Abstract
Changes in pituitary-ovarian hormones across the menopausal transition have multiple physiological consequences. However, little is known about how the major types of postmenopausal hormone therapy (HT) affect pituitary-ovarian hormonal relationships. This study evaluated these relationships in recently menopausal women (52.45 ± 2.49 yr of age) in the Kronos Early Estrogen Prevention Study (KEEPS) who were compliant to randomized, double-blinded treatment with oral conjugated equine estrogen (o-CEE; n = 109), transdermal 17β-estradiol (t-E2; n = 107), or placebo (n = 146). Androstenedione, testosterone, 17β-estradiol, estrone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) were measured in serum before (baseline) and 48 mo after randomization to treatment. Descriptive summaries of hormone levels were performed, and multiple regression analyses were used to examine the effects of o-CEE, t-E2, and placebo on these hormone levels at 48 mo, adjusting for baseline levels. A network analysis examined the covariance of changes in hormone levels over the 48 mo within treatment groups. As expected, at 48 mo of treatment, hormone levels differed between women in the two active treatment groups compared with placebo, and network analysis indicated stronger relationships among hormone levels in the t-E2 and o-CEE groups compared with placebo. Associations among testosterone, 17β-estradiol, FSH, and LH differed between the o-CEE group compared with t-E2 and placebo groups. Thus, two common HT regimens differentially alter pituitary-ovarian hormone levels, altering feedback cycles and interhormonal associations in recently menopausal women. These interactions provide the basis for future studies investigating the impact of hormonal modulation of aging, including cognitive decline in women.
Keywords: androgen, estrogen, follicle-stimulating hormone, hormone therapy, menopause, pituitary-ovarian hormones
INTRODUCTION
The relationship among pituitary-ovarian hormones represents a classical negative feedback control of hormone release, i.e., the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH) released from the pituitary stimulates synthesis and secretion of 17β-estradiol (E2) by the ovaries, which in turn inhibits further release of LH and FSH. With advancing age the feedback system gradually changes, and with menopause ovarian follicle depletion is accompanied by dramatically decreased production of E2 and progesterone, subsequently increasing secretion of FSH and LH. In contrast, levels of androgens, including androstenedione and testosterone, remain at their relatively low premenopausal levels being released from the follicle-depleted ovary (2, 19, 24, 29, 31, 41) and the adrenal glands (12). Despite some aromatization of testosterone to E2, total gonadal hormone levels remain low and do not impact the increased secretion of FSH and LH.
Several studies have evaluated the impact of exogenous menopausal hormone therapy (HT) to relieve specific outcomes of menopause such as vasomotor hot flashes and night sweats, changes in cognition, depressed mood, and osteoporosis (4, 9, 20, 21, 23, 36, 39), but few have evaluated the effects of HT on hormones of the hypothalamic-pituitary axis (34). Nevertheless, changes in levels of endogenous pituitary-ovarian hormones resulting from exogenous HT may have important health-related effects. For example, lower relative levels of FSH are associated with an increased risk of cardiovascular disease, diabetes, and prediabetes in postmenopausal women, and higher levels of pituitary LH secretion may be associated with reduced intracerebral LH production, leading to adverse changes in cognitive function (6, 44, 45). Furthermore, HT may impact production of androgens (testosterone and androstenedione) from the postmenopausal ovary, which could be associated with physiological (2, 18) and neurobehavioral (8, 32) consequences. Thus, it is important to better understand the relationships among different formulations of HT and endogenous pituitary hormone levels to explore possible long-term effects of HT in postmenopausal women.
To address this question, this study analyzed serum collected from participants in the Kronos Early Estrogen Prevention Study (KEEPS) to investigate the associations among pituitary gonadotropins and ovarian hormones during up to 4 yr of treatment with either oral conjugated equine estrogen (o-CEE), transdermal 17β-estradiol (t-E2), or placebo in recently menopausal women.
METHODS
The Kronos Early Estrogen Prevention Study (KEEPS; NCT00154180) was a randomized, placebo-controlled, double-blinded multicenter clinical trial to evaluate the cardiovascular and cognitive effects of o-CEE, transdermal 17β-estradiol, and placebo in healthy women who experienced natural menopause between 42 and 58 yr of age. Enrollment occurred at nine US sites between August 2005 and July 2008, with final visits completed in 2012. To be eligible for KEEPS, women could not have taken estrogen- or progestin-containing medications within 6 mo of randomization and had to have had their last menstrual period within 6–36 mo, also having a plasma follicle-stimulating hormone (FSH) level of ≥35 ng/mL and plasma E2 level of <40 pg/mL. The women were generally healthy, as determined by exclusion criteria published previously (21).
Institutional Review Board numbers for KEEPS institutions.
The central KEEPS and Phoenix KEEPS [Institutional Review Board (IRB) protocol by the Western IRB] numbers were as follows: study no. 1058663 and Western IRB pro no. 20040792KEEPS (main study and cognitive substudy), no. 10-02980 and MDBHAS no. 11-05383; Brigham and Women’s Hospital (Partners): no. 2004-P-002144 BWH; Mayo Clinic: no. 2241-04; Columbia: IRB no. AAAA-8062; Yale: 0409027022; University of Utah: 13257; Einstein/Montefiore: 04–08–213; University of Wisconsin: H-2005–0059; University of California San Francisco: KEEPS (main study and cognitive substudy) no. 10-02980; University of Washington IRB no. 26702; Phoenix Veterans Affairs Health Care System IRB no. 01048.
Participants were randomized to receive ≤4 yr of double-blinded treatment with 0.45 mg/day o-CEE daily, 50 µg/day t-E2 twice weekly with both oral 200 mg/day micronized progesterone for the first 12 days of each month to protect the uterus, or placebo pills and patches. Institutional review boards (IRBs) at the participating sites approved the study procedures and all participants provided written, informed consent.
Of the 727 women participating in KEEPS, serum was evaluated from 362 women who had samples available and who were deemed compliant to treatment (by counts of pills and patches) from all enrollment centers. Androstenedione (ng/dL), testosterone (ng/dL), E2 (pg/mL), estrone (pg/mL), FSH (IU/L), and LH (IU/L) were measured in serum samples collected at baseline (before randomization) and 48 mo after randomization. The protocol for the blood drawing included that it was in the week before the addition of progesterone, so the progesterone effect contaminated the results least. Hormone levels were measured at the clinical core laboratory at the Mayo Clinic, Rochester, MN.
Seventeen β-estradiol and estrone were extracted with methylene chloride, and after derivatization with dansyl chloride, high-pressure liquid chromatography (HPLC) was used before introduction of the derivatized sample extract into the tandem mass spectrometry (LC-MS/MS) (Agilent Technologies, Santa Clara, CA). Testosterone was measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS; Agilent Technologies). FSH and LH were measured by respective, specific, two-site immunoenzymatic assays performed on a DxI 800 automated immunoassay system (Beckman Instruments, Chaska, MN). The intra-assay and interassay coefficients of variability (CVs) were within acceptable ranges for high-sensitivity testosterone, E2, estrone, FSH, and LH (Table 1).
