Abstract
Extracellular matrix hyaluronan is increased in skeletal muscle of high-fat-fed insulin-resistant mice, and reduction of hyaluronan by PEGPH20 hyaluronidase ameliorates diet-induced insulin resistance (IR). CD44, the main hyaluronan receptor, is positively correlated with type 2 diabetes. This study determines the role of CD44 in skeletal muscle IR. Global CD44-deficient (cd44−/−) mice and wild-type littermates (cd44+/+) were fed a chow diet or 60% high-fat diet for 16 wk. High-fat-fed cd44−/− mice were also treated with PEGPH20 to evaluate its CD44-dependent action. Insulin sensitivity was measured by hyperinsulinemic-euglycemic clamp (ICv). High-fat feeding increased muscle CD44 protein expression. In the absence of differences in body weight and composition, despite lower clamp insulin during ICv, the cd44−/− mice had sustained glucose infusion rate (GIR) regardless of diet. High-fat diet-induced muscle IR as evidenced by decreased muscle glucose uptake (Rg) was exhibited in cd44+/+ mice but absent in cd44−/− mice. Moreover, gastrocnemius Rg remained unchanged between genotypes on chow diet but was increased in high-fat-fed cd44−/− compared with cd44+/+ when normalized to clamp insulin concentrations. Ameliorated muscle IR in high-fat-fed cd44−/− mice was associated with increased vascularization. In contrast to previously observed increases in wild-type mice, PEGPH20 treatment in high-fat-fed cd44−/− mice did not change GIR or muscle Rg during ICv, suggesting a CD44-dependent action. In conclusion, genetic CD44 deletion improves muscle IR, and the beneficial effects of PEGPH20 are CD44-dependent. These results suggest a critical role of CD44 in promoting hyaluronan-mediated muscle IR, therefore representing a potential therapeutic target for diabetes.
Keywords: extracellular matrix, hyaluronan, insulin resistance
INTRODUCTION
Extracellular matrix (ECM) remodeling is attracting considerable interest in the pathogenesis of several metabolic disorders such as cancer (48), obesity, insulin resistance, and diabetes (33, 36). Chronic inflammation in response to high-fat (HF) diet leads to increased deposition of ECM components such as collagens and hyaluronan (7, 23–25, 42). Hyaluronan, a major glycosaminoglycan component of the ECM, is increased in skeletal muscle of insulin-resistant mice, possibly because of increased activity of hyaluronan synthase or decreased degradation by hyaluronidase (24). Reduction of muscle hyaluronan by a long-acting PEGylated human recombinant hyaluronidase PH-20 enzyme (PEGPH20) has previously been shown to ameliorate skeletal muscle insulin resistance in HF-fed mice (24). Apart from muscle, increased hyaluronan deposition also occurs in a variety of tissues in diabetic rodents, including aorta (16), pancreatic islets (10, 54), and kidneys (32). In addition, elevated hyaluronan content is also seen in serum of patients with type 2 diabetes (37, 38).
CD44 is the main hyaluronan receptor, which is present in most tissues including adipose tissue, muscle, liver, pancreas, and endothelium (3, 29). The main biological functions of CD44 are cell aggregation, angiogenesis, endothelial cell proliferation, and immune cell migration and activation (45). An expression-based genome-wide association study revealed that cd44 gene is implicated in the molecular pathogenesis of type 2 diabetes (29). Previous studies have positively correlated CD44 expression in adipose tissue/liver and CD44 levels in serum with insulin resistance, adipose tissue inflammation, and hepatic steatosis in diet-induced obese mice and humans who are obese (18, 29, 30, 34). Genetic deletion of CD44 and CD44-receptor antagonism using anti-CD44 monoclonal antibody has been shown to reduce obesity-mediated glucose and insulin tolerance in HF-fed mice (22, 30). CD44 antagonism also displayed anti-diabetic effects in nonobese diabetic mice (54).
Taken together, increased hyaluronan and CD44 expression are implicated in the pathogenesis of insulin resistance and type 2 diabetes. However, research so far has mainly focused on the role of CD44 in adipose inflammation and hepatic steatosis, with little research into the role of CD44 signaling in insulin resistance, specifically in skeletal muscle. The present study aimed at deciphering the link between hyaluronan and CD44 interaction and skeletal muscle insulin resistance. Therefore, the hypotheses that 1) genetic deletion of CD44 prevents diet-induced skeletal muscle insulin resistance and 2) improved insulin resistance by PEGPH20 treatment is dependent on the presence of CD44 were tested in the present study. Global cd44-deficient mice and their wild-type littermates fed either chow diet or HF diet were used to study the potential role of CD44 in insulin-resistant skeletal muscle. HF-fed cd44-deficient mice were also treated with PEGPH20 to evaluate its CD44-dependent action. Insulin action was measured by hyperinsulinemic-euglycemic clamp (ICv) coupled with radioisotopes in stress-free mice using the dual, arterial-venous catheter protocol (5).
MATERIALS AND METHODS
Mouse models.
Mice lacking cd44 (cd44−/−) were obtained on a C57BL/6J background from Jackson Laboratory (Maine; stock no. 005085). The homozygous cd44-null mice (cd44−/−) and their wild-type littermate controls (cd44+/+), both males and females, were fed either an HF diet (60% calories as fat, BioServ; product no. F3282) or kept on a chow diet (13% calories as fat, LabDiet; product no. 5001) starting at 3 wk of age for 16 wk. All mice were studied at 19 wk of age. Body composition was determined by EchoMRI (Echo Medical Systems, TX). ICvs were performed to measure insulin sensitivity. All animals were housed in an air-conditioned room at 22 ± 2°C with a 12-h light/12-h dark cycle. Animal experiments were carried out in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986, the Vanderbilt Animal Care and Use Committee, and the University of Dundee ethics committee.
Chronic PEGPH20 treatment.
HF-fed cd44−/− (60% calories as fat, Special Diet Services, UK; product no. 824054) mice (18 wk old) received injections of either vehicle (10 mmol/L histidine, 130 mmol/L NaCl at pH 6.5) or PEGPH20 (Halozyme Therapeutics, San Diego, CA) at 1 mg/kg through the tail vein, once every 3 days for 24 days (q3dx9). Body weight was monitored at 3-day intervals. Food intake, locomotor activity, respiratory exchange ratio (RER), and energy expenditure (EE) were assessed for 48 h, using Comprehensive Laboratory Animal Monitoring System metabolic chambers (Columbus Instruments, OH). Oral glucose tolerance test (2 g/kg body wt) was performed on the 18th treatment day. Intraperitoneal insulin tolerance test (0.75 U/kg body wt) and ICv were performed at the end of the 24-day treatment period on different cohorts of mice.
Hyperinsulinemic-euglycemic clamp using a dual arterial-venous catheter protocol.
Catheters were implanted in the carotid artery and jugular vein of mice for sampling and intravenous infusions (4 mU·kg−1·min−1 insulin, donor erythrocytes and [3-3H]glucose), 5–7 days before the ICv (5). ICv was performed on 5-h fasted mice as described previously (25). Glucose flux rates were determined by isotope dilution using [3-3H]glucose. All mice were clamped at euglycemia using a variable glucose infusion rate (GIR). Euglycemia during ICv was achieved by assessments of arterial glucose every 10 min and adjustments of GIR as needed. Blood was collected during steady state at 80–120 min for the determination of [3-3H]-glucose. Clamp insulin was determined at t = 100 and 120 min. At 120 min, 0.481 MBq [14C]2-deoxyglucose was administered as an intravenous bolus. Blood was taken from 2 to 35 min for arterial [14C]2-deoxyglucose determination. After the last sample, mice were culled and tissues were excised.
