Abstract
Extracts from white birch have been reported to possess antimicrobial properties, but no study has linked the chemical composition of bark extract with antimicrobial activity. This study aimed to identify white birch (Betula papyrifera Marshall) bark extracts with antimicrobial activity and elucidate its composition. In order to obtain the highest extraction yield, bark residues >3 mm were retained for extraction. A total of 10 extraction solvents were used to determine the extraction yield of each of them. Methanol and ethanol solvents extracted a greater proportion of molecules. When tested on eight microorganism species, the water extract proved to have the best antimicrobial potential followed by the methanol extract. The water extract inhibited all microorganisms at low concentration with minimal inhibitory concentration between 0.83 and 1.67 mg/ml. Using ultraperformance liquid chromatography coupled to a time‐of‐flight quadrupole mass spectrometer, several molecules that have already been studied for their antimicrobial properties were identified in water and methanol extracts. Catechol was identified as one of the dominant components in white birch bark water extract, and its antimicrobial activity has already been demonstrated, suggesting that catechol could be one of the main components contributing to the antimicrobial activity of this extract. Thus, extractives from forestry wastes have potential for new applications to valorize these residues.
Keywords: antimicrobial activity, bark extracts, Betula papyrifera Marshall, mass spectrometry, white birch
We have shown that several extracts of white birch bark residues display natural antimicrobial activity. Among them, the water extract followed by the methanol extract proved to have the best antimicrobial potential. For the first time, extracts were characterized using ultrahigh‐performance liquid chromatography‐quadrupole time‐of‐flight mass spectrometry, which led to the identification of several molecules including catechol, predominant in the water extract, which is known to have antimicrobial activity.
1. INTRODUCTION
More than 50 million tons of bark, mainly derived from pulp and wood industries, are produced annually in North America (Gupta, 2009). In Canada, only a fraction of the bark is used as an energy source by direct combustion, and the rest of the bark is incinerated or landfilled as waste (Cheng, Deng, Zhang, Riedl, & Cloutier, 2006). Both incineration and landfilling are nonsustainable avenues, whereas the combustion of bark is not ideal for energy production as it contains a high ash content that lower its heating values. Thus, the combustion of bark for energy recovery is not economically advantageous. In addition to ash, cellulose, hemicellulose, and lignin, bark also contains small amounts of bioactive compounds called extractives, which have potential to provide value‐added coproducts to bark. Recent studies have revealed that forestry wastes such as bark possess potentially important properties for new applications, which are related to their chemical content (Feng, Cheng, Yuan, Leitch, & Xu, 2013; Jablonsky et al., 2017).
The biodiversity of the boreal forest constitutes an important pool of natural bioactive compounds (Royer, Ben Amor, Boucher, & Les, 2016; Royer, Houde, Viano, & Stevanovic, 2012). For example, the white birch (Betula papyrifera Marshall), one of the broad‐leaved species widely present in the boreal forest of North America, contains a large amount of bioactive molecules including terpenoids and phenolic compounds known to have antimicrobial and antioxidant properties (Mshvildadze, Legault, Lavoie, Gauthier, & Pichette, 2007; Royer et al., 2012). Bark is a set of dead tissues, developed after the primary and secondary growth of bark, which together form the protective layers of branches and the trunks of woody plants. The bark inhibits water loss through evaporation and has a protective role against overheating, frost, herbivores, or pathogens. It comprises up to 20% of the dry weight of woody plants and contains various molecules (Tanase, Coșarcă, & Muntean, 2019). The extraction of such molecules for the valorization of bark residues prior to thermal energy production is an interesting way to make it economically and environmentally advantageous for several industries (e.g., pharmaceutical, cosmetic, food, and sanitary industries). This important and inexpensive source of bioactive molecules can be used in the formulation of biosourced products, thus promoting applications for forest industries. As a result of the industrial method of wood processing, bark residues are removed from the sapwood and discarded (Celhay, Mathieu, Candy, Vilarem, & Rigal, 2014). For these reasons, residues are present in large quantities, thus representing an abundant and currently underutilized natural resource (Zhao, Yan, & Cao, 2007). Currently in Québec, the only valorization of bark is its unefficient transformation into heat and electricity by cogeneration central. It is therefore necessary to find new ways to valorize this biomass, including the production of biosourced products.
The use and research for new drugs, food supplements, and sanitary products derived from plants has continuously increased in recent years (Jamshidi‐Kia, Lorigooini, & Amini‐Khoei, 2018). In particular, natural products represent new avenues for the treatment of infectious diseases (Saleem et al., 2010). While 25%–50% of current pharmaceuticals come from plants, few are used as antimicrobial agents (Gurib‐Fakim, 2006). Plants are known to be rich in a wide variety of specialized (aka secondary) metabolites (e.g., phenolics, terpenoids, and alkaloids), often studied in vitro for their antimicrobial properties (Cowan, 1999; Royer, Prado, García‐Pérez, Diouf, & Stevanovic, 2013). These specialized metabolites are produced in various plant tissues whose main function is to protect the plant against fungi, bacteria, and insect attacks (Omar et al., 2000; Wink, 1988). For example, the antimicrobial activity of plant phenolic compounds such as catechin and ellagitannins has been intensively studied (Chandra et al., 2017; Daglia, 2012; Puupponen‐Pimiä, Nohynek, Alakomi, & Oksman‐Caldentey, 2005; Zhang et al., 2016). In addition to controlling invasion and growth of plant pathogens, terpenoids (thymol and carvacrol) were found to be active against human pathogens as well (Barbieri et al., 2017; Moon & Rhee, 2016). The use of plant specialized metabolites as antimicrobial compounds tends to increase due to the constant emergence of microorganisms resistant to current antimicrobial agents (Amábile‐Cuevas, 2003; Saleem et al., 2010). However, the majority of these studies focused on flowering plants and only few studied woody plants or forest residues (Annabelle, Dorian, Nathalie, Julien, & Isabel, 2019; Papuc, Goran, Predescu, Nicorescu, & Stefan, 2017; St‐Pierre et al., 2018; Tanase et al., 2018). It is therefore essential to investigate the antimicrobial potential of specialized metabolites present in bark residues of Québec's forest industries to valorize these residues and to expend the repertoire of antimicrobial compounds.
White birch is an important source of extractives with many interesting biological properties for the formulation of high value‐added coproduct (Krasutsky, 2006). Birch bark is a low‐value waste product in the forest industry (Ekman, 1983). In fact, 96,000 tons of paper birch bark is produced annually in Québec province (Pedieu, Riedl, & Pichette, 2009). The majority of extractives from this species is obtained from its bark with a yield of 22 g/100 g of dry bark (Krasutsky, 2006). Previous studies on white birch showed that specialized metabolites (e.g., terpenoids and polyphenols) from this tree possess several beneficial pharmacological properties (Gauthier, Legault, Lebrun, Dufour, & Pichette, 2006; Krasutsky, 2006; Vandal, Abou‐Zaid, Ferroni, & Leduc, 2015). For example, pentacyclic triterpenoids, mainly of the lupane and oleanane types, have been isolated from the outer bark of various species of birch, including Betula papyrifera Marshall. These triterpenoids displayed diversified biological activities such as bactericidal, antiviral, anti‐inflammatory, cytotoxic, and anticancer (Gauthier et al., 2006; O'Connell, Bentley, Campbell, & Cole, 1988; Omar et al., 2000). Specifically, betulin found in large quantities in the bark of white birch (72.4%) and betulinic acid (5.4%) are two lupane triterpenoids with great interest for the pharmaceutical sector because of their antimicrobial, anticancer, and anti‐HIV properties (Krasutsky, 2006; Royer et al., 2016). In addition, birch phenolic compound platyphylloside was shown to exert anticancer activity in vitro against lung carcinoma (A‐549) and colorectal adenocarcinoma (DLD‐1) human cell lines (Mshvildadze et al., 2007). Although the antimicrobial potential of a few specialized metabolites from white birch has been reported, the composition of bark extractives and its correlation with antimicrobial activity has not yet been investigated.
The objective of this research was to extract the specialized metabolites present in the bark of white birch and to determine the optimal conditions to maximize extraction yields either by using different solvents or different bark granulometries. Initial screening of specialized metabolites was performed using thin‐layer chromatography coupled with different colorimetric revelation methods. In addition, the antimicrobial activity of the extracts was measured against eight different microorganisms using the broth microdilution method. The minimal inhibitory concentration (MIC) as well as the minimal bactericidal/fungicidal concentration (MBC/MFC) was evaluated. Finally, the chemical composition of birch extracts with antimicrobial potential was determined using ultraperformance liquid chromatography coupled to a time‐of‐flight quadrupole mass spectrometry (UPLC‐QTOF‐MS). The characterization of the compounds present in each extract allows to identify molecules with an associated antimicrobial activity. Consequently, biologically active molecules obtained from the bark of white birch could be exploited on an industrial scale in order to valorize these abundant residues from forest wastes.
2. MATERIALS AND METHODS
2.1. Material and reagent
A total of 10 solvents were used for the extraction of bark residues. The majority were purchased from Fisher Scientific (methanol HPLC Grade, ethanol denatured, acetone certified ACS, hexane HPLC Grade), while the others (chloroform 99.9%, methylene chloride >99%, ethyl acetate 99.6%) were obtained from Acros Organics. For the acid–base extraction, hydrochloric acid (Fisher certified ACS) was used to acidify methanol at pH 4, as well as 7 N ammonia (Acros organics) to alkalize the aqueous fraction during liquid–liquid extraction. For the revelation of thin‐layer chromatography (TLC) plates, reagents were purchased from Fisher Chemical. All extracts were dissolved in dimethyl sulfoxide (DMSO), certified ACS from Fisher Chemical.
2.2. Plant material and granulometric fraction
White birch (B. papyrifera Marshall) bark was collected from the Thomas‐Louis Tremblay industry, a sawmill from Ste‐Monique in Lac‐St‐Jean (Québec, Canada), during the winter of 2016. The collected bark residues were immediately dried at room temperature. Before starting the laboratory tests, we determined the contribution of bark residues particle size to the total extractives. To do this, bark residues, which also contained wood particles, were sieved with different sizes of strand (3 mm, 7 mm, 45 mm). Fraction sizes were classified in four groups: smaller than 3 mm (<3 mm), between 3 and 7 mm (3–7 mm), between 7 and 45 mm (7–45 mm) and over 45 mm (>45 mm). The largest bark residues in this last group were approximately 300 mm. All fractions were extracted with either of the three solvents: water, water–ethanol, and ethanol. The results are reported as g of extractives in a given fraction group per gram of total mass of the bark residues before sieving. This is to reflect the weight of the fraction in addition to the extractives yield of the fraction (ex. g of 3–7mm extractives/total mass of bark residues batch obtained from the sawmill).
2.3. Bark composition
The general composition of the bark residues, which comprised wood particles, ash, extractives, lignin, cellulose, and hemicellulose content, was determined using the National Renewable Energy Laboratory (NREL) protocols: TP‐510‐42618, TP‐510‐42619, TP‐510‐42620, and TP‐510‐42622 (Hames et al., 2008; Sluiter, Hames, et al., 2005; Sluiter et al., 2008b; Sluiter, Ruiz, Scarlata, Sluiter, & Templeton, 2005). Briefly, the method for determining the ash composition was based on the percentage of residue remaining after dry oxidation at 550–600°C. All results are reported relative to the 105°C oven dry weight of the sample. The extractive composition was carried out using a Soxhlet apparatus with ethanol at reflux for 16–24 hr. The lignin content was measured by UV‐Vis spectroscopy (Hach DR6000) after a two‐step acid hydrolysis to fractionate the biomass into forms that are more easily quantified. The monomeric sugars constituting cellulose and hemicellulose were quantified by ion chromatography (Dionex ICS‐5000) with the Dionex CarboPac SA‐10 column and the electrochemical detector.
2.4. Bark extraction
The bark was grounded into a fine powder (0.425 mm) by using a Wiley mill. Ten different solvents were used with three solvent‐dependent extraction techniques: water (1), methanol (2), ethanol (3), acetone (4), methylene chloride (5), ethyl acetate (6), chloroform (7), hexane (8), water–ethanol (9), and acid–base (10). The last solvent corresponds to a liquid–liquid extraction technique using several solvents described by Yubin, Miao, Bing, and Yao (Yubin, Miao, Bing, & Yao, 2014). This method allows mainly the extraction of alkaloids from the bark. The majority of the extracts (2, 3, 4, 5, 6, 7, 8) were obtained using the soxhlet extraction technique during 7 hr of reflux. The other two extracts (1, 9) were obtained using an accelerated solvent extractor (Dionex™ ASE™ 350; ThermoFisher). The solid bark was extracted with distilled water at 100°C and 1,500 psi over six cycles of 10 min each. For the water–ethanol extract (9), a liquid extraction with ethanol was carried out following a first extraction with water. The liquid extract was re‐extracted with ethanol at 120°C and 1,500 psi over six cycles of 5 min. Finally, all extracts were evaporated to dryness in a low temperature oven.
2.5. Thin‐layer chromatography method
The 10 extracts were dissolved in their respective solvents to a final concentration of 10 mg/ml, and these solutions were used for preparative thin‐layer chromatography. For that purpose, TLC (Aluminum TLC Silica gel 60 F254) plates of size 14 × 20 cm were used. Drops (7 μl) of each extract solution were loaded individually onto the baseline of the layer, which was then developed with chloroform: methanol (9:1 v/v). A solution containing several standards concentrated at 1,000 ppm (piperine, vanillin, ferulic acid, glucose, and betulin) was prepared. On each TLC, 7 μl of this solution was deposited in order to visualize the separation of different families of compounds. TLC was dried, and observed in a UV chamber at 254 nm, which revealed the presence of several chemical revelators. The revelators used were a ρ‐anisaldehyde sulfuric acid solution to visualize all types of compounds present in the extract (Jork, Funk, Fischer, Wimmer, & Burns, 1990), iron chloride reagent (FeCl3) to observe the presence of phenols (Jork et al., 1990), and Dragendorff reagent for alkaloid compounds (Sasidharan, Chen, Saravanan, Sundram, & Latha, 2011).