Table 1.
Hormone intra- and interassay CVs
| Hormone | Intra-Assay CV | Interassay CV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17β-Estradiol, pg/mL | 0.23 | 0.50 | 0.74 | 35 | 151 | 405 | 0.29 | 0.50 | 0.77 | 32 | 140 | 382 |
| 17β-Estradiol variation, % | 11.8 | 7.3 | 6.0 | 1.6 | 1.5 | 1.4 | 10.8 | 8.5 | 6.9 | 5.1 | 4.6 | 4.8 |
| Estrone, pg/mL | 0.30 | 0.50 | 0.84 | 32 | 142 | 389 | 0.25 | 0.51 | 0.85 | 30 | 131 | 355 |
| Estrone variation, % | 17.8 | 7.5 | 6.1 | 2.5 | 1.7 | 1.2 | 12.0 | 9.5 | 7.9 | 7.4 | 7.1 | 6.6 |
| Follicle-stimulating hormone, mIU/mL | 8.6 | 47.1 | 6.5 | 16.7 | 58.0 | |||||||
| Follicle-stimulating hormone variation, % | 32. | 2.8 | 3.6 | 3.2 | 4.7 | |||||||
| Luteinizing hormone, mIU/mL | 1.2 | 38.5 | 1.4 | 15.6 | 48.8 | |||||||
| Luteinizing hormone variation, % | 4.3 | 4.0 | 9.3 | 6.0 | 6.0 | |||||||
| Testosterone, high sensitivity, ng/dL | 0.65 | 4.3 | 48 | 118 | 832 | 0.69 | 4.3 | 45 | 117 | 841 | ||
| Testosterone, high sensitivity, variation, % | 7.4 | 6.1 | 9.0 | 2.3 | 0.9 | 8.9 | 6.9 | 4.0 | 3.6 | 3.5 | ||
CV, coefficient of variability.
Androstenedione was assayed by adding a known amount of deuterated d7-androstenedione to a 0.1-mL plasma sample as an internal standard, followed by extraction of androstenedione and the internal standard using a solid-phase cartridge, from which they were eluted with methanol. The extracts were then dried down under nitrogen reconstituted with 75 µL of 70/30 methanol/H2O containing 1 µg/mL of estriol to prevent adsorption to equipment components and analyzed by liquid chromatography-tandem mass spectrometry using multiple-reaction monitoring (3, 11, 22, 37, 43, 46).
Data analysis.
Multiple regression analyses were conducted to examine the effects of o-CEE and t-E2 therapy on hormone levels in month 48, adjusting for baseline hormone levels. FSH and LH levels were normally distributed at both data collection time points, but androstenedione, testosterone, E2, and estrone measures required log transformation to normalize their distributions. The treatment variable was coded using two indicator variables: o-CEE (1 = yes, 0 = no) and t-E2 (1 = yes, 0 = no); the placebo group was the “reference” group represented by zeroes for all two indicator variables. To evaluate the influence of time since menopause on therapy response, time in months since menopause was included in the regression models. Linearity assumptions underlying the regression model were checked by using scatterplots and partial regression plots. Plots of the studentized residuals against the unstandardized predicted values were used to confirm homoscedasticity. Normality of residuals was inspected using Q-Q plots of the studentized residuals. An adjustment for multiple comparisons was taken into account using the Benjamini and Hochberg (5) method with a false discovery rate set at 0.05. All models were estimated using R 3.5.1 (35).
Exploring associations among gonadotropins and ovarian hormones: network analysis.
A network analysis technique (14, 16) was used to gain insight into the covariance of changes from baseline in hormone levels and describe the pattern of relationships within treatment groups. That is, the network approach allowed us to visualize the “interconnectivity” of the hormones or how these hormones are related among themselves within each HT group. It also permitted the identification of specific hormones that were more interconnected within group. The analysis produced a network of associations comprised of nodes representing hormone changes from baseline and edges representing the associations between the changes in hormones. The edges can directly be interpreted as non-zero partial correlation coefficients; thus, for example, the edge between hormone A and hormone B is their association after controlling for all other non-zero edges in the group. In the network plot, thicker (or saturated) edges represent stronger associations. Positive and negative associations were plotted in different colors to facilitate interpretation. The Fruchterman-Reingold algorithm (17) was used to plot nodes with more connections closer together. The partial correlations were estimated using a Gaussian Graphical Model and a least absolute shrinkage and selection operator (LASSO) (40) regularization technique that estimates all pairwise associations that are relevant (i.e., non-zero) to the underlying network structure (16) and the covariation in the data. The tuning parameter that was used with LASSO to control for the applied regularization was selected by minimizing the extended Bayesian information criterion (10, 15). The EBIC criterion was used to select the best-fitting regression function defining each node in the final network structure. Using 1,000 samples, we calculated nonparametric bootstrapped confidence intervals for the estimated edges (partial correlations). The structure of the multigroup networks was estimated using the software package igraph (22a). A residualized change model was used instead of a difference score model to estimate change from baseline using data from 362 participants with hormone data at both time points (baseline and month 48). That is, a linear regression model was applied using baseline hormone level as a predictor. This model has a higher power than difference scores (42, 48). The unstandardized residuals for each of the six hormones were used as outcomes to conduct the network analysis.
RESULTS
Of the 362 women who were compliant with treatment at 48 mo of the study, 109 were in the o-CEE group, 107 were in the t-E2 group, and 146 were in the placebo group. Demographics of the participating cohort in this analysis are presented in Table 2. The demographic and clinical characteristics of the sample included in the present study were not significantly different from the sample of participants enrolled in the KEEPS study who were noncompliant and/or missing hormone level data either at baseline or in month 48, Also, they did not differ in apolipoprotein E-ε4 (APOE-ε4) carrier status [χ2 (1), P = 0.728], ethnic/racial background [χ2(7), P = 0.051)], or years since menopause [t(723) = 0.171, P = 0.864]. The participants included in this study were slightly younger (M = 52.48, SD = 2.48) compared with those in the primary KEEPS study (52.88, SD = 2.63) [t(725) = 2.390, P = 0.017].
Table 2.