Plasma and tissue sample processing for isotopic analysis.
Plasma and tissue radioactivity of [3-3H]glucose, [14C]2-deoxyglucose, and [14C]2-deoxyglucose-6-phosphate was determined by liquid scintillation counting (4). Whole body glucose turnover rates, including glucose appearance (Ra) and glucose disappearance (Rd) rates, were determined using non-steady-state equations as described by Steele et al. (49). Endogenous glucose appearance (EndoRa) was determined by subtracting the GIR from total Ra. The glucose metabolic index (Rg), which measured glucose uptake into specific tissues, was calculated as previously described (31).
Non-steady-state calculation of glucose flux.
where Ra, Rd indicate glucose appearance and disappearance rates (mg·kg−1·min−1); I indicates tracer infusion rate (disintegrations/min); Vd indicates volume distribution of glucose; A indicates concentration of glucose (mg/dL); SA indicates specific activity of glucose (disintegrations·min−1·mg−1); and T indicates time (min).
Tissue-specific glucose uptake calculation.
where Rg is glucose uptake, [14C]2DGP is 14C-2-deoxyglucose phosphate, AUC is area under the curve, and [14C]2DG is 2-deoxyglucose.
Biochemical analysis.
Blood was collected in EDTA-coated tubes at the time points indicated in the figures. Blood glucose was measured directly using a handheld Ascensia Contour Blood Glucose Meter (Bayer Healthcare). For plasma insulin and non-esterified fatty acid (NEFA) analyses, blood samples were immediately centrifuged using a microcentrifuge for 1 min at 16,000 g and stored at −20°C. Plasma insulin and NEFA concentrations were measured using commercially available ELISA (90060, Crystal Chem) and enzymatic colorimetric NEFA C assay (436–91995, Wako Chemicals, Germany) kits per manufacturer’s instructions.
Immunohistochemistry.
Hyaluronan and CD31 were assessed by immunohistochemistry in paraffin-embedded gastrocnemius sections (5 μm) with the following primary antibodies: biotinylated hyaluronan-binding protein (AMS.HKD-BC41, AMS Biotechnology, UK) or anti-CD31 (NBP1–49805, Novus Biologicals, UK). Slides were lightly counterstained with Mayer’s hematoxylin. The EnVision +HRP/DAB System (DakoCytomation) was used to produce visible staining. Ten to twelve images per animal were used in the quantification of hyaluronan and CD31 staining. Bowel samples were used as positive control for CD31 staining. To confirm the specificity of hyaluronan immunohistochemistry, sections were treated with or without recombinant human hyaluronidase PH-20.
The negative controls without primary antibodies were carried out in parallel for the staining of both hyaluronan and CD31. All staining procedures and image analysis were carried out in a blinded manner. Images were captured using camera mounted on an Axiovision microscope (Zeiss Axioscope, Germany). Immunostaining was quantified by the ImageJ Software. Hyaluronan content was measured by the integrated intensity of staining. Muscle vascularity was determined by counting CD31-positive structures.
Western blotting.
Gastrocnemius, superficial vastus lateralis (SVL), and liver were homogenized as described previously (25). Protein (40–70 μg) was applied to SDS-PAGE gel. The following antibodies were used to detect respective proteins using Western blotting: CD44 (cat. no. AF6127, 1:1,000; R&D Systems, UK) (21); p-Akt(Ser473) (9), p-Akt(Thr308) (9), total Akt (9), p-GSK-3β(Ser9) (43), total GSK-3β (53) (cat. nos. 9271, 4056, 9272, 8566, and 5676; 1:1,000; Cell Signaling, UK); and insulin-degrading enzyme (IDE) (55) (cat. no. ab32216, 1:1,000; Abcam, UK). GAPDH (19) (cat. no. 5174, 1:1,000; Cell Signaling) and Ponceau were used as loading controls.
Statistical analysis.
Data are expressed as mean ± SE. Statistical analyses were performed using Student’s t test or two-way ANOVA followed by Bonferroni post hoc test as appropriate. Groups of data were significantly different if P < 0.05.
RESULTS
CD44 protein expression in muscle.
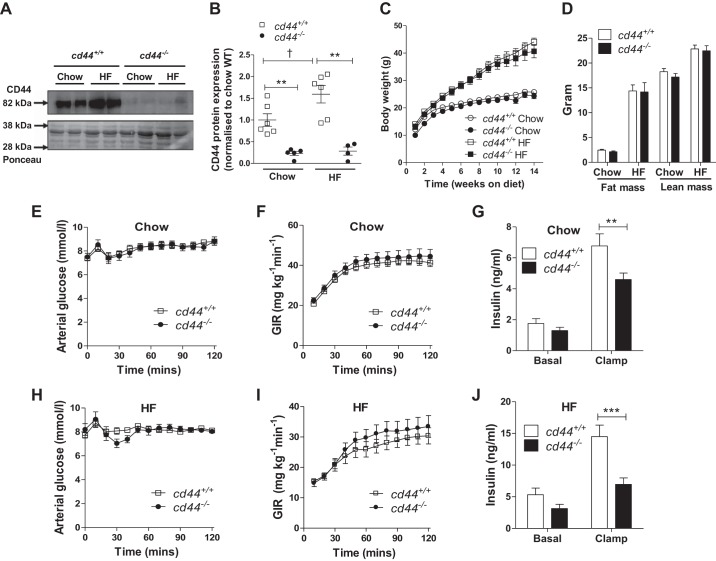
Global deletion of cd44 gene significantly reduced protein expression of CD44 in muscle of chow- and HF-fed mice (Fig. 1, A and B). HF feeding increased the muscle CD44 protein (~1.6-fold) in cd44+/+ mice when compared with lean cd44+/+ mice (Fig. 1, A and B).
Fig. 1.
Protein expression of CD44 in skeletal muscle, growth curve, body composition, and hyperinsulinemic-euglycemic clamp (ICv) measurements in chow-fed and high-fat (HF)-fed mice. A and B: CD44 protein expression was measured in superficial vastus lateralis muscle harvested from male mice following ICv. A: representative bands. B: quantitative data. Data were normalized to Ponceau staining and presented as fold of chow-fed cd44+/+ mice. (n = 4–6 male mice). C and D: mice were fed either chow or HF-diet starting at 3 wk of age. C: body weights were monitored weekly, up to 14 wk on respective diets [n = 12 (5–7 females, 5–7 males) for chow-fed mice and n = 11–13 (4–6 females, 5–9 males) for HF-fed mice]. D: body composition (fat mass and lean mass) was determined at 18 wk of age [n = 12 (5–7 females, 5–7 males) for chow-fed mice and n = 8–13 (3–4 females, 5–9 males) for HF-fed mice]. ICvs were performed in chow-fed and HF-fed mice to determine insulin action. E and H: arterial glucose level. F and I: glucose infusion rate (GIR); 50% glucose was infused to maintain euglycemia throughout the ICv. G and J: insulin levels were measured in chow-fed and HF-fed mice at baseline (−5) and during the ICv clamp (100 and 120). E–J: n = 12 (5–7 females, 5–7 males) for chow-fed mice and n = 11–13 (4–6 females, 5–9 males) for HF-fed mice. All data are represented as mean ± SE. Data represent values from pooled female and male mice. Statistical analysis was performed by two-way ANOVA followed by Bonferroni post hoc test. **P < 0.01, ***P < 0.001 when compared with cd44+/+ mice with the same diet (B) and compared with cd44+/+ mice during clamp (G and J). †P < 0.05 when compared with chow-fed mice with the same genotype (B).
