Abstract
Pyridoxine (vitamin B6), an essential micronutrient for normal cell physiology, plays an important role in the function of the exocrine pancreas. Pancreatic acinar cells (PACs) obtain vitamin B6 from circulation, but little is known about the mechanism involved in the uptake process; limited information also exists on the effect of pyridoxine availability on the gene expression profile in these cells. We addressed both these issues in the current investigation using mouse-derived pancreatic acinar 266-6 cells (PAC 266-6) and human primary PACs (hPACs; obtained from organ donors), together with appropriate physiological and molecular (RNA-Seq) approaches. The results showed [3H]pyridoxine uptake to be 1) pH and temperature (but not Na+) dependent, 2) saturable as a function of concentration, 3) cis-inhibited by unlabeled pyridoxine and its close structural analogs, 4) trans-stimulated by unlabeled pyridoxine, 5) regulated by an intracellular Ca2+/calmodulin-mediated pathway, 6) adaptively-regulated by extracellular substrate (pyridoxine) availability, and 7) negatively impacted by exposure to cigarette smoke extract. Vitamin B6 availability was found (by means of RNA-Seq) to significantly (FDR < 0.05) modulate the expression profile of many genes in PAC 266-6 cells (including those that are relevant to pancreatic health and development). These studies demonstrate, for the first time, the involvement of a regulatable and specific carrier-mediated mechanism for pyridoxine uptake by PACs; the results also show that pyridoxine availability exerts profound effects on the gene expression profile in mammalian PACs.
Keywords: carrier-mediated uptake, pancreatic acinar cells, pyridoxine, RNA-Seq, vitamin B6
INTRODUCTION
Vitamin B6, a member of the water-soluble family of vitamins, is represented by a group of three related compounds pyridoxine, pyridoxal, and pyridoxamine. These compounds exist in varying proportions in the diet in both phosphorylated and non-phosphorylated forms. Vitamin B6 plays an important role as a cofactor for enzymes that catalyze transaminase, decarboxylase, and synthetase reactions involved in the metabolism of carbohydrate, lipid, and protein (including metabolism of the amino acid homocysteine) and in the synthesis of neurotransmitters (13). Deficiency of vitamin B6 leads to a variety of clinical abnormalities that include microcytic anemia, dermatitis/glossitis, and neurological disorders. Deficiency/suboptimal levels of vitamin B6 occur in conditions like chronic alcoholism, diabetes, in patients with celiac and renal diseases, and following long-term use of hydrazines (e.g., isoniazid) and penicillamine (13). Low blood levels of vitamin B6 have also been reported in patients with vitamin B6-dependent seizure, an autosomal-recessive disorder believed to be caused by an abnormality in pyridoxine transport into cells (6).
The pancreas is a complex organ with important exocrine and endocrine functions, and diseases of this organ lead to significant morbidity and mortality. Previous studies have shown that vitamin B6 deficiency negatively impacts the exocrine function of the pancreas (4) and also affects the expression of enzymes like amylase (4, 22). Like all other mammalian cells, pancreatic cells cannot synthesize vitamin B6 endogenously and, thus, must obtain it from their surroundings (circulation) via transport across their cell membranes. We (17, 19, 20) have previously shown that uptake of pyridoxine by other cell types (e.g., intestinal and renal epithelia) is via specific carrier-mediated mechanisms. There is nothing currently known about the mechanism involved in vitamin B6 uptake in pancreatic acinar cells (PACs). Little is also known about the effect of vitamin B6 levels on gene expression profile in PACs. Our aim in this study was to delineate the physiological mechanism involved in vitamin B6 (pyridoxine) uptake as well as to examine the effect of pyridoxine level on the gene expression profile in PACs. We used mouse-derived PAC 266-6 and freshly isolated human primary PACs (hPACs) as models; we also used RNA-Seq to uncover the effect of pyridoxine availability on gene expression profile in PACs. The results showed the existence of a specific and regulatable carrier-mediated mechanism for pyridoxine uptake by mouse/human PACs. The results also showed that pyridoxine availability exerts significant modulatory effect on expression profile of many genes in PACs.
MATERIALS AND METHODS
[3H]pyridoxine (specific activity = 20 Ci/mmol; radiochemical purity >98%) was obtained from ARC (St. Louis, MO). Unlabeled pyridoxine and all other chemicals and reagents were purchased from commercial sources and were of analytical grade.
Cell Culture and Uptake Assays
The mouse-derived PAC 266-6 were obtained from American Type Tissue Collection (ATCC; Rockville, MD) and were cultured in DMEM growth medium containing 10% FBS with an antibiotic cocktail (unless otherwise stated). Cells used in this study were between passages 2 and 20. The 266-6 cells are not polarized, but they form a confluent monolayer and are consistent during passages. Primary hPACs were isolated from pancreatic tissues of organ donors at the Islet Cell Laboratory of the University of Louisville (Louisville, KY) and City of Hope (Duarte, CA). Briefly, after written informed consents were obtained, pancreata of subjects were removed from brain-dead donors and digested to separate islets from acinar cells and purified as described previously (25, 26). The resulting primary hPACs were transported to the University of California, Irvine (in less than 48 h after isolation), and cultured in Ham’s F-12K medium with 10% FCS, 5% BSA, 10 ng/mL epidermal growth factor (EGF), and 0.1 mg/mL soybean trypsin inhibitor, as described by us and others previously (21, 25, 26). The viability of the hPACs was also assessed on the day of culture and on the day of use by using the trypan blue exclusion method and found to be >80%. The study protocols were approved by the institutional review boards of the City of Hope, Duarte, CA, the University of Louisville, Louisville, KY, and the University of California, Irvine, CA (Institutional Review Board: 2017-1593).
