Abstract
This study explored the mechanism by which Ca2+-activated Cl− channels (CaCCs) encoded by the Tmem16a gene are regulated by calmodulin-dependent protein kinase II (CaMKII) and protein phosphatases 1 (PP1) and 2A (PP2A). Ca2+-activated Cl− currents (IClCa) were recorded from HEK-293 cells expressing mouse TMEM16A. IClCa were evoked using a pipette solution in which free Ca2+ concentration was clamped to 500 nM, in the presence (5 mM) or absence of ATP. With 5 mM ATP, IClCa decayed to <50% of the initial current magnitude within 10 min after seal rupture. IClCa rundown seen with ATP-containing pipette solution was greatly diminished by omitting ATP. IClCa recorded after 20 min of cell dialysis with 0 ATP were more than twofold larger than those recorded with 5 mM ATP. Intracellular application of autocamtide-2-related inhibitory peptide (5 µM) or KN-93 (10 µM), two specific CaMKII inhibitors, produced a similar attenuation of TMEM16A rundown. In contrast, internal application of okadaic acid (30 nM) or cantharidin (100 nM), two nonselective PP1 and PP2A blockers, promoted the rundown of TMEM16A in cells dialyzed with 0 ATP. Mutating serine 528 of TMEM16A to an alanine led to a similar inhibition of TMEM16A rundown to that exerted by either one of the two CaMKII inhibitors tested, which was not observed for three putative CaMKII consensus sites for phosphorylation (T273, T622, and S730). Our results suggest that TMEM16A-mediated CaCCs are regulated by CaMKII and PP1/PP2A. Our data also suggest that serine 528 of TMEM16A is an important contributor to the regulation of IClCa by CaMKII.
Keywords: Ano-1, calcium-activated chloride channel, CaMKII, regulation, TMEM16A
INTRODUCTION
Ca2+-activated Cl− currents (IClCa) recorded from many cell types have been shown to exhibit rundown following membrane rupture in the whole cell recording mode or after patch excision to monitor single CaCC activity, which led investigators to speculate that the channels are the target of posttranslational regulation (15, 22, 23, 29, 33). Wang and Kotlikoff (46) demonstrated for the first time that the rundown of IClCa in airway smooth muscle cells was attributed to phosphorylation involving the serine-threonine kinase calmodulin-dependent protein kinase II (CaMKII). A later study by our group showed that CaMKII-induced phosphorylation similarly reduced IClCa in rabbit coronary and pulmonary arterial smooth muscle cells but not in portal vein myocytes (13). In accordance with the hypothesis that CaCCs in arterial smooth muscle cells are regulated by phosphorylation was the demonstration that the rundown of IClCa was as follows: 1) reversed by omitting ATP in the pipette solution or by replacing the nucleotide with a nonhydrolyzable form of ATP, AMP-PNP (1, 2, 47); and 2) antagonized by the Ca2+-dependent serine-threonine protein phosphatase calcineurin (2, 14, 24) and protein phosphatases 1 (PP1) and 2A (PP2A) (2). Because CaCCs exert a powerful depolarizing stimulus that can sustain Ca2+ influx through L-type Ca2+ channels (CaL) that itself reinforces CaCC activation, it is hypothesized that Ca2+-dependent downregulation by CaMKII-induced phosphorylation may offer a means to attenuate the impact of the positive feedback loop between CaL and CaCCs (4a, 13, 22, 23, 36).
The discovery of the Tmem16 or Anoctamin gene family encoding for CaCCs by three independent groups (5, 34, 49) paved the way for the molecular characterization of their biophysical properties, molecular assembly and membrane topology, functional role, and regulation in various cell types. The first two members of this family of 10 paralogs, TMEM16A (anoctamin-1 or ANO1) and TMEM16B (anoctamin-2 or ANO2), are confirmed CaCC pore-forming subunits whose properties resemble those of CaCCs characterized in native cells (16, 22, 31, 43). While the expression of ANO2 is low in VSMCs (7), TMEM16A is abundantly expressed in these cells and is believed to be the major CaCC contributing to IClCa and its impact on the membrane depolarization and contraction of VSMCs mediated by endogenous vasoconstrictors (7, 8, 11, 17, 22, 26, 38, 41, 45).
The molecular mechanism(s) responsible for the regulation and more specifically the rundown of TMEM16A remains largely undefined. A recent study provided evidence that serine 727 of mouse TMEM16A is the target amino acid responsible for the downregulation of CaCCs by CaMKIIγ in basilar artery smooth muscle cells, a posttranslational modification suggested to be involved in regulating smooth muscle cell growth in cerebral blood vessels in an animal model of systemic hypertension (25). The goals of the present study were as follows: 1) to assess whether TMEM16A-induced IClCa is regulated by ATP, CaMKII, and PP1/PP2A in a manner analogous to that of CaCCs in arterial myocytes (2, 13, 14, 24, 47); and 2) to identify the amino acid residue(s) that may be targeted by CaMKII and responsible, at least in part, for TMEM16A-induced CaCC inhibition by phosphorylation.
MATERIALS AND METHODS
Cell culture.
HEK-293 cells (ATCC, Manassas, VA) were maintained in DMEM (Thermo Fisher Scientific, Waltham, MA) with 10% (vol/vol) heat-inactivated fetal bovine serum (Thermo Fisher Scientific) and 1% (vol/vol) penicillin/streptomycin (Thermo Fisher Scientific). All cells were incubated in a humidified environment containing 5% CO2 at 37°C. Once the cells were 80–100% confluent, they were then trypsinized with 0.05% trypsin-EDTA (Thermo Fisher Scientific) and plated into T25 cell culture flasks to maintain the cell line for transient transfections of mouse TMEM16A for electrophysiological experiments.
Immunocytochemical staining and confocal microscopy imaging.
Nontransfected HEK-293 cells grown on collagen I-coated glass coverslips (Neuvitro, Vancouver, WA) were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature. After washing in PBS, the cells were blocked and permeabilized with 1% (wt/vol) bovine serum albumin and 0.01% Triton X-100 (Sigma-Aldrich, St. Louis, MO) in PBS for 1 h before incubation overnight at 4°C with primary goat polyclonal antibodies to PP1α (sc-6105; Santa Cruz Biotechnology, Dallas, TX), PP1β (sc-6107; Santa Cruz Biotechnology), and PP1γ (sc-6109; Santa Cruz Biotechnology) or rabbit monoclonal PP2A (52F8; Cell Signaling Technology, Danvers, MA) and rabbit polyclonal CaMKII (sc-9035; Santa Cruz Biotechnology). For control experiments (2nd antibody only), the primary antibody was omitted. After repeated washing with PBS, the coverslips were subsequently incubated with goat anti-rabbit-IgG Alexa Fluor 546 (A-11010; Invitrogen, Grand Island, NY) or donkey anti-goat-IgG Alexa Fluor 488 (A-21447; Invitrogen) diluted in blocking buffer (1:500) for 1 h at room temperature and washed again in PBS. Fluoroshield mounting medium with 4′,6-diamino-2-phenylindole (DAPI; Abcam, Cambridge, UK) was used to mount coverslips onto precleaned microscope slides. Control slides were processed using secondary antibodies only. Slides were viewed and photographed using an Olympus FluoView FV 1000 laser scanning confocal microscope with an Olympus 60X PlanApo_N 1.42 NA oil immersion objective. DAPI and secondary antibodies were visualized using 405 nm and 488 or 546 lines, respectively.
Western blot analysis.
Nontransfected HEK-293 cells were collected and lysed in RIPA lysis buffer containing a cocktail of protease inhibitors. Proteins were separated in 4–12% NuPAGE (Thermo Fisher Scientific) and transferred into nitrocellulose membranes (0.45 μm; cat. no. 162-0215; Bio-Rad, Hercules, CA). The membranes were blocked with LI-COR Odyssey blocking buffer (part no. 92740000; Lincoln, NE) in PBS (1:1) for 1 h at room temperature and incubated overnight at 4°C with the following primary antibodies [all diluted 1:1000 in LI-COR Odyssey blocking buffer in PBS (1:1) with 0.01% Tween-20]: rabbit polyclonal CaMKII (sc-9035; Santa Cruz Biotechnology, Dallas, TX), rabbit monoclonal PP2A (52F8D8; Cell Signaling Technology, Danvers, MA), goat polyclonal PP1α (sc-6105; Santa Cruz Biotechnology), goat polyclonal PP1β (Santa Cruz Biotechnology, sc-6107), or goat polyclonal PP1γ (sc-6109; Santa Cruz Biotechnology) and rabbit polyclonal GAPDH (sc-25778; Santa Cruz Biotechnology) or goat polyclonal β-actin (sc-1616; Santa Cruz Biotechnology). The membranes were incubated with Alexa Fluor 680 or 800 conjugated goat anti-rabbit IgG or donkey anti-goat (diluted 1:25,000 in LI-COR Odyssey blocking buffer in PBS (1:1) with 0.01% Tween-20) for 1 h at room temperature. Signals were detected using Odyssey Infrared Imaging System (LI-COR) near infrared wavelengths using the 700 nm and 800 nm channels. All experiments were performed in triplicate.
RNA isolation and cDNA synthesis from HEK-293 cells.
mRNA was isolated from cultured nontransfected HEK-293 cells using PureZol (Bio-Rad). Prior to cDNA preparation, RNA was treated with DNase I (Invitrogen) to prevent genomic DNA contamination. cDNA was prepared using oligo(dT) and dNTP mixtures with Superscript III (Invitrogen). PCR primers were designed against the association domain for all four isoforms of CaMKII (α, β, γ, and δ), and all primer sets spanned multiple exons (Table 1). All primers were synthesized by Integrated DNA Technologies (Coralville, IA). Amplification of cDNA was performed using GoTaqHot Start Polymerase (Promega, Madison, WI), which had an amplification profile consisting of an initial step to 95°C for 2 min to activate the Amplitaq polymerase, followed by 36 cycles of denaturation at 95°C for 30 s, annealing at Ta (in °C; the optimal annealing temperature for each primer pair; range: 60–63°C) for 30 s, and extension at 72°C for 5 min. The amplified products (10 µl) were separated by electrophoresis on a 2% agarose/Tris, acetic acid, EDTA gel, and the DNA bands were visualized by ethidium bromide staining. A nontemplate control for the master mix of reagents made for each primer pair was run on the gels to ensure lack of contaminants in the sample.
Table 1.
Primer sequences for detection of CaMKII transcripts
| Gene | Primer Sequence | GenBank Accession No. | Amplicon, bp | Region Spanned |
|---|---|---|---|---|
| CaMKIIα | ||||
| Forward | 5′-TCCACCGTGGCATCCTGCAT-3′ | NM_015981.3 | 452 | 1,038–1,489 |
| Reverse | 5′-ATGGTGGTGTGCACGGGCTT-3′ | |||
| CaMKIIβ | ||||
| Forward | 5′-TGCATCCTCCTGCCAACGCA-3′ | NM_001220.4 | 396 | 2,654–3,049 |
| Reverse | 5′-TCGGCGAGAGGCCCAAAACT-3′ | |||
| CaMKIIγ | ||||
| Forward | 5′-AAGCGCATCACGGCTGACCA-3′ | NM_172171.2 | 413 | 905–1,317 |
| Reverse | 5′-ACCGAGCTGCCATTCCCAGT-3′ | |||
| CaMKIIδ | ||||
| Forward | 5′-TCGCAACTGCTTGCCACTCGT-3′ | NM_172127.2 | 396 | 479–874 |
| Reverse | 5′-TGTGGTCGAAGCCATCCTCGGT-3′ |
CaMKII, calmodulin-dependent protein kinase II.
