long Island, NY). After the final tree was built, variables were deleted (“pruned”) on the importance score and the sensitivity into two groups: severe postoperative pain group or no severe postoperative pain group. The Hosmer-Lemeshow goodness-of-fit test confirmed the suitability of the trees.
Electrophysiological data analysis.
Na+ currents were analyzed with the IgorPro software (WaveMetrics, Oswego, OR). Na+ channel activation curves were determined by converting the peak Na+ currents (INa) to conductance employing the chord conductance equation:
where GNa is the peak membrane conductance at potential EM and ENa is the Na+ reversal potential. Thereafter, the data was fit to a modified Boltzmann function employing nonlinear regression analysis with GraphPad Prism software (GraphPad, San Diego, CA):
where GNa/Gmax is the fractional peak membrane conductance, VH is the half-activation potential, VM is the membrane potential, and k is a slope factor. The inactivation curves were obtained by employing a steady-state inactivation protocol (see Fig. 6), consisting of a 1-s conditioning prepulse over the potential range (−80 to 0 mV) followed by a constant test pulse to the peak test potential. The curves were also fitted to a modified Boltzmann equation using nonlinear regression analysis (see above).
Fig. 6.
Inactivation of NaV1.8 currents in rat superior cervical ganglion (SCG) neurons. A: family of wild-type NaV1.8 current traces recorded from an SCG neuron using the inactivation protocol (inset). B: NaV1.8 A1073 current traces acquired using the voltage protocol in A. C: superimposed inactivation curves for both groups of neurons. Solid lines represent the best fit of a modified Boltzmann equation. Data were normalized to the fitted maximum current. D: summary dot plot of the mean (±SE) current density of SCG neurons expressing wild type (n = 21) or NaV1.8 A1073V (n = 15). VH, half-inactivation potential.
RESULTS
Patient Cohort Characteristics and Postoperative Opioid Use
In the present retrospective study, we examined whether there was an association between pain severity following sigmoid colectomy in homozygous carriers of the SCN10A polymorphism (rs6795970, A1073V) and heterozygous or homozygous noncarriers. We identified an initial cohort of 294 patients who had undergone sigmoid colectomy under general anesthesia. Thirty-seven patients who received a thoracic epidural were subsequently removed from the study cohort. An additional 91 patients were excluded from the study because they underwent additional significant surgical procedures at the same time as the sigmoid resection. Consequently, a total of 166 patients were included in this study (Fig. 1).
Table 1 lists the various demographic and clinical characteristics of the total study cohort, including the subcohorts that were either homozygous carriers for rs6795970 or demonstrated “other” genotypes (e.g., heterozygous for rs6795970 or wild type). A statistical comparison for these characteristics indicated there was not a significant difference between subcohorts when age, sex, race, surgery type, and preoperative incidence of tobacco, alcohol, opiates, nonsteroidal anti-inflammatory drugs (NSAIDs), and other pain medications are compared (Table 1). Of note, the mean allelic frequency (MAF) of rs6795970 in our study population was 0.404. This is comparable to the upper range of MAFs reported in other genomic sequence databases [e.g., 0.17 (Vietnamese database) to 0.404 (Avon Longitudinal Study of Parents and Children) previously reported (dbSNP; https://www.ncbi.nlm.nih.gov/snp/rs6795970).
Table 1.
Study participant clinical and demographic characteristics
| Total Cohort | Rs6795970 Homozygote | Heterozygote and Wild Type | P Value | |
|---|---|---|---|---|
| n | 166 | 27 | 139 | |
| Age, yr | 61.5 ± 1.0 | 61 ± 2.6 | 61.6 ± 1.1 | 0.89 |
| Sex, female/male | 104/62 | 17/10 | 87/52 | 0.99 |
| Body weight, kg | 83 ± 1.7 | 88 ± 4.2 | 83 ± 1.9 | 0.25 |
| Body mass index, kg/m2 | 29.4 ± 0.5 | 29.4 ± 1.3 | 29.5 ± 0.6 | 0.81 |
| Race/Ethnicity | 164 White, 2 Hispanic |
27 White | 137 White, 2 Hispanic |
0.99 |
| Active tobacco use, yes/no | 32/134 | 4/23 | 28/111 | 0.61 |
| Active alcohol use, yes/no | 92/74 | 17/10 | 75/64 | 0.41 |
| Opiate before surgery, yes/no | 10/156 | 2/25 | 8/131 | 0.67 |
| NSAID before surgery, yes/no | 60/106 | 12/15 | 48/91 | 0.38 |
| Other pain medication use before surgery, yes/no | 63/103 | 10/17 | 53/86 | 0.99 |
| Surgery type, laparoscopic/open | 116/50 | 19/8 | 97/42 | 0.99 |
| Intraoperative opiate use (morphine equivalent), mg | 26.8 ± 0.9 | 28.1 ± 2.5 | 26.6 ± 1.0 | 0.48 |
| Intraoperative opiate use normalized to body weight, mg/kg | 0.34 ± 0.01 | 0.33 ± 0.03 | 0.34 ± 0.01 | 0.85 |
| Postoperative (24 h) opiate use normalized to body weight, mg/kg | 0.83 ± 0.05 | 0.65 ± 0.09 | 0.86 ± 0.06 | 0.12 |
Values are means ± SE or number of patients with the indicated characteristic.