Body weight and body composition of cd44-null mice.
Both male and female mice comparable in numbers were studied (Supplemental Table S1; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.9585287.v2). Since we did not observe a difference in genotype effect between genders, we pooled data from both male and female mice unless noted otherwise. The growth curve of chow-fed cd44−/− mice was similar to their diet-matched cd44+/+ controls (Fig. 1C). HF feeding increased weight gain in both cd44+/+ and cd44−/− mice equivalently (Fig. 1C). The cd44+/+ and cd44−/− mice displayed comparable body composition on both diets (Fig. 1D).
Effect of global cd44 deletion on insulin action.
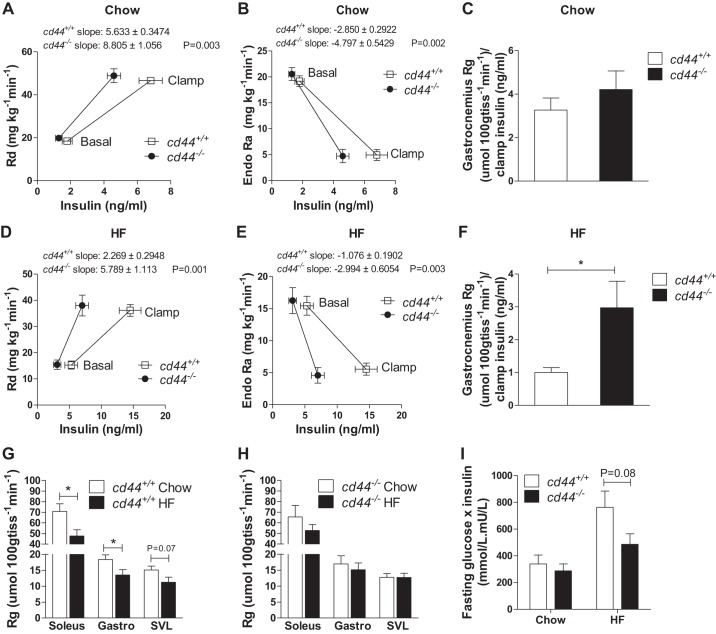
ICv was performed in cd44−/− mice and cd44+/+ littermates. During ICv, all groups of mice were clamped at an arterial glucose level of ~8 mmol/L (Fig. 1, E and H). GIRs were similar between cd44−/− and cd44+/+ mice in both chow-fed and HF-fed states (Fig. 1, F and I). The ICv insulin levels, however, were substantially lower in cd44−/− mice on both chow and HF diets (32% and 50% decrease, respectively), relative to their diet-matched cd44+/+ littermates (Fig. 1, G and J). Rd and EndoRa rates were represented in relation to basal and clamp insulin concentrations (Fig. 2, A, B, D, and E). The rates of stimulation of Rd and suppression of EndoRa by insulin (slopes of the lines) were higher in cd44−/− mice than those in cd44+/+ mice regardless of diet (Fig. 2, A, B, D, and E). Upon normalization to insulin concentrations during clamp, gastrocnemius Rg was not different between genotypes in chow-fed mice (Fig. 2C); however, HF-fed cd44−/− mice exhibited higher gastrocnemius muscle Rg (3-fold increase) compared with HF-fed cd44+/+ mice (Fig. 2F). These results suggest that during the insulin clamp, cd44−/− mice were able to sustain GIR and glucose turnover despite responding to lower plasma insulin levels compared with wild-type littermates regardless of diet. Moreover, increased glucose uptake in muscle of HF-fed cd44−/− mice after normalizing to clamp insulin levels suggests an improved muscle insulin action compared with HF-fed cd44+/+ mice.
Fig. 2.
Insulin sensitivity in chow-fed and high-fat (HF)-fed mice. A and D: rate of glucose disappearance (Rd) was calculated using non-steady-state equations. B and E: rate of endogenous glucose appearance (EndoRa) was calculated by subtracting glucose infusion rate from total glucose appearance rate (Ra). Intravenous bolus of nonmetabolizable glucose analog [14C]2-deoxyglucose was administered at 120 min of hyperinsulinemic-euglycemic clamp to determine glucose uptake in tissues. C and F: glucose metabolic index (Rg) in gastrocnemius muscle was normalized to clamp insulin levels. G and H: unnormalized Rg, an index of muscle glucose uptake, is represented to show diet effect in cd44+/+ and cd44−/− mice. A–H: n = 12 (5–7 females, 5–7 males) for chow-fed mice and n = 11–13 (4–6 females, 5–9 males) for HF-fed mice. I: fasting insulin (mU/L) × fasting glucose (mmol/L) was calculated [n = 11 (5–7 females, 4–6 males) for chow-fed mice and n = 14–19 (5–6 females, 9–11 males) for HF-fed mice]. All data are represented as mean ± SE. Statistical analysis was performed by linear regression and unpaired t test as appropriate. *P < 0.05 when compared with HF-fed cd44+/+ mice (F) or chow-fed cd44+/+ mice (H).
To further confirm the protective role of cd44 deletion in diet-induced insulin resistance specifically in muscle, independent of ICv insulin normalization, we looked at the diet effect of muscle Rg in both cd44+/+ and cd44−/− mice, respectively. We observed that HF feeding-induced muscle insulin resistance in cd44+/+ mice was evidenced by decreased muscle Rg in soleus and gastrocnemius muscles (Fig. 2G), but this effect was absent in cd44−/− mice (Fig. 2H). Although insulin sensitivity index, calculated by fasting glucose × fasting insulin, displayed no significant differences between genotypes regardless of diet, a statistical P value of 0.08 between HF-fed cd44+/+ and cd44−/− mice indicates a more insulin-sensitive state in cd44−/− mice (Fig. 2I).
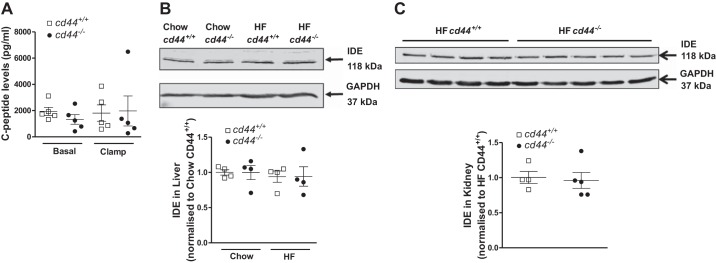
We further explored the potential mechanisms of lower ICv insulin levels in cd44-deficient mice. C-peptide levels in both basal and insulin-clamped states were similar between genotypes in HF-fed mice (Fig. 3A). This indicates a potential increased clearance of insulin in the cd44-deficient mice. However, this was not associated with changes in insulin-degrading enzyme protein expression levels in liver or kidney (Fig. 3, B and C).
Fig. 3.
Endogenous insulin production and metabolic activity. For determining endogenous insulin production, C-peptide levels were measured during basal (−5 and −15) and clamp (100 and 120) state in high-fat (HF)-fed mice (n = 5 male mice) (A). Protein expression of insulin-degrading enzyme (IDE) was measured in liver collected at the end of the hyperinsulinemic-euglycemic clamp (n = 4 male mice) (B) and kidney collected at 30 min post-insulin bolus (0.75 U/kg, ip) (n = 2 female and 2–3 male mice) (C). All data are represented as mean ± SE.