Uptake of [3H]pyridoxine by PAC 266-6 was performed in a 12-well plate (37°C) in Krebs-Ringer buffer containing (in mM) 123 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, and 10 MES at pH 7.4 (unless otherwise stated). [3H]pyridoxine was added to the incubation buffer at the onset of incubation, and uptake was terminated at the desired time by the addition of 1 mL of ice-cold buffer and subsequent immediate removal by aspiration. The cells were then rinsed twice with ice-cold buffer, digested with 1 mL of 1 N NaOH, neutralized by HCl, and counted for radioactivity in a liquid scintillation counter. Protein contents of cell digests were estimated on parallel wells using a Bio-Rad protein assay kit. Uptake of [3H]pyridoxine by primary hPACs was done as with PAC 266-6, except that a rapid-filtration technique was used as described by us previously (26). Uptake by the carrier-mediated component at any given substrate concentration was determined by subtracting the diffusion component [calculated from the slope of the line between uptake at high concentration (200 μM) and the point of origin] from total uptake. For determination of kinetic parameters (the apparent Km and Vmax), carrier-mediated uptake component at each concentration was applied to a computerized model of the Michaelis-Menten equation as described previously (29).
In studying the effect of pyridoxine levels, PAC 266-6 were maintained in a vitamin B6-deficient or -oversupplemented (1 mM) growth medium for 1 (or 14) days. In both cases, dialyzed fetal bovine serum (10%) was used.
RNA Isolation and RNA-Seq
PAC 266-6 were maintained in vitamin B6-deficient and -oversupplemented (1 mM) growth medium for 14 days. RNA extraction was performed using an RNeasy mini kit (Qiagen, Hilden, Germany). The concentration and integrity of the mRNA were analyzed using the Agilent BioAnalyzer (Agilent Technologies). Library preparation was initiated with 1 μg of mRNA using an IlluminaTruSeq RNA protocol. Each cDNA library was subjected to Illumina (HiSeq 4000) single-read (SR), 100-cycle sequencing to obtain ~25–35 M reads. The raw reads were QC analyzed (FastQC: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/), quality and adapter trimmed (Trimmomatic: http://www.usadellab.org/cms/?page=trimmomatic) and aligned to mouse mm10 reference genome with transcriptome annotation using a splice-aware short-read aligner Tophat2 (https://ccb.jhu.edu/software/tophat/index.shtmL). Gene expression was quantified using raw counts with featureCounts (http://bioinf.wehi.edu.au/featureCounts/) and RPKMs (reads per kilobase per million mapped reads) with Cufflinks (http://cole-trapnell-lab.github.io/cufflinks/). Differential gene expression statistical analysis was done using the R package DESeq2 (https://bioconductor.org/packages/release/bioc/htmL/DESeq2.htmL).
Statistical Analysis
Uptake results are of multiple separate determinations and are expressed as means ± SE (in pmol·mg protein−1·unit time−1) obtained from completely independent wells processed separately throughout the experiment. Statistical analysis was performed using ANOVA or Student’s t test, with a significant P value set at <0.05. We observed some variations in the absolute amount of pyridoxine uptake with different cell batches; we therefore performed simultaneous controls with each set of experiments, and the data obtained were compared with those of the control, as done previously (17).
RESULTS
Physiology of Pyridoxine Uptake by Mouse-Derived PAC 266-6, and by Primary hPACs
General characteristics of pyridoxine uptake by PAC 266-6.
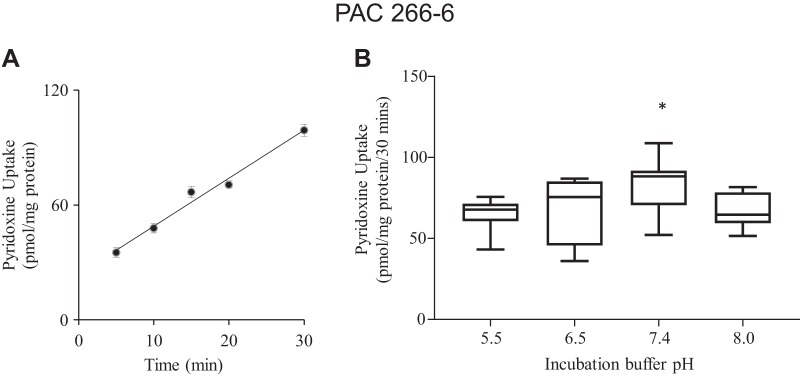
[3H]pyridoxine (7.5 nM) uptake by PAC 266-6 was examined as a function of incubation time and found to be linear for up to 30 min of incubation (rate = 99 ± 3.1 pmol·mg protein−1·30 min−1; r = 0.98; Fig. 1A). A 30-min incubation time was therefore chosen to represent the initial rate of uptake in all subsequent investigations. The effect of incubation buffer pH on initial rate of pyridoxine (7.5 nM) uptake by PAC 266-6 was also examined, with the results showing a significantly (P < 0.05) higher uptake at pH 7.4, but the uptake was decreased at lower and higher buffer pHs (Fig. 1B). Incubation buffer of pH 7.4 was chosen for all subsequent studies. We also examined the effect of incubation temperature on pyridoxine (7.5 nM) uptake by PAC 266-6 and observed a significantly (P < 0.05) higher uptake at 37°C compared with 4°C (95.0 ± 18.1 and 45.5 ± 2.5 pmol·mg protein−1·30 min−1, respectively).
Fig. 1.
Uptake of pyridoxine by pancreatic acinar cells (PAC) 266-6 as a function of time, and effect of incubation buffer pH. A: cells were incubated in Krebs-Ringer buffer (pH 7.4, 37°C) for different periods of time. B: initial rate (total) of pyridoxine uptake (30 min) at different incubation buffer pHs. Cells were maintained in DMEM growth medium, and before uptake experiments they were washed with Krebs-Ringer buffer. [3H]pyridoxine (7.5 nM) was added at onset of incubation. Data are means ± SE of 8–10 separate uptake determinations, respectively. When not shown, error bars are smaller than the symbols. *P < 0.05.
In other studies, we examined the role of Na+ in the incubation buffer on pyridoxine uptake. In these studies, Na+ in the Krebs-Ringer incubation buffer was isoosmotically replaced with an equimolar concentration of either choline or mannitol. The results showed pyridoxine (7.5 nM) initial rate of uptake to be similar in the presence and absence of Na+ (Fig. 2). In a related study, we pretreated PAC 266-6 (for 30 min) with the Na-K-ATPase inhibitor ouabain (1 mM) and examined the effect of that pretreatment on the initial rate of pyridoxine (7.5 nM) uptake. No significant inhibition was observed following such a pretreatment (uptake of 94.3 ± 2.9 and 87.2 ± 4.0 pmol·mg protein−1·30 min−1 in the presence and absence of ouabain, respectively). This further supports the Na-independent nature of the vitamin B6 uptake process.