Transient transfection of TMEM16A in HEK-293 cells.
The “a” variant of mouse TMEM16A (excluding exon 0, which encodes for an additional 57 amino acids upstream of the sequence used) was kindly provided by Dr. Lily Y. Jan, Howard Hughes Medical Institute, University of California, San Francisco. The full sequence used in our study is shown in Fig. 1. The full-length open reading frame was subcloned into pIRES-hrGFP-1a vector (Agilent Technologies, Santa Clara, CA). Two transfection methods were used during the course of this study. The first method involved transiently transfecting HEK-293 cells with 1–3 µg of mTMEM16A in a T25 cell culture flask using a 1:3 ratio of the X-tremeGENE 9 DNA Transfection Reagent (Roche Applied Science, Indianapolis, IN). Patch-clamp experiments were carried out 48–72 h posttransfection. In other experiments, cells grown at 80% confluency were transfected with 1.5 μg of plasmid DNA using the Lipofectamine 3000 Transfection Kit (Thermo Fisher Scientific) and OPTI-MEM (1×; Thermo Fisher Scientific) in a 35-mm dish. Because of the hightransfection efficiency of the latter protocol, electrophysiological experiments were carried out after only 24 h posttransfection and for a period not exceeding 48 h after transfection.
Fig. 1.
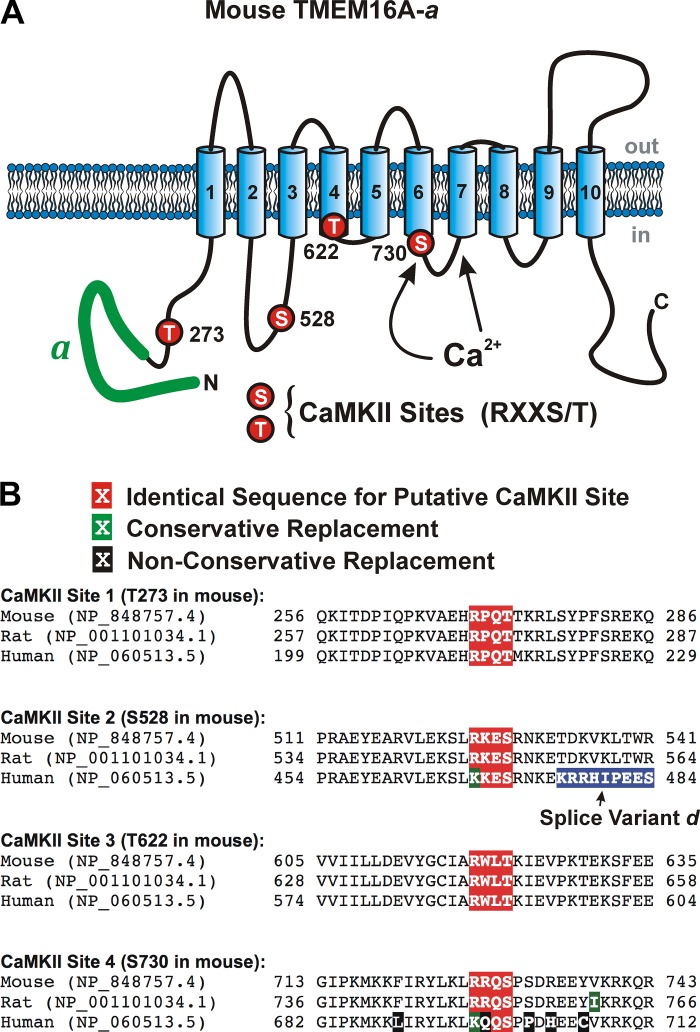
Mouse anoctamin-1 sequence used in our study (TMEM16A-a) with the position of the four predicted calmodulin-dependent protein kinase II (CaMKII) sites (RXXT/S). The mouse clone used in our study only included splice variant “a.” The position of the four hypothetical CaMKII phosphorylation sites shown in white font over a red background are relative to the National Center for Biotechnology Information protein database sequence NP_848757.4. Intracellular and extracellular domains are shown in blue and black fonts, respectively. Transmembrane domains are labeled with underlined green fonts. The sequence of splice variant “a” is shown in black font over a yellow background. TMDX, transmembrane domain X. The position of the transmembrane domains is based on the recent Cryo-EM study by Paulino et al. (30) revealing the quaternary structure of mouse TMEM16A.
Site-directed mutagenesis.
For the TMEM16A mutants S528A and T622A, single-point mutations and amino acid insertions were performed using the QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA). Mutagenic primers were designed against the putative CaMKII phosphorylation sites S528 and T622 of mouse TMEM16A replacing the serine residue with alanine leaving the flanking regions unmodified. Amplified products were purified using the QIAquick Gel Extraction Kit (Qiagen, Valencia, CA) and sequenced at the Nevada Genomic Center (Reno, NV) for verification. Thermal cycling parameters were set to conditions according to the manufacturers’ instructions. In short this included an initial step at 95°C for 1 min, followed by 18 cycles of denaturation at 95°C for 50 s, annealing at 60°C for 50 s, and extension at 68°C for 1 min/kb of plasmid length. The amplified products were treated with the Dpn I restriction enzyme (10 U/μl) for ~1 h at 37°C to digest parental methylated and hemimethylated DNA. The Dpn I-treated DNA was transformed using XL10-Gold ultracompetent cells (Agilent Techonologies) and optimized in NZY+ broth for 1 h before plating the reaction on ampicillin-treated agar plates. Agar plates were incubated for 12 h at 37°C. For the T273A and S730A mutations, those plasmids were generated by Mutagenex (Ohio State University, Columbus, OH), a company specializing in creating new plasmid constructs and mutations.
Whole cell patch-clamp electrophysiology.
IClCa resulting from mouse TMEM16A transiently transfected into HEK 293 cells were recorded using the conventional whole cell configuration of the patch-clamp technique using an Axopatch 200A amplifier (Molecular Devices-Axon, Foster City, CA), Digidata 1440A acquisition system (Molecular Devices-Axon), and pCLAMP 10 software (Molecular Devices-Axon). To examine the effects of phosphorylation-induced rundown, IClCa were elicited by pipette solutions containing either 0 or 5 mM ATP. The composition of the intracellular pipette solution also contained 10 mM BAPTA as the Ca2+ buffer, and free Ca2+concentration ([Ca2+]) was set to 500 nM by the addition of 7.08 mM CaCl2. Intracellular free [Ca2+] was calculated using the calcium chelator program Maxchelator (https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/). To reduce contamination of IClCa from other types of currents in our recordings, CsCl and TEA were added to the pipette solution and TEA was added to the K+-free external solution (see composition below). Series resistance compensation was performed in all experiments. Cells were continuously superfused in the recording chamber with the external solution at a flow rate of ~1 ml/min. All electrophysiological recordings were obtained at room temperature.
Experimental protocols.
IClCa were evoked immediately upon rupture of the cell membrane and its voltage-dependent properties were monitored every 10 s by stepping from a holding potential (HP) of −50 to +90 mV for 1 s, followed by repolarization to −80 mV for 1 s. Current-voltage (I-V) relationships were constructed after 10–15 min of cell dialysis by stepping in 10-mV increments from HP to test potentials between −100 and +140 mV for 1 s, each step followed by a 1 s repolarizing step to −80 mV. For I-V relationships, IClCa were expressed as current density (pA/pF) by dividing the current amplitude measured at the end of each voltage clamp step by the cell capacitance. For all figures showing a time course of IClCa changes, the late current measured at +90 mV was usually normalized to the amplitude of the initial current elicited at time = 0 (this is ~30 s after the membrane patch and measuring cell capacitance). After cell capacitance was measured, the constant step protocol described above was initiated to monitor the changes of IClCa over a 10- to 15-min period of intracellular dialysis.
Solutions.
The external solution used in all patch-clamp experiments had the following composition (in mM): 126 NaCl, 10 HEPES, 8.4 TEA, 20 glucose, 1.2 MgCl2, and 1.8 CaCl2. The pH was adjusted to 7.35 with NaOH. The internal pipette solution was composed of the following (in mM): 20 TEA, 106 CsCl, 10 HEPES, 10 BAPTA, 7.08 CaCl2, 0 or 5 ATP, 0.545 (0 ATP) or 3 (5 mM ATP) MgCl2 and 0.2 guanosine-triphosphate. All enzymes and analytical grade reagents were purchased from Sigma-Aldrich, St. Louis, MO. Drugs and peptides were purchased from the following sources: okadaic acid (OA), KN-93, and cantharidin from Sigma-Aldrich and autocamtide-2-related inhibitory peptide (ARIP) from Enzo Life Science (Farmingdale, NY).
Statistical analysis.
All pooled data are expressed as means with error bars representing the SE. Raw data were imported into Excel and the means were exported to OriginLab 9.3 software (OriginLab Corp., Northampton, MA) for plotting and testing of statistical significance between groups. Statistical significance between means was determined using paired or unpaired Student’s t test when two groups were compared or one-way ANOVA for multiple group comparisons and the Tukey post hoc test to determine which groups were statistically significant from each other. P < 0.05 was considered to be statistically significant. All graphs and current traces were uploaded to CorelDraw 12 (Ottawa, ON, Canada) for final processing of the figures.
RESULTS
We transiently expressed the mouse TMEM16A isoform containing only the “a” splice variant (see Fig. 1 for the complete amino acid sequence) in HEK-293 cells. A recent study by Paulino et al. (30) revealed a detailed structure of mouse TMEM16A using a Cryo-EM approach and proposed that the protein is composed of 10 instead of 8 transmembrane domains, which is consistent with that suggested for the fungal TMEM16 protein from Nectria haematococca (3). The clone used in our study comprises a 956 amino acid sequence that lacks the 57 amino acid segment recently reported by Mazzone et al. (28) (encoded by the so-called exon 0), which is upstream of splice variant “a.” Figure 1 also highlights the four putative CaMKII sites analyzed in our study (all serine or threonine positions are relative to the National Center for Biotechnology Information protein sequence NP_848757.4).