Participant demographics
| Characteristic | Total Sample | o-CEE | t-E2 | Placebo |
|---|---|---|---|---|
| Age [means (SD)] | 52.45 (2.49) | 52.51 (2.62) | 52.53 (2.50) | 52.35 (2.38) |
| Years since menopause at baseline [means (SD)] | 1.43 (0.719) | 1.48 (0.78) | 1.49 (0.65) | 1.35 (0.71) |
| Ethnicity [n (%)] | ||||
| Asian/Indian | 3 (0.9) | 2 (1.9) | 1 (0.7) | |
| Black/African American | 19 (5.5) | 4 (3.8) | 3 (3.1) | 12 (8.5) |
| Caucasian/White | 293 (85.4) | 91 (87.5) | 85 (86.7) | 117 (83.0) |
| Chinese | 4 (1.2) | 1 (1.0) | 2 (2.0) | 1 (0.7) |
| Filipino | 1 (0.3) | – | – | 1 (0.7) |
| Hispanic | 18 (5.2) | 5 (4.8) | 5 (5.1) | 8 (5.7) |
| Japanese | 1 (0.3) | 1 (1.0) | ||
| Other | 4 (1.2) | 1 (1.0) | 2 (2.0) | 1 (0.7) |
| APOE-ε4 [n (%)] | 86 (25.9) | 32 (32.8) | 29 (30.5) | 25 (18.0) |
APOE-ε4, apolipoprotein E-ε4; o-CEE, oral conjugated equine estrogen; t-E2, transdermal 17β-estradiol. Note that treatment groups did not differ in age (F = 0.22, P = 0.81), years since menopause at baseline (F = 1.50, P = 0.26), ethnic composition (χ2 = 12.14, P = 0.60), or educational attainment (χ2 = 14.70, P = 0.14). The proportion of APOE-ε4 carriers differed by treatment group (χ2 = 7.92, P = 0.02).
At baseline (before initiation of study treatment), both mean and median hormone levels were similar among the assigned treatment groups (Table 3). After 48 mo of treatment, observed hormone levels (Table 3) varied with treatment compared with placebo. Parameter estimates for the separate multiple regression models evaluating the differential effects of o-CEE and t-E2 therapy on individual hormone levels in month 48 after adjusting for baseline hormone levels are shown in Table 4. Compared with the placebo group, in month 48 the t-E2 group had significantly lower levels of FSH (β = −24.703, P < 0.001) and LH (β = −4.557, P < 0.001) and higher levels of estrone (β = 0.583, P < 0.001) and 17β-estradiol (β = 1.511, P < 0.001). Androstendione levels in month 48 were marginally higher for the E2 group compared with the placebo group, but the difference was not statistically significant after adjustment for multiple comparisons (P = 0.044 > 0.039). The o-CEE group had significantly higher levels of testosterone (β = 0.158, P < 0.001), as well as estrone (β = 1.123, P < 0.001) and E2 (β = 0.707, P < 0.001), than the placebo group. Androstenedione levels in the o-CEE group were similar to those of the placebo group. The levels of FSH in month 48 were significantly lower for the o-CEE treatment group (β = 19.360, P < 0.001), but LH levels did not differ (β = 0.198, P = 0.879) compared with placebo. There was a marginal average effect of time since menopause on FSH in month 48 (Table 3). However, interactions between treatment groups and time since menopause for FSH were not significant, indicating that time since menopause did not have an independent effect on hormone levels in month 48.
Table 3.
Hormone levels at baseline and month 48 by time since menopause at baseline and treatment group
| Baseline Hormone Levels |
Hormone Levels in Month 48 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Andro, ng/dL | FSH, IU/L | LH, IU/L | Testo, ng/dL | E1, pg/mL | E2, pg/mL | Andro, ng/dL | FSH, IU/L | LH, IU/L | Testo, ng/dL | E1, pg/mL | E2, pg/mL |
| 6–10.8 mo Since menopause (n = 114) | ||||||||||||
| o-CEE (n = 32) | ||||||||||||
| Mean | 75.61 | 77.78 | 36.94 | 20.74 | 42.94 | 25.07 | 66.00 | 62.91 | 34.79 | 21.50 | 103.32 | 15.72 |
| SD | 35.31 | 27.30 | 13.38 | 13.51 | 24.80 | 32.54 | 27.96 | 23.57 | 10.92 | 9.54 | 71.32 | 10.60 |
| t-E2 (n = 25) | ||||||||||||
| Mean | 62.96 | 80.64 | 38.28 | 16.00 | 36.60 | 26.03 | 69.50 | 60.95 | 30.74 | 17.56 | 52.16 | 44.09 |
| SD | 27.25 | 41.22 | 16.63 | 6.64 | 17.83 | 34.07 | 40.07 | 27.04 | 9.63 | 9.03 | 22.75 | 29.50 |
| Placebo (n = 56) | ||||||||||||
| Mean | 61.23 | 76.27 | 37.44 | 15.77 | 43.70 | 32.89 | 59.76 | 85.58 | 36.12 | 16.61 | 29.19 | 6.94 |
| SD | 26.94 | 32.39 | 16.82 | 6.03 | 34.67 | 54.23 | 22.84 | 31.89 | 14.33 | 11.08 | 12.83 | 3.48 |
| 12–21.6 mo Since menopause (n = 134) | ||||||||||||
| o-CEE (n = 42) | ||||||||||||
| Mean | 62.07 | 91.09 | 41.77 | 16.56 | 32.35 | 11.52 | 53.55 | 68.31 | 33.28 | 18.95 | 118.19 | 16.99 |
| SD | 25.29 | 32.58 | 14.48 | 7.42 | 14.87 | 14.76 | 20.92 | 33.47 | 14.38 | 8.66 | 118.16 | 10.98 |
| t-E2 (n = 45) | ||||||||||||
| Mean | 69.18 | 99.29 | 44.09 | 18.67 | 34.53 | 19.62 | 67.20 | 70.92 | 32.84 | 18.52 | 53.91 | 48.04 |
| SD | 34.77 | 40.23 | 14.24 | 10.10 | 20.48 | 30.82 | 36.07 | 35.94 | 12.29 | 9.78 | 28.05 | 44.56 |
| Placebo (n = 47) | ||||||||||||
| Mean | 57.21 | 83.51 | 39.23 | 18.04 | 31.82 | 12.89 | 55.32 | 81.92 | 32.74 | 22.05 | 28.79 | 7.58 |
| SD | 25.46 | 31.60 | 15.54 | 9.04 | 24.10 | 28.38 | 20.68 | 31.78 | 11.98 | 27.67 | 10.81 | 3.35 |
| 22.8–36 mo Since menopause (n = 119) | ||||||||||||
| o-CEE (n = 37) | ||||||||||||
| Mean | 52.51 | 105.99 | 43.88 | 16.17 | 25.92 | 6.79 | 54.00 | 74.41 | 38.49 | 21.34 | 93.47 | 13.72 |
| SD | 18.13 | 37.05 | 15.95 | 8.05 | 10.99 | 3.37 | 17.70 | 26.16 | 14.61 | 11.75 | 47.85 | 6.32 |
| t-E2 (n = 37) | ||||||||||||
| Mean | 53.92 | 97.49 | 42.42 | 14.87 | 26.88 | 11.24 | 58.19 | 60.08 | 29.82 | 15.37 | 51.54 | 47.89 |
| SD | 22.02 | 40.88 | 17.07 | 6.92 | 22.00 | 31.90 | 21.28 | 20.92 | 10.67 | 6.88 | 27.63 | 40.50 |
| Placebo (n = 45) | ||||||||||||
| Mean | 54.86 | 95.33 | 40.20 | 17.79 | 28.62 | 7.34 | 51.36 | 87.16 | 34.07 | 15.77 | 27.58 | 7.07 |
| SD | 24.15 | 35.02 | 17.05 | 16.18 | 10.93 | 3.79 | 25.10 | 35.55 | 13.54 | 7.34 | 11.13 | 4.45 |
Andro, androstenedione; E1, estrone; E2, estradiol; FSH, follicle-stimulating hormone; LH; luteinizing hormone; o-CEE, oral conjugated equine estrogen; SD, standard deviation; t-E2, transdermal estradiol; Testo, testosterone.
