Abstract
Marine purple photosynthetic bacteria are ideal organisms for the production of useful materials at reduced costs and contributing to a sustainable society because they can utilize sunlight, seawater, and components of air, including carbon dioxide and nitrogen gases, for their growth. However, conjugation is the only applicable method for the transformation of marine purple photosynthetic bacteria so far. Here, we examined a calcium chloride‐mediated method for the transformation of marine purple photosynthetic bacteria. Plasmid DNAs containing the kanamycin resistance gene were successfully transferred into chemically competent cells of two strains of marine purple photosynthetic bacteria (Rhodovulum sulfidophilum and Roseospira marina). Heat shock treatment increased the transformation efficiency in R. sulfidophilum, whereas the addition of cell‐penetrating peptide did not improve it. We also found that prolonged incubation in agar plates containing kanamycin led to spontaneous mutation of the 16S rRNA, resulting in kanamycin resistance in R. marina. Thus, we developed an efficient and facile transformation method using chemically competent cells of marine purple photosynthetic bacteria with calcium chloride.
Keywords: 16S rRNA, calcium chloride method, chemically competent cells, marine purple photosynthetic bacteria, transformation
Marine purple photosynthetic bacteria are expected to be ideal microbial hosts for the industrial production by utilization of their carbon dioxide and nitrogen fixation abilities. However, only conjugation is an applicable method of transformation in marine purple photosynthetic bacteria and genetic transformation methods have not been developed well. Here, we examined chemical competence method by CaCl2 treatment for marine purple photosynthetic bacteria.
1. INTRODUCTION
Transformation, which introduces foreign DNA into cells, is an essential technology for genetic engineering. A number of transformation methods have been established (Aune & Aachmann, 2010). In the case of bacteria, electroporation, conjugation, natural competence, and chemical competence methods have been used to transfer foreign DNA into the cells. Electroporation is a physical method that temporally permeabilizes the cell membranes by applying a short electrical pulse. Conjugation is the transfer of DNA between donor and recipient cells via direct cell‐to‐cell contact. Natural competence is the ability to uptake DNA from the environment and has been utilized for transformation in many bacterial species (Lorenz & Wackernagel, 1994). Competency is known to be induced by chemical treatment, such as treatment with dimethyl sulfoxide (DMSO)(Hanahan, 1983), divalent cations (Dagert & Ehrlich, 1979a; Mandel & Higa, 1970), or polyethylene glycol (PEG)(Klebe, Harriss, Sharp, & Douglas, 1983). Applicable and efficient transformation methods have been selected according to the physiological and biological properties of bacterial species.
The use of photosynthetic organisms to produce value‐added materials and chemicals is one of the potential methods to reduce costs and can contribute to a sustainable social system because these organisms can utilize sunlight and carbon dioxide (CO2) in the air for their growth and producing activity. Some species of purple photosynthetic bacteria are known to fix nitrogen in addition to CO2 (Gallon, 2001; McKinlay & Harwood, 2010). This means that they can use nitrogen gas (N2) and CO2 in the air as nitrogen and carbon sources for their growth, respectively. Marine bacteria have other advantages as host organisms of biological production because high‐salinity culture medium can reduce the risk of biological contamination, and the highly abundant seawater can be used as a growth medium. We focused on marine purple photosynthetic bacteria as host microorganisms for the production of polyhydroxyalkanoates, which are a family of biopolyesters (Foong, Higuchi‐Takeuchi, & Numata, 2019; Higuchi‐Takeuchi, Morisaki, Toyooka, & Numata, 2016; Higuchi‐Takeuchi & Numata, 2019). However, genetic transformation technologies, such as the use of high‐expression promoters and transformation methods for marine purple photosynthetic bacteria, have not been well established to date.
Although conjugation methods are widely used for transformation in purple photosynthetic bacteria, the protocol for conjugation contains many steps and is time‐consuming. Moreover, there are a limited number of vectors for the conjugation method. Another way of transformation in purple photosynthetic bacteria is the electroporation method. However, high‐salt medium, which is necessary for marine bacteria, reduces the electroporation efficiency. Therefore, only conjugation is an applicable method of transformation for marine purple photosynthetic bacteria.
In this study, we examined using the chemical competence method by CaCl2 treatment for the transformation of marine purple photosynthetic bacteria. As a result, plasmid DNA (pDNA) has been successfully introduced into two marine purple photosynthetic bacterial strains. Heat shock treatment was effective in the transformation of CaCl2 competent cells, while cell‐penetrating peptide (CPP) did not enhance the efficiency. We also found that prolonged incubation for more than 30 days led to mutation of the 16S rRNA gene, resulting in kanamycin resistance.
