Abstract
Skeletal muscle myosin heavy chain (MyHC) fiber type composition is a critical determinant of overall muscle function and health. Various approaches interrogate fiber type at the single cell, but the two most commonly utilized are single-muscle fiber sodium dodecyl sulfate-polyacrylamide gel electrophoresis (smfSDS-PAGE) and fluorescent immunohistochemistry (IHC). Although smfSDS-PAGE is generally considered the “gold standard,” IHC is more commonly used because of its time-effectiveness and relative ease. Unfortunately, there is lingering inconsistency on how best to accurately and quickly determine fiber type via IHC and an overall misunderstanding regarding pure fiber type proportions, specifically the abundance of fibers exclusively expressing highly glycolytic MyHC IIX in humans. We therefore 1) present information and data showing the low abundance of pure MyHC IIX muscle fibers in healthy human skeletal muscle and 2) leverage this information to provide straightforward protocols that are informed by human biology and employ inexpensive, easily attainable antibodies for the accurate determination of fiber type.
Keywords: immunohistochemistry, MyHC, SDS-PAGE
BRIEF BACKGROUND ON HUMAN SKELETAL MUSCLE FIBER TYPE
There are three primary myosin heavy chain (MyHC) proteins expressed in human skeletal muscle that determine fiber type: MyHC I, MyHC IIA, and MyHC IIX. In sequential order, each fiber type possesses distinct contractile characteristics ranging from slow (less powerful) to fast (more powerful) and metabolic profiles ranging from oxidative to glycolytic. Fiber type composition can exist along a continuum, where fibers express a single MyHC isoform or coexpress multiple MyHC isoforms (MyHC I/IIA, IIA/IIX, and I/IIA/IIX), yielding six different MyHC fiber isotypes (6, 26). In healthy individuals, roughly equal proportions of MyHC I and IIA fibers are present in biopsy samples of vastus lateralis and lateral gastrocnemius muscle, the two most abundant and commonly studied human muscles. However, this distribution is not static and can be altered by muscle use patterns (9). For instance, endurance athletes generally possess more MyHC I fibers (9, 24, 32, 33, 37), which is likely a consequence of the training stimulus (9, 29, 48, 55). Conversely, MyHC IIA fibers may become more prevalent in athletes participating in strength/power activities (5, 7, 25, 38, 47, 59). The appearance of “hybrid” fibers that coexpress MyHC IIA and IIX and a general shift to a more fast-twitch contractile phenotype often accompany sedentary behavior (20, 27, 59). A transition from comparatively more oxidative MyHC I and IIA fibers toward glycolytic coexpressing IIA/IIX fibers could characterize elite strength and power performance (16, 47) but is generally linked to poor health outcomes (14, 20, 52); for example, the abundance of IIA/IIX hybrid fibers is inversely correlated with aerobic fitness (19). An extreme manifestation of muscle disuse in the form of spinal cord injury (SCI) leads to an accumulation of pure MyHC IIX fibers (3, 12, 35). Despite these examples of how fiber type can differ under various physiological and pathological conditions, there is currently no consensus on the relative abundance of pure MyHC IIX fibers in healthy human skeletal muscle. Our position is that this confusion largely arises from methodological constraints and not biological variability.
Fiber type proportions can provide insight into overall muscle health and function, so accurate classification is important for research purposes. The goal of this Cores of Reproducibility in Physiology (CORP) article is to 1) provide data and perspectives on the abundance of pure MyHC IIX muscle fibers in muscle samples from healthy humans and 2) outline detailed, validated, and simple fluorescent immunohistochemistry (IHC) approaches for accurately assessing skeletal muscle fiber type, with specific focus on assessing the prevalence of pure MyHC IIX fibers. This article serves to explain fiber typing discrepancies among laboratories and techniques and offers several approaches to fiber typing via IHC that cater to specific research questions based on human biology, thereby expanding on prior foundational work (11).
LACK OF CONSENSUS IN REPORTING ABUNDANCE OF MYHC IIX-EXPRESSING FIBERS IN HUMAN SKELETAL MUSCLE BIOPSIES
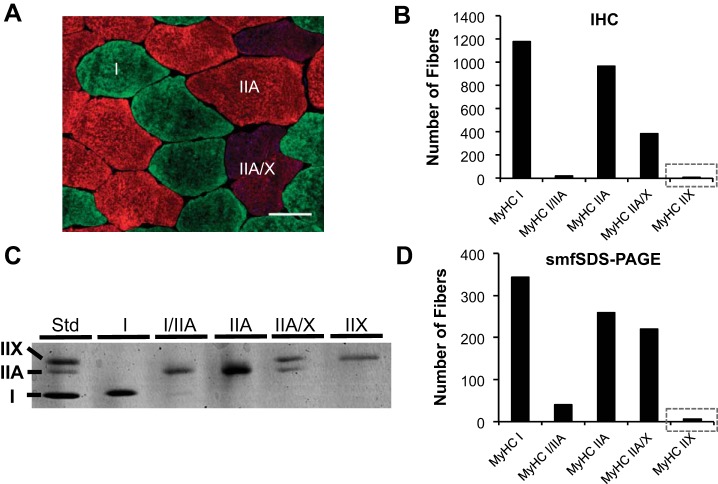
IHC involves the acquisition of thin muscle cross sections via cryosectioning, followed by incubation with isoform-specific MyHC antibodies and fiber type identification via fluorescent microscopy; this is the most commonly used modern approach for determining fiber type and can also afford the advantage of distinguishing anatomical distribution of fiber type as well as fiber size. In 2012, Bloemberg and Quadrilatero published a comprehensive IHC methods paper in which they tested a variety of antibody combinations and provided a recommendation for determining fiber type in human vastus lateralis muscle samples (11). Their recommendation was to use a cocktail of primary antibodies available from the Developmental Studies Hybridoma Bank (DSHB; University of Iowa) that detect MyHC I (BA-D5 or BA-F8), IIA (SC-71), and IIX (6H-1) fibers, followed by isotype-specific fluorescent-conjugated secondary antibodies. With this strategy, the authors reported that pure MyHC IIX fibers comprise 6.2 ± 1.6% of total fibers in young healthy human muscle samples (n = 7 subjects). In contrast, ongoing studies among our laboratories in which vastus lateralis muscle samples from a cohort of young healthy individuals (n = 22 subjects, 10 men and 12 women, age 25 ± 2 yr; Fig. 1A) were fiber typed with Bloemberg and Quadrilatero’s antibody guidelines showed very few pure MyHC IIX fibers (3 of 2,552 fibers analyzed, or 0.1%; Fig. 1B). Using a similar IHC approach, Fry et al. recently reported that pure MyHC IIX fibers were undetectable in healthy adults (19), whereas Roberts et al. reported up to 20% (44). This lack of consensus regarding MyHC IIX fiber type percentage in healthy human skeletal muscle via IHC is disconcerting, and may reflect variation in analytical approach rather than true biological variability.