2.6. Microorganism cultures
The white birch extracts were individually tested against a panel of microorganisms including Gram‐negative strains Escherichia coli ATCC 35218, Salmonella enterica ATCC 10708, and Pseudomonas aeruginosa ATCC 1542; Gram‐positive strains Staphylococcus aureus ATCC 6538 and Enterococcus faecalis ATCC 29212; fungal strains Aspergillus niger ATCC 10535; and yeast strains Candida albicans and Saccharomyces cerevisiae. The two yeast strains were supplied by the microbiology laboratory of the Université du Québec in Trois‐Rivières (Québec, Canada). Bacterial strains were cultured overnight at 37°C in Mueller Hinton agar (MHA) prior to the antimicrobial tests. Fungus and yeast (fungi) strains were cultured for 72 hr at 37°C in Sabouraud dextrose agar (SDA).
2.7. Antimicrobial assays
The antimicrobial activity of the extracts was studied using the broth microdilution method, with appropriate culture media. For example, Mueller Hinton broth (MHB) was used for bacteria and Sabouraud dextrose broth (SDB) for fungi. Microorganism suspensions were prepared in sterile 0.9% saline solution to obtain a final inoculum estimated at 1.5 × 108 cfu/ml for bacteria, 1.5 × 106 cfu/ml for yeast, and 1.5 × 104 cfu/ml for fungus, according to 0.5 McFarland turbidity value as measured using turbidimeter (Hach, 2100AN). Extracts were prepared and dissolved in DMSO at a concentration of 10 mg/ml. According to the antimicrobial testing method, the highest DMSO concentration found in the microplate well was 26%, which had no influence on microbial growth of the tested microorganisms.
To test the antimicrobial properties of the extracts, 96‐well plates were prepared according to a modified method mentioned in Balouiri, Sadiki, and Ibnsouda (2016). Initially, screening of the extracts using 4.5% was carried out in order to quickly identify the potential extracts that allow inhibition of the microbial growth. These extracts were further tested using microdilution technique. The microdilution method was initiated by dispensing 100 μl of each extract in the first column of a 96‐well plate containing 50 μl of broth (MHB or SDB). Serial dilutions of the extracts were carried out in order to obtain a final concentration between 4.44 and 0.01 mg/ml. An equal volume (50 μl) of microbial suspension was added into the wells. Negative and positive controls were prepared without antimicrobial agent and with quaternary ammonium solution (BTC 2125M‐80%) obtained from Sani Marc Group, respectively. The plates were incubated at 37°C during 3 hr for bacteria and 6 hr for fungi. Then, to indicate the metabolic activity of the microorganisms, 40 μl/well of INT (2‐p‐iodophenyl‐3‐p‐nitrophenyl‐5‐phenyl tetrazolium chloride; Sigma) dissolved in water (2.85 mg/ml) was added to each well. This tetrazolium salt is reduced to red formazan dye by the active dehydrogenases of living cells. The visual development of color was observed after incubation under appropriate cultivation conditions during 1 hr for bacteria and 16 hr for fungi. MIC values were defined as the lowest concentration of each natural product which resulted in no color formation, meaning that microbial growth was inhibited. Results were expressed in milligrams per milliliters. All measurements of MIC values were repeated in duplicate. To determine minimum bactericidal/fungicidal concentration (MBC/MFC), an aliquot (100 μl) of each incubated well, with concentration equal or over the MIC, was plated onto MHA for bacteria and SDA for fungi. MBC and MFC were defined as the lowest concentrations that allow no visible growth on agar after 24 hr of incubation for bacteria and 48 hr for fungi.
2.8. UPLC‐QTOF‐MS analysis
These analyses were carried out externally by the Centre de Recherche Industrielle du Québec (CRIQ). Briefly, a UPLC analysis was performed using a Waters Acquity Ultraperformance LC system (Waters), equipped with a binary pump system (Waters). An Acquity Ethylene Bridged Hybrid (BEH) C18 column (100 mm_2.mm id, 1.7 mm particle size) from Waters was used. The molecules were separated with a mobile phase that consisted of 0.2% acetic acid (eluent A) and acetonitrile (eluent B). The flow rate was 0.2 ml/min and the gradient elution was initial, 2% B; 0–1 min, 2%–100% B; 1–30 min, isocratic 100% B; 30–33 min, 100%–2% B; 33–33.5 min, isocratic 2% B; 33–40 min. The mass spectrometry (MS) analyses were carried out on a QTOF Micro mass spectrometer (Waters) equipped with a Z‐spray electrospray interface. Each analysis was performed in both positive and negative mode, and the data were acquired through a mass scan from 100 to 1,250 m/z. The ionization occurred at 120°C using a cone gas flow rate of 50 L/hr, desolvation gas flow rate of 350 L/hr, and a desolvation temperature set at 200°C. Nitrogen (99% purity) was used as a nebulizing gas. Data interpretation was carried out with the MassLynx 4.1 software. Mass extraction, deconvolution, and isotope and library search were performed using MZMine 2 (Pluskal, Castillo, Villar‐Briones, & Orešič, 2010).
2.9. Statistical analysis
Extraction experiments were carried out in triplicate. Data were expressed as mean ± standard deviation. Significant differences (p < .05) among the mean values of extraction results were determined by one‐way analysis of variance (ANOVA) followed by Tukey test, using the Past 3 statistical software (version 3.16; Hammer, Harper, & Ryan, 2001).
3. RESULTS
3.1. Granulometry, composition, and extraction yields
The residues of white birch bark were sieved using different sieve sizes to obtain four fractions of bark residue particle sizes. The different fractions were crushed and extracted using three solvents: water, water–ethanol, and ethanol. The extraction yields (g/100 g of total bark residues) obtained for each granulometric fraction of bark are summarized in Figure 1a. The fraction >3 mm represents the mix of three fraction groups: 3–7, 7–45, and >45 mm. In general, extraction with ethanol yielded a greater extractive content compared to other solvents (Figure 1a). With regard to the extraction yield by function of the particle size distribution, smaller fractions (3–7 and <3 mm) contributed to a smaller amount of the extractive yield compared to the larger ones (7–45 and >45 mm). For example, the fraction 7–45 and >45 mm extracted with ethanol yielded, respectively, 8.82 and 5.76 g of extractives/100 g of bark, whereas the fractions <3 and 3–7 mm generated 8 times less with only 1.12 and 0.88 g/100 g of bark, respectively (Figure 1a).
Figure 1.
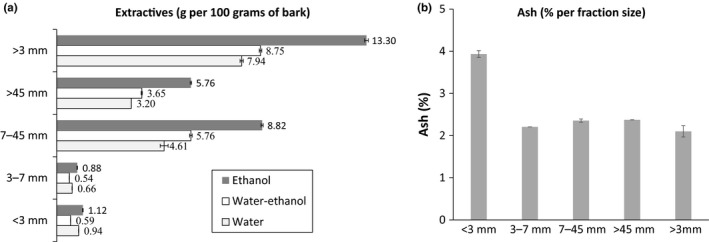
Analysis of bark biomass. Table of the optimal granulometric fraction to be used for the extraction of the bark (a), the graph shows the extractive concentration (g) per 100 g of bark batch among fraction sizes following extraction with ethanol, water–ethanol, and water. The graph (b) represents the percentage of ash in the bark fraction <3 and 3–7 mm
Next, we quantified the ash content present in each particle size fraction of bark residues, and the values obtained are presented in Figure 1b. The ash content of fraction <3 mm (3.94%) was significantly higher compared to others, which were 2.21% for 3–7 mm, 2.36% for 7–45 mm, and 2.37% for >45 mm.
Next, the general composition of white birch bark residues was assessed. The values are reported as a percentage of the total mass of the whole fraction >3 mm. The largest proportion of the composition corresponded to lignin (36.55%), followed by cellulose (21.70%) and hemicellulose (14.70%). In addition, the average extractive content following ethanol extraction was 13.05% (Figure 2).
Figure 2.
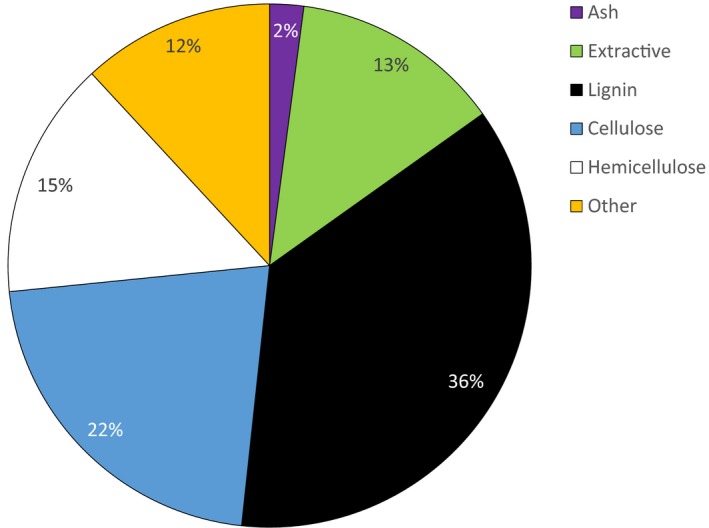
Pie chart of bark components. The pie chart represents the analysis of the composition of the bark in fraction >3 mm
The extraction yields of the residues as a function of the solvent used are reported in Figure 3. The results show that alcohol solvents were more effective and extracted more compounds altogether, with the methanol and ethanol extracts having a respective percent extraction of 16.10% and 14.56% (Figure 3). These two polar solvents extracted more compounds present in residues, whereas the less polar solvents (e.g., hexane 5.64% yield) had lower extraction yields. In addition, the acid–base extraction protocol aimed to extract alkaloid compounds displayed the lowest extraction efficiency with 3.09% and appeared to be the least optimal condition to obtain the highest weight of dry extractive (Figure 3).
Figure 3.
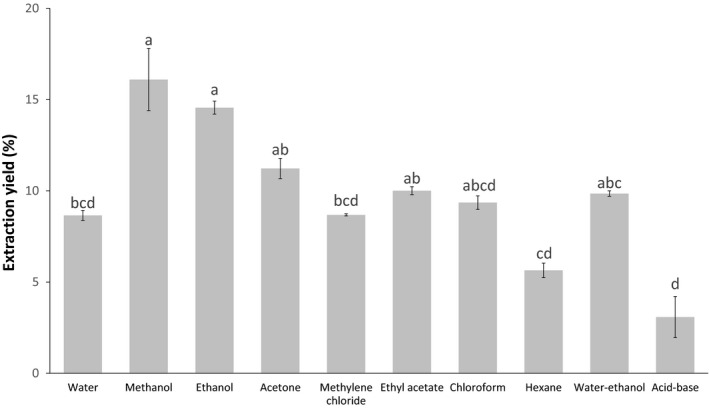
Bark extraction yields. Graph of the extraction yield according to the solvent used
3.2. Metabolite profile—Thin‐layer chromatography
Next, we assessed the general chemical composition of crude white birch bark extracts using thin‐layer chromatography (TLC) (Figure 4). The TLC plates correspond to the chromatogram showing the separation of compounds present in each extract using different revelation reagents. Based on the mobile phase used, most of the polar compounds present in the extracts were found at the bottom of the TLC plate, while less polar compounds migrated to the top of the silica TLC plate. With the help of standards, this technique made it possible to roughly visualize which types of molecules were present in each extract according to their polarity. Each TLC plate showed a lane of each extract (1–10) and a lane containing four standards (S) (P: piperine, V: vanillin, B: betulin, F: ferulic acid, G: glucose) (Figure 4). The first TLC plate showed compounds that reacted with UV (λ = 254 nm), in other words, aromatic compounds having a strong conjugation in their molecular structure (Figure 4a). On this plate, only three standards were visualized, piperine (P), vanillin (V), and ferulic acid (F), which can be attributed to their chemical structure. On the second TLC plate, separated compounds reacted with the ρ‐anisaldehyde reagent to demonstrate the presence of different families of compounds (Figure 4b). Each of these families reacted differently and can be distinguished by their different colors. The colored compounds at the bottom of the plate are strongly polar. For example, sugars such as glucose (G) show a green spot in the standard column (S), while the compounds higher on the plate are less polar, such as betulin (B), a triterpenoid characterized by a purple spot. Results showed that extracts using polar solvents such as water, methanol, and ethanol contained a greater proportion of sugars (green‐brown spots) than the other extracts. Moreover, all extracts appeared to have several nonpolar compounds including terpenoids (purple spot) and phenolic compounds. The third TLC plate showed the phenolic compounds which reacted with iron chloride (FeCl3; Figure 4c). Phenolic compounds in the extracts had more affinity for the mobile phase and were concentrated at the top of the TLC plate. By comparing the last two TLC plates, it is interesting to note that the two lines of purple spots at the top of the second plate likely corresponded to phenolic compounds, since they are found at the same position (Rf of 0.8 and 0.9; Figure 2b) than the spots at the top of the third plate that reacted with FeCl3 (Figure 4c). In addition, the Dragendorff reagent, used to visualize the presence of alkaloids, was applied to a TLC plate. However, the results showed a very low presence (accumulation and number) of these compounds in the white birch extracts.
Figure 4.