Mechanisms of ameliorated insulin resistance in the HF-fed cd44-null mice.
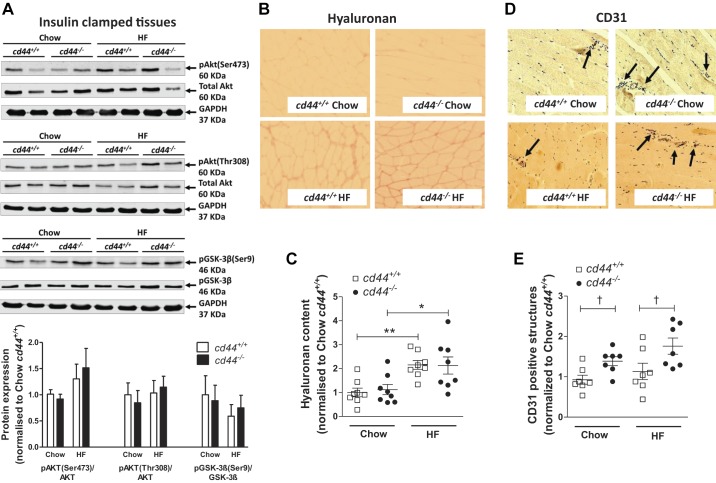
After insulin clamp, insulin signaling measured by p-Akt(Ser472 or Thr308)/Akt and p-GSK-3β/GSK-3β ratio was unchanged between groups (Fig. 4A), suggesting that cd44−/− mice were able to sustain the insulin signaling pathway despite responding to lower plasma insulin levels. HF diet feeding increased hyaluronan content in gastrocnemius muscle in both genotypes (Fig. 4, B and C). However, the hyaluronan content was not different between genotypes on respective diets (Fig. 4C). Muscle vascularization, examined by the number of CD31-positive structures, was increased in cd44−/− mice compared with diet-matched cd44+/+ littermates (Fig. 4D). cd44 deletion increased muscle CD31 expression by ~1.5-fold in both chow-fed and HF-fed mice (Fig. 4E).
Fig. 4.
Hyaluronan deposition, insulin signaling, and vascularization in gastrocnemius muscle of mice. A–E: gastrocnemius muscle was collected at the end of the hyperinsulinemic-euglycemic clamp, and hyaluronan content and protein expression of CD31 were measured by immunohistochemistry. p-Akt (Ser473 or Thr308)/Akt and p-GSK-3β/GSK-3β were measured by Western blotting. A: representative bands and integrated intensity of respective protein expression when normalized to chow-fed cd44+/+ mice (n = 4–6 male mice). B and C: immunohistochemical detection of hyaluronan was quantified by measuring the integrated intensity of staining. D and E: muscle vascularity was determined by counting CD31-positive structures (denoted by arrows). B and D: representative images (×20 magnification). C and E: quantitative data when normalized to chow-fed cd44+/+ mice (n = 4 female, 3–4 male mice). All data are represented as mean ± SE. Statistical analysis were performed by two-way ANOVA followed by Benferroni post hoc test and unpaired t test as appropriate. *P < 0.05, **P < 0.01 when compared with chow-fed mice with the same genotype (C). †P < 0.05 when compared with cd44+/+ mice on respective diets (E). HF, high fat.
Effects of chronic PEGPH20 treatment in HF-fed cd44-null mice.
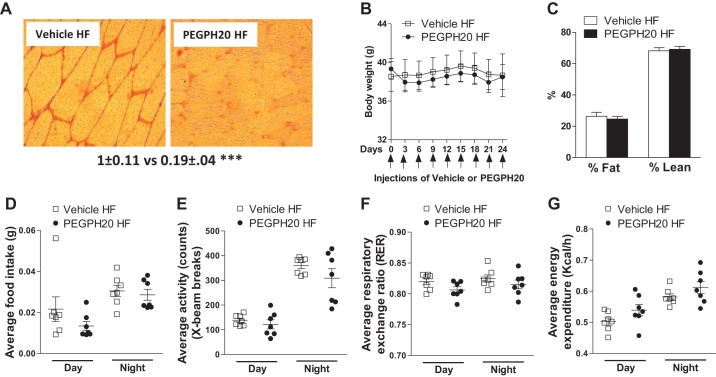
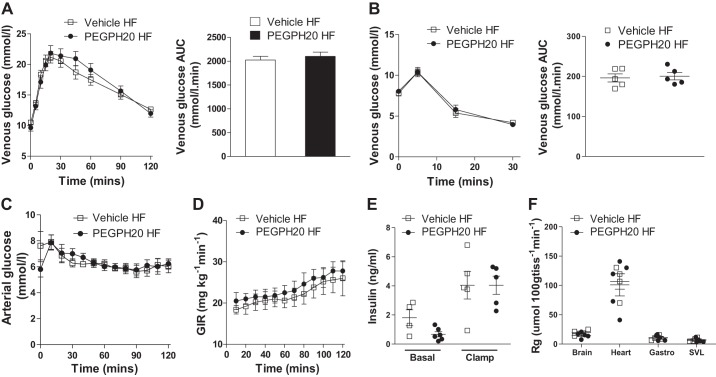
PEGPH20 treatment has been previously reported to reduce muscle hyaluronan and reverse insulin resistance in HF-fed wild-type mice in a dose-dependent manner (24). In HF-fed wild-type mice, chronic PEGPH20 at 1 mg/kg decreases muscle hyaluronan by 70% and improves muscle insulin resistance by increasing GIR and gastrocnemius and SVL muscle Rg during an ICv when compared with vehicle-treated wild-type mice (24). To test whether this beneficial effect of PEGPH20 is dependent on the presence of CD44, we treated cd44−/− mice with either vehicle or 1 mg/kg PEGPH20 as previously described (24). Chronic PEGPH20 treatment decreased muscle hyaluronan content by 80% in HF-fed cd44−/− mice compared with vehicle controls (Fig. 5A). PEGPH20 had no effect on body weight or body composition in HF-fed cd44−/− mice (Fig. 5, B and C). Food intake, locomotor activity, RER, and EE were similar in both groups of mice (Fig. 5, D–G). PEGPH20 treatment did not alter glucose or insulin tolerance in HF-fed cd44−/− mice (Fig. 6, A and B). During ICv, both groups of mice were clamped at an arterial glucose level of ~6 mmol/L (Fig. 6C). The ICv showed similar GIRs, basal and clamp insulin, and muscle glucose uptake between vehicle- and PEGPH20-treated HF-fed cd44−/− mice (Fig. 6, D–F).
Fig. 5.
Effects of chronic PEGPH20 treatment in high-fat (HF)-fed cd44 null mice. Following 21-day treatment, vehicle and PEGPH20 treated mice underwent hyperinsulinemic-euglycemic clamp, gastrocnemius muscle was collected, and hyaluronan content was measured using immunohistochemistry; representative image (×20 magnification) and hyaluronan content as measured by the integrated intensity of staining (n = 6 male mice) (A). Body weight was measured at 3-day interval during treatment with either vehicle or PEGPH20 (1 mg/kg) (B). Body composition (percentage body fat mass and lean mass) was measured at the end of treatment period (n = 12 male mice) (C). Following 21-day treatment, mice were placed in Comprehensive laboratory animal monitoring system (CLAMS) metabolic chambers and parameters were measured. The overall effect for food intake (D), locomotor activity (E), respiratory exchange ratio (RER) (F), and energy expenditure (EE) (G) during 48-h recording period (n = 7 male mice). All data are represented as mean ± SE. Statistical analysis was performed by unpaired Student’s t test. ***P < 0.001 when compared with vehicle-treated HF-fed cd44−/− mice.
