Abstract
Growth and Differentiation Factor 5 (GDF5) is a key risk locus for osteoarthritis (OA). However, little is known regarding regulation of Gdf5 expression following joint tissue damage. Here, we employed Gdf5-LacZ reporter mouse lines to assess the spatiotemporal activity of Gdf5 regulatory sequences in experimental OA following destabilisation of the medial meniscus (DMM) and after acute cartilage injury and repair. Gdf5 expression was upregulated in articular cartilage post-DMM, and was increased in human OA cartilage as determined by immunohistochemistry and microarray analysis. Gdf5 expression was also upregulated during cartilage repair in mice and was switched on in injured synovium in prospective areas of cartilage formation, where it inversely correlated with expression of the transcriptional co-factor Yes-associated protein (Yap). Indeed, overexpression of Yap suppressed Gdf5 expression in chondroprogenitors in vitro. Gdf5 expression in both mouse injury models required regulatory sequence downstream of Gdf5 coding exons. Our findings suggest that Gdf5 upregulation in articular cartilage and synovium is a generic response to knee injury that is dependent on downstream regulatory sequence and in progenitors is associated with chondrogenic specification. We propose a role for Gdf5 in tissue remodelling and repair after injury, which may partly underpin its association with OA risk.
Subject terms: Musculoskeletal models, Stem cells
Introduction
Growth and Differentiation Factor 5 (GDF5) is a major risk locus for osteoarthritis (OA), the most common joint disease characterised by progressive loss of articular cartilage, remodelling of subchondral bone, chondro-osteophyte formation and synovitis. Common variants spanning a large 130 kb interval confer risk of hip and knee OA1–3. A well-studied SNP is located in the 5′ UTR of the GDF5 gene (rs143383), with the OA susceptibility allele resulting in decreased GDF5 expression2,4–6.
Gdf5 plays important roles during joint formation. It is one of the earliest genes expressed in the embryonic joint interzone7–10, fated to give rise to joint tissues including articular cartilage, synovium, menisci, and ligaments11,12. Gdf5-expressing progenitors are continuously recruited into joint interzones throughout development13 and their progeny retain skeletal joint stem/progenitor activity in adulthood14. Following injury to the joint surface, Gdf5-lineage mesenchymal stromal/stem cells (MSCs) proliferate to underpin synovial hyperplasia and migrate to the site of injury, through the activity of the transcriptional co-factor Yes-associated protein (Yap), where they repair cartilage14.
Loss-of-function mutations in GDF5 have been linked to congenital disorders including Hunter-Thompson syndrome15, brachydactyly type C16, and DuPan syndrome17. These syndromes are partly phenocopied in brachypodism (bp) mice, which harbour Gdf5 coding mutations7. Homozygous bp mice have dysmorphic knees lacking cruciate ligaments18,19. Heterozygous bp mice, which model human GDF5 variants that cause decreased GDF5 expression, display no overt phenotype19–21 but show increased susceptibility to OA under experimental challenges21.
Recent studies using mice harbouring BAC transgenes have revealed a conserved cis-regulatory architecture for GDF5 between humans and mice19,22–24. Regulatory sequences that control Gdf5 expression in developing and adult joints are distributed over a hundred kilobases, including regions both upstream and downstream of its coding exons22. While Gdf5 expression in the developing knee is driven by both upstream and downstream regulatory sequences, in adulthood downstream regulatory regions are uniquely used19,22, suggesting that the genomic sequences regulating continued expression of Gdf5/GDF5 in the adult knee during homeostasis may be distinct. Of note, these downstream regions harbour a number of genetic risk variants for knee OA3.
In this study, we used BAC Gdf5-LacZ reporter mice19,22 to map Gdf5 expression during adult knee joint tissue remodelling associated with OA development or acute cartilage injury and repair, and to determine whether a differential regulation of Gdf5 expression is associated with such events.
Methods
Mice
All methods were carried out in accordance with relevant guidelines and regulations. All animal experimental protocols were approved by the UK Home Office and the Animal Welfare and Ethical Review Committee of the University of Aberdeen. Two Gdf5 BAC transgenic mouse lines were used19,22,23. They both harbour a BAC transgene containing mouse Gdf5 with an IRES-LacZ cassette in the 3’UTR. Gdf5UP-LacZ mice contain a modified BAC extending 110 kb upstream to 30 kb downstream of Gdf5 coding exons, which includes a conserved regulatory region adjacent to the promoter upstream of the Gdf5 coding exons. Gdf5DOWN-LacZ mice contain a modified BAC extending a further 109 kb downstream, which includes additional regulatory regions downstream of the Gdf5 coding exons. Both lines were maintained as heterozygotes on an FVB background. Gdf5-CreER mice13 were provided by Dr. Elazar Zelzer (Weizmann Institute of Science, Israel) and crossed with Cre-inducible tdTomato (tdTom) reporter mice (Jackson Laboratory; B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J)25. Mice were group-housed in conventional cages on a 12:12 light-dark cycle, in a temperature-controlled room with water and food ad libitum and environmental enrichment provided. Tamoxifen (Sigma) dissolved in corn oil was administered by gavage at 6 weeks of age (180 mg/kg daily for 5 days), or to the pregnant dam at E11.5 (120 mg/kg), E13.5 (160 mg/ml) and E15.5 (160 mg/ml), and embryos were collected following euthanasia of the pregnant dam at E19.0.
Surgical procedures
Male mice, 11–12 weeks old, underwent surgical unilateral destabilisation of the medial meniscus (DMM) on the left knee26 while the right knee served as internal control, and mice were euthanised 2 or 8 weeks later. Female mice, 9–11 weeks old, underwent surgery to induce unilateral joint surface injury by medial parapatellar arthrotomy as previously described14, and were euthanised 6–7 days or 4 weeks later. For all surgeries, isoflurane inhalation anaesthesia was used, and mice received a subcutaneous injection of 0.1 mg/kg Vetergesic (containing 0.3 mg/ml Buprenorphine) on the day of surgery and the following day. Mice were kept group-housed.