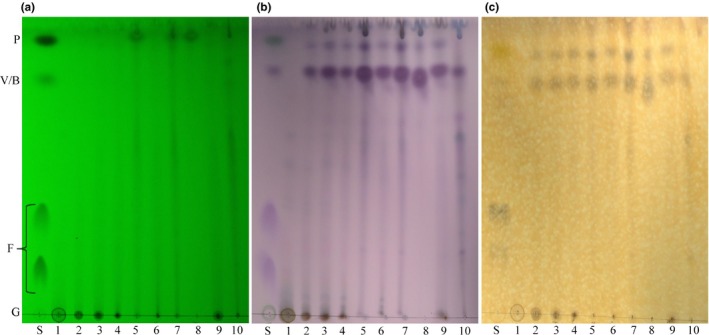
Thin‐layer chromatography of bark extracts. Thin‐layer chromatography of bark extracts with chloroform: methanol (9:1) and visualized under UV 254 nm (a), stained with ρ‐anisaldehyde (b) and with FeCl3 (c). Lane S corresponds to a mixture of standards (P, piperine; V, vanillin; B, botulin; F, ferulic acid; and G, glucose). The samples from left to right were extracts from different solvents: water (1), methanol (2), ethanol (3), acetone (4), methylene chloride (5), ethyl acetate (6), chloroform (7), hexane (8), ethanol–water (9), and acid–base (10)
3.3. Antimicrobial activity
Using the broth microdilution method, the antimicrobial activity of the ten extracts was determined on eight microorganisms. Table 1 shows the results obtained in regard to the antimicrobial power of bark extracts on five bacteria and three fungal species according to the extraction solvent used. A quaternary ammonium compound (QAC, BTC 2125M‐80%, Stepan®) was used as a positive control because it is a well‐known antimicrobial agent often used as an active ingredient in the formulation of several biocidal products such as surface disinfectants. In addition, for each test, a negative control was included, which contained no agent or extract and resulted in the growth of each microorganism.
Table 1.
Antimicrobial activity of white birch bark extracts against different strains of microorganisms
| Extracts | Escherichia coli | Salmonella enterica | Pseudomonas aeruginosa | Staphylococcus aureus | ||||
|---|---|---|---|---|---|---|---|---|
| MICa | MBC/MFCb | MIC | MBC/MFC | MIC | MBC/MFC | MIC | MBC/MFC | |
| Water | 1.67 | −c | 0.83 | − | 0.83 | − | 1.67 | 1.67 |
| Methanol | − | − | 1.67 | 4.44 | − | − | 4.44 | 4.44 |
| Ethanol | − | − | 1.67 | − | 1.67 | − | − | − |
| Acetone | − | − | 4.44 | − | − | − | − | − |
| Methylene chloride | 0.83 | − | 1.67 | 4.44 | 0.21 | 0.21 | − | − |
| Ethyl acetate | − | − | 1.67 | − | − | − | − | − |
| Chloroform | − | − | 1.67 | 4.44 | 0.21 | 0.83 | − | − |
| Hexane | − | − | 1.67 | − | − | − | − | − |
| Water–ethanol | 1.67 | − | 1.67 | − | 1.67 | − | 4.44 | − |
| Acid–base | 4.44 | − | 1.67 | − | − | − | − | − |
| QACd | 2.60 | 5.21 | 0.65 | 0.65 | 10.42 | 10.42 | 5.21 | 5.21 |
| Extracts | Enterococcus faecalis | Aspergillus niger | Candida albicans | Saccharomyces cerevisiae | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC/MFC | MIC | MBC/MFC | MIC | MBC/MFC | MIC | MBC/MFC | |
| Water | 1.67 | − | 0.83 | − | 1.67 | − | 1.11 | 4.44 |
| Methanol | 1.67 | 4.44 | 1.67 | − | − | − | 2.22 | − |
| Ethanol | − | − | 1.67 | − | − | − | 2.22 | − |
| Acetone | − | − | 1.67 | − | − | − | 2.22 | − |
| Methylene chloride | − | − | − | − | − | − | 1.11 | 4.44 |
| Ethyl acetate | − | − | − | − | − | − | 2.22 | − |
| Chloroform | − | − | − | − | − | − | 1.11 | 4.44 |
| Hexane | − | − | − | − | − | − | − | − |
| Water–ethanol | − | − | − | − | − | − | − | − |
| Acid–base | − | − | 2.22 | − | 1.67 | − | 2.22 | 4.44 |
| QACd | 2.60 | 2.60 | 10.42 | 10.42 | 5.21 | 5.21 | 2.60 | 2.60 |
MIC, minimum inhibitory concentration. Values are given as mg/ml.
MBC/MFC, minimum bactericidal concentration/minimum fungicidal concentration. Values are given as mg/ml.
Not active at maximum concentration (4.44 mg/ml)
QAC, quaternary ammonium cation is used as a positive control (BTC® 2125M‐80%). Values are given as mg/l.
All extracts of white birch bark residues inhibited the growth of S. enterica, a Gram‐negative bacterium (Table 1). For this bacteria, the MIC of each extract was 1.67 mg/ml, except for the water and acetone extracts which had a MIC value of 0.83 and 4.44 mg/ml, respectively. In addition, only the extracts with methanol, methylene chloride, and chloroform displayed bactericidal effect on S. enterica at a concentration of 4.44 mg/ml. For E. faecalis and C. albicans, only the water and methanol extracts promoted inhibition, and none caused total kill. The acid–base extract inhibited the growth of five out of eight microorganisms tested and was fungicidal for C. albicans with a MFC value of 4.44 mg/ml (Table 1).
Looking at the full range of antimicrobial test results, few extracts stood out and displayed greater potential as an antimicrobial agent. For example, the water extract inhibited all eight microorganisms tested and showed bactericidal effect on bacteria S. aureus (MBC 1.67 mg/ml) as well as on yeast S. cerevisiae (MFC 4.44 mg/ml). It was therefore the most promising extract because of its broad spectrum of antimicrobial activity at low concentrations, since its MIC was found to be between 0.83 and 1.67 mg/ml. The methanol extract was another promising one, because it inhibited the growth of five microorganisms (S. enterica, S. aureus, E. faecalis, A. niger, and S. cerevisiae) at MIC of 1.67 mg/ml except for S. aureus and S. cerevisiae that have MIC values of 4.44 and 2.22 mg/ml, respectively. In addition, this extract displayed bactericidal effect on S. enterica, S. aureus, and E. faecalis at a concentration of 4.44 mg/ml. The extract obtained using the acid–base extraction method, which is specific to enrich alkaloids, also shows a strong antimicrobial activity. It inhibits the growth of five microorganisms (E. coli, S. enterica, A. niger, C. albicans, and S. cerevisiae) and kills only one group of microorganisms, S. cerevisiae, at a concentration of 4.44 mg/ml.
3.4. Characterization using UPLC‐QTOF‐MS
After selection of the extracts with the best antimicrobial activities, it was important to identify their exact chemical composition to help target molecules that may be responsible for the biological activity.
The chemical composition of the white birch bark water and methanol extracts analyzed by UPLC‐QTOF‐MS is presented in Tables 2 and 3, respectively. These tables show the different molecules identified using database based on their exact mass. The compounds in the tables were selected on the basis of their relevance and percentage of area under the peak (% area) ≥0.50. The identified compounds were classified into families of molecules. Using this method of analysis, it was possible to notice that the retention time was not similar or even close together between the same family of compounds. This value derives from the affinity of each constituent for the stationary phase, which depends on its solubility in this phase and its polarity. Two modes of ionization were used, namely, the positive mode (M+H) and the negative mode (M−H) to ensure that the majority of compounds were detected and identified during the analysis.
Table 2.
Composition of white birch bark water extract using UPLC‐QTOF‐MS analyses
| Compoundsa | Rt b | Exact mass (m/z)c | Aread (%) | |
|---|---|---|---|---|
| [M+H] | [M−H] | |||
| Phenols | ||||
| Epirosmanol | 5.08 | — | 345.1393 | 5.88 |
| Sakuranetin | 5.64 | — | 285.1237 | 1.13 |
| Catechol | 5.65 | — | 109.0359 | 6.47 |
| 4‐Hydroxybenzaldehyde | 6.84 | — | 121.0385 | 1.65 |
| Fisetin | 9.52 | 287.1035 | — | 3.75 |
| Scutellarein | 12.41 | — | 285.0584 | 2.48 |
| Oleuropein‐aglycone | 14.61 | — | 377.1308 | 0.51 |
| 16.12 | — | 0.67 | ||
| Kaempferol | 15.55 | — | 285.0992 | 3.70 |
| 15.57 | 287.1035 | — | 2.86 | |
| Piceatannol | 16.24 | — | 243.0846 | 0.53 |
| Terpenoids | ||||
| Terpendole C | 7.87 | 520.3510 | — | 2.35 |
| Artabsin | 8.40 | 249.1232 | — | 4.43 |
| Confertiflorin | 11.10 | 307.1928 | — | 0.88 |
| p‐Menthane‐3,8‐diol | 11.63 | — | 171.1150 | 1.02 |
| Phytuberin | 14.30 | 295.1483 | — | 1.03 |
| Alkaloids | ||||
| Gentianaine | 0.98 | 142.0390 | — | 2.25 |
| Berberine | 4.52 | — | 335.1533 | 2.27 |
| Tubulosine | 7.53 | 476.3204 | — | 2.03 |
| Avenanthramide 2f | 13.55 | — | 329.2584 | 0.88 |
| Macarpine | 14.29 | — | 391.1157 | 0.56 |
| (–)‐Solenopsin A | 23.78 | 254.2584 | — | 4.48 |
| Glycosylated molecules | ||||
| 3‐Hydroxyphloretin 2'‐O‐glucoside | 7.16 | — | 451.1916 | 0.57 |
| Phlorin | 9.51 | — | 287.0781 | 1.59 |
| Grandidentatin | 9.80 | — | 423.1843 | 3.09 |
| 10.15 | 2.51 | |||
| Eriodictyol 7‐O‐glucoside | 10.78 | — | 449.1708 | 3.28 |
| 11.93 | 3.16 | |||
| Arbutin | 12.20 | — | 271.0804 | 3.16 |
| 12.22 | 273.0878 | — | 3.59 | |
| Acids | ||||
| Muconic acid | 1.12 | — | 141.0299 | 0.69 |
| Caffeic acid | 1.19 | — | 179.0726 | 0.96 |
| Hydroxycaffeic acid | 1.21 | — | 195.0641 | 0.59 |
| 3‐p‐Coumaroylquinic acid | 5.00 | — | 337.1743 | 2.05 |
| 5‐O‐Galloylquinic acid | 5.85 | — | 343.1214 | 1.03 |
| Feruloyl tartaric acid | 6.23 | — | 325.1221 | 0.55 |
| 6.54 | 325.1133 | 0.77 | ||
| Vanillic acid | 6.36 | — | 167.0478 | 0.50 |
| p‐Coumaric acid | 7.59 | — | 163.0480 | 3.05 |
| 4‐Hydroxybenzoic acid | 9.14 | — | 137.0339 | 3.85 |
| Pisiferic acid | 9.21 | 317.1948 | — | 0.73 |
| Ellagic acid | 13.14 | — | 301.0882 | 0.61 |
| Sugars | ||||
| l‐Rhamnose | 0.98 | 165.0511 | — | 0.20 |
| (+)‐Catechin 3‐O‐gallate | 7.75 | — | 441.1687 | 0.72 |
| d‐Glucose | 34.86 | 181.0349 | — | 0.21 |
| Others | ||||
| 4‐Nitrophenol | 1.02 | 140.0050 | — | 0.80 |
| 1,4‐Naphthoquinone | 3.02 | — | 157.0604 | 1.20 |
| Marchantin A | 8.92 | — | 439.1857 | 1.03 |
| Picrasin C | 9.33 | — | 421.1777 | 2.61 |
| 34.89 | 421.2571 | 0.50 | ||
| Myristamide | 23.05 | 228.2430 | — | 3.02 |
| Heptadecylamine | 26.02 | 256.2760 | — | 3.87 |
| 2‐Nitrophenol | 34.87 | 139.9936 | — | 1.36 |
The compounds were selected on the basis of their relevance and having a % area ≥0.50. The compounds were determined by comparing the exact masses in a data bank. Potential identities are presented.
Retention time (min).
Exact mass depending on the ionization mode of the analysis, either positive [M+H] or negative [M−H].
The percentage of area is relative to the ionization mode of the analysis used.
Table 3.
Chemical composition of white birch bark methanol extract using UPLC‐QTOF‐MS analyses
| Compoundsa | Rt b | Exact mass (m/z)c | Aread (%) | |
|---|---|---|---|---|
| [M+H] | [M−H] | |||
| Phenols | ||||
| Pentacosyl resorcinol | 7.32 | — | 459.1974 | 2.78 |
| Hydroxymatairesinol | 7.44 | — | 373.1544 | 3.66 |
| Anhydro‐secoisolariciresinol | 7.99 | — | 343.1340 | 1.36 |
| Taxifolin | 8.48 | — | 303.0838 | 0.82 |
| Ligstroside | 9.26 | — | 523.2272 | 2.30 |
| 9.40 | 2.35 | |||
| Phloridzin | 10.83 | — | 435.1445 | 1.44 |
| Dimethylquercetin | 13.64 | — | 329.2446 | 3.24 |
| Kaempferid | 14.20 | — | 299.1744 | 3.33 |
| Acacetine | 15.69 | — | 285.0872 | 3.30 |
| Oleuropein‐aglycone | 16.26 | — | 377.1339 | 0.65 |
| Terpenoids | ||||
| Perilloside | 11.61 | — | 313.1478 | 5.54 |
| Deoxystansioside | 14.53 | — | 295.1493 | 3.76 |
| Alkaloids | ||||
| Annotinine | 7.40 | 276.1790 | — | 2.35 |
| Methylconiine | 10.76 | 142.1624 | — | 1.74 |
| Isocorypalmine | 11.14 | 342.2122 | — | 4.19 |
| Pinidine | 11.65 | 140.1349 | — | 6.57 |
| Coniine | 14.57 | 128.1771 | — | 4.95 |
| Glycosylated molecules | ||||
| Galloyl glucose | 5.47 | — | 331.1245 | 1.20 |
| Hydroxyphloretin‐glucoside | 9.65 | — | 451.1421 | 1.12 |
| Apigenin 6‐glucoside | 10.32 | — | 431.1400 | 2.18 |
| Apigenin‐diglucoside | 11.13 | — | 593.2709 | 2.96 |
| Acids | ||||
| Ibotenic acid | 1.38 | 159.0468 | — | 0.61 |
| Caffeic acid | 1.55 | — | 179.0905 | 0.84 |
| Hydroxybenzoic acid | 6.05 | — | 137.0502 | 0.57 |
| 9.89 | — | 137.0389 | 0.67 | |
| Coumaric acid | 7.85 | — | 163.0530 | 0.78 |
| Valoneic acid dilactone | 8.55 | — | 469.1547 | 1.15 |
| Chicoric acid | 9.16 | — | 473.1739 | 1.74 |
| 9.52 | 2.80 | |||
| 12‐Hydroxydodecanoic acid | 9.58 | 217.1958 | — | 0.68 |
| 9.87 | 0.66 | |||
| Others | ||||
| Carbophenothion | 11.14 | 342.9818 | — | 1.52 |
| Butonate | 12.99 | 326.9941 | — | 3.73 |
| Asparagusate | 13.64 | 150.9699 | — | 3.93 |
| Retinal | 20.07 | 285.1934 | — | 1.41 |
The compounds were selected on the basis of their relevance and having a % area ≥0.50. The compounds were determined by comparing the exact masses in a data bank. Potential identities are presented.
