Abstract
Muscle is a heterogeneous tissue composed of multiple fiber types. Earlier research revealed fiber type-selective postexercise effects on insulin-stimulated glucose uptake (ISGU) from insulin-resistant rats (increased for type IIA, IIB, IIBX, and IIX, but not type I). In whole muscle from insulin-resistant rats, the exercise increase in ISGU is accompanied by an exercise increase in insulin-stimulated AS160 phosphorylation (pAS160), an ISGU-regulating protein. We hypothesized that, in insulin-resistant muscle, the fiber type-selective exercise effects on ISGU would correspond to the fiber type-selective exercise effects on pAS160. Rats were fed a 2-wk high-fat diet (HFD) and remained sedentary (SED) or exercised before epitrochlearis muscles were dissected either immediately postexercise (IPEX) or at 3 h postexercise (3hPEX) using an exercise protocol that previously revealed fiber type-selective effects on ISGU. 3hPEX muscles and SED controls were incubated ± 100µU/mL insulin. Individual myofibers were isolated and pooled on the basis of myosin heavy chain (MHC) expression, and key phosphoproteins were measured. Myofiber glycogen and MHC expression were evaluated in muscles from other SED, IPEX, and 3hPEX rats. Insulin-stimulated pAktSer473 and pAktThr308 were unaltered by exercise in all fiber types. Insulin-stimulated pAS160 was greater for 3hPEX vs. SED on at least one phosphosite (Ser588, Thr642, and/or Ser704) in type IIA, IIBX, and IIB fibers, but not in type I or IIX fibers. Both IPEX and 3hPEX glycogen were decreased versus SED in all fiber types. These results provided evidence that fiber type-specific pAS160 in insulin-resistant muscle may play a role in the previously reported fiber type-specific elevation in ISGU in some, but not all, fiber types.
Keywords: exercise, fiber type, high-fat diet, insulin resistance, TBC1D4
INTRODUCTION
Type 2 diabetes is a global health epidemic, which costs the United States upward of $133 billion annually (27). Even in the absence of diabetes, insulin resistance is associated with many negative health outcomes (16). Skeletal muscle accounts for up to 85% of insulin-mediated glucose disposal, making it a prime target for combating insulin resistance (15). It is well documented that exercise can enhance insulin-stimulated glucose uptake into skeletal muscle from either healthy or insulin-resistant individuals (47–49, 56). The robust impact of exercise on insulin-stimulated glucose uptake by skeletal muscle has prompted the pursuit of mechanisms that link exercise to enhanced insulin sensitivity.
Extensive research has previously evaluated the potential effects of acute exercise on insulin signaling in skeletal muscle tissue as a possible mechanism for enhanced insulin sensitivity. The results of numerous studies suggest that insulin signaling events distal to Akt phosphorylation are likely important for increased insulin sensitivity after exercise (3, 11, 20, 21, 28, 32, 35, 44, 52, 58, 60, 66). In particular, the Rab GAP protein, AS160 (Akt substrate of 160 kDa; also known as TBC1D4), has emerged as a leading candidate for mediating the effects of prior exercise on insulin sensitivity. In an unphosphorylated state, AS160 is believed to restrain GLUT4 translocation by maintaining Rab proteins in an inactive GDP-bound state. Site-selective phosphorylation of AS160 results in GTP-bound Rab, leading to greater GLUT4 vesicle exocytosis (33) and increased glucose uptake. The insulin-stimulated phosphorylation of AS160 on Ser588 (11, 44, 52, 59) and Thr642 (20, 21, 52, 66) has been reported to be enhanced in skeletal muscle several hours after exercise, concomitant with elevated insulin-stimulated glucose uptake. In addition, the time course of the reversal of enhanced postexercise insulin-stimulated AS160 phosphorylation tracks with the reversal of glucose uptake (21). Recent research has shown that, at least in response to 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) or in situ contractions, AS160 is essential for increased insulin-stimulated glucose uptake by skeletal muscle (30). Taken together, these previous findings support the idea that greater pAS160Ser588 and pAS160Thr642 may be important for enhanced insulin sensitivity observed in the hours after exercise. Recent research has also reported increased insulin-stimulated pAS160Ser704 several hours after exercise in human and rat skeletal muscle (44, 54, 60, 62). These results are intriguing given that Kjøbsted et al. (32) found that mutating Ser704 on AS160 to Ala704, thereby preventing phosphorylation on this site, reduces the insulin-stimulated increase in pAS160Thr642 in mouse skeletal muscle. These observations suggest the possibility that phosphorylation of AS160Ser704 may contribute to enhanced phosphorylation of AS160Thr642 and insulin-stimulated glucose uptake (12, 50).
Although there is substantial evidence suggesting that increased site-selective AS160 phosphorylation may play a role in mediating the effect of exercise on insulin sensitivity, almost all of this previous research has been performed in whole muscle tissue. Skeletal muscle is a heterogeneous tissue composed of multiple fiber types, characterized by myosin heavy chain (MHC) isoforms that vary in metabolic and contractile properties (45). Recent research has indicated that the effect of acute exercise on insulin-stimulated glucose uptake is fiber type-specific in skeletal muscle from healthy rats (9). A subsequent study evaluated fiber type-selective AS160 phosphorylation in insulin-stimulated muscles from healthy rats and reported that those fiber types with exercise-induced improvement in insulin-stimulated glucose uptake were also characterized by greater AS160 phosphorylation (61). These findings provided evidence at the fiber type-specific and cellular levels consistent with the idea that AS160 plays a role in the increased insulin sensitivity after exercise in insulin-sensitive muscle. Although studying exercise’s effects on insulin sensitivity in healthy muscle is important, a more urgent need is to understand these processes in insulin resistance.
We recently found that a 2-wk high-fat diet (HFD) can induce significant insulin resistance in the whole rat epitrochlearis muscle (11, 42), but analysis of single fibers from rats revealed that the insulin resistance was not uniform across all fiber types (42, 43). Insulin resistance was detected in type II fibers (including IIA, IIAX, IIX, IIBX, and IIB), but not in type I fibers (42). Furthermore, when HFD-fed rats performed an acute bout of exercise, insulin-stimulated glucose uptake was increased in whole epitrochlearis muscles (11). However, this exercise-benefit on insulin-stimulated glucose uptake occurred only in type II fibers, and not in type I fibers in HFD-fed rats (43). Earlier research indicated that acute exercise can increase AS160 phosphorylation in muscles from insulin-resistant rats and humans (11, 44), but the effects of exercise on fiber type-specific AS160 phosphorylation have not been reported. The evidence that exercise effects on AS160 phosphorylation are fiber type-specific in muscles from healthy rats (61) and that exercise effects on insulin-stimulated glucose uptake is fiber type-specific in muscles from insulin-resistant rats (43), provides a clear rationale for evaluating fiber-type-specific signaling processes in insulin-resistant muscle after exercise. Therefore, our first aim was to evaluate fiber-type-specific effects of exercise (both immediately postexercise and 3-h postexercise with and without insulin) on the phosphorylation of key signaling proteins (AMPK, Akt, and AS160) that have been implicated in the regulation of exercise effects on skeletal muscle glucose uptake. We also evaluated the phosphorylation of acetyl CoA-carboxylase, an AMPK substrate, which is often used as an indicator of AMPK activity (5, 32, 34).
We recently found that type II fibers (including IIA, IIB, IIBX, and IIX) from HFD-fed versus low-fat diet (LFD)-fed rats had lower insulin-stimulated glucose uptake (42), and acute exercise resulted in elevated insulin-stimulated glucose uptake by each of the type II fibers from HFD-fed rats (43). Surprisingly, in HFD-fed rats, exercise did not result in enhanced insulin-stimulated glucose uptake by type I fibers, which was the only fiber type that did not become insulin resistant with the HFD (43). The exercise protocol resulted in a significant reduction in muscle glycogen in all fiber types of HFD-fed rats immediately postexercise, but glycogen concentration was not determined 3 h postexercise, when insulin-stimulated glucose uptake was measured (43). Previous research has suggested that the postexercise restoration of muscle glycogen concentration may be important for the reversal of the postexercise increase in insulin sensitivity (10, 21, 23). Therefore, it seemed possible that in type I fibers, glycogen concentration might have recovered by 3 h postexercise. Accordingly, our second aim was to determine fiber type-specific glycogen content in epitrochlearis muscles from HFD-fed rats both immediately and 3-h postexercise compared with sedentary (SED) controls.
Hexokinase II (HKII) abundance has been suggested to be important for insulin-stimulated glucose uptake by skeletal muscle (18). HKII mRNA expression (37, 46) and activity (37) of HKII have been shown to be increased in whole muscle several hours after one exercise session. Furthermore, HKII protein abundance is elevated in all fiber types after acute exercise in insulin-sensitive rats (61). Therefore, our final aim was to assess the abundance of HKII after acute exercise in different fiber types from insulin-resistant rat skeletal muscle.
METHODS
Materials.