2. MATERIALS AND METHODS
2.1. Culture conditions
The marine purple photosynthetic bacteria investigated in this study were obtained from biological resource centers (RIKEN BioResource Center and ATCC). Marine purple photosynthetic bacteria were cultured in Marine Broth, Marine Agar 2,216 (Difco, Detroit, USA), and JCM 568 medium (https://www.jcm.riken.jp/cgi-bin/jcm/jcm_grmd?GRMD=568), the last of which contains the following components per liter: KH2PO4 0.5 g, CaCl2.2H2O 0.15 g, MgSO4 0.7H2O 1.5 g, NH4Cl 0.6 g, NaCl 20 g, sodium pyruvate 3.0 g, yeast extract 0.4 g, ferric citrate 0.25 mg, ZnCl2.5H2O 70 μg, MnCl2.4H2O 100 μg, H3BO3 60 μg, CoCl2.6H2O 200 μg, CuCl2.2H2O 20 μg, NiCl2.6H2O 20 μg, Na2MoO4·H2O 40 μg, vitamin B12 2 mg, 0.1% NaHCO3, and 2 mM Na2S2O3. The pH was adjusted to 6.8 with NaOH. Marine Broth and Marine Agar 2,216 were used for the growth of Rhodovulum sulfidophilum and Roseospira marina. The 568 medium was used as the growth medium of Roseospira visakhapatnamensis and Roseospira goensis cells. Marine purple photosynthetic bacteria were grown under continuous far‐red LED light (730 nm, VBP‐L24‐C3, Valore, Tokyo, Japan) at 30°C in plastic tubes filled with medium to the tops of the necks.
2.2. Preparation of chemically competent cells
Cells were harvested in the log phase (OD660 = approximately 2.0), diluted with growth media to OD660 = 0.1, and cultured for approximately 20 hr (OD660 = approximately 0.6 to 0.8). Cells were transferred to a 50‐mL plastic tube and incubated on ice for 10 min. Cooled cell cultures were centrifuged at 3,000 rpm without braking at 0°C for 5 min, and the supernatant was discarded. Cells were suspended in 8 ml of cooled 50 mM CaCl2 and centrifuged at 2,500 rpm without braking at 0°C for 3 min. The supernatant was discarded, and cells were suspended in 8 ml of cooled 50 mM CaCl2. The resulting cell suspensions were incubated on ice for more than 30 min and centrifuged at 2,500 rpm without braking at 0°C for 3 min. The supernatant was discarded, and finally, the cells were suspended in 4 ml of cooled 50 mM CaCl2/ 15% glycerol and incubated on ice for more than 2 hr. Then, 100 μl of chemically competent cells were transferred into 1.5‐mL tubes and frozen in liquid nitrogen. Chemically competent cells were kept at −80°C until use.
2.3. Preparation of peptide–pDNA complex and transformation
Plasmid (pBBR1MCS‐2)(Kovach et al., 1995) carrying the kanamycin‐resistant gene npt II was prepared using a QIAprep Spin Miniprep Kit (QIAGEN, Dusseldorf, Germany) according to the standard protocol. When we used a low‐purity plasmid with a 260/280 ratio of ~ 1.7, the transformation efficiency was low compared to that with a highly pure plasmid (260/280 ratio of approximately 1.9). Then, 1 μg of pDNA was mixed with peptides (BP100)2K8: KKLFKKILKYLKKLFKKILKYLKKKKKKKK at an N/P ratio of 0.1 and incubated at room temperature for 10 min to form peptide–pDNA complexes (Lakshmanan, Kodama, Yoshizumi, Sudesh, & Numata, 2013). Competent cells were thawed on ice and mixed with 1 μg of pDNA or peptide–pDNA complexes. Cells were incubated for more than 30 min on ice. For heat treatment, cells were incubated for 1 min at 42°C followed by incubation on ice for 2 min. After incubation on ice, 1.5 ml of growth medium without antibiotics was added to cells, and cells were preincubated at 30°C overnight under far‐red illumination. After preculture, cell cultures were spread in Marine Agar 2,216 (Difco, Detroit, USA) or 568‐medium agar plates containing 100 mg/L kanamycin. Cells were cultured at 30°C under far‐red light for 14 days (short‐term incubation) and up to 37 days (long‐term incubation). For the long‐term incubation study, transformations of R. marina were carried out two times under four transformation conditions (with or without CPP and heat shock treatment). Eight kanamycin‐resistant colonies were examined after 20 days of incubation (long‐term incubation) for sequencing analysis of 16S ribosomal RNA (16S rRNA).
2.4. RT‐PCR analysis
Total RNA was extracted using the RNeasy Mini Kit (QIAGEN, Dusseldorf, Germany) according to the manufacturer's protocol. Using 0.1 μg of RNA as a template, cDNA was synthesized by a QuantiTect Reverse Transcription Kit (QIAGEN, Dusseldorf, Germany) following the manufacturer's protocol. To determine the expression levels of the npt II gene, the following sets of primers were used: npt‐F (5’‐ AGA CAA TCG GCT GCT CTG AT −3’) and npt‐R (5’‐ CTC GTC CTG CAG TTC ATT CA −3’). Primer mix from the bacterial 16S TaKaRa rDNA PCR Kit (TaKaRa, Shiga, Japan) was used for amplification of the 16S rRNA gene.