Retention time (min).
Exact mass depending on the ionization mode of the analysis, either positive [M+H] or negative [M−H].
The percentage of area is relative to the ionization mode of the analysis used.
The water and methanol extracts contained a large number of phenolic compounds and acids. With regard to the water extract (Table 2), the presence of 9 phenolics and 11 acids was noted, including epirosmanol, which is present in large proportion (5.88%), and catechol (6.47%). The methanol extract (Table 3) contained a total of 10 phenolic compounds and 7 acids. In addition, among the nonvolatile components identified, pinidine (6.57%) was found to be most dominant compound in the methanol extract of white birch bark. Some acids were present in both extracts including caffeic acid, hydrobenzoic acid, and coumaric acid. However, these three acids were present in higher proportion in the extract with water. Finally, as anticipated it is observed that the water extract contained more sugars and glycosylated molecules compared to the methanol extract (Tables 2 and 3).
Despite several articles reporting the presence of high betulin content of white birch and its bark (Krasutsky, 2006; O'Connell et al., 1988), no betulin and betulinic acid were detected in water and methanol extracts. One possible explanation is that betulin (if present) is at lower concentration, and we did not derivatize prior to analysis. However, betulin was detected in samples extracted with less polar solvent, including the acid–base, ethyl acetate, and hexane extracts.
The chemical characterization of white birch bark extracts made it possible to target molecules with reported antimicrobial properties (Table 4). A total of six phenolics, one alkaloid and five acids, were listed in either the water or methanol extract. However, most of these molecules were found to be more abundant in the water extract. Catechol, also known as pyrocatechol or 1,2‐dihydroxybenzene, is a phenolic organic compound present at 6.47% concentration in water bark extract. This molecule is a monomer of flavan‐3‐ol, one of the components of proanthocyanidin polymers (condensed tannins).
Table 4.
Compounds present in water and methanol extracts of white birch bark with known antimicrobial activity as reported in the literature
| Compound class | Compound name | Extraction solvent | References | |
|---|---|---|---|---|
| Water | Methanol | |||
| Phenols | Catechol | + (6.47)a | − | Jeong et al. (2009), Kocaçalışkan et al. (2006) |
| 4‐Hydroxybenzaldehyde | + (1.65) | − | Chang, Chen, and Chang (2001), Friedman, Henika, and Mandrell (2003) | |
| Fisetin | + (3.75) | − | da Costa et al. (2014), Gabor and Eperjessy (1966) | |
| Phloridzin | − | + (1.44) | Barreca, Bellocco, Laganà, Ginestra, and Bisignano (2014), Zhang et al. (2016) | |
| Kaempferol | + (3.70) | − | Cai and Wu (1996), Calderon‐Montano, Burgos‐Morón, Pérez‐Guerrero, and López‐Lázaro (2011), Tatsimo et al. (2012) | |
| Piceatannol | + (0.53) | − | Plumed‐Ferrer et al. (2013), Yim et al. (2010) | |
| Alkaloids | Berberine | + (2.27) | − | IgbaláChoudhary (1995), Stermitz, Kamm, and Tawara (2000), Yu et al. (2005) |
| Acids | Caffeic acid | + (0.96) | + (0.84) | Aziz, Farag, Mousa, and Abo‐Zaid (1998), Jeong‐Yong, Jae‐Hak, Seong, and Keun‐Hyung (1998), Merkl, Hrádková, Filip, and Smidrkal (2010), Özçelik, Kartal, and Orhan (2011), Stojković et al. (2013) |
| Hydrobenzoic acid | + (3.85) | + (0.67) | Jeong‐Yong et al. (1998), Merkl et al. (2010) | |
| Vanillic acid | + (0.50) | − | Aziz et al. (1998), Delaquis, Stanich, and Toivonen (2005), Merkl et al. (2010) | |
| Coumaric acid | + (3.05) | + (0.78) | Aziz et al. (1998), Lou et al. (2012) | |
| Pisiferic acid | + (0.73) | − | Fukui, Koshimizu, and Egawa (1978), Kobayashi, Nishino, Fukushima, Shiobara, and Kodama (1988) | |
−, not present; +, present in the extract.
% of area in the extract.
4. DISCUSSION
4.1. Granulometry, composition, and extraction yields
The first step of the study was to analyze the crude bark residues of white birch. Since these are postindustrial residues, the bark samples received were not perfectly homogeneous and the chips were of different sizes. Pieces of wood could be found in the residues considering the bark has been removed from the sapwood directly in sawmill following industrial mechanical processes. Since the presence of wood in the bark residues can affect the extractives yield, it was therefore essential to determine the yield for each fraction size. The bark particle size fractioning is generally used in the treatment of biomass and may be used for selective enrichment of specific components (Miranda, Gominho, Mirra, & Pereira, 2012, 2013; Silva, Guilbert, & Rouau, 2011). The results presented the extractives yield in proportion to the mass fraction of the total mass of the bark residues batch, reflecting the real contribution to the total yield (Figure 1a,b). Although the contribution of fractions <3 and 3–7 mm to the total yield was lower than fractions over 7 mm, the 3–7 mm fraction has been retained for the study because its lower contribution was mainly due to its lower mass in the total mass of bark residues batch, as well as the <3 mm fraction. The <3 mm was, however, removed because of its higher ash content. Ash is an inorganic, undesirable material produced during extraction process and tends to accumulate in smaller fractions during biomass processing due to its fine size and fragility (Bridgeman et al., 2007; Liu & Bi, 2011). In addition, the smaller particles may correspond to particles (e.g., sand) that can damage the process equipment. On the other hand, it has been shown that the extent of mineral accumulation in bark fractions depends on the species (Miranda, Gominho, Mirra, & Pereira, 2012). The ash content of B. papyrifera Marshall was reported as 1.8% of the bark biomass by Corder (1976). This value is similar to that obtained in our study (2.10%).
Although the composition of white birch extracts has been studied before, the composition of raw bark has been much less studied. Harkin and Rowe (1971) reported that the total extractive content is 22.4% and lignin 37.8% (Krasutsky, 2006). Our results are consistent with these studies since the extractive composition obtained is 13.05% and the lignin content is 36.55% (Figure 2). The composition of the bark has not been fully characterized, and a certain proportion (11.89%) corresponds to other components. These compounds include suberinic acids, waxes, and degradation products present in the bark such as hydroxymethylfurfural (HMF) and furfural, as well as other components of interest, such as organic acids and sugar alcohols (Sluiter et al., 2008a).
Methanol and ethanol solvents significantly extracted a greater percentage of molecules (Figure 3). These two solvents allow the extraction of polar compounds, which include polyphenols, sugars, and some organic acids corresponding to a significant proportion of the total content of the extracts (Tables 2 and 3) (Royer et al., 2012). Less polar solvents such as chloroform and hexane extracted waxes and nonpolar compounds such as triterpenoids present at 20%–35% in white birch bark (Krasutsky, 2006). The extraction with hexane (5.64%) and the acid–base extraction (3.09%) are the least optimal methods for obtaining the highest dry weight in extractives. On the other hand, despite the fact that the methanol and ethanol extracts have higher extraction yields, the eight other solvents should not be eliminated since there are no significant differences between these two and those with acetone, ethyl acetate, chloroform, and water–ethanol. The choice of the best solvent should be determined by the molecules it can extract, those that will provide an antimicrobial effect, and its potential uses in industry. It is certain that a very low extraction yield would be unfavorable, since it would require a huge amount of bark residues to achieve an effective concentration of extract to act as antimicrobial agent. However, if the quantity of extract that can inhibit the growth of microorganisms is low, it is possible that this extraction will be still profitable.
4.2. Antimicrobial activity
Plants contain bioactive molecules in different tissues and one of the functions of these specialized metabolites is to protect plants against microbial invaders (Vardar‐Ünlü, Silici, & Ünlü, 2008). A study about the antimicrobial activity of extracts of eastern North American hardwood trees demonstrates that bark extracts had higher inhibitory effect against both bacterial and fungal strains tested than wood extracts (Omar et al., 2000). The bark is the outer most protective part of the tree, so it has a greater amount of specialized metabolites with antimicrobial properties since bark is the first barrier to defend the tree against biotic and abiotic stress.
Among the 10 bark extracts studied, the water extract had the most extensive antimicrobial activity on the eight microbial strains tested followed by methanol extract (Table 1). The water extract had a bactericidal effect on two microorganisms in addition to inhibiting the growth of all tested strains. This is linked to the greater capacity of the water to concentrate the pool of active molecules in this extract. In fact, specialized metabolites with an antimicrobial activity appeared to have more affinity for polar solvents such as water and methanol than nonpolar solvents. Furthermore, as water is a low‐cost “green” solvent (better for environment), it would therefore be a solvent of choice for extraction in the industry (Capello, Fischer, & Hungerbühler, 2007). In addition, the acid–base extract, specific to alkaloids, also demonstrated a strong antimicrobial activity. However, this extraction protocol uses solvents harmful to the environment including hexane and chloroform, which is why this extract is considered less interesting in an industrial context of green chemistry compared to the extract using water or methanol.
Aforementioned, not much is known about the antimicrobial properties of white birch extracts and even less from the bark (Vandal et al., 2015). However, it should be noted that Omar et al. (2000) have shown that ethanol extracts of white birch bark exhibited antimicrobial properties against four bacterial species (two Gram‐positive and two Gram‐negative), but no antifungal activity was observed. In our study, we show that the ethanol extract inhibits microbial proliferation of S. enterica and P. aeruginosa, but no effect was observed on E. coli and C. albicans. In contrast, several studies highlighted this biological activity in the extracts of yellow birch (Betula alleghaniensis) also present in North America. For example, the aqueous bark extract of yellow birch showed antifungal activity against S. cerevisiae (MIC 0.1 mg/ml) as well as antibacterial activity against E. coli (Royer et al., 2013; Webster, Taschereau, Belland, Sand, & Rennie, 2008). Furthermore, various studies reported on the antimicrobial activity of different parts of white birch, other than bark. For example, Vandal et al. (2015) demonstrated the antimicrobial activity of ethanol extracts of white birch foliage, twigs, branches, and phloem against S. aureus and C. albicans. It was further shown that methanol extract of air‐dried white birch branches had activity against 6 of 11 tested bacteria (four Gram‐positive, two Gram‐negative) and three fungal species (Borchardt et al., 2008; McCutcheon, Ellis, Hancock, & Towers, 1992, 1994).
In most studies, the antimicrobial activity of white birch is related to the high abundance of triterpenoids such as betulin and lupeol extract with a less polar solvent such as hexane (Krasutsky, 2006; Krasutsky, Carlson, Nesterenko, Kolomitsyn, & Edwardson, 2007). However, the presence of phenolic compounds in water and methanol extracts (more polar solvents) could also be attributed to greater antimicrobial activity as demonstrated by our results also supported by Royer et al. (2012). In agreement with our results, studies on other North American trees such as Populus species have shown that flavonoid and esters of phenolic acids are generally regarded to be responsible for the antimicrobial activity (Vardar‐Ünlü et al., 2008). Kedzia, Geppert, and Iwaszkiewicz (1990) reported that the mechanism of antimicrobial activity is complex and could be attributed to synergism between flavonoids, hydroxyacids, and sesquiterpenes.
4.3. Characterization of extracts
The water and methanol extracts contained a large proportion of phenolic compounds as well as many organic acids such as hydrobenzoic acid and coumaric acid present in both extracts (Tables 2 and 3). Catechol, a phenolic compound synthesized by the shikimate pathway in plants, was widely present in the water extract (Kocaçalışkan, Talan, & Terzi, 2006). Catechol has been isolated by Kuiters and Sarink (1986) from leaf and needle litter of several deciduous (beech, birch, oak, hazelnut, maple, willow, and poplar) and coniferous trees (spruce‐fir, douglas‐fir, and larch), and there is also an indication that it is synthesized abundantly in onions and released by their outer layer cells (Farkas & Kiraaly, 1962). Furthermore, catechol has been shown to have antifungal effects on Colletotrichum circinans (Farkas & Kiraaly, 1962) and significantly inhibited the growth of Clostridium difficile and moderately inhibited the growth of E. coli ( Jeong, Jeon, Lee, & Lee, 2009). The results of several previous studies indicated that catechol and its derivatives act as antioxidants in eukaryotic cells, thereby preventing degenerative diseases such as cancer and heart disease (Berberian et al., 2007). Thus, the present results suggested that catechol could be a major active component in white birch bark extract contributing to its antimicrobial activity.
More sugars were present in the water extract than methanol, and this is consistent with the findings of Sjöström and Alén (2013), which suggested that carbohydrates have more affinity for water than other solvents because of its strong polarity. In an antimicrobial agent context, it is preferable to use an extract with less proportion of sugars. Indeed, it has been shown that sugars increase microbial growth because it serves as food for bacteria and fungi. On the other hand, when looking at the results obtained from the antimicrobial assays with each extract, we found that the water extract had a strong antimicrobial activity on the majority of the microorganisms tested. So, despite the presence of sugars, the specialized metabolites present in the extract must have a sufficiently high antimicrobial power to allow the inhibition and the death of microbial strains.
