Abstract
Veno-occlusive disease (VOD), also called sinusoidal obstruction syndrome (SOS), is a potentially life-threatening complication of hematopoietic stem cell transplantation (HSCT) conditioning or high-dose nontransplant chemotherapy. VOD/SOS with multi-organ dysfunction (MOD) is associated with a mortality rate of > 80%. Defibrotide (25 mg/kg/day) is approved to treat hepatic VOD/SOS with renal or pulmonary dysfunction post HSCT in the United States and to treat severe hepatic VOD/SOS in patients > 1 month of age in the European Union. A random effects model was used for pooling data from 17 systematically chosen defibrotide studies. For patients in these reports (n = 2598), and those in the subset of 10 reports of patients treated with ~ 25 mg/kg/day (n = 1691), estimated Day + 100 survival rates were 54% and 56%, respectively. Among those patients treated with ~ 25 mg/kg/day, estimated Day + 100 survival was 44% among patients with MOD and 71% in patients without MOD; survival was 41% and 70%, respectively, for the population of patients receiving any dose of defibrotide. Safety results were not pooled owing to differences in reporting methodology but were generally consistent with the known tolerability profile of defibrotide. This analysis provides the largest assessment of survival in patients treated with defibrotide for VOD/SOS with or without MOD.
Subject terms: Drug therapy, Cancer
Introduction
Hepatic veno-occlusive disease (VOD), also called sinusoidal obstruction syndrome (SOS), is a potentially life-threatening complication of conditioning regimens for hematopoietic stem cell transplantation (HSCT) and also of chemotherapy alone [1–4]. Risk factors may be related to transplant (eg, the toxicity of chemotherapy or the conditioning regimen, allogeneic vs autologous transplant, immunosuppressive regimen), patient characteristics (eg, age, underlying disease, genetic predisposition), and health status of the liver (eg, immature liver function in infants, iron overload, liver fibrosis, hepatitis) [5, 6].
The pathogenesis of VOD/SOS involves multiple thrombotic and inflammatory factors that initially trigger damage to the endothelial cells lining the sinusoids of the hepatic acinus. Damaged endothelial cells may show cytopathic effects by rounding up, forming gaps in the sinusoidal barrier that allow passage of erythrocytes, leukocytes, and cellular debris into the space of Disse. As the venous lumen narrows and reduces the effluent from the sinus, post-sinusoidal portal hypertension occurs and can progress to the clinical symptoms of VOD/SOS [5–8].
Diagnosis of VOD/SOS has historically been based on clinical examination by either Baltimore criteria (bilirubin ≥ 2 mg/dL plus 2 or more of hepatomegaly, ascites, or ≥ 5% weight gain by Day 21 post HSCT) [9] or modified Seattle criteria (two or more of the following: bilirubin > 2 mg/dL, hepatomegaly or right upper quadrant pain, 2% weight gain [sometimes revised as 5% by Day 20 post HSCT [10]]) [11]. However, those criteria lack sensitivity and specificity, making early identification of VOD/SOS difficult. In addition, particularities in the presentation of VOD/SOD in children are not reflected in these diagnostic criteria. More recently, the European Society for Blood and Marrow transplantation (EBMT) has proposed new diagnostic guidelines and prospective severity grading criteria for adults and for children [7, 8]. The adult diagnostic criteria from EBMT encompass classical VOD/SOS as defined by Baltimore criteria but also include late-onset VOD/SOS (VOD/SOS developing after 21 days post HSCT). The new pediatric diagnostic criteria from EBMT include differences from the traditional criteria, such as having no defined timeframe of onset and the presence of unexplained consumptive/transfusion-refractory thrombocytopenia, otherwise unexplained weight gain for 3 consecutive days despite diuretic use, and rising bilirubin from baseline value on 3 consecutive days or bilirubin ≥ 2 mg/dL within 72 h. These new diagnostic guidelines are designed to support earlier diagnosis and treatment with greater specificity, and to highlight substantial differences in presentation between adult and pediatric patients (eg, anicteric presentation in ~ 30% of children which may be less common in adults presenting by Day 21 post HSCT) [7, 8].
VOD/SOS develops in ~ 10–15% of adult patients who receive myeloablative conditioning followed by allogeneic HSCT [1, 5, 12, 13]. In patients receiving autologous HSCT or reduced intensity conditioning with allogeneic HSCT, incidence may be ~ 5% [14], although a rate of 8.8% post–reduced intensity conditioning was reported in the past few years by one center [15]. Overall incidence in pediatric patients post HSCT has been reported between 22 and 30%, and in high-risk pediatric patients may increase to 60% [8]. The incidence in pediatric patients of VOD/SOS post-autologous HSCT for neuroblastoma is ~ 30%, likely owing to a busulfan–melphalan myeloablative conditioning [8].
VOD/SOS with multi-organ dysfunction (MOD; typically defined by renal and/or pulmonary dysfunction and sometimes referred to as multi-organ failure) may be associated with survival of 20–30% in HSCT patients receiving supportive care alone [1, 3, 4].
Owing to the progressive pathophysiology of VOD/SOS and the high mortality associated with VOD/SOS and MOD, the EBMT recommends that early diagnosis and treatment of VOD/SOS should be a priority, and they note that the “only proven” treatment is defibrotide [5]. For adult and pediatric patients, defibrotide (25 mg/kg/day intravenously in four divided doses) is approved to treat hepatic VOD/SOS with renal or pulmonary dysfunction post HSCT in the United States [16], and to treat severe hepatic VOD/SOS post HSCT in patients over 1 month of age in the European Union [17]. Defibrotide’s mechanism of action, as elucidated in preclinical studies, centers on protection of endothelial cells and anti-inflammatory effects, which together help restore thrombo-fibrinolytic balance [18–23].
To provide an estimate of overall survival in patients with VOD/SOS treated with defibrotide, we pooled systematically collected Day + 100 survival analysis data from published studies on the use of defibrotide to treat patients with VOD/SOS, post HSCT or post-nontransplant chemotherapy, with or without MOD.
Materials and methods
Search criteria
A systematic review of Medline and Medline In-Process for journal articles, and Embase for journal articles and conference abstracts, until July 10, 2017, was performed per a prespecified and clearly defined protocol based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Owing to the lag time of 3–6 months for conference abstracts in Embase, abstracts from the more recent 2017 EBMT and European Hematology Association meetings were also searched using the conference websites. The search term for all databases was “defibrotide” in the title or abstract. Duplicate results from these searches were removed.
Criteria for study selection
Prospective and retrospective studies of defibrotide for treatment of VOD/SOS post HSCT or post-nontransplant chemotherapy were selected for inclusion. Excluded were reviews, prophylaxis or prevention studies, post hoc analyses, nonclinical studies, letters, clinical studies without primary efficacy data, no defibrotide treatment, and use in patients without VOD/SOS. Results from the initial keyword literature searches were screened, and full-length text for each report was evaluated for eligibility.