Fig. 6.
Effects of chronic PEGPH20 treatment on insulin sensitivity in high-fat (HF)-fed cd44 null mice. Oral glucose tolerance (A) and intraperitoneal insulin tolerance (B) tests were performed in 5-h fasted mice. Blood glucose was measured before and after oral gavage of glucose (2 g/kg) at the time points indicated (A). Blood glucose area under the curve (AUC) is also shown (n = 12 male mice). Blood glucose was measured before and 5, 15, and 30 min after intraperitoneal injection of insulin (0.75 U/kg) in 5-h fasted mice previously treated with either vehicle or PEGPH20 for 24 days (B). AUC is also shown (n = 5 female mice). Data represent values from pooled female and male mice. Hyperinsulinemic-euglycemic clamp (ICv) was performed in vehicle- and PEGPH20-treated mice at the end of treatment period; blood glucose (C), glucose infusion rate (GIR) (D), baseline (−5) and ICv clamp insulin levels (100 and 120) (E), and glucose uptake in tissues (Rg) (F) are shown (n = 5–6 male mice). Open squares and open bars indicate vehicle-treated HF-fed cd44−/− mice; closed circles and closed bars indicate PEGPH20-treated HF-fed cd44−/− mice. All data are represented as mean ± SE.
DISCUSSION
ECM remodeling is a hallmark of insulin-resistant skeletal muscle characterized by increased deposition of ECM components, such as collagens and hyaluronan, and altered activity of ECM enzymes, such as matrix metalloproteinase 9 (7, 23, 24, 47). We have previously reported that insulin resistance in skeletal muscle of HF-fed mice is tightly associated with increased hyaluronan content, as reduction of hyaluronan by PEGPH20 dose-dependently ameliorates skeletal muscle insulin resistance (24). CD44, the main hyaluronan receptor, has been manifested to be associated with the pathogenesis of type 2 diabetes (22, 30). It is to be noted that elevated CD44 expression is not merely an attribute of diet-induced diabetic rodents but also is an established characteristic in humans who are obese(29, 34), thus affirming relevance to the human condition. In the present study, by utilizing global cd44−/− mice, we demonstrate a link between hyaluronan deposition and CD44 interaction in regulating skeletal muscle insulin resistance. The cd44−/− mice were less susceptible to the development of diet-induced skeletal muscle insulin resistance as evident by increased muscle glucose uptake and vascularization. Furthermore, an unchanged insulin-resistant state in PEGPH20-treated cd44−/− mice suggests that the link between normalized hyaluronan deposition and ameliorated skeletal muscle insulin resistance is uncoupled in the absence of hyaluronan-CD44 interaction (Fig. 7).
Fig. 7.
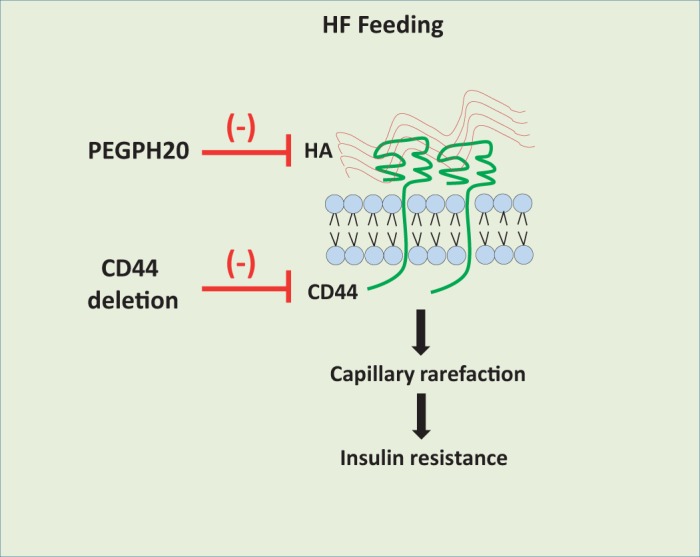
Working model that links hyaluronan-CD44 signaling to skeletal muscle insulin resistance. It is hypothesized that high-fat (HF) feeding in mice increases extracellular matrix hyaluronan (HA) content and CD44 expression, which activates CD44 signaling and causes muscle capillary rarefaction, subsequently leading to the development of insulin resistance. Disruption of this pathway at various steps, such as reduction of hyaluronan by PEGPH20 treatment and/or CD44 deletion, helps in alleviating insulin resistance. Furthermore, PEGPH20 treatment does not further ameliorate insulin resistance post-deletion of CD44, suggesting that hyaluronan is upstream of CD44 and that CD44 is essential for the beneficial effects of PEGPH20. Further investigation using conditional mouse approach will help in identifying CD44 expression site responsible for the beneficial metabolic effects.
CD44 is important for lymphocyte trafficking; however, global cd44 deletion did not affect mouse embryonic development and showed no abnormalities in adults as to viability and differentiation of tissues and organs (46). Here we have used HF feeding to induce insulin resistance in mice, as HF-fed mice are a robust model for studying pathophysiology of type 2 diabetes (23, 24). Increased CD44 protein levels occur in liver and adipose tissue of diet-induced obese mice (22). In line with elevated hyaluronan deposition, HF feeding also increased muscle CD44 expression. We show that genetic deletion of cd44 did not affect weight gain in lean mice, and in the presence of HF diet for up to 14 wk, cd44−/− mice displayed similar weight gain and adiposity as their diet-matched controls. Fasting insulin level is an index of insulin-resistant state in research and clinical settings (22, 50); hence the lower fasting insulin concentrations in cd44−/− mice support improved insulin sensitivity. In addition, the diet-induced obese cd44−/− mice have been previously shown to exhibit lower fasting glucose and serum insulin concentrations, reduced macrophage infiltration into adipose tissue, and improved glucose and insulin tolerance (22, 29). Anti-CD44 antibody treatment suppressed adipose tissue inflammation, weight gain, and liver steatosis to levels comparable to conventional diabetes treatments (30). Taken together, our data with those of Kang et al. (22) suggest that cd44 deletion protected mice from developing HF diet-induced insulin resistance in major peripheral tissues including muscle, liver, and adipose tissue.
The cd44−/− mice also had lower insulin levels during the ICv despite the same insulin infusion rate of 4 mU·kg−1·min−1, indicating either a reduced endogenous insulin secretion and/or an increased insulin clearance. Deletion of CD44 did not alter arterial C-peptide levels either at baseline or during the ICv, suggesting comparable insulin secretion. Although IDE expression in liver and kidney was not altered by cd44 deletion, the reduced insulin levels could be attributed to differential enzymatic activity of IDE rather than its protein expression (28). Indeed, insulin clearance is inversely related to insulin sensitivity (2), and pharmacological rectification of peripheral insulin sensitivity has been shown to reduce metabolic demand of insulin and preserve β-cell compensatory response to chronic insulin resistance (11, 14).