X-gal staining
Whole-mount staining with X-gal to detect β-galactosidase (β-gal) activity was performed as described27, with modifications. Limbs were fixed in 4% PFA for 2 h at 4 °C, washed 3x in wash buffer (0.1 M phosphate buffer supplemented with 2 mM MgCl2, 0.01% sodium deoxycholate and 0.02% Igepal), stained with 0.75 mg/ml X-gal in staining solution (wash buffer supplemented with 4 mM potassium ferrocyanide, 4 mM potassium ferricyanide and 20 mM Tris buffer, pH 7.4) for 6 days at room temperature, then washed 3x in PBS. Limbs from wild-type mice were stained as controls.
Human tissue collection
All human cartilage samples were obtained after informed consent and in accordance with the relevant guidelines and regulations, with approval from the NHS Grampian Biorepository Tissue Bank Committee. OA samples were obtained from five patients (47 to 79 years old, all female) undergoing knee arthroplasty. Normal samples were obtained from five joints (two knee joints, 1st metatarsal phalangeal joint, ankle joint, talo-calcaneal joint) donated by three patients (40 to 59 years old, two males, one female) undergoing excision or amputation surgery for tumours unrelated to the joint sampled.
Histology and immunohistochemistry
Samples were fixed in 4% PFA at 4 °C and decalcified in 10% EDTA in PBS. Samples were embedded and sectioned as described14. Sections were stained with Nuclear Fast Red (Vector Laboratories, UK) to stain nuclei, or with safranin-O (Sigma) to stain glycosaminoglycans in the cartilage matrix red, with fast green (Sigma) counterstain, following standard protocols. TRAP staining to detect osteoclasts was carried out using a TRAP staining kit (Sigma). Immunohistochemistry was performed as described28,29 using antibodies listed in Supplementary Table 1. Collagen type II was detected following enzyme-based antigen retrieval with 1.5 mg/ml porcine pepsin (Sigma) for 45 min at 37 °C. Yap and GDF5 were detected following antigen retrieval for 4 hours at 80 °C in antigen unmasking citrate buffer solution (pH 6, Vector Laboratories, UK). Stained sections were imaged using a Zeiss Axioscan Z1 slide scanner (Carl Zeiss Ltd, UK), Zeiss Axioskop 40 (Zeiss) with Progress XT Core 5 colour digital camera and ProgRes CapturePro 2.9.0.1 software (JenOptik, Germany), or 710 META Laser-Scanning Confocal Microscope with ZEN software (Zeiss) and analysed using ZEN2 (blue edition, Carl Zeiss Ltd). Cartilage damage of the tibial plateau was assessed using the Osteoarthritis Research Society International (OARSI) scoring system30.
Quantification of X-gal staining
Colour deconvolution was applied to images of X-gal-stained sections to remove the Nuclear Fast Red counterstaining using ImageJ with Fiji package and Colour Deconvolution Plugin (Dr. Gabriel Landini, University of Birmingham, UK) based on published methods31. All images were acquired with the same magnification, resolution and light settings. The number, size and staining intensity of X-gal-stained chondrocytes in the tibial cartilage was then determined by creating a binary image using thresholding and watershedding, and analysing particles by redirecting measurements to matching greyscale images. Four sections per sample were analysed. Total X-gal staining was calculated by multiplying the number and staining intensity of X-gal-stained chondrocytes.
Primary cell isolation and in vitro chondrogenesis
Cells were isolated from Gdf5 BAC mouse knees as described14. Chondrogenesis was induced in high-cell density pellet culture (2.5–3 × 105 cells) with 10 ng/ml TGFβ1 (Gibco) or 300 ng/ml BMP-2 (Prospec) for 21 days, as described14. Pellets were fixed in 4% PFA for 15 min, X-gal-stained for 4 h and post-fixed for 15 min, cryoprocessed, sectioned and stained with Toluidine Blue or Nuclear Fast Red.
Overexpression and knockdown experiments
C3H10T1/2 cells (American Type Culture Collection, USA) were retrovirally transduced to express wildtype or constitutively active YAP1, as described32. Cells were seeded in monolayer (15,000/cm2), transduced the next day, and RNA extracted 2 days later. Alternatively, transduced cells were seeded in high-cell density micromass culture (4 × 105 cells) in chemically-defined serum-free medium (high-glucose DMEM with glutamine, supplemented with 50 μg/ml ascorbic acid, 1 mg/ml recombinant human insulin, 0.55 mg/ml transferrin, 0.5 ug/ml sodium selenite, 50 mg/ml BSA and 470 µg/ml linoleic acid)32, and the next day RNA was extracted. For knockdown experiments, cells were seeded at 42,000/cm2 and transfected the next day with DsiRNA (Supplementary Table 2) (Integrated DNA Technologies, USA) using Mirus TransIT-X2 reagent (Mirus Bio LLC, USA). The following day, cells were seeded in micromass culture (2.5–3 × 105 cells) and cultured under chondrogenic conditions by treatment with 300 ng/ml BMP-2, as described32. After 4 days, RNA was extracted for analysis of gene expression.
Gene expression analysis
Total RNA was extracted using TRIzol reagent (Invitrogen, Paisley, UK) according to standard protocols, and RNA was quantified using a NanoDrop ND-1000 spectrophotometer (Labtech, Uckfield, UK). cDNA was synthesised using random hexamer primers and SuperScript Reverse Transcriptase (Invitrogen), according to manufacturer’s instructions. Quantitative PCR (qPCR) was performed with a Roche LightCycler 480 using SYBR Green Master (Roche), according to standard protocols. Expression of genes of interest was normalised to expression of Hprt1. Primer sequences are listed in Supplementary Table 3.