The reagents and apparatus for SDS-PAGE, nonfat dry milk (no. 170-6404), and Clarity Max Western ECL substrate (no. 1705062) were from Bio-Rad (Hercules, CA). Tissue protein extraction reagent T-PER (no. PI78510), Simply Blue SafeStain (no. LC6065), Alexa Fluor 555 IgM (no. A-21043), Alexa Fluor 647 IgG1 (no. A-21240), Alexa Fluor 350 IgG2b (no. A-21140), and WGA-Alexa Fluor 488 (no. W11261) were from Thermo Fisher Scientific (Pittsburgh, PA). Anti-MHC type IIB IgM (no. BF-F3), anti-MHC type IIA IgG1 (no. SC-71), and anti-MHC type I IgG2b (no. BA-D5) were obtained from Developmental Studies Hybridoma Bank, University of Iowa (Iowa City, IA). PhosStop (no. 4906845001), protease inhibitor cocktail (no. P8340), periodic acid-Schiff (PAS) kit (no. 395B), and bovine liver glycogen type IX (no. G0885) were obtained from Sigma-Aldrich (St. Louis, MO). Anti-rabbit IgG horseradish peroxidase conjugate (no. 7074), anti-phospho Akt Ser473 (pAktSer473; no. 4060), anti-phospho Akt Thr308 (pAktThr308; no. 13038), anti-Akt (no. 4691), anti-phospho AS160 Thr642 (pAS160Thr642; no. 4288), anti-phospho AS160 Ser588 (pAS160Ser588; no. 8730), anti-AS160 (AS160; no. 2447), anti-phospho AMPKα Thr172 (pAMPKαThr172; no. 2535), anti-AMP-activated protein kinase-α (AMPKα; no. 2532), anti-acetyl CoA carboxylase (ACC; no. 3676), anti-phospho ACC Ser79 (pACCSer79; no. 3661), and anti-Hexokinase II (HKII; no. 2867) were from Cell Signaling Technology (Danvers, MA). Skeletal muscle expresses two isoforms of ACC (ACC1 and ACC2). ACC1 has a relatively low expression in skeletal muscle and is phosphorylated by AMPK on Ser79. ACC2 has a relatively high expression in skeletal muscle and is phosphorylated by AMPK on Ser212 (1). Since the pACCSer79 antibody (no. 3661) detects both pACC1Ser79 and pACC2Ser212 (38); hereafter, we refer to the results with this antibody as pACCSer79/212. Anti-phospho-AS160Ser704 was custom made by Capra Science (Ängelholm, Sweden).
Animal treatment and muscle preparation.
Procedures for animal care were approved by the University of Michigan Committee on Use and Care of Animals. Male Wistar rats (6–7 wk old; Charles River Laboratories, Wilmington, MA) were individually housed and were provided high-fat chow (Laboratory Diet no. D12492; Research Diets, New Brunswick, NJ) and water ad libitum for 2 wk until they were fasted on the night before the experiment at ~1700. Caloric intake for each rat during the 2-wk diet period was estimated on the basis of the difference between the food provided on day 1 and the food remaining at ~1700 on the night before the experiment. At ~0700 on the day of the experiment, rats either remained sedentary or swam in a barrel filled with water (35°C) for four 30-min bouts with 5 min of rest between bouts. Rats were anesthetized (intraperitoneal injection: 50 mg/kg ketamine, 5 mg/kg xylazine) either immediately postexercise (IPEX) or at 3 h postexercise (3hPEX) with time-matched sedentary controls. Rats were then weighed, and epitrochlearis muscles were dissected. After muscle dissections, the epididymal fat pads were dissected and weighed.
Muscle storage and incubation.
Muscles that were dissected IPEX (and sedentary controls) were immediately frozen in liquid N2 and stored at −80°C until further processing. In the 3hPEX experiment, after dissection, muscles underwent a two-step incubation process. First, muscles were incubated in media (Krebs Henseleit buffer, supplemented with 0.1% BSA, 2 mM sodium pyruvate, and 6 mM mannitol) for 30 min in glass vials gassed (95% O2-5% CO2) in a temperature-controlled bath (35°C) with or without 100 µU/mL insulin. Then muscles were transferred to other vials (35°C) containing fresh media at the same insulin concentration (0 or 100 µU/mL) for 20 min. After the second incubation, muscles were blotted, frozen in liquid N2, and stored at −80°C until further processing.
Freeze-drying and isolation of single muscle fibers.
Frozen muscles were processed as previously described (2, 61). In brief, muscles were freeze-dried for 48 h, and then individual fiber segments (~1–5 mm in length) were isolated under a dissecting microscope (EZ4D; Leica; Buffalo Grove, IL) using fine forceps at room temperature in a humidity-controlled room (30–35% humidity) to prevent freeze-dried fiber rehydration and possible alterations in protein phosphorylation status. Fibers were placed in individual PCR tubes and then in 6 µl of lysis buffer (T-PER, 1 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate tetrabasic decahydrate, 1 mM β-glycerophosphate, 1 μg/mL leupeptin, 1 mM phenylmethylsulfonyl fluoride, 100 μL/mL protease inhibitor cocktail, and 1 tablet/5 mL PhosSTOP) and 6 µL of 6× Laemmli sample buffer were added to each tube. Tubes were placed on ice, and fiber lysates were solubilized for at least 15 min before heating at 95–100°C for 5 min. Fiber lysates were then stored at −80°C until further processing.
Pooled freeze-dried fiber preparation and protein quantification.
An aliquot (6 µL) of each fiber lysate was used to determine fiber type by myosin heavy chain (MHC) isoform expression using SDS-PAGE, as previously described (36, 41). The remaining fiber lysate (6 µL) of each sample was then pooled with other samples from the same muscle that expressed the same fiber type. This resulted in five pools of lysed fiber from each muscle containing samples, which expressed type I, IIA, IIX, IIBX, and IIB MHC. Although type IIAX hybrid fibers were detected, there was an insufficient amount of fibers (and, thus, protein) for subsequent immunoblotting.
A total of 1,503 fibers isolated from 16 muscles (one muscle per rat in eight SED and eight IPEX rats) was analyzed for the IPEX experiment. The means ± SE for the number of pooled fibers used from each muscle of each fiber type from SED/IPEX groups were type I (12 ± 2/12 ± 1), type IIA (13 ± 2/12 ± 1), type IIX (24 ± 5/17 ± 3), type IIBX (14 ± 1/18 ± 2), and type IIB (35 ± 5/34 ± 4). A total of 4,084 fibers isolated from 40 muscles (paired muscles from each rat were incubated with or without insulin; 10 SED and 10 3hPEX rats) was analyzed for the 3hPEX experiment. The means ± SE for number of pooled fibers used from each muscle of each fiber type from SED no insulin/SED insulin and 3hPEX no insulin/3hPEX insulin groups were type I (16 ± 1/18 ± 1 and 18 ± 2/23 ± 2), type IIA (17 ± 2/20 ± 2 and 22 ± 2/23 ± 2), type IIX (21 ± 2/22 ± 2 and 25 ± 2/26 ± 2), type IIBX (20 ± 2/21 ± 2 and 23 ± 1/21 ± 2), and type IIB (38 ± 3/30 ± 3 and 39 ± 4/39 ± 3).
A 10-µL aliquot of each pooled sample was loaded on 10% SDS-PAGE gels to determine the MHC protein concentration. On each gel, there was a standard curve of known protein concentration (ranging from 0.2 µg to 3.2 µg) made from homogenized epitrochlearis muscle. After electrophoresis, gels were stained for 1 h using Simply Blue SafeStain and destained for 2–3 h in deionized water. Stained MHC band intensity was quantified by densitometry, and protein concentration was determined using the standard curve (R2 = 0.95–0.99).
Immunoblotting.
Samples were heated (95–100°C) for 5 min, an equal amount of pooled fiber lysate protein was subjected to SDS-PAGE, and then samples were transferred to polyvinylidene difluoride membranes. Following electrotransfer, gels were stained for 1 h (Simply Blue SafeStain) and destained for 2–3 h (deionized water); then MHC bands were quantified by densitometry to serve as loading controls. Membranes were blocked (3% BSA or nonfat milk in TBS-Tween (TBST) for 45 min at room temperature), incubated in appropriate primary antibodies (3% BSA or nonfat milk in TBST overnight at 4°C), and washed (3 × 5 min with TBST) before incubation in secondary antibody (3% BSA or nonfat milk in TBST for 1 h at room temperature). Membranes were then washed in TBST (3 × 5 min) and TBS (3 × 5 min) before exposure to Clarity Max Western ECL Substrate for 5 min. After imaging (Fluorchem E Imager, ProteinSimple, San Leandro, CA), proteins were quantified by densitometry, and each sample value was expressed relative to the normalized average of all samples on the blot and divided by the respective MHC loading control. Phosphorylated protein data were expressed as a ratio of phosphorylated to total protein. The signal for pAS160Ser588 in type I fibers was insufficient to quantify; therefore, the data are not shown.
Histochemical glycogen analysis.
External standards of known glycogen concentration were created using bovine liver glycogen type IX, as previously described (43, 51). Serial muscle cross sections (10 µm thick) were used for periodic acid-Schiff (PAS) staining and MHC detection. External standards of known glycogen concentrations (2, 10, 25, 50, 100, and 150 mmol/L) were used, as previously described (43, 51). Each PAS slide also contained these external glycogen standards (10 µm thick) to reliably compare staining of muscle sections on separate slides. Slides were fixed in 10% formalin, treated with PAS reagent, washed, and mounted, as previously described (43). MHC identification was performed using standard procedures, as previously described (6). Images of cross-sectionally oriented muscles were captured using a Keyence BZ-X700 microscope capable of fluorescent and bright-field illumination. DAPI, GFP, TRITC, and Cy5 filter cubes were used for the fluorescent detection of MHC isoforms and extracellular matrix at ×10 magnification. PAS-stained sections were captured using bright-field illumination at ×10 magnification and converted to 8-bit images for quantification of stain intensity. Image capture settings and conditions remained constant for PAS slide imaging to minimize variability between slides. Two researchers independently identified MHC isoform expression of all analyzed fibers to confirm accurate fiber typing. After fiber type was identified, the corresponding fibers in PAS images were manually traced and quantified by mean grayscale value using ImageJ.