Fig. 1.
Fluorescent immunohistochemistry (IHC; A and B) and single-muscle fiber sodium dodecyl sulfate-polyacrylamide gel electrophoresis (smfSDS-PAGE; C and D) for myosin heavy chain (MyHC) fiber typing in vastus lateralis biopsy samples from a cohort of healthy adult men and women (n = 22; 10 men/12 women). A: representative IHC image of MyHC I (green), MyHC IIA (red), and MyHC IIA/IIX (blue/red) fibers. Scale bar, 50 µm. B: quantification of muscle fiber type via IHC (2,552 total fibers), with gray box emphasizing low MyHC IIX abundance. C: representative image of a smfSDS-PAGE gel showing the continuum of MyHC fiber types. D: quantification of smfSDS-PAGE data from mechanically dissected single muscle fibers (876 total fibers) from the same 22 subjects analyzed in B, with gray box emphasizing low MyHC IIX abundance. Skeletal muscle biopsy samples were obtained under resting conditions, and smfSDS-PAGE was conducted as described by our laboratories in detail elsewhere (36). IHC was conducted in the Toth laboratory according to the methods described in the text, and images were captured at ×20 magnification. Std, standard.
The “gold standard” technique for determining fiber type proportion in skeletal muscle samples is sodium dodecyl sulfate-polyacrylamide gel electrophoresis on isolated single muscle fiber segments (smfSDS-PAGE). In brief, smfSDS-PAGE involves manual dissection of individual muscle fibers, denaturation of muscle proteins contained in those fibers, and then electrophoretic separation and direct visualization of MyHC proteins on a polyacrylamide gel via silver or Coomassie staining (10, 36, 52). Because a larger section of the muscle fiber is analyzed relative to IHC (e.g., 1–3 mm in length), this technique provides a measure that better reflects MyHC expression throughout the fiber. Using this approach (Fig. 1C), we assessed a sizable number of manually dissected muscle fibers from the same 22 subjects reported above and confirmed that <1% of these muscle fibers expressed pure MyHC IIX (6 of 876 fibers analyzed, or 0.6%; Fig. 1D). Our data are congruent with numerous other smfSDS-PAGE investigations (1, 9, 17, 20–22, 31, 33, 39, 41, 42, 55, 59, 61). To further validate the scarcity of pure MyHC IIX fibers in healthy adults, we pooled data on single-muscle fiber MyHC isoform expression from a number of published studies (13, 36, 53). This analysis included 38 healthy older individuals (18 men and 20 women, age 69 ± 6 yr) and 1,761 fibers (Table 1). The conclusion from these data was the same, <1% pure MyHC IIX fibers, consistent with the majority of studies that have evaluated fiber type via smfSDS-PAGE in older subjects (4, 23, 28–30, 49, 60) as well as a recent single-muscle fiber proteomics study (40). Taken together, these data argue strongly that pure MyHC IIX fibers are rare in healthy humans throughout a broad age range. This conclusion may not apply to certain pathological conditions, such as SCI patients, in whom a much higher proportion of pure MyHC IIX (>20%) has been reported (3, 12, 35). Additionally, elite athletes who compete in high-power sports/activities may have a genetic predisposition toward a greater abundance of pure MyHC IIX fibers, as detailed in a recent case report profiling an Olympic-caliber sprinter (56); however, other elite strength/power athletes lack pure MyHC IIX (47), indicating that the Olympic sprinter was a very unique case. The data from strength/power athletes also points to how IIA/IIX fibers may be highly functional and not exclusively a by-product of sedentary behavior (47), which is consistent with findings in other mammals that display high IIA/IIX proportions along with superior speed performance and fatigue resistance (15).
Table 1.