Fig. 2.
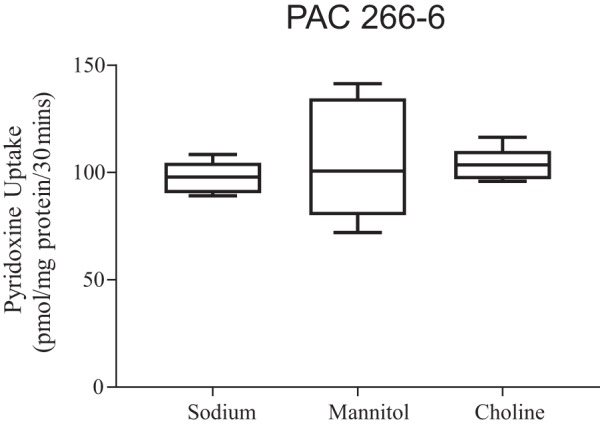
Role of Na+ in pyridoxine uptake by pancreatic acinar cells (PAC) 266-6. Cells were incubated in Krebs-Ringer buffer (pH 7.4) in the presence of Na+ and after its isoosmotic replacement with mannitol or choline. Initial rate (total) of [3H]pyridoxine (7.5 nM) uptake was then determined (30 min, 37°C). Data are means ± SE of 8 separate uptake determinations.
Involvement of a specific carrier-mediated mechanism for pyridoxine uptake by PAC 266-6.
In these investigations we first examined the effect of adding unlabeled pyridoxine (1 mM) to the incubation medium on an initial rate of [3H]pyridoxine uptake (influx) by PAC 266-6. The results showed significant (P < 0.01) inhibition in [3H]pyridoxine uptake in the presence of the unlabeled pyridoxine compared with its absence (91.20 ± 1.51 and 32.14 ± 1.02 pmol·mg protein−1·30 min−1 for controls and in the presence of unlabeled pyridoxine, respectively. We also examined possible trans-stimulation in [3H]pyridoxine transport (efflux) across the PAC 266-6 cell membrane by unlabeled pyridoxine. Here, we first preloaded the cells with 7.5 nM [3H]pyridoxine (for 30 min at 37°C) and then incubated the cells (for 10 min) in the absence and presence of 100 μM of unlabeled pyridoxine in the incubation buffer. The results showed the cellular content of 3H radioactivity to be significantly (P < 0.01) lower in PAC 266-6 incubated in the presence of unlabeled pyridoxine in the incubation buffer compared with those incubated in its absence (cell content of 3H radioactivity was 42 ± 3.9 and 69 ± 4.3 pmol·mg protein−1·30 min−1, respectively). These findings suggest the existence of a specific carrier-mediated mechanism for pyridoxine uptake by PAC 266-6.
In another study, we investigated the specificity of the pyridoxine uptake process of PAC 266-6. This was done by examining the effect of the pyridoxine-related compounds pyridoxal, pyridoxal 5-phosphate (P-5-P), pyridoxamine, 4-deoxypyridoxine, and pyridoxic acid as well as that of the unrelated isoniazid, penicillamine, and biotin on initial rate of [3H]pyridoxine (7.5 nM) uptake. The results showed pyridoxal, P-5-P, and pyridoxamine to cause significant (P < 0.01 for all) inhibition in [3H]pyridoxine uptake, whereas 4-pyridoxic acid (the principle urinary metabolite of vitamin B6) as well as the unrelated penicillamine, isoniazid, and biotin were without any effect (Table 1).
Table 1.
Effect of pyridoxine-related and unrelated compounds on uptake of [3H]pyridoxine by PAC 266-6
| Compound | Pyridoxine Uptake, pmol·mg protein−1·30 min−1 | P Value |
|---|---|---|
| Control | 94.70 ± 2.2 | |
| Pyridoxine | 38.10 ± 3.9 | <0.01 |
| Pyridoxal | 31.98 ± 0.8 | <0.01 |
| P-5-P | 34.96 ± 1.1 | <0.01 |
| Pyridoxamine | 35.49 ± 0.5 | <0.01 |
| 4-Pyridoxic acid | 91.31 ± 1.5 | NS |
| Penicillamine | 87.17 ± 0.25 | NS |
| Biotin | 93.15 ± 4.1 | NS |
Data are means ± SE of 6–8 separate uptake determinations. Cells were incubated in Krebs-Ringer buffer (pH 7.4, 30 min, 37°C) in the presence of [3H]pyridoxine (7.5 nM) and 1 mM of the compound under investigation. PAC, pancreatic acinar cells; P-5-P, pyridoxal 5-phosphate; NS, not significant.
To determine the kinetic parameters of the carrier-mediated pyridoxine uptake process, we examined the initial rate of uptake as a function of substrate concentration (0.05 to 6 μM). The results showed clear saturation in the vitamin uptake as a function of concentration, further confirming the involvement of a carrier-mediated mechanism in the uptake process (Fig. 3). Kinetic parameters of the saturable component were determined as described in materials and methods and found to be 0.17 ± 0.04 μM and 3.2 ± 0.17 nmol·mg protein−1·30 min−1 for the apparent Km and Vmax, respectively. Collectively, the above findings demonstrate the existence of a specific carrier-mediated uptake process for pyridoxine in PAC 266-6.
Fig. 3.