Data collection
Full versions of the selected studies were assessed to determine study design, sample size, dose, treatment duration, patient characteristics (age, post-transplant or post-chemotherapy onset, and underlying disease), comparator(s), Day + 100 survival, and safety outcomes. When necessary, subgroup data were sourced from clinical study reports. For case series reporting patient-level data, the overall efficacy outcomes were estimated. Where reported, patients were divided into subgroups to analyze data for those with and without MOD, and for adult and pediatric patients.
Biostatistical analysis
A random effects model was used for pooling data for survival. Interstudy heterogeneity was assessed with Cochran’s Q-test. The percentage of total variation across studies owing to heterogeneity was evaluated by the I2 measure. Owing to differences in defining and reporting adverse events (AEs) in the individual studies, pooling the data may have been misleading; therefore, safety was evaluated qualitatively. Pooled survival was estimated using StataSE software (StataCorp, College Station, TX).
Results
The literature search based on the keyword “defibrotide” identified a total of 606 publications, which included 367 PubMed records, 225 Embase records, and 14 abstracts from the EBMT and European Hematology Association conferences (Fig. 1). Duplicates from these searches were removed.
Fig. 1.
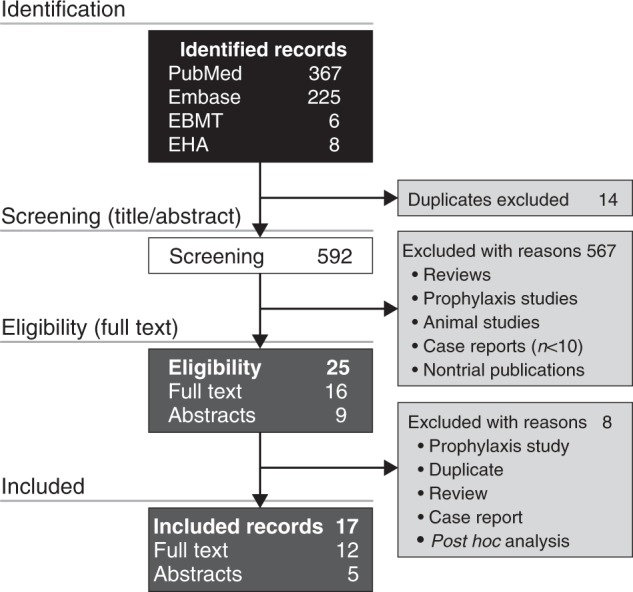
Study selection
After screening titles and abstracts for exclusion criteria, the remaining 25 complete records (16 full-text articles and nine conference abstracts) were analyzed and further refined by excluding eight reports that were post hoc analyses, reviews, duplicates, case reports, or a prevention trial. The 17 records chosen for pooled analysis were 12 full-length articles [3, 4, 24–33] and five abstracts [34–38] (Table 1). The study quality and types included retrospective case studies, single-center studies, registry reports, prospective multicenter compassionate use and treatment IND studies, and prospective multicenter two-arm phase 2 and 3 studies (Table 1). Patient ages ranged from 0.1 to 77 years, with median ages of 8.2–60.5 years. The combined studies included 2598 VOD/SOS patients treated with defibrotide, 1260 of whom had MOD (the precise definition of MOD varied among the studies). Most patients with VOD/SOS had received HSCT, and the most common primary diseases were acute leukemias [3, 4, 24, 26–33, 35, 37, 38] (Table 1). Defibrotide doses ranged from 5 mg/kg/day to 110 mg/kg/day, and the duration of defibrotide treatment ranged from 1–139 days with median duration ranging from 14–21.5 days (Table 2). Ten of the 17 reports included patients treated with approximately the approved 25 mg/kg/day defibrotide dose (n = 1691) [3, 25–27, 30, 34–38]. Seven of the 17 reports included other dosages, or the dosage was not reported [4, 24, 28, 29, 31–33].
Table 1.
Summary of report characteristics, patient demographics, transplant type, and disease parameters
| Study quality factors | Defibrotide-treated | Age | Transplant | Common underlying diseases | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Prospective? | Type | Control | Total | MOD | No MOD | Median | ≤ 16 years | > 16 years | Auto | Allo | ALL | AML | Other | |
| Richardson 1998 [33] | Yes | Single center | No | 19 | 19 | 0 | 40 | 3 | 16 | 11 | 8 | 1 | 3 | 15 | |
| Chopra 2000 [31] | Yes | CU | No | 40 | 26 | 14 | 30 | 11 | 29 | 14 | 26 | 6 | 14 | 20 | |
| Richardson 2002 [28] | Yes | CU | No | 88 | 88 | 0 | 35 | 29a | 59a | 28 | 60 | 6 | 22 | 60 | |
| Corbacioglu 2004 [29] | No | CU | No | 45 | 22 | 23 | 8.2 | 40 | 5 | 8 | 37 | 6 | 10 | 29 | |
| Bulley 2007 [32] | No | Single center | No | 14 | NR | NR | 10.2 | 14b | 0b | 0 | 14 | 3 | 1 | 10 | |
| Sucak 2007 [26] | No | Single center | No | 14 | 6 | 8 | 40.5 | 0b | 14b | 1 | 13 | 4 | 5 | 5 | |
| Richardson 2010 [27] | Yes | Phase 2, dose finding | No | 149 | 149 | 0 | 34 | 48a | 101a | 20 | 129 | 15 | 47 | 87 | |
| Ruiz Ramos 2014 [34] | No | Observational | No | 11 | NR | NR | NR | 4 | 5 | NR | NR | NR | NR | NR | |
| Locatelli 2015 [35] | Yes | CU | No | 98 | 17 | 77 | 13.4 | 52a | 42a | 10 | 75 | 18 | 23 | 57 | |
| Triplett 2015 [24] | Yes | Single center | No | 34 | 22 | 12 | 8.9 | 31a | 3a | 2 | 29 | 10 | 10 | 14 | |
| Balade Martinez 2016 [36] | No | Observational | No | 42 | 42 | 0 | 46 | 12 | 30 | NR | NR | NR | NR | NR | |
| Corbacioglu 2016 [30] | Yes | CU | No | 710 | 261 | 348 | 25 | 303a | 407a | 112 | 499 | 120 | 177 | 413 | |
| Pol 2016 [25] | No | Single center | No | 13 | 12 | 1 | 60.5 | 0 | 13 | 0 | 13 | 0 | 7 | 6 | |
| Richardson 2016 [3] | Yes | Phase 3 | Historical | 102 | 102 | 0 | 21 | 44 | 58 | 12 | 90 | 17 | 29 | 56 | |
| Strouse 2016 [4] | No | Registry | Not treated | 41 | 41 | 0 | 11 | 25 (61) | 16 (39) | 2 (5) | 39 (95) | 19c | 22 | ||
| Yakushijin 2016 [37] | No | Registry | Thrombo-modulin | 24 | NR | NR | 40 | NR | NR | 0 | 65 | NR | NR | NR | |
| Richardson 2017 (T-IND) [38] | Yes | T-IND | No | 1154 | 571 | NR | 12 | 691 | 463 | 155 | 843 | 279 | 279 | 596 | |
ALL, acute lymphocytic leukemia; Allo, allogeneic; AML, acute myelogenous leukemia; Auto, autologous; CU, compassionate use; MOD, multi-organ dysfunction; NR, not reported; T-IND, defibrotide expanded access program
aPediatric defined as ≤ 18 years and adults as > 18 years
bInclusive of age 16 years
cAcute leukemias
Table 2.