Fiber type via smfSDS-PAGE in healthy aged individuals
| MyHC |
||||||
|---|---|---|---|---|---|---|
| I | I/IIA | IIA | IIA/IIX | IIX | I/IIA/IIX | |
| No. of fibers (%) | 1,094 (62.1) | 66 (3.7) | 434 (24.6) | 143 (8.1) | 7 (0.4) | 17 (1.0) |
Values are from n = 38 subjects. MyHC, myosin heavy chain; smfSDS-PAGE, single-muscle fiber sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
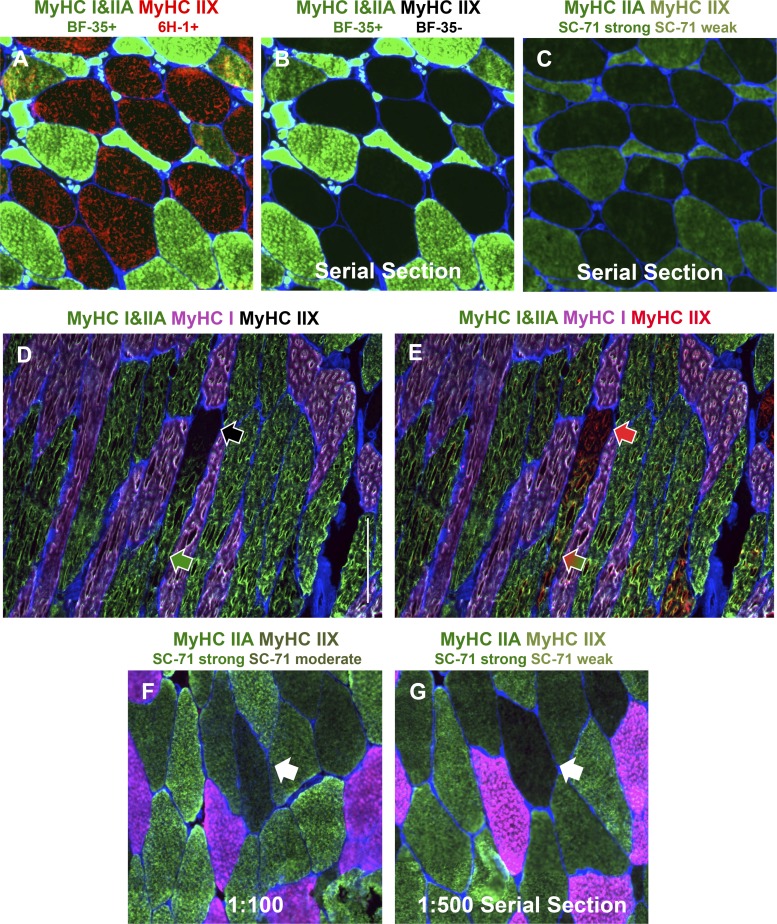
With the knowledge that SCI patients consistently present with pure MyHC IIX fibers, we validated the specificity of DSHB antibodies, using a vastus lateralis muscle sample from an individual who was 13 yr postinjury (American Spinal Cord Injury Association classification “A”). Using the DSHB antibody BF-35, which specifically recognizes MyHC I and IIA but not IIX (11, 50), we confirmed the appearance of frequent pure MyHC IIX fibers (labeled with 6H-1 in Fig. 2A and unlabeled in Fig. 2B). A serial section incubated with SC-71 shows that this antibody has some affinity for MyHC IIX protein in human samples (Fig. 2C), in agreement with previous reports using Western blots (8, 50). By sufficiently diluting SC-71, Bloemberg and Quadrilatero reduced the appearance of cross-reactivity and reported pure MyHC IIX fibers to be 5–10% of total fibers in healthy muscle samples (11), whereas essentially none is detected by smfSDS-PAGE (Fig. 1).
Fig. 2.
Immunohistochemistry (IHC) demonstrating specificity of myosin heavy chain (MyHC) antibodies and regional MyHC accumulation. A–C: representative images of sections from a muscle biopsy from a spinal cord-injured man (50 yr old, American Spinal Cord Injury Association classification “A”, 13 yr postinjury), taken at the same exposure. A and B: fiber typing using BF-35 (MyHC I and IIA, green) and 6H-1 (MyHC IIX, red) (A) compared with BF-35 only (B) on the same section. C: a serial section of the same region as in A and B showing fibers strongly (MyHC IIA) and weakly (MyHC IIX) positive for SC-71 (green), demonstrating the preferential affinity of the SC-71 antibody for MyHC IIA but weak affinity for MyHC IIX. D and E: representative images of a longitudinal muscle section from a young healthy man showing a muscle fiber that transitions from pure MyHC IIX [negative for BF-35, no fluorescence (black arrow in D), positive for 6H-1 (red arrow in E)] to MyHC IIA/IIX [positive for BF-35 (green arrow in D) and 6H-1 (green and red arrow in E)]. Scale bar, 300 µm. F and G: SC-71 applied to a muscle sample from a healthy young subject at 1:100 (F) and 1:500 (G) dilutions, illustrating the effect of antibody concentration on cross-reactivity (white arrows point to differential reactivity in the same fiber on serial sections). MyHC I is pink in D–G. Muscle tissue was obtained from the Bamman laboratory (A–C) and the University of Kentucky Center for Muscle Biology Tissue Bank (https://www.uky.edu/chs/muscle/bank), and IHC was conducted in the Peterson laboratory (D and E). Fiber borders (laminin) are in blue, and all images were captured at ×10 magnification.
What might account for the discrepancy in relative abundance of pure MyHC IIX fibers? One possibility is that this discrepancy reflects a fundamental limitation of all IHC approaches for fiber typing muscle samples, specifically, that only a very small length of the muscle is evaluated. In most instances IHC muscle fiber sections are 6–10 µm thick, and in mice, rats, rabbits, amphibians, and birds MyHC expression can vary along the length of a muscle fiber (18, 34, 43, 45, 46, 51, 62, 63). We speculated that this same pattern of expression could occur in human muscle fibers and may in part explain why IHC yields results discrepant from smfSDS-PAGE. By applying BF-35 and 6H-1 antibodies (along with the MyHC I-specific BA-D5 antibody) to longitudinal muscle sections from a young healthy subject, we observed that muscle fiber type may transform along the length of the fiber (Fig. 2, D and E), similar to reports in muscle from smaller mammals. Thus a single fiber segment from a human biopsy sample could present as a MyHC IIA/IIX hybrid via smfSDS-PAGE but could appear as pure MyHC IIA or IIX on cross section depending on where along the longitudinal axis of the fiber the cross section was obtained; indeed, a larger proportion of hybrid fibers manifested via smfSDS-PAGE compared with IHC in the data presented here (Fig. 1). Other factors associated with the analytical methods could also impact relative abundance of MyHC isotypes in muscle fibers via IHC. These factors include exposure time during imaging and contrast adjustments after imaging, whether analysis is performed by a trained technician or with automated software (58), the dilution/concentration at which the antibody is used, as well as the duration of antibody incubation. Figure 2, F and G, illustrate how different dilutions of SC-71 alter the degree of reactivity of this antibody with MyHC IIX; 1:100 results in noticeable fluorescence in a pure MyHC IIX fiber, whereas 1:500 on a serial section shows that same fiber is almost devoid of fluorescence. Antibody reactivity could be further manipulated by using undiluted supernatant antibodies that have very high concentrations or by incubating for a short period of time (e.g., 1 h) versus overnight (11, 19, 44). All of these factors combined can contribute to discrepant results between smfSDS-PAGE and IHC, further emphasizing the necessity of understanding the prevalence of pure MyHC IIX in human muscle and arriving at consensus methods for accurate fiber type identification.