Statistics.
Data are expressed as means ± SE, with two-tailed significance levels of α ≤ 0.05. Two-tailed t-tests were used to determine the exercise effect in IPEX samples compared with sedentary. Two-way ANOVA was used to determine the effect of exercise and insulin on protein phosphorylation or abundance in the 3hPEX experiments, and Bonferroni post-hoc tests were performed to identify the source of significant differences. Because glycogen was collected from multiple individual fibers per rat, we analyzed these data using mixed-effects linear regression models, incorporating fixed parameters evaluating the contributions of treatment group (SED, IPEX, or 3hPEX) interaction effects, and random y-intercepts to account for multiple observations within each rat. Analyses were performed using StataIC 14.2 (College Station, TX).
RESULTS
Signaling immediately postexercise.
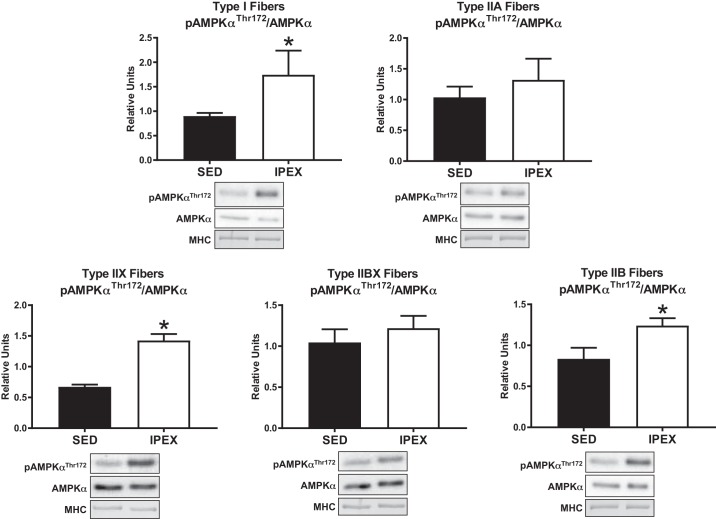
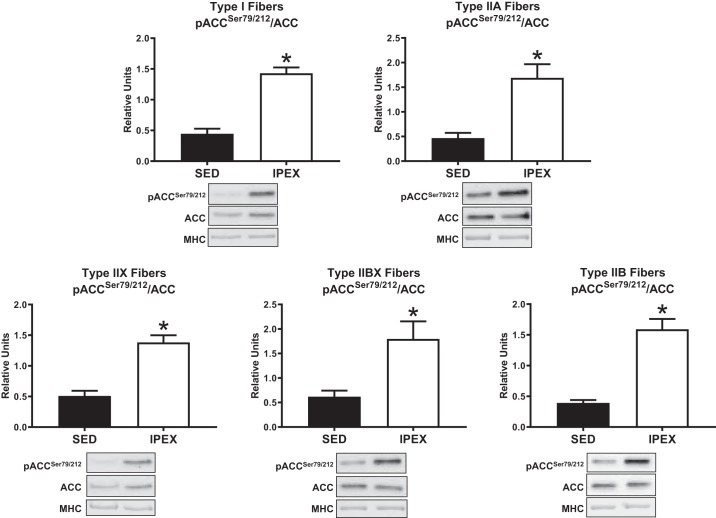
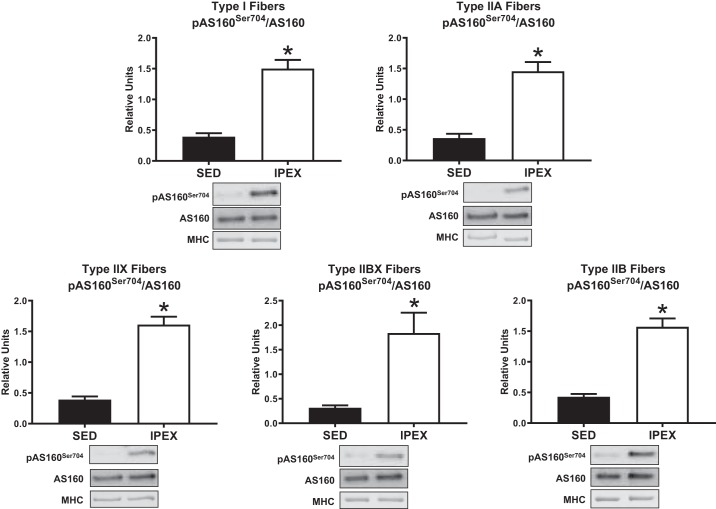
Phosphorylation of AMPKαThr172 divided by total AMPKα (pAMPKαThr172/AMPKα) was greater (P < 0.05) immediately postexercise (IPEX) compared with sedentary (SED) controls in type I, IIX, and IIB fibers (Fig. 1). There was no IPEX-induced increase in pAMPKαThr172/AMPKα in type IIA or IIBX fibers IPEX. The phosphorylation of ACCSer79/212 divided by total ACC (pACCSer79/212/ACC) was greater (P < 0.05) in all fiber types IPEX versus SED controls (Fig. 2). Phosphorylation of AS160Ser704 divided by total AS160 (pAS160Ser704/AS160) was greater (P < 0.01) in all fiber types IPEX versus SED controls (Fig. 3). The abundance of AMPKα, ACC, or AS160 was not significantly altered IPEX compared with SED in any fiber type (data not shown).
Fig. 1.
Fiber type-specific pAMPKαThr172/AMPKα immediately postexercise from high-fat diet-fed rats. *P < 0.05, indicates IPEX significantly greater than SED. Values are expressed as means ± SE; n = 7 or 8 per group. Representative blots and loading controls are included for each fiber type. IPEX, immediately postexercise; MHC, myosin heavy chain; SED, sedentary.
Fig. 2.
Fiber-type-specific pACCSer79/212/ACC immediately postexercise from high-fat diet-fed rats. *P < 0.05, indicates IPEX significantly greater than SED. Values are expressed as means ± SE; n = 6–8 per group. Representative blots and loading controls are included for each fiber type. IPEX, immediately postexercise; MHC, myosin heavy chain; SED, sedentary.
Fig. 3.
Fiber type-specific pAS160Ser704/AS160 immediately postexercise from high-fat diet-fed rats. *P < 0.01, indicates IPEX significantly greater than SED. Values are expressed as means ± SE; n = 7 or 8 per group. Representative blots and loading controls are included for each fiber type. IPEX, immediately postexercise; MHC, myosin heavy chain; SED, sedentary.
Insulin signaling 3 h postexercise.
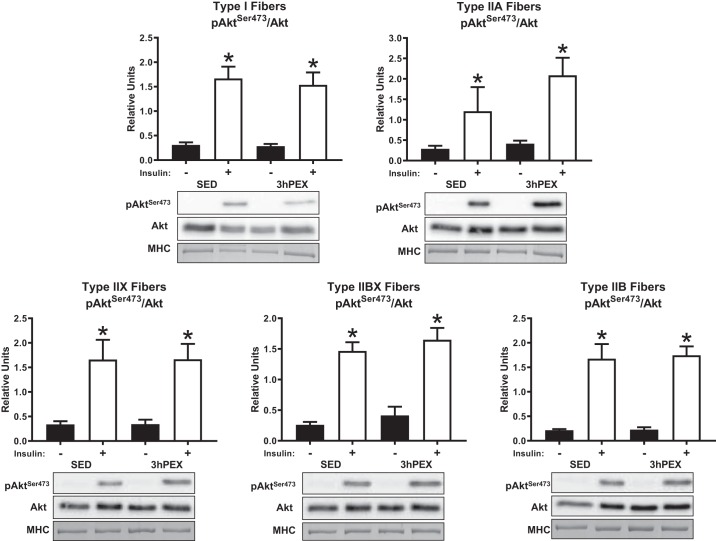
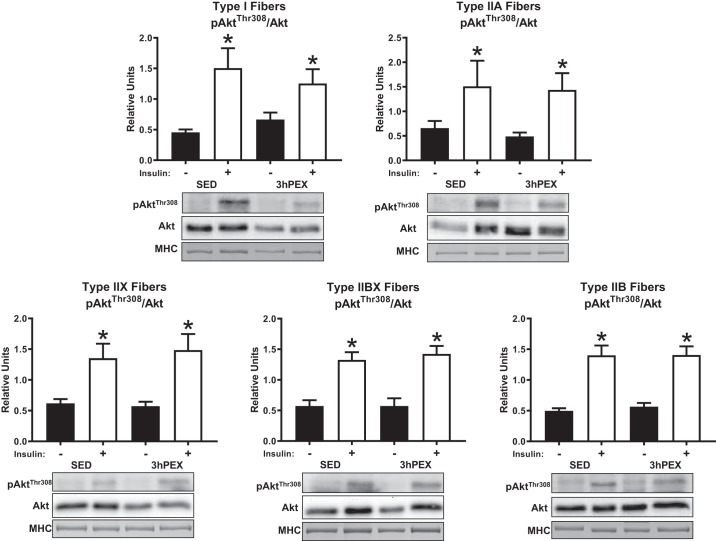
There was a main effect of insulin (P < 0.001) on the phosphorylation of AktSer473 divided by total Akt (pAktSer473/Akt) in all fiber types. Post hoc analyses revealed effects of insulin on pAktSer473/Akt within both SED and 3hPEX muscles of all fiber types (P < 0.05) (Fig. 4). In addition; there was a main effect of insulin (P < 0.005) on the phosphorylation of AktThr308 divided by total Akt (pAktThr308/Akt) in all fiber types. Post hoc analyses revealed effects of insulin on pAktThr308/Akt within both SED and 3hPEX muscles of all fiber types (P ≤ 0.05) (Fig. 5). We did not detect significant exercise-induced changes in Akt phosphorylation (on Ser473 or Thr308) in any fiber type. The abundance of Akt was not significantly altered 3hPEX compared with SED in any fiber type (data not shown).