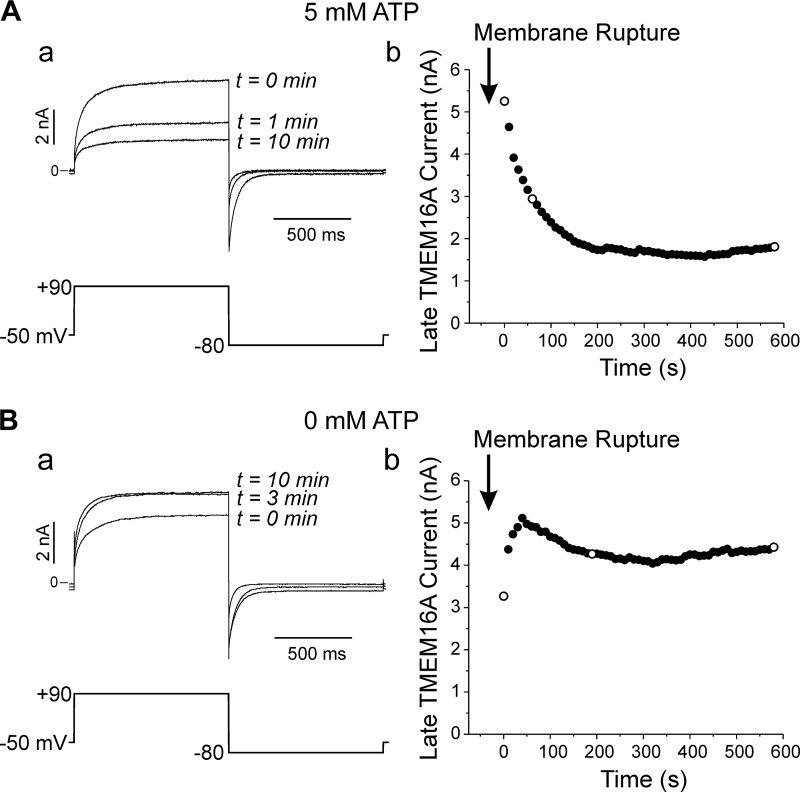
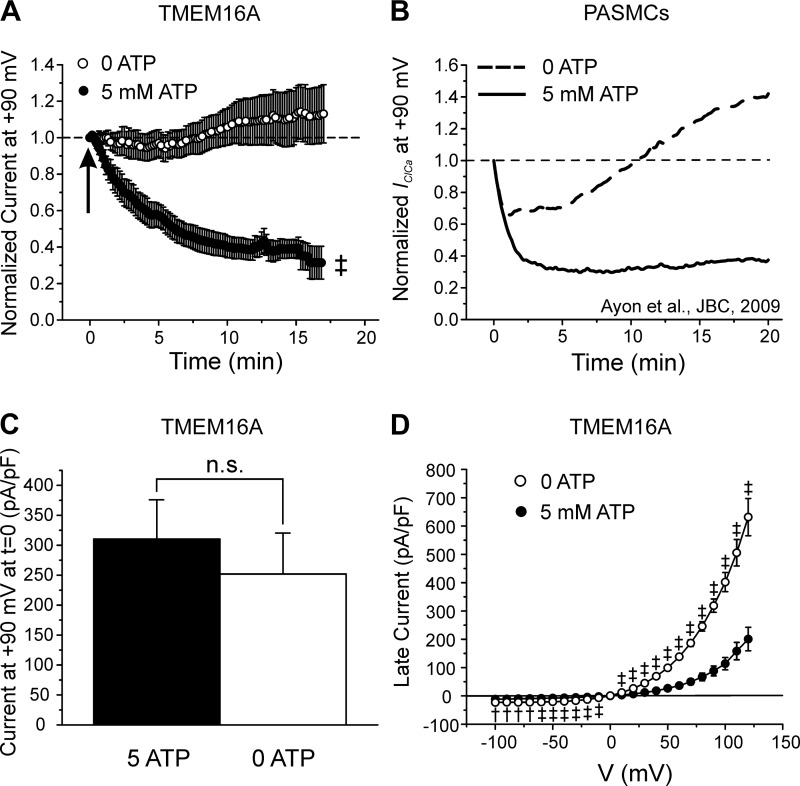
Figure 2Aa shows TMEM16A-mediated IClCa recorded at various times after seal rupture in a cell dialyzed with 500 nM free Ca2+ and 5 mM ATP. The currents were elicited by a repetitive voltage clamp protocol applied every 10 s (displayed below the current traces). A significant rundown was observed that reached a steady state within 5 to 10 min (Fig. 2Ab). In contrast, the experiment in Fig. 2B shows that removal of ATP from the pipette solution led to an initial increase in IClCa, which stabilized to a level that was higher than the initial current recorded immediately after breaking the seal. On average, TMEM16A-mediated IClCa decreased to 25.4 ± 2.8% (n = 5) relative to the initial current recorded after seal rupture over the course of 17 min of cell dialysis in the presence of 5 mM ATP, whereas the current in cells lacking ATP displayed little rundown and stabilized to 113 ± 16% (n = 9) relative to the initial current recorded at time = 0 (Fig. 3, A and B; 0 ATP vs. 5 mM ATP, P < 0.01). Although TMEM16A-induced IClCa in HEK-293 cells ran down more slowly, these data display remarkable similarity with the behavior of native IClCa in rabbit pulmonary artery smooth muscle cells (PASMCs) (1, 2, 47). TMEM16A currents were similar in magnitude at time = 0 (P > 0.05; Fig. 3C) but were more than twofold greater in magnitude in cells dialyzed with 0 ATP than those supplied with ATP after 20 min of cell dialysis and displayed strong outwardly rectifying properties as previously reported for this channel when elicited by [Ca2+]i <1 μM (Fig. 3D). These results demonstrate that Cl− currents produced by expression of TMEM16A displays ATP-dependent rundown that is similar to the phosphorylation-mediated regulation of CaCCs in vascular smooth muscle cells.
Fig. 2.
Ca2+-activated Cl− currents (IClCa) elicited by mouse TMEM16A expression in HEK-293 cell rundown in the presence of intracellular ATP. A: typical experiment illustrating the rundown of TMEM16A-induced IClCa in a HEK-293 cell dialyzed with 5 mM ATP and 500 nM free Ca2+. A-a: superimposed current traces (top) recorded at selected times shown to the right of each trace. The currents were elicited by the voltage-clamp protocol depicted below the traces and applied to the cell at a frequency of one step every 10 s. A-b: plot of the time course of changes of the current measured at the end of the pulse to +90 mV for the experiment in Aa. Open circles report measurements for the traces depicted in A-a. B: a similar experiment carried out in a different cell dialyzed with 0 mM ATP and 500 nM free Ca2+. The nomenclature of both panels is identical to that of A.
Fig. 3.
ATP dependence of TMEM16A-mediated Ca2+-activated Cl− currents (IClCa) and comparison with native IClCa. A: mean time courses of changes of normalized TMEM16A-induced IClCa measured at +90 mV in HEK-293 cells dialyzed with 0 (open circles; n = 14) or 5 mM ATP (closed circles; n = 26). Arrow indicates when current density was measured to compare the magnitude of the initial IClCa recorded at time = 0 in cells dialyzed with 0 or 5 mM ATP, for which mean data are reported in C. B: mean time courses of changes of native IClCa recorded in rabbit pulmonary artery smooth muscle cells (PASMCs) dialyzed with 0 (dashed line) or 5 mM ATP (solid line). [Plot was adapted from research originally published by our group in the Journal of Biological Chemistry (2), with permission. © the American Society for Biochemistry and Molecular Biology.] A and B emphasize the remarkable similarity in the response of TMEM16A and native IClCa to intracellular ATP. C: mean bar graph showing the magnitude of the initial late TMEM16A current (in pA/pF) recorded immediately after seal rupture (t = 0) in cells dialyzed with 5 (closed bar; n = 23) or 0 mM (open bar; n = 14) ATP. D: mean current-voltage (I-V) relationships for TMEM16A-induced IClCa recorded 20 min after seal rupture in HEK-293 cells dialyzed with 0 (open circles; n = 15) and 5 mM ATP (closed circles; n = 17). Both I-Vs displayed pronounced outward rectification and reversed around 0 mV, which is near the predicted equilibrium potential for Cl− in our conditions. For all panels: †P < 0.01; ‡P < 0.001; n.s., not significant.
Rundown of TMEM16A-induced IClCa is attenuated by CaMKII inhibition.
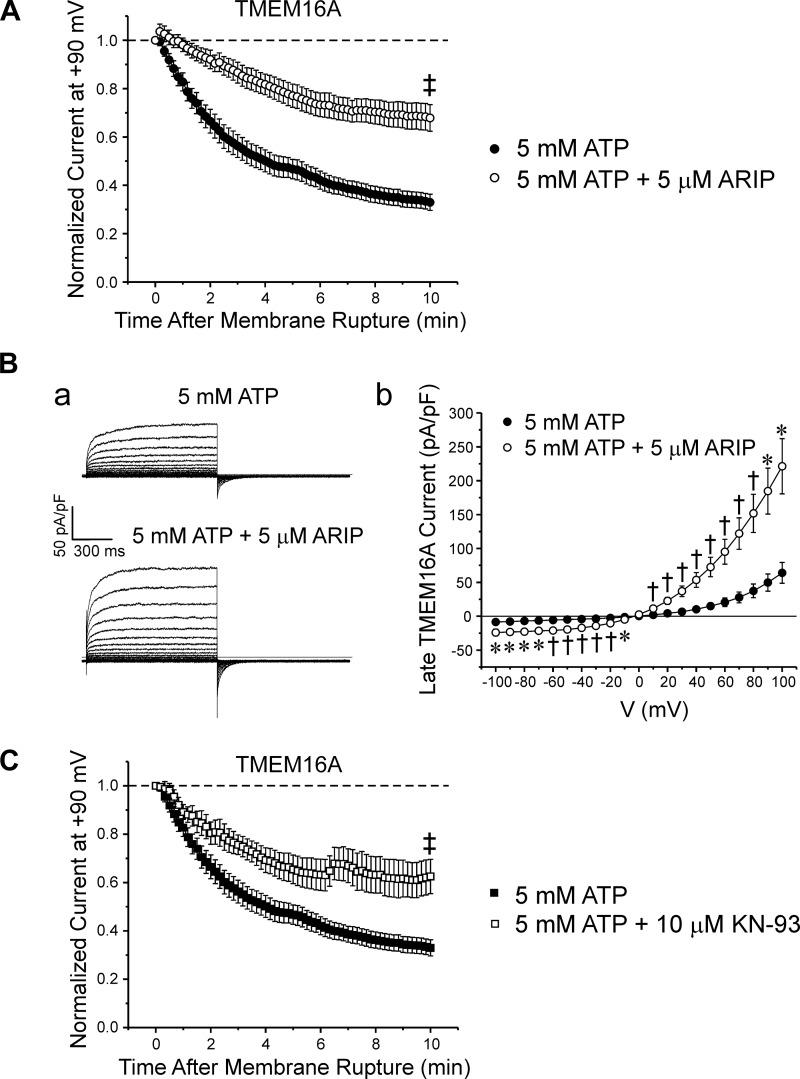
Previous studies showed that calmodulin-dependent protein kinase II (CaMKII) mediated phosphorylation-induced rundown of IClCa in airway and arterial myocytes (13, 46). We next assessed whether the rundown of TMEM16A-induced IClCa observed in cells dialyzed with ATP was sensitive to CaMKII inhibition. We tested the contribution of CaMKII activity by dialyzing HEK-293 cells with the autocamtide-2-related inhibitory peptide (ARIP), a 13 amino acid synthetic peptide that is a potent and selective inhibitor of CaMKII (19). Application of 5 µM ARIP significantly attenuated the rundown seen in the presence of 5 mM ATP over the course of a 10-min period of cell dialysis (Fig. 4A; n = 8). Enhancement of the TMEM16A-induced IClCa by the peptide inhibitor was also evident when comparing typical families of membrane currents recorded 10 min after cell rupture to determine the voltage dependence of the currents with or without CaMKII inhibition (Fig. 4Ba). Figure 4Bb shows mean I-V curves for late currents (measured at the end of 1-s steps) registered with pipette solutions containing 5 mM ATP (n = 17), with (n = 6) or without 5 µM ARIP (n = 17). Similar to data obtained in the absence of drug, cells dialyzed with 5 µM ARIP reversed near the predicted ECl (~0 mV) and displayed outward rectification at positive potentials. A marked enhancement of current density at both negative and positive potentials was clearly evident in ARIP-treated cells.