RECOMMENDATIONS FOR ACCURATELY FIBER TYPING HUMAN SKELETAL MUSCLE VIA IHC
The method used for fiber typing human skeletal muscle samples must consider time, resources, capabilities, and the research question at hand. SmfSDS-PAGE is the gold standard technique, but it is time consuming and tedious, requires considerable resources, and is low throughput. For these reasons, IHC is the more desirable choice for fiber typing human muscle samples. In agreement with Bloemberg and Quadrilatero (11), one can utilize an antibody cocktail containing BA-D5 (or BA-F8), SC-71 (or A4.74, which yields essentially identical results at low concentrations), and 6H-1 from DSHB at the concentrations indicated in Table 2 to detect MyHC I, MyHC IIA, and MyHC IIX, respectively. The entire continuum of fiber types, including MyHC I/IIA and IIA/IIX hybrids, can be visualized with this approach, with the exception of pure MyHC IIX because of cross-reactivity of the SC-71 antibody. The result will nevertheless be consistent with smfSDS-PAGE, as we have demonstrated above, and we further suggest that MyHC IIA/IIX and MyHC IIX fibers be classified as MyHC IIAX/IIX (57); this prudent, conservative nomenclature accounts for the range of IIA and IIX proportions in hybrid fibers while acknowledging the low and method-dependent abundance of MyHC IIX. To reliably detect pure MyHC IIX on cross sections (e.g., if evaluating injured/diseased/unique populations or wanting to visualize segmental MyHC expression), we recommend cutting a serial section and using BF-35 (Fig. 3), which identifies MyHC I and IIA proteins. Alternatively, BF-35 can be substituted for SC-71 in the antibody cocktail, which only limits detection of MyHC I/IIA hybrid fibers. MyHC I/IIA is a comparatively lower-abundance fiber type (generally <10%; see Fig. 1 and Table 1 and Refs. 1, 2, 11, 54, 61), but if it is of interest it can be detected by using BA-D5 and SC-71 (see above). We do not recommend further diluting the MyHC IIA antibodies beyond 1:500, as this may incorrectly reflect a complete lack of reactivity with MyHC IIX (in our hands) and may also affect detection of MyHC IIA.
Table 2.
Antibodies for IHC fiber typing
| Antibody | Dilution (Concentration) | Source | Notes |
|---|---|---|---|
| 1° Antibodies to match smfSDS-PAGE | |||
| BA-D5 (MyHC I) | 1:100 (3.05 µg/mL) | DSHB | αMs IgG2B |
| SC-71 (MyHC IIA) | 1:500 (0.85 µg/mL) | DSHB | αMs IgG1 |
| 6H-1 (MyHC IIX) | 1:1 in PBS (10.5 µg/mL) | DSHB | αMs IgM, supernatant |
| Laminin | 1:100 | Sigma-Aldrich | αRb IgG |
| 1° Antibody to detect pure MyHC IIX | |||
| BF-35 (MyHC I and IIA) | 1:100 | DSHB | αMs IgG1 |
| 2° Antibodies | |||
| Fluor 647 | 1:200 | Invitrogen A21242 | GtαMs IgG2B |
| Fluor 488 | 1:200 | Invitrogen A21121 | GtαMs IgG1 |
| Fluor 555 | 1:200 | Invitrogen A21426 | GtαMs IgGM |
| Fluor 350 | 1:100 | Vector CI-1000 | GtαRb IgG |
| Alternative 1° antibodies | |||
| BA-F8 (MyHC I) | 1:100 | DSHB | αMs IgG2B |
| A4.74 (MyHC IIA)* | 1:500 | DSHB | αMs IgG1 |
DSHB, Developmental Studies Hybridoma Bank; Gt, goat; IHC, immunohistochemistry; Ms, mouse; MyHC, myosin heavy chain; Rb, rabbit.
Fig. 3.
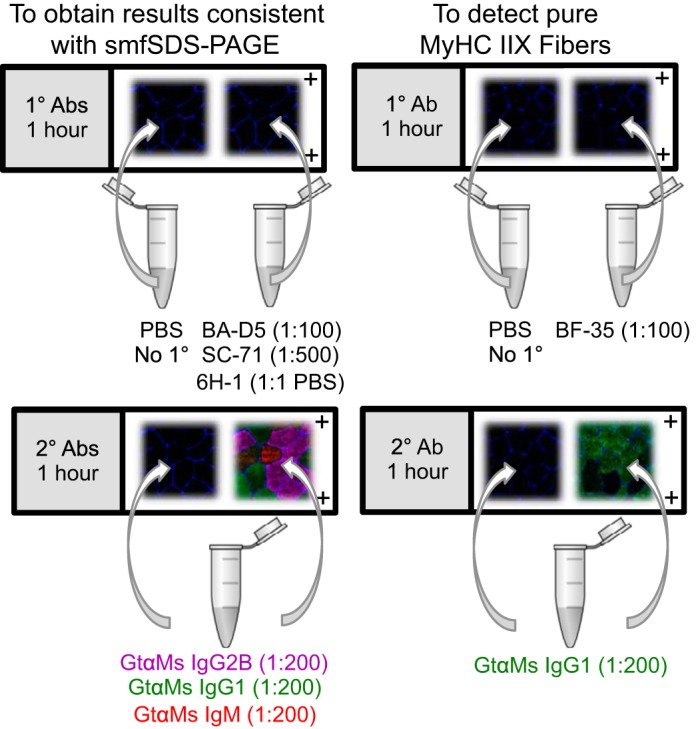
Visual representations of recommended immunohistochemistry (IHC) fiber-typing protocols. Muscle fiber borders (laminin, blue) are shown on all images for illustrative purposes. Left: pink is myosin heavy chain (MyHC) I, green is MyHC IIA, and red is MyHC IIX. Right: green is MyHC I and/or IIA, and no fluorescence is MyHC IIX.