Fig. 4.
Insulin-stimulated pAktSer473/Akt at 3hPEX vs. SED controls in different fiber types from high-fat diet-fed rats. *P < 0.05, indicates insulin significantly greater than no insulin within the same treatment group (SED or 3hPEX). Values are expressed as means ± SE; n = 7–10 per group. Representative blots and loading controls are included for each fiber type. 3hPEX, 3 h postexercise; MHC, myosin heavy chain; SED, sedentary.
Fig. 5.
Insulin-stimulated pAktThr308/Akt at 3hPEX vs. SED controls in different fiber types from high-fat diet-fed rats. *P ≤ 0.05, indicates insulin significantly greater than no insulin within the same treatment group (SED or 3hPEX). Values are expressed as means ± SE; n = 7–10 per group. Representative blots and loading controls are included for each fiber type. MHC, myosin heavy chain; SED, sedentary; 3hPEX, 3 h postexercise.
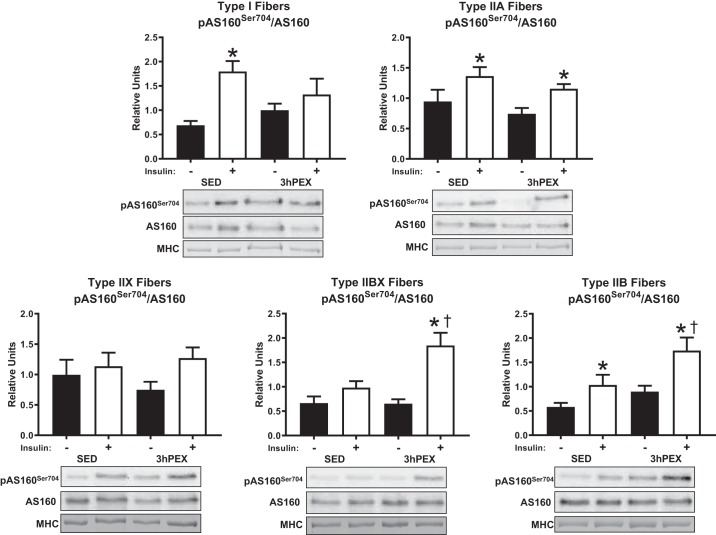
There was a main effect of insulin (P < 0.005) on the phosphorylation of AS160Ser704 divided by total AS160 (pAS160Ser704/AS160) in type I, IIA, IIBX, and IIB fibers. A main effect of exercise (P < 0.05) was detected for pAS160Ser704/AS160 in type IIBX and IIB fibers. An insulin by exercise interaction (P < 0.05) was detected for pAS160Ser704/AS160 in type I and IIBX fibers. Post hoc analyses revealed that insulin-stimulated pAS160Ser704/AS160 at 3hPEX was greater versus SED in type IIBX and IIB fibers (P < 0.05). The insulin-stimulated increase in pAS160Ser704/AS160 was significant in type I, IIA, and IIB fiber from SED rats (P < 0.05), and in type IIA, IIBX, and IIB fibers from 3hPEX rats (P < 0.05) (Fig. 6).
Fig. 6.
Insulin-stimulated pAS160Ser704/AS160 at 3hPEX vs. SED controls in different fiber types from high-fat diet fed rats. *P < 0.05, indicates insulin significantly greater than no insulin within the same treatment group (SED or 3hPEX). †P < 0.05, indicates 3hPEX significantly different than SED within insulin treated fibers. Values are expressed as means ± SE; n = 6–10 per group. Representative blots and loading controls are included for each fiber type. MHC, myosin heavy chain; SED, sedentary; 3hPEX, 3 h postexercise.
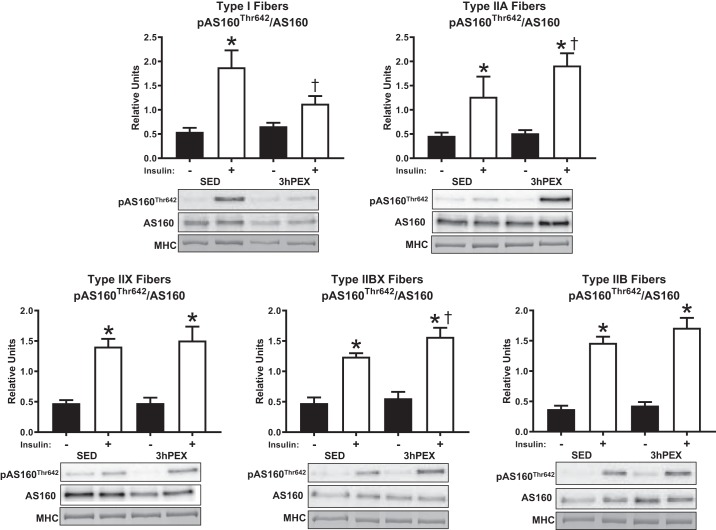
There was a main effect of insulin (P < 0.001) on the phosphorylation of AS160Thr642 divided by total AS160 (pAS160Thr642/AS160) in all fiber types at 3hPEX. An insulin by exercise interaction (P < 0.05) was detected for pAS160Thr642/AS160 in type I fibers. Post hoc analyses revealed that insulin-stimulated pAS160Thr642/AS160 at 3hPEX was greater versus SED in type IIA and IIBX fibers (P < 0.05) but was lower versus SED in type I fibers (P < 0.05). Insulin-stimulated pAS160Thr642/AS160 was greater in all fiber types from SED rats (P < 0.005), and in type IIA, IIX, IIBX, and IIB fibers from 3hPEX rats (P < 0.001) (Fig. 7).
Fig. 7.
Insulin-stimulated pAS160Thr642/AS160 at 3hPEX vs. SED controls in different fiber types from high-fat diet-fed rats. *P < 0.05, indicates insulin significantly greater than no insulin within the same treatment group (SED or 3hPEX). †P < 0.05, indicates 3hPEX significantly different than SED within insulin treated fibers. Values are expressed as means ± SE; n = 6–10 per group. Representative blots and loading controls are included for each fiber type. MHC, myosin heavy chain; SED, sedentary; 3hPEX, 3 h postexercise.
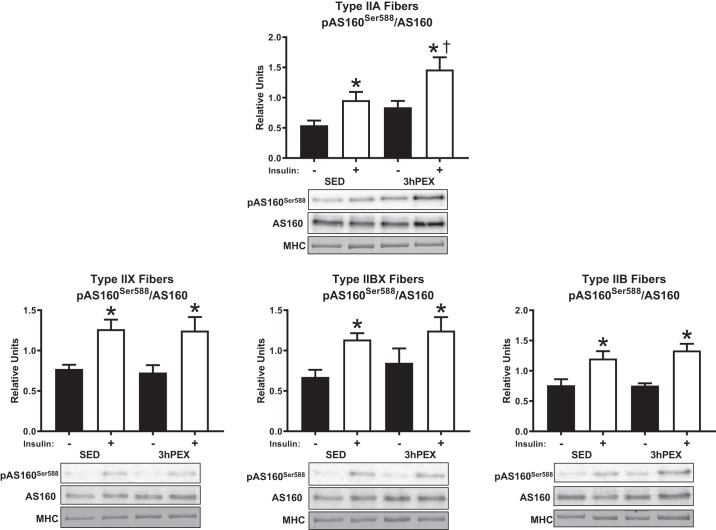
There was a main effect of insulin (P < 0.005) on the phosphorylation of AS160Ser588 divided by total AS160 (pAS160Ser588/AS160) in type IIA, IIX, IIBX, and IIB fibers at 3hPEX. The signal for pAS160Ser588 in type I fibers was insufficient to quantify; therefore, the data are not shown. A main effect of exercise (P < 0.01) was detected for pAS160Ser588/AS160 in type IIA fibers. Post hoc analyses revealed that insulin-stimulated pAS160Ser588/AS160 at 3hPEX was greater versus SED in type IIA fibers (P < 0.05). Insulin-stimulated pAS160Ser588/AS160 was greater in type IIA, IIX, IIBX, and IIB fiber from SED rats (P < 0.05), and 3hPEX rats (P < 0.05) (Fig. 8). The abundance of AS160 was not significantly altered 3hPEX compared with SED in any fiber type (data not shown).
Fig. 8.
Insulin-stimulated pAS160Ser588/AS160 at 3hPEX vs. SED controls in different fiber types from high-fat diet fed rats. The signal for pAS160Ser588 in type I fibers was insufficient to quantify; therefore, the data are not shown. *P < 0.05, indicates insulin significantly greater than no insulin within the same treatment group (SED or 3hPEX). †P < 0.05, indicates 3hPEX significantly different than SED within insulin treated fibers. Values are expressed as means ± SE; n = 7–10 per group. Representative blots and loading controls are included for each fiber type. MHC, myosin heavy chain; SED, sedentary; 3hPEX, 3 h postexercise.