Compilation of defibrotide treatment parameters
| Reference | Total treated patients, n | Patients treated with ~ 25 mg/kg/d defibrotide, n | Median duration, days (range) | Defibrotide start time from VOD/SOS diagnosis | Mean dosage (mg/kg/d) | Median dosage (mg/kg/d) | Dosage range (mg/kg/d) |
|---|---|---|---|---|---|---|---|
| Richardson 1998 [33] | 19 | NR | NR | Median 6 days (range, 0–47 days) | NR | NR | 5–60 |
| Chopra 2000 [31] | 40 | NR | 18 (2–71) | NR | NR | NR | 10–40 |
| Richardson 2002 [28] | 88 | NR | 15 (1–139) | Median 3 days (range, 0–46 days) | NR | NR | 5–60 |
| Corbacioglu 2004 [29] | 45 | NR | 17 (1–83) | Median 1 day (range, 0–12 days) | 45 in the CR group; 27 in the no responder group | 40 | 10–110 |
| Bulley 2007 [32] | 14 | NR | 16 (4–37) | Median 1 day (range, 0–33 days) | 25 (starting dosage) | 33–38.5 | 11–81 |
| Sucak 2007 [26] | 14 | 14 | 21.5 (4–39) | As soon as possible | NR | NR | 10–25 |
| Richardson 2010 [27] | 149 | 75 | 19 (2–82)a | Day of randomization (n = 119 [80%]) | 25a | 25a | 25, 40 |
| Ruiz Ramos 2014 [34] | 11 | 11 | 9 (5–25) | NR | NR | 25 | 25–40 |
| Locatelli 2015 [35] | 98 | 94 | 14 (1–84) | NR | NR | 25 | 6.15–40.0 |
| Triplett 2015 [24] | 34 | NR | 15 (1–102) | Median 0 days | NR | 60 | 6.25–110 |
| Balade Martinez 2016 [36] | 42 | 42 | 11 (1–40) | NR | 25 | NR | 10–45 |
| Corbacioglu 2016 [30] | 710 | 227 | 15 (1–119) | NR | NR | 25 | 10–80 |
| Pol 2016 [25] | 13 | 13 | 14 (6–21) | Within 24 h | 25 | 25 | 25 |
| Richardson 2016 [3] | 102b | 102b | 21.5 (1–58) | NR | 25 | 25 | 25 |
| Strouse 2016 [4] | 41 | NR | NR | NR | NR | NR | NR |
| Yakushijin 2016 [37] | 24 | 24 | 15 (1–46) | NR | NR | 24c | 7–80c |
| Richardson 2017 (T-IND) [38] | 1154 | 1089 | 21 | NR | 25 | 25 | 25 |
CI, confidence interval; CR, complete responder; NR, not reported; VOD/SOS, veno-occlusive disease/sinusoidal obstruction syndrome
aAmong the 75 patients who received defibrotide 25 mg/kg/d (treatment arm A)
bAmong the 102 patients who received defibrotide 25 mg/kg/d in the active treatment group (not including the 32 historical controls)
cMedian dose or dose range (mg/kg)
Efficacy
The estimated Day + 100 survival rate for 2598 patients receiving defibrotide at any dose across all studies [3, 4, 24–38] was 54% (Fig. 2a), and a 56% rate was shown among the 1691 patients in 10 reports of treatment at ~25 mg/kg/day (Fig. 2b) [3, 25–27, 30, 34–38].
Fig. 2.
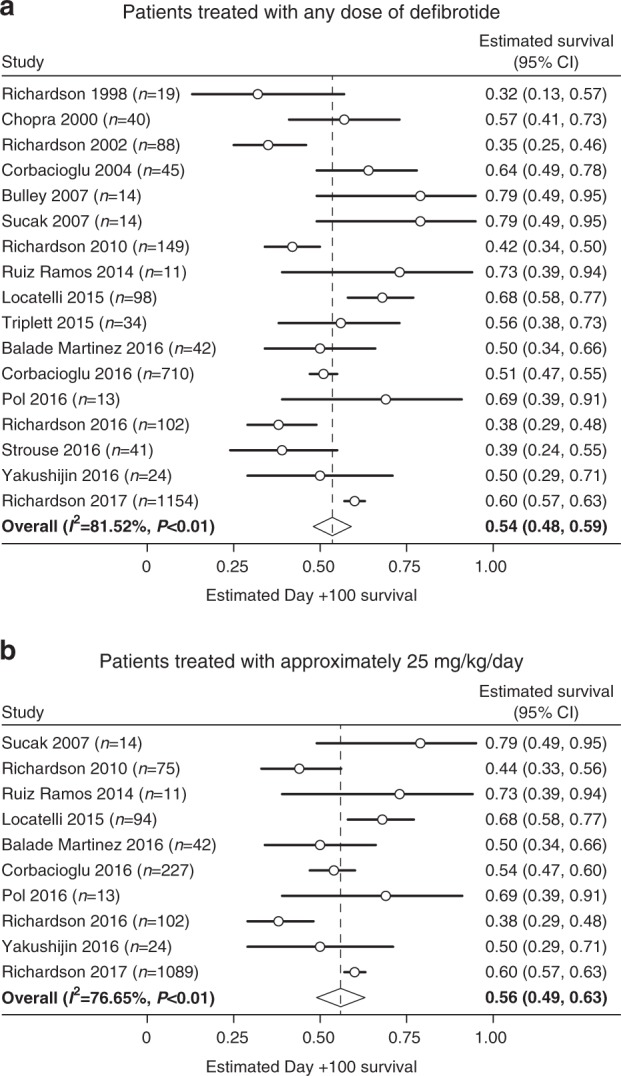
Pooled analysis of the estimated Day + 100 survival rates of the overall patient populations treated with any defibrotide dose or ~ 25 mg/kg/day
Pooled subgroup results showed that patients with MOD (n = 1260) who received any of the defibrotide doses [3, 27, 28, 30, 31, 33, 35, 36, 38] had an estimated Day + 100 survival rate of 41% (Fig. 3a), and a 44% rate was shown in the ~ 25 mg/kg/day subgroup (n = 792; Fig. 3b) [3, 27, 35, 36, 38].
Fig. 3.