2.5. Southern blotting analysis
Genomic DNA was isolated from R. marina cells using the CTAB method (C. N. Jr. Stewart & L. E. Via, 1993). Approximately 10 μg of genomic DNA was digested with BamHI, run on a 1% agarose gel, and transferred to a Hybond N + nylon membrane (GE Healthcare, Chicago, IL, USA). The GAPDH gene was used as an internal reference gene. Probe preparation for GAPDH and npt II genes and detection were performed using the CDP‐Star AlkPhos Direct Labeling and Detection system (GE Healthcare, Chicago, IL, US) according to the manufacturer's protocol. The following primer sets were used for probe preparation: for GAPDH, GAPDH‐Fw (5’‐ CAA GGT AGC AAT CAA CGG CT −3’) and GAPDH‐Re (5’‐ TGT GGA TCG TGG TCA TGA AG −3’); for npt II, nptR‐F2 (5’‐ TGC TCC TGC CGA GAA AGT AT −3’) and npt‐R2 (5’‐ AGA ACT CGT CAA GAA GGC GA −3’). R. sulfidophilum genomic DNA and pBBR1MCS‐2 were used for PCR templates.
2.6. Sequence analysis of the 16S rRNA
Genomic DNA of R. marina cells was extracted using DNase Blood and Tissue Kit (QIAGEN, Dusseldorf, Germany) according to the manufacturer's protocol. A bacterial 16S TaKaRa rDNA PCR Kit (TaKaRa, Shiga, Japan) was used for amplification of the 16S rRNA and sequencing.
3. RESULTS AND DISCUSSION
3.1. Antibiotic resistance of marine purple photosynthetic bacteria
To develop a transformation method for marine purple photosynthetic bacteria, the plasmid pBBR1MCS‐2 (Kovach et al., 1995), carrying the kanamycin‐resistant gene npt II, was selected in this study. pBBR1MCS is a broad‐host range plasmid and was used in some previous studies of purple photosynthetic bacteria (Garcia‐Contreras, Celis, & Romero, 2004; Pinta, Picaud, Reiss‐Husson, & Astier, 2002). We examined the kanamycin sensitivity of several strains of marine purple photosynthetic bacteria. As a result, four strains (R. sulfidophilum, R. marina, R. visakhapatnamensis, and R. goensis) exhibited resistance to more than 100 mg/L kanamycin (Figure A1). These strains were examined for transformation using kanamycin resistance as a selectable marker.
First, we tried a natural transformation method using R. sulfidophilum, which is a representative strain of marine purple photosynthetic bacteria. pDNA was mixed with cell cultures, and cells were plated on agar plates containing kanamycin. We examined several conditions, such as high pDNA content and various concentrations of cell cultures. However, the natural transformation method was not successful in R. sulfidophilum.
The natural transformation method was also investigated using the CPP‐based technique. CPPs are composed of 5–30 amino acids and have the ability to penetrate the cells. We developed a peptide‐based gene delivery system using fusion peptides consisting of CPP and lysine‐ and histidine‐rich polycationic peptides. In this system, negatively charged plasmid DNA preferentially interacts with polycationic peptides and condenses through electrostatic interactions, and the CPP region functions as a cell‐penetrating motif. This fusion peptide carrier was applied to DNA, RNA, and protein delivery into plants, tobacco BY‐2 cells, and human cells (Lakshmanan et al., 2013; Ng et al., 2016; Numata, Ohtani, Yoshizumi, Demura, & Kodama, 2014). BP100 (KKLFKKILKYL) was originally designed as an antimicrobial peptide (Badosa et al., 2007) and shown to have cell penetration ability in tobacco BY‐2 cells (Eggenberger, Mink, Wadhwani, Ulrich, & Nick, 2011). Previously, we compared the penetration efficiency of BP100 monomer and dimer in E. coli and demonstrated that the dimer of BP100 exhibited higher penetration efficiency than the monomer (Oikawa, Islam, Horii, Yoshizumi, & Numata, 2018). Moreover, (BP100)2K8 showed better protein delivery into the cells of plant leaves than BP100(KH)9 (Ng et al., 2016). Therefore, we evaluated the effect of (BP100)2K8 on the natural transformation of R. sulfidophilum cells.
Addition of more than 10 g/L CPP led to severe growth inhibition of R. sulfidophilum (Figure A2). However, the CPP‐pDNA complex in which CPP was mixed with plasmid DNA in solution did not affect the growth of R. sulfidophilum cells. The CPP‐pDNA complex prepared at an N/P ratio of 0.1 was shown to be effective for transformation in Escherichia coli in a previous study (Islam et al., 2019). The CPP‐pDNA complex was prepared with an N/P ratio of 0.1 using (BP100)2K8 and pBBR1MCS‐2 and mixed with R. sulfidophilum cell cultures. These cell cultures containing peptide–pDNA complexes were selected on plates containing kanamycin after preincubation. However, transformants could not be obtained despite repeated experiments. We previously demonstrated that the optimal CPP was variable among the plant species using a library containing 55 types of CPP (Numata et al., 2018). Although (BP100)2K8 is one of the best CPPs against E. coli, the CPP did not function with R. sulfidophilum in this study. CPPs other than (BP100)2K8 might be effective for marine purple photosynthetic bacteria.