Upon normalization to clamp insulin levels, HF-fed cd44−/− mice were less insulin resistant compared with their littermate controls, evident by increased [14C]2-deoxyglucose uptake in the muscle of HF-fed cd44−/− mice. Moreover, independent of normalization to clamp insulins, HF feeding-induced skeletal muscle insulin resistance in cd44+/+ mice was shown by decreases in muscle Rg. This was completely prevented in cd44−/− mice, further emphasizing the protective role of cd44 deletion in muscle insulin resistance. Several previous studies have demonstrated the role of CD44 signaling in hepatic and adipose tissue-specific insulin resistance (22, 29, 30), and this is the first time the CD44 receptor has been implicated in hyaluronan-mediated skeletal muscle insulin resistance. Although a clear increment of muscle glucose uptake is evident in HF-fed cd44−/− mice, this was not the case in lean mice. Chow-fed cd44−/− mice had unchanged muscle insulin sensitivity. However, their responses of EndoRa inhibition and Rd stimulation to insulin during the ICv were increased. These data suggest that the CD44 signaling may be able to promote insulin resistance in lean mice.
CD44 is expressed in endothelial cells (3) and has been shown to be involved in the regulation of angiogenesis and endothelial cell proliferation by modulating CD31 and VE-cadherin expression (15, 52). ECM-mediated muscle insulin resistance occurs mainly because of a reduction in blood capillaries (12) and impaired vascular endothelial dysfunction (20), in turn reducing insulin or glucose delivery from blood to skeletal muscle cells (6). Increased muscle capillaries have been associated with improved muscle insulin resistance in several HF-fed mouse models, including PEGPH20-treated mice (by increasing cardiac output to muscle) (24), muscle-specific mitochondrial-targeted catalase transgenic mice, and sildenafil-treated mice (by reducing muscle inflammation and collagen expansion) (23). In contrast, HF-fed mmp9−/− mice displayed exacerbated muscle insulin resistance in association with decreased numbers of muscle capillaries (25). Our data demonstrate that mice lacking cd44 maintained their ability to generate an adaptive response to chronic HF feeding, leading to increased vascularization and thereby preserving muscle glucose uptake. CD44 receptor in both muscle and endothelium is proposed to contribute toward muscle insulin resistance by binding to hyaluronan. Further investigation using conditional cd44 knockout mice would help in identifying the expression site of CD44 responsible for these improved metabolic effects.
Chronic PEGPH20 treatment dose-dependently decreases muscle hyaluronan content and improves insulin resistance in skeletal muscle of HF-fed mice (24). Therefore, to test whether these beneficial effects of PEGPH20 were dependent on CD44, we treated HF-fed cd44−/− mice with PEGPH20. Owing to its extended half-life of >10 h (51), the PEGPH20 treatment every third day led to sustained reduction of hyaluronan content in HF-fed cd44−/− mice. The dose of 1 mg/kg was used, as it has been shown to normalize muscle hyaluronan content to levels seen in chow-fed mice and improve muscle glucose uptake in HF-fed wild-type mice (24). No adverse effect of drug treatment was reported on body weight, adiposity, food intake, locomotor activity, or EE. PEGPH20 treatment at 1 mg/kg in cd44−/− mice achieved a similar reduction in muscle hyaluronan content as seen in HF-fed wild-type mice yet did not improve diet-induced insulin resistance in cd44−/− mice, as evident by similar glucose and insulin tolerance as well as the same insulin sensitivity determined by the ICv. A clear augmentation of insulin action was seen in HF-fed wild-type mice after chronic 1 mg/kg PEGPH20 treatment (24). These results suggest that the beneficial effects of PEGPH20 on diet-induced insulin resistance require the presence of CD44 receptor. In addition to hyaluronan, CD44 may also interact with osteopontin to activate intracellular signaling involved in insulin resistance. Osteopontin expression is upregulated in obese mice and humans (8, 27), and both genetic (40) or pharmacological (26) reduction of osteopontin signaling have been shown to improve glucose tolerance and insulin sensitivity via decreased adipose tissue inflammation. The essential role of CD44 in ostepontin-mediated insulin resistance remains to be determined.
Intracellular mediators of the CD44 signaling are not fully understood. In cancerous tissues, hyaluronan-mediated activation of CD44 can regulate various oncogenic signals to promote tumor progression and metastasis, including RhoGTPases, TGFβ, Src/MAPK, Ras/MAPK, Snail/β-catenin, and phosphatidylinositol 3-kinase/AKT pathways (1, 13, 39, 41, 57). The activation of CD44 COOH-terminal-bound proteins such as ankyrin, merlin, and ERM (ezrin, radixin, and moesin) has been postulated to promote cytoskeletal changes and initiate downstream cell signaling pathways of cancer cell growth, survival, and invasion (35, 44, 56). Moreover, various CD44 variants such as CD44v3 and CD44v6 have also been shown to be associated with breast cancer progression and survival rate (17). Despite the emerging role of CD44 in cancers, the involvement of these downstream intracellular pathways of CD44 in metabolic regulation is unknown and remains to be investigated.
Our present data from global knockout mice demonstrate that CD44 plays an important role in the regulation of skeletal muscle insulin resistance; however, it is possible that these effects in skeletal muscle could be secondary to adaptations that occur as a result of loss of CD44 in other insulin-sensitive tissues, such as liver and adipose. Indeed, CD44 deletion has been previously shown to play an important role in liver and adipose tissue inflammation (22, 34). This is a limitation of our study that can be addressed by further investigation using adipose tissue and skeletal muscle-specific knockout mice to confirm the comprehensive tissue-specific effects of CD44 in mediating hyaluronan-mediated insulin resistance. In conclusion, we establish for the first time a pivotal role of hyaluronan-CD44 signaling in mediating skeletal muscle insulin resistance during HF feeding. Our study demonstrates that mice deficient in the cd44 gene are resistant to diet-induced skeletal muscle insulin resistance and exhibit increased muscle vascularization. Through a pharmacological hyaluronidase intervention, we further confirm that the association between increased hyaluronan deposition and worsened skeletal muscle insulin resistance is uncoupled in the absence of the CD44 receptor. These results support the concept that increased hyaluronan content contributes to skeletal muscle insulin resistance by interacting with the CD44 receptor, in turn modulating pathways such as muscle vascularization leading to reduced vascular glucose and insulin delivery through the ECM (Fig. 7). In conclusion, this study provides evidence that manipulation of hyaluronan-CD44 signaling may provide a potential therapeutic target for insulin resistance and type 2 diabetes.
GRANTS
This work was supported by National Institutes of Health Grants DK-059637, DK-054902, and DK-050277 (to D. H. Wasserman); Diabetes UK 15/0005256 (to L. Kang, R. J. McCrimmon, M. L. J. Ashford, F. Khan); European Commission Marie Curie International Incoming Fellowship 625119 (to L. Kang); and funding from the Diabetes Research and Wellness Foundation (to L. Kang) and Tenovus Scotland (to L. Kang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.H., D.H.W., and L.K. conceived and designed research; A.H., C.K.H., D.P.B., A.R.B.-L., and L.K. performed experiments; A.H. and L.K. analyzed data; A.H., L.L., F.K., M.L.J.A., R.J.M., D.H.W., and L.K. interpreted results of experiments; A.H. prepared figures; A.H. drafted manuscript; A.H., L.L., F.K., M.L.J.A., R.J.M., D.H.W., and L.K. edited and revised manuscript; A.H., C.K.H., D.P.B., A.R.B.-L., L.L., F.K., M.L.J.A., R.J.M., D.H.W., and L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
PEGPH20 was provided by Halozyme Therapeutics, Inc. The authors thank Susan Bray (Tayside Biorepository) for help on the CD31 staining of gastrocnemius muscle. Vanderbilt Mouse Metabolic Phenotyping Centre (DK059637) cores are acknowledged.