Fig. 4.
Two structurally unrelated specific calmodulin-dependent protein kinase II (CaMKII) inhibitors similarly attenuated the rundown of TMEM16A-induced Ca2+-activated Cl− currents (IClCa). A: intracellular application of autocamtide-2-related inhibitory peptide (ARIP; 5 µM) significantly reduced the rundown of TMEM16A-induced IClCa in myocytes dialyzed with 5 mM ATP. Graph shows the mean time course of changes for TMEM16A-induced IClCa in HEK-293 cells dialyzed over 10 min with 5 mM ATP in the presence (open circles; n = 8) or absence of ARIP (closed circles, n = 23). B-a: representative families of currents recorded from cells dialyzed with 5 mM ATP (top traces) or 5 mM ATP + 5 µM ARIP (bottom traces). B-b: mean current-voltage (I-V) curves generated from families of currents evoked by intracellular pipette solutions containing 5 mM ATP (n = 17) or 5 mM ATP plus 5 µM ARIP (n = 6). Similar to control, cells dialyzed with 5 µM ARIP reversed near equilibrium potential for Cl− and displayed outward rectification at positive potentials. However, TMEM16A currents measured in cells treated with ARIP were significantly larger than those recorded in the absence of the inhibitor. C: graph shows the mean time courses of changes of normalized TMEM16A-generated IClCa for control cells dialyzed with 5 mM ATP (closed squares, n = 23) and cells dialyzed with 5 mM ATP + 10 µM KN-93 (open circles, n = 8). As for ARIP, KN-93 significantly mitigated the rundown seen in control cells dialyzed with 5 mM ATP alone. For all panels: *P < 0.05; †P < 0.01; ‡P < 0.001.
Intracellular application of KN-93 (10 µM), another potent and highly selective inhibitor of CaMKII (IC50 = 0.37 µM) (37), to HEK-293 cells overexpressing TMEM16A attenuated the rundown of IClCa to a level that was quantitatively similar to that produced by ARIP (Fig. 4C; ARIP: 0.679 ± 0.06, n = 8; KN-93: 0.625 ± 0.071, n = 8; P = 0.562). While currents recorded in the absence of the drug ran down to ~33% of their initial amplitude, those from cells treated with KN-93 only decayed to ~63% of their initial level at the end of a 10-min period of cell dialysis (Control: 0.327 ± 0.03, n = 23; KN-93: 0.625 ± 0.071; n = 8; P < 0.001). Taken together these data indicate that a significant portion of the rundown of TMEM16A-induced IClCa in cells supplied with intracellular ATP may involve at least one phosphorylation step mediated by CaMKII activity.
Effects of PP1/PP2A inhibitors on TMEM16A-induced IClCa.
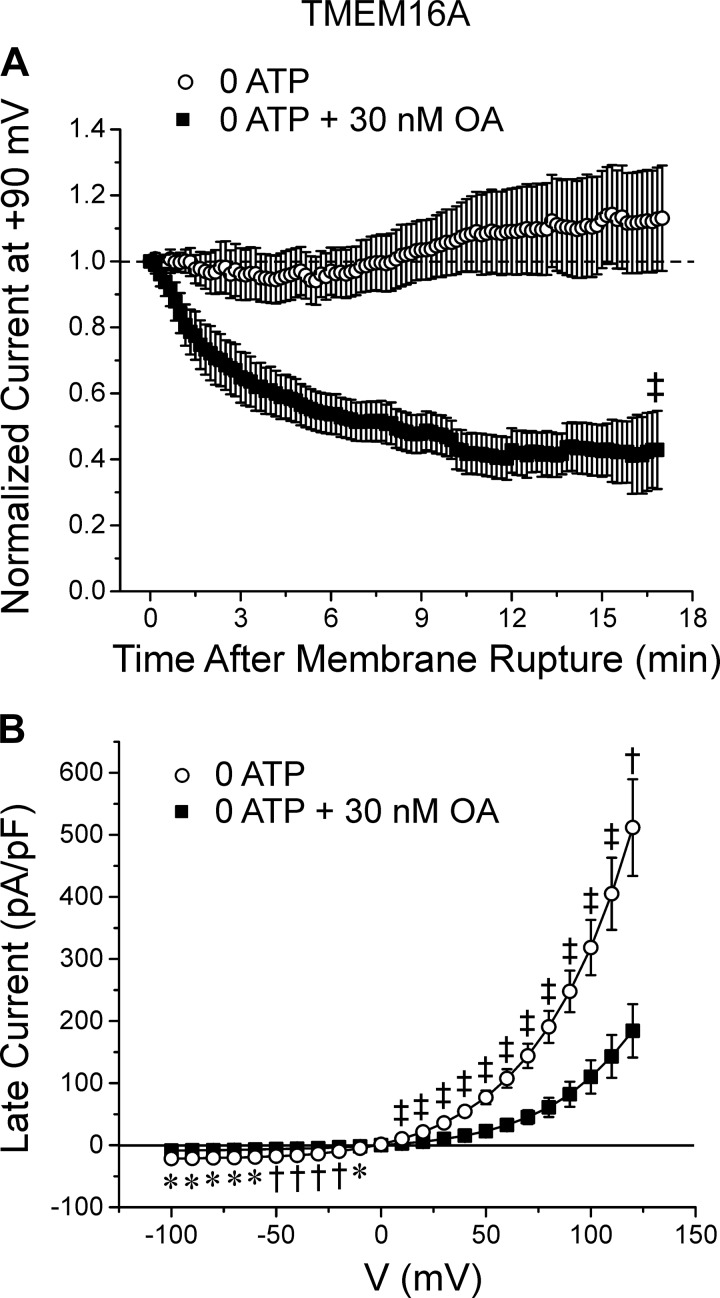
Studies in PASMCs revealed that both Ca2+-dependent and Ca2+-independent phosphatases augmented IClCa (2, 14). We therefore performed experiments to ascertain if a similar type of regulation occurred for TMEM16A. We utilized okadaic acid (OA), a nonspecific PP1/PP2A inhibitor, to determine whether TMEM16A-induced IClCa are modulated by the Ca2+-independent serine/threonine protein phosphatases PP1 and PP2A. As shown in Fig. 5A, an intracellular application of 30 nM OA, a concentration that would potently block both PP1 and PP2A (6, 35), promoted significant rundown of TMEM16A-induced IClCa with normalized late IClCa stabilizing at ~40% of the initial current (0 ATP: 113 ± 16%, n = 9; 0 ATP + 30 nM OA: 42.9 ± 11.8%, n = 10; P < 0.001), which was similar to the behavior of the current in cells intracellularly supplied with 5 mM ATP (Figs. 2 and 3). Analysis of the I-V relationships for late IClCa in the two conditions showed that OA inhibited TMEM16A current density by >50% at all test potentials (Fig. 5B). Use of cantharidin (100 nM), another nonselective PP1/PP2A inhibitor, produced relatively similar effects on the time course of changes for IClCa during dialysis with no ATP when compared with cells treated with OA (data not shown; 0 ATP: 109 ± 5.6%, n = 11; 0 ATP + cantharidin: 73.3 ± 7.9%, n = 6; P < 0.001). These data suggest that TMEM16A channels are modulated by PP1/PP2A in a manner that is remarkably similar to native IClCa in PASMCs (2).
Fig. 5.
Modulation of TMEM16A-mediated Ca2+-activated Cl− currents (IClCa) by Ca2+-independent serine/threonine protein phosphatase activity. A: plot of averaged time courses of changes of normalized TMEM16A-induced IClCa in HEK-293 cells dialyzed with 0 ATP, with (closed squares) or without (open circles) 30 nM okadaic acid (OA), a nonselective inhibitor of protein phosphatases PP1 and PP2A. The graph shows that in the absence of ATP, TMEM16A-mediated IClCa displayed a small but significant transient rundown followed by a delayed recovery current recovery phase after ~9 min. B: mean current-voltage (I-V) curves generated from families of currents evoked by intracellular pipette solutions containing 0 mM ATP (open circles; n = 14) or 0 mM ATP plus 30 nM OA (closed squares; n = 9), both after 20 min of cell dialysis. For both panels: *P < 0.05; †P < 0.01; ‡P < 0.001.
Expression of CaMKII, PP1, and PP2A in HEK-293 cells.
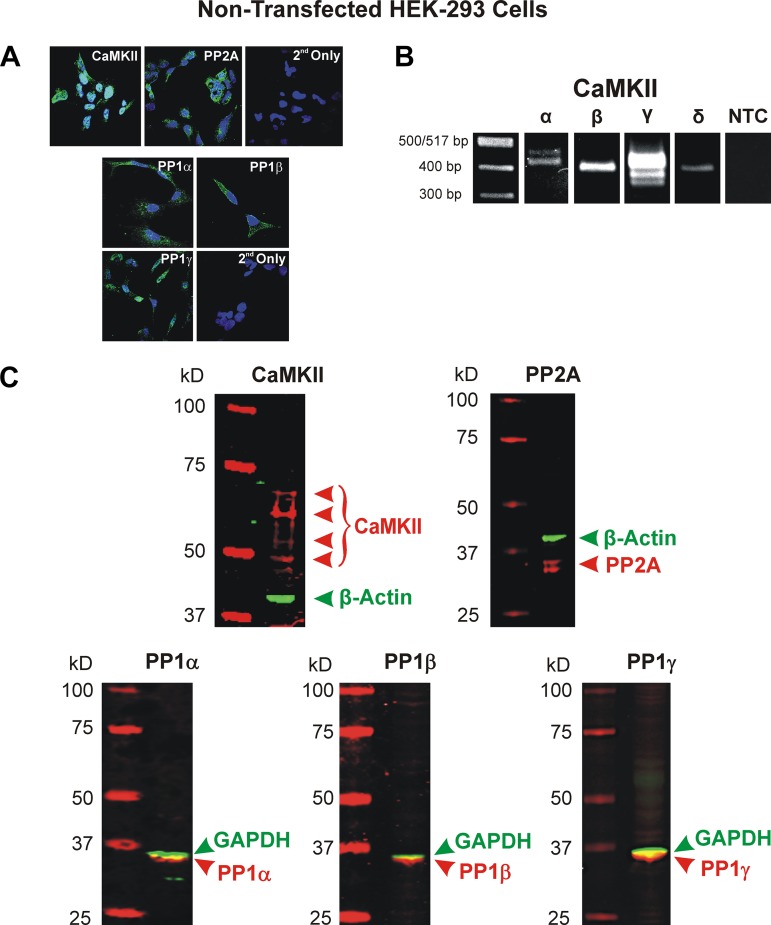
Using a rabbit anti-CaMKII pan antibody, we were able to successfully detect CaMKII in cultured HEK-293 cells (Fig. 6A). The cells displayed a fairly uniform expression of CaMKII within the cytosol. Immunocytochemical experiments were also performed to determine the endogenous expression of PP1 and PP2A. This was achieved by using isoform-specific antibodies for PP1 (PP1α, PP1β/δ, and PP1γ) as well as a nonisoform specific antibody for PP2A. These experiments revealed fluorescent signals for PP2A as well as the three isoforms of PP1, all of which were primarily localized in the cytosol (Fig. 6A). Additionally, CaMKII expression was also detected at the mRNA level. Using primers designed against nonconserved sequences of the CaMKIIα, -β, -γ, and -δ isoforms (see Table 1), we found that all four isoforms (and some alternatively spliced CaMKIIα and -γ transcripts) were expressed (Fig. 6B). Finally, Western blot experiments were also carried out and confirmed the expression of several isoforms of the catalytic subunits of CaMKII (predicted molecular mass ranging from between ~50 and 70 kDa and single bands for all three isoforms of PP1 (predicted molecular mass between ~35 and 40 kDa) and two isoforms of PP2A (predicted molecular mass between ~35 and 40 kDa). These results confirmed the presence of CaMKII, PP1, and PP2A in the HEK-293 cell line used in our electrophysiological experiments.