Statistical analysis
Microarray data were analysed using Bioconductor (Affy package for pre-processing and normalization and Limma for statistical comparison of expression levels using a false-discovery-rate of 5%). Principal component analysis was performed using the prcomp package in R. All other data were analysed using GraphPad Prism v5 and SigmaPlot v13. A p-value ≤0.05 was considered statistically significant. For comparison of two groups, two-tailed t-test was used. For comparison of ≥3 groups, one-way or two-way ANOVA with Holm-Sidak post-test was used. Data following a lognormal distribution were log-transformed for statistical testing. N-numbers and data points on graphs represent individual mice, patients, or in vitro experiments, with horizontal lines indicating mean.
Results
Gdf5 expression in OA
To investigate Gdf5 expression in experimentally induced OA, we used two Gdf5-LacZ reporter mouse lines22. Gdf5UP-LacZ mice contain a BAC extending 110 kb upstream to 30 kb downstream of Gdf5 coding exons, which includes a conserved regulatory region adjacent to the promoter upstream of the Gdf5 coding exons. Gdf5DOWN-LacZ mice contain a BAC extending a further 109 kb downstream, which includes additional regulatory regions downstream of the Gdf5 coding exons that are not present in the Gdf5UP-LacZ BAC. Both BACs were modified to contain an IRES-LacZ cassette in the 3′UTR of the Gdf5 gene, thus LacZ expression is indicative of the activity of the Gdf5 regulatory regions contained within the BAC22. While both mouse lines express LacZ in the knee during development19,22, only Gdf5DOWN-LacZ mice express LacZ in the knee in adulthood (Supplementary Fig. 1)19. The Gdf5DOWN-LacZ BAC is also able to rescue the knee phenotype in bp mice, indicating it contains the regulatory regions necessary for adequate expression in the knee19. Here, we found that the LacZ expression pattern in Gdf5DOWN-LacZ adult knees resembled the tdTom labelling pattern in knees from adult mice with a knock-in of CreER at the endogenous Gdf5 locus13 crossed with Cre-inducible tdTom reporter mice25 shortly after tamoxifen induction (Supplementary Fig. 2A,B). TdTom labelling was sparse, likely due to inefficient Cre-recombination as observed in embryos (Supplementary Fig. 2C–E)13. Nonetheless, these data support LacZ expression in knees from adult Gdf5DOWN-LacZ mice as reflecting transcriptional activity of endogenous Gdf5.
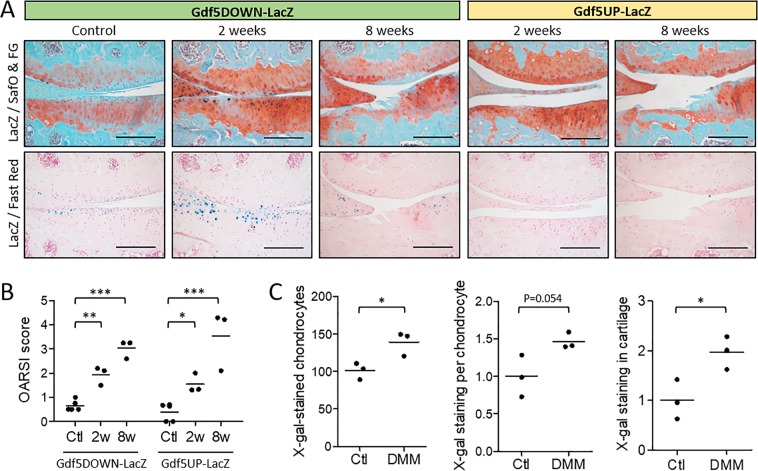
We analysed LacZ expression in the knees of Gdf5-LacZ mice after DMM (Fig. 1A,B). In Gdf5DOWN-LacZ mice, increased LacZ expression was observed in medial compartment articular cartilage at 2 weeks, particularly in areas with early signs of damage, as shown by loss of Safranin O staining which stains proteoglycans in the cartilage extracellular matrix (Fig. 1A). Quantification showed an increase in both the number of LacZ-expressing chondrocytes and average X-gal staining intensity per chondrocyte (Fig. 1C), resulting in a significantly higher overall LacZ-expression in the medial tibial plateau cartilage in DMM knees. At 8 weeks after DMM, LacZ expression persisted in articular cartilage of Gdf5DOWN-LacZ mice but was less pronounced and undetectable in areas of severe damage (Fig. 1A). In Gdf5UP-LacZ mice, no LacZ expression was detectable in the cartilage at either time-point (Fig. 1A). These data indicate that Gdf5 downstream regulatory elements are activated in articular chondrocytes in the early phase of OA.
Figure 1.
Gdf5 expression in articular cartilage following DMM. (A) LacZ expression (blue X-gal staining) in the articular cartilage of the medial femoral condyle (top of images) and tibial plateau (bottom of images) at 2 and 8 weeks after DMM, or in contralateral control knee, in Gdf5DOWN-LacZ and Gdf5UP-LacZ mice (n = 3 for both strains and both timepoints). At 2 weeks, cartilage shows focal loss of proteoglycan staining (red Safranin O staining) and minor fibrillations at the surface, while at 8 weeks it is severely damaged. LacZ, whole-mount X-gal staining to detect LacZ expression; SafO & FG, Safranin O and Fast Green counterstaining; Fast Red, Nuclear Fast Red counterstaining. Scale bars, 200 μm. (B) OARSI histopathological scores of cartilage damage of the medial tibial plateau at 2 weeks (2w, n = 3) or 8 weeks (8w, n = 3) after DMM surgery, or no surgery (Ctl, n = 5). *p < 0.05; **p < 0.01; ***p < 0.001, two-way ANOVA with Holm-Sidak post-test for comparisons against control. There were no significant differences between the two mouse lines. (C) Number of counted X-gal-stained chondrocytes, average X-gal staining intensity per chondrocyte, and X-gal staining in cartilage calculated by multiplying number and staining intensity of X-gal-stained chondrocytes, in tibial articular cartilage of Gdf5DOWN-LacZ mice at 2 weeks after DMM. Data are expressed relative to the internal contralateral control knees (Ctl). *p < 0.05, two-tailed Student’s t-test.