IHC METHODS FOR FIBER TYPING
The antibodies for fiber typing can be found in Table 2. To maintain consistency across laboratories, we suggest using concentrated antibodies (if available) at the recommended dilutions/concentrations; supernatant antibodies can be diluted to similar concentrations if necessary but are unfiltered and could introduce variability. The protocol is optimized for a four-channel fluorescent microscope but can be modified as needed if fewer channels are available. We also recommend using automated detection software, such as MyoVision (58), for quantification of fiber type distribution since this removes subjectivity and promotes consistency. As with any IHC protocol, the results are largely dependent on how well the tissue was cryopreserved and the quality of histological sectioning; freeze-damaged tissue and/or poor sectioning will yield unfavorable results.
Step 1
Allow muscle samples to equilibrate to the temperature of the cryostat (−20 to −23°C, 20–30 min). Mount muscle sample and cut 6- to 10-µm sections with a fresh blade.
Step 2
Collect each section on charged glass slides. Dry at room temperature for a minimum of 1 h (sections can be dried for up to 16 h and still be used for fiber typing). Use a PAP pen to circle the sections, and let the PAP dry for at least 15 min.
Note.
On each slide, cut one section specifically for use as a no-primary/secondary-only control, so that background fluorescence can be assessed and corrected at the time of imaging.
Note.
If antibody application commences before samples are dry, the sections tend to wrinkle and/or develop air bubbles, which affect image quality.
Note.
If sections were cut previously, dried, and stored at −20 to −80°C, slides should be dried again for at least an hour after thawing.
Step 3
Make a primary antibody cocktail containing BA-D5, SC-71, and laminin diluted into 6H-1 supernatant-PBS (1:1; can substitute BF-35 for SC-71 if pure MyHC IIX is of interest, such as when fiber typing SCI patients or sprint athletes). Apply to sections and incubate in a humidified slide box at room temperature for 1 h. Incubate no-primary control sections in PBS.
Step 4
Wash sections in a Coplin jar for 5 min three separate times at room temperature, using fresh PBS for each wash.
Step 5
Make a secondary antibody cocktail (all diluted in PBS), apply to sections, and incubate in a humidified slide box at room temperature for 1 h. Apply secondary antibodies to no-primary control sections at this time as well.
Step 6
Wash sections in a Coplin jar for 5 min three separate times at room temperature, using fresh PBS for each wash.
Step 7
Mount slides with VectaShield (long term) or glycerol-PBS (1:1; immediate imaging) under a glass coverslip (#1.5 thickness).
Step 8
When imaging, the no-primary control sections can be used to help inform exposure times for the sections that received primary antibody. The 6H-1 antibody (MyHC IIX) sometimes underperforms, especially in low-quality tissue, so using the amount of staining present in BA-D5+ (MyHC I) fibers can inform where to set the exposure time.
Technical Notes Regarding Reproducibility
Every laboratory conducting IHC likely has different equipment and may deploy certain stylistic approaches that are difficult to document. These discrepancies could produce different results and/or interpretation of results between laboratories. Thus, to provide a basis for comparison to the results presented above, we offer a few technical notes for consideration.
Regarding tissue handling, all three laboratories involved in this work mount their muscle samples in tragacanth gum or Optimal Cutting Temperature compound and freeze the tissue mounts with fresh liquid nitrogen-cooled isopentane; reusing isopentane dilutes it and results in poor tissue quality. The quality of cryosections is usually readily apparent upon visual inspection with a light microscope at the time of sectioning; poor tissue quality will manifest as indistinguishable muscle fiber borders and/or holes within muscle fibers. The best muscle sections are usually obtained when using a fresh blade. Exposure times used to obtain fluorescent images are highly dependent on the microscope itself and the usage history of the light source, so it is challenging to offer guidance in this area. With that said, the Peterson laboratory uses a Zeiss Axio Scope upright microscope with a DAPI/Hoechst/aminomethylcoumarin acetate filter (exciter 350/50, emitter 460/50), a FITC/green fluorescent protein filter (exciter 480/30, emitter 535/40), a tetramethylrhodamine isothiocyanate/Cy3 filter (exciter 545/30, emitter 610/75), and a Cy5 filter (exciter 620/60, emitter 700/75), and exposure times are typically in the 200–800 ms range; filters with broader or narrower excitation and emission spectra could yield slightly different results. ×10 or ×20 images, depending on the size of the section, are sufficient to obtain high-quality fiber type data. Images are preprocessed with ZEN software where background is manually corrected based on fluorescence in no-primary control samples.
CONCLUSIONS
Using the simple and rapid IHC protocol above, accurate fiber typing in human samples can be obtained that will be reproducible and comparable across laboratories. Our recommended methods have the potential to overestimate the abundance of pure MyHC IIX fibers compared with smfSDS-PAGE, perhaps because of regional differences in MyHC abundance along the fiber. However, as we demonstrate, this overestimation is likely quite small in healthy adults throughout a broad age range. Accordingly, our data support the conclusion that pure MyHC IIX fibers are relatively rare in healthy human skeletal muscle, and we recommend usage of the nomenclature MyHC IIAX/IIX to describe IIX-containing fibers. Our hope is that the methods presented in this CORP article will be widely adopted to advance further understanding of skeletal muscle phenotype in health and disease and facilitate comparison of data across research laboratories.
GRANTS
This work was supported by grants from the National Institutes of Health to K. A. Murach (AR-071753), C. A. Peterson (AR-060701 and AG-046920), M. S. Miller (AG-031303), T. W. Tourville (AR-066729), and M. J. Toth (AG-033547, HL-077418, AR-069199).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.P. and M.J.T. conceived and designed research; K.A.M., C.M.D., K.K., T.B.V., T.W.T., and M.S.M. performed experiments; K.A.M., C.M.D., K.K., T.B.V., T.W.T., M.S.M., M.M.B., C.A.P., and M.J.T. analyzed data; K.A.M., C.M.D., K.K., T.B.V., T.W.T., M.S.M., M.M.B., C.A.P., and M.J.T. interpreted results of experiments; K.A.M. prepared figures; K.A.M. and C.A.P. drafted manuscript; K.A.M., C.M.D., K.K., T.B.V., T.W.T., M.S.M., M.M.B., C.A.P., and M.J.T. edited and revised manuscript; K.A.M., C.M.D., K.K., T.B.V., T.W.T., M.S.M., M.M.B., C.A.P., and M.J.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all the volunteers who dedicated valuable time to these studies.