Table 1 summarizes the acute exercise effect on fiber type-specific insulin-stimulated phosphorylation of Akt and AS160 in HFD-fed rats. Additionally, Table 1 presents the postexercise results for fiber type-specific insulin-stimulated glucose uptake from our previously published data in rats following the identical HFD and exercise protocol (43). There were striking fiber type-specific differences in the phosphorylation of various phosphosites of AS160.
Table 1.
Summary of the acute exercise effect on fiber type-specific-stimulated glucose uptake, pAkt, and pAS160 at 3hPEX in HFD rat epitochlearis muscle
| Glucose Uptake | pAktSer473 | pAktThr308 | pAS160Ser704 | pAS160Thr642 | pAS160Ser588 | |
|---|---|---|---|---|---|---|
| Type I fibers | ↔ | ↔ | ↔ | ↔ | ↓ | Insufficient Signal |
| Type IIA fibers | ↑ | ↔ | ↔ | ↔ | ↑ | ↑ |
| Type IIX fibers | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Type IIBX fibers | ↑ | ↔ | ↔ | ↑ | ↑ | ↔ |
| Type IIB fibers | ↑ | ↔ | ↔ | ↑ | ↔ | ↔ |
↑, ↓, and ↔ indicate that the insulin-stimulated value at 3 h postexercise (3hPEX) was significantly greater, significantly less, or unchanged compared with the insulin-stimulated value in the sedentary condition for the same fiber type, respectively. Reported changes in the fiber-type specific glucose uptake values are from our previously published article (43). HFD, high-fat diet.
Glycogen.
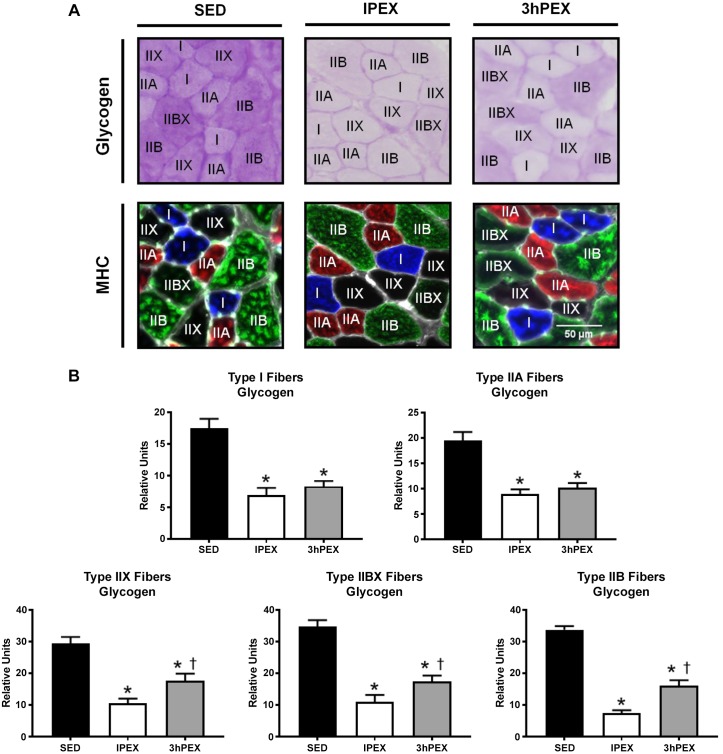
Glycogen was decreased (P < 0.001) at both IPEX and 3hPEX groups when compared with SED in all fiber types (Fig. 9). At 3hPEX versus IPEX, the type IIX, IIBX, and IIB fibers had greater glycogen values (P < 0.001).
Fig. 9.
Postexercise fiber type-specific glycogen content. A: representative images of serially cross-sectioned rat muscle for identification of glycogen content and fiber type. Glycogen content was determined by PAS stain intensity (darker stain represents higher glycogen content). MHC type I is shown in blue, MHC type IIA is shown in red, MHC type IIB is shown in green, and MHC type IIX is represented by the absence of signal (black). B: quantification of fiber type-specific glycogen content by PAS stain intensity. *P < 0.001, indicates SED significantly greater than both IPEX and 3hPEX. †P < 0.001, indicates 3hPEX significantly greater than IPEX. Values are expressed as means ± SE. The number of rats used for each group in this experiment was n = 6; 51–108 fibers/muscle were used to determine glycogen content. Numbers of fibers (SED, IPEX, 3hPEX) used for glycogen measurement of each fiber type were type I (n = 100, 76, 106), type IIA (n = 117, 133, 123), type IIX (n = 45, 83, 81), type IIBX (n = 42, 37, 54), type IIB (n = 146, 163, 175). MHC, myosin heavy chain; IPEX, immediately postexercise; PAS, periodic acid-Schiff; SED, sedentary; 3hPEX, 3 h postexercise.
Hexokinase II abundance.
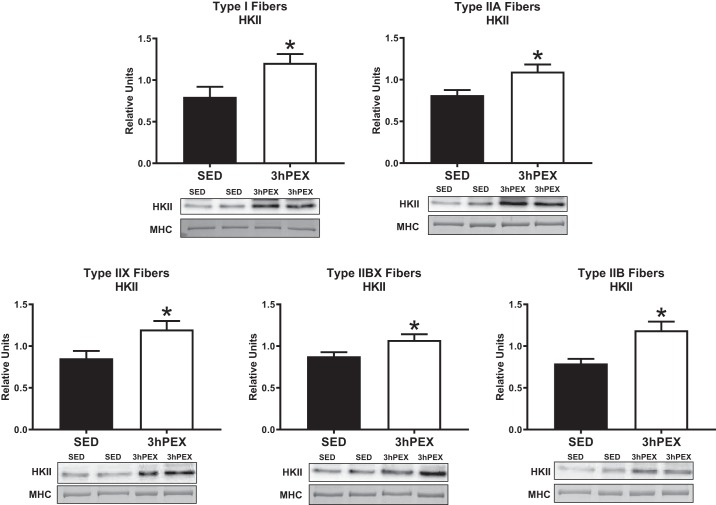
The abundance of hexokinase II (HKII) was greater (P < 0.05) at 3hPEX versus SED in all fiber types (Fig. 10).
Fig. 10.
Fiber-type-specific hexokinase II (HKII) abundance 3-h postexercise. *P < 0.05, indicates 3hPEX significantly greater than SED. Values are expressed as means ± SE; n = 12–20 per group. Representative blots and loading controls are included for each fiber type. MHC, myosin heavy chain; SED, sedentary; 3hPEX, 3 h postexercise.
DISCUSSION
Improved insulin-stimulated glucose uptake is a critical health benefit of exercise that is observed in both healthy and insulin-resistant skeletal muscle. The specific molecular and cellular processes responsible for this important outcome are not completely understood. In whole muscle tissue, increased AS160 phosphorylation has emerged as an attractive candidate for mediating the effects of exercise on insulin-stimulated glucose uptake. Recently, we observed in healthy rats that exercise-induced fiber type-specific increases in insulin-stimulated glucose uptake correspond to fiber type-specific insulin-stimulated AS160 phosphorylation on multiple sites (61). However, the mechanisms that lead to increased insulin-stimulated glucose uptake after exercise in insulin-resistant muscle fibers may differ from those in insulin-sensitive muscle. Therefore, we assessed the effects of exercise and insulin on signaling differences at a fiber type-specific level in HFD-fed rats using a diet protocol that we have repeatedly shown to produce skeletal muscle insulin resistance (11, 40, 42, 43). In different fiber types from rats fed a HFD, we discovered: 1) recruitment of all fiber types was strongly evidenced by decreased glycogen along with increased pACCSer79/212 and pAS160Ser704 for IPEX versus SED rats; 2) insulin-stimulated pAktSer473 and pAktThr308 were unaffected by exercise in all fiber types; 3) a fiber type-specific effect of exercise on insulin-stimulated pAS160Ser704, pAS160Thr642, and pAS160Ser588 for 3hPEX versus SED; 4) glycogen levels were lower for 3hPEX compared with SED in all fiber types; and 5) HKII abundance was greater in all fiber types for 3hPEX versus SED.
Multiple lines of evidence indicate that all fiber types were recruited by the exercise protocol. Glycogen levels were significantly reduced for all fiber types in IPEX compared with SED rats. We also observed increased pAMPKαThr172 IPEX in type IIB, IIX, and I fibers, but unaltered pAMPKαThr172 IPEX in type IIBX, and IIA fibers. Although pAMPKαThr172 was not elevated IPEX in every fiber type, the phosphorylation of AMPK may not completely reflect its enzymatic activity because AMPK can be allosterically activated by AMP binding (26). In this context, there is evidence that AMPK was activated in all fiber types from IPEX versus SED rats based on an increase in pACCSer79/212, an AMPK substrate and widely used indicator for greater AMPK activity (25, 53). Greater activation of AMPK was also evidenced by enhanced pAS160Ser704 in all fiber types from IPEX compared with SED rats. An alternative possible explanation for the increased pACCSer79/212 and pAS160Ser704 IPEX is that some other currently unidentified exercise-responsive kinase is responsible for the greater pACCSer79/212 and pAS160Ser704 in these fiber types. The current results, along with our earlier report that the same exercise protocol significantly increased insulin-independent glucose uptake in all fiber types of IPEX versus SED rats (43), provide substantial evidence that all fiber types were recruited by the exercise protocol. It is unlikely that the lack of an exercise-induced increase in AS160 phosphorylation at 3hPEX in type I fibers was attributable to a lack of type I fiber recruitment by the exercise protocol.