Table 4.
Model estimates by outcome measure after adding time since menopause (in mo)
| 95% CI |
||||||
|---|---|---|---|---|---|---|
| Model (Outcome) | Estimate | SE | t | P Value† | Lower | Upper |
| Model 1 (LnAndro_48) | ||||||
| (Intercept) | 1.709 | 0.176 | 9.701 | <0.001 | 1.363 | 2.056 |
| LnAndro_0 | 0.567 | 0.041 | 13.676 | <0.001 | 0.486 | 0.649 |
| o-Cee | −0.010 | 0.043 | −0.223 | 0.823 | −0.094 | 0.074 |
| t-E2 | 0.087 | 0.043 | 2.023 | 0.044 | 0.002 | 0.171 |
| Time since menopause | −0.001 | 0.002 | −0.619 | 0.536 | −0.005 | 0.003 |
| Model 2 (FSH_48) | ||||||
| (Intercept) | 43.524 | 3.993 | 10.899 | <0.001 | 35.670 | 51.377 |
| FSH_0 | 0.546 | 0.036 | 15.372 | <0.001 | 0.476 | 0.616 |
| o-Cee | −19.360 | 3.037 | −6.375 | <0.001 | −25.332 | −13.388 |
| t-E2 | −24.703 | 3.061 | −8.071 | <0.001 | −30.722 | −18.683 |
| Time since menopause | −0.308 | 0.149 | −2.062 | 0.040 | −0.601 | −0.014 |
| Model 3 (LH_48) | ||||||
| (Intercept) | 16.884 | 1.817 | 9.291 | <0.001 | 13.310 | 20.457 |
| LH_0 | 0.491 | 0.034 | 14.295 | <0.001 | 0.424 | 0.559 |
| o-Cee | 0.198 | 1.305 | 0.152 | 0.879 | −2.368 | 2.764 |
| t-E2 | −4.557 | 1.315 | −3.466 | <0.001 | −7.143 | −1.972 |
| Time Since Menopause | −0.097 | 0.063 | −1.535 | 0.126 | −0.221 | 0.027 |
| Model 4 (LnTesto_48) | ||||||
| (Intercept) | 0.726 | 0.125 | 5.803 | <0.001 | 0.480 | 0.972 |
| Lntesto_0 | 0.733 | 0.042 | 17.602 | <0.001 | 0.651 | 0.815 |
| o-Cee | 0.158 | 0.047 | 3.380 | <0.001 | 0.066 | 0.251 |
| t-E2 | 0.010 | 0.047 | 0.206 | 0.837 | −0.083 | 0.102 |
| Time since menopause | 0.001 | 0.002 | 0.202 | 0.840 | −0.004 | 0.005 |
| Model 5 (LnE1_48) | ||||||
| (Intercept) | 2.113 | 0.216 | 9.798 | <0.001 | 1.689 | 2.537 |
| LnE1_0 | 0.317 | 0.056 | 5.630 | <0.001 | 0.207 | 0.428 |
| o-Cee | 1.123 | 0.070 | 16.148 | <0.001 | 0.987 | 1.260 |
| t-E2 | 0.583 | 0.070 | 8.325 | <0.001 | 0.445 | 0.720 |
| Time since menopause | 0.005 | 0.003 | 1.411 | 0.159 | −0.002 | 0.012 |
| Model 6 (LnE2_48) | ||||||
| (Intercept) | 1.609 | 0.154 | 10.425 | <0.001 | 1.305 | 1.912 |
| LnE2_0 | 0.086 | 0.044 | 1.944 | 0.053 | −0.001 | 0.174 |
| o-Cee | 0.707 | 0.096 | 7.370 | <0.001 | 0.518 | 0.895 |
| t-E2 | 1.511 | 0.096 | 15.671 | <0.001 | 1.321 | 1.700 |
| Time since menopause | 0.004 | 0.005 | 0.777 | 0.437 | −0.006 | 0.013 |
CI, confidence interval; FSH, follicle stimulating hormone, LnAndro, natural logarithm of androstenedione; LH, luteinizing hormone; LnE1, natural logarithm of estrone; LnE2, natural logarithm of estradiol; o-CEE, oral conjugated equine estrogen; t-E2, transdermal estradiol; Testo, testosterone.
Unadjusted P values.
Exploratory network analysis results.
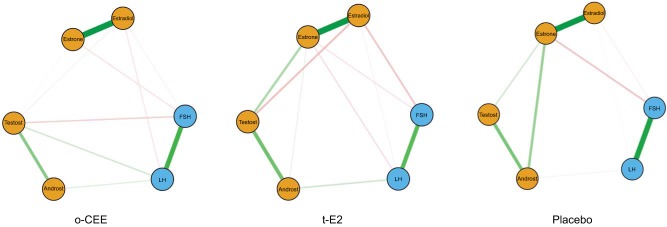
Associations among the changes in gonadotropins and steroid hormones after 48 mo of randomization differed by treatment assignment (Fig. 1), as tested by network analysis of the associations (partial correlations) among all of the hormones across HT groups. More hormone interconnectivity was observed in the t-E2 and the o-CEE groups compared with the placebo group. In the t-E2 group, changes in estrone levels showed more interconnection with other hormone levels, as demonstrated by more edges/lines in Fig. 1. In the placebo group, there was a stronger negative association between changes in estrone and FSH than in both the t-E2 and o-CEE groups. The association between estrone and testosterone was positive for all of the groups but was weaker in the o-CEE group.
Fig. 1.
Empirical network structure for pituitary and ovarian hormones. Blue circles represent pituitary hormones, and orange circles represent ovarian hormones. Thickness of the lines indicates the strength of the association between the variables. Thicker lines represent stronger associations. Line colors indicate the direction of the association; green lines are positive associations, and red lines are negative associations. o-CEE, oral conjugated equine estrogen; t-E2, transdermal 17β-estradiol.