REFERENCES
- 1.Andersen JL, Klitgaard H, Bangsbo J, Saltin B. Myosin heavy chain isoforms in single fibres from m. vastus lateralis of soccer players: effects of strength-training. Acta Physiol Scand 150: 21–26, 1994. doi: 10.1111/j.1748-1716.1994.tb09655.x. [DOI] [PubMed] [Google Scholar]
- 2.Andersen JL, Klitgaard H, Saltin B. Myosin heavy chain isoforms in single fibres from m. vastus lateralis of sprinters: influence of training. Acta Physiol Scand 151: 135–142, 1994. doi: 10.1111/j.1748-1716.1994.tb09730.x. [DOI] [PubMed] [Google Scholar]
- 3.Andersen JL, Mohr T, Biering-Sørensen F, Galbo H, Kjaer M. Myosin heavy chain isoform transformation in single fibres from m. vastus lateralis in spinal cord injured individuals: effects of long-term functional electrical stimulation (FES). Pflugers Arch 431: 513–518, 1996. doi: 10.1007/BF02191897. [DOI] [PubMed] [Google Scholar]
- 4.Andersen JL, Terzis G, Kryger A. Increase in the degree of coexpression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve 22: 449–454, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 5.Arevalo JA, Lynn SK, Bagley JR, Brown LE, Costa PB, Galpin AJ. Lower-limb dominance, performance, and fiber type in resistance-trained men. Med Sci Sports Exerc 50: 1054–1060, 2018. doi: 10.1249/MSS.0000000000001533. [DOI] [PubMed] [Google Scholar]
- 6.Bagley J, Murach K, Trappe S. Microgravity-induced fiber type shift in human skeletal muscle. Gravit Space Biol 26: 34–40, 2012. [Google Scholar]
- 7.Bagley JR, McLeland KA, Arevalo JA, Brown LE, Coburn JW, Galpin AJ. Skeletal muscle fatigability and myosin heavy chain fiber type in resistance trained men. J Strength Cond Res 31: 602–607, 2017. doi: 10.1519/JSC.0000000000001759. [DOI] [PubMed] [Google Scholar]
- 8.Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen MC. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol (1985) 84: 157–163, 1998. doi: 10.1152/jappl.1998.84.1.157. [DOI] [PubMed] [Google Scholar]
- 9.Bathgate KE, Bagley JR, Jo E, Talmadge RJ, Tobias IS, Brown LE, Coburn JW, Arevalo JA, Segal NL, Galpin AJ. Muscle health and performance in monozygotic twins with 30 years of discordant exercise habits. Eur J Appl Physiol 118: 2097–2110, 2018. doi: 10.1007/s00421-018-3943-7. [DOI] [PubMed] [Google Scholar]
- 10.Biral D, Betto R, Danieli-Betto D, Salviati G. Myosin heavy chain composition of single fibres from normal human muscle. Biochem J 250: 307–308, 1988. doi: 10.1042/bj2500307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7: e35273, 2012. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broos S, Malisoux L, Theisen D, Francaux M, Deldicque L, Thomis MA. Role of alpha-actinin-3 in contractile properties of human single muscle fibers: a case series study in paraplegics. PLoS One 7: e49281, 2012. doi: 10.1371/journal.pone.0049281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callahan DM, Miller MS, Sweeny AP, Tourville TW, Slauterbeck JR, Savage PD, Maugan DW, Ades PA, Beynnon BD, Toth MJ. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J Physiol 592: 4555–4573, 2014. doi: 10.1113/jphysiol.2014.279034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll CC, Gallagher PM, Seidle ME, Trappe SW. Skeletal muscle characteristics of people with multiple sclerosis. Arch Phys Med Rehabil 86: 224–229, 2005. doi: 10.1016/j.apmr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Curry JW, Hohl R, Noakes TD, Kohn TA. High oxidative capacity and type IIx fibre content in springbok and fallow deer skeletal muscle suggest fast sprinters with a resistance to fatigue. J Exp Biol 215: 3997–4005, 2012. doi: 10.1242/jeb.073684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Antona G, Lanfranconi F, Pellegrino MA, Brocca L, Adami R, Rossi R, Moro G, Miotti D, Canepari M, Bottinelli R. Skeletal muscle hypertrophy and structure and function of skeletal muscle fibres in male body builders. J Physiol 570: 611–627, 2006. doi: 10.1113/jphysiol.2005.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickinson JM, Lee JD, Sullivan BE, Harber MP, Trappe SW, Trappe TA. A new method to study in vivo protein synthesis in slow- and fast-twitch muscle fibers and initial measurements in humans. J Appl Physiol (1985) 108: 1410–1416, 2010. doi: 10.1152/japplphysiol.00905.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edman KA, Reggiani C, Schiaffino S, te Kronnie G. Maximum velocity of shortening related to myosin isoform composition in frog skeletal muscle fibres. J Physiol 395: 679–694, 1988. doi: 10.1113/jphysiol.1988.sp016941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fry CS, Noehren B, Mula J, Ubele MF, Westgate PM, Kern PA, Peterson CA. Fibre type-specific satellite cell response to aerobic training in sedentary adults. J Physiol 592: 2625–2635, 2014. doi: 10.1113/jphysiol.2014.271288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallagher P, Trappe S, Harber M, Creer A, Mazzetti S, Trappe T, Alkner B, Tesch P. Effects of 84-days of bedrest and resistance training on single muscle fibre myosin heavy chain distribution in human vastus lateralis and soleus muscles. Acta Physiol Scand 185: 61–69, 2005. doi: 10.1111/j.1365-201X.2005.01457.x. [DOI] [PubMed] [Google Scholar]
- 21.Galpin AJ, Raue U, Jemiolo B, Trappe TA, Harber MP, Minchev K, Trappe S. Human skeletal muscle fiber type specific protein content. Anal Biochem 425: 175–182, 2012. doi: 10.1016/j.ab.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garner DJ, Widrick JJ. Cross-bridge mechanisms of muscle weakness in multiple sclerosis. Muscle Nerve 27: 456–464, 2003. doi: 10.1002/mus.10346. [DOI] [PubMed] [Google Scholar]