The following four polyphenols were found across the genus Betula: (+)‐catechin, salidroside, (+)‐rhododendrin, and platyphylloside (Royer et al., 2012). The (+)‐catechin 3‐O‐gallate molecule was identified in our water extract. Several studies have shown that (+)‐catechin, a flavonoid produced by the plant was found to have strong antimicrobial properties (Bais, Walker, Stermitz, Hufbauer, & Vivanco, 2002). (+)‐Catechin is also a well‐known antioxidant and acts as a free radical scavenger, antifungal and antitumor agent, and insect repellant agent (Fukuhara et al., 2002; Veluri, Weir, Bais, Stermitz, & Vivanco, 2004). Several glycosylated molecules have been identified in the extracts such as arbutin, a glycosylated hydroquinone (Table 2). Glycosylation is a widespread modification of plant specialized metabolites. It is involved in various functions, including the regulation of hormone homeostasis, detoxification of xenobiotics, and biosynthesis and storage of specialized compounds. From a chemical point of view, sugar conjugation results in both increased stability (through the protection of reactive nucleophilic groups) and water solubility (Gachon, Langlois‐Meurinne, & Saindrenan, 2005). Several of these molecules have some interesting biological activity without its sugar molecule. For example, apigenin, a chemical compound of the family of flavones, a subclass of flavonoids, has been studied for its multiple biological activities such as anti‐inflammatory and antimicrobial properties (Akroum, Bendjeddou, Satta, & Lalaoui, 2009; Basile, Giordano, López‐Sáez, & Cobianchi, 1999; Funakoshi‐Tago, Nakamura, Tago, Mashino, & Kasahara, 2011; Kukić et al., 2008). The apigenin‐6‐glucoside and apigenin diglucoside were identified in our methanol extract (Table 3). In few articles, only some glycosylated molecules have also demonstrated antimicrobial activity, for example, apigenin‐7‐glucoside in Kukić et al. (2008) as well as kaempferol‐3‐O‐glucoside in Akroum et al. (2009). Therefore, we cannot exclude the possibility that these molecules may play a role in the antimicrobial activity of the extracts, despite their association to a sugar moiety.
The antimicrobial properties of the molecules present either in the water or methanol extracts, as shown in Table 4, have been identified before (see reference in Table 4), but the mechanisms underlying these properties have seldom been studied. For example, berberine present at 2.27% in the water extract is an alkaloid known as an antimicrobial agent due to its quaternary ammonium salt structure. This compound acts as an antimicrobial agent by binding minor groves of DNA and by regulating the gene expression of microorganisms (Yu et al., 2005). In contrast, caffeic acid, listed in a lower proportion in both extracts (0.96% in water extract and 0.84% in methanol extract), has an antimicrobial mechanism that acts on the cell wall and the cytoplasmic membrane of microorganisms (Perumal, Mahmud, & Ismail, 2017). Several mechanisms of action of antimicrobial agents are currently known, but the ones involved in white birch bark extracts have yet to be elucidated. Each of the targeted molecules in white birch bark extracts may therefore have different antimicrobial mechanisms and these may have synergistic effects. It is well‐known that plant extracts are complex mixtures of phytochemical compounds, which act synergistically together to achieve antimicrobial effect (Burt, 2004; Cushnie & Lamb, 2011).
The composition of the extracts determined by UPLC‐QTOF‐MS makes it possible to better understand which molecules contribute to their antimicrobial action. However, it is very important to note that the proportion of each component may vary from one tissue specimen to the other within a single species, and vary also due to other parameters such as harvest time, geographical location, or other environmental conditions (Royer et al., 2012; Vardar‐Ünlü et al., 2008). Furthermore, although several samples were pooled, only one method of analysis was performed to identify the compounds present in the extracts (UPLC‐QTOF‐MS). Hence, it is possible that some volatile compounds have not been identified and may play a role in the antimicrobial activity of the extract. In addition, the percentages of area are relative to the mode of analysis used (positive or negative) as well as the ability of a molecule to ionize easily or not. The compounds were identified by comparing the exact masses (m/z) and retention time (Rt) obtained in a database; these are only potential identities of molecules with respect to the percentage of similarity. The complete lists of all the molecules identified for the water extract and the methanol extract can be found in additional material (Tables A1‐A4 in Appendix).
Until today, the main way to valorize bark residues is to burn them to generate heat and electricity. Therefore, it is important to ensure that the added valorization step, that is, extraction of bioactive compounds from residues, will be more efficient or will not affect energy yield. It is thus primordial that the calorific value of the material before and after the extraction is similar or only slightly modified. For this reason, heating value tests should be carried out on bark residues after extraction. When wood is burned, it is the structural components of the wood that burn and produce heat. The high proportions of lignin, cellulose, and hemicellulose (Figure 2) suggest a high calorific value of the material as well as low ash content. In addition, in the case of birch bark, the extractive compounds (Figure 2) may include suberin and betulin, which are the main components giving 1.5 times higher heating value in comparison with wood components (Ferreira et al., 2013; Heinamaki et al., 2017). Several studies have shown that there was a highly significant linear correlation between the higher heating value of the extractive‐free wood and lignin content (Demirbaş, 2001; White, 1987) and another one showed that high ash content in parts of a plant makes it less desirable as fuel (Demirbas, 2002). Despite the fact that some articles demonstrate a slight decrease in the calorific value of wood after extraction, the energy loss is, however, low compared to the added value of the extracted molecules. The production of high value‐added products from white birch bark extract will promote an innovating industrial sector, generating wealth and sustainable jobs, which will encourage wider opportunities to use wood as a renewable crude material (Royer et al., 2012).
To our knowledge, the present study is first to highlight the potential of white birch bark extracts, obtained from sawmill bark residues, as a natural source of antimicrobial agents which could be considered as good candidates for the development of high value products such as new cosmetics, nutraceuticals, or sanitary products. The water extract had the best antimicrobial potential followed by methanol extract. In an industrial context, the water extract is to be prioritized because of its low environmental impact. Using UPLC‐QTOF‐MS, catechol was identified as one of the main components in white birch bark water extract, and its antimicrobial activity has already been studied, suggesting that catechol could be one of the components contributing to the antimicrobial activity of this extract. However, the extract is a complex mixture of phytochemical compounds, which act certainly synergistically together to achieve antimicrobial effect. These results offer the possibility to valorize the bark residues produced in huge quantities by the Canadian forest industry, using the concept of extractives. In addition, the extractive can be obtained while maintaining the biomass that can subsequently be used to energy production and other applications. Nevertheless, further investigations would be required to determine the cytotoxicity of the extracts, their biological efficacies in vivo, and their stability in the context of pharmaceutical, cosmetic, food, or sanitary formulations.
With the current challenges that the industrial world faces regarding the unavoidable environmental impact of manufactured goods, industries are turning to sustainable means to reduce this impact and to minimize the damage to the environment while at the same time reaping the marketing bonus that is the claim of a greener product. The bark of woody plants is often considered a forest waste, but it can be an important source of bioactive molecules with a high potential for capitalization. While many studies have focussed on optimizing extraction methods and the identification of bioactive molecules, our study offers additional information on newly identified antimicrobial molecules in white birch bark residues confirming their importance and their value. Future research directions should be directed toward the description of the mechanism of action of these molecules in living systems. Consequently, biologically active molecules obtained from the bark residues could be exploited on an industrial scale.
CONFLICT OF INTERESTS
None declared.
AUTHOR CONTRIBUTIONS
DB and AS‐P performed the investigation, analyzed the data, and wrote the original draft. JB and AL provided resources and input on the experimental design, methodology and data analyses. NB and ID‐P conceived, designed the study, and acquired funding. ID‐P revised and edited the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT
None required.
ACKNOWLEDGMENTS
This work was supported by Forêt modèle du Lac‐Saint‐Jean, Groupe Sani Marc, and Coopérative pour la valorisation de la biomasse. The authors gratefully acknowledge the Consortium de Recherche et Innovations en Bioprocédés Industriels au Québec (CRIBIQ), grant reference number 2015‐033‐C17, and Natural Sciences and Engineering Research Council of Canada (NSERC) for financial support. Also, Mitacs—Acceleration (grant number IT IT07562) scholarships supported ASP and DB.
The authors would like to thank Hugo Germain, Gervais Bérubé, Simon Barnabé, Patrick Marchand, and Dominic Desrosiers for providing the equipment and access to laboratories, and Stephanie Blais and Josée Doucet for handling microdilution and extraction techniques. They also extend their thanks to Pascal Dubé from the Centre de Recherche Industrielle du Québec (CRIQ) for carrying out the chemical characterization of the extracts.
APPENDIX 1.
Crude chemical composition of white birch bark water and methanol extract using UPLC‐QTOF‐MS in positive and negative ionization mode
Table A1.
Crude chemical composition of white birch bark water extract using UPLC‐QTOF‐MS analyses in positive mode
| Exact mass [M+H] | Retention time (Rt) | Compounds | Area under the curve |
|---|---|---|---|
| 282.2869 | 26.5 | 9‐Octadecenoate | 11,588 |
| 214.9984 | 35.6 | 2,5‐Dioxo‐2,5‐dihydrofuran‐3,4‐diyl diacetate | 4,220 |
| 254.2584 | 23.8 | (–)‐Solenopsin A | 3,785 |
| 249.1232 | 8.4 | Artabsin | 3,742 |
| 563.5732 | 26.6 | Oleic acid, eicosyl ester | 3,659 |
| 256.2760 | 26.0 | 1‐Heptadecanamine | 3,269 |
| 297.1591 | 14.4 | 4‐Prenylresveratrol | 3,203 |
| 287.1035 | 9.5 | Fisetin | 3,166 |
| 165.0511 | 1.0 | l‐Rhamnose | 2,980 |
| 228.2430 | 23.0 | Myristamide | 2,545 |
| 287.1035 | 15.6 | Kaempferol | 2,413 |
| 564.3776 | 8.2 | Tris(1‐hydroxy‐2,2,6,6‐tetramethyl‐4‐piperidinyl) phosphate | 2,120 |
| 184.0484 | 1.0 | 3‐Carboxy‐4‐methoxy‐N‐methyl‐2‐pyridone | 2,029 |
| 520.3510 | 7.9 | Terpendole C | 1,984 |
| 142.0390 | 1.0 | Gentianaine | 1,899 |
| 608.4124 | 8.4 | C35H61NO7 | 1,870 |
| 476.3204 | 7.5 | Tubulosine | 1,715 |
| 214.9984 | 36.2 | 1‐Deoxy‐d‐xylulose 5‐phosphate | 1,636 |
| 107.0503 | 14.4 | d‐Glycerate | 1,521 |
| 267.1002 | 10.8 | Carbamazepine‐o‐quinone | 1,516 |
| 301.0838 | 15.9 | 3‐Methoxyapigenin | 1,507 |
| 652.4316 | 8.7 | 1‐Deoxy‐1‐[dodecanoyl(nonyl)amino]‐4‐O‐hexopyranosylhexitol | 1,478 |
| 503.3298 | 7.9 | 4a,7b‐Dihydroxy‐3‐(hydroxymethyl)‐1,1,6,8‐tetramethyl‐5‐oxo‐1,1a,1b,4,4a,5,7a,7b,8,9‐decahydro‐9aH‐cyclopropa[3,4]benzo[1,2‐e]azulen‐9a‐yl decanoate | 1,233 |
| 273.0878 | 12.2 | Arbutin | 1,225 |
| 139.9936 | 34.9 | 2‐Nitrophenol | 1,145 |
| 459.3102 | 7.5 | Phthalic acid—(8xi,13xi)‐abietan‐18‐ol (1:1) | 1,136 |
| 547.3581 | 8.2 | Trioctyl trimellitate | 1,128 |
| 432.3024 | 7.2 | (2beta,3alpha,5alpha,16alpha)‐3‐Hydroxy‐16‐methyl‐2‐(4‐morpholinyl)pregnane‐11,20‐dione | 1,125 |
| 154.9842 | 1.0 | 3,4‐Dihydroxybenzoate | 1,108 |
| 268.2797 | 25.2 | 9‐Octadecen‐1‐amine | 959 |
| 415.2808 | 7.2 | 1‐Methyl‐3‐oxoandrost‐1‐en‐17‐yl heptanoate | 911 |
| 202.0671 | 1.0 | 2'‐Aminobiphenyl‐2,3‐diol | 906 |
| 295.1483 | 14.3 | Phytuberin | 867 |
| 696.4679 | 8.9 | quinetolate | 830 |
| 161.0354 | 1.0 | 2‐Oxoadipate | 770 |
| 296.2758 | 21.0 | N‐Hexadecylacrylamide | 768 |
| 271.1084 | 13.6 | 2'‐O‐Methylisoliquiritigenin | 755 |
| 307.1928 | 11.1 | Confertiflorin | 745 |
| 343.1249 | 10.0 | Coniferin | 686 |
| 140.0050 | 1.0 | 4‐Nitrophenol | 673 |
| 181.0349 | 34.9 | d‐Glucose | 672 |
| 149.0252 | 22.4 | trans‐Cinnamate | 630 |
| 317.1948 | 9.2 | Pisiferic acid | 615 |
| 124.0296 | 1.0 | Nitrobenzene | 580 |
| 546.2267 | 12.9 | alpha‐d‐Galactosyl‐N‐acetyllactosamine | 571 |
| 119.0215 | 1.0 | Succinate | 516 |
Table A2.