Pain Scores and Opiate Use
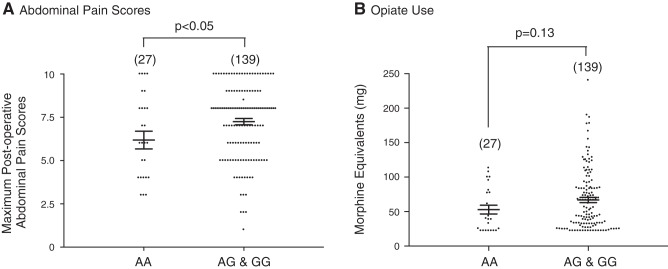
During the 24-h period following surgery, the study participants were asked to score their abdominal pain severity using a Likert-based scale (0–10). Figure 2A is a summary plot depicting the mean abdominal pain scores for the homozygote (AA) and heterozygote/wild-type (AG+GG) cohorts. Homozygous (AA) carriers demonstrated a significantly (P < 0.05) lower mean maximum abdominal pain score during the pos-operative period compared with the combined heterozygote/wild-type cohort.
Fig. 2.
Postoperative patient abdominal pain scores and opiate use. A: summary bar graph indicating mean (±SE) maximum postoperative abdominal pain scores reported by patients (0–10 scale; 0 = none and 10 = most severe), comparing homozygous carriers of the NaV1.8 A1073V gene (AA) and heterozygous and homozygous wild-type (AG & GG). B: summary bar graph comparing mean opiate use (morphine equivalent, in mg) 24 h postoperatively in homozygous mutant carriers (GG) with that in both homozygous wild-type and heterozygous carriers (AG & GG). Numbers in parenthesis indicate the number of patients tested.
The mean (±SE) dose (mg of morphine equivalents) used by all patients within the 24-h period following surgery was 67.1 ± 50.1 mg, whereas the median dose was 48.5 mg (lower and upper quartiles were 28.0 and 85.8 mg, respectively). The 95% confidence interval for the observed doses (14.7−331.7 mg) ranged from 22.2 to 159.4 mg. Comparison of the mean opiate dose administered to patients within the 24-h period postsurgery demonstrated that the combined AG+GG cohort trended toward requiring more opiate medication than AA carriers (Fig. 2B). However, this difference did not reach statistical significance (P = 0.13). Of note, maximum reported abdominal pain scores and total morphine equivalent doses demonstrated a statistically significant (P < 0.0001) but weak (r = 0.38) correlation with one another.
We also examined the frequency of patient genotype cohort within the lowest and highest quartiles of opiate use. Although no statistically significant differences were found, the box plot in Fig. 3 suggests that homozygous carriers of the NaV1.8 variant (AA) had a higher propensity to fall within the lowest quartile of opiate use (37.0% vs. 21.9%, P = 0.09). On the other hand, wild-type and heterozygous carriers exhibited an increased likelihood of falling within the highest quartile (22.2% vs. 39.0%, P = 0.14).
Fig. 3.
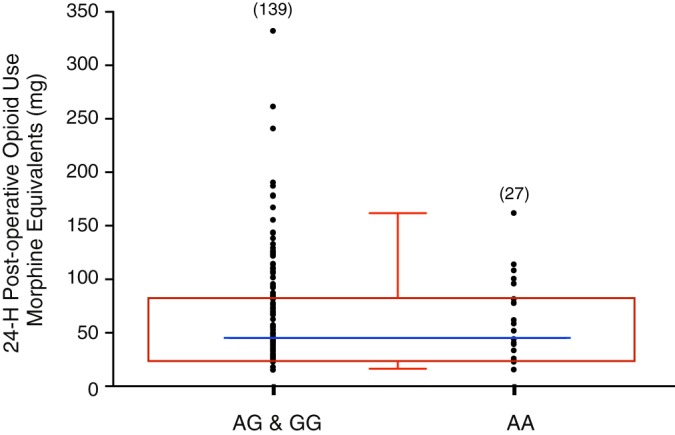
Dot plot of individual opiate doses (morphine equivalent, in mg) 24 h postsurgery for heterozygous and homozygous wild-type (combined, AG & GG) and homozygous NaV1.8 A1073V carrier (AA) genotype groups. Box and whisker plot (red) depicts the median (blue line) with the 5th−95th percentiles (red) for the three genotypes.