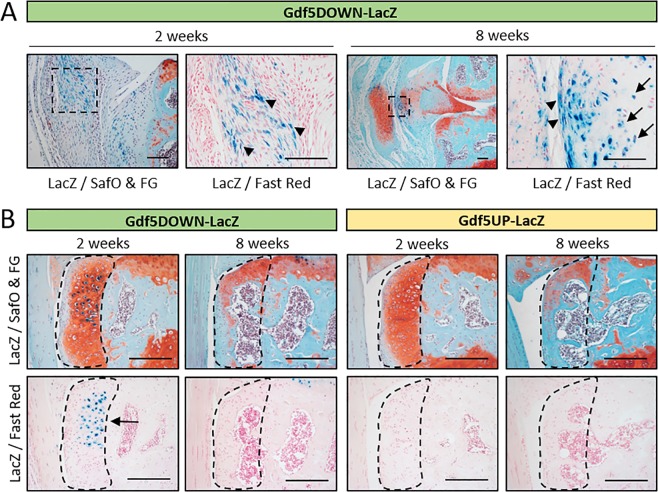
LacZ expression was also detected in the medial synovium of Gdf5DOWN-LacZ mice at 2 weeks post-DMM and remained detectable at 8 weeks, specifically in ectopic chondrocytes and surrounding fibroblast-like cells (Fig. 2A). In addition, LacZ was expressed in chondrophytes at 2 weeks post-DMM but was no longer detectable in mature osteophytes at 8 weeks (Fig. 2B). LacZ expression was not detected in knees from Gdf5UP-LacZ mice at either time-point (Fig. 2B). We infer that Gdf5 is expressed in areas of forming ectopic cartilage during OA.
Figure 2.
Gdf5 expression during ectopic cartilage formation in vivo. (A) LacZ expression in fibroblast-like cells (blue, arrowheads) in medial synovium at 2 and 8 weeks post-DMM in Gdf5DOWN-LacZ mice (n = 3). At 8 weeks post-DMM, ectopic cartilage in synovium was observed with LacZ-expressing chondrocytes (blue, arrows) and surrounding LacZ-expressing fibroblast-like cells (blue, arrowheads). (B) Chondrophytes at 2 weeks post-DMM and mature osteophytes at 8 weeks post-DMM (indicated by dashed lines) showing LacZ-expressing chondrocytes (blue) in the chondrophytes in Gdf5DOWN-LacZ mice, but not Gdf5UP-LacZ mice, at 2 weeks post-DMM (n = 3 for both strains and both time points). LacZ, whole-mount X-gal staining to detect LacZ expression; SafO & FG, Safranin O and Fast Green counterstaining; Fast Red, Nuclear Fast Red counterstaining. Scale bars, (A) 100 μm, (B) 200 μm.
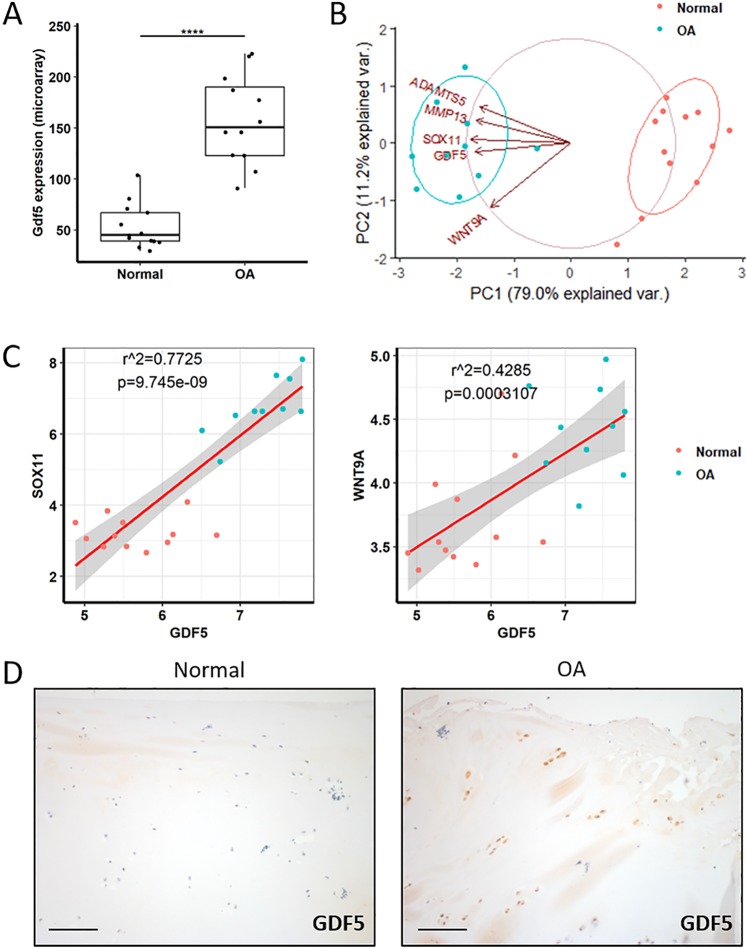
For clinical relevance, we analysed data from published microarrays of human cartilage from knees of normal donors and OA patients33. GDF5 expression was upregulated in the cartilage of OA patients (Fig. 3A), alongside increased expression of cartilage degrading proteins known to be upregulated in OA (MMP13, ADAMTS5) (Fig. 3B). GDF5 expression correlated with expression of SOX11 and WNT9A (Fig. 3B,C), known upstream regulators of Gdf5 expression during development34–36, indicating these factors may also modulate GDF5 expression in human articular cartilage during OA. Immunohistochemistry for GDF5 on articular cartilage samples from a distinct cohort of OA patients and controls confirmed GDF5 was upregulated in OA cartilage (Fig. 3D and Supplementary Fig. 3).