Crude chemical composition of white birch bark water extract using UPLC‐QTOF‐MS analyses in negative mode
| Exact mass [M−H] | Retention time (Rt) | Compounds | Area under the curve |
|---|---|---|---|
| 109.0359 | 5.7 | Catechol | 4,973 |
| 345.1393 | 5.1 | Epirosmanol | 4,517 |
| 137.0339 | 9.1 | 4‐Hydroxybenzoic acid | 2,960 |
| 285.0992 | 15.6 | Kaempferol | 2,842 |
| 271.0804 | 12.2 | Arbutin | 2,762 |
| 449.1708 | 10.8 | Eriodictyol 7‐O‐glucoside | 2,523 |
| 449.1708 | 11.9 | Eriodictyol 7‐O‐glucoside | 2,431 |
| 423.1843 | 9.8 | Grandidentatin | 2,374 |
| 163.0480 | 7.6 | p‐Coumaric acid | 2,344 |
| 571.2244 | 5.1 | C23H40O16 | 2,174 |
| 431.1579 | 10.3 | 5‐Tricosenylresorcinol | 2,089 |
| 421.1777 | 9.3 | Picrasin C | 2,009 |
| 423.1843 | 10.1 | Grandidentatin | 1,927 |
| 285.0584 | 12.4 | Scutellarein | 1,904 |
| 299.0783 | 15.9 | Kaempferide | 1,763 |
| 335.1533 | 4.5 | Berberine | 1,742 |
| 337.1743 | 5.0 | 3‐p‐Coumaroylquinic acid | 1,572 |
| 121.0385 | 6.8 | 4‐Hydroxybenzaldehyde | 1,267 |
| 191.0339 | 1.3 | Scopoletin | 1,256 |
| 287.0781 | 9.5 | Phlorin | 1,222 |
| 121.0385 | 9.4 | 3‐Hydroxybenzaldehyde | 1,116 |
| 465.1720 | 9.5 | Dihydromyricetin 3‐O‐rhamnoside | 1,064 |
| 133.0284 | 1.3 | Malate | 996 |
| 521.1940 | 7.5 | Isobrucein A | 971 |
| 507.2056 | 4.8 | Gibberellin 2‐O‐beta‐d‐glucoside | 927 |
| 157.0604 | 3.0 | 1,4‐Naphthoquinone | 926 |
| 285.1237 | 5.6 | Sakuranetin | 870 |
| 439.1857 | 8.9 | Marchantin A | 795 |
| 343.1214 | 5.9 | 5‐O‐Galloylquinic acid | 791 |
| 171.1150 | 11.6 | p‐Menthane‐3,8‐diol | 781 |
| 179.0726 | 1.2 | Caffeic acid | 738 |
| 175.1160 | 8.4 | Prenylbenzoquinone | 685 |
| 329.2584 | 13.5 | Avenanthramide 2f | 679 |
| 587.2112 | 12.9 | Phthalic acid—1,2‐diphenylguanidine (1:2) | 603 |
| 325.1133 | 6.5 | Feruloyl tartaric acid | 590 |
| 373.1419 | 7.1 | 5‐Nonadecenylresorcinol | 550 |
| 441.1687 | 7.7 | (+)‐Catechin 3‐O‐gallate | 550 |
| 141.0299 | 1.1 | muconic acid | 528 |
| 631.2736 | 5.1 | Methyl 1,7,12‐triacetoxy‐17‐(3‐furyl)‐3,11‐dihydroxy‐4,8‐dimethyl‐14,15‐epoxyandrostane‐4‐carboxylate | 518 |
| 527.1934 | 12.9 | Tremulacin | 517 |
| 361.1462 | 2.7 | p‐HPEA‐EA | 516 |
| 377.1308 | 16.1 | Oleuropein‐aglycone | 515 |
| 433.1378 | 9.2 | 5‐Tricosylresorcinol | 508 |
| 117.0299 | 2.0 | Succinate | 489 |
| 501.2240 | 8.9 | 6''‐O‐Malonyldaidzin | 486 |
| 301.0882 | 13.1 | Ellagic acid | 472 |
| 195.0641 | 1.2 | Hydroxycaffeic acid | 455 |
| 451.1916 | 7.2 | 3‐Hydroxyphloretin 2'‐O‐glucoside | 434 |
| 391.1157 | 14.3 | Macarpine | 431 |
| 423.2042 | 9.1 | Didrovaltratum | 430 |
| 485.1945 | 7.9 | Rutaevin | 427 |
| 393.1261 | 13.8 | Podolactone B | 423 |
| 325.1221 | 6.2 | Feruloyl tartaric acid | 423 |
| 405.1508 | 9.9 | Piceatannol 3‐O‐glucoside | 416 |
| 423.1942 | 9.4 | Nigakilactone H | 409 |
| 239.0911 | 1.2 | 4'‐Hydroxyflavanone | 409 |
| 447.1648 | 11.4 | 6‐Hydroxyluteolin 7‐O‐rhamnoside | 407 |
| 243.0846 | 16.2 | Piceatannol | 407 |
| 377.1308 | 14.6 | Oleuropein‐aglycone | 388 |
| 421.2571 | 34.9 | Picrasin C | 382 |
| 167.0478 | 6.4 | Vanillic acid | 381 |
| 509.1783 | 17.0 | C30H26N2O6 | 377 |
| 187.1118 | 9.6 | cis‐2,3‐Dihydro‐2,3‐dihydroxybiphenyl | 352 |
| 425.1757 | 7.9 | Abscisic acid glucose ester | 344 |
| 483.1732 | 8.4 | C21H24N8O6 | 343 |
| 447.1648 | 8.6 | Kaempferol 7‐O‐glucoside | 338 |
| 319.1264 | 3.8 | 5‐Pentadecylresorcinol | 338 |
| 201.0444 | 1.0 | Bergaptol | 330 |
| 145.1007 | 9.9 | Coumarin | 326 |
| 205.1783 | 22.6 | Acetyl eugenol | 320 |
| 545.1999 | 11.1 | Flavonol 3‐O‐[alpha‐l‐rhamnosyl‐(1‐>6)‐beta‐d‐glucoside] | 318 |
| 389.1581 | 10.7 | Laserolide | 317 |
| 315.0974 | 3.9 | Avenanthramide 2c | 301 |
| 153.0332 | 4.4 | Gallic aldehyde | 291 |
| 123.0574 | 5.9 | 3‐Methylcatechol | 287 |
| 391.1730 | 7.1 | Viguiestenin | 250 |
Table A3.
Crude chemical composition of white birch bark methanol extract using UPLC‐QTOF‐MS analyses in positive mode
| Exact mass [M+H] | Retention time (Rt) | Compounds | Area under the curve |
|---|---|---|---|
| 140.1349 | 11.6 | Pinidine | 5,190 |
| 128.1771 | 14.6 | Coniine | 3,912 |
| 150.9699 | 13.6 | Asparagusate | 3,106 |
| 377.3593 | 11.1 | C20H40O6 | 3,809 |
| 326.9941 | 13.0 | Butonate | 2,951 |
| 122.8746 | 9.7 | C6H2O3 | 2,613 |
| 342.2122 | 11.1 | Isocorypalmine | 3,314 |
| 242.4491 | 7.4 | C16H49 | 3,462 |
| 216.4768 | 9.6 | C6H47O6 | 3,991 |
| 215.0140 | 5.6 | 2‐Deoxy‐d‐ribose 1‐phosphate | 1,947 |
| 276.1790 | 7.4 | Annotinine | 1,859 |
| 378.1298 | 11.2 | 6‐(2‐Hydroxyethyl)‐5,6‐dihydrosanguinarine | 1,398 |
| 142.1624 | 10.8 | Methylconiine | 1,374 |
| 290.2941 | 9.3 | 17a‐Aza‐d‐homoandrost‐5‐en‐3beta‐ol | 1,608 |
| 342.9818 | 11.1 | Carbophenothion | 1,201 |
| 300.0028 | 9.3 | 1‐Methylseleno‐N‐acetyl‐d‐galactosamine | 1,181 |
| 285.1934 | 20.1 | Retinal | 1,112 |
| 352.8556 | 9.4 | C8O16 | 935 |
| 388.1222 | 9.4 | C12H19O14 | 823 |
| 243.1798 | 7.4 | Falcarinone | 878 |
| 217.1958 | 9.6 | 12‐Hydroxydodecanoic acid | 541 |
| 251.3133 | 20.1 | C9H46O6 | 939 |
| 217.1958 | 9.9 | 12‐Hydroxydodecanoic acid | 523 |
| 227.9633 | 8.8 | C13H7O4 | 709 |
| 159.0468 | 1.4 | Ibotenic acid | 482 |
| 276.9267 | 7.4 | C15O6 | 228 |
| 285.1934 | 20.3 | (+)‐Larreatricin | 337 |
| 251.3133 | 20.5 | C10H34O6 | 256 |
| 126.1111 | 3.3 | C4H13O4 | 301 |
Table A4.
Crude chemical composition of white birch bark methanol extract using UPLC‐QTOF‐MS analyses in negative mode
| Exact mass [M−H] | Retention time (Rt) | Compounds | Area under the curve |
|---|---|---|---|
| 313.1478 | 11.6 | Perilloside | 1,893 |
| 271.0771 | 12.3 | Naringenin, Phloretin, Butein ou Arbutin | 1,594 |
| 295.1493 | 14.5 | Deoxystansioside | 1,284 |
| 373.1544 | 7.4 | Hydroxymatairesinol | 1,249 |
| 639.2776 | 11.1 | C42H40O6 | 1,162 |
| 299.1744 | 14.2 | Kaempferid | 1,138 |
| 315.1700 | 10.8 | Sesquiterpene | 1,128 |
| 285.0872 | 15.7 | Acacetine | 1,128 |
| 329.2446 | 13.6 | Dimethylquercetin | 1,107 |
| 521.2055 | 9.7 | C33H30O6 | 1,035 |
| 593.2709 | 11.1 | Apigenin‐diglucoside | 1,013 |
| 655.2672 | 8.9 | Methyl 3‐O‐benzyl‐4‐O‐(2,3,4‐tri‐O‐benzyl‐b‐d‐xylopyranosyl)‐b‐d‐xylopyranoside | 997 |
| 473.1739 | 9.5 | Chicoric acid isomer | 958 |
| 459.1974 | 7.3 | Pentacosyl Resorcinol | 950 |
| 573.1666 | 13.0 | C35H26O8 | 936 |
| 287.0742 | 9.6 | Eriodictylol isomer ou Phlorin | 844 |
| 523.2272 | 9.4 | Ligstroside isomer | 804 |
| 523.2272 | 9.3 | Ligstroside | 785 |
| 431.1400 | 10.3 | Apigenin 6‐glucoside | 746 |
| 319.0613 | 7.1 | 3,4‐DHPEA‐EDA | 693 |
| 421.1602 | 9.1 | C21H26O9 | 670 |
| 423.1767 | 9.6 | C21H28O9 | 657 |
| 473.1739 | 9.2 | Chicoric acid | 594 |
| 423.1767 | 9.9 | C21H28O9 | 580 |
| 543.1417 | 12.3 | Diferuloylquinic acid | 567 |
| 435.1445 | 10.8 | Phloridzin | 492 |
| 343.1340 | 8.0 | Anhydro‐secoisolariciresinol | 464 |
| 327.1602 | 7.4 | C20H24O4 | 450 |
| 421.1305 | 5.6 | C27H18O5 | 444 |
| 627.3060 | 11.6 | C31H48O13 | 437 |
| 331.1245 | 5.5 | Galloyl glucose | 412 |
| 295.1576 | 16.5 | C12H24O8 | 397 |
| 507.2391 | 11.5 | C30H36O7 | 395 |
| 469.1547 | 8.6 | Valoneic acid dilactone | 394 |
| 314.1710 | 11.6 | C16H27O6 | 389 |
| 451.1421 | 9.6 | Hydroxyphloretin‐glucoside | 383 |
| 505.2050 | 7.3 | C26H34O10 | 359 |
| 297.1543 | 15.3 | C15H22O6 | 356 |
| 507.1955 | 10.0 | C32H28O6 | 352 |
| 393.1194 | 13.9 | C22H18O7 | 351 |
| 287.0824 | 11.0 | Eriodictyol isomer or Phlorin | 333 |
| 423.1866 | 10.3 | C22H32O8 | 327 |
| 301.1008 | 13.3 | Hesperetin | 306 |
| 477.1890 | 10.2 | Taramixin | 304 |
| 179.0905 | 1.5 | Caffeic acid | 286 |
| 303.0838 | 8.5 | Taxifolin | 280 |
| 163.0530 | 7.9 | Coumaric acid | 268 |
| 377.1246 | 14.7 | 3,4‐DHPEA‐EA | 239 |
| 296.1717 | 14.5 | C16H25O5 | 236 |
| 137.0389 | 9.9 | Hydroxybenzoic acid | 228 |
| 377.1339 | 16.3 | Oleuropein‐aglycone | 223 |
| 137.0502 | 6.0 | Hydroxybenzoic acid | 195 |
| 327.1514 | 9.2 | C12H24O10 | 133 |
| 393.1385 | 12.7 | C23H22O6 | 118 |
| 521.2055 | 7.5 | C33H30O6 | 105 |
Blondeau D, St‐Pierre A, Bourdeau N, Bley J, Lajeunesse A, Desgagné‐Penix I. Antimicrobial activity and chemical composition of white birch (Betula papyrifera Marshall) bark extracts. MicrobiologyOpen. 2020;9:e944 10.1002/mbo3.944
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are included in this published article and available from the corresponding author on reasonable request.