REFERENCES
- 1.Acharya PS, Majumdar S, Jacob M, Hayden J, Mrass P, Weninger W, Assoian RK, Puré E. Fibroblast migration is mediated by CD44-dependent TGF β activation. J Cell Sci 121: 1393–1402, 2008. doi: 10.1242/jcs.021683. [DOI] [PubMed] [Google Scholar]
- 2.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in african-american children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 51: 3014–3019, 2002. doi: 10.2337/diabetes.51.10.3014. [DOI] [PubMed] [Google Scholar]
- 3.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell 61: 1303–1313, 1990. doi: 10.1016/0092-8674(90)90694-A. [DOI] [PubMed] [Google Scholar]
- 4.Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 56: 1025–1033, 2007. doi: 10.2337/db06-0883. [DOI] [PubMed] [Google Scholar]
- 5.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 6.Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 52: 752–764, 2009. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berria R, Wang L, Richardson DK, Finlayson J, Belfort R, Pratipanawatr T, De Filippis EA, Kashyap S, Mandarino LJ. Increased collagen content in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab 290: E560–E565, 2006. doi: 10.1152/ajpendo.00202.2005. [DOI] [PubMed] [Google Scholar]
- 8.Bertola A, Deveaux V, Bonnafous S, Rousseau D, Anty R, Wakkach A, Dahman M, Tordjman J, Clément K, McQuaid SE, Frayn KN, Huet PM, Gugenheim J, Lotersztajn S, Le Marchand-Brustel Y, Tran A, Gual P. Elevated expression of osteopontin may be related to adipose tissue macrophage accumulation and liver steatosis in morbid obesity. Diabetes 58: 125–133, 2009. doi: 10.2337/db08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bery N, Legg S, Debreczeni J, Breed J, Embrey K, Stubbs C, Kolasinska-Zwierz P, Barrett N, Marwood R, Watson J, Tart J, Overman R, Miller A, Phillips C, Minter R, Rabbitts TH. KRAS-specific inhibition using a DARPin binding to a site in the allosteric lobe. Nat Commun 10: 2607, 2019. doi: 10.1038/s41467-019-10419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogdani M, Johnson PY, Potter-Perigo S, Nagy N, Day AJ, Bollyky PL, Wight TN. Hyaluronan and hyaluronan-binding proteins accumulate in both human type 1 diabetic islets and lymphoid tissues and associate with inflammatory cells in insulitis. Diabetes 63: 2727–2743, 2014. doi: 10.2337/db13-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boland BB, Brown C Jr, Boland ML, Cann J, Sulikowski M, Hansen G, Grønlund RV, King W, Rondinone C, Trevaskis J, Rhodes CJ, Grimsby JS. Pancreatic β-cell rest replenishes insulin secretory capacity and attenuates diabetes in an extreme model of obese type 2 diabetes. Diabetes 68: 131–140, 2019. doi: 10.2337/db18-0304. [DOI] [PubMed] [Google Scholar]
- 12.Bonner JS, Lantier L, Hasenour CM, James FD, Bracy DP, Wasserman DH. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes 62: 572–580, 2013. doi: 10.2337/db12-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol 18: 251–259, 2008. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 51: 2796–2803, 2002. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 15.Cao G, Savani RC, Fehrenbach M, Lyons C, Zhang L, Coukos G, Delisser HM. Involvement of endothelial CD44 during in vivo angiogenesis. Am J Pathol 169: 325–336, 2006. doi: 10.2353/ajpath.2006.060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chajara A, Raoudi M, Delpech B, Leroy M, Basuyau JP, Levesque H. Increased hyaluronan and hyaluronidase production and hyaluronan degradation in injured aorta of insulin-resistant rats. Arterioscler Thromb Vasc Biol 20: 1480–1487, 2000. doi: 10.1161/01.ATV.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Zhao S, Karnad A, Freeman JW. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol 11: 64, 2018. doi: 10.1186/s13045-018-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egan CE, Daugherity EK, Rogers AB, Abi Abdallah DS, Denkers EY, Maurer KJ. CCR2 and CD44 promote inflammatory cell recruitment during fatty liver formation in a lithogenic diet fed mouse model. PLoS One 8: e65247, 2013. doi: 10.1371/journal.pone.0065247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo X, Tao X, Tong Q, Li T, Dong D, Zhang B, Zhao M, Song T. Impaired AMPK–CGRP signaling in the central nervous system contributes to enhanced neuropathic pain in high–fat diet–induced obese rats, with or without nerve injury. Mol Med Rep 20: 1279–1287, 2019. doi: 10.3892/mmr.2019.10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansson PA. Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med 262: 173–183, 2007. doi: 10.1111/j.1365-2796.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 21.Jin F, Jiang K, Ji S, Wang L, Ni Z, Huang F, Li C, Chen R, Zhang H, Hu Z, Zha X. Deficient TSC1/TSC2-complex suppression of SOX9-osteopontin-AKT signalling cascade constrains tumour growth in tuberous sclerosis complex. Hum Mol Genet 26: 407–419, 2017. doi: 10.1093/hmg/ddw397. [DOI] [PubMed] [Google Scholar]
- 22.Kang HS, Liao G, DeGraff LM, Gerrish K, Bortner CD, Garantziotis S, Jetten AM. CD44 plays a critical role in regulating diet-induced adipose inflammation, hepatic steatosis, and insulin resistance. PLoS One 8: e58417, 2013. doi: 10.1371/journal.pone.0058417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang L, Ayala JE, Lee-Young RS, Zhang Z, James FD, Neufer PD, Pozzi A, Zutter MM, Wasserman DH. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin α2β1 in mice. Diabetes 60: 416–426, 2011. doi: 10.2337/db10-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang L, Lantier L, Kennedy A, Bonner JS, Mayes WH, Bracy DP, Bookbinder LH, Hasty AH, Thompson CB, Wasserman DH. Hyaluronan accumulates with high-fat feeding and contributes to insulin resistance. Diabetes 62: 1888–1896, 2013. doi: 10.2337/db12-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang L, Mayes WH, James FD, Bracy DP, Wasserman DH. Matrix metalloproteinase 9 opposes diet-induced muscle insulin resistance in mice. Diabetologia 57: 603–613, 2014. doi: 10.1007/s00125-013-3128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiefer FW, Zeyda M, Gollinger K, Pfau B, Neuhofer A, Weichhart T, Säemann MD, Geyeregger R, Schlederer M, Kenner L, Stulnig TM. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes 59: 935–946, 2010. doi: 10.2337/db09-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiefer FW, Zeyda M, Todoric J, Huber J, Geyeregger R, Weichhart T, Aszmann O, Ludvik B, Silberhumer GR, Prager G, Stulnig TM. Osteopontin expression in human and murine obesity: extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology 149: 1350–1357, 2008. doi: 10.1210/en.2007-1312. [DOI] [PubMed] [Google Scholar]
- 28.Kim M, Hersh LB, Leissring MA, Ingelsson M, Matsui T, Farris W, Lu A, Hyman BT, Selkoe DJ, Bertram L, Tanzi RE. Decreased catalytic activity of the insulin-degrading enzyme in chromosome 10-linked Alzheimer disease families. J Biol Chem 282: 7825–7832, 2007. doi: 10.1074/jbc.M609168200. [DOI] [PubMed] [Google Scholar]