In each of the three randomized groups, the associations among testosterone, androstenedione, and estrone were positive, and a slight positive association was seen between LH and androstenedione as well. Overall, the number of nonzero associations between the hormones was greater in the t-E2 group than in the placebo group, such that associations emerged in the t-E2 group that were not present in the placebo group. In the t-E2 group, the negative association between FSH and E2 was stronger, whereas the positive association between androstenedione and estrone was weaker, than that seen in the placebo group. There was a negative association in the t-E2 group between estrone and LH that was not noted in the placebo group. The association between estrone and androstenedione was weaker in the t-E2 group than in the placebo group. Additionally, in the t-E2 group, a negative association was found between testosterone and E2 that was not observed in the placebo group.
In the o-CEE group, the negative association between FSH and 17β-estradiol was weaker than the same association in the t-E2 group. Also, in the o-CEE group, a negative association was found between FSH and testosterone, whereas a positive association emerged between testosterone and LH; these effects were not seen in the other groups. Finally, a weak positive association was found between E2 and testosterone, whereas no association was found between estrone and androstenedione in the o-CEE group.
DISCUSSION
The current analysis provides the first graphical depiction and overview of the impact of two different types of HT on associations among circulating gonadotropic and reproductive hormones in recently menopausal women. The relationships among the changing hormone levels across two time points demonstrated expected negative feedback control between E2 and FSH. Time since menopause at enrollment did not appear to have an independent effect on hormone levels in month 48. Although the expected negative association between E2 and FSH was seen in both treated groups, there was a weaker association in the o-CEE group. o-CEE consists of numerous biologically active hormones, some of which are nonhuman (33). As seen in oral preparations, some of these components would undergo metabolism in the liver before reaching the peripheral circulation due to the gastric-hepatic circulation. The principle estrogen measured in the serum of the o-CEE group was estrone, which is less biologically active than E2. Thus, these estrogen metabolites may alter the relationship between E2 and FSH. It is not surprising that it is difficult to fully explicate the hormonal associations for the o-CEE group, given the interconversion between estrone and E2.
Many of the associations seen in the t-E2 group would be expected based on established hormonal regulatory processes in premenopausal women (38). For example, the associations among testosterone, androstenedione, and estrone were, as expected, positive, since androstenedione converts to estrone via the aromatase enzyme, and it is also an intermediate in the conversion of dehydroepiandrosterone (DHEA) to testosterone. Also, as expected, FSH levels were lower in month 48 than at baseline in both treatment groups compared with the placebo group. Other studies have shown associations between FSH levels and various disease states such as cardiovascular disease, diabetes, and adiposity (28, 30, 44, 45, 47). Elevated FSH levels are not known to have direct effects on these adverse outcomes in the postmenopausal state, but instead, elevated FSH levels seem more likely to be a marker of other causal factors. The greater reduction in circulating LH with t-E2 than with o-CEE treatment may have implications for preservation of cognitive function by HT (6), but further research is needed. It is unclear whether pituitary hormone levels and their association with outcomes are related solely to levels of hormone and/or to alterations in hypothalamic-pituitary-adrenal axis responsiveness.
In experimentally induced ovarian follicle-depleted rats, higher levels of circulating androstenedione were associated with cognitive deficits (1, 8). In the current study, androstenedione levels differed by treatment group; however, after adjustment, no statistically significant change was seen in the treatment groups compared with placebo in month 48. How circulating levels of androstenedione associate with cognitive function or menopausal symptoms in postmenopausal women is an important question that remains to be answered.
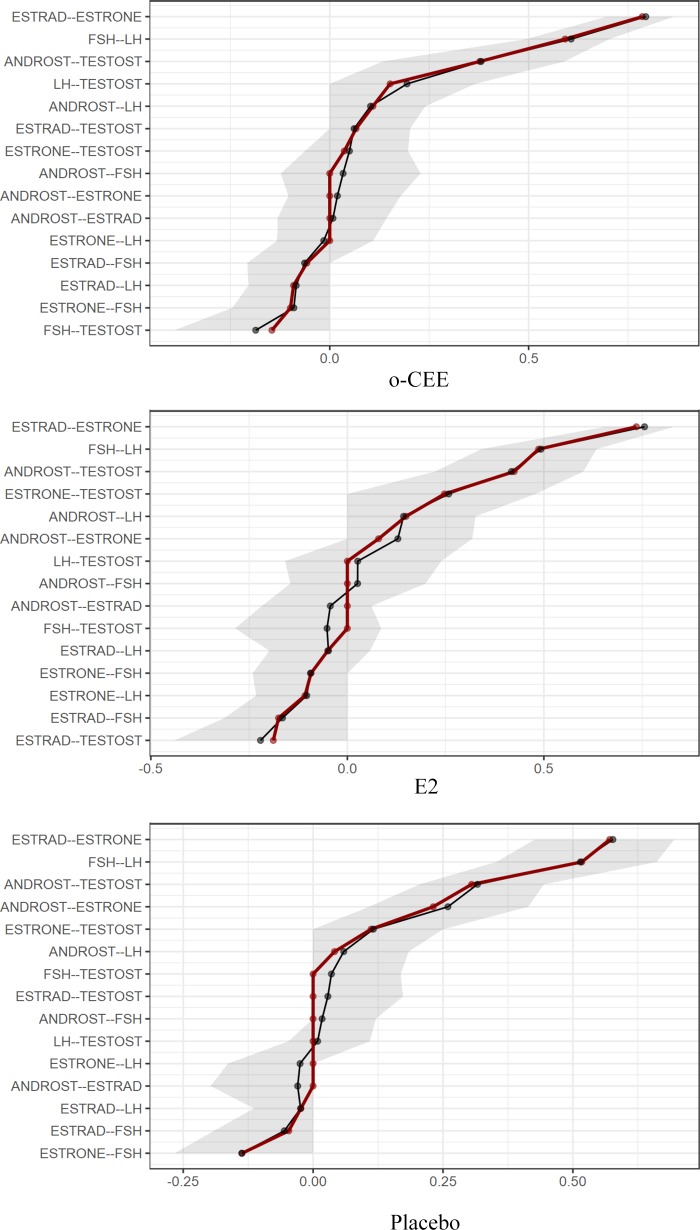
The exploratory graphical network analyses across treatment groups reveal very strong connections (partial correlations) between estrone and E2, testosterone, and androstenedione as well as LH and FSH. (The bootstrapped confidence intervals are shown in Fig. 2.) The first two relationships are not surprising from a biochemical point of view, given the equilibrium interconversion of the two estrogens and the fact that androstenedione is a precursor directly converted to testosterone. In the third case, FSH and LH are secretory products of the same pituitary cells (gonadotrophs) and can be secreted in tandem. Particularly striking is the observation of moderate to strong relationships of E2 to testosterone in the t-E2 group and of estrone to androstenedione in the placebo group, especially given that these relationships are reciprocally present, albeit weaker, in the opposite groups. These relationships may be explained by androstenedione being an immediate precursor of estrone and testosterone being an immediate precursor of E2. The relative strengths of the relationships may reflect the greater abundance of estrone in the placebo group and, after treatment, the greater abundance of E2 in the t-E2 group. It is noteworthy that neither of these estrogen-to-androgen relationships was detected in the o-CEE group most likely influenced by the o-CEE, resulting in high circulating levels that would interfere with feedback mechanisms. Collectively, these outcomes suggest that treatment with o-CEE disrupts the pathways connecting these two important classes of sex steroids.