REFERENCES
- Akroum, S. , Bendjeddou, D. , Satta, D. , & Lalaoui, K. (2009). Antibacterial activity and acute toxicity effect of flavonoids extracted from Mentha longifolia . American‐Eurasian Journal of Scientific Research, 4(2), 93–96. [Google Scholar]
- Amábile‐Cuevas, C. (2003). New antibiotics and new resistance in many ways, the fight against antibiotic resistance is already lost; preventing bacterial disease requires thoughtful new approaches. American Scientist, 91(2), 138–149. 10.1511/2003.2.138 [DOI] [Google Scholar]
- Annabelle, S.‐P. , Dorian, B. , Nathalie, B. , Julien, B. , & Isabel, D.‐P. (2019). Chemical composition of black spruce (Picea mariana) bark extracts and their potential as natural disinfectant. Industrial Biotechnology, 15(3), 219–231. [Google Scholar]
- Aziz, N. , Farag, S. , Mousa, L. , & Abo‐Zaid, M. (1998). Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios, 93(374), 43–54. [PubMed] [Google Scholar]
- Bais, H. P. , Walker, T. S. , Stermitz, F. R. , Hufbauer, R. A. , & Vivanco, J. M. (2002). Enantiomeric‐dependent phytotoxic and antimicrobial activity of (±)‐catechin. A rhizosecreted racemic mixture from spotted knapweed. Plant Physiology, 128(4), 1173–1179. 10.1104/pp.011019 [DOI] [PubMed] [Google Scholar]
- Balouiri, M. , Sadiki, M. , & Ibnsouda, S. K. (2016). Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis, 6(2), 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri, R. , Coppo, E. , Marchese, A. , Daglia, M. , Sobarzo‐Sánchez, E. , Nabavi, S. F. , & Nabavi, S. M. (2017). Phytochemicals for human disease: An update on plant‐derived compounds antibacterial activity. Microbiological Research, 196, 44–68. [DOI] [PubMed] [Google Scholar]
- Barreca, D. , Bellocco, E. , Laganà, G. , Ginestra, G. , & Bisignano, C. (2014). Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chemistry, 160, 292–297. 10.1016/j.foodchem.2014.03.118 [DOI] [PubMed] [Google Scholar]
- Basile, A. , Giordano, S. , López‐Sáez, J. A. , & Cobianchi, R. C. (1999). Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry, 52(8), 1479–1482. 10.1016/S0031-9422(99)00286-1 [DOI] [PubMed] [Google Scholar]
- Berberian, V. , Allen, C. C. R. , Sharma, N. D. , Boyd, D. R. , & Hardacre, C. (2007). A comparative study of the synthesis of 3‐substituted catechols using an enzymatic and a chemoenzymatic method. Advanced Synthesis & Catalysis, 349(4–5), 727–739. 10.1002/adsc.200600437 [DOI] [Google Scholar]
- Borchardt, J. R. , Wyse, D. L. , Sheaffer, C. C. , Kauppi, K. L. , Ehlke, R. G. F. N. J. , Biesboer, D. D. , & Bey, R. F. (2008). Antimicrobial activity of native and naturalized plants of Minnesota and Wisconsin. Journal of Medicinal Plants Research, 2(5), 098–110. [Google Scholar]
- Bridgeman, T. , Darvell, L. , Jones, J. , Williams, P. , Fahmi, R. , Bridgwater, A. , … Thain, S. (2007). Influence of particle size on the analytical and chemical properties of two energy crops. Fuel, 86(1), 60–72. 10.1016/j.fuel.2006.06.022 [DOI] [Google Scholar]
- Burt, S. (2004). Essential oils: Their antibacterial properties and potential applications in foods—A review. International Journal of Food Microbiology, 94(3), 223–253. [DOI] [PubMed] [Google Scholar]
- Cai, L. , & Wu, C. D. (1996). Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. Journal of Natural Products, 59(10), 987–990. [DOI] [PubMed] [Google Scholar]
- Calderon‐Montano, J. M. , Burgos‐Morón, E. , Pérez‐Guerrero, C. , & López‐Lázaro, M. (2011). A review on the dietary flavonoid kaempferol. Mini Reviews in Medicinal Chemistry, 11(4), 298–344. [DOI] [PubMed] [Google Scholar]
- Capello, C. , Fischer, U. , & Hungerbühler, K. (2007). What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chemistry, 9(9), 927–934. 10.1039/b617536h [DOI] [Google Scholar]
- Celhay, C. , Mathieu, C. E. , Candy, L. , Vilarem, G. , & Rigal, L. (2014). Aqueous extraction of polyphenols and antiradicals from wood by‐products by a twin‐screw extractor: Feasibility study. Comptes Rendus Chimie, 17(3), 204–211. [Google Scholar]
- Chandra, H. , Bishnoi, P. , Yadav, A. , Patni, B. , Mishra, A. P. , & Nautiyal, A. R. (2017). Antimicrobial resistance and the alternative resources with special emphasis on plant‐based antimicrobials—A review. Plants, 6(2), 16 10.3390/plants6020016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, S.‐T. , Chen, P.‐F. , & Chang, S.‐C. (2001). Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum . Journal of Ethnopharmacology, 77(1), 123–127. 10.1016/S0378-8741(01)00273-2 [DOI] [PubMed] [Google Scholar]
- Cheng, X. , Deng, J. , Zhang, S. , Riedl, B. , & Cloutier, A. (2006). Impact of bark content on the properties of medium density fiberboard (MDF) in four species grown in eastern Canada. Forest Products Journal, 56(3), 64. [Google Scholar]
- Corder, S. E. (1976). Properties and uses of bark as an energy source. Corvallis, OR: Forest Research Laboratory, Oregon State University. [Google Scholar]
- Cowan, M. M. (1999). Plant products as antimicrobial agents. Clinical Microbiology Reviews, 12(4), 564–582. 10.1128/CMR.12.4.564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushnie, T. T. , & Lamb, A. J. (2011). Recent advances in understanding the antibacterial properties of flavonoids. International Journal of Antimicrobial Agents, 38(2), 99–107. 10.1016/j.ijantimicag.2011.02.014 [DOI] [PubMed] [Google Scholar]
- da Costa, M. P. , Bozinis, M. C. V. , Andrade, W. M. , Costa, C. R. , da Silva, A. L. , de Oliveira, C. M. A. , … Silva, M. R. R. (2014). Antifungal and cytotoxicity activities of the fresh xylem sap of Hymenaea courbaril L. and its major constituent fisetin. BMC Complementary and Alternative Medicine, 14(1), 245 10.1186/1472-6882-14-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daglia, M. (2012). Polyphenols as antimicrobial agents. Current Opinion in Biotechnology, 23(2), 174–181. 10.1016/j.copbio.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Delaquis, P. , Stanich, K. , & Toivonen, P. (2005). Effect of pH on the inhibition of Listeria spp. by vanillin and vanillic acid. Journal of Food Protection, 68(7), 1472–1476. 10.4315/0362-028X-68.7.1472 [DOI] [PubMed] [Google Scholar]
- Demirbaş, A. (2001). Relationships between lignin contents and heating values of biomass. Energy Conversion and Management, 42(2), 183–188. 10.1016/S0196-8904(00)00050-9 [DOI] [Google Scholar]
- Demirbas, A. (2002). Relationships between heating value and lignin, moisture, ash and extractive contents of biomass fuels. Energy Exploration & Exploitation, 20(1), 105–111. 10.1260/014459802760170420 [DOI] [Google Scholar]
- Ekman, R. (1983). The suberin monomers and triterpenoids from the outer bark of Betula verrucosa Ehrh. Holzforschung‐International Journal of the Biology, Chemistry, Physics and Technology of Wood, 37(4), 205–211. [Google Scholar]
- Farkas, G. , & Kiraaly, Z. (1962). Role of phenolic compounds in the physiology of plant diseases and disease resistance. Journal of Phytopathology, 44(2), 105–150. 10.1111/j.1439-0434.1962.tb02005.x [DOI] [Google Scholar]
- Feng, S. , Cheng, S. , Yuan, Z. , Leitch, M. , & Xu, C. (2013). Valorization of bark for chemicals and materials: A review. Renewable and Sustainable Energy Reviews, 26, 560–578. 10.1016/j.rser.2013.06.024 [DOI] [Google Scholar]
- Ferreira, R. , Garcia, H. , Sousa, A. F. , Freire, C. S. R. , Silvestre, A. J. D. , Rebelo, L. P. N. , & Silva Pereira, C. (2013). Isolation of suberin from birch outer bark and cork using ionic liquids: A new source of macromonomers. Industrial Crops and Products, 44, 520–527. 10.1016/j.indcrop.2012.10.002 [DOI] [Google Scholar]
- Friedman, M. , Henika, P. R. , & Mandrell, R. E. (2003). Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica . Journal of Food Protection, 66(10), 1811–1821. 10.4315/0362-028X-66.10.1811 [DOI] [PubMed] [Google Scholar]
- Fukuhara, K. , Nakanishi, I. , Kansui, H. , Sugiyama, E. , Kimura, M. , Shimada, T. , … Miyata, N. (2002). Enhanced radical‐scavenging activity of a planar catechin analogue. Journal of the American Chemical Society, 124(21), 5952–5953. 10.1021/ja0178259 [DOI] [PubMed] [Google Scholar]
- Fukui, H. , Koshimizu, K. , & Egawa, H. (1978). A new diterpene with antimicrobial activity from Chamaecyparis pisifera Endle. Agricultural and Biological Chemistry, 42(7), 1419–1423. 10.1271/bbb1961.42.1419 [DOI] [Google Scholar]
- Funakoshi‐Tago, M. , Nakamura, K. , Tago, K. , Mashino, T. , & Kasahara, T. (2011). Anti‐inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. International Immunopharmacology, 11(9), 1150–1159. 10.1016/j.intimp.2011.03.012 [DOI] [PubMed] [Google Scholar]
- Gabor, M. , & Eperjessy, E. (1966). Antibacterial effect of fisetin and fisetinidin. Nature, 212(5067), 1273–1273. 10.1038/2121273a0 [DOI] [PubMed] [Google Scholar]
- Gachon, C. M. , Langlois‐Meurinne, M. , & Saindrenan, P. (2005). Plant secondary metabolism glycosyltransferases: The emerging functional analysis. Trends in Plant Science, 10(11), 542–549. [DOI] [PubMed] [Google Scholar]
- Gauthier, C. , Legault, J. , Lebrun, M. , Dufour, P. , & Pichette, A. (2006). Glycosidation of lupane‐type triterpenoids as potent in vitro cytotoxic agents. Bioorganic & Medicinal Chemistry, 14(19), 6713–6725. 10.1016/j.bmc.2006.05.075 [DOI] [PubMed] [Google Scholar]
- Gupta, G. K. (2009). Development of bark‐based environmental‐friendly composite panels (p. 133). Toronto, ON: Faculty of Forestry, University of Toronto. [Google Scholar]
- Gurib‐Fakim, A. (2006). Medicinal plants: Traditions of yesterday and drugs of tomorrow. Molecular Aspects of Medicine, 27(1), 1–93. [DOI] [PubMed] [Google Scholar]
- Hames, B. , Ruiz, R. , Scarlata, C. , Sluiter, A. , Sluiter, J. , & Templeton, D. (2008). Preparation of samples for compositional analysis, laboratory analytical procedure (LAP). Lakewood, CO: NREL. [Google Scholar]
- Hammer, O. , Harper, D. A. T. , & Ryan, P. D. (2001). PAST: PaAeotological STatistics software package for education an data analysis. Palaeontologia Electronica, 4(1), 1–9. [Google Scholar]
- Harkin, J. M. , & Rowe, J. W. (1971). Bark and its possible uses. Madison, WI: U.S. Department of Agriculture, Forest Products Laboratory. [Google Scholar]
- Heinamaki, J. , Pirttimaa, M. M. , Alakurtti, S. , Pitkanen, H. P. , Kanerva, H. , Hulkko, J. , … Kogermann, K. (2017). Suberin fatty acids from outer birch bark: Isolation and physical material characterization. Journal of Natural Products, 80(4), 916–924. [DOI] [PubMed] [Google Scholar]
- IgbaláChoudhary, M. (1995). Diterpenoid and steroidal alkaloids. Natural Product Reports, 12(4), 361–379. 10.1039/np9951200361 [DOI] [PubMed] [Google Scholar]
- Jablonsky, M. , Nosalova, J. , Sladkova, A. , Haz, A. , Kreps, F. , Valka, J. , … Surina, I. (2017). Valorisation of softwood bark through extraction of utilizable chemicals. A review. Biotechnology Advances, 35(6), 726–750. 10.1016/j.biotechadv.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Jamshidi‐Kia, F. , Lorigooini, Z. , & Amini‐Khoei, H. (2018). Medicinal plants: Past history and future perspective. Journal of Herbmed Pharmacology, 1, 1–7. [Google Scholar]
- Jeong, E.‐Y. , Jeon, J.‐H. , Lee, C.‐H. , & Lee, H.‐S. (2009). Antimicrobial activity of catechol isolated from Diospyros kaki Thunb. roots and its derivatives toward intestinal bacteria. Food Chemistry, 115(3), 1006–1010. 10.1016/j.foodchem.2009.01.021 [DOI] [Google Scholar]
- Jeong‐Yong, C. , Jae‐Hak, M. , Seong, K.‐Y. , & Keun‐Hyung, P. (1998). Antimicrobial activity of 4‐hydroxybenzoic acid and trans 4‐hydroxycinnamic acid isolated and identified from rice hull. Bioscience, Biotechnology, and Biochemistry, 62(11), 2273–2276. [DOI] [PubMed] [Google Scholar]
- Jork, H. , Funk, W. , Fischer, W. , Wimmer, H. , & Burns, D. T. (1990). Thin‐layer chromatography. Reagents and detection methods. Physical and chemical detection methods: fundamentals, reagents I. Volume 1a: VCH, Weinheim, 1990 (ISBN 3‐527‐27834‐6). xv+ 464 pp. Price DM 148.00, Analytica Chimica Acta, 237, 511–512. [Google Scholar]
- Kedzia, B. , Geppert, B. , & Iwaszkiewicz, J. (1990). Pharmacological investigations of ethanolic extract of propolis. Phytotherapie, 6, 7–10. [Google Scholar]
- Kobayashi, K. , Nishino, C. , Fukushima, M. , Shiobara, Y. , & Kodama, M. (1988). Antibacterial activity of pisiferic acid and its derivatives against Gram‐negative and‐positive bacteria. Agricultural and Biological Chemistry, 52(1), 77–83. 10.1080/00021369.1988.10868599 [DOI] [Google Scholar]