Figure 3.
Expression of GDF5 and upstream regulators in human OA cartilage. Gene expression data were obtained by mining a previously published microarray comparing normal versus OA human knee cartilage33. (A) Gdf5 expression was higher in OA cartilage compared to normal cartilage. Linear modelling (limma) with Benjamini-Hochberg correction for multiple comparisons. ****p < 0.0001. (B) Principal component analysis including the genes indicated showed complete separation of the normal samples from the OA samples. PCA was performed with the prcomp function in R. (C) Linear regression modelling showed GDF5 expression to strongly correlate with the expression of SOX11 (left) and WNT9a (right). (D) IHC staining for GDF5 in articular cartilage samples from patients with OA (n = 5 donors) in comparison to normal cartilage (n = 3 donors; 5 joints). Scale bars, 100 μm. See also Supplementary Fig. 3.
Gdf5 expression following joint surface injury
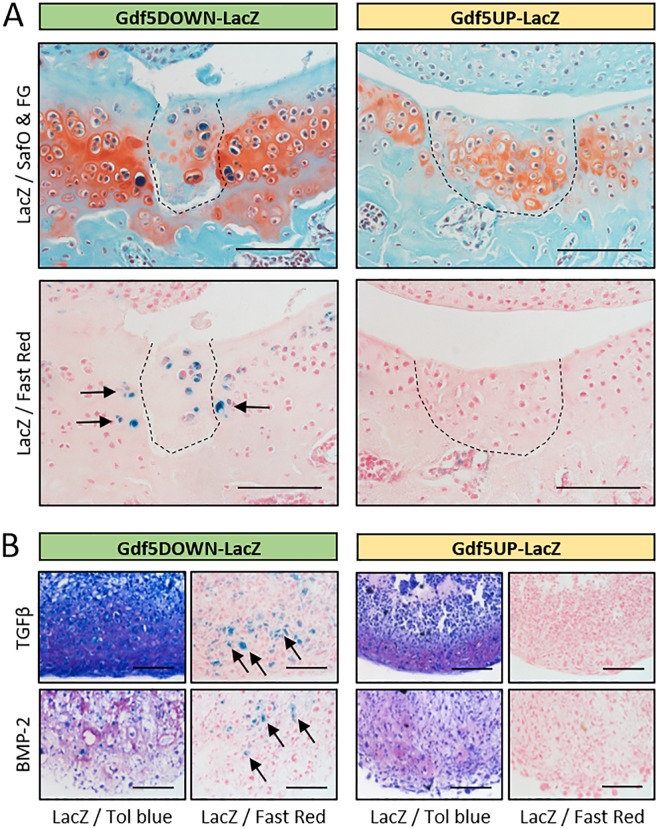
To investigate Gdf5 expression during cartilage repair, we analysed LacZ expression in the Gdf5-LacZ transgenic mice 4 weeks after joint surface injury. In Gdf5DOWN-LacZ mice, chondrocytes in the repair tissue strongly expressed LacZ. We also detected prominent LacZ expression in chondrocytes in the native cartilage immediately adjacent to the repair tissue (Fig. 4A). In contrast, no staining was observed in repaired cartilage in Gdf5UP-LacZ mice (Fig. 4A). In support of these findings, while undetectable in monolayer culture, LacZ expression was detected in MSCs isolated from the knees of Gdf5DOWN-LacZ mice following chondrogenic differentiation in pellet culture, but not in chondrogenic pellets of Gdf5UP-LacZ MSCs (Fig. 4B). These data indicate upregulation of Gdf5 expression, mediated by downstream regulatory regions, during articular cartilage repair.
Figure 4.
LacZ expression is upregulated during cartilage repair and in vitro chondrogenesis. (A) Areas of healed cartilage (dashed line) in the patellar groove of the femur of Gdf5DOWN-LacZ (n = 4/10) and Gdf5UP-LacZ (n = 3/10) mice, with LacZ-expressing chondrocytes (blue, arrows) detected in Gdf5DOWN-LacZ mice 4 weeks post-injury. LacZ, whole-mount X-gal staining to detect LacZ expression; SafO & FG, Safranin O and Fast Green counterstaining; Fast Red, Nuclear Fast Red counterstaining. Scale bars, 100 μm. (B) Histological sections of chondrogenic cell pellets. Synovial cells were isolated from Gdf5DOWN-LacZ and Gdf5UP-LacZ mice and treated in vitro for 21 days with TGFβ (10 ng/ml) or BMP-2 (300 ng/ml) to induce chondrogenesis, followed by X-gal staining to detect LacZ expression. Tol blue, Toluidine blue metachromatic staining indicates deposition of cartilage proteoglycans; Fast Red, Nuclear Fast Red counterstaining. LacZ-expressing chondrocytes (blue, arrows) were observed in Gdf5DOWN-LacZ cell pellets, but not Gdf5UP-LacZ pellets, under both culture conditions. Scale bars, 100 μm.