Fig. 2.
Bootstrapped confidence intervals of estimated edges (partial correlations) for the estimated network of 6 hormones by randomized group. Black line indicates the bootstrapped values, red line shows the sample values, and gray area shows the bootstrapped confidence intervals. The x-axis shows the partial correlations. E2, 17β-estradiol; o-CEE, oral conjugated equine estrogen.
Because the relationships of the hormone associations in the network analysis are different within the three treatment groups, it is likely that the hormone treatments (or lack thereof in the placebo) have influenced the connections. Together, the observed differences in the hormonal network connections may help explain differences in both the short- and long-term outcomes associated with different HT formulations. For example, brain ventricular volumes increased and cortical volumes decreased to a greater extent in women on o-CEE than on placebo (26). Furthermore, in the subset of women who were APOE-ε4 carriers, those randomized to transdermal E2 had less accumulation of β-amyloid (a putative marker of Alzheimer’s disease) than those on placebo and o-CEE (25, 27). As expected, women treated with hormones report improvement in vasomotor symptoms and in the o-CEE group, an additional improvement in mood (21). Systematic study of the associated changes with cognitive and mood data could reveal which hormones, or relationships among hormones, associate with cognitive and mood changes.
Limitations.
Women enrolled in KEEPS had undergone natural menopause and had their uteruses and ovaries at the time of study; thus, the relationships noted here may not apply to women who underwent oophorectomy or hysterectomy before or after the age of natural menopause. Also, reproductive history was not collected on this cohort, nor was a history of other conditions that might have affected ovarian function before menopause (e.g., polycystic ovarian syndrome, endometriosis). Additionally, other hormones such as progesterone, thyroid hormones, sex hormone-binding globulin, and corticosteroids, which might also affect the interpretability of the observed associations and menopausal state of the gonadotropic-pituitary axis, were not included in this analysis. Because sex hormone-binding globulin was not included in the analysis, serum hormone levels reflect the total hormone level and not the free or bioavailable state. Finally, our findings are based on observation of associations; thus, the results are hypothesis generating and may set the stage for future studies that could lead to enhanced understanding of hormonal interactions in women, with and without exogenous hormone treatment, as they age.
Perspectives and Significance
Levels of ovarian and pituitary hormones and their interrelationships differ among women who have recently undergone natural menopause during administration of two common regimens of menopausal HT. Further studies evaluating the relationships among these changes in circulating hormones with other postmenopausal changes, such as cognition, neuroimaging, and menopausal symptomology, may help elucidate the mechanisms of differential effects of various HT regimens on these clinical outcomes. Importantly, further definition of the interrelationships among hormonal and clinical changes with HT could identify new approaches to managing menopause-related symptoms and pathophysiological events.
GRANTS
This work was supported in part by a Mayo Clinic Mentored Research Award as well as Grant National Institute on Aging (NIA) RF1 AG057547 to K. Kantarci, C. E. Gleason, and V. M. Miller; NIA AG-028084, ADHS14-052688, and the National Institutes of Health (NIH) Alzheimer’s Disease Centers P30-AG-019610 to H. A. Bimonte-Nelson; P50-AG-033514 to C. E. Gleason; and the Mayo Clinic Foundation for Research and Education. KEEPS was funded by grants from the Aurora Foundation to the Kronos Longevity Research Institute, from National Heart, Lung, and Blood Institute Grant HL-90639 to V. M. Miller, Mayo Clinic CTSA 1 UL1 RR024150, the Mayo Foundation, Brigham and Women’s Hospital/Harvard Medical School CTSA, CTSA UL1 RR024139 and UCSF CTSA UL1 RR024131 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH and NIH Roadmap for Medical Research.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official view of the NCATS or NIH. Study medications were supplied in part by Bayer Health Care and by Abbott Pharmaceuticals. Funding for FSH/LH assays was made available through support offered by the Department of Obstetrics, Gynecology, and Reproductive Sciences at Yale and the Benneck-Polan Family Foundation.
DISCLOSURES
Kejal Kantarci serves on the data safety monitoring board for Takeda Global Research and Development Center, Inc., and receives research support from Avid Radiopharmaceuticals and Eli Lilly and funding from NIH and the Alzheimer’s Drug Discovery Foundation. Other authors have nothing additional to disclose.
The Aurora Foundation, Bayer HealthCare, Abbott Pharmaceuticals, and Pfizer Pharmaceuticals had no input into the design or conduct of the study or the writing, review, or approval of this article.
AUTHOR CONTRIBUTIONS
J.M.K., H.A.B.-N., and V.M.M. conceived and designed research; N.M.D. performed experiments; N.M.D. analyzed data; J.M.K., N.M.D., H.A.B.-N., C.E.G., K.K., J.E.M., H.S.T., E.A.B., R.A.L., M.L.C., L.P., G.N.-P., F.N., S.M.H., and V.M.M. interpreted results of experiments; N.M.D. prepared figures; J.M.K. drafted manuscript; J.M.K., N.M.D., H.A.B.-N., C.E.G., K.K., J.E.M., H.S.T., E.A.B., R.A.L., M.L.C., L.P., G.N.-P., F.N., S.M.H., and V.M.M. edited and revised manuscript; J.M.K., N.M.D., H.A.B.-N., C.E.G., K.K., J.E.M., H.S.T., E.A.B., R.A.L., M.L.C., L.P., G.N.-P., F.N., S.M.H., and V.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
All serum assays were performed at the Immunochemical Core Laboratory at the Mayo Clinic, Rochester, MN.
We gratefully acknowledge the dedicated efforts of all of the investigators and staff at the KEEPS clinical centers, the KEEPS Data Coordinating Center at Kronos Longevity Research Institute (KLRI), and the National Institutes of Health (NIH) institutes supporting ancillary studies. Above all, we recognize and thank the KEEPS participants for their dedication and commitment to the KEEPS research program.