- 23.Grosicki GJ, Standley RA, Murach KA, Raue U, Minchev K, Coen PM, Newman AB, Cummings S, Harris T, Kritchevsky S, Goodpaster BH, Trappe S; Health ABC Study . Improved single muscle fiber quality in the oldest-old. J Appl Physiol (1985) 121: 878–884, 2016. doi: 10.1152/japplphysiol.00479.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harber MP, Gallagher PM, Trautmann J, Trappe SW. Myosin heavy chain composition of single muscle fibers in male distance runners. Int J Sports Med 23: 484–488, 2002. doi: 10.1055/s-2002-35067. [DOI] [PubMed] [Google Scholar]
- 25.Kesidis N, Metaxas TI, Vrabas IS, Stefanidis P, Vamvakoudis E, Christoulas K, Mandroukas A, Balasas D, Mandroukas K. Myosin heavy chain isoform distribution in single fibres of bodybuilders. Eur J Appl Physiol 103: 579–583, 2008. doi: 10.1007/s00421-008-0751-5. [DOI] [PubMed] [Google Scholar]
- 26.Klitgaard H, Bergman O, Betto R, Salviati G, Schiaffino S, Clausen T, Saltin B. Co-existence of myosin heavy chain I and IIa isoforms in human skeletal muscle fibres with endurance training. Pflugers Arch 416: 470–472, 1990. doi: 10.1007/BF00370757. [DOI] [PubMed] [Google Scholar]
- 27.Klitgaard H, Zhou M, Richter EA. Myosin heavy chain composition of single fibres from m. biceps brachii of male body builders. Acta Physiol Scand 140: 175–180, 1990. doi: 10.1111/j.1748-1716.1990.tb08989.x. [DOI] [PubMed] [Google Scholar]
- 28.Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand 140: 55–62, 1990. doi: 10.1111/j.1748-1716.1990.tb08975.x. [DOI] [PubMed] [Google Scholar]
- 29.Konopka AR, Trappe TA, Jemiolo B, Trappe SW, Harber MP. Myosin heavy chain plasticity in aging skeletal muscle with aerobic exercise training. J Gerontol A Biol Sci Med Sci 66A: 835–841, 2011. doi: 10.1093/gerona/glr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol Cell Physiol 272: C638–C649, 1997. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- 31.Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol 472: 595–614, 1993. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luden N, Hayes E, Galpin A, Minchev K, Jemiolo B, Raue U, Trappe TA, Harber MP, Bowers T, Trappe S. Myocellular basis for tapering in competitive distance runners. J Appl Physiol (1985) 108: 1501–1509, 2010. doi: 10.1152/japplphysiol.00045.2010. [DOI] [PubMed] [Google Scholar]
- 33.Luden N, Hayes E, Minchev K, Louis E, Raue U, Conley T, Trappe S. Skeletal muscle plasticity with marathon training in novice runners. Scand J Med Sci Sports 22: 662–670, 2012. doi: 10.1111/j.1600-0838.2011.01305.x. [DOI] [PubMed] [Google Scholar]
- 34.Lutz GJ, Bremner SN, Bade MJ, Lieber RL. Identification of myosin light chains in Rana pipiens skeletal muscle and their expression patterns along single fibres. J Exp Biol 204: 4237–4248, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Malisoux L, Jamart C, Delplace K, Nielens H, Francaux M, Theisen D. Effect of long-term muscle paralysis on human single fiber mechanics. J Appl Physiol (1985) 102: 340–349, 2007. doi: 10.1152/japplphysiol.00609.2006. [DOI] [PubMed] [Google Scholar]
- 36.Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME 2nd, Ades PA, Maughan DW, Palmer BM, Toth MJ. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol (1985) 115: 1004–1014, 2013. doi: 10.1152/japplphysiol.00563.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murach K, Raue U, Wilkerson B, Minchev K, Jemiolo B, Bagley J, Luden N, Trappe S. Single muscle fiber gene expression with run taper. PLoS One 9: e108547, 2014. doi: 10.1371/journal.pone.0108547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murach KA, Bagley JR, McLeland KA, Arevalo JA, Ciccone AB, Malyszek KK, Wen Y, Galpin AJ. Improving human skeletal muscle myosin heavy chain fiber typing efficiency. J Muscle Res Cell Motil 37: 1–5, 2016. doi: 10.1007/s10974-016-9441-9. [DOI] [PubMed] [Google Scholar]
- 39.Murach KA, Minchev K, Grosicki GJ, Lavin KM, Perkins RK, Ryder JW, Scott J, Ploutz-Snyder L, Trappe TA, Trappe S. Myocellular responses to concurrent flywheel training during 70 days of bed rest. Med Sci Sports Exerc 50: 1950–1960, 2018. doi: 10.1249/MSS.0000000000001620. [DOI] [PubMed] [Google Scholar]
- 40.Murgia M, Toniolo L, Nagaraj N, Ciciliot S, Vindigni V, Schiaffino S, Reggiani C, Mann M. Single muscle fiber proteomics reveals fiber-type-specific features of human muscle aging. Cell Rep 19: 2396–2409, 2017. doi: 10.1016/j.celrep.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 41.Parcell AC, Sawyer RD, Craig Poole R. Single muscle fiber myosin heavy chain distribution in elite female track athletes. Med Sci Sports Exerc 35: 434–438, 2003. doi: 10.1249/01.MSS.0000053735.99344.C0. [DOI] [PubMed] [Google Scholar]
- 42.Parcell AC, Sawyer RD, Drummond MJ, O’Neil B, Miller N, Woolstenhulme MT. Single-fiber MHC polymorphic expression is unaffected by sprint cycle training. Med Sci Sports Exerc 37: 1133–1137, 2005. doi: 10.1249/01.mss.0000170123.27209.e1. [DOI] [PubMed] [Google Scholar]
- 43.Peuker H, Pette D. Quantitative analyses of myosin heavy-chain mRNA and protein isoforms in single fibers reveal a pronounced fiber heterogeneity in normal rabbit muscles. Eur J Biochem 247: 30–36, 1997. doi: 10.1111/j.1432-1033.1997.00030.x. [DOI] [PubMed] [Google Scholar]