Independent Associations with Postoperative Opioid Use
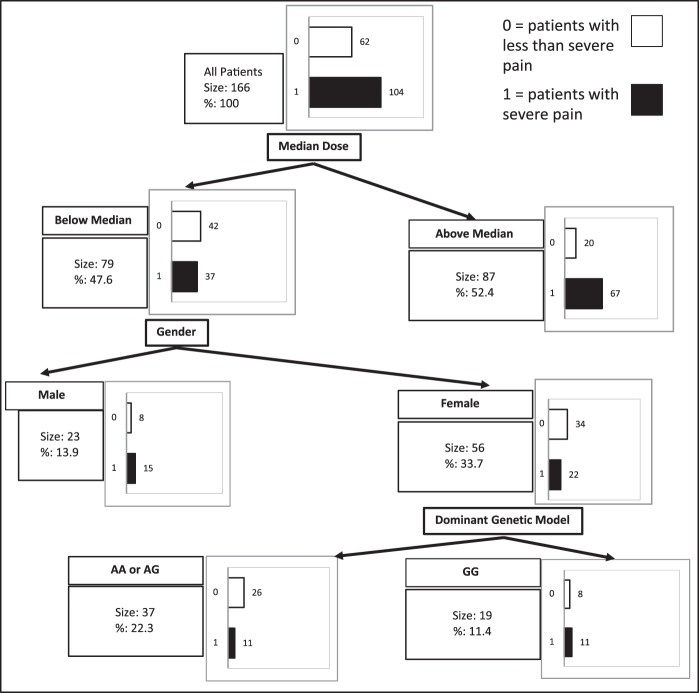
We also employed CART analysis to search for independent predictors of severe postoperative abdominal pain (e.g., abdominal pain scores of 8−10 only). Unlike multivariable logistic regression models, CART analysis allowed us to obtain a final tree that contained complex interactions among the variables. It employs recursive partitioning to obtain a series of dichotomous splits (i.e., presence or absence of variables under study) to create a decision tree and correctly classify members of the group under study. With this approach, complex interactions among variables in the final tree can be identified (Fig. 4). The first split occurred at the level of frequency of patients with severe pain who used less or more than the median opioid dose postoperatively (P < 0.05). That is, severe pain occurred more frequently in patients that required more than the median dose and less frequently in patients that required less opioid amounts below the median dose. The next split occurred in patients requiring less than the median dose of opioid and was sex based (e.g., the frequency of severe abdominal pain was higher in males when compared with females, P < 0.05). The final split occurred at the level of females who used less than the median postoperative opioid dose. The female AA or AG carriers reported severe pain less frequently than GG carriers. No other analyzed variables had significant effect on the frequency of the patients reporting at least one severe pain episode during the 24-h period immediately following surgery.
Fig. 4.
Classification and regression tree (CART) analysis of factors involved in the probability of severe postoperative pain (pain scores of 8–10). Open and closed bars indicate the frequency of patients without severe postoperative pain and with severe postoperative pain, respectively (highest pain score noted within the 24-h postoperative period). AA, homozygous NaV1.8 A1073V genotype; AG, heterozygous genotype; GG, homozygous wild-type genotype.
NaV1.8 Currents in SCG Neurons
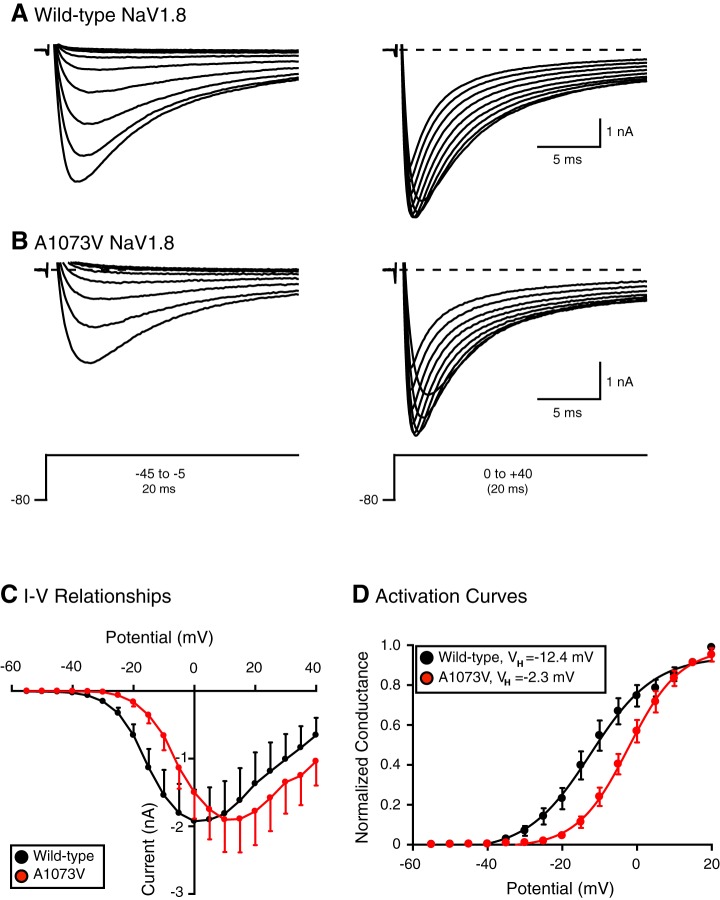
In the next set of experiments, we recorded NaV1.8 currents in the presence of TTX (to block native TTX-sensitive channels) in adult rat SCG neurons, which do not express NaV1.8 channels and provide a suitable null background (Cummins et al. 2009; Schofield et al. 2008). Figure 5A shows superimposed inward currents elicited from a holding potential (VH) of −80 with test potentials from −45 to +40 mV. The currents began to activate at approximately −35 mV and reached a peak at ~0 to +5 mV. The superimposed NaV1.8 current traces shown in Fig. 5B were obtained from an SCG neuron expressing NaV1.8 A1073V channels. The Na+ currents began to activate at approximately −25 mV and reached the peak current near +10 to +15 mV. The averaged current-voltage (I-V) relationships for both group of neurons are shown in Fig. 5C. The I-V relationship for the mutant channels was shifted ~10 mV toward more depolarized potentials.