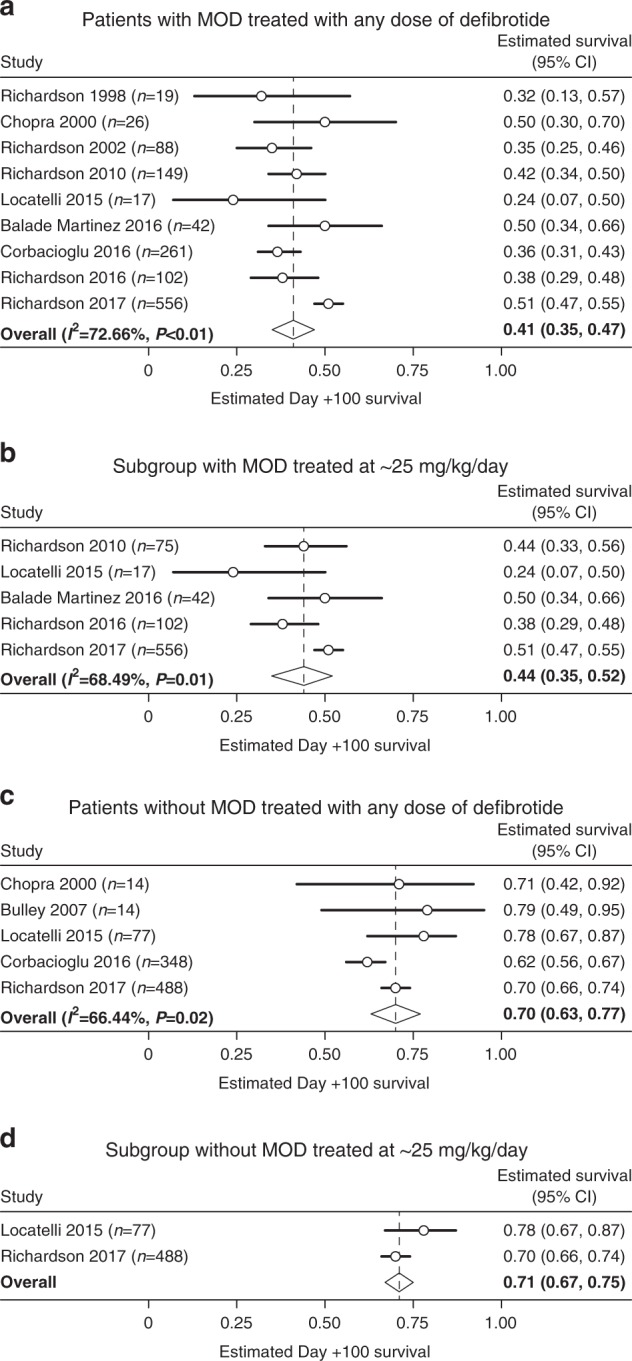
Estimated Day + 100 survival for patients with MOD and without MOD
Among the subgroup of patients without MOD (n = 941) receiving defibrotide at any dose [30–32, 35, 38], estimated Day + 100 survival was 70% for those receiving any dose (Fig. 3c), and 71% in those receiving ~ 25 mg/kg/day (n = 565; Fig. 3d) [35, 38].
The pediatric subgroup was defined as patients aged ≤ 16 years in three studies [3, 4, 38] and ≤ 18 years in five studies [27, 28, 30, 31, 35]. Pediatric patients with VOD/SOS, regardless of MOD status and dose (n = 1036) [3, 4, 27, 28, 30, 31, 35, 38] had an estimated Day + 100 survival rate of 60% (Fig. 4a), whereas the subgroup that received ~ 25 mg/kg/day dose (n = 792) [3, 27, 30, 35, 38] had a 68% estimated Day + 100 survival rate (Fig. 4b). Three of the 25 mg/kg/day studies included patients with MOD, with pooled Day + 100 survival of 58% (95% CI: 51–66%) [3, 27, 38].
Fig. 4.
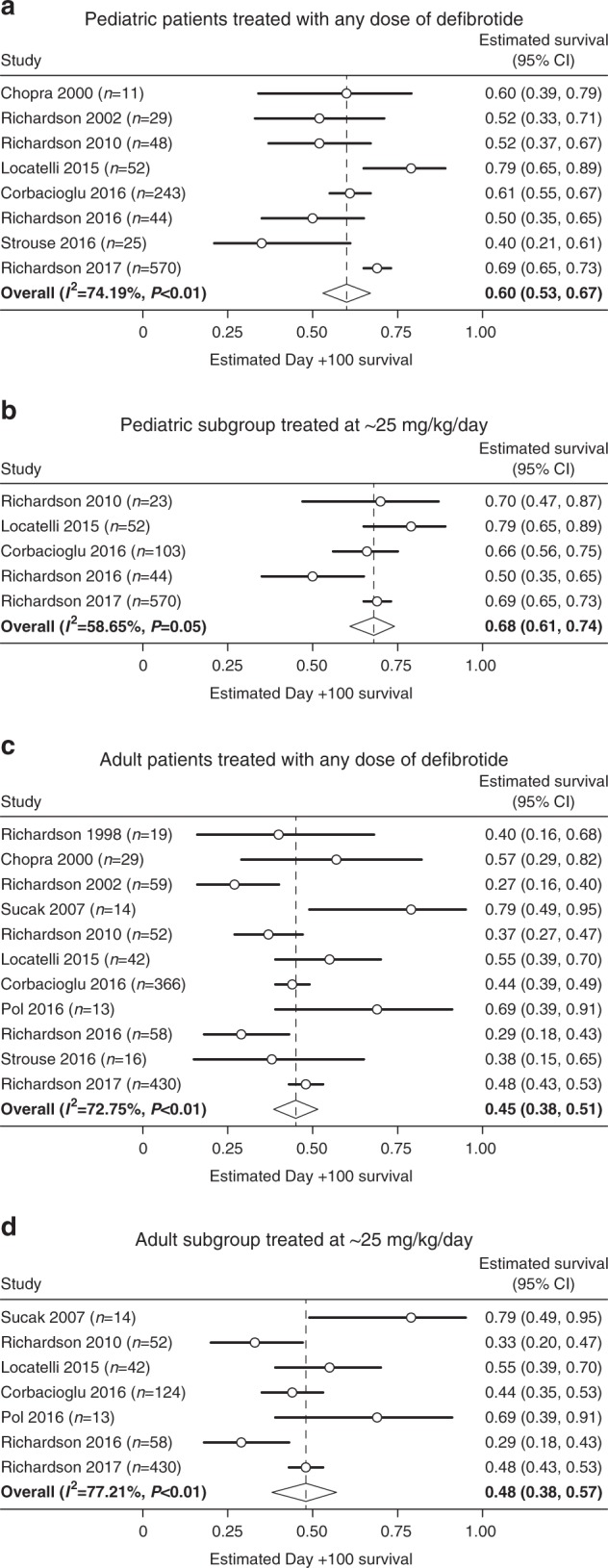
Estimated Day + 100 survival rates in pediatric and adult subgroups
Adults were defined as patients aged > 16 years in six studies [3, 4, 25, 26, 35, 38] and as > 18 years in five studies [27, 28, 30, 31, 33]. The adult subgroup, regardless of MOD status and dose (n = 1128) [3, 4, 25–28, 31, 33, 35, 38, 39] had an estimated Day + 100 survival rate of 45% (Fig. 4c), whereas the subgroup that received the defibrotide dose of ~ 25 mg/kg/day (n = 773) [3, 25–27, 30, 35, 38] had an estimated Day + 100 survival rate of 48% (Fig. 4d). Three of the 25 mg/kg/day studies included patients with MOD, with pooled Day + 100 survival of 36% (95% CI: 29–42%) [3, 27, 38].