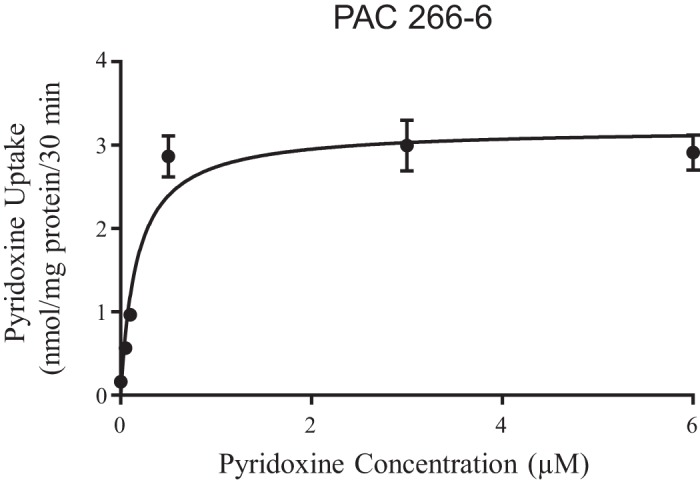
Uptake of pyridoxine as a function of concentration in pancreatic acinar cells (PAC) 266-6. PAC 266-6 cells were maintained in DMEM growth media and before uptake experiments they were washed with Krebs-Ringer buffer. Cells were incubated at 37°C in Krebs-Ringer buffer at pH 7.4 in the presence of different concentrations of pyridoxine. Uptake by the saturable component was calculated as detailed in materials and methods. Data are means ± SE of 8 separate uptake determinations.
Pyridoxine by primary hPACs: involvement of a specific carrier-mediated mechanism.
In these studies, we extended our findings with the mouse-derived PAC 266-6 described above to the human situation. For this, we used primary hPACs obtained from organ donors (see materials and methods (23, 24, 26). We focused on determining whether the primary hPACs also possess a carrier-mediated mechanism for pyridoxine uptake, and if so, to determine its specificity and kinetic parameters. Thus, we examined the effect of adding unlabeled pyridoxine (1 mM) to the incubation medium on initial rate of [3H]pyridoxine (7.5 nM) uptake by primary hPACs. The results showed a significant (P < 0.01) inhibition in [3H]pyridoxine uptake by cells incubated in the presence of unlabeled pyridoxine compared with its absence (16.75 ± 1.25 and 5.85 ± 0.34 pmol·mg protein−1·30 min−1 for controls and presence of unlabeled pyridoxine, respectively). We then examined the specificity of the pyridoxine uptake process of primary hPACs. This was done by examining the effects of pyridoxine structural analogs and unrelated compounds on initial rate of [3H]pyridoxine (7.5 nM) uptake. The results showed a significant inhibition in [3H]pyridoxine uptake by P-5-P and pyridoxamine but not by 4-pyridoxic acid and penicillamine (Table 2). These findings indicate the involvement of a specific carrier-mediated mechanism for pyridoxine uptake by human primary PACs.
Table 2.
Effect of pyridoxine-related and unrelated compounds on uptake of [3H]pyridoxine by primary hPACs
| Compound | Pyridoxine Uptake, pmol·mg protein−1·30 min−1 | P Value |
|---|---|---|
| Control | 16.20 ± 0.7 | |
| Pyridoxine | 5.41 ± 0.30 | <0.01 |
| P-5-P | 8.57 ± 0.71 | <0.01 |
| Pyridoxamine | 7.07 ± 0.58 | <0.01 |
| 4-Pyridoxic acid | 16.83 ± 0.68 | NS |
| Penicillamine | 17.51 ± 1.01 | NS |
Data are means ± SE of 8 separate uptake determinations. Cells were incubated in Krebs-Ringer buffer (pH 7.4; 30 min; 37°C) in the presence of [3H] pyridoxine (7.5 nM) and 1 mM of the compound under investigation. PAC, pancreatic acinar cells; P-5-P, pyridoxal 5-phosphate; NS, not significant.
To determine the kinetic parameters (apparent Km and Vmax) of the pyridoxine uptake process of primary hPACs, we examined the initial rate of pyridoxine uptake as a function of concentration (0.05 to 10 μM). The results (Fig. 4) showed a clear saturation in the substrate uptake. Kinetic parameters of the saturable process were then determined and found to be 1.84 ± 0.35 μM and 8.97 ± 0.63 nmol·mg protein−1·30 min−1for the apparent Km and Vmax, respectively.
Fig. 4.
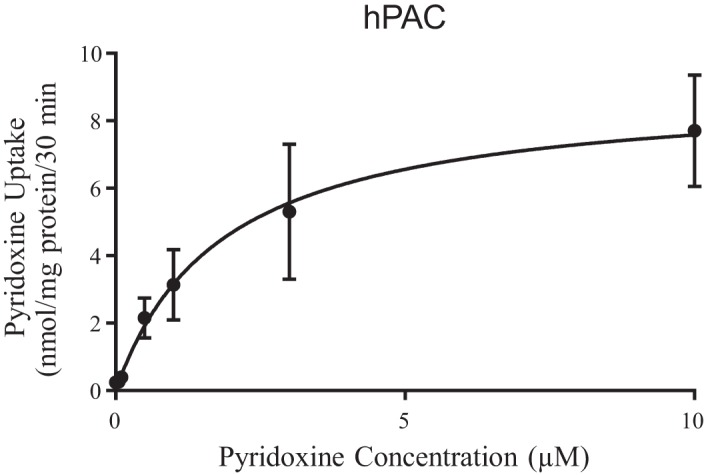
Uptake of pyridoxine as a function of concentration in primary human pancreatic acinar cells (hPACs). Cells were incubated at 37°C in Krebs-Ringer buffer at pH 7.4 in the presence of different concentrations of pyridoxine. Uptake by the saturable component was calculated as detailed in materials and methods. Data are means ± SE of 3–4 separate uptake determinations.
Collectively, these above-mentioned observations with primary hPACs demonstrate the involvement of a specific carrier-mediated mechanism for pyridoxine uptake; the results also confirm the suitability of the mouse-derived PAC 266-6 as a model system to delineate cellular and molecular aspects of vitamin B6 uptake by PACs.
Regulation of the Pyridoxine Uptake Process of PACs: Studies Utilizing PAC 266-6 and Primary hPACs
Regulation by intracellular regulatory pathways.