Our recent determination of both glucose uptake and fiber type in single fibers from rats undergoing the same diet and exercise treatment as in the current study revealed that the exercise effect on insulin-stimulated glucose uptake is not uniform across all fiber types (43). In that study, insulin-stimulated glucose uptake in type II fibers (IIA, IIB, IIBX and IIX) from HFD-3hPEX rats exceeded HFD-SED values, but no exercise effect on insulin-stimulated glucose uptake was evident for type I fibers (Table 1) (43). The current study offers new insights into possible reasons for the differential fiber-type specific increases in insulin-stimulated glucose uptake after acute exercise by HFD-fed rats. In type IIB, IIBX, and IIA fibers, we observed postexercise enhancement of insulin-stimulated AS160 phosphorylation on one or more phosphosites (IIB on Ser704; IIBX on Ser704 and Thr642; and IIA on Thr642 and Ser588) in HFD-fed rats at 3hPEX. The elevated AS160 phosphorylation may play a role in the enhanced postexercise insulin-stimulated glucose uptake found in these fiber types. In contrast, in type IIX fibers, the insulin-stimulated AS160 phosphorylation was unaltered after exercise on all of the measured phosphosites in HFD-fed rats. We previously observed the same lack of a postexercise effect on insulin-stimulated AS160 phosphorylation in type IIX fibers from low fat-fed rats (61). Together, these findings suggest that other mechanisms are responsible for the improved postexercise insulin-stimulated glucose uptake in IIX fibers. In type I fibers, the current study found decreased insulin-stimulated pAS160Thr642 and unaltered insulin-stimulated pAS160Ser704 after exercise in HFD-fed rats. The lack of an increase in insulin-stimulated AS160 site-selective phosphorylation in type I fibers after exercise may contribute to the absence of an effect of exercise on insulin-stimulated glucose uptake.
A striking observation from our previous study using the same diet and exercise protocol was that insulin-stimulated glucose uptake was not increased in type I fibers from HFD-3hPEX versus HFD-SED rats (43). Because it has been suggested that recovery of muscle glycogen after exercise may be important for the reversal of insulin-stimulated glucose uptake (10, 21, 23), it seemed possible that glycogen might have been rapidly restored in type I fibers from HFD-3hPEX rats. Accordingly, we tested this idea by measuring fiber type-specific glycogen values of epitrochlearis muscles from SED, IPEX, and 3hPEX rats. Glycogen levels at 3hPEX in type I fibers remained significantly below SED values and not different from IPEX levels. The current glycogen results together with the previously reported fiber type-specific glucose uptake data in HFD-fed rats after the same exercise protocol are consistent with the results from earlier research, suggesting that a sustained decrease in glycogen after exercise may be necessary, but is not sufficient for the postexercise increase in insulin sensitivity (29, 32).
Given that glycogen was not resynthesized to SED control levels at 3hPEX in type I fibers, what other possible mechanisms could account for the absence of improved insulin-stimulated glucose uptake in type I fibers? AMPK activation has been proposed to play an important role in triggering events that lead to insulin-stimulated glucose uptake. AMPK is a heterotrimeric protein complex composed of one catalytic (α) and two regulatory (β and γ) subunits. γ3-containing AMPK is essential for the effect of AICAR on insulin-stimulated glucose uptake (32). Furthermore, substantial evidence has consistently shown that γ3-containing AMPK is activated in response to exercise (5, 31, 34, 57) and remains elevated hours later concomitant with elevated insulin-stimulated glucose uptake in both chow-fed (62) and HFD-fed rats (40). It is possible that type I fibers from HFD-fed rats do not have sustained postexercise elevations in γ3-AMPK activity, which, in turn, might be responsible for the lack of a postexercise effect on insulin-stimulated glucose uptake. Accordingly, it will be important to evaluate γ3-AMPK activity in pooled fiber samples in future experiments.
Earlier research on rats with normal insulin sensitivity subjected to the same exercise protocol as used in the current study found that insulin-stimulated glucose uptake was increased at 3hPEX above SED values for type I, IIA, IIB, and IIBX fibers, but not for type IIX fibers (9). AS160 phosphorylation was also increased in each of the fiber types with postexercise elevation in insulin-stimulated glucose uptake (I, IIA, IIB, and IIBX), but not in type IIX fibers from 3hPEX compared with SED rats with normal insulin sensitivity (61). Thus, type IIA, IIB, and IIBX fibers from either insulin sensitive or insulin-resistant rats had increased postexercise insulin-stimulated glucose uptake concomitant with greater AS160 phosphorylation. In contrast, exercise effects on type I fibers were different for insulin-sensitive (both insulin-stimulated glucose uptake and AS160 phosphorylation increased) compared with insulin-resistant (insulin-stimulated glucose uptake unchanged and AS160 phosphorylation decreased) rats. However, the results for type I fibers in both diet groups support the idea that increased insulin-stimulated AS160 phosphorylation is linked to postexercise effects on insulin sensitivity. The results for type IIX fibers in insulin-sensitive rats provide further evidence for an association between greater insulin-stimulated AS160 phosphorylation and greater insulin-stimulated glucose uptake (i.e., neither was increased after exercise). Among the five fiber types evaluated in both normal and insulin-resistant rats, only type IIX fibers from HFD rats were characterized by greater insulin-stimulated glucose uptake for 3hPEX versus diet-matched SED controls without an exercise-induced improvement in AS160 phosphorylation. Taking together all of the observations from the earlier and current research provides considerable evidence for a relationship between postexercise effects on AS160 phosphorylation and glucose uptake in insulin-stimulated fibers.
What might account for the improved insulin-stimulated glucose uptake in type IIX fibers from the HFD-3hPEX rats in which insulin-stimulated AS160 phosphorylation was not further increased compared with the SED rats? Overexpression of HKII can lead to elevated postexercise glucose uptake in mouse skeletal muscle (19). In chow-fed rats with normal insulin sensitivity performing the same exercise protocol, as used in this study, HKII protein expression was either significantly increased (type I, IIB, IIBX, and IIX) or tended to increase (type IIA) at 3hPEX compared with SED controls (61). Similarly, HKII abundance of HFD-3hPEX rats exceeded HFD-SED values in all fiber types. Taken together, the exercise-induced increase in HKII abundance in type I fibers in the current results and the earlier studies indicating that insulin-stimulated glucose uptake was not increased in type I fibers of HFD-3hPEX rats suggests that increased HKII expression after exercise is not sufficient for the elevation in insulin-stimulated glucose uptake in type I fibers. However, it remains possible that greater HKII abundance has a role in the improved insulin-stimulated glucose uptake that was previously reported for IIX fibers, and possibly other type II fiber types, from HFD-3hPEX rats (43).
Although Akt plays a crucial role in regulating insulin-stimulated glucose uptake, insulin resistance can occur under some conditions by Akt-independent mechanisms (14). In this context, it is interesting that exercise did not significantly alter insulin-stimulated pAktSer473 or pAktThr308 in any fiber type, indicating that enhanced Akt phosphorylation was not responsible for the elevated insulin-stimulated glucose uptake in any of the fiber types. The lack of an effect of exercise on insulin-stimulated Akt phosphorylation in all fiber types is consistent with the results from previous studies that analyzed whole muscle tissue (7, 17, 24, 44, 60, 63). However, we recently observed in whole epitrochlearis muscle from rats undergoing the same diet and exercise protocols as the current study that insulin-stimulated pAktSer473 and pAktThr308 were increased at 3hPEX (40). Skeletal muscle includes many cell types other than muscle fibers, so whole muscle analysis reflects the sum total for all cell types. Other cell types that are included in whole skeletal muscle analysis (including adipocytes, smooth muscle cells, endothelial cells, nerve cells, fibroblasts, erythrocytes, platelets, and neutrophils) are known to express Akt (13, 22, 39, 55, 65, 67). Thus, interpreting together the results of the current study and our data from HFD-fed whole muscles (40), an intriguing possibility emerges: acute exercise may enhance Akt phosphorylation in cell types other than myofibers. Future research will be required to test this provocative idea.
TBC1D1 and AS160 are paralog proteins that share 50% identity (8). Therefore, it is reasonable to consider the possibility that TBC1D1 might play a role in postexercise improvement in insulin sensitivity. However, whereas AS160-null rats have substantial whole body insulin resistance and reduced insulin-stimulated glucose uptake by skeletal muscle (4), TBC1D1-null rats had normal whole body insulin tolerance, and unaltered insulin-stimulated GLUT4 translocation in skeletal muscle (64). Furthermore, there was no increase in TBC1D1 phosphorylation in insulin-stimulated rat epitrochlearis several hours postexercise (21). TBC1D1 phosphorylation was also not different for exercised compared with nonexercised insulin-stimulated muscles sampled from humans several hours after acute exercise (44). Substantial evidence has implicated a role for greater AS160 phosphorylation in the increased insulin-stimulated glucose uptake by skeletal muscle after acute exercise, but available information has not supported a similar role for TBC1D1.