Safety results for the included reports are summarized in Table 3. Safety results were not pooled for these studies owing to differences in safety reporting methodology; however, the results of individual studies were generally consistent with the previously reported safety profiles, such as in the phase 3 historically controlled trial in VOD/SOS patients with MOD [3]. That trial reported that 101/102 defibrotide-treated patients and all 32 historical control patients experienced ≥ 1 adverse event (AE). Hypotension was the most frequent AE (39% for defibrotide, 50% for controls), and common hemorrhagic AEs, which included pulmonary alveolar and gastrointestinal hemorrhage, occurred in 64% of defibrotide-treated patients and 75% of controls. Treatment-related AEs in the defibrotide arm included hemorrhagic events and hypotension [3].
Table 3.
Adverse event summaries extracted from the published articles
| Reference | Total treated patients | Adverse events | Treatment-related AEs |
|---|---|---|---|
| Richardson 1998 [33] | 19 | Grade 1/2 transient mild systolic hypotension (n = 5 [26%]) and grade 3/4 hypotension (n = 2 [11%]); but causal relationship not reported | None caused defibrotide discontinuation. No severe treatment-related hemorrhage was reported |
| Chopra 2000 [31] | 40 | Not reported | Not reported |
| Richardson 2002 [28] | 88 | No worsening of clinical bleeding was seen. No other grade 3/4 toxicity related to DF was reported. Grade 1/2 AEs included transient mild systolic hypotension (treatment attribution not reported) | No grade 3/4 treatment-related AEs were reported |
| Corbacioglu 2004 [29] | 45 | Not reported | 1 patient discontinued DF owing to diarrhea. Mild (grades 0–2) coagulation abnormalities (n = 16 [36%]); none led to discontinuation |
| Bulley 2007 [32] | 14 | GI bleed (n = 2 [14%]; 1 patient had GI bleed 1 day prior to initiation of defibrotide) and intracranial bleed (n = 1 [7%]; likely owing to disseminated fungal infection) | Not reported |
| Sucak 2007 [26] | 14 | Mild-to-moderate AEs included abdominal pain (n = 4 [28.6%]) and mild mucosal bleeding (n = 5 [35.7%]); none led to discontinuation | No significant drug-related toxicities. As patients were thrombocytopenic, it was not clear whether mild mucosal bleeding events were related to defibrotide) |
| Richardson 2010 [27] | 149 | 71/75 (95%; treatment arm A [25 mg/kg/d defibrotide]) and 73/74 (99%; treatment arm B [40 mg/kg/d defibrotide]) patients reported ≥ 1 treatment-emergent AE | Grades 3–5 AEs were in arm A (5/75, 7%) and arm B patients (7/74, 10%). Discontinuations from TRAE were owing to hypotension in 1 patient in each arm ( ≤ 1.4%); 1 grade 3–5 bleeding event in arm B; and alveolar bleeding (n = 1 [1.4%] in arm A) and GI and pulmonary bleedings (n = 1 [1.3%] each in arm B) |
| Ruiz Ramos 2014 [34] | 11 | 3 (27.3%) patients with hemorrhagic episodes (2 GI and 1 nasal) | Not reported |
| Locatelli 2015 [35] | 98 | 21 (21%) patients with ≥ 1 treatment-emergent AE; most common ( ≥ 2 patients) were MOD (9%), VOD/SOS (6%), and CMV infection and acute respiratory distress syndrome (2% each) | 5 (5.3%) patients had hemorrhagic events. 1 patient each had hemorrhagic cystitis, urinary tract hemorrhage, hemorrhages (unspecified), hemorrhagic diathesis, and pulmonary hemorrhage |
| Triplett 2015 [24] | 34 | Patients receiving ≤ 100 mg/kg/day had average of 3 bleeding episodes/100 days; 13.2 bleeding episodes/100 days in patients receiving > 100 mg/kg/d | Not reported |
| Balade Martinez 2016 [36] | 42 | Not reported | Not reported |
| Corbacioglu 2016 [30] | 710 | 53% of patients reported an AE. The most common AEs ( ≥ 4%) included new or worsening MOD, progression of hepatic VOD/SOS, sepsis, and GVHD | The relationship to defibrotide was not available for the majority of AEs |
| Pol 2016 [25] | 13 | Not reported | Not reported |
| Richardson 2016 [3] | 102 | 101/102 (99%; DF treatment group) and 32/32 (100%; historical controls) patients experienced ≥ 1 AE. Incidence of common hemorrhagic AEs (pulmonary alveolar [11.8% and 15.6%] and GI [7.8% and 9.4%]) was similar between defibrotide and control groups, respectively | 11 (10.7%) patients discontinued defibrotide owing to a possible drug-related toxicity |
| Strouse 2016 [4] | 41 | Not reported | Not reported |
| Yakushijin 2016 [37] | 24 | Not reported | 1 (4%) patient experienced 2 treatment-related AEs (GI and pulmonary bleeding) |
| Richardson 2017 (T-IND) [38] | 1154 | 810 (70.2%) patients reported ≥ 1 treatment-emergent AE | 248 (21.5%) patients; led to discontinuation in 12% and death in 2.7% (pulmonary hemorrhage, 1.0%, was most common). Hypotension in 2.1% patients, and hemorrhagic events in pulmonary (4.3%), GI (3.0%), and epistaxis (2.3%) |
AEs, adverse events; ALL, acute lymphocytic leukemia; CMV, cytomegalovirus; DF, defibrotide; GI, gastrointestinal; GVHD, graft-vs-host disease; MOD, multi-organ dysfunction; T-IND, defibrotide expanded access program; TRAE, treatment-related adverse event; VOD/SOS, veno-occlusive disease/sinusoidal obstruction syndrome
Discussion
This systematic, pooled analysis of currently available evidence for defibrotide efficacy in the treatment of patients with VOD/SOS included 17 studies, representing 2598 patients. Estimated Day + 100 survival rates in the 17 studies ranged from 35 to 79%, and the pooled survival rate at Day + 100 was 54%. The approved 25 mg/kg/day dose was administered to patients in 10 of 17 studies (n = 1691), and its estimated survival rate at Day + 100 was 56%. This pooled analysis further supports the efficacy found for the 25 mg/kg/day dose of defibrotide in the phase 3 study [3] that was approved by regulatory authorities.
Patients with VOD/SOS and MOD, regardless of treatment dose, had lower estimated survival rates at Day + 100 than those without MOD: estimated survival rates for patients treated with any defibrotide dose or treated with the ~ 25 mg/kg/day defibrotide dose were 41% and 44%, respectively. As points of comparison, Day + 100 survival in the historical control population (rigorously selected owing to ethical concerns regarding withholding a supposed beneficial treatment from very sick patients with a dismal prognosis) in the phase 3 study was 25% [3], and in prior reports, patients with MOD who did not receive defibrotide were shown to have Day + 100 survival results of 30.9% [4] and 15.7% [1]. Conversely, the estimated survival rates at Day + 100 for patients without MOD were higher than those for the overall population: 70% for patients treated with any dose and 71% for those treated with ~ 25 mg/kg/day. Of note, the definition of MOD varied among studies, and the large sponsored studies used a standard that represented the most severe forms (renal dysfunction typically defined by creatinine ≥ 3 × level at time of transplant or creatinine clearance/glomerular filtration rate ≤ 40% of baseline, or dialysis dependence; pulmonary dysfunction typically defined by oxygen saturation ≤ 90% on room air or need for supplemental oxygen/ventilator dependence [3, 38]).