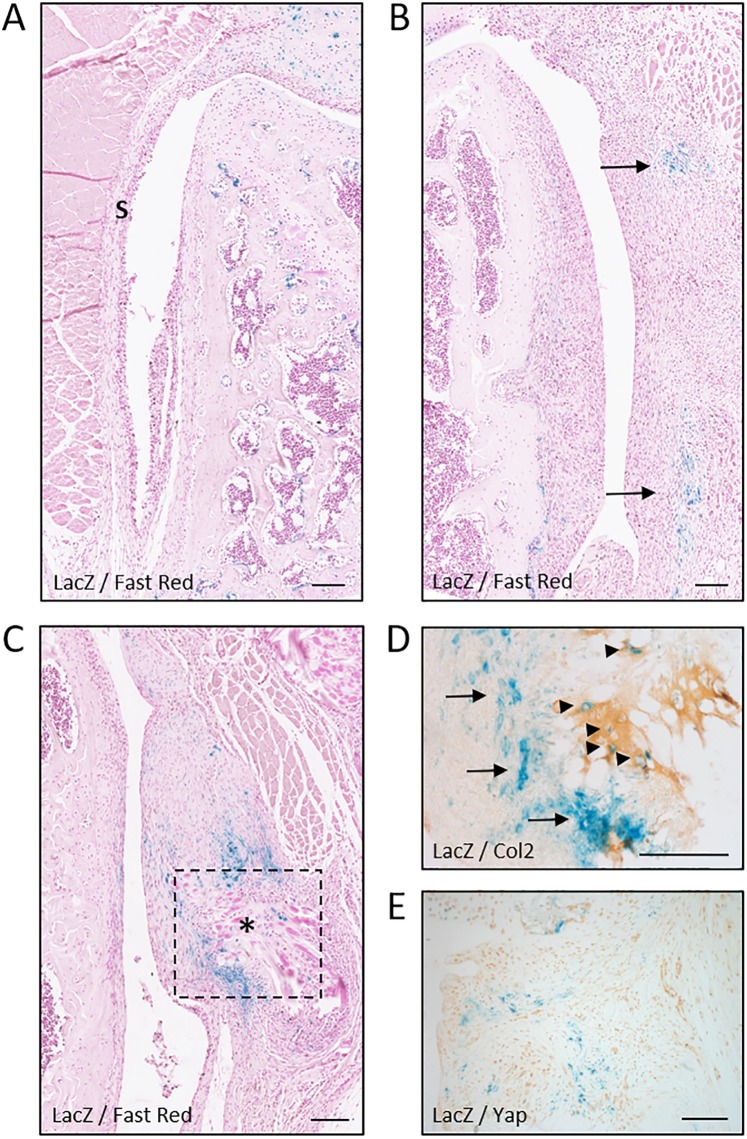
Since LacZ was switched on in Gdf5DOWN-LacZ MSCs during chondrogenesis, we next analysed the synovium, which contains stem/progenitor cells that can undergo chondrogenic differentiation following injury and are postulated to repair injured cartilage14,28,37. LacZ was not detectable in synovium during homeostasis in either model (Supplementary Fig. 1). One week after joint surface injury, the synovium was hyperplastic, as expected28,38. In the synovium on the lateral side of the knee, not incised during surgery, LacZ remained undetectable in both mouse lines at both time-points (Fig. 5A and not shown), indicating that Gdf5 expression is not switched on in synovium in response to cartilage injury. However, in synovium on the medial side, which was incised during surgery, small clusters of LacZ-expressing cells with a fibroblast-like morphology were detected in Gdf5DOWN-LacZ mice (Fig. 5B), and such cells persisted at 4 weeks after injury (Fig. 5C). They were predominantly localized near surgical sutures, where fibroblast-like cells that stained strongly for β-gal were observed around small clusters of LacZ-expressing chondrocytes embedded in a matrix containing collagen type II (Fig. 5D). Thus, as in DMM mice, Gdf5 expression is upregulated in synovium in areas of prospective cartilage formation, suggesting a role for Gdf5 in chondrogenic specification and differentiation.
Figure 5.
Gdf5 is switched on in areas undergoing ectopic cartilage formation in synovium. LacZ expression in lateral (A) and medial synovium (B–E) from Gdf5DOWN-LacZ mice 1 week (A,B) or 4 weeks (C–E) after joint surface injury (n = 4 for both timepoints). (A) LacZ expression was not detected in synovium (S) on the lateral side. (B) Clusters of LacZ-expressing fibroblast-like cells (blue, arrows) were found in the medial synovium, near the site of surgical incision. (C) LacZ expression in medial synovium persisted at 4 weeks after injury, particularly near surgical sutures (asterisk). Dotted line indicates area shown in (D) in a consecutive section. (D) IHC staining for Collagen type II (Col2; light brown) revealing LacZ-expressing chondrocytes (blue, arrowheads) embedded in a cartilage matrix surrounded by LacZ-expressing fibroblast-like cells (blue, arrows). (E) IHC staining for Yap showing LacZ-expressing cells (blue) with little or no Yap interspersed between Yap-expressing cells (light brown) that did not detectably express LacZ. LacZ, whole-mount X-gal staining to detect LacZ expression; Fast Red, Nuclear Fast Red counterstaining. Scale bars, 100 μm.