We examined possible involvement of intracellular regulatory pathways in the regulation of pyridoxine uptake by PACs. We focused on those pathways that have been shown to play a role in regulating uptake of other substrates (including other members of the water-soluble family of vitamins) in PAC and other cellular systems, e.g., Ca2+/calmodulin-, protein kinase A (PKA)-, and protein kinase C (PKC)-mediated pathways (5, 15, 17–19). The role for the Ca2+/calmodulin-mediated pathway was tested by examining the effect of pretreating PAC 266-6 (for 1 h) with specific inhibitors of this pathway, i.e., trifluoperazine (TFP) and calmidazolium (both at 50 μM), on the carrier-mediated pyridoxine (7.5 nM) uptake. The results showed that these compounds caused a significant (P < 0.01 for both) inhibition in initial rate of pyridoxine uptake (115.9 ± 4.3, 61.9 ± 6.7 and 73.1 ± 5.8 pmol·mg protein−1·30 min−1 for control and following pretreatment with TFP and calmidazolium, respectively). We also examined the effects of pretreatment with calmidazolium (50 µM) on kinetic parameters (Vmax and apparent Km) of the pyridoxine-uptake process of PAC 266-6. The results (Fig. 5) showed calmidazolium to cause a significant inhibition in the apparent Km and Vmax of the pyridoxine-uptake process [Km of 0.88 ± 0.22 and 0.23 ± 0.07 μM (P < 0.05); Vmax of 7.9 ± 0.62 and 2.59 ± 0.19 nmol·mg protein−1·30 min−1 (P < 0.05) for control and calmidazolium-treated cells, respectively). Similarly, treating primary hPACs with TFP and calmidazolium (both at 50 μM) led to a significant (P < 0.01 for both) inhibition in the carrier-mediated pyridoxine uptake (12.48 ± 1.1, 4.45 ± 0.47 and 5.67 ± 0.64 pmol·mg protein−1·30 min−1 for controls, in the presence of TFP and calmidazolium, respectively).
Fig. 5.
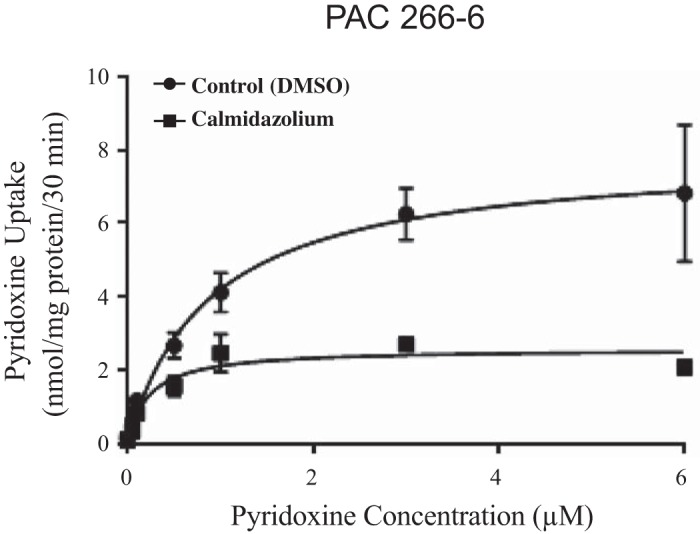
Effect of calmidazolium on kinetic parameters of pyridoxine uptake by pancreatic acinar cells (PAC) 266-6. Cells were pretreated (for 1 h) with 50 µM calmidazolium (control cells were treated with DMSO only). Uptake was performed (for 30 min) in Krebs-Ringer buffer (pH 7.4, 37°C) in the presence of different concentrations of pyridoxine. Uptake by the saturable component was calculated as detailed in materials and methods. Data are means ± SE of 6–8 separate uptake determinations.
In contrast to the role of the Ca2+/calmodulin-mediated pathway in regulating pyridoxine uptake by PACs, no role for the PKA-mediated pathway was observed. This is based on the observation that treatment of PAC 266 cells with dibutyryl cAMP (DBcAMP; 5 mM) failed to affect pyridoxine uptake (107.3 ± 7.7 and 118.8 ± 6.6 pmol·mg protein−1·30 min−1 for control and presence of DBcAMP, respectively). Similarly, modulators of the PKC-mediated pathway (all at 1 μM) failed to affect the initial rate of pyridoxine (7.5 nM) uptake (109.4 ± 9.3, 100.6 ± 2.7, 96.7 ± 6.7, and 96.1 ± 7.7 pmol·mg protein−1·30 min−1 for controls and in the presence of PMA, Bisindolylmaleimide I, and chelerythrin, respectively).
Adaptive regulation of pyridoxine uptake by PACs by extracellular substrate level.
In this study, we examined the effect of pyridoxine availability in the extracellular environment on carrier-mediated pyridoxine uptake by PAC 266-6 as well as primary hPACs. In both cases, cells were maintained in pyridoxine-deficient and -oversupplemented (1 mM) culture medium followed by examination of the initial rate of [3H]pyridoxine (7.5 nM) uptake. In the case of PAC 266-6, the results showed a significantly (P < 0.01) lower [3H]pyridoxine uptake by cells maintained (for 24 h) under the pyridoxine-oversupplemented condition compared with those maintained under the deficient condition (68.6 ± 3.7 and 181.3 ± 1.8 pmol·mg protein−1·30 min−1, respectively; similar results were obtained when PAC 266-6 were maintained under the pyridoxine-oversupplemented and -deficient conditions for 14 days; data not shown).
Similarly, maintaining (for 24 h) primary hPACs in pyridoxine-oversupplemented culture medium led to a significantly (P < 0.01) lower initial rate of [3H]pyridoxine uptake compared with uptake by cells maintained in a pyridoxine-deficient medium (4.71 ± 1.06 and 11.17 ± 0.49 pmol·mg protein−1·30 min−1, respectively).