3.2. CaCl2‐induced transformation
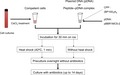
Next, we examined calcium chloride‐induced transformation in R. sulfidophilum. Calcium chloride treatment is widely used for the transformation of E. coli (Dagert & Ehrlich, 1979b; Weston, Brown, Perkins, Saunders, & Humphreys, 1981) and other bacteria, including purple photosynthesis bacteria (Fornari & Kaplan, 1982; Russo, Panangala, Wood, & Klesius, 2009). The transformation procedure is summarized in Figure 1. Chemically competent cells were prepared using R. sulfidophilum cells by calcium chloride treatment. Chemically competent cells were mixed with plasmid DNA and preincubated overnight without kanamycin followed by incubation with kanamycin. Kanamycin‐resistant colonies appeared after approximately 7–14 days of incubation, as shown in Figure 2a.
Figure 1.
Scheme for the transformation of marine purple photosynthetic bacteria. Chemically competent cells were prepared by the CaCl2 method. Plasmid DNA (pBBR1MCS‐2) was mixed with competent cells in the presence or absence of CPP ((BP100)2K8). After incubation on ice for 30 min, competent cells were treated with or without heat shock. Four transformation conditions (with or without CPP and heat shock treatment) were examined. Common (solid lines) and alternative (dashed lines) procedures are shown in arrows
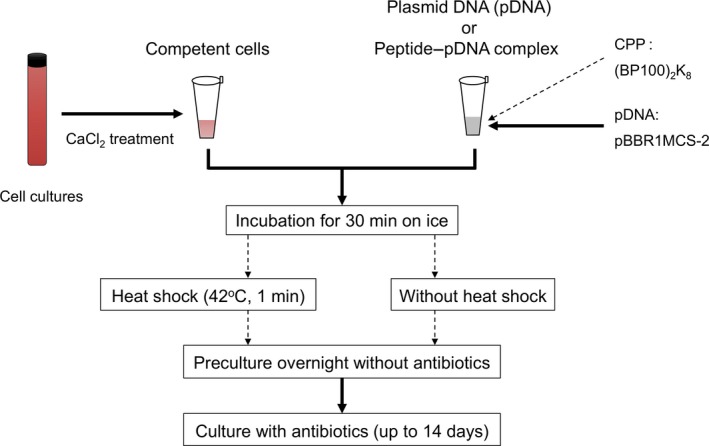
Figure 2.
Transformation of marine purple photosynthetic bacteria. (a) Kanamycin‐resistant colonies of R. sulfidophilum and R. marina. Photographs were taken after 14 days of incubation. Transformation was carried out by heat shock treatment in the presence of peptide. (b) Effects of CPP and heat shock on the transformation efficiency. Transformations were carried out in the presence or absence of CPP and heat shock treatment. The number of kanamycin‐resistant colonies was counted after 14 days of incubation. The asterisk indicates a significant difference (p < .05). Plasmid extraction from kanamycin‐resistant colonies of R. sulfidophilum (c) and R. marina (d). Plasmid solutions extracted from kanamycin‐resistant colonies were analyzed by 1% agarose gel. Transformations were carried out in the presence or absence of CPP and heat shock treatment. Three kanamycin‐resistant colonies (R. sulfidophilum) and two colonies (R. marina) from each set of conditions were analyzed in agarose gels
Heat shock treatment is commonly used in CaCl2‐mediated transformation (Mandel & Higa, 1970). We examined the effects of heat shock treatment at 42°C for 1 min and the CPP‐pDNA complex on the transformation efficiency of R. sulfidophilum (Figure 2b). The number of transformants increased significantly by heat shock treatment in the presence and absence of CPP. Thus, the transformation efficiency was not improved by the addition of CPP but by the heat shock treatment. Transformation was also performed using another plasmid, pJRD215, which has a broad‐host range (Davison, Heusterspreute, Chevalier, Hathi, & Brunel, 1987). The size of pJRD215 is 10.2 kb, and it is larger than pBBR1MCS‐2 (5.1 kb). Kanamycin‐resistant colonies could be obtained by heat shock treatment after 14 days of incubation. Colony‐forming units (CFU) per μg DNA were 1.8 ± 1.5 and 3.4 ± 4.4 (n = 5) in the presence or absence of CPP, respectively. A large plasmid size could be introduced into R. sulfidophilum cells in our method, although the transformation efficiency of pJRD215 was low compared to that of pBBR1MCS‐2.
Chemically competent cells of another marine purple photosynthetic bacteria, R. marina, were prepared and investigated for transformation efficiency using pBBR1MCS‐2. As a result, only several kanamycin‐resistant colonies were observed for R. marina (Figure 2a, Table A1). The presence of plasmid was confirmed by extraction of plasmid from kanamycin‐resistant cells of R. sulfidophilum (Figure 2c) and R. marina (Figure 2d), indicating that pDNAs were successfully transferred into the cells.