Yap suppresses Gdf5 expression in chondroprogenitors
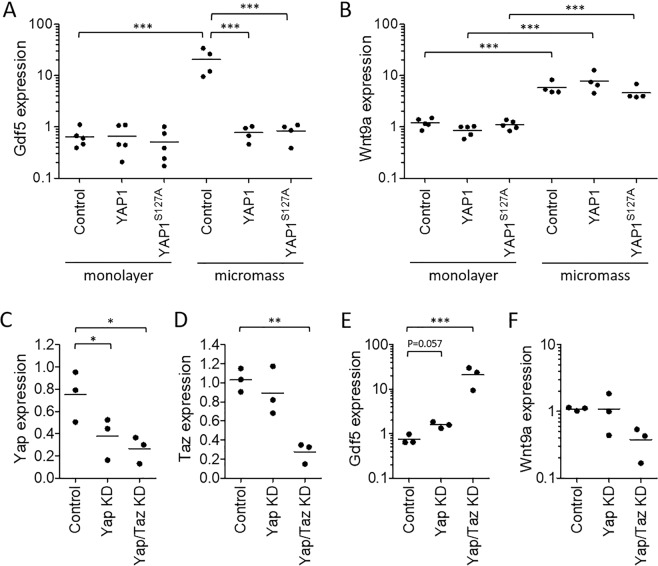
We previously reported that Yap is upregulated in synovium after joint surface injury and is required for the local expansion of Gdf5-lineage MSCs and their recruitment to the cartilage defect14, whereas Yap prevents chondrogenic differentiation32. Here, we compared expression of LacZ and Yap in Gdf5DOWN-LacZ mouse knees after joint surface injury and observed areas in synovium where Yap and LacZ showed an inverse expression pattern, with cells that expressed LacZ showing diminished Yap compared to surrounding cells (Fig. 5E). We hypothesized that high Yap activity during cell proliferation inhibits chondrogenic differentiation, as reported32, by actively suppressing chondrogenic factors including Gdf5. Hence, we determined the effect of overexpression of Yap on Gdf5 expression in high-cell-density cultures using murine C3H10T1/2 MSCs. After one day of high-cell-density micromass culture, Gdf5 expression was upregulated approximately 20-fold when compared to cells in monolayer (Fig. 6A), as previously reported with human synovial MSCs39. Strikingly, overexpression of YAP1 prevented the upregulation of Gdf5 in micromass (Fig. 6A). In contrast, YAP1 overexpression failed to prevent the upregulation of Wnt9a, known to be upstream of Gdf535, even when cells were transduced to express constitutively active YAP1S127A (Fig. 6B). Conversely, knockdown of Yap in C3H10T1/2 MSCs in micromass increased Gdf5 expression, an effect that was synergistically enhanced with concomitant knockdown of the paralog of Yap, Transcriptional Co-Activator with PDZ binding motif (Taz) (Fig. 6C–E). Wnt9a expression was not similarly modulated by Yap and Taz knockdown (Fig. 6F). Altogether, these data identify Yap as a negative regulator of Gdf5 expression in chondrogenic MSCs, and indicate that Yap acts downstream of Wnt9a, possibly by directly modulating the activity of one or more transcription factors acting on Gdf5 cis-regulatory elements.
Figure 6.
Yap supresses Gdf5 expression. (A,B) C3H10T1/2 cells were transduced with retrovirus encoding YAP1 or constitutively active YAP1S127A, or with empty vector (Control), and cultured in standard monolayer (n = 5 experiments), or in high-density micromass for 1 day (n = 4 experiments; data from separate experiments). (C–F) C3H10T1/2 cells were transfected with DsiRNA to knock down Yap, or Yap and Taz, or with mismatch DsiRNA (Control), and cultured for 4 days in chondrogenic micromass culture. Gdf5 (A,E), Wnt9A (B,F), Yap (C) and Taz (D) expression were determined by quantitative RT-PCR. All data were normalised to expression of Hprt1, and are shown relative to expression of the gene of interest in non-transduced cells in monolayer (A,B), or in micromass (C–F). *p < 0.05; **p < 0.01; ***p < 0.001, based on two-way ANOVA with Holm-Sidak post-test for pairwise comparisons (A,B) or one-way ANOVA with Holm-Sidak post-test for comparisons against control (C–E).
Discussion
Allelic variants at the GDF5 locus have been linked to OA risk, suggesting GDF5 plays important roles in joint maintenance throughout life. Expression of Gdf5 in adult articular cartilage has been reported in mice40 and humans5,41, with upregulation in OA41. Little was known regarding Gdf5 expression in response to acute joint surface defects, which can progress to OA in the absence of repair42, or during the different stages of OA. Here, we show Gdf5 expression in remodelling joint tissues, using two BAC LacZ reporter mouse strains harbouring distinct yet partially overlapping regions of the Gdf5 locus19,22. After joint surface injury, Gdf5 was highly expressed in chondrocytes both inside the newly formed cartilage repair tissue and in the adjacent stressed cartilage. Similarly, Gdf5 was upregulated in cartilage during early-stage OA, particularly in areas of initial damage, and was detected in forming chondrophytes. Given the known chondrogenic activity of Gdf512,43 our findings implicate a role for Gdf5 in new cartilage formation following injurious events in adulthood, possibly representing an attempt to repair joint damage. During late-stage OA, areas of advanced cartilage damage displayed markedly reduced LacZ staining, in line with previous studies reporting decreased Gdf5 expression in extensively damaged cartilage in mice with inflammatory or degenerative arthritis34,40. These data support a role for Gdf5 in the maintenance and repair of articular cartilage in adult life, and provide a rationale for the administration of exogenous Gdf5 to aid cartilage repair in OA treatment44.
We show that Gdf5 expression after injury and during OA is dependent on DNA sequence more than 30 kb downstream from the Gdf5 coding region. This downstream sequence contains joint-specific regulatory elements22, and is both capable of, and necessary for, rescuing the bp knee phenotype in mice19,22–24. Importantly, it harbours many common risk variants for OA, of which several reside in known enhancers. Our findings indicate that such downstream variants may confer OA risk partly through modulating Gdf5 expression in the adult knee in response to injurious events, thereby impacting on joint maintenance and reparative processes. They further indicate that the effect of a human variant such as the rs143383 SNP in the 5′UTR2,4–6 is likely to be dependent on cis-acting variants present in downstream cis-regulatory elements that are critical to drive adequate expression of Gdf5. Whether downstream regulatory elements involved in repair are different from those involved in OA development remains to be determined.