Fig. 5.
TTX-resistant Na+ currents carried by heterologously expressed wild-type and NaV1.8 A1073V channels in rat superior cervical ganglion (SCG) neurons. A and B: representative family of Na+ currents evoked with the voltage protocol shown in an SCG neuron expressing wild-type (A) and A1073V NaV1.8 channels (B). Recordings were elicited in the presence of 0.0003 mM TTX to block endogenous NaV channels. C: mean current-voltage (I-V) relationships for peak TTX-resistant Na+ currents in wild-type (n = 20)- and A1073V-expressing neurons (n = 15). Data are means ± SE of SCG neurons tested. D: voltage-dependent activation curves of wild-type (black circles, n = 20) and A1073V NaV1.8 (red circles, n = 9). Solid lines represent the nonlinear regression fits of a modified Boltzmann equation to the data (mean ± SE) obtained at each step potential.
Figure 5D is a comparison of the activation curves of wild-type and NaV1.8 variant heterologously expressed in SCG neurons. After the data were fit to a modified Boltzmann equation, the best fits for both groups of neurons were significantly different (P < 0.0001). The wild-type NaV1.8 channels began to activate near −35 mV, reached half activation (VH) at −12.4 ± 1.4 mV, and had a slope (k) of 8.5 mV ± 1.5 (n = 20). On the other hand, the NaV1.8 A1073V current began to activate near −25 mV, but VH shifted to −2.3 ± 0.9 mV toward depolarizing potentials and was significantly different (P < 0.05) from that for the wild-type NaV1.8 channels. The slope value was 6.9 ± 0.9 mV (n = 9).
We next determined the steady-state inactivation parameters of Na+ currents with the protocol shown in Fig. 6A (inset). The voltage protocol consisted of a 0.5-s conditioning pulse to progressively more depolarized potentials followed by a constant test pulse (10 ms) to the peak potential. The representative traces for wild-type- and NaV1.8 A1073V-expressing neurons are illustrated in Fig. 6, A and B, respectively. Figure 6C depicts the normalized inactivation curves for both groups of SCG neurons. The solid lines represent the nonlinear least-square fits of the averaged data to a modified Boltzmann equation. In the wild-type-expressing group of neurons, the mean (±SE) half-inactivation (VH) and slope (k) were −41.3 ± 1.1 and −11.7 ± 1.2 mV (n = 9), respectively. Furthermore, VH and k values for the mutant-expressing neurons were −38.0 ± 1.0 and −12.1 ± 1.1 mV (n = 9), respectively. The data indicate that inactivation kinetics of NaV1.8 A1073V-expressing neurons were not overtly affected by the polymorphism. In addition, we tested whether channel expression differed between both groups of neurons. Figure 6D is a summary plot of the Na+ current density (normalized to cell capacitance) for both wild-type- and A1073V-expressing SCG neurons. A statistical comparison of both values indicated no significant difference (P = 0.76) in current density. Furthermore, the mean cell membrane capacitance values for all wild-type- and A1073V NaV1.8-expressing neurons were 45.2 ± 4.4 (n = 21) and 52.8 ± 3.9 (n = 15), respectively (P = 0.23, unpaired t test).