KEEPs Investigators and Staff are as follows: Albert Einstein College of Medicine: Genevieve Neal-Perry, Ruth Freeman, Hussein Amin, Barbara Isaac, Maureen Magnani, and Rachel Wildman; Brigham and Women’s Hospital/Harvard Medical School: JoAnn Manson, Maria Bueche, Marie Gerhard-Herman, Kate Kalan, Jan Lieson, Kathryn M. Rexrode, Barbara Richmond, Frank Rybicki, and Brian Walsh; Columbia College of Physicians and Surgeons: Rogerio Lobo, Luz Sanabria, Maria Soto, Michelle P. Warren, and Ralf C. Zimmerman; KLRI: S. Mitchell Harman, Mary Dunn, Panayiotis D. Tsitouras, and Viola Zepeda; Mayo Clinic: Virginia M. Miller, Philip A. Araoz, Rebecca Beck, Dalene Bott-Kitslaar, Sharon L. Mulvagh, Lynne T. Shuster, and Teresa G. Zais (deceased); University of California Los Angeles, CAC Reading Center: Matthew Budoff, Chris Dailing, Yanlin Gao, and Angel Solano; University of California San Francisco (UCSF) Medical Center: Marcelle I. Cedars, Nancy Jancar, Jean Perry, Rebecca S. Wong, Robyn Pearl, Judy Yee, Brett Elicker, and Gretchen A. W. Gooding; UCSF Statistical Center: Dennis Black, Eric Vittinghof, and Lisa Palermo; University of Southern California, Atherosclerosis Research Unit/Core Imaging and Reading Center: Howard N. Hodis, Yanjie Li, and Mingzhu Yan; University of Utah School of Medicine and Utah Lipid Center: Eliot A. Brinton, Paul N. Hopkins, M. Nazeem Nanjee, Kirtly Jones, Timothy Beals, and Stacey Larrinaga-Shum; Veterans Affairs Puget Sound Health Care System and University of Washington School of Medicine: George R. Merriam, Pamela Asberry, Sue Ann Brickle, Colleen Carney, Molly Carr, Monica Kletke, and Lynna C. Smith; Yale University School of Medicine: Hugh Taylor, Kathryn Czarkowski, Lubna Pal, Linda McDonald, Mary Jane Minkin, Diane Wall, and Erin Wolff (now at National Institute of Child Health and Human Development); others: Frederick Naftolin (New York University) and Nanette Santoro (University of Colorado).
REFERENCES
- 1.Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology 151: 3795–3804, 2010. doi: 10.1210/en.2010-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adashi EY. The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertil Steril 62: 20–27, 1994. doi: 10.1016/S0015-0282(16)56810-1. [DOI] [PubMed] [Google Scholar]
- 3.Allolio B, Arlt W. DHEA treatment: myth or reality? Trends Endocrinol Metab 13: 288–294, 2002. doi: 10.1016/S1043-2760(02)00617-3. [DOI] [PubMed] [Google Scholar]
- 4.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S; Women’s Health Initiative Steering Committee . Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 291: 1701–1712, 2004. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 5.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300, 1995. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 6.Bhatta S, Blair JA, Casadesus G. Luteinizing hormone involvement in aging female cognition: not all is estrogen loss. Front Endocrinol (Lausanne) 9: 544, 2018. doi: 10.3389/fendo.2018.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camp BW, Gerson JE, Tsang CW, Villa SR, Acosta JI, Blair Braden B, Hoffman AN, Conrad CD, Bimonte-Nelson HA. High serum androstenedione levels correlate with impaired memory in the surgically menopausal rat: a replication and new findings. Eur J Neurosci 36: 3086–3095, 2012. doi: 10.1111/j.1460-9568.2012.08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick MLO, Wactawski-Wende J, Watts NB; Women’s Health Initiative Investigators . Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA 290: 1729–1738, 2003. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Chen Z. Extended Bayesian information criteria for model selection with large model spaces. Biometrika 95: 759–771, 2008. doi: 10.1093/biomet/asn034. [DOI] [Google Scholar]
- 11.Collett-Solberg PF. Congenital adrenal hyperplasia: from genetics and biochemistry to clinical practice, Part 1. Clin Pediatr (Phila) 40: 1–16, 2001. doi: 10.1177/000992280104000101. [DOI] [PubMed] [Google Scholar]
- 12.Couzinet B, Meduri G, Lecce MG, Young J, Brailly S, Loosfelt H, Milgrom E, Schaison G. The postmenopausal ovary is not a major androgen-producing gland. J Clin Endocrinol Metab 86: 5060–5066, 2001. doi: 10.1210/jcem.86.10.7900. [DOI] [PubMed] [Google Scholar]
- 14.Epskamp S, Rhemtulla M, Borsboom D. Generalized network pschometrics: combining network and latent variable models. Psychometrika 82: 904–927, 2017. doi: 10.1007/s11336-017-9557-x. [DOI] [PubMed] [Google Scholar]
- 15.Foygel R, Drton M. Extended Bayesian information criteria for Gaussian graphical models. In: Advances in Neural Information Processing Systems 23 (NIPS 2010), edited by Lafferty JD, Williams CKI, Shawe-Taylor J, Zemel RS, and Culotta A. Red Hook, NY: Curran Associates, 2010, vol. 23, p. 604–612. [Google Scholar]
- 16.Fried EI, van Borkulo CD, Cramer AO, Boschloo L, Schoevers RA, Borsboom D. Mental disorders as networks of problems: a review of recent insights. Soc Psychiatry Psychiatr Epidemiol 52: 1–10, 2017. doi: 10.1007/s00127-016-1319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fruchterman TM, Reingold EM. Graph drawing by force-directed placement. Softw Pract Exper 21: 1129–1164, 1991. doi: 10.1002/spe.4380211102. [DOI] [Google Scholar]
- 18.Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, Brinton EA, Cedars MI, Lobo RA, Merriam GR, Neal-Perry G, Santoro NF, Taylor HS, Black DM, Budoff MJ, Hodis HN, Naftolin F, Harman SM, Asthana S. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med 12: e1001833, 2015. doi: 10.1371/journal.pmed.1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenblatt RB, Colle ML, Mahesh VB. Ovarian and adrenal steroid production in the postmenopausal woman. Obstet Gynecol 47: 383–387, 1976. [PubMed] [Google Scholar]
- 20.Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology 72: 1850–1857, 2009. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric 8: 3–12, 2005. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- 22.Ibáñez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche–normal variant or forerunner of adult disease? Endocr Rev 21: 671–696, 2000. doi: 10.1210/er.21.6.671. [DOI] [PubMed] [Google Scholar]
- 22a.igraph Igraph–The network analysis package. The igraph Core Team, 2003–2015http://http://igraph.sf.net. [Google Scholar]
- 23.Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, Yood RA, Watts NB, Robbins JA, Lewis CE, Beresford SA, Ko MG, Naughton MJ, Satterfield S, Bassford T; Women’s Health Initiative Investigators . Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the women’s health initiative randomized trial. J Bone Miner Res 21: 817–828, 2006. doi: 10.1359/jbmr.060312. [DOI] [PubMed] [Google Scholar]