Effect of Environmental Factors on Pyridoxine Uptake by PACs: Studies Utilizing PAC 266-6 and Primary hPACs
Cigarette smoke has been shown to negatively impact the physiology of PACs as well as that of other cells, including transport events across the cell membrane (1, 11, 12, 26, 30). In this study, we examined the effect of chronic exposure of PAC 266-6 to cigarette smoke extract (CSE) on the carrier-mediated uptake of pyridoxine (7.5 nM). For this, cells were pretreated with 20 ng/mL CSE for 48 h and then used for uptake investigations. The results showed a significant (P < 0.01) decrease in initial rate of pyridoxine uptake (125.6 ± 9.2 and 53.8 ± 8.6 pmol·mg protein−1·30 min−1 for control cells and those exposed with CSE, respectively). Similarly, exposing primary hPACs to CSE (20 μg/mL, 48 h) led to a significant (P < 0.01) inhibition in initial rate of carrier-mediated pyridoxine (7.5 nM) uptake (14.39 ± 0.56 and 7.02 ± 0.65 pmol·mg protein−1·30 min−1 for control cells and those treated with CSE, respectively).
In other studies, we examined the effect of prolonged exposure of PAC 266-6 to the bacterial endotoxin lipopolysaccharide (LPS) and to bacterial flagellin on carrier-mediated uptake of pyridoxine (7.5 nM) by PAC 266-6. These bacterial products exert profound effects on the physiology of a variety of cell types including PACs (3, 23, 27). For this, cells were exposed to various concentrations of LPS (0.1, 1, and 10 μg/mL) for 48 h followed by examination of [3H]pyridoxine uptake. The results showed a lack of effect of LPS on carrier-mediated pyridoxine uptake (109.3 ± 6.8, 105.6 ± 15.8, 116.2 ± 6.3, and 123.7 ± 11.1 pmol·mg protein−1·30 min−1 for controls and LPS- (0.1, 1, and 10 μg/mL) exposed cells, respectively). Similarly, treating PAC 266-6 with flagellin (100 ng/mL, 24 h) was without a significant effect on [3H]pyridoxine uptake (93.2 ± 2.4 and 114.4 ± 6.0 pmol·mg protein−1·30 min−1 for control cells and those treated with flagellin, respectively).
Effect of Pyridoxine Level on Gene Expression in PACs
In this study, we aimed to understand the role of pyridoxine availability in the regulation of gene expression of PACs. Previous studies have examined the effect of pyridoxine availability on gene expression of selective pancreatic enzymes (4). To acquire a comprehensive view of gene expression changes of the whole transcriptome based on availability of pyridoxine, we performed next-generation RNA-Seq with mRNA libraries generated from PAC 266-6 maintained under pyridoxine-deficient and -oversupplemented conditions for 14 days. The total RNA isolated from these cells was subjected to RNA-Seq analysis. The results were analyzed as mentioned in materials and methods.
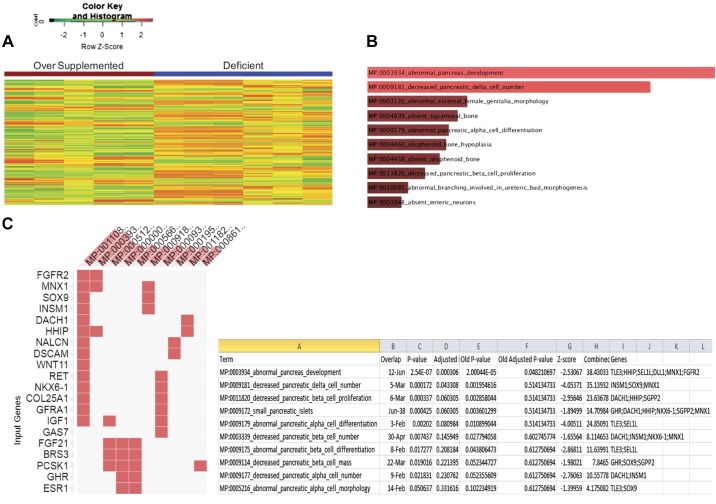
Differential gene expression analysis showed a change in the expression profile of the mRNA in the oversupplemented group versus the deficient group. In particular, we detected 525 genes differentially expressed in pyridoxine-deficient cells (Fig. 6A, and see Supplemental Table S1: https://dx.doi.org/10.17504/protocols.io.3zwgp7e). This includes genes that are involved in pancreatic health and development as shown by the EnrichR Pathway Analysis (http://amp.pharm.mssm.edu/Enrichr/) (Fig. 6, B and C).
Fig. 6.
RNA-Seq analysis of mouse pancreatic acinar cells (PAC) 266-6 maintained in pyridoxine-deficient and -oversupplemented media. A: heatmap of differentially expressed genes in pyridoxine-oversupplemented and -deficient samples. B: mouse phenotype gene set enrichment analysis in enrichR using differentially expressed genes. C: differentially expressed genes in the enriched mouse phenotype.
DISCUSSION
As an essential micronutrient, vitamin B6 plays an important role in the exocrine function of the pancreas (4, 22). However, the physiological aspects/mechanism of vitamin B6 uptake and the effect of its availability on the gene expression profile of PACs have not been explored previously. The aim of this study was to address these issues using mouse-derived PAC 266-6 and primary hPACs. The use of these cell models to investigate different aspects of PAC physiology and molecular biology has been well established by us and others previously (11, 21, 23–25). Our results showed pyridoxine uptake by PAC 266-6 to be pH dependent and temperature sensitive; it was, however, Na+ independent in nature. In addition, pyridoxine uptake was found to be carrier mediated, as indicated by the cis-inhibition in [3H]pyridoxine uptake by unlabeled substrate, the trans-stimulation in [3H]pyridoxine efflux by unlabeled pyridoxine, and the clear saturation in the initial rate of uptake as a function of substrate concentration (apparent Km of 0.17 ± 0.06 μM).
To confirm and extend our findings on pyridoxine uptake observed with the mouse-derived PAC-266 to the human situation, we used primary hPACs obtained from organ donors (see materials and methods). The results again showed the involvement of a carrier-mediated mechanism in the uptake process, as indicated by the saturation as a function of substrate concentration (apparent Km 1.32 ± 0.28 μM). It is interesting to note that the level of uptake of pyridoxine by primary hPAC is lower than its level of uptake by mouse PAC 266-6. This is most likely due to differences in cells maintained in culture indefinitely compared with primary cells. Also, the uptake process was found to be specific for pyridoxine, as suggested by the inhibition caused by related (but not unrelated) compounds. The similarities observed in the uptake of pyridoxine by the murine-derived PAC 266-6 and primary hPACs justify the use of the former as a model for further characterization of pyridoxine physiology in PACs. The molecular identity of the pyridoxine uptake system of mammalian PACs (or of any other mammalian cell type) is not known at present; this issue awaits elucidation.