DISCUSSION
The first part of this study demonstrated that homozygous carriers of the functionally relevant NaV1.8 polymorphism, rs6795970 (G>A, A1073V), describe reduced severity in postoperative abdominal pain compared with heterozygous or wild-type carriers of this gene after undergoing similar intra-abdominal surgeries. Homozygous carriers also exhibited a trend trends toward using less analgesic medication (e.g., opiates) in the postoperative period (although this finding did not reach statistical significance). No other clinical or demographic characteristics were significantly associated with abdominal pain scores or opiate use, although CART analysis demonstrated that female homozygous carriers of the wild-type gene were more likely to demonstrate severe abdominal pain. Although polymorphisms for at least two other voltage-gated Na+ channels (e.g., NaV1.7 and NaV1.9) have been linked to alterations in perioperative pain experience (Duan et al. 2013; Sun et al. 2017), to our knowledge this is the first report of a NaV1.8 genetic variant being implicated in this regard. Our results support the findings of prior investigations demonstrating that the A1073V variant has a significant impact on NaV1.8 function and human pain experience in general (Duan et al. 2016; Gonzalez-Lopez et al. 2018). They also support previous work demonstrating that the homozygous genotype is required to manifest differences in individual pain experience (Duan et al. 2016; Gonzalez-Lopez et al. 2018) and that abdominal pain perception can be specifically altered in this context.
The second part of our study demonstrated that SCG neurons transfected with the A1073V variant exhibited a shift in the I-V curve of ~10 mV toward more depolarizing potentials. Additionally, the activation of the mutant channels was also shifted by 10 mV compared with that of the wild-type-expressing neurons. These differences were not a result of differences in channel expression levels, because the Na+ current density was not significantly different between both groups of neurons. Unlike our results, activation kinetics of the variant Na+ channel have been reported to be shifted to more hyperpolarizing potentials (~4 to 12 mV) in DRG neurons transfected with NaV1.8 A1073V from NaV1.8 knockout mice (Duan et al. 2016), Neuro 2A cells (Jabbari et al. 2015), and ND7/23 cells (Behr et al. 2015). A shift to more hyperpolarizing potentials, however, can lead to hyperexcitability and is difficult to reconcile with a loss-of-function phenotype. It should be noted that the study in mouse DRG neurons also showed that the mutant NaV1.8 channels exhibited significantly faster open-state inactivation (i.e., indicative of channels transitioning from open to inactivated states) than wild-type channels (Duan et al. 2016). This change in inactivation is thought to counteract the mutant channel’s leftward shift in activation. Furthermore, the authors reported that the mutant Na+ channel-expressing neurons produced a lower number of action potentials during application of depolarizing stimuli compared with wild-type-expressing neurons.
Similarly to the present study, a previous report also heterologously expressed wild-type mouse NaV1.8 in rat SCG neurons and compared these currents with those of native mouse DRG neurons (Schofield et al. 2008). The authors also found a 10-mV depolarizing shift of the activation parameters of the heterologously expressed NaV1.8 channel compared with native Na+ currents in DRG neurons. The VH for both activation and inactivation of the present study and that by Schofield et al. (2008) are comparable but not identical. Additionally, the slope factor (k) for both parameters obtained in the present study exhibited a shallower voltage dependence.
Comparison of the inactivation properties of both wild-type and mutant NaV1.8 channels revealed a slight shift toward depolarizing potentials of the latter (~3 mV). Similar results were obtained by others (Duan et al. 2016; Jabbari et al. 2015), whereas Behr et al. (2015) observed a >20-mV hyperpolarizing shift. It is possible that SCG neurons do not express proteins that confer NaV1.8 biophysical properties native to sensory neurons. SCG neurons do not natively express NaV1.8 channels, which makes them a good expression model (Cummins et al. 2009; Schofield et al. 2008). However, their physiological cell background is different from that of sensory neurons. Additionally, differences in recording conditions can account for the observed results. We recorded Na+ currents within 18–24 h posttransfection, whereas the aforementioned studies obtained their recordings 40−48 h posttransfection. We also omitted trophic factors from the cell culture media to avoid upregulation of endogenous Na+ channels and to obviate induction of neurite formation, which can affect the voltage clamp, as recommended by Cummins et al. (2009). Finally, we should point out that our external recording solution, like that of others (Duan et al. 2016; Schofield et al. 2008), contained lower Na+ concentrations (i.e., 70–100 mM) than in previous studies (Behr et al. 2015; Jabbari et al. 2015) that employed amounts in the physiological range.
Taken together, these findings reinforce the concept that the NaV1.8 variant, A1073V, is functionally relevant to human pain perception. Although we did not specifically evaluate patient visceral pain experience alone in this study, our findings provide evidence that abdominal pain perception is significantly impacted by this polymorphism, providing at least circumstantial evidence that human intestinal (visceral) nociception might be influenced, as well. We also provide confirmatory electrophysiological evidence demonstrating how this variant may impart a hypoalgesic effect in humans. Given the substantial shift in voltage-dependent activation, it would be less likely for nociceptive neurons to relay a signal after experiencing a noxious stimulus that would otherwise result in pain perception (from the gut or otherwise).