Fig. 6.
Endogenous expression of calmodulin-dependent protein kinase II (CaMKII) and the Ca2+-independent protein phosphatases PP1 and PP2A in nontransfected HEK-293 cells. A: immunocytochemical detection of CaMKII (top left) and PP2A (top middle) protein expression was respectively performed using a primary rabbit polyclonal antibody against CaMKII from Santa Cruz Biotechnology (Dallas, TX; sc-9035) and a rabbit monoclonal antibody against the catalytic subunit of PP2A from Cell Signaling Technology (Danvers, MA; 52F8). Detection of both enzymes was carried out by incubating cells with a secondary anti-rabbit IgG Alexa Fluor 546 (Thermo Fisher Scientific, Waltham, MA; A-11010). Cells serving as negative controls were incubated with the secondary antibody only (2nd Only; top right). Immunocytochemical studies were also carried out to determine the endogenous expression of the catalytic subunits of PP1α (bottom left top), PP1β (bottom right top), and PP1γ (bottom left lower) in cultured HEK-293 cells. All antibodies were goat antibodies from Santa Cruz Biotechnology (PP1α: sc-6105; PP1β: sc-6107; PP1γ: sc-6109). All cells were incubated with the goat anti-rabbit IgG Alexa Fluor 488 (Thermo Fisher Scientific; A-11008) secondary antibody. Negative controls were labeled with secondary antibody only (2nd Only; bottom right lower). A ×60 oil immersion objective was used for detection of all enzymes. For all images in A, nuclei were stained with DAPI (Abcam, Cambridge, UK). B: expression of CaMKII isoforms at the mRNA level was determined using specific primers designed against each of the 4 CaMKII isoforms (see Table 1). For each set of primers pairs, a nontemplate control (NTC) was performed; however, for the sake of space, this figure shows a single representative NTC. PCR amplified products were resolved on a 2% agarose gel. Single bands for the CaMKIIβ and -δ isoforms were detected in HEK-293 cells, which fall within the expected amplicon size (396 bp for both) based on primer design. Multiple bands were detected in HEK-293 cells for CaMKIIα and -γ, which is most likely the result of alternative splicing. Predicted amplicon size for CaMKIIα: 452 bp; CaMKIIγ: 413 bp; CaMKIIδ: 396 bp. C: Western blot analysis of nontransfected HEK-293 cells demonstrated the expression of several isoforms of CaMKII, all three known catalytic PP1 isoforms, and two isoforms of the catalytic subunit of PP2A. Proteins of interest and ladder appear in red while the housekeeping proteins GAPDH and β-actin appear in green. 20 μg of lysate protein were loaded in each lane.
Mutational analysis of potential TMEM16A phosphorylation sites by CaMKII.
The data presented so far provide strong evidence that TMEM16A is regulated by kinase and phosphatase activity. Several putative serine/threonine phosphorylation sites have been predicted based on sequence analysis, including PKA, PKC, PKG, casein kinase, and CaMKII (18, 21). By far the most common consensus sequences for phosphorylation by CaMKII are RXXT*/S* (21). We identified four such putative CaMKII phosphorylation sites (Fig. 7) on TMEM16A segments previously or currently believed to be located on the cytoplasmic side of the membrane (44, 50). Based on the original eight transmembrane TMEM16A protein model (5, 16, 34, 49), we had identified threonine 622 as a potential consensus site for phosphorylation by CaMKII bearing the RXXT signature sequence. This residue was particularly appealing due to its apparent proximity to the pore-forming domain and recent evidence derived from the crystal structure of the fungal TMEM16 protein from Nectria haematococca (3). Experiments with wild-type TMEM16A and this mutant were performed on the same batch of cells, alternating between the two isoforms. TMEM16A bearing the T622A mutation displayed a nearly superimposable rundown relative to that of cells expressing wild-type TMEM16A (data not shown; P > 0.05). Analysis of the voltage dependence of maximal current after 5 min of cell dialysis with 5 mM ATP showed that the relationships were statistically superimposable, suggesting that T622 is not involved in the regulation of TMEM16A by CaMKII (P > 0.05). These results are consistent with the revised structure of TMEM16A where this residue is now thought to be embedded in transmembrane domain 4 of mouse TMEM16A (30).
Fig. 7.
Proposed membrane topology and location of the four putative calmodulin-dependent protein kinase II (CaMKII) phosphorylation sites identified on mouse TMEM16A. A: proposed secondary structure of mouse TMEM16A highlighting the alternatively spliced variant “a” located in the NH2 terminus (thick green solid line) and approximate location of the proposed binding sites for Ca2+ based on the structure proposed by Paulino et al. (30), and the position of the four consensus sites for phosphorylation by CaMKII that obey the basic sequence RXXS/T (T273, S528, T561, and S730 all labeled with red circles). B: sequence homology among mouse, rat, and human TMEM16A in the region surrounding each of the four identified potential CaMKII phosphorylation targets. The putative CaMKII RXXT/S sequence and conservative and nonconservative amino acid replacements are highlighted in white font over a red, green, or black background, respectively, as shown above the sequences. The amino acids highlighted in blue for the human sequence corresponds to splice variant “d,” which is not present in the mouse and rat sequences defined by the two National Center for Biotechnology Information protein accession numbers. However, the human sequence downstream of splice variant “d” (not shown) defined by accession no. NP_060513.5 is identical to the remaining sequence shown for mouse and rat (TDKVKLTWR).
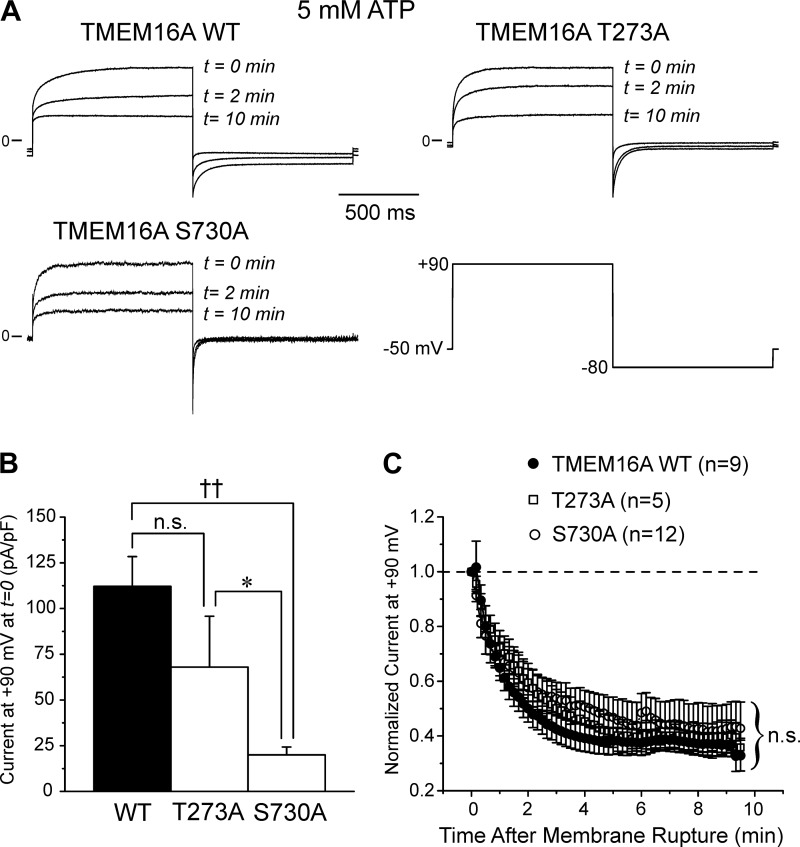
We next sought to examine the other three CaMKII phosphorylation sites (T273, S528 and S730) now believed to lie on the cytoplasmic side of the protein by mutating each site to a neutral alanine, one at a time. Figure 8A shows typical currents (normalized to an identical arbitrary scale) recorded at three different times following seal rupture for wild-type TMEM16A and TMEM16A carrying the T273A or S730A mutation. These experiments show that the current displayed very similar rundown over a 10-min time period. Figure 8B shows that the initial current amplitude at time = 0 of the S730A mutant was significantly smaller than that of wild-type TMEM16A and the T273A mutant. A reduction in current density associated with this mutation is not an uncommon observation, even for single-point mutagenesis experiments. We can only speculate that the mutation may have altered transfection, transcription, translation, and/or trafficking efficiency, which will require further investigation. The mean data for such experiments clearly demonstrate however that the ~70% rundown of TMEM16A-induced current was unaffected by mutating T273 or S730 to an alanine, suggesting that either residue is not involved in the regulation of TMEM16A by CaMKII. Our data with S730A contrast with those of Lin et al. (25), who showed that the corresponding residue (S727) of native TMEM16A in mouse basilar artery smooth muscle cells (BASMCs) was the residue phosphorylated by CaMKIIγ.
Fig. 8.
Substitution of threonine 273 or serine 528 to a neutral alanine had no impact on the regulation of TMEM16A-induced Ca2+-activated Cl− currents (IClCa). A: representative current traces recorded immediately (t = 0 min) and after 2 (t = 2 min) and 10 min (t = 10 min) following membrane rupture demonstrating the time-dependent rundown of IClCa recorded from HEK-293 cells overexpressed with wild-type (WT) TMEM16A (TMEM16A WT; top left), the T273A (TMEM16A T273A; top right) or the S730A mutant (TMEM16A S730A; bottom left), all recorded in the presence of 5 mM ATP in the pipette solution. The currents were evoked by 1 s steps to +90 mV from a holding potential of −50 mV; the inward tail current was elicited by a 1 s return step to −80 mV (bottom right). B: mean bar graph showing the amplitude of the initial current recorded at +90 mV at time = 0 from a holding potential of −50 mV (protocol identical to that shown in A, bottom right). Each bar is a mean ± SE current density in pA/pF. C: graph showing the mean time courses of changes of normalized late IClCa elicited by depolarizing steps to +90 mV applied at a frequency of 0.1 Hz for WT TMEM16A (closed circles) and the T273A (open squares) and S730A (open circles) mutants. Each data point is a means ± SE; n, number of cells. Mutation of the threonine at position 273 or serine at position 730 to an alanine did not alter the time course and magnitude of TMEM16A-mediated IClCa rundown. For B and C: *P < 0.05; ††P < 0.001; n.s., not significant.