In contrast to R. sulfidophilum and R. marina, no kanamycin‐resistant colony was found in R. visakhapatnamensis and R. goensis under all conditions (Table A1). Both R. visakhapatnamensis and R. goensis showed poor growth compared to R. sulfidophilum and R. marina. In these experiments, we found that the purity of pDNA is important for efficient transformation. Further optimization of various parameters, such as growth conditions and cell culture volume, is required to achieve transformation.
3.3. Long‐term incubation
Time‐dependent changes in the number of kanamycin‐resistant colonies of R. marina are shown in Table A2. When the incubation period with kanamycin was extended to more than 20 days during long incubation times, additional kanamycin‐resistant colonies appeared (Table A2, white arrow in Figure 3a). An average of 1.0 colony per plate was observed from 8 agar plates during long‐term incubation. Plasmid extraction from kanamycin‐resistant cells was carried out after a long period of incubation. However, no plasmid could be extracted and detected by agarose gel electrophoresis. Gene expression of npt II was analyzed by RT‐PCR analysis (Figure 3b). RT‐PCR products were observed in short‐term cultured kanamycin‐resistant cells. On the other hand, npt II expression was hardly detected or quite low in long‐term cultured cells. To check the possibility that the npt II gene was integrated into the genome, Southern blotting analysis was carried out using the npt II gene probe (Figure 3c). A hybridization signal was not observed in long‐term cultured cells, suggesting that the npt II gene was not integrated into the genome.
Figure 3.

Kanamycin‐resistant R. marina cells after long‐term incubation. (a) Kanamycin‐resistant colonies of R. marina after 30 days of incubation. Kanamycin‐resistant colonies appeared after 30 days of incubation, and they are shown in white arrows. (b) RT‐PCR analysis of the npt II gene. Transformations were carried out in the presence or absence of CPP and heat shock treatment. RNA was extracted from short‐term and long‐term incubated kanamycin‐resistant colonies. RT‐PCR was carried out using the npt II gene‐specific primer and the 16S rRNA gene (internal standard)‐specific primer sets. (c) Southern blotting analysis of the npt II gene. Transformations were carried out in the presence or absence of CPP and heat shock treatment. Genomic DNA was extracted from long‐term incubated kanamycin‐resistant colonies of R. marina. Southern blotting analysis was carried out using npt II‐ and the 16S rRNA gene (internal standard)‐specific probes. The PCR product of the npt II gene was used as a positive control. (d) Alignment of the 16S rRNA sequences between WT and long‐term incubated kanamycin‐resistant colonies of R. marina. 16S rRNA sequences of WT and eight long‐term incubated kanamycin‐resistant colonies of R. marina were determined. Sequences show a 3’ part of the 16S rRNA. The red triangle shows position 1,325 of the 16S rRNA
It has been reported that mutation of 16S rRNA conferred kanamycin resistance in E. coli and Mycobacterium tuberculosis (Apirion & Schlessinger, 1968; De Stasio, Moazed, Noller, & Dahlberg, 1989; Suzuki et al., 1998). The sequences of the 16S rRNA in R. marina long‐term incubated kanamycin‐resistant cells were determined and compared to those of WT (Figure 3d). An A‐to‐G mutation was found at position 1,325 among all kanamycin‐resistant R. marina cells. Mutations at position 1,408 in E. coli and 1,389 in M. tuberculosis lead to kanamycin and other antibiotic resistance (De Stasio et al., 1989; Suzuki et al., 1998), and this position is reported to be an important region of antibiotic binding (Fourmy, Recht, Blanchard, & Puglisi, 1996). Multiple sequence alignment revealed that the mutations at position 1,408 in E. coli and 1,389 in M. tuberculosis corresponded to position 1,325 in R. marina. These results suggest that spontaneous mutation of the 16S rRNA occurred during a long incubation period of more than 30 days to lead to kanamycin resistance in R. marina. Thus, our observations demonstrated that long‐term incubation with antibiotics for transformation should be avoided due to spontaneous mutation.
4. CONCLUSIONS
Marine purple photosynthetic bacteria have the potential to be biological production systems contributing to the sustainable development of society. However, biotechnological tools have not been well established. In this study, we examined a transformation method for marine purple photosynthetic bacteria using competent cells treated with CaCl2. pDNA with a kanamycin resistance gene was successfully transferred into two strains of marine purple photosynthetic bacteria. Heat shock treatment improved the transformation efficiency of competent cells of R. sulfidophilum. Long‐term incubation with kanamycin led to spontaneous mutation of the 16S rRNA in R. marina, resulting in kanamycin resistance. Chemically competent cells can be stored at −80°C and used for transformation at any time. Thus, our method developed here generates ready‐to‐use competent cells of marine purple photosynthetic bacteria, which will expand the science and technology applications involving marine purple photosynthetic bacteria.
CONFLICT OF INTERESTS
None declared.
AUTHOR CONTRIBUTIONS
M.H.‐T. and K. N. conceived and designed the study. K.M. performed the experiments. M.H.‐T. analyzed and interpreted the data and prepared the manuscript. M.H.‐T. and K.N. approved the manuscript.