There are several potential limitations to this work. First, we collected clinical data in a retrospective fashion, and although we attempted to control for several clinical and demographic factors that have a potential influence on patient pain experience, there could be other relevant variables that we did not account for. For example, CART analysis suggested that female wild-type carriers of the NaV1.8 gene are more prone to exhibiting severe abdominal pain after colectomy. Thus it would be reasonable to study larger groups of both male and female patients to further evaluate for sex-based differences in pain experience in this setting. Second, although patients were asked standardized questions about their pain experience, this approach could only provide a subjective evaluation of pain, and we did not perform an objective assessment of somatosensory or visceral nociception. Third, we did not genotype patients for other Na+ channel polymorphisms (or variants of other pain-modifying gene targets) that could have influenced pain experience. Finally, we evaluated the biophysical impact of rs6795970 in rat sympathetic neurons, which has limitations mentioned above. Moving forward, it will be important to confirm the functional consequence of this variant in native visceral nociceptive neurons, including the use of NaV1.8 knockout animals (Duan et al. 2016).
Nevertheless, our findings provide new insights into human pain physiology and VGSC function. Specifically, we believe that the results outlined above represent an important first step demonstrating the potential importance of NaV1.8 and the A1073V variant to human abdominal pain perception and perioperative symptom experience. Similarly, a polymorphism of the NaV1.9 channel (Leu811Pro), expressed primarily in nociceptive neurons, has been reported to exhibit a gain of function in electrophysiological assays (Leipold et al. 2013). However, carriers of the mutation were reported to be insensitive to pain while also being affected with “gastrointestinal disturbances.” It should be noted that loss-of-function NaV polymorphisms have been reported to be linked to chronic pain patients (Kaluza et al. 2018). That is, the in vitro experiments demonstrated that the NaV1.8 D1639N variant exhibited decreased current density compared with wild-type-expressing cells as a result of impaired channel trafficking. In considering our results and other studies demonstrating the influence of VGSC polymorphisms on human pain experience in the perioperative setting, it is conceivable that (among other potential applications) these genes could eventually be used to screen patients to stratify their relative risk for postoperative pain and analgesic use. These insights could also potentially be used to eventually develop more targeted methods of pain control. However, to optimize these approaches, it will be important to further refine our understanding regarding how NaV1.8 and other VGSC polymorphisms integrate to impact patient pain experience. Future investigations should consider use of larger patient cohorts that allow for evaluation of multiple genetic targets simultaneously in prospectively designed investigations that incorporate objective measures of both somatosensory and visceral pain end points. These efforts will be critical to finding better-targeted, less toxic therapies to help manage pain in the perioperative setting (and elsewhere).
GRANTS
This research was supported by the Peter and Marsha Carlino Early Career Professorship in Inflammatory Bowel Disease (to M. D. Coates), the Margot E. Walrath Career Development Professorship in Gastroenterology (to M. D. Coates), and the Elliot S. Vesell Professorship in Pharmacology (to K. E. Vrana).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.D.C., J.S.K., N.C.-S., K.E.V., W.A.K., H.L.P., S.D.A., P.K.J., and V.R.-V. conceived and designed research; M.D.C., J.S.K., N.C.-S., S.D.A., P.K.J., and V.R.-V. performed experiments; M.D.C., J.S.K., P.K.J., and V.R.-V. analyzed data; M.D.C., K.E.V., S.D.A., P.K.J., and V.R.-V. interpreted results of experiments; M.D.C., P.K.J., and V.R.-V. prepared figures; M.D.C., K.E.V., P.K.J., and V.R.-V. drafted manuscript; M.D.C., J.S.K., N.C.-S., K.E.V., H.L.P., P.K.J., and V.R.-V. edited and revised manuscript; M.D.C., J.S.K., N.C.-S., K.E.V., H.L.P., S.D.A., P.K.J., and V.R.-V. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Susan Deiling, Leonard Harris, and Jeff Small for assistance in identifying, retrieving, and processing tissue samples associated with this study.