- 44.Roberts BM, Lavin KM, Many GM, Thalacker-Mercer A, Merritt EK, Bickel CS, Mayhew DL, Tuggle SC, Cross JM, Kosek DJ, Petrella JK, Brown CJ, Hunter GR, Windham ST, Allman RM, Bamman MM. Human neuromuscular aging: sex differences revealed at the myocellular level. Exp Gerontol 106: 116–124, 2018. doi: 10.1016/j.exger.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosser B, Bandman E. Heterogeneity of protein expression within muscle fibers. J Anim Sci 81: E94–E101, 2003. [Google Scholar]
- 46.Sawano S, Komiya Y, Ichitsubo R, Ohkawa Y, Nakamura M, Tatsumi R, Ikeuchi Y, Mizunoya W. A one-step immunostaining method to visualize rodent muscle fiber type within a single specimen. PLoS One 11: e0166080, 2016. doi: 10.1371/journal.pone.0166080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrano N, Colenso-Semple LM, Lazauskus KK, Siu JW, Bagley JR, Lockie RG, Costa PB, Galpin AJ. Extraordinary fast-twitch fiber abundance in elite weightlifters. PLoS One 14: e0207975, 2019. doi: 10.1371/journal.pone.0207975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simoneau JA, Lortie G, Boulay MR, Marcotte M, Thibault MC, Bouchard C. Human skeletal muscle fiber type alteration with high-intensity intermittent training. Eur J Appl Physiol Occup Physiol 54: 250–253, 1985. doi: 10.1007/BF00426141. [DOI] [PubMed] [Google Scholar]
- 49.Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol 295: R273–R280, 2008. doi: 10.1152/ajpregu.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smerdu V, Soukup T. Demonstration of myosin heavy chain isoforms in rat and humans: the specificity of seven available monoclonal antibodies used in immunohistochemical and immunoblotting methods. Eur J Histochem 52: 179–190, 2008. doi: 10.4081/1210. [DOI] [PubMed] [Google Scholar]
- 51.Staron RS, Pette D. Nonuniform myosin expression along single fibers of chronically stimulated and contralateral rabbit tibialis anterior muscles. Pflugers Arch 409: 67–73, 1987. doi: 10.1007/BF00584751. [DOI] [PubMed] [Google Scholar]
- 52.Toth MJ, Callahan DM, Miller MS, Tourville TW, Hackett SB, Couch ME, Dittus K. Skeletal muscle fiber size and fiber type distribution in human cancer: effects of weight loss and relationship to physical function. Clin Nutr 35: 1359–1365, 2016. doi: 10.1016/j.clnu.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toth MJ, Miller MS, VanBuren P, Bedrin NG, LeWinter MM, Ades PA, Palmer BM. Resistance training alters skeletal muscle structure and function in human heart failure: effects at the tissue, cellular and molecular levels. J Physiol 590: 1243–1259, 2012. doi: 10.1113/jphysiol.2011.219659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trappe S, Costill D, Gallagher P, Creer A, Peters JR, Evans H, Riley DA, Fitts RH. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. J Appl Physiol (1985) 106: 1159–1168, 2009. doi: 10.1152/japplphysiol.91578.2008. [DOI] [PubMed] [Google Scholar]
- 55.Trappe S, Harber M, Creer A, Gallagher P, Slivka D, Minchev K, Whitsett D. Single muscle fiber adaptations with marathon training. J Appl Physiol (1985) 101: 721–727, 2006. doi: 10.1152/japplphysiol.01595.2005. [DOI] [PubMed] [Google Scholar]
- 56.Trappe S, Luden N, Minchev K, Raue U, Jemiolo B, Trappe TA. Skeletal muscle signature of a champion sprint runner. J Appl Physiol (1985) 118: 1460–1466, 2015. doi: 10.1152/japplphysiol.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walton RG, Dungan CM, Long DE, Tuggle SC, Kosmac K, Peck BD, Bush HM, Villasante Tezanos AG, McGwin G, Windham ST, Ovalle F, Bamman MM, Kern PA, Peterson CA. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: a randomized, double-blind, placebo-controlled, multicenter trial: the MASTERS trial. Aging Cell 18: e13039, 2019. doi: 10.1111/acel.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen Y, Murach KA, Vechetti IJ Jr, Fry CS, Vickery C, Peterson CA, McCarthy JJ, Campbell KS. MyoVision: software for automated high-content analysis of skeletal muscle immunohistochemistry. J Appl Physiol (1985) 124: 40–51, 2018. doi: 10.1152/japplphysiol.00762.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williamson DL, Gallagher PM, Carroll CC, Raue U, Trappe SW. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol (1985) 91: 1955–1961, 2001. doi: 10.1152/jappl.2001.91.5.1955. [DOI] [PubMed] [Google Scholar]
- 60.Williamson DL, Godard MP, Porter DA, Costill DL, Trappe SW. Progressive resistance training reduces myosin heavy chain coexpression in single muscle fibers from older men. J Appl Physiol (1985) 88: 627–633, 2000. doi: 10.1152/jappl.2000.88.2.627. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Jemiolo B, Trappe S. Proteolytic mRNA expression in response to acute resistance exercise in human single skeletal muscle fibers. J Appl Physiol (1985) 101: 1442–1450, 2006. doi: 10.1152/japplphysiol.00438.2006. [DOI] [PubMed] [Google Scholar]
- 62.Zhang M, Gould M. Segmental distribution of myosin heavy chain isoforms within single muscle fibers. Anat Rec (Hoboken) 300: 1636–1642, 2017. doi: 10.1002/ar.23578. [DOI] [PubMed] [Google Scholar]
- 63.Zhang MY, Zhang WJ, Medler S. The continuum of hybrid IIX/IIB fibers in normal mouse muscles: MHC isoform proportions and spatial distribution within single fibers. Am J Physiol Regul Integr Comp Physiol 299: R1582–R1591, 2010. doi: 10.1152/ajpregu.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]