- 24.Judd HL, Fournet N. Changes of ovarian hormonal function with aging. Exp Gerontol 29: 285–298, 1994. doi: 10.1016/0531-5565(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 25.Kantarci K, Lowe VJ, Lesnick TG, Tosakulwong N, Bailey KR, Fields JA, Shuster LT, Zuk SM, Senjem ML, Mielke MM, Gleason C, Jack CR, Rocca WA, Miller VM. Early postmenopausal transdermal 17β-estradiol therapy and amyloid-β deposition. J Alzheimers Dis 53: 547–556, 2016. doi: 10.3233/JAD-160258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kantarci K, Tosakulwong N, Lesnick TG, Zuk SM, Gunter JL, Gleason CE, Wharton W, Dowling NM, Vemuri P, Senjem ML, Shuster LT, Bailey KR, Rocca WA, Jack CR Jr, Asthana S, Miller VM. Effects of hormone therapy on brain structure: a randomized controlled trial. Neurology 87: 887–896, 2016. doi: 10.1212/WNL.0000000000002970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantarci K, Tosakulwong N, Lesnick TG, Zuk SM, Lowe VJ, Fields JA, Gunter JL, Senjem ML, Settell ML, Gleason CE, Shuster LT, Bailey KR, Dowling NM, Asthana S, Jack CR Jr, Rocca WA, Miller VM. Brain structure and cognition 3 years after the end of an early menopausal hormone therapy trial. Neurology 90: e1404–e1412, 2018. doi: 10.1212/WNL.0000000000005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohrt WM, Wierman ME. Preventing fat gain by blocking follicle-stimulating hormone. N Engl J Med 377: 293–295, 2017. doi: 10.1056/NEJMcibr1704542. [DOI] [PubMed] [Google Scholar]
- 29.Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Mühlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab 85: 645–651, 2000. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- 30.Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, Abu-Amer W, Izadmehr S, Zhou B, Shin AC, Latif R, Thangeswaran P, Gupta A, Li J, Shnayder V, Robinson ST, Yu YE, Zhang X, Yang F, Lu P, Zhou Y, Zhu LL, Oberlin DJ, Davies TF, Reagan MR, Brown A, Kumar TR, Epstein S, Iqbal J, Avadhani NG, New MI, Molina H, van Klinken JB, Guo EX, Buettner C, Haider S, Bian Z, Sun L, Rosen CJ, Zaidi M. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 546: 107–112, 2017. doi: 10.1038/nature22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meldrum DR, Davidson BJ, Tataryn IV, Judd HL. Changes in circulating steroids with aging in postmenopausal women. Obstet Gynecol 57: 624–628, 1981. [PubMed] [Google Scholar]
- 32.Mennenga SE, Koebele SV, Mousa AA, Alderete TJ, Tsang CW, Acosta JI, Camp BW, Demers LM, Bimonte-Nelson HA. Pharmacological blockade of the aromatase enzyme, but not the androgen receptor, reverses androstenedione-induced cognitive impairments in young surgically menopausal rats. Steroids 99: 16–25, 2015. doi: 10.1016/j.steroids.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Connell MB. Pharmacokinetic and pharmacologic variation between different estrogen products. J Clin Pharmacol 35: 18S–24S, 1995. doi: 10.1002/j.1552-4604.1995.tb04143.x. [DOI] [PubMed] [Google Scholar]
- 34.Odell WD, Swerdloff RS. Progestogen-induced luteinizing and follicle-stimulating hormone surge in postmenopausal women: a simulated ovulatory peak. Proc Natl Acad Sci USA 61: 529–536, 1968. doi: 10.1073/pnas.61.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2018. http://www.R-project.org/. [Google Scholar]
- 36.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women’s Health Initiative Investigators . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288: 321–333, 2002. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 37.Sciarra F, Tosti-Croce C, Toscano V. Androgen-secreting adrenal tumors. Minerva Endocrinol 20: 63–68, 1995. [PubMed] [Google Scholar]
- 38.Sherman BM, Korenman SG. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest 55: 699–706, 1975. doi: 10.1172/JCI107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sluijmer AV, Heineman MJ, De Jong FH, Evers JL. Endocrine activity of the postmenopausal ovary: the effects of pituitary down-regulation and oophorectomy. J Clin Endocrinol Metab 80: 2163–2167, 1995. doi: 10.1210/jcem.80.7.7608272. [DOI] [PubMed] [Google Scholar]
- 40.Tibshirani R. Regression shrinkage and selection via the lasso. J Royal Stat Soc B (Methodological) 58: 267–288, 1996. doi: 10.1111/j.2517-6161.1996.tb02080.x. [DOI] [Google Scholar]
- 41.Vermeulen A. The hormonal activity of the postmenopausal ovary. J Clin Endocrinol Metab 42: 247–253, 1976. doi: 10.1210/jcem-42-2-247. [DOI] [PubMed] [Google Scholar]
- 42.Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol 1: 6–9, 2001. doi: 10.1186/1471-2288-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Schnakenburg K, Bidlingmaier F, Knorr D. 17-hydroxyprogesterone, androstenedione, and testosterone in normal children and in prepubertal patients with congenital adrenal hyperplasia. Eur J Pediatr 133: 259–267, 1980. doi: 10.1007/BF00496086. [DOI] [PubMed] [Google Scholar]
- 44.Wang N, Kuang L, Han B, Li Q, Chen Y, Zhu C, Chen Y, Xia F, Cang Z, Zhu C, Lu M, Meng Y, Guo H, Chen C, Lin D, Lu Y. Follicle-stimulating hormone associates with prediabetes and diabetes in postmenopausal women. Acta Diabetol 53: 227–236, 2016. doi: 10.1007/s00592-015-0769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang N, Shao H, Chen Y, Xia F, Chi C, Li Q, Han B, Teng Y, Lu Y. Follicle-stimulating hormone, its association with cardiometabolic risk factors, and 10-year risk of cardiovascular disease in postmenopausal women. J Am Heart Assoc 6: e005918, 2017. doi: 10.1161/JAHA.117.005918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young WF., Jr Management approaches to adrenal incidentalomas. A view from Rochester, Minnesota. Endocrinol Metab Clin North Am 29: 159–185, 2000. doi: 10.1016/S0889-8529(05)70122-5. [DOI] [PubMed] [Google Scholar]
- 47.Zhu LL, Blair H, Cao J, Yuen T, Latif R, Guo L, Tourkova IL, Li J, Davies TF, Sun L, Bian Z, Rosen C, Zallone A, New MI, Zaidi M. Blocking antibody to the β-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci USA 109: 14574–14579, 2012. doi: 10.1073/pnas.1212806109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zumbo BD. The simple difference score as an inherently poor measure of change: some reality, much mythology. In: Advances in Social Science Methodology, edited by Thompson B. Greenwich, CT: JAI Press, 1999, vol. 5, p. 269–304. [Google Scholar]