- Kocaçalışkan, I. , Talan, I. , & Terzi, I. (2006). Antimicrobial activity of catechol and pyrogallol as allelochemicals. Zeitschrift für Naturforschung C, 61(9–10), 639–642. 10.1515/znc-2006-9-1004 [DOI] [PubMed] [Google Scholar]
- Krasutsky, P. A. (2006). Birch bark research and development. Natural Product Reports, 23(6), 919–942. 10.1039/b606816b [DOI] [PubMed] [Google Scholar]
- Krasutsky, P. A. , Carlson, R. M. , Nesterenko, V. V. , Kolomitsyn, I. V. , & Edwardson, C. F. (2007). Birch bark processing and the isolation of natural products from birch bark. US: University of Minnesota. Google Patents. US6815553B2. [Google Scholar]
- Kuiters, A. , & Sarink, H. (1986). Leaching of phenolic compounds from leaf and needle litter of several deciduous and coniferous trees. Soil Biology and Biochemistry, 18(5), 475–480. 10.1016/0038-0717(86)90003-9 [DOI] [Google Scholar]
- Kukić, J. , Popović, V. , Petrović, S. , Mucaji, P. , Ćirić, A. , Stojković, D. , & Soković, M. (2008). Antioxidant and antimicrobial activity of Cynara cardunculus extracts. Food Chemistry, 107(2), 861–868. 10.1016/j.foodchem.2007.09.005 [DOI] [Google Scholar]
- Liu, X. , & Bi, X. T. (2011). Removal of inorganic constituents from pine barks and switchgrass. Fuel Processing Technology, 92(7), 1273–1279. 10.1016/j.fuproc.2011.01.016 [DOI] [Google Scholar]
- Lou, Z. , Wang, H. , Rao, S. , Sun, J. , Ma, C. , & Li, J. (2012). p‐Coumaric acid kills bacteria through dual damage mechanisms. Food Control, 25(2), 550–554. 10.1016/j.foodcont.2011.11.022 [DOI] [Google Scholar]
- McCutcheon, A. , Ellis, S. , Hancock, R. , & Towers, G. (1992). Antibiotic screening of medicinal plants of the British Columbian native peoples. Journal of Ethnopharmacology, 37(3), 213–223. 10.1016/0378-8741(92)90036-Q [DOI] [PubMed] [Google Scholar]
- McCutcheon, A. , Ellis, S. , Hancock, R. , & Towers, G. (1994). Antifungal screening of medicinal plants of British Columbian native peoples. Journal of Ethnopharmacology, 44(3), 157–169. 10.1016/0378-8741(94)01183-4 [DOI] [PubMed] [Google Scholar]
- Merkl, R. , Hrádková, I., Filip, V., & Smidrkal, J. (2010). Antimicrobial and antioxidant properties of phenolic acids alkyl esters, Czech. Journal of Food Science, 28(4), 275–279. 10.17221/132/2010-CJFS [DOI] [Google Scholar]
- Miranda, I. , Gominho, J. , Mirra, I. , & Pereira, H. (2012). Chemical characterization of barks from Picea abies and Pinus sylvestris after fractioning into different particle sizes. Industrial Crops and Products, 36(1), 395–400. 10.1016/j.indcrop.2011.10.035 [DOI] [Google Scholar]
- Miranda, I. , Gominho, J. , Mirra, I. , & Pereira, H. (2013). Fractioning and chemical characterization of barks of Betula pendula and Eucalyptus globulus . Industrial Crops and Products, 41, 299–305. 10.1016/j.indcrop.2012.04.024 [DOI] [Google Scholar]
- Moon, H. , & Rhee, M. S. (2016). Synergism between carvacrol or thymol increases the antimicrobial efficacy of soy sauce with no sensory impact. International Journal of Food Microbiology, 217, 35–41. 10.1016/j.ijfoodmicro.2015.10.009 [DOI] [PubMed] [Google Scholar]
- Mshvildadze, V. , Legault, J. , Lavoie, S. , Gauthier, C. , & Pichette, A. (2007). Anticancer diarylheptanoid glycosides from the inner bark of Betula papyrifera . Phytochemistry, 68(20), 2531–2536. 10.1016/j.phytochem.2007.05.018 [DOI] [PubMed] [Google Scholar]
- O'Connell, M. M. , Bentley, M. D. , Campbell, C. S. , & Cole, B. J. (1988). Betulin and lupeol in bark from four white‐barked birches. Phytochemistry, 27(7), 2175–2176. 10.1016/0031-9422(88)80120-1 [DOI] [Google Scholar]
- Omar, S. , Lemonnier, B. , Jones, N. , Ficker, C. , Smith, M. , Neema, C. , … Arnason, J. (2000). Antimicrobial activity of extracts of eastern North American hardwood trees and relation to traditional medicine. Journal of Ethnopharmacology, 73(1), 161–170. 10.1016/S0378-8741(00)00294-4 [DOI] [PubMed] [Google Scholar]
- Özçelik, B. , Kartal, M. , & Orhan, I. (2011). Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharmaceutical Biology, 49(4), 396–402. 10.3109/13880209.2010.519390 [DOI] [PubMed] [Google Scholar]
- Papuc, C. , Goran, G. V. , Predescu, C. N. , Nicorescu, V. , & Stefan, G. (2017). Plant polyphenols as antioxidant and antibacterial agents for shelf‐life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Comprehensive Reviews in Food Science and Food Safety, 16(6), 1243–1268. [DOI] [PubMed] [Google Scholar]
- Pedieu, R. , Riedl, B. , & Pichette, A. (2009). Properties of mixed particleboards based on white birch (Betula papyrifera) inner bark particles and reinforced with wood fibres. European Journal of Wood and Wood Products, 67(1), 95–101. 10.1007/s00107-008-0297-6 [DOI] [Google Scholar]
- Perumal, S. , Mahmud, R. , & Ismail, S. (2017). Mechanism of action of isolated caffeic acid and epicatechin 3‐gallate from Euphorbia hirta against Pseudomonas aeruginosa . Pharmacognosy Magazine, 13(Suppl 2), S311–S315. 10.4103/pm.pm_309_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumed‐Ferrer, C. , Väkeväinen, K. , Komulainen, H. , Rautiainen, M. , Smeds, A. , Raitanen, J.‐E. , … Saarela, M. (2013). The antimicrobial effects of wood‐associated polyphenols on food pathogens and spoilage organisms. International Journal of Food Microbiology, 164(1), 99–107. 10.1016/j.ijfoodmicro.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Pluskal, T. , Castillo, S. , Villar‐Briones, A. , & Orešič, M. (2010). MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry‐based molecular profile data. BMC Bioinformatics, 11(1), 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puupponen‐Pimiä, R. , Nohynek, L. , Alakomi, H.‐L. , & Oksman‐Caldentey, K.‐M. (2005). Bioactive berry compounds—Novel tools against human pathogens. Applied Microbiology and Biotechnology, 67(1), 8–18. 10.1007/s00253-004-1817-x [DOI] [PubMed] [Google Scholar]
- Royer, M. , Ben Amor, A. , & Boucher, M.‐A. (2016). Les extractibles forestiers. Biosourcé, 3, 18. [Google Scholar]
- Royer, M. , Houde, R. , Viano, Y. , & Stevanovic, T. (2012). Non‐wood forest products based on extractives – A new opportunity for the Canadian forest industry part 1: Hardwood forest species. Journal of Food Research, 1(3), 8. [Google Scholar]
- Royer, M. , Prado, M. , García‐Pérez, M. E. , Diouf, P. N. , & Stevanovic, T. (2013). Study of nutraceutical, nutricosmetics and cosmeceutical potentials of polyphenolic bark extracts from Canadian forest species. PharmaNutrition, 1(4), 158–167. 10.1016/j.phanu.2013.05.001 [DOI] [Google Scholar]
- Saleem, M. , Nazir, M. , Ali, M. S. , Hussain, H. , Lee, Y. S. , Riaz, N. , & Jabbar, A. (2010). Antimicrobial natural products: An update on future antibiotic drug candidates. Natural Product Reports, 27(2), 238–254. [DOI] [PubMed] [Google Scholar]
- Sasidharan, S. , Chen, Y. , Saravanan, D. , Sundram, K. , & Latha, L. Y. (2011). Extraction, isolation and characterization of bioactive compounds from plants’ extracts. African Journal of Traditional, Complementary and Alternative Medicines, 8(1), 1-10. 10.4314/ajtcam.v8i1.60483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, G. G. D. , Guilbert, S. , & Rouau, X. (2011). Successive centrifugal grinding and sieving of wheat straw. Powder Technology, 208(2), 266–270. 10.1016/j.powtec.2010.08.015 [DOI] [Google Scholar]
- Sjöström, E. , & Alén, R. (2013). Analytical methods in wood chemistry, pulping, and papermaking. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- Sluiter, A. , Hames, B. , Ruiz, R. , Scarlata, C. , Sluiter, J. , & Templeton, D. (2005). Determination of ash in biomass, laboratory analytical procedure (LAP). Lakewood, CO: NREL. [Google Scholar]
- Sluiter, A. , Hames, B. , Ruiz, R. , Scarlata, C. , Sluiter, J. , & Templeton, D. (2008a). Determination of sugars, byproducts, and degradation products in liquid fraction process samples, laboratory analytical procedure (LAP). Lakewood, CO: NREL. [Google Scholar]
- Sluiter, A. , Hames, B. , Ruiz, R. , Scarlata, C. , Sluiter, J. , Templeton, D. , & Crocker, D. (2008b). Determination of structural carbohydrates and lignin in biomass, laboratory analytical procedure (LAP). Lakewood, CO: NREL. [Google Scholar]
- Sluiter, A. , Ruiz, R. , Scarlata, C. , Sluiter, J. , & Templeton, D. (2005). Determination of extractives in biomass, laboratory analytical procedure (LAP). Lakewood, CO: NREL. [Google Scholar]
- Stermitz, F. R. , Kamm, C. D. , & Tawara, J. N. (2000). Piperidine alkaloids of spruce (Picea) and fir (Abies) species. Biochemical Systematics and Ecology, 28(2), 177–181. 10.1016/S0305-1978(99)00054-X [DOI] [Google Scholar]
- Stojković, D. , Petrović, J. , Soković, M. , Glamočlija, J. , Kukić‐Marković, J. , & Petrović, S. (2013). In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p‐coumaric acid and rutin, using food systems. Journal of the Science of Food and Agriculture, 93(13), 3205–3208. [DOI] [PubMed] [Google Scholar]
- St‐Pierre, A. , Blondeau, D. , Lajeunesse, A. , Bley, J. , Bourdeau, N. , & Desgagné‐Penix, I. (2018). Phytochemical screening of quaking aspen (Populus tremuloides) extracts by UPLC‐QTOF‐MS and evaluation of their antimicrobial activity. Molecules, 23, 1739 10.3390/molecules23071739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanase, C. , Coșarcă, S. , & Muntean, D.‐L. (2019). A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules, 24(6), 1182 10.3390/molecules24061182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanase, C. , Coșarcă,S. , Toma, F. , Mare, A. , Cosarca, A. , Man, A. , … Imre, S. (2018). Antibacterial activities of spruce bark (Picea abies L.) extract and its components against human pathogens. Revista de Chimie, 69(6), 1462–1467. [Google Scholar]
- Tatsimo, S. J. N. , de Dieu Tamokou, J. , Havyarimana, L. , Csupor, D. , Forgo, P. , Hohmann, J. , … Tane, P. (2012). Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum . BMC Research Notes, 5(1), 158 10.1186/1756-0500-5-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandal, J. , Abou‐Zaid, M. M. , Ferroni, G. , & Leduc, L. G. (2015). Antimicrobial activity of natural products from the flora of Northern Ontario. Canada, Pharmaceutical Biology, 53(6), 800–806. 10.3109/13880209.2014.942867 [DOI] [PubMed] [Google Scholar]
- Vardar‐Ünlü, G. , Silici, S. , & Ünlü, M. (2008). Composition and in vitro antimicrobial activity of Populus buds and poplar‐type propolis. World Journal of Microbiology and Biotechnology, 24(7), 1011–1017. [Google Scholar]
- Veluri, R. , Weir, T. L. , Bais, H. P. , Stermitz, F. R. , & Vivanco, J. M. (2004). Phytotoxic and antimicrobial activities of catechin derivatives. Journal of Agricultural and Food Chemistry, 52(5), 1077–1082. 10.1021/jf030653+ [DOI] [PubMed] [Google Scholar]
- Webster, D. , Taschereau, P. , Belland, R. J. , Sand, C. , & Rennie, R. P. (2008). Antifungal activity of medicinal plant extracts; preliminary screening studies. Journal of Ethnopharmacology, 115(1), 140–146. 10.1016/j.jep.2007.09.014 [DOI] [PubMed] [Google Scholar]
- White, R. (1987). Effect of lignin content and extractives on the higher heating value of wood. Wood and Fiber Science, 19(4), 446–452. [Google Scholar]
- Wink, M. (1988). Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. TAG Theoretical and Applied Genetics, 75(2), 225–233. [Google Scholar]
- Yim, N. , Trung, T. N. , Kim, J. P. , Lee, S. , Na, M. , Jung, H. , … Bae, K. (2010). The antimicrobial activity of compounds from the leaf and stem of Vitis amurensis against two oral pathogens. Bioorganic & Medicinal Chemistry Letters, 20(3), 1165–1168. 10.1016/j.bmcl.2009.12.020 [DOI] [PubMed] [Google Scholar]
- Yu, H.‐H. , Kim, K.‐J. , Cha, J.‐D. , Kim, H.‐K. , Lee, Y.‐E. , Choi, N.‐Y. , & You, Y.‐O. (2005). Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin‐resistant Staphylococcus aureus . Journal of Medicinal Food, 8(4), 454–461. [DOI] [PubMed] [Google Scholar]
- Yubin, J. , Miao, Y. , Bing, W. , & Yao, Z. (2014). The extraction, separation and purification of alkaloids in the natural medicine. Journal of Chemical and Pharmaceutical Research, 6(1), 338–345. [Google Scholar]
- Zhang, T. , Wei, X. , Miao, Z. , Hassan, H. , Song, Y. , & Fan, M. (2016). Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chemistry Central Journal, 10(1), 47 10.1186/s13065-016-0195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, G. , Yan, W. , & Cao, D. (2007). Simultaneous determination of betulin and betulinic acid in white birch bark using RP‐HPLC. Journal of Pharmaceutical and Biomedical Analysis, 43(3), 959–962. 10.1016/j.jpba.2006.09.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are included in this published article and available from the corresponding author on reasonable request.