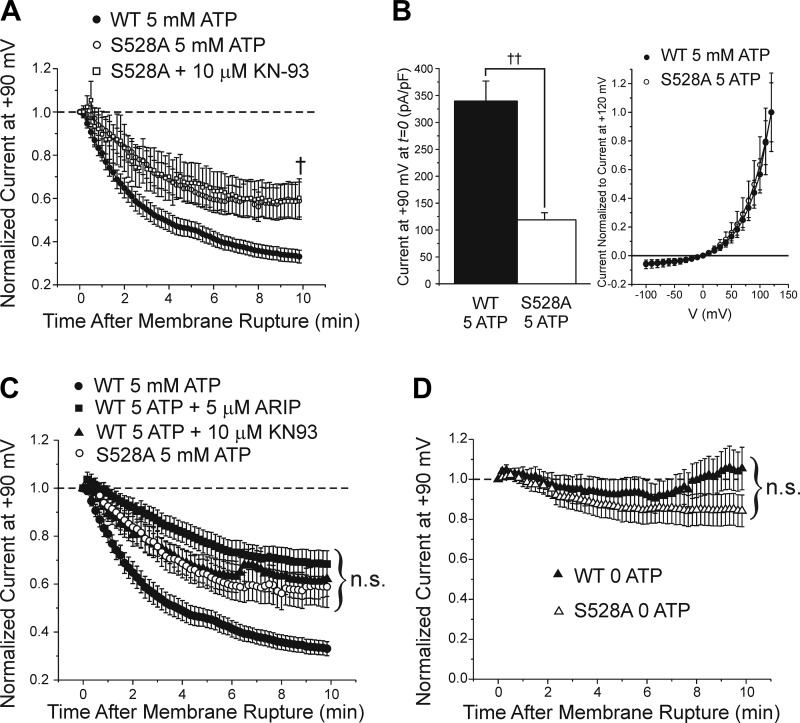
We next investigated the serine residue at position 528, which is located in the first intracellular loop of TMEM16A (Fig. 1). This amino acid is interesting because of its proximity to the peptide segments encoded by exons 13 (splice variant “c”) and 15 (splice variant “d”), which have been shown to alter the Ca2+ and voltage dependence, as well as activation and deactivation kinetics of TMEM16A-induced IClCa expressed in HEK-293 cells (10, 27, 48). We performed site-directed mutagenesis on this serine again by replacing it with the nonpolar amino acid alanine (S528A). In contrast to the other mutated residues, rundown of TMEM16A-induced IClCa was partially but significantly attenuated compared with wild-type TMEM16A current in HEK-293 cells expressing the S528A mutant dialyzed with 5 mM ATP, an effect that was not affected by including KN-93 (10 μM) in the pipette solution (Fig. 9A). Late TMEM16A-induced current at +90 mV recorded from the S528A mutant, with or without KN-93, dialyzed with 5 mM ATP ran down to ~60% of the initial amplitude registered at time = 0, whereas currents recorded from wild-type TMEM16A-overexpressing cells displayed a more pronounced rundown with levels stabilizing to ~33% of the initial amplitude (S528A: 0.589 ± 0.087, n = 19; S528A + KN-93: 0.602 ± 0.088, n = 9; wild type: 0.327 ± 0.03, n = 23; P < 0.01). These data suggest that serine at position 528 may provide the necessary substrate for CaMKII leading to partial downregulation of TMEM16A-induced IClCa by phosphorylation. Associated with the diminished rundown of TMEM16A-induced IClCa was the observation that outwardly rectifying IClCa measured at positive potentials for the S528A mutant were lower than the wild-type TMEM16A-induced current. The mean late IClCa for the S528A mutant dialyzed with 5 mM ATP for 10 min was 64.8 ± 10.5 pA/pF at +120 mV (n = 8) compared with 200.8 ± 41.3 pA/pF (n = 14) for the wild-type isoform (P < 0.05). This led us to examine whether the current density of IClCa for HEK-293 cells expressing this mutant was of lower magnitude than that of control cells immediately after seal rupture. We found that the current density recorded immediately upon membrane rupture (t = 0) was significantly reduced in the S528A mutants (118.8 ± 13.3 pA/pF) compared with current density of wild-type TMEM16A (339.0 ± 37.2 pA/pF; P < 0.001; Fig. 9B, left). To determine whether this difference in current density was due to differences in voltage sensitivity, we normalized the family of currents generated in wild-type TMEM16A and S528A mutant-expressing cells (both group of cells dialyzed with 5 mM ATP) to the maximal current recorded at +120 mV in their respective group. Analysis of the voltage dependence of normalized current indicated that the voltage sensitivity was not significantly different between the two isoforms (Fig. 9B, right). These data show that similar to the S730A mutant, the current density of IClCa was significantly reduced in HEK-293 cells expressing TMEM16A S528A but this was not associated with a change in voltage sensitivity.
Fig. 9.
Mutation of serine 528 to alanine attenuates TMEM16A rundown to a level that is quantitatively similar to that exerted by specific calmodulin-dependent protein kinase II (CaMKII) inhibitors. A: graph showing the mean time courses of changes of normalized late Ca2+-activated Cl− currents (IClCa) elicited by depolarizing steps to +90 mV applied at a frequency of 0.1 Hz from holding potential of −50 mV for wild-type (WT) TMEM16A (closed circles) or TMEM16A T528A mutant in the absence (open circles) or presence of 10 μM KN-93 in the pipette solution (open squares). All currents were normalized to the initial current recorded immediately upon cell membrane rupture. The S528A mutation significantly reduced the rundown of TMEM16A-induced IClCa HEK-293 cells over the course of a 10-min period of cell dialysis with 5 mM ATP (S528A: 0.589 ± 0.087, n = 19; S528A + KN-93: 0.602 ± 0.088, n = 9; WT: 0.327 ± 0.030, n = 23; P < 0.01). B, left: mean bar graph showing the magnitude of the initial late TMEM16A current density ± SE (in pA/pF) recorded after seal rupture (t = 0) in cells dialyzed with 5 mM ATP for wild-type TMEM16A (closed bar; n = 14) and the S528A mutant (open bar; n = 8). B, right: mean current-voltage relationships for late IClCa recorded from wild-type TMEM16A (closed circles) and the S528A mutant after seal rupture (t = 0). Currents in each group were normalized to that recorded at +120 mV in their respective group. Data points in each group are means ± SE. C: the time course plots shown in Fig. 4, A and C, were superimposed to those of A to illustrate the similarity between the attenuation of rundown of TMEM16A produced by the S528A mutation and that elicited by the 2 CaMKII inhibitors autocamtide-2-related inhibitory peptide (ARIP) and KN-93. One-way ANOVA analysis revealed no significant difference between the 3 groups of data after 10 min of cell dialysis with 5 mM ATP (P > 0.05). D: graph showing the mean time courses of changes of normalized late IClCa elicited by depolarizing steps to +90 mV applied at a frequency of 0.1 Hz from holding potential of −50 mV for WT TMEM16A (closed triangles) or TMEM16A S528A mutant (open triangles) dialyzed with 0 ATP. The TMEM16A S528A mutant decayed over a 10 min period to a level that was ~16% of the initial amplitude, which was not significantly different from WT TMEM16A currents (1.038 ± 0.098, n = 13; P = 0.13). For all panels: †P < 0.01; ††P < 0.001; n.s., not significant.
Cells expressing the S528A mutant displayed current rundown that was similar to the partial rundown observed for wild-type TMEM16A current in the presence of either one of the two specific CaMKII inhibitors tested (Fig. 4). To confirm this observation, we superimposed the mean time courses for S528A-induced currents with wild-type TMEM16A-induced currents recorded in cells incubated with or without KN-93 or ARIP (Fig. 9C). Analysis of these results revealed that the rundown of TMEM16A-induced IClCa in the three conditions was not significantly different (one-way ANOVA, P = 0.76). Currents from S528A mutants were also recorded in the absence of ATP to minimize phosphorylation. When global phosphorylation was suppressed, rundown of IClCa from the S528A mutant was not significantly different from that of wild-type TMEM16A also recorded in the absence of ATP (S528A: 0.842 ± 0.08, n = 21, vs. wild type: 1.038 ± 0.098, n = 13; P = 0.13; Fig. 9D).
DISCUSSION
CaCC activity is suppressed in airway and vascular smooth muscle cells by mechanisms involving at least one phosphorylation step mediated, at least in part, by CaMKII (13, 22, 23, 46). In the present study, we sought to determine whether TMEM16A is regulated in a similar manner to native CaCCs. We show that currents resulting from expressing mouse TMEM16A in HEK-293 cells display remarkably similar properties to those of vascular myocytes: 1) TMEM16A-induced IClCa quickly runs down after seal rupture; 2) the rundown is largely prevented by omitting ATP from the pipette solution; 3) the rundown is partially prevented by inhibition of CaMKII; and 4) the upregulation of TMEM16A seen in the absence of ATP is suppressed by inhibition of the Ca2+-independent phosphatases PP1/PP2A. Moreover, our study identified a serine residue (serine 528) in the first intracellular loop of TMEM16A that is potentially involved in the phosphorylation of the anion channel by CaMKII.
Mouse TMEM16A activity is suppressed by CaMKII.
Our study demonstrated that the Cl− current produced by the expression of mouse TMEM16A “a” splice variant consistently ran down after seal rupture in HEK-293 cells supplied with 5 mM ATP and 500 nM free [Ca2+]i to activate the channels. Although this phenomenon displayed a slightly slower time course and was less pronounced than that previously described by our group in rabbit PASMCs (Figs. 2 and 3) (1, 2, 14, 47), the overall behavior was very similar with the anion current stabilizing to a new steady-state level within 5 to 10 min and remaining stable for more than 20 min.
The key observation that current rundown was abolished by removing ATP is reminiscent of that shown for IClCa in arterial myocytes (1, 2, 47). To our knowledge, only one other study reported a spontaneous decay of TMEM16A currents after membrane rupture in HEK-293 cells under whole cell recording conditions similar to ours (43). In this report, the authors also showed that the rundown was potently inhibited by a 2-h preincubation with the CaMKII inhibitor KN-62 (25 µM) and was increased by the intracellular application of a Ca2+-independent constitutive active form of CaMKII.
Application of two specific CaMKII inhibitors KN-93 (10 μM) and ARIP (5 μM) led to a significant but partial attenuation of the rundown of TMEM16A currents. These data also fall in line with previous studies indicating that CaMKII is involved in the regulation of IClCa in airway (46) and arterial myocytes (2, 13, 24). Immunocytochemical and Western blot experiments confirmed the presence of CaMKII at the protein level in HEK-293 cells, while RT-PCR studies identified transcripts for all four known isoforms (CaMKIIα, CaMKIIβ, CaMKIIγ, and CaMKIIδ), which indicates that this cell line was suitable for engaging in experiments evaluating the regulation of expressed TMEM16A by endogenous CaMKII. At the concentrations used, both inhibitors would be expected to exert maximal or near maximal inhibition of the enzyme (19, 37). Interestingly, the magnitude of their effect on current decay was statistically indistinguishable. These results (along with our site-directed mutagenesis experiments discussed below) suggest that the latter experiments most likely revealed the full extent of the response and indicate that phosphorylation by CaMKII only partially (~40–50%) contributes to the rundown of TMEM16A currents, suggesting the involvement of another mechanism also relying on intracellular ATP.