- 29.Kodama K, Horikoshi M, Toda K, Yamada S, Hara K, Irie J, Sirota M, Morgan AA, Chen R, Ohtsu H, Maeda S, Kadowaki T, Butte AJ. Expression-based genome-wide association study links the receptor CD44 in adipose tissue with type 2 diabetes. Proc Natl Acad Sci USA 109: 7049–7054, 2012. doi: 10.1073/pnas.1114513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kodama K, Toda K, Morinaga S, Yamada S, Butte AJ. Anti-CD44 antibody treatment lowers hyperglycemia and improves insulin resistance, adipose inflammation, and hepatic steatosis in diet-induced obese mice. Diabetes 64: 867–875, 2015. doi: 10.2337/db14-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraegen EW, James DE, Jenkins AB, Chisholm DJ. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol Endocrinol Metab Physiol 248: E353–E362, 1985. doi: 10.1152/ajpendo.1985.248.3.E353. [DOI] [PubMed] [Google Scholar]
- 32.Lewis A, Steadman R, Manley P, Craig K, de la Motte C, Hascall V, Phillips AO. Diabetic nephropathy, inflammation, hyaluronan and interstitial fibrosis. Histol Histopathol 23: 731–739, 2008. doi: 10.14670/HH-23.731. [DOI] [PubMed] [Google Scholar]
- 33.Lin D, Chun TH, Kang L. Adipose extracellular matrix remodelling in obesity and insulin resistance. Biochem Pharmacol 119: 8–16, 2016. doi: 10.1016/j.bcp.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu LF, Kodama K, Wei K, Tolentino LL, Choi O, Engleman EG, Butte AJ, McLaughlin T. The receptor CD44 is associated with systemic insulin resistance and proinflammatory macrophages in human adipose tissue. Diabetologia 58: 1579–1586, 2015. doi: 10.1007/s00125-015-3603-y. [DOI] [PubMed] [Google Scholar]
- 35.Martin TA, Harrison G, Mansel RE, Jiang WG. The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol 46: 165–186, 2003. doi: 10.1016/S1040-8428(02)00172-5. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Huenchullan S, McLennan SV, Verhoeven A, Twigg SM, Tam CS. The emerging role of skeletal muscle extracellular matrix remodelling in obesity and exercise. Obes Rev 18: 776–790, 2017. doi: 10.1111/obr.12548. [DOI] [PubMed] [Google Scholar]
- 37.Mine S, Okada Y, Kawahara C, Tabata T, Tanaka Y. Serum hyaluronan concentration as a marker of angiopathy in patients with diabetes mellitus. Endocr J 53: 761–766, 2006. doi: 10.1507/endocrj.K05-119. [DOI] [PubMed] [Google Scholar]
- 38.Morita M, Yano S, Ishibashi Y, Nakata N, Kurioka S, Sugimoto T. Close relationship between serum hyaluronan levels and vascular function in patients with type 2 diabetes. Biomarkers 19: 493–497, 2014. doi: 10.3109/1354750X.2014.940502. [DOI] [PubMed] [Google Scholar]
- 39.Nam K, Oh S, Lee KM, Yoo SA, Shin I. CD44 regulates cell proliferation, migration, and invasion via modulation of c-Src transcription in human breast cancer cells. Cell Signal 27: 1882–1894, 2015. doi: 10.1016/j.cellsig.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschöp MH, Bruemmer D. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest 117: 2877–2888, 2007. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouhtit A, Rizeq B, Saleh HA, Rahman MM, Zayed H. Novel CD44-downstream signaling pathways mediating breast tumor invasion. Int J Biol Sci 14: 1782–1790, 2018. doi: 10.7150/ijbs.23586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab 94: 5155–5162, 2009. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phelps CA, Lindsey-Boltz L, Sancar A, Mu D. Mechanistic study of TTF-1 modulation of cellular sensitivity to cisplatin. Sci Rep 9: 7990, 2019. doi: 10.1038/s41598-019-44549-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pokharel D, Padula MP, Lu JF, Jaiswal R, Djordjevic SP, Bebawy M. The role of CD44 and ERM proteins in expression and functionality of P-glycoprotein in breast cancer cells. Molecules 21: 290, 2016. doi: 10.3390/molecules21030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4: 33–45, 2003. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 46.Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol 163: 4917–4923, 1999. [PubMed] [Google Scholar]
- 47.Richardson DK, Kashyap S, Bajaj M, Cusi K, Mandarino SJ, Finlayson J, DeFronzo RA, Jenkinson CP, Mandarino LJ. Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J Biol Chem 280: 10290–10297, 2005. doi: 10.1074/jbc.M408985200. [DOI] [PubMed] [Google Scholar]
- 48.Skhinas JN, Cox TR. The interplay between extracellular matrix remodelling and kinase signalling in cancer progression and metastasis. Cell Adhes Migr 12: 529–537, 2018. doi: 10.1080/19336918.2017.1405208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 187: 15–24, 1956. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- 50.ter Horst KW, Gilijamse PW, Koopman KE, de Weijer BA, Brands M, Kootte RS, Romijn JA, Ackermans MT, Nieuwdorp M, Soeters MR, Serlie MJ. Insulin resistance in obesity can be reliably identified from fasting plasma insulin. Int J Obes 39: 1703–1709, 2015. doi: 10.1038/ijo.2015.125. [DOI] [PubMed] [Google Scholar]
- 51.Thompson CB, Shepard HM, O’Connor PM, Kadhim S, Jiang P, Osgood RJ, Bookbinder LH, Li X, Sugarman BJ, Connor RJ, Nadjsombati S, Frost GI. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther 9: 3052–3064, 2010. doi: 10.1158/1535-7163.MCT-10-0470. [DOI] [PubMed] [Google Scholar]
- 52.Tsuneki M, Madri JA. CD44 regulation of endothelial cell proliferation and apoptosis via modulation of CD31 and VE-cadherin expression. J Biol Chem 289: 5357–5370, 2014. doi: 10.1074/jbc.M113.529313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang D, Jin M, Zhao X, Zhao T, Lin W, He Z, Fan M, Jin W, Zhou J, Jin L, Zheng C, Jin H, Zhao Y, Li X, Ying L, Wang Y, Zhu G, Huang Z. FGF1ΔHBS ameliorates chronic kidney disease via PI3K/AKT mediated suppression of oxidative stress and inflammation. Cell Death Dis 10: 464, 2019. doi: 10.1038/s41419-019-1696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss L, Slavin S, Reich S, Cohen P, Shuster S, Stern R, Kaganovsky E, Okon E, Rubinstein AM, Naor D. Induction of resistance to diabetes in non-obese diabetic mice by targeting CD44 with a specific monoclonal antibody. Proc Natl Acad Sci USA 97: 285–290, 2000. doi: 10.1073/pnas.97.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Z, Kuboyama T, Tohda C. Naringenin promotes microglial M2 polarization and Aβ degradation enzyme expression. Phytother Res 33: 1114–1121, 2019. doi: 10.1002/ptr.6305. [DOI] [PubMed] [Google Scholar]
- 56.Zhu D, Bourguignon LY. Interaction between CD44 and the repeat domain of ankyrin promotes hyaluronic acid-mediated ovarian tumor cell migration. J Cell Physiol 183: 182–195, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 57.Zhu X, Morales FC, Agarwal NK, Dogruluk T, Gagea M, Georgescu MM. Moesin is a glioma progression marker that induces proliferation and Wnt/β-catenin pathway activation via interaction with CD44. Cancer Res 73: 1142–1155, 2013. doi: 10.1158/0008-5472.CAN-12-1040. [DOI] [PubMed] [Google Scholar]