Estimated survival rates at Day + 100 for pediatric patients with and without MOD, treated with any defibrotide dose or treated with the approximately 25 mg/kg/day defibrotide dose were 60% and 68%, respectively, with 58% survival in the MOD subgroup receiving 25 mg/kg/day; in the T-IND study, survival at Day + 100 for pediatric patients with MOD was 58.1% [40]. In comparison, the US registry included in the pooled analysis also reported Day + 100 survival among patients not receiving defibrotide to be 45.5% among pediatric patients with MOD [4].
Overall, estimated Day + 100 survival rates for adults were 45% for those who received any dose and 48% for those receiving ~25 mg/kg/day, with 36% survival in the MOD subgroup receiving 25 mg/kg/day; in the T-IND study, survival at Day + 100 for adults with MOD was 39.0% [41]. In comparison, a Japanese registry of primarily adult patients (84.2% aged ≥ 16 years), 95% of whom did not receive defibrotide, had a Day + 100 survival rate of 32% in patients with and without MOD, and a rate of 15% in the MOD subgroup [13]. In the US registry, Day + 100 survival was 27.3% among adults with MOD not receiving defibrotide [4].
The AE reports for the 17 studies could not be pooled across studies owing to distinct reporting schemes. However, the overall AE profiles in the 17 studies were similar to those reported in the defibrotide phase 3 study [3]. In that study, AEs assessed by investigators as at least possibly related to defibrotide included hemorrhagic events and hypotension. Of note, however, overall rates of hypotension and hemorrhage (regardless of relatedness) were similar between arms. Hypotension was the most frequently reported AE (39% for defibrotide, 50% for controls), and hemorrhagic AEs, which included pulmonary alveolar hemorrhage (11.8% vs 15.6%, respectively) and gastrointestinal hemorrhage (7.8% vs 9.4%, respectively), occurred in 64% of defibrotide-treated patients and 75% of controls [3].
Safety results from the two large single-arm studies, both of which included patients without MOD, found no novel AEs [30, 38]. Final results from the T-IND showed an AE rate of 70.2%, with serious AEs reported in 51.8%, whereas AEs considered treatment-related were most commonly hemorrhage (pulmonary, 4.3%; gastrointestinal, 3.0%; epistaxis, 2.3%) and hypotension (2.0%); serious treatment-related AEs occurred in 11.5% of patients [38]. In the compassionate use program, data reporting was not required owing to the nature of the study [30]. AEs were reported in 53% of patients, and causes of death (frequently reported as AEs) were primarily owing to progressive VOD/SOS with MOD.
A key strength of this analysis is that it represents the largest, most comprehensive review of Day + 100 survival in patients with VOD/SOS who were treated with defibrotide. In most cases, however, patient-level data were not available to control for heterogeneity between studies, including differences in baseline characteristics, such as severity of MOD (eg, reduced pulmonary/renal function vs ventilator/dialysis dependence), which represents a limitation in that it was not possible to retrospectively apply the new severity criteria proposed by EBMT or to pool safety data. Another consideration in interpretation of these results is that the largest reports were from single-arm studies designed to provide access to defibrotide [30, 38]; however, the estimated Day + 100 survival results are comparable to those of the phase 3 historically controlled trial (in patients with MOD only, with a propensity-adjusted number-needed-to-treat of five to prevent one death) [3, 42, 43], and the safety profile in the phase 3 study helps illustrate the range of AEs associated with VOD/SOS and MOD irrespective of treatment with defibrotide.
The results in the patients without MOD may be supportive of treatment earlier in the pathophysiologic cascade of VOD/SOS. An exploratory analysis from the T-IND on the impact of timing of initiation with defibrotide on outcome found that earlier treatment initiation was associated with improved survival [44], which is consistent with what is known about the pathophysiologic cascade of VOD/SOS progression and the treatment recommendations from the EBMT [5]. Although mortality of VOD/SOS without prospectively identified MOD has not been well studied, a Japanese registry reported that VOD/SOS without MOD was associated with Day + 100 mortality > 50% [13]. Indeed, prevention of development of both MOD and VOD/SOS itself are important areas for research. At present, no medications are approved for the prevention of VOD/SOS, although defibrotide has been investigated in several studies [45–47] including a phase 3 study in pediatric patients, which suggest that defibrotide may reduce the risk for development of VOD/SOS compared with supportive care only (12% vs 20% incidence, respectively; P = 0.0488, competing risk analysis, P = 0.0507 log rank test) [48]. Hemorrhage was the AE most commonly attributed by investigators to defibrotide; however, the incidence was similar between groups: 22% in the defibrotide arm and 21% in the control arm [49]. The HARMONY clinical trial (NCT02851407) to compare efficacy and safety of defibrotide versus best supportive care in the prevention of VOD/SOS in pediatric and adult patients is continuing to recruit patients [49]. Also, awareness of the importance of early intervention for improved outcomes may lead to a shift in the application of diagnostic criteria, from emphasis on the more exclusionary Baltimore and Seattle criteria to the more age-specific fit of the EBMT criteria. Finally, early use of magnetic resonance imaging for evaluating iron overload and/or ultrasound imaging to confirm such clinical criteria as ascites or hepatomegaly for VOD/SOS diagnosis and intervention may be of benefit to high-risk patients [5]. Elastographic methods also are under investigation to detect early markers of VOD/SOS, which may lead to earlier diagnosis and treatment [50].
In this pooled analysis of studies of defibrotide given approximately at the approved dose of 25 mg/kg/day for the treatment of VOD/SOS, estimated Day + 100 survival was 56% in the 2073 patients with or without MOD. As expected, survival in patients with VOD/SOS without MOD was greater at Day + 100 (69%) than in patients with VOD/SOS with MOD (42%). Safety results in the individual studies were generally consistent with the known safety profile of defibrotide. Taken together, these results show a largely consistent treatment effect for defibrotide in the broad population of patients treated with defibrotide for VOD/SOS, with or without MOD.
Acknowledgements
Clinical analysis was funded by Jazz Pharmaceuticals. We thank Katherine Molnar-Kimber, PhD, and John Norwood of The Curry Rockefeller Group, LLC, of Tarrytown, NY, USA for providing medical writing and editorial support, which were funded by Jazz Pharmaceuticals in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Jazz Pharmaceuticals also reviewed and edited the manuscript for scientific accuracy. We thank all of the study investigators, study staff, nursing teams, and patients for their participation in the clinical studies cited in this pooled analysis.