ETHICS APPROVAL
None required.
ACKNOWLEDGMENTS
We thank Dr. K.V.P. Nagashima for providing plasmid pJRRD215. We also thank the RIKEN BioResource Center and Global Bioresource Center (ATCC) for providing the marine purple photosynthetic bacteria strains. This work was supported by JSPS KAKENHI grant number JP16K00593, JST ERATO grant number JPMJER1602, and the Institute for Fermentation.
APPENDIX A.
Table A1.
Transformation efficiencies (CFU/μg DNA) of marine purple photosynthetic bacteria
| Peptide | + | + | ‐ | ‐ |
|---|---|---|---|---|
| Heat shock | + | ‐ | + | ‐ |
| R. marina | 1.1 ± 1.9 | 1.1 ± 1.3 | 1.7 ± 1.1 | 0.7 ± 1.1 |
| R. visakhapatnamensis | 0 | 0 | 0 | 0 |
| R. goensis | 0 | 0 | 0 | 0 |
Four transformation conditions (with or without CPP peptide and heat shock treatment) were examined using pBBR1MSC‐2 plasmid in three strains (R. marina, R. visakhapatnamensis and R. goensis). Transformation was carried out in the presence (+) or absence (‐) of CPP peptide. After incubation on ice, cells were treated with (+) or without (‐) heat shock.
Table A2.
Time course changes in number of kanamycin resistant colonies of R. marina
| (a) 1st transformation | ||||||
|---|---|---|---|---|---|---|
| Time of incubation (days) | 6 | 10 | 20 | 27 | 38 | Total |
| +CPP, +Heat shock | 4 | 1 | 0 | 1 (1–6) | 0 | 6 |
| +CPP | 1 | 0 | 1 (2–2) | 0 | 0 | 2 |
| +Heat shock | 1 | 0 | 1 (3–2) | 0 | 1 (3–3) | 3 |
| DNA only | 0 | 0 | 0 | 0 | 1 (4–1) | 1 |
| (b) 2nd transformation | ||||
|---|---|---|---|---|
| Time of incubation (days) | 6 | 11 | 27 | Total |
| +CPP, +Heat shock | 0 | 2 | 0 | 2 |
| +CPP | 2 | 2 | 0 | 4 |
| +Heat shock | 3 | 0 | 1 (3–4) | 4 |
| DNA only | 2 | 1 | 2 (4–4,4–5) | 5 |
Transformations of R. marina were carried out two times under four transformation conditions (with or without CPP peptide and heat shock treatment). Eight kanamycin resistant colonies found after 20‐days of incubation (long‐term incubation) shown in bold were examined for sequencing analysis of 16S rRNA. The number in parenthesis correspond to the 16S rRNA sequences in Figure 3d.
Figure A1.
Kanamycin resistance tests of marine purple photosynthetic bacteria. Four strains of marine purple photosynthetic bacteria (R. sulfidophilum, R. marina, R. visakhapatnamensis and R. goensis) cells were cultured in the absence or the presence of 100, 200, 300 and 400 mg/L of kanamycin
Figure A2.
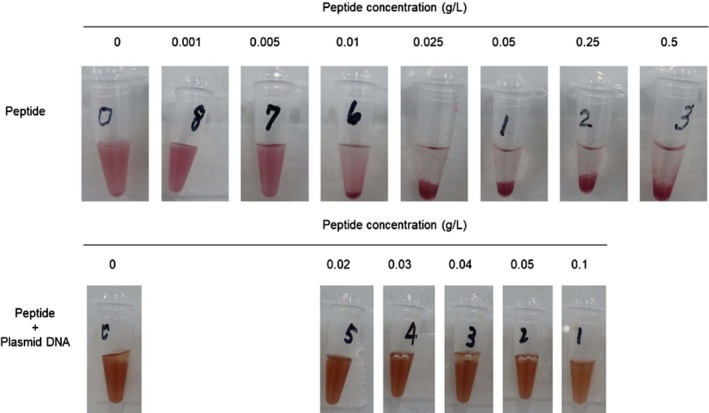
Cytotoxic effects of CPP peptide and peptide‐pDNA complex. Various concentrations of CPP peptide ((BP100)2K8) ranging from 0.001 to 0.5 g/L were added to R. sulfidophilum cell cultures and incubated for 30 min at room temperature. The 1 μg of plasmid DNA was mixed with different concentrations of CPP peptides (0, 0.02, 0.03, 0.04, 0.05 and 0.1 g/L) and incubated at room temperature for 10 min. The peptide‐pDNA complexes were added to R. sulfidophilum cell cultures and incubated for 30 min at room temperature
Higuchi‐Takeuchi M, Morisaki K, Numata K. Method for the facile transformation of marine purple photosynthetic bacteria using chemically competent cells. MicrobiologyOpen. 2020;9:e953 10.1002/mbo3.953
Contributor Information
Mieko Higuchi‐Takeuchi, Email: mieko.higuchi@riken.jp.