REFERENCES
- Ahmed A, Latif N, Khan R. Post-operative analgesia for major abdominal surgery and its effectiveness in a tertiary care hospital. J Anaesthesiol Clin Pharmacol 29: 472–477, 2013. doi: 10.4103/0970-9185.119137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379: 257–262, 1996. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Behr ER, Savio-Galimberti E, Barc J, Holst AG, Petropoulou E, Prins BP, Jabbari J, Torchio M, Berthet M, Mizusawa Y, Yang T, Nannenberg EA, Dagradi F, Weeke P, Bastiaenan R, Ackerman MJ, Haunso S, Leenhardt A, Kääb S, Probst V, Redon R, Sharma S, Wilde A, Tfelt-Hansen J, Schwartz P, Roden DM, Bezzina CR, Olesen M, Darbar D, Guicheney P, Crotti L, Jamshidi Y; UK10K Consortium . Role of common and rare variants in SCN10A: results from the Brugada syndrome QRS locus gene discovery collaborative study. Cardiovasc Res 106: 520–529, 2015. doi: 10.1093/cvr/cvv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Woods CG. Painful and painless channelopathies. Lancet Neurol 13: 587–599, 2014. doi: 10.1016/S1474-4422(14)70024-9. [DOI] [PubMed] [Google Scholar]
- Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol 287: G845–G855, 2004. doi: 10.1152/ajpgi.00154.2004. [DOI] [PubMed] [Google Scholar]
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Brain Res Mol Brain Res 43: 117–131, 1996. doi: 10.1016/S0169-328X(96)00163-5. [DOI] [PubMed] [Google Scholar]
- Blum R, Kafitz KW, Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel NaV1.9. Nature 419: 687–693, 2002. doi: 10.1038/nature01085. [DOI] [PubMed] [Google Scholar]
- Carli F, Lattermann R, Schricker T. Epidural analgesia and postoperative lipid metabolism: stable isotope studies during a fasted/fed state. Reg Anesth Pain Med 27: 132–138, 2002. doi: 10.1097/00115550-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Rush AM, Estacion M, Dib-Hajj SD, Waxman SG. Voltage-clamp and current-clamp recordings from mammalian DRG neurons. Nat Protoc 4: 1103–1112, 2009. doi: 10.1038/nprot.2009.91. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol 550: 739–752, 2003. doi: 10.1113/jphysiol.2003.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth JP, Waxman SG. Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J Clin Invest 117: 3603–3609, 2007. doi: 10.1172/JCI33297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan G, Han C, Wang Q, Guo S, Zhang Y, Ying Y, Huang P, Zhang L, Macala L, Shah P, Zhang M, Li N, Dib-Hajj SD, Waxman SG, Zhang X. A SCN10A SNP biases human pain sensitivity. Mol Pain 12: 1744806916666083, 2016. doi: 10.1177/1744806916666083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan G, Xiang G, Zhang X, Yuan R, Zhan H, Qi D. A single-nucleotide polymorphism in SCN9A may decrease postoperative pain sensitivity in the general population. Anesthesiology 118: 436–442, 2013. doi: 10.1097/ALN.0b013e31827dde74. [DOI] [PubMed] [Google Scholar]
- Garimella V, Cellini C. Postoperative pain control. Clin Colon Rectal Surg 26: 191–196, 2013. doi: 10.1055/s-0033-1351138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Zhang L, Wrigley DL, Traub RJ. Prostaglandin E2 modulates TTX-R INa in rat colonic sensory neurons. J Neurophysiol 88: 1512–1522, 2002. doi: 10.1152/jn.2002.88.3.1512. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lopez E, Imamura Kawasawa Y, Walter V, Zhang L, Koltun WA, Huang X, Vrana KE, Coates MD. Homozygosity for the SCN10A polymorphism rs6795970 is associated with hypoalgesic inflammatory bowel disease phenotype. Front Med (Lausanne) 5: 324, 2018. doi: 10.3389/fmed.2018.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Yang Y, Zhao P, Gerrits MM, Hoeijmakers JG, Bekelaar K, Merkies IS, Faber CG, Dib-Hajj SD, Waxman SG. Small-fiber neuropathy Nav1.8 mutation shifts activation to hyperpolarized potentials and increases excitability of dorsal root ganglion neurons. J Neurosci 33: 14087–14097, 2013. doi: 10.1523/JNEUROSCI.2710-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari J, Olesen MS, Yuan L, Nielsen JB, Liang B, Macri V, Christophersen IE, Nielsen N, Sajadieh A, Ellinor PT, Grunnet M, Haunsø S, Holst AG, Svendsen JH, Jespersen T. Common and rare variants in SCN10A modulate the risk of atrial fibrillation. Circ Cardiovasc Genet 8: 64–73, 2015. doi: 10.1161/CIRCGENETICS.113.000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, Kort M, Carroll W, Marron B, Atkinson R, Thomas J, Liu D, Krambis M, Liu Y, McGaraughty S, Chu K, Roeloffs R, Zhong C, Mikusa JP, Hernandez G, Gauvin D, Wade C, Zhu C, Pai M, Scanio M, Shi L, Drizin I, Gregg R, Matulenko M, Hakeem A, Gross M, Johnson M, Marsh K, Wagoner PK, Sullivan JP, Faltynek CR, Krafte DS. A-803467, a potent and selective NaV1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA 104: 8520–8525, 2007. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza L, Meents JE, Hampl M, Rösseler C, Hautvast PA, Detro-Dassen S, Hausmann R, Schmalzing G, Lampert A. Loss-of-function of Nav1.8/D1639N linked to human pain can be rescued by lidocaine. Pflugers Arch 470: 1787–1801, 2018. doi: 10.1007/s00424-018-2189-x. [DOI] [PubMed] [Google Scholar]