We also examined the regulation of pyridoxine uptake by intracellular and extracellular factors. Intracellular pathways like Ca2+/calmodulin-, PKA-, and PKC-mediated pathways have been shown to regulate various membrane transport events, including those involved in the uptake of other water-soluble vitamins (5, 15, 17–19). Our results showed a role for the Ca2+/calmodulin pathway in regulating pyridoxine uptake in both PAC-266-6 as well as the primary hPACs. Pretreatment of PACs with specific modulators of the Ca2+/calmodulin pathway, namely calmidazolium and TFP, was found to lead to a significant inhibition in carrier-mediated pyridoxine uptake; this inhibition appeared to affect both the apparent Km and Vmax of the pyridoxine uptake process. No role for PKA- and PKC-mediated pathways in the regulation of pyridoxine uptake by PAC 266-6, however, was observed. The lack of a role for the PKA-mediated pathway in regulating pyridoxine uptake by PACs is in contrast to its involvement in the regulation of the vitamin uptake by intestinal epithelial cells (17). The pyridoxine uptake process of PAC 266-6 and primary hPACs was also found to be adaptively regulated by substrate availability in the extracellular media. Maintaining these cells in pyridoxine-deficient medium was found to lead to a significant induction in initial rate of [3H]pyridoxine uptake compared with uptake by cells maintained in a pyridoxine-oversupplemented medium.
Environmental factors like cigarette smoke and its components as well as bacterial toxins have been shown to negatively impact the physiology of PACs, including their ability to take in other water-soluble vitamins (23–26). Our findings in the current investigation showed that exposure of both PAC-266-6 and primary hPACs to CSE (but not to the bacterial LPS and flagellin) led to a significant inhibition in pyridoxine uptake compared with untreated controls. Further studies are required to uncover the molecular mechanism(s) that mediated this inhibition.
Availability of vitamins has been shown to affect the expression of a variety of genes in different cellular systems (2, 7, 9, 16); however, there is limited knowledge regarding the effect of pyridoxine availability on global gene expression. Although an effect of pyridoxine availability on expression of total RNA in PACs (22) and a few selected genes (amylase and trypsin) has been reported (4), a comprehensive analysis of the whole transcriptome under conditions of altered pyridoxine level has not been investigated. We addressed this issue using RNA-Seq analysis of RNA from PAC 266-6 maintained for 14 days in pyridoxine-deficient and -oversupplemented conditions. Pyridoxine availability was found to significantly affect the expression of 525 genes, including genes that are important for pancreatic health (e.g., PRSS1) as well as those that encode transcriptional regulators (e.g., Tle3, HHIP, and Sel1) that are relevant to pancreatic development (8, 10, 14, 28).
In summary, the results of this study show, for the first time, the existence of a specific and regulatable carrier-mediated mechanism for pyridoxine uptake by mammalian PACs. The results also demonstrate that pyridoxine availability affects the gene expression profile of PACs, including genes that are relevant to pancreatic health and development.
GRANTS
This study was supported by grants from the National Institutes of Health (AA-018071, DK-56061, and DK-58057) and the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.S., V.R., and H.M.S. conceived and designed research; P.S., V.R., C.W.H., and B.D.C. performed experiments; P.S., V.R., and J.W. analyzed data; P.S., V.R., and J.W. interpreted results of experiments; P.S., V.R., and J.W. prepared figures; P.S. drafted manuscript; P.S., V.R., J.W., and H.M.S. edited and revised manuscript; P.S., V.R., J.W., C.W.H., B.D.C., and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Alexandre M, Pandol SJ, Gorelick FS, Thrower EC. The emerging role of smoking in the development of pancreatitis. Pancreatology 11: 469–474, 2011. doi: 10.1159/000332196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canali R, Natarelli L, Leoni G, Azzini E, Comitato R, Sancak O, Barella L, Virgili F. Vitamin C supplementation modulates gene expression in peripheral blood mononuclear cells specifically upon an inflammatory stimulus: a pilot study in healthy subjects. Genes Nutr 9: 390, 2014. doi: 10.1007/s12263-014-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding SP, Li JC, Jin C. A mouse model of severe acute pancreatitis induced with caerulein and lipopolysaccharide. World J Gastroenterol 9: 584–589, 2003. doi: 10.3748/wjg.v9.i3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubick MA, Gretz D, Majumdar AP. Overt vitamin B-6 deficiency affects rat pancreatic digestive enzyme and glutathione reductase activities. J Nutr 125: 20–25, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Ghosal A, Said HM. Mechanism and regulation of vitamin B2 (riboflavin) uptake by mouse and human pancreatic β-cells/islets: physiological and molecular aspects. Am J Physiol Gastrointest Liver Physiol 303: G1052–G1058, 2012. doi: 10.1152/ajpgi.00314.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gospe SM., Jr Pyridoxine-dependent seizures: findings from recent studies pose new questions. Pediatr Neurol 26: 181–185, 2002. doi: 10.1016/S0887-8994(01)00407-6. [DOI] [PubMed] [Google Scholar]
- 7.Han SN, Adolfsson O, Lee CK, Prolla TA, Ordovas J, Meydani SN. Vitamin E and gene expression in immune cells. Ann N Y Acad Sci 1031: 96–101, 2004. doi: 10.1196/annals.1331.010. [DOI] [PubMed] [Google Scholar]