By definition, glucose uptake is a cellular process, so evaluating fiber-type differences is essential to fully understand the regulation of glucose uptake. Previous data using whole muscle (3, 21, 52, 62) or single fibers (61) from insulin-sensitive rats demonstrated a strong relationship between increased AS160 phosphorylation and improved insulin-stimulated glucose uptake after exercise. This relationship was also observed in whole muscle from insulin-resistant rats (11). However, the lack of a uniform effect of prior exercise on insulin-stimulated AS160 phosphorylation across all fiber types in the current study using insulin-resistant muscle revealed novel and important information about the complexity of this relationship that could not have been discovered using only whole muscle analysis. These data provide further evidence for a possible role of AS160 in postexercise insulin-stimulated glucose uptake, but this relationship was notably absent in insulin-resistant type IIX fibers. The underlying biological basis for the different postexercise responses in various fiber types with regard to insulin-stimulated glucose uptake or pAS160 remains to be elucidated, but the current results indicate that the fiber type differences are not attributable to differences in postexercise effects on Akt phosphorylation, glycogen levels, or HKII abundance. The current results reveal the need for future research to test the possible causal relationships between postexercise outcomes (including site-selective AS160 phosphorylation, increased HKII abundance, and other postexercise events, such as γ3-AMPK activity) and postexercise insulin-stimulated glucose uptake in each fiber type from insulin-resistant animals.
GRANTS
These experiments were supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK-71771).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.W.P. and G.D.C. conceived and designed research; M.W.P., S.L.V.A., R.D., M.M.F., E.B.A., K.O., H.W., and J.T.T. performed experiments; M.W.P. analyzed data; M.W.P. and G.D.C. interpreted results of experiments; M.W.P. prepared figures; M.W.P. and G.D.C. drafted manuscript; M.W.P., S.L.V.A., R.D., M.M.F., E.B.A., K.O., H.W., J.T.T., and G.D.C. edited and revised manuscript; M.W.P., S.L.V.A., R.D., M.M.F., E.B.A., K.O., H.W., J.T.T., and G.D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Manak Singh and Jalal Almallouhi for technical assistance.
REFERENCES
- 1.Abu-Elheiga L, Almarza-Ortega DB, Baldini A, Wakil SJ. Human acetyl-CoA carboxylase 2. Molecular cloning, characterization, chromosomal mapping, and evidence for two isoforms. J Biol Chem 272: 10669–10677, 1997. doi: 10.1074/jbc.272.16.10669. [DOI] [PubMed] [Google Scholar]
- 2.Albers PH, Pedersen AJ, Birk JB, Kristensen DE, Vind BF, Baba O, Nøhr J, Højlund K, Wojtaszewski JF. Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes 64: 485–497, 2015. doi: 10.2337/db14-0590. [DOI] [PubMed] [Google Scholar]
- 3.Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab 292: E1191–E1200, 2007. doi: 10.1152/ajpendo.00602.2006. [DOI] [PubMed] [Google Scholar]
- 4.Arias EB, Zheng X, Agrawal S, Cartee GD. Whole body glucoregulation and tissue-specific glucose uptake in a novel Akt substrate of 160 kDa knockout rat model. PLoS One 14: e0216236, 2019. doi: 10.1371/journal.pone.0216236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birk JB, Wojtaszewski JF. Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J Physiol 577: 1021–1032, 2006. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7: e35273, 2012. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartee GD. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am J Physiol Endocrinol Metab 309: E949–E959, 2015. doi: 10.1152/ajpendo.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartee GD. Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle. Diabetologia 58: 19–30, 2015. doi: 10.1007/s00125-014-3395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartee GD, Arias EB, Yu CS, Pataky MW. Novel single skeletal muscle fiber analysis reveals a fiber type-selective effect of acute exercise on glucose uptake. Am J Physiol Endocrinol Metab 311: E818–E824, 2016. doi: 10.1152/ajpendo.00289.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol Endocrinol Metab 256: E494–E499, 1989. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- 11.Castorena CM, Arias EB, Sharma N, Cartee GD. Postexercise improvement in insulin-stimulated glucose uptake occurs concomitant with greater AS160 phosphorylation in muscle from normal and insulin-resistant rats. Diabetes 63: 2297–2308, 2014. doi: 10.2337/db13-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Wasserman DH, MacKintosh C, Sakamoto K. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab 13: 68–79, 2011. doi: 10.1016/j.cmet.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cong L-N, Chen H, Li Y, Zhou L, McGibbon MA, Taylor SI, Quon MJ. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol 11: 1881–1890, 1997. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 14.Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med 23: 804–814, 2017. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30: 1000–1007, 1981. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 16.Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab 86: 3574–3578, 2001. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- 17.Frøsig C, Sajan MP, Maarbjerg SJ, Brandt N, Roepstorff C, Wojtaszewski JF, Kiens B, Farese RV, Richter EA. Exercise improves phosphatidylinositol-3,4,5-trisphosphate responsiveness of atypical protein kinase C and interacts with insulin signalling to peptide elongation in human skeletal muscle. J Physiol 582: 1289–1301, 2007. doi: 10.1113/jphysiol.2007.136614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fueger PT, Hess HS, Bracy DP, Pencek RR, Posey KA, Charron MJ, Wasserman DH. Regulation of insulin-stimulated muscle glucose uptake in the conscious mouse: role of glucose transport is dependent on glucose phosphorylation capacity. Endocrinology 145: 4912–4916, 2004. doi: 10.1210/en.2004-0465. [DOI] [PubMed] [Google Scholar]
- 19.Fueger PT, Hess HS, Posey KA, Bracy DP, Pencek RR, Charron MJ, Wasserman DH. Control of exercise-stimulated muscle glucose uptake by GLUT4 is dependent on glucose phosphorylation capacity in the conscious mouse. J Biol Chem 279: 50956–50961, 2004. doi: 10.1074/jbc.M408312200. [DOI] [PubMed] [Google Scholar]
- 20.Funai K, Schweitzer GG, Castorena CM, Kanzaki M, Cartee GD. In vivo exercise followed by in vitro contraction additively elevates subsequent insulin-stimulated glucose transport by rat skeletal muscle. Am J Physiol Endocrinol Metab 298: E999–E1010, 2010. doi: 10.1152/ajpendo.00758.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab 297: E242–E251, 2009. doi: 10.1152/ajpendo.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grote CW, Groover AL, Ryals JM, Geiger PC, Feldman EL, Wright DE. Peripheral nervous system insulin resistance in ob/ob mice. Acta Neuropathol Commun 1: 15, 2013. doi: 10.1186/2051-5960-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulve EA, Cartee GD, Zierath JR, Corpus VM, Holloszy JO. Reversal of enhanced muscle glucose transport after exercise: roles of insulin and glucose. Am J Physiol Endocrinol Metab 259: E685–E691, 1990. doi: 10.1152/ajpendo.1990.259.5.E685. [DOI] [PubMed] [Google Scholar]
- 24.Hamada T, Arias EB, Cartee GD. Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise. J Appl Physiol (1985) 101: 1368–1376, 2006. doi: 10.1152/japplphysiol.00416.2006. [DOI] [PubMed] [Google Scholar]
- 25.Hardie DG. Regulation of fatty acid and cholesterol metabolism by the AMP-activated protein kinase. Biochim Biophys Acta 1123: 231–238, 1992. doi: 10.1016/0005-2760(92)90001-C. [DOI] [PubMed] [Google Scholar]
- 26.Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda) 29: 99–107, 2014. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang ES, Basu A, O’Grady M, Capretta JC. Projecting the future diabetes population size and related costs for the U.S. Diabetes Care 32: 2225–2229, 2009. doi: 10.2337/dc09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwabe M, Kawamoto E, Koshinaka K, Kawanaka K. Increased postexercise insulin sensitivity is accompanied by increased AS160 phosphorylation in slow-twitch soleus muscle. Physiol Rep 2: e12162, 2014. doi: 10.14814/phy2.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Solis RS, Arias EB, Cartee GD. Postcontraction insulin sensitivity: relationship with contraction protocol, glycogen concentration, and 5′ AMP-activated protein kinase phosphorylation. J Appl Physiol (1985) 96: 575–583, 2004. doi: 10.1152/japplphysiol.00909.2003. [DOI] [PubMed] [Google Scholar]
- 30.Kjøbsted R, Chadt A, Jørgensen NO, Kido K, Larsen JK, de Wendt C, Al-Hasani H, Wojtaszewski JFP. TBC1D4 is necessary for enhancing muscle insulin sensitivity in response to AICAR and contraction. Diabetes 68: 1756–1766, 2019. doi: 10.2337/db18-0769. [DOI] [PubMed] [Google Scholar]
- 31.Kjøbsted R, Pedersen AJ, Hingst JR, Sabaratnam R, Birk JB, Kristensen JM, Højlund K, Wojtaszewski JF. Intact regulation of the AMPK signaling network in response to exercise and insulin in skeletal muscle of male patients with type 2 diabetes: illumination of AMPK activation in recovery from exercise. Diabetes 65: 1219–1230, 2016. doi: 10.2337/db15-1034. [DOI] [PubMed] [Google Scholar]