Keiji Numata, Email: keiji.numata@riken.jp.
DATA AVAILABILITY STATEMENT
All data are provided in full in the results section of this paper and available from the corresponding author on reasonable request.
REFERENCES
- Apirion, D. , & Schlessinger, D. (1968). Coresistance to neomycin and kanamycin by mutations in an Escherichia Coli locus that affects ribosomes. Journal of Bacteriology, 96(3), 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune, T. E. V. , & Aachmann, F. L. (2010). Methodologies to increase the transformation efficiencies and the range of bacteria that can be transformed. Applied Microbiology and Biotechnology, 85(5), 1301–1313. 10.1007/s00253-009-2349-1 [DOI] [PubMed] [Google Scholar]
- Badosa, E. , Ferre, R. , Planas, M. , Feliu, L. , Besalú, E. , Cabrefiga, J. , … Montesinos, E. (2007). A library of linear undecapeptides with bactericidal activity against phytopathogenic bacteria. Peptides, 28(12), 2276–2285. 10.1016/j.peptides.2007.09.010 [DOI] [PubMed] [Google Scholar]
- Dagert, M. , & Ehrlich, S. D. (1979a). Prolonged incubation in calcium‐chloride improves the competence of Escherichia‐coli‐cells. Gene, 6(1), 23–28. 10.1016/0378-1119(79)90082-9 [DOI] [PubMed] [Google Scholar]
- Dagert, M. , & Ehrlich, S. D. (1979b). Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene, 6(1), 23–28. 10.1016/0378-1119(79)90082-9 [DOI] [PubMed] [Google Scholar]
- Davison, J. , Heusterspreute, M. , Chevalier, N. , Hathi, V. , & Brunel, F. (1987). Vectors with restriction site banks.5. Pjrd215, a wide‐host‐range cosmid vector with multiple cloning sites. Gene, 51(2–3), 275–280. 10.1016/0378-1119(87)90316-7 [DOI] [PubMed] [Google Scholar]
- De Stasio, E. A. , Moazed, D. , Noller, H. F. , & Dahlberg, A. E. (1989). Mutations in 16S ribosomal RNA disrupt antibiotic–RNA interactions. EMBO Journal, 8(4), 1213–1216. 10.1002/j.1460-2075.1989.tb03494.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenberger, K. , Mink, C. , Wadhwani, P. , Ulrich, A. S. , & Nick, P. (2011). Using the peptide bp100 as a cell‐penetrating tool for the chemical engineering of actin filaments within living plant cells. ChemBioChem, 12(1), 132–137. 10.1002/cbic.201000402 [DOI] [PubMed] [Google Scholar]
- Foong, C. P. , Higuchi‐Takeuchi, M. , & Numata, K. (2019). Optimal iron concentrations for growth‐associated polyhydroxyalkanoate biosynthesis in the marine photosynthetic purple bacterium Rhodovulum sulfidophilum under photoheterotrophic condition. PLoS ONE, 14(4), e0212654 10.1371/journal.pone.0212654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornari, C. S. , & Kaplan, S. (1982). Genetic‐transformation of rhodopseudomonas‐sphaeroides by plasmid DNA. Journal of Bacteriology, 152(1), 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourmy, D. , Recht, M. I. , Blanchard, S. C. , & Puglisi, J. D. (1996). Structure of the a site of Escherichia coli 16s ribosomal RNA complexed with an aminoglycoside antibiotic. Science, 274(5291), 1367–1371. [DOI] [PubMed] [Google Scholar]
- Gallon, J. R. (2001). N2 fixation in phototrophs: Adaptation to a specialized way of life. Plant and Soil, 230(1), 39–48. 10.1023/A:1004640219659 [DOI] [Google Scholar]
- Garcia‐Contreras, R. , Celis, H. , & Romero, I. (2004). Importance of Rhodospirillum rubrum H+‐pyrophosphatase under low‐energy conditions. Journal of Bacteriology, 186(19), 6651–6655. 10.1128/JB.186.19.6651-6655.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D. (1983). Studies on transformation of Escherichia coli with plasmids. Journal of Molecular Biology, 166(4), 557–580. 10.1016/S0022-2836(83)80284-8 [DOI] [PubMed] [Google Scholar]
- Higuchi‐Takeuchi, M. , Morisaki, K. , Toyooka, K. , & Numata, K. (2016). Synthesis of high‐molecular‐weight polyhydroxyalkanoates by marine photosynthetic purple bacteria. PLoS ONE, 11(8), e0160981 10.1371/journal.pone.0160981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi‐Takeuchi, M. , & Numata, K. (2019). Acetate‐inducing metabolic states enhance polyhydroxyalkanoate production in marine purple non‐sulfur bacteria under aerobic conditions. Frontiers in Bioengineering and Biotechnology, 7, 118 10.3389/fbioe.2019.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, M. M. , Odahara, M. , Yoshizumi, T. , Oikawa, K. , Kimura, M. , Su'etsugu, M. , & Numata, K. (2019). Cell‐penetrating peptide‐mediated transformation of large plasmid DNA into Escherichia coli . ACS Synthetic Biology, 10.1021/acssynbio.9b00055 [DOI] [PubMed] [Google Scholar]