- 8.Kawahira H, Ma NH, Tzanakakis ES, McMahon AP, Chuang PT, Hebrok M. Combined activities of hedgehog signaling inhibitors regulate pancreas development. Development 130: 4871–4879, 2003. doi: 10.1242/dev.00653. [DOI] [PubMed] [Google Scholar]
- 9.León-Del-Río A. Biotin-dependent regulation of gene expression in human cells. J Nutr Biochem 16: 432–434, 2005. doi: 10.1016/j.jnutbio.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Francisco AB, Munroe RJ, Schimenti JC, Long Q. SEL1L deficiency impairs growth and differentiation of pancreatic epithelial cells. BMC Dev Biol 10: 19, 2010. doi: 10.1186/1471-213X-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lugea A, Gerloff A, Su HY, Xu Z, Go A, Hu C, French SW, Wilson JS, Apte MV, Waldron RT, Pandol SJ. The combination of alcohol and cigarette smoke induces endoplasmic reticulum stress and cell death in pancreatic acinar cells. Gastroenterology 153: 1674–1686, 2017. doi: 10.1053/j.gastro.2017.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malfertheiner P, Schütte K. Smoking—a trigger for chronic inflammation and cancer development in the pancreas. Am J Gastroenterol 101: 160–162, 2006. doi: 10.1111/j.1572-0241.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 13.Merrill AH Jr, Henderson JM. Diseases associated with defects in vitamin B6 metabolism or utilization. Annu Rev Nutr 7: 137–156, 1987. doi: 10.1146/annurev.nu.07.070187.001033. [DOI] [PubMed] [Google Scholar]
- 14.Metzger DE, Gasperowicz M, Otto F, Cross JC, Gradwohl G, Zaret KS. The transcriptional co-repressor Grg3/Tle3 promotes pancreatic endocrine progenitor delamination and β-cell differentiation. Development 139: 1447–1456, 2012. doi: 10.1242/dev.072892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reidling JC, Subramanian VS, Dahhan T, Sadat M, Said HM. Mechanisms and regulation of vitamin C uptake: studies of the hSVCT systems in human liver epithelial cells. Am J Physiol Gastrointest Liver Physiol 295: G1217–G1227, 2008. doi: 10.1152/ajpgi.90399.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Melendez R, Zempleni J. Regulation of gene expression by biotin. J Nutr Biochem 14: 680–690, 2003. doi: 10.1016/j.jnutbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Said HM, Ortiz A, Ma TY. A carrier-mediated mechanism for pyridoxine uptake by human intestinal epithelial Caco-2 cells: regulation by a PKA-mediated pathway. Am J Physiol Cell Physiol 285: C1219–C1225, 2003. doi: 10.1152/ajpcell.00204.2003. [DOI] [PubMed] [Google Scholar]
- 18.Said HM, Ortiz A, McCloud E, Dyer D, Moyer MP, Rubin S. Biotin uptake by human colonic epithelial NCM460 cells: a carrier-mediated process shared with pantothenic acid. Am J Physiol Cell Physiol 275: C1365–C1371, 1998. doi: 10.1152/ajpcell.1998.275.5.C1365. [DOI] [PubMed] [Google Scholar]
- 19.Said HM, Ortiz A, Vaziri ND. Mechanism and regulation of vitamin B(6) uptake by renal tubular epithelia: studies with cultured OK cells. Am J Physiol Renal Physiol 282: F465–F471, 2002. doi: 10.1152/ajprenal.00267.2001. [DOI] [PubMed] [Google Scholar]
- 20.Said ZM, Subramanian VS, Vaziri ND, Said HM. Pyridoxine uptake by colonocytes: a specific and regulated carrier-mediated process. Am J Physiol Cell Physiol 294: C1192–C1197, 2008. doi: 10.1152/ajpcell.00015.2008. [DOI] [PubMed] [Google Scholar]
- 21.Singh L, Bakshi DK, Vasishta RK, Arora SK, Majumdar S, Wig JD. Primary culture of pancreatic (human) acinar cells. Dig Dis Sci 53: 2569–2575, 2008. doi: 10.1007/s10620-007-0162-1. [DOI] [PubMed] [Google Scholar]
- 22.Singh M. Effect of vitamin B6 deficiency on pancreatic acpinar cell function. Life Sci 26: 715–724, 1980. doi: 10.1016/0024-3205(80)90261-1. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan P, Anandam KY, Ramesh V, Geltz ET, Said HM. Effect of bacterial flagellin on thiamin uptake by human and mouse pancreatic acinar cells: inhibition mediated at the level of transcription of thiamin transporters 1 and 2. Am J Physiol Gastrointest Liver Physiol 316: G735–G743, 2019. doi: 10.1152/ajpgi.00048.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasan P, Nabokina S, Said HM. Chronic alcohol exposure affects pancreatic acinar mitochondrial thiamin pyrophosphate uptake: studies with mouse 266-6 cell line and primary cells. Am J Physiol Gastrointest Liver Physiol 309: G750–G758, 2015. doi: 10.1152/ajpgi.00226.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivasan P, Thrower EC, Gorelick FS, Said HM. Inhibition of pancreatic acinar mitochondrial thiamin pyrophosphate uptake by the cigarette smoke component 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Am J Physiol Gastrointest Liver Physiol 310: G874–G883, 2016. doi: 10.1152/ajpgi.00461.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasan P, Thrower EC, Loganathan G, Balamurugan AN, Subramanian VS, Gorelick FS, Said HM. Chronic nicotine exposure in vivo and in vitro inhibits vitamin b1 (thiamin) uptake by pancreatic acinar cells. PLoS One 10: e0143575, 2015. doi: 10.1371/journal.pone.0143575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaccaro MI, Calvo EL, Suburo AM, Sordelli DO, Lanosa G, Iovanna JL. Lipopolysaccharide directly affects pancreatic acinar cells: implications on acute pancreatitis pathophysiology. Dig Dis Sci 45: 915–926, 2000. doi: 10.1023/A:1005521007609. [DOI] [PubMed] [Google Scholar]
- 28.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 14: 141–145, 1996. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson GN. Statistical estimations in enzyme kinetics. Biochem J 80: 324–332, 1961. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittel UA, Singh AP, Henley BJ, Andrianifahanana M, Akhter MP, Cullen DM, Batra SK. Cigarette smoke-induced differential expression of the genes involved in exocrine function of the rat pancreas. Pancreas 33: 364–370, 2006. doi: 10.1097/01.mpa.0000240601.80570.31. [DOI] [PubMed] [Google Scholar]