Compliance with ethical standards
Conflict of interest
Paul G. Richardson has served on advisory committees and as a consultant, and received research funding from Jazz Pharmaceuticals. Saurabh Aggarwal and Ozlem Topaloglu are employees of Novel Health Strategies, which received funding from Jazz Pharmaceuticals for this analysis. Kathleen Villa is an employee of Jazz Pharmaceuticals and holds stock and/or stock options in Jazz Pharmaceuticals plc. Selim Corbacioglu has served as a consultant to and received honoraria from Gentium/Jazz Pharmaceuticals.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/26/2019
The original version of this article included some minor typesetting errors in Table 1 – a corrected version of which is provided in the linked correction article and has been corrected in this article – and one error in text, which has been corrected in this article. Furthermore, the article was erroneously published with the copyright holders as ‘Springer Nature Limited’, however, since the article is Open Access, the copyright holders should in fact be ‘The Author(s)’. This has now been corrected in this article.
Change history
4/1/2019
The original version of this article included some minor typesetting errors in Table 1–a corrected version of which is provided in this correction article and has been corrected in the original article–and one error in text, which has been corrected in the original article. Furthermore, the article was erroneously published with the copyright holders as ‘Springer Nature Limited’, however, since the article is Open Access, the copyright holders should in fact be ‘The Author(s)’. This has also been corrected in the original article.
References
- 1.Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157–68. doi: 10.1016/j.bbmt.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corbacioglu S, Hönig M, Lahr G, Stöhr S, Berry G, Friedrich W, et al. Stem cell transplantation in children with infantile osteopetrosis is associated with a high incidence of VOD, which could be prevented with defibrotide. Bone Marrow Transplant. 2006;38:547–53. doi: 10.1038/sj.bmt.1705485. [DOI] [PubMed] [Google Scholar]
- 3.Richardson PG, Riches ML, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127:1656–65. doi: 10.1182/blood-2015-10-676924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strouse C, Richardson P, Prentice G, Korman S, Hume R, Nejadnik B, et al. Defibrotide for treatment of severe veno-occlusive disease in pediatrics and adults: an exploratory analysis using data from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2016;22:1306–12. doi: 10.1016/j.bbmt.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT) Bone Marrow Transplant. 2015;50:781–9. doi: 10.1038/bmt.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011;46:1495–502. doi: 10.1038/bmt.2011.65. [DOI] [PubMed] [Google Scholar]
- 7.Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51:906–12. doi: 10.1038/bmt.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbacioglu S, Carreras E, Ansari M, Balduzzi A, Cesaro S, Dalle JH, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2018;53:138–45. doi: 10.1038/bmt.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, et al. Haemato-oncology Task Force of British Committee for Standards in Haematology; British Society for Blood and Marrow Transplantation. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163:444–57. doi: 10.1111/bjh.12558. [DOI] [PubMed] [Google Scholar]
- 11.McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–67. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 12.Carreras E, Bertz H, Arcese W, Vernant JP, Tomás JF, Hagglund H, et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood. 1998;92:3599–604. [PubMed] [Google Scholar]
- 13.Yakushijin K, Atsuta Y, Doki N, Yokota A, Kanamori H, Miyamoto T, et al. Sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors and outcomes. Bone Marrow Transplant. 2016;51:403–9. doi: 10.1038/bmt.2015.283. [DOI] [PubMed] [Google Scholar]
- 14.Carreras E, Díaz-Beyá M, Rosiñol L, Martínez C, Fernández-Avilés F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant. 2011;17:1713–20. doi: 10.1016/j.bbmt.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Tsirigotis PD, Resnick IB, Avni B, Grisariu S, Stepensky P, Or R, et al. Incidence and risk factors for moderate-to-severe veno-occlusive disease of the liver after allogeneic stem cell transplantation using a reduced intensity conditioning regimen. Bone Marrow Transplant. 2014;49:1389–92. doi: 10.1038/bmt.2014.168. [DOI] [PubMed] [Google Scholar]
- 16.Defitelio® (defibrotide sodium) injection [package insert on the Internet]. Palo Alto (CA): Jazz Pharmaceuticals, 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208114lbl.pdf.
- 17.Defitelio® (defibrotide) [package insert on the Internet]. Villa Guardia (Italy): Gentium, 2016. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002393/WC500153150.pdf.
- 18.Pescador R, Capuzzi L, Mantovani M, Fulgenzi A, Ferrero ME. Defibrotide: properties and clinical use of an old/new drug. Vasc Pharmacol. 2013;59:1–10. doi: 10.1016/j.vph.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Palomo M, Diaz-Ricart M, Rovira M, Escolar G, Carreras E. Defibrotide prevents the activation of macrovascular and microvascular endothelia caused by soluble factors released to blood by autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:497–506. doi: 10.1016/j.bbmt.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Benimetskaya L, Wu S, Voskresenskiy AM, Echart C, Zhou JF, Shin J, et al. Angiogenesis alteration by defibrotide: implications for its mechanism of action in severe hepatic veno-occlusive disease. Blood. 2008;112:4343–52. doi: 10.1182/blood-2008-04-149682. [DOI] [PubMed] [Google Scholar]
- 21.Echart CL, Graziadio B, Somaini S, Ferro LI, Richardson PG, Fareed J, et al. The fibrinolytic mechanism of defibrotide: effect of defibrotide on plasmin activity. Blood Coagul Fibrinolysis. 2009;20:627–34. doi: 10.1097/MBC.0b013e32832da1e3. [DOI] [PubMed] [Google Scholar]
- 22.Palomo M, Mir E, Rovira M, Escolar G, Carreras E, Diaz-Ricart M. What is going on between defibrotide and endothelial cells? Snapshots reveal the hot spots of their romance. Blood. 2016;127:1719–27. doi: 10.1182/blood-2015-10-676114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbacioglu S, Richardson PG. Defibrotide for children and adults with hepatic veno-occlusive disease post hematopoietic cell transplantation. Expert Rev Gastroenterol Hepatol. 2017;11:885–98. doi: 10.1080/17474124.2017.1370372. [DOI] [PubMed] [Google Scholar]
- 24.Triplett BM, Kuttab HI, Kang G, Leung W. Escalation to high-dose defibrotide in patients with hepatic veno-occlusive disease. Biol Blood Marrow Transplant. 2015;21:2148–53. doi: 10.1016/j.bbmt.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pol RR, Russell N, Das-Gupta E, Watson L, Rachael L, Byrne J. Incidence and management of hepatic severe veno-occlusive disease in 273 patients in a single centre with defibrotide. Bone Marrow Transplant. 2016;51:1262–4. doi: 10.1038/bmt.2016.99. [DOI] [PubMed] [Google Scholar]