Regulation of TMEM16A-induced IClCa by Ca2+-independent serine/threonine phosphatases.
We used the specific PP1/PP2A inhibitor OA to determine whether TMEM16A-induced IClCa were regulated by the Ca2+-independent serine/threonine protein phosphatases PP1 and PP2A. Intracellular application of OA (30 nM), a strategy previously used to examine the regulation of IClCa by these phosphatases in rabbit PASMCs (2), significantly attenuated TMEM16A-induced IClCa in HEK-293 cells dialyzed with no ATP. This time course is similar to the results obtained in rabbit PASMCs (2), which suggests that TMEM16A-mediated IClCa are similarly modulated by protein phosphatase activity. Another nonselective PP1/PP2A blocker, cantharidin, also promoted the rundown of TMEM16A-induced currents in cells dialyzed with no ATP and reduced TMEM16A channel conductance by approximately half. As for CaMKII, immunolabeling and Western blot experiments confirmed the presence of PP1α, PP1β, PP1γ, as well as PP2A in this cell line.
In contrast to our observations, Tian et al. (42) reported no effect of a 2-h preincubation with 10 nM OA on expressed TMEM16A recorded under similar conditions with the exception that their pipette solution contained 3 mM ATP. We can only speculate that the inclusion of ATP promoted phosphorylation by CaMKII and perhaps other enzymes, which opposed the effects of phosphatases. Because TMEM16A-induced current (and native IClCa in PASMCs) always displays significant rundown in the presence of ATP, its regulation must be dominated by kinase activity, which is most likely sufficiently potent to overwhelm phosphatase activity, but answering this question will require more investigation.
Serine 528 is a target for CaMKII-mediated phosphorylation.
Several putative serine/threonine phosphorylation sites were identified in mouse TMEM16A based on sequence analysis (18, 21). We initially identified three sites bearing the well-known consensus sequence “RXXS*/T*” for phosphorylation by CaMKII on the domains of TMEM16A predicted to lie on the cytoplasmic side of the membrane; this early assessment was based on the first model of this anion channel that predicted an eight transmembrane domain topology (5, 34, 44, 49, 50). Sequence analysis of the region surrounding these three sites (T273, S528, and T622) showed a high degree of homology among mouse, rat, and human (Fig. 7B). The only difference is a conservative mutation of the arginine in mouse (and rat) at position 525 for a lysine at the –3 position in human (relative to the mouse sequence). The “KXXS*/T*” sequence has recently been documented to be a substrate for phosphorylation and regulation by CaMKII of the metabotropic glutamate receptor type Ia (20). The recent findings that the distant relative of TMEM16A in the fungus Nectria haematococca (3) and mouse TMEM16A (30), were both found to exhibit 10 instead of 8 transmembrane domains unraveled the existence of a new CaMKII phosphorylation site (S730) previously thought to lie outside the membrane but now localizing within the third intracellular loop between TMD6 and TMD7 (Fig. 1). Notably, the region surrounding this potential site is not well conserved when rodent TMEM16A sequences are compared with the human sequence, which contains several nonconservative mutations (Fig. 7B).
Site-directed mutagenesis experiments were thus undertaken to identify the possible CaMKII phosphorylation site(s) involved in regulating TMEM16A. Mutating threonine at position 622 (now considered to lie within TMD4), or serine residues at positions 273 (NH2-terminal domain) and 730 to a neutral alanine, did not affect current rundown suggesting that these amino acids are not involved in the regulation of TMEM16A by CaMKII. In contrast, mutating the serine at position 528 to an alanine partially attenuated IClCa rundown; this reduction of rundown was unaffected by including the CaMKII inhibitor KN-93 in the pipette solution indicating that the significant effect of the latter (and likely ARIP) seen on wild-type TMEM16A was not due to an off-target effect on another signaling pathway indirectly regulating TMEM16A. Interestingly, the time course and magnitude of IClCa rundown with this TMEM16A mutant were statistically indistinguishable to those exerted by two structurally unrelated CaMKII inhibitors on wild-type TMEM16A, which supports the hypothesis that S528 is a major site targeted by CaMKII. This site also scored highest of the four (T273: 3.4; S528: 7.231; T622: not scored; and S730: 3.82) based on bioinformatics analysis using Group-based Prediction System software (v. 3.0; source: http://gps.biocuckoo.org). In a recent study, Lin et al. (25) instead proposed that serine 730 (serine 727 in their case), but not S528 (S525 in their case), is the site phosphorylated by CaMKIIγ in mouse basilar artery smooth muscle cells (BASMCs). We can only speculate to the use of different cell types and experimental conditions to explain such differences. In their study, the investigators did not provide time courses of changes in the current after seal rupture so it is not possible to know if those recordings were obtained after membrane rupture or following a period of dialysis with elevated Ca2+ and ATP and if this impacted their results. In all our previous studies using vascular myocytes and in this study with expressed mouse TMEM16A, the regulation of this channel by intracellular ATP and enzymes took several minutes to develop after seal rupture. When dialyzed with 500 nM Ca2+, native IClCa in BASMCs or BASMCs transfected with an empty plasmid vector were extremely small or inexistent (25), which is at odd with other studies in mouse cerebral myocytes, which exhibited robust native IClCa (4, 41). Although the results of Lin et al. (25) strongly supported the hypothesis that S730 is the residue involved in the regulation of TMEM16A by CaMKII, an unexplained finding from their study was the fact that transfection of BASMCs with TMEM16A carrying the phosphomimetic mutation S528 to aspartate (S525D in their case) led to inhibited currents relative to those recorded from the expression of S528A. Clearly, additional experiments are required to clarify these apparent discrepancies.
In summary, data presented in this report show that overexpressed TMEM16A-induced IClCa in HEK-293 cells display rundown and are regulated by CaMKII and PP1/PP2A in a manner that is similar to native IClCa in rabbit PASMCs (2, 13, 14, 47). Our data also suggest that serine 528 is an important contributor to the regulation of TMEM16A. It will be of great interest to assess the potential role of other splice variants in this process as the S528 is located between splice variants “c” and “d,” both of which were shown to alter the voltage and Ca2+ dependence, kinetics, and pharmacology of TMEM16A (12, 27, 39, 48). Our results also suggest that another unidentified kinase(s) or mechanism(s) is involved in the regulation of TMEM16A because blocking CaMKII specifically or suppressing a potential phosphorylation site by a single-point mutation led to a similar partial attenuation of the rundown. This additional CaMKII-independent fraction of TMEM16A regulation was demonstrated by removing ATP from the pipette solution. Whether PIP2 regulation of native CaCCs (32) and expressed TMEM16A (9, 40), or one or several other kinases, is involved in this process remains to be determined.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants R01 HL075477, R01 HL091238, and R01 HL146054 and University of Nevada, Reno Vice President for Research and Innovation Grant REG 1200-121-1207 to N. Leblanc and by NHLBI Grant R01 HL127192 to C. A. Singer. The publication was also made possible by a grant to N. Leblanc (NCRR 5 P20 RR15581) from the National Center for Research Resources (NCRR), now part of the National Institute of General Medical Sciences (NIGMS), which supported a Center of Biomedical Research Excellence (COBRE) at the University of Nevada, Reno School of Medicine from 2000 to 2010. Confocal imaging experiments were supported by the High Spatial and Temporal Resolution Imaging Core of the new Center of Biomedical Research Excellence for Molecular and Cellular Signal Transduction in the Cardiovascular System funded by NIGMS Grant 1 P20 GM130459 (N. Leblanc). Support was also provided by NHLBI Ruth L. Kirschstein National Research Service Predoctoral Fellowship Grant F31 HL090023 (to R. J. Ayon).
DISCLAIMERS
The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J.A., J.A., A.S.F., C.A.S., M.L.V., I.A.G., and N.L. conceived and designed research; R.J.A., M.B.H., J.A., M.W., A.S.F., F.C., C.A.S., M.L.V., and N.L. performed experiments; R.J.A., M.B.H., J.A., M.W., A.S.F., F.C., C.A.S., M.L.V., I.A.G., and N.L. analyzed data; R.J.A., M.B.H., J.A., M.W., A.S.F., F.C., C.A.S., M.L.V., I.A.G., and N.L. interpreted results of experiments; R.J.A., J.A., M.W., F.C., and N.L. prepared figures; R.J.A. and N.L. drafted manuscript; R.J.A., M.B.H., J.A., M.W., A.S.F., F.C., C.A.S., M.L.V., I.A.G., and N.L. edited and revised manuscript; R.J.A., M.B.H., J.A., M.W., A.S.F., F.C., C.A.S., M.L.V., I.A.G., and N.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Talia Joyce for technical support and Dr. Lily Jan from the University of California, San Francisco, for gracefully providing the mouse TMEM16A clone.