- Klebe, R. J. , Harriss, J. V. , Sharp, Z. D. , & Douglas, M. G. (1983). A general‐method for polyethylene‐glycol‐induced genetic‐transformation of bacteria and yeast. Gene, 25(2–3), 333–341. 10.1016/0378-1119(83)90238-X [DOI] [PubMed] [Google Scholar]
- Kovach, M. E. , Elzer, P. H. , Hill, D. S. , Robertson, G. T. , Farris, M. A. , Roop, R. M. 2nd , & Peterson, K. M. (1995). Four new derivatives of the broad‐host‐range cloning vector pBBR1MCS, carrying different antibiotic‐resistance cassettes. Gene, 166(1), 175–176. 10.1016/0378-1119(95)00584-1 [DOI] [PubMed] [Google Scholar]
- Lakshmanan, M. , Kodama, Y. , Yoshizumi, T. , Sudesh, K. , & Numata, K. (2013). Rapid and efficient gene delivery into plant cells using designed peptide carriers. Biomacromolecules, 14(1), 10–16. 10.1021/bm301275g [DOI] [PubMed] [Google Scholar]
- Lorenz, M. G. , & Wackernagel, W. (1994). Bacterial gene‐transfer by natural genetic‐transformation in the environment. Microbiological Reviews, 58(3), 563–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel, M. , & Higa, A. (1970). Calcium‐dependent bacteriophage DNA infection. Journal of Molecular Biology, 53(1), 159–162. 10.1016/0022-2836(70)90051-3 [DOI] [PubMed] [Google Scholar]
- McKinlay, J. B. , & Harwood, C. S. (2010). Photobiological production of hydrogen gas as a biofuel. Current Opinion in Biotechnology, 21(3), 244–251. 10.1016/j.copbio.2010.02.012 [DOI] [PubMed] [Google Scholar]
- Ng, K. K. , Motoda, Y. , Watanabe, S. , Sofiman Othman, A. , Kigawa, T. , Kodama, Y. , & Numata, K. (2016). Intracellular delivery of proteins via fusion peptides in intact plants. PLoS ONE, 11(4), e0154081 10.1371/journal.pone.0154081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata, K. , Horii, Y. , Oikawa, K. , Miyagi, Y. , Demura, T. , & Ohtani, M. (2018). Library screening of cell‐penetrating peptide for BY‐2 cells, leaves of Arabidopsis, tobacco, tomato, poplar, and rice callus. Scientific Reports, 8(1), 10966 10.1038/s41598-018-29298-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata, K. , Ohtani, M. , Yoshizumi, T. , Demura, T. , & Kodama, Y. (2014). Local gene silencing in plants via synthetic dsRNA and carrier peptide. Plant Biotechnology Journal, 12(8), 1027–1034. 10.1111/pbi.12208 [DOI] [PubMed] [Google Scholar]
- Oikawa, K. , Islam, M. M. , Horii, Y. , Yoshizumi, T. , & Numata, K. (2018). Screening of a cell‐penetrating peptide library in Escherichia coli: Relationship between cell penetration efficiency and cytotoxicity. Acs Omega, 3(12), 16489–16499. 10.1021/acsomega.8b02348 [DOI] [Google Scholar]
- Pinta, V. , Picaud, M. , Reiss‐Husson, F. , & Astier, C. (2002). Rubrivivax gelatinosus acsF (previously orf358) codes for a conserved, putative binuclear‐iron‐cluster‐containing protein involved in aerobic oxidative cyclization of Mg‐protoporphyrin IX monomethylester. Journal of Bacteriology, 184(3), 746–753. 10.1128/JB.184.3.746-753.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, R. , Panangala, V. S. , Wood, R. R. , & Klesius, P. H. (2009). Chemical and electroporated transformation of Edwardsiella ictaluri using three different plasmids. Fems Microbiology Letters, 298(1), 105–110. 10.1111/j.1574-6968.2009.01702.x [DOI] [PubMed] [Google Scholar]
- Stewart Jr, C. N. , & Via, L. E. (1993). A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. BioTechniques, 14(5), 748. [PubMed] [Google Scholar]
- Suzuki, Y. , Katsukawa, C. , Tamaru, A. , Abe, C. , Makino, M. , Mizuguchi, Y. , & Taniguchi, H. (1998). Detection of kanamycin‐resistant Mycobacterium tuberculosis by identifying mutations in the 16S rRNA gene. Journal of Clinical Microbiology, 36(5), 1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston, A. , Brown, M. G. , Perkins, H. R. , Saunders, J. R. , & Humphreys, G. O. (1981). Transformation of Escherichia coli with plasmid deoxyribonucleic acid: Calcium‐induced binding of deoxyribonucleic acid to whole cells and to isolated membrane fractions. Journal of Bacteriology, 145(2), 780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are provided in full in the results section of this paper and available from the corresponding author on reasonable request.