- 26.Sucak GT, Aki ZS, Yagcí M, Yegin ZA, Ozkurt ZN, Haznedar R. Treatment of sinusoidal obstruction syndrome with defibrotide: a single-center experience. Transplant Proc. 2007;39:1558–63. doi: 10.1016/j.transproceed.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 27.Richardson PG, Soiffer RJ, Antin JH, Uno H, Jin Z, Kurtzberg J, et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant. 2010;16:1005–17. doi: 10.1016/j.bbmt.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson PG, Murakami C, Jin Z, Warren D, Momtaz P, Hoppensteadt D, et al. Multi-institutional use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high-risk population and factors predictive of outcome. Blood. 2002;100:4337–43. doi: 10.1182/blood-2002-04-1216. [DOI] [PubMed] [Google Scholar]
- 29.Corbacioglu S, Greil J, Peters C, Wulffraat N, Laws HJ, Dilloo D, et al. Defibrotide in the treatment of children with veno-occlusive disease (VOD): a retrospective multicentre study demonstrates therapeutic efficacy upon early intervention. Bone Marrow Transplant. 2004;33:189–95. doi: 10.1038/sj.bmt.1704329. [DOI] [PubMed] [Google Scholar]
- 30.Corbacioglu S, Carreras E, Mohty M, Pagliuca A, Boelens JJ, Damaj G, et al. Defibrotide for the treatment of hepatic veno-occlusive disease: final results from the international compassionate-use program. Biol Blood Marrow Transplant. 2016;22:1874–82. doi: 10.1016/j.bbmt.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Chopra R, Eaton JD, Grassi A, Potter M, Shaw B, Salat C, et al. Defibrotide for the treatment of hepatic veno-occlusive disease: results of the European compassionate-use study. Br J Haematol. 2000;111:1122–9. doi: 10.1046/j.1365-2141.2000.02475.x. [DOI] [PubMed] [Google Scholar]
- 32.Bulley SR, Strahm B, Doyle J, Dupuis LL. Defibrotide for the treatment of hepatic veno-occlusive disease in children. Pediatr Blood Cancer. 2007;48:700–4. doi: 10.1002/pbc.20934. [DOI] [PubMed] [Google Scholar]
- 33.Richardson PG, Elias AD, Krishnan A, Wheeler C, Nath R, Hoppensteadt D, et al. Treatment of severe veno-occlusive disease with defibrotide: compassionate use results in response without significant toxicity in a high-risk population. Blood. 1998;92:737–44. [PubMed] [Google Scholar]
- 34.Ruiz Ramos J, Company Albir MJ, Favieres Puigcerver C, Marrero Álvarez P, Megias Vericat JE, Valero García S, et al. CP-129 Defibrotide for sinusoidal obstruction syndrome: a single centre experience. Eur J Hosp Pharm. 2014;21:A52. doi: 10.1136/ejhpharm-2013-000436.127. [DOI] [Google Scholar]
- 35.Locatelli F, Faraci M, Cesaro S, Pagliara D, Fagioli F, Zecca Marco, et al. Defibrotide for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome following hematopoietic stem cell transplantation or chemotherapy―results from the Italian Therapeutic Use Protocol. Blood. 2015;126:3107. doi: 10.1182/blood.V126.23.3107.3107. [DOI] [Google Scholar]
- 36.Balade Martinez L, Valle LGD, Martin ER, De Sebastian Rueda M, Molia Cabezuelo M, Herrero Ambrosio A. CP-062 Defibrotide for the treatment of severe hepatic veno-occlusive disease. A single centre experience. Eur J Hosp Pharm. 2016;23:A27–A28. doi: 10.1136/ejhpharm-2018-001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yakushijin K, Ikezoe T, Ohwade C, Kudo K, Okamura H, Goto H, et al. Nationwide survey of defibrotide and recombinant human soluble thrombomodulin for treatment of sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51:S77–S78. doi: 10.1038/bmt.2015.283. [DOI] [Google Scholar]
- 38.Richardson P, Smith A, Triplett B, Kernan N, Grupp S, Antin J, et al. Final efficacy and safety results from a defibrotide expanded-access program for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Bone Marrow Transplant. 2017;52:S64–S65. [Google Scholar]
- 39.Corbacioglu S, Grupp SA, Pagliuca A, Hume RL, Zhu J, Tappe W, et al. Protocol for a phase 3, randomized, adaptive study comparing the efficacy and safety of defibrotide vs best supportive care to prevent hepatic veno-occlusive disease/sinusoidal obstruction syndrome in adult and pediatric patients undergoing hematopoietic stem cell transplant. Paper presented at ASH Meeting on Hematologic Malignancies; Chicago, IL; 2016; 16–17.
- 40.Kernan NA, Smith A, Triplett B, Lehmann L, Ryan RJ, Liang W, et al. Efficacy and safety of defibrotide in pediatric patients with veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) after hematopoietic stem cell transplantation (HSCT): final results from the expanded-access program. Blood. 2017;130:1948. [Google Scholar]
- 41.Richardson PG, Antin JH, Giralt SA, Ryan RJ, Liang W, Hume RL, et al. Adults receiving defibrotide for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) after hematopoietic stem cell transplantation (HSCT): final results from the expanded-access program. Blood. 2017;130:3225. [Google Scholar]
- 42.Richardson PG, Grupp SA, Pagliuca A, Krishnan A, Ho VT, Corbacioglu S. Defibrotide for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome with multiorgan failure. Int J Haematol Oncol. 2017;6:75–93. doi: 10.2217/ijh-2017-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grupp S, Richardson PG, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM, et al. Defibrotide for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction –syndrome with multi-organ dysfunction: NNT for Day+100 survival and CR based on results from a pivotal, historically controlled, phase 3 trial. Pediatr Blood Cancer. 2016;63:S93. doi: 10.1002/pbc.25691. [DOI] [Google Scholar]
- 44.Kernan NA, Grupp S, Smith AR, Arai S, Triplett B, Antin JH, et al. Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Br J Haematol. 2018;181:816–27. doi: 10.1111/bjh.15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chalandon Y, Roosnek E, Mermillod B, Newton A, Ozsahin H, Wacker P, et al. Prevention of veno-occlusive disease with defibrotide after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:347–54. doi: 10.1016/j.bbmt.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Dignan F, Gujral D, Ethell M, Evans S, Treleaven J, Morgan G, et al. Prophylactic defibrotide in allogeneic stem cell transplantation: minimal morbidity and zero mortality from veno-occlusive disease. Bone Marrow Transplant. 2007;40:79–82. doi: 10.1038/sj.bmt.1705696. [DOI] [PubMed] [Google Scholar]
- 47.Qureshi A, Marshall L, Lancaster D. Defibrotide in the prevention and treatment of veno-occlusive disease in autologous and allogeneic stem cell transplantation in children. Pediatr Blood Cancer. 2008;50:831–2. doi: 10.1002/pbc.21425. [DOI] [PubMed] [Google Scholar]
- 48.Corbacioglu S, Cesaro S, Faraci M, Balteau-Couanet D, Gruhn B, Rovelli A, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379:1301–9. doi: 10.1016/S0140-6736(11)61938-7. [DOI] [PubMed] [Google Scholar]
- 49.Clinicaltrials.gov. NCT02851407: Study comparing efficacy and safety of defibrotide vs best supportive care in the prevention of hepatic veno-occlusive disease in adult and pediatric patients. Available from: https://clinicaltrials.gov/ct2/show/NCT02851407.
- 50.Dietrich CF, Trenker C, Fontanilla T, Görg C, Hausmann A, Klein S, et al. New ultrasound techniques challenge the diagnosis of sinusoidal obstruction syndrome. Ultrasound Med Biol. 2018;44:2171–82. doi: 10.1016/j.ultrasmedbio.2018.06.002. [DOI] [PubMed] [Google Scholar]