- 32.Kjøbsted R, Treebak JT, Fentz J, Lantier L, Viollet B, Birk JB, Schjerling P, Björnholm M, Zierath JR, Wojtaszewski JF. Prior AICAR stimulation increases insulin sensitivity in mouse skeletal muscle in an AMPK-dependent manner. Diabetes 64: 2042–2055, 2015. doi: 10.2337/db14-1402. [DOI] [PubMed] [Google Scholar]
- 33.Klip A, Sun Y, Chiu TT, Foley KP. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am J Physiol Cell Physiol 306: C879–C886, 2014. doi: 10.1152/ajpcell.00069.2014. [DOI] [PubMed] [Google Scholar]
- 34.Kristensen DE, Albers PH, Prats C, Baba O, Birk JB, Wojtaszewski JF. Human muscle fibre type-specific regulation of AMPK and downstream targets by exercise. J Physiol 593: 2053–2069, 2015. doi: 10.1113/jphysiol.2014.283267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levinger I, Jerums G, Stepto NK, Parker L, Serpiello FR, McConell GK, Anderson M, Hare DL, Byrnes E, Ebeling PR, Seeman E. The effect of acute exercise on undercarboxylated osteocalcin and insulin sensitivity in obese men. J Bone Miner Res 29: 2571–2576, 2014. doi: 10.1002/jbmr.2285. [DOI] [PubMed] [Google Scholar]
- 36.Mizunoya W, Wakamatsu J, Tatsumi R, Ikeuchi Y. Protocol for high-resolution separation of rodent myosin heavy chain isoforms in a mini-gel electrophoresis system. Anal Biochem 377: 111–113, 2008. doi: 10.1016/j.ab.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 37.O’Doherty RM, Bracy DP, Osawa H, Wasserman DH, Granner DK. Rat skeletal muscle hexokinase II mRNA and activity are increased by a single bout of acute exercise. Am J Physiol Endocrinol Metab 266: E171–E178, 1994. doi: 10.1152/ajpendo.1994.266.2.E171. [DOI] [PubMed] [Google Scholar]
- 38.O’Neill HM, Lally JS, Galic S, Pulinilkunnil T, Ford RJ, Dyck JR, van Denderen BJ, Kemp BE, Steinberg GR. Skeletal muscle ACC2 S212 phosphorylation is not required for the control of fatty acid oxidation during exercise. Physiol Rep 3: e12444, 2015. doi: 10.14814/phy2.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park CS, Schneider IC, Haugh JM. Kinetic analysis of platelet-derived growth factor receptor/phosphoinositide 3-kinase/Akt signaling in fibroblasts. J Biol Chem 278: 37064–37072, 2003. doi: 10.1074/jbc.M304968200. [DOI] [PubMed] [Google Scholar]
- 40.Pataky MW. Mechanisms for exercise, high-fat diet, and muscle fiber-type effects on insulin-stimulated glucose uptake in skeletal muscle (doctoral dissertation). Ann Arbor, MI: School of Kinesiology, University of Michigan, 2019. https://deepblue.lib.umich.edu/handle/2027.42/151424. [Google Scholar]
- 41.Pataky MW, Arias EB, Cartee GD. Measuring both glucose uptake and myosin heavy chain isoform expression in single rat skeletal muscle fibers. Methods Mol Biol 1889: 283–300, 2019. doi: 10.1007/978-1-4939-8897-6_17. [DOI] [PubMed] [Google Scholar]
- 42.Pataky MW, Wang H, Yu CS, Arias EB, Ploutz-Snyder RJ, Zheng X, Cartee GD. High-fat diet-induced insulin resistance in single skeletal muscle fibers is fiber type selective. Sci Rep 7: 13642, 2017. doi: 10.1038/s41598-017-12682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pataky MW, Yu CS, Nie Y, Arias EB, Singh M, Mendias CL, Ploutz-Snyder RJ, Cartee GD. Skeletal muscle fiber type-selective effects of acute exercise on insulin-stimulated glucose uptake in insulin-resistant, high-fat-fed rats. Am J Physiol Endocrinol Metab 316: E695–E706, 2019. doi: 10.1152/ajpendo.00482.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pehmøller C, Brandt N, Birk JB, Høeg LD, Sjøberg KA, Goodyear LJ, Kiens B, Richter EA, Wojtaszewski JF. Exercise alleviates lipid-induced insulin resistance in human skeletal muscle-signaling interaction at the level of TBC1 domain family member 4. Diabetes 61: 2743–2752, 2012. doi: 10.2337/db11-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol 116: 1–76, 1990. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- 46.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol 546: 851–858, 2003. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest 69: 785–793, 1982. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol (1985) 66: 876–885, 1989. doi: 10.1152/jappl.1989.66.2.876. [DOI] [PubMed] [Google Scholar]
- 49.Ropelle ER, Pauli JR, Prada PO, de Souza CT, Picardi PK, Faria MC, Cintra DE, Fernandes MF, Flores MB, Velloso LA, Saad MJ, Carvalheira JB. Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation. J Physiol 577: 997–1007, 2006. doi: 10.1113/jphysiol.2006.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 51.Schaart G, Hesselink RP, Keizer HA, van Kranenburg G, Drost MR, Hesselink MK. A modified PAS stain combined with immunofluorescence for quantitative analyses of glycogen in muscle sections. Histochem Cell Biol 122: 161–169, 2004. doi: 10.1007/s00418-004-0690-0. [DOI] [PubMed] [Google Scholar]
- 52.Schweitzer GG, Arias EB, Cartee GD. Sustained postexercise increases in AS160 Thr642 and Ser588 phosphorylation in skeletal muscle without sustained increases in kinase phosphorylation. J Appl Physiol (1985) 113: 1852–1861, 2012. doi: 10.1152/japplphysiol.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott JW, Norman DG, Hawley SA, Kontogiannis L, Hardie DG. Protein kinase substrate recognition studied using the recombinant catalytic domain of AMP-activated protein kinase and a model substrate. J Mol Biol 317: 309–323, 2002. doi: 10.1006/jmbi.2001.5316. [DOI] [PubMed] [Google Scholar]
- 54.Sjøberg KA, Frøsig C, Kjøbsted R, Sylow L, Kleinert M, Betik AC, Shaw CS, Kiens B, Wojtaszewski JFP, Rattigan S, Richter EA, McConell GK. Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes 66: 1501–1510, 2017. doi: 10.2337/db16-1327. [DOI] [PubMed] [Google Scholar]
- 55.Suhr F, Brenig J, Müller R, Behrens H, Bloch W, Grau M. Moderate exercise promotes human RBC-NOS activity, NO production and deformability through Akt kinase pathway. PLoS One 7: e45982, 2012. doi: 10.1371/journal.pone.0045982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka S, Hayashi T, Toyoda T, Hamada T, Shimizu Y, Hirata M, Ebihara K, Masuzaki H, Hosoda K, Fushiki T, Nakao K. High-fat diet impairs the effects of a single bout of endurance exercise on glucose transport and insulin sensitivity in rat skeletal muscle. Metabolism 56: 1719–1728, 2007. doi: 10.1016/j.metabol.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 57.Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of α2β2γ1- but not α2β2γ3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab 292: E715–E722, 2007. doi: 10.1152/ajpendo.00380.2006. [DOI] [PubMed] [Google Scholar]
- 58.Treebak JT, Frøsig C, Pehmøller C, Chen S, Maarbjerg SJ, Brandt N, MacKintosh C, Zierath JR, Hardie DG, Kiens B, Richter EA, Pilegaard H, Wojtaszewski JF. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia 52: 891–900, 2009. doi: 10.1007/s00125-009-1294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Treebak JT, Pehmøller C, Kristensen JM, Kjøbsted R, Birk JB, Schjerling P, Richter EA, Goodyear LJ, Wojtaszewski JF. Acute exercise and physiological insulin induce distinct phosphorylation signatures on TBC1D1 and TBC1D4 proteins in human skeletal muscle. J Physiol 592: 351–375, 2014. doi: 10.1113/jphysiol.2013.266338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vendelbo MH, Møller AB, Treebak JT, Gormsen LC, Goodyear LJ, Wojtaszewski JF, Jørgensen JOL, Møller N, Jessen N. Sustained AS160 and TBC1D1 phosphorylations in human skeletal muscle 30 min after a single bout of exercise. J Appl Physiol (1985) 117: 289–296, 2014. doi: 10.1152/japplphysiol.00044.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Arias EB, Oki K, Pataky MW, Almallouhi JA, Cartee GD. Fiber type-selective exercise effects on AS160 phosphorylation. Am J Physiol Endocrinol Metab 316: E837–E851, 2019. doi: 10.1152/ajpendo.00528.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Arias EB, Pataky MW, Goodyear LJ, Cartee GD. Postexercise improvement in glucose uptake occurs concomitant with greater γ3-AMPK activation and AS160 phosphorylation in rat skeletal muscle. Am J Physiol Endocrinol Metab 315: E859–E871, 2018. doi: 10.1152/ajpendo.00020.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H, Sharma N, Arias EB, Cartee GD. Insulin signaling and glucose uptake in the soleus muscle of 30-month-old rats after calorie restriction with or without acute exercise. J Gerontol A Biol Sci Med Sci 71: 323–332, 2016. doi: 10.1093/gerona/glv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitfield J, Paglialunga S, Smith BK, Miotto PM, Simnett G, Robson HL, Jain SS, Herbst EAF, Desjardins EM, Dyck DJ, Spriet LL, Steinberg GR, Holloway GP. Ablating the protein TBC1D1 impairs contraction-induced sarcolemmal glucose transporter 4 redistribution but not insulin-mediated responses in rats. J Biol Chem 292: 16653–16664, 2017. doi: 10.1074/jbc.M117.806786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woulfe DS. Akt signaling in platelets and thrombosis. Expert Rev Hematol 3: 81–91, 2010. doi: 10.1586/ehm.09.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao Y, Sharma N, Arias EB, Castorena CM, Cartee GD. A persistent increase in insulin-stimulated glucose uptake by both fast-twitch and slow-twitch skeletal muscles after a single exercise session by old rats. Age (Dordr) 35: 573–582, 2013. doi: 10.1007/s11357-012-9383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu H, Littlewood T, Bennett M. Akt isoforms in vascular disease. Vascul Pharmacol 71: 57–64, 2015. doi: 10.1016/j.vph.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]