REFERENCES
- 1.Angermann JE, Sanguinetti AR, Kenyon JL, Leblanc N, Greenwood IA. Mechanism of the inhibition of Ca2+-activated Cl− currents by phosphorylation in pulmonary arterial smooth muscle cells. J Gen Physiol 128: 73–87, 2006. doi: 10.1085/jgp.200609507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayon R, Sones W, Forrest AS, Wiwchar M, Valencik ML, Sanguinetti AR, Perrino BA, Greenwood IA, Leblanc N. Complex phosphatase regulation of Ca2+-activated Cl−currents in pulmonary arterial smooth muscle cells. J Biol Chem 284: 32507–32521, 2009. doi: 10.1074/jbc.M109.050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 516: 207–212, 2014. doi: 10.1038/nature13984. [DOI] [PubMed] [Google Scholar]
- 4.Bulley S, Neeb ZP, Burris SK, Bannister JP, Thomas-Gatewood CM, Jangsangthong W, Jaggar JH. TMEM16A/ANO1 channels contribute to the myogenic response in cerebral arteries. Circ Res 111: 1027–1036, 2012. doi: 10.1161/CIRCRESAHA.112.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Bulley S, Jaggar JH. Cl− channels in smooth muscle cells. Pflugers Arch 466: 861–872, 2014. [Erratum in Pflugers Arch 466: 873, 2014.] doi: 10.1007/s00424-013-1357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 6.Cohen P, Holmes CF, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci 15: 98–102, 1990. doi: 10.1016/0968-0004(90)90192-E. [DOI] [PubMed] [Google Scholar]
- 7.Davis AJ, Forrest AS, Jepps TA, Valencik ML, Wiwchar M, Singer CA, Sones WR, Greenwood IA, Leblanc N. Expression profile and protein translation of TMEM16A in murine smooth muscle. Am J Physiol Cell Physiol 299: C948–C959, 2010. doi: 10.1152/ajpcell.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis AJ, Shi J, Pritchard HA, Chadha PS, Leblanc N, Vasilikostas G, Yao Z, Verkman AS, Albert AP, Greenwood IA. Potent vasorelaxant activity of the TMEM16A inhibitor T16A(inh) -A01. Br J Pharmacol 168: 773–784, 2013. doi: 10.1111/j.1476-5381.2012.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Jesús-Pérez JJ, Cruz-Rangel S, Espino-Saldaña AE, Martínez-Torres A, Qu Z, Hartzell HC, Corral-Fernandez NE, Pérez-Cornejo P, Arreola J. Phosphatidylinositol 4,5-bisphosphate, cholesterol, and fatty acids modulate the calcium-activated chloride channel TMEM16A (ANO1). Biochim Biophys Acta Mol Cell Biol Lipids 1863: 299–312, 2018. doi: 10.1016/j.bbalip.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrera L, Caputo A, Ubby I, Bussani E, Zegarra-Moran O, Ravazzolo R, Pagani F, Galietta LJ. Regulation of TMEM16A chloride channel properties by alternative splicing. J Biol Chem 284: 33360–33368, 2009. doi: 10.1074/jbc.M109.046607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrest AS, Joyce TC, Huebner ML, Ayon RJ, Wiwchar M, Joyce J, Freitas N, Davis AJ, Ye L, Duan DD, Singer CA, Valencik ML, Greenwood IA, Leblanc N. Increased TMEM16A-encoded calcium-activated chloride channel activity is associated with pulmonary hypertension. Am J Physiol Cell Physiol 303: C1229–C1243, 2012. doi: 10.1152/ajpcell.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galietta LJ. The TMEM16 protein family: a new class of chloride channels? Biophys J 97: 3047–3053, 2009. doi: 10.1016/j.bpj.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenwood IA, Ledoux J, Leblanc N. Differential regulation of Ca2+-activated Cl− currents in rabbit arterial and portal vein smooth muscle cells by Ca2+-calmodulin-dependent kinase. J Physiol 534: 395–408, 2001. doi: 10.1111/j.1469-7793.2001.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenwood IA, Ledoux J, Sanguinetti A, Perrino BA, Leblanc N. Calcineurin Aα but not Aβ augments ICl(Ca) in rabbit pulmonary artery smooth muscle cells. J Biol Chem 279: 38830–38837, 2004. doi: 10.1074/jbc.M406234200. [DOI] [PubMed] [Google Scholar]
- 15.Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol 67: 719–758, 2005. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- 16.Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J Physiol 587: 2127–2139, 2009. doi: 10.1113/jphysiol.2008.163709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinze C, Seniuk A, Sokolov MV, Huebner AK, Klementowicz AE, Szijártó IA, Schleifenbaum J, Vitzthum H, Gollasch M, Ehmke H, Schroeder BC, Hübner CA. Disruption of vascular Ca2+-activated chloride currents lowers blood pressure. J Clin Invest 124: 675–686, 2014. doi: 10.1172/JCI70025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang RY, Laing JG, Kanter EM, Berthoud VM, Bao M, Rohrs HW, Townsend RR, Yamada KA. Identification of CaMKII phosphorylation sites in Connexin43 by high-resolution mass spectrometry. J Proteome Res 10: 1098–1109, 2011. doi: 10.1021/pr1008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun 212: 806–812, 1995. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- 20.Jin DZ, Guo ML, Xue B, Fibuch EE, Choe ES, Mao LM, Wang JQ. Phosphorylation and feedback regulation of metabotropic glutamate receptor 1 by calcium/calmodulin-dependent protein kinase II. J Neurosci 33: 3402–3412, 2013. doi: 10.1523/JNEUROSCI.3192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem 266: 15555–15558, 1991. [PubMed] [Google Scholar]
- 22.Leblanc N, Forrest AS, Ayon RJ, Wiwchar M, Angermann JE, Pritchard HA, Singer CA, Valencik ML, Britton F, Greenwood IA. Molecular and functional significance of Ca2+-activated Cl− channels in pulmonary arterial smooth muscle. Pulm Circ 5: 244–268, 2015. doi: 10.1086/680189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O’Driscoll K, Britton F, Perrino BA, Greenwood IA. Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can J Physiol Pharmacol 83: 541–556, 2005. doi: 10.1139/y05-040. [DOI] [PubMed] [Google Scholar]
- 24.Ledoux J, Greenwood I, Villeneuve LR, Leblanc N. Modulation of Ca2+-dependent Cl− channels by calcineurin in rabbit coronary arterial myocytes. J Physiol 552: 701–714, 2003. doi: 10.1113/jphysiol.2003.043836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin CX, Lv XF, Yuan F, Li XY, Ma MM, Liu CZ, Zhou JG, Wang GL, Guan YY. Ca2+/calmodulin-dependent protein kinase II γ-dependent serine727 phosphorylation is required for TMEM16A Ca2+-activated Cl− channel regulation in cerebrovascular cells. Circ J 82: 903–913, 2018. doi: 10.1253/circj.CJ-17-0585. [DOI] [PubMed] [Google Scholar]
- 26.Manoury B, Tamuleviciute A, Tammaro P. TMEM16A/anoctamin 1 protein mediates calcium-activated chloride currents in pulmonary arterial smooth muscle cells. J Physiol 588: 2305–2314, 2010. doi: 10.1113/jphysiol.2010.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzone A, Bernard CE, Strege PR, Beyder A, Galietta LJ, Pasricha PJ, Rae JL, Parkman HP, Linden DR, Szurszewski JH, Ördög T, Gibbons SJ, Farrugia G. Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem 286: 13393–13403, 2011. doi: 10.1074/jbc.M110.196089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzone A, Gibbons SJ, Bernard CE, Nowsheen S, Middha S, Almada LL, Ordog T, Kendrick ML, Reid Lombardo KM, Shen KR, Galietta LJ, Fernandez-Zapico ME, Farrugia G. Identification and characterization of a novel promoter for the human ANO1 gene regulated by the transcription factor signal transducer and activator of transcription 6 (STAT6). FASEB J 29: 152–163, 2015. doi: 10.1096/fj.14-258541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris AP, Frizzell RA. Ca2+-dependent Cl− channels in undifferentiated human colonic cells (HT-29). II. Regulation and rundown. Am J Physiol Cell Physiol 264: C977–C985, 1993. doi: 10.1152/ajpcell.1993.264.4.C977. [DOI] [PubMed] [Google Scholar]
- 30.Paulino C, Kalienkova V, Lam AK, Neldner Y, Dutzler R. Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature 552: 421–425, 2017. doi: 10.1038/nature24652. [DOI] [PubMed] [Google Scholar]
- 31.Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins). Physiol Rev 94: 419–459, 2014. doi: 10.1152/physrev.00039.2011. [DOI] [PubMed] [Google Scholar]
- 32.Pritchard HA, Leblanc N, Albert AP, Greenwood IA. Inhibitory role of phosphatidylinositol 4,5-bisphosphate on TMEM16A-encoded calcium-activated chloride channels in rat pulmonary artery. Br J Pharmacol 171: 4311–4321, 2014. doi: 10.1111/bph.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reisert J, Bauer PJ, Yau KW, Frings S. The Ca-activated Cl channel and its control in rat olfactory receptor neurons. J Gen Physiol 122: 349–363, 2003. doi: 10.1085/jgp.200308888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheppeck JE 2nd, Gauss CM, Chamberlin AR. Inhibition of the Ser-Thr phosphatases PP1 and PP2A by naturally occurring toxins. Bioorg Med Chem 5: 1739–1750, 1997. doi: 10.1016/S0968-0896(97)00146-6. [DOI] [PubMed] [Google Scholar]
- 37.Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun 181: 968–975, 1991. doi: 10.1016/0006-291X(91)92031-E. [DOI] [PubMed] [Google Scholar]
- 38.Sun H, Xia Y, Paudel O, Yang XR, Sham JS. Chronic hypoxia-induced upregulation of Ca2+-activated Cl− channel in pulmonary arterial myocytes: a mechanism contributing to enhanced vasoreactivity. J Physiol 590: 3507–3521, 2012. doi: 10.1113/jphysiol.2012.232520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sung TS, O’Driscoll K, Zheng H, Yapp NJ, Leblanc N, Koh SD, Sanders KM. Influence of intracellular Ca2+ and alternative splicing on the pharmacological profile of ANO1 channels. Am J Physiol Cell Physiol 311: C437–C451, 2016. doi: 10.1152/ajpcell.00070.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ta CM, Acheson KE, Rorsman NJG, Jongkind RC, Tammaro P. Contrasting effects of phosphatidylinositol 4,5-bisphosphate on cloned TMEM16A and TMEM16B channels. Br J Pharmacol 174: 2984–2999, 2017. doi: 10.1111/bph.13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas-Gatewood C, Neeb ZP, Bulley S, Adebiyi A, Bannister JP, Leo MD, Jaggar JH. TMEM16A channels generate Ca2+-activated Cl− currents in cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol 301: H1819–H1827, 2011. doi: 10.1152/ajpheart.00404.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian Y, Kongsuphol P, Hug M, Ousingsawat J, Witzgall R, Schreiber R, Kunzelmann K. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J 25: 1058–1068, 2011. doi: 10.1096/fj.10-166884. [DOI] [PubMed] [Google Scholar]
- 43.Tian Y, Schreiber R, Kunzelmann K. Anoctamins are a family of Ca2+-activated Cl− channels. J Cell Sci 125: 4991–4998, 2012. doi: 10.1242/jcs.109553. [DOI] [PubMed] [Google Scholar]
- 44.Tien J, Peters CJ, Wong XM, Cheng T, Jan YN, Jan LY, Yang H. A comprehensive search for calcium binding sites critical for TMEM16A calcium-activated chloride channel activity. eLife 3: e02772, 2014. doi: 10.7554/eLife.02772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang M, Yang H, Zheng LY, Zhang Z, Tang YB, Wang GL, Du YH, Lv XF, Liu J, Zhou JG, Guan YY. Downregulation of TMEM16A calcium-activated chloride channel contributes to cerebrovascular remodeling during hypertension by promoting basilar smooth muscle cell proliferation. Circulation 125: 697–707, 2012. doi: 10.1161/CIRCULATIONAHA.111.041806. [DOI] [PubMed] [Google Scholar]
- 46.Wang YX, Kotlikoff MI. Inactivation of calcium-activated chloride channels in smooth muscle by calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci USA 94: 14918–14923, 1997. doi: 10.1073/pnas.94.26.14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiwchar M, Ayon R, Greenwood IA, Leblanc N. Phosphorylation alters the pharmacology of Ca2+-activated Cl− channels in rabbit pulmonary arterial smooth muscle cells. Br J Pharmacol 158: 1356–1365, 2009. doi: 10.1111/j.1476-5381.2009.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao Q, Yu K, Perez-Cornejo P, Cui Y, Arreola J, Hartzell HC. Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop. Proc Natl Acad Sci USA 108: 8891–8896, 2011. doi: 10.1073/pnas.1102147108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 50.Yu K, Duran C, Qu Z, Cui YY, Hartzell HC. Explaining calcium-dependent gating of anoctamin-1 chloride channels requires a revised topology. Circ Res 110: 990–999, 2012. doi: 10.1161/CIRCRESAHA.112.264440. [DOI] [PMC free article] [PubMed] [Google Scholar]