- Kirchhoff P, Clavien PA, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg 4: 5, 2010. doi: 10.1186/1754-9493-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird JM, Souslova V, Wood JN, Cervero F. Deficits in visceral pain and referred hyperalgesia in Nav1.8 (SNS/PN3)-null mice. J Neurosci 22: 8352–8356, 2002. doi: 10.1523/JNEUROSCI.22-19-08352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipold E, Liebmann L, Korenke GC, Heinrich T, Giesselmann S, Baets J, Ebbinghaus M, Goral RO, Stödberg T, Hennings JC, Bergmann M, Altmüller J, Thiele H, Wetzel A, Nürnberg P, Timmerman V, De Jonghe P, Blum R, Schaible HG, Weis J, Heinemann SH, Hübner CA, Kurth I. A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat Genet 45: 1399–1404, 2013. doi: 10.1038/ng.2767. [DOI] [PubMed] [Google Scholar]
- Major CP Jr, Greer MS, Russell WL, Roe SM. Postoperative pulmonary complications and morbidity after abdominal aneurysmectomy: a comparison of postoperative epidural versus parenteral opioid analgesia. Am Surg 62: 45–51, 1996. [PubMed] [Google Scholar]
- Mann C, Pouzeratte Y, Boccara G, Peccoux C, Vergne C, Brunat G, Domergue J, Millat B, Colson P. Comparison of intravenous or epidural patient-controlled analgesia in the elderly after major abdominal surgery. Anesthesiology 92: 433–441, 2000. doi: 10.1097/00000542-200002000-00025. [DOI] [PubMed] [Google Scholar]
- Margas W, Zubkoff I, Schuler HG, Janicki PK, Ruiz-Velasco V. Modulation of Ca2+ channels by heterologously expressed wild-type and mutant human micro-opioid receptors (hMORs) containing the A118G single-nucleotide polymorphism. J Neurophysiol 97: 1058–1067, 2007. doi: 10.1152/jn.01007.2006. [DOI] [PubMed] [Google Scholar]
- Nilsen KB, Nicholas AK, Woods CG, Mellgren SI, Nebuchennykh M, Aasly J. Two novel SCN9A mutations causing insensitivity to pain. Pain 143: 155–158, 2009. doi: 10.1016/j.pain.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Pöpping DM, Zahn PK, Van Aken HK, Dasch B, Boche R, Pogatzki-Zahn EM. Effectiveness and safety of postoperative pain management: a survey of 18 925 consecutive patients between 1998 and 2006 (2nd revision): a database analysis of prospectively raised data. Br J Anaesth 101: 832–840, 2008. doi: 10.1093/bja/aen300. [DOI] [PubMed] [Google Scholar]
- Reimann F, Cox JJ, Belfer I, Diatchenko L, Zaykin DV, McHale DP, Drenth JP, Dai F, Wheeler J, Sanders F, Wood L, Wu TX, Karppinen J, Nikolajsen L, Männikkö M, Max MB, Kiselycznyk C, Poddar M, Te Morsche RH, Smith S, Gibson D, Kelempisioti A, Maixner W, Gribble FM, Woods CG. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc Natl Acad Sci USA 107: 5148–5153, 2010. doi: 10.1073/pnas.0913181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Waxman SG. Contribution of Nav1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol 86: 629–640, 2001. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- Salicath JH, Yeoh EC, Bennett MH. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. Cochrane Database Syst Rev 8: CD010434, 2018. doi: 10.1002/14651858.CD010434.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangameswaran L, Fish LM, Koch BD, Rabert DK, Delgado SG, Ilnicka M, Jakeman LB, Novakovic S, Wong K, Sze P, Tzoumaka E, Stewart GR, Herman RC, Chan H, Eglen RM, Hunter JC. A novel tetrodotoxin-sensitive, voltage-gated sodium channel expressed in rat and human dorsal root ganglia. J Biol Chem 272: 14805–14809, 1997. doi: 10.1074/jbc.272.23.14805. [DOI] [PubMed] [Google Scholar]
- Schofield GG, Puhl HL 3rd, Ikeda SR. Properties of wild-type and fluorescent protein-tagged mouse tetrodotoxin-resistant sodium channel (NaV1.8) heterologously expressed in rat sympathetic neurons. J Neurophysiol 99: 1917–1927, 2008. doi: 10.1152/jn.01170.2007. [DOI] [PubMed] [Google Scholar]
- Sun J, Duan G, Li N, Guo S, Zhang Y, Ying Y, Zhang M, Wang Q, Liu JY, Zhang X. SCN11A variants may influence postoperative pain sensitivity after gynecological surgery in Chinese Han female patients. Medicine (Baltimore) 96: e8149, 2017. doi: 10.1097/MD.0000000000008149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 447: 855–858, 2007. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]