The identification of molecules that regulate Gdf5 expression will provide critical insights into joint formation, maintenance and disease. We have unveiled a regulatory mechanism, to our knowledge hitherto unreported, that links Yap activity to Gdf5 expression. Undetectable in quiescent synovium, Gdf5 was switched on in activated chondroprogenitors in synovium following injury, concomitant with Yap downregulation. In chondrogenic MSCs, Yap suppressed expression of Gdf5 but not Wnt9a, known to induce Gdf5 expression35,36. Our data indicate that Yap negatively regulates Gdf5 expression, possibly downstream of Wnt9a, and we propose that Yap needs to be down-regulated to enable Gdf5 expression to prime progenitors towards chondrogenesis. Indeed, Yap prevents MSC chondrogenic differentiation in vitro32. Candidate transcription factors that could partner with Yap to regulate Gdf5 include Sox11, reported to directly regulate Gdf5 expression34 and found here to correlate with GDF5 expression in human OA cartilage, and ZEB1, since ZEB1 binding sites are present in the enhancer upstream of the Gdf5 promoter region22 and a direct interaction between ZEB1 and Yap has been reported45.
In conclusion, Gdf5 is upregulated in stressed cartilage, switched on in chondroprogenitors and expressed in newly forming cartilage during tissue remodelling following knee injury. This is dependent on activity of downstream regulatory sequence and occurs irrespective of whether the injury is acute or the result of chronic joint instability, indicating that Gdf5 modulation is not linked to a specific injurious event. An understanding of the regulation of Gdf5 in the context of remodelling, repair and OA pathogenesis will have important implications for joint surface regenerative therapies and OA treatment.
Supplementary information
Acknowledgements
The authors thank all members of the Arthritis & Regenerative Medicine Laboratory at the University of Aberdeen, particularly Dr. Sadaf Ashraf for the TRAP staining, and Dr. Alison Richmond, Ausra Lionikiene and Susan Clark for technical assistance; Cameron Simpson and Donald Menzies for their contributions to data collection and analysis; Dr. Elazar Zelzer for providing the Gdf5-CreER mice; Dr. Mark Behlke and the R&D team at IDT for providing the validated gene knockdown reagents; Staff at the University of Aberdeen’s Animal Facility and Microscopy & Histology Facility for their support. Arthritis Research UK (Grants No. 20775, 19667, 20865, 21156); European Union’s Horizon 2020 research and innovation programme under Marie Sklodowska Curie Grant Agreement No. 642414; Medical Research Council (Grant MR/L022893/1); A.H.K.R. was supported by the Wellcome Trust through the Scottish Translational Medicine and Therapeutics Initiative (Grant No. WT 085664).
Author contributions
A.J.R. and C.D.B. conceived and oversaw the project. K.K., A.J.R. and C.D.B. designed experiments and interpreted data, with input from T.D.C. T.D.C. provided BAC transgenic mice. K.K. oversaw mouse breeding and performed and analysed in vivo experiments. H.W., A.J.R. and K.K. performed mouse surgeries. F.C., K.K. and A.J.R. performed and analysed in vitro experiments. A.H.K.R. collected human tissues. K.A.H. advised on knockdown experiments. T.A. and F.D.A. provided and analysed microarray data. K.K., A.J.R. and C.D.B. wrote the manuscript, with input from T.D.C. All authors critically reviewed the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Anke J. Roelofs and Cosimo De Bari.
Supplementary information
is available for this paper at 10.1038/s41598-019-57011-8.
References
- 1.Loughlin J. Genetic contribution to osteoarthritis development. Curr. Opin. Rheumatol. 2015;27:284–288. doi: 10.1097/BOR.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang R, et al. A comprehensive meta-analysis of association between genetic variants of GDF5 and osteoarthritis of the knee, hip and hand. Inflamm. Res. 2015;64:405–414. doi: 10.1007/s00011-015-0818-9. [DOI] [PubMed] [Google Scholar]
- 3.Zengini E, et al. Genome-wide analyses using UK Biobank data provide insights into the genetic architecture of osteoarthritis. Nat. Genet. 2018;50:549–558. doi: 10.1038/s41588-018-0079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyamoto Y, et al. A functional polymorphism in the 5’ UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat. Genet. 2007;39:529–533. doi: 10.1038/2005. [DOI] [PubMed] [Google Scholar]
- 5.Southam L, et al. An SNP in the 5′-UTR of GDF5 is associated with osteoarthritis susceptibility in Europeans and with in vivo differences in allelic expression in articular cartilage. Hum. Mol. Genet. 2007;16:2226–2232. doi: 10.1093/hmg/ddm174. [DOI] [PubMed] [Google Scholar]
- 6.Egli RJ, et al. Functional analysis of the osteoarthritis susceptibility-associated GDF5 regulatory polymorphism. Arthritis Rheum. 2009;60:2055–2064. doi: 10.1002/art.24616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storm EE, et al. Limb alterations in brachypodism mice due to mutations in a new member of the TGFβ-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- 8.Chang SC, et al. Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-β superfamily predominantly expressed in long bones during human embryonic development. J. Biol. Chem. 1994;269:28227–28234. [PubMed] [Google Scholar]
- 9.Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 10.Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res. Part C Embryo Today Rev. 2003;69:144–155. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- 11.Rountree RB, et al. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004;2:e355. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koyama E, et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev. Biol. 2008;316:62–73. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shwartz Y, Viukov S, Krief S, Zelzer E. Joint Development Involves a Continuous Influx of Gdf5-Positive Cells. Cell Rep. 2016;15:2577–2587. doi: 10.1016/j.celrep.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roelofs AJ, et al. Joint morphogenetic cells in the adult mammalian synovium. Nat. Commun. 2017;8:15040. doi: 10.1038/ncomms15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas JT, et al. Human chondrodysplasia due to a mutation in a TGF-β superfamily member. Nat. Genet. 1996;12:315–317. doi: 10.1038/ng0396-315. [DOI] [PubMed] [Google Scholar]
- 16.Polinkovsky A, et al. Mutations in CDMP1 cause autosomal dominant brachydactyly type C. Nat. Genet. 1997;17:18–19. doi: 10.1038/ng0997-18. [DOI] [PubMed] [Google Scholar]
- 17.Faiyaz-Ul-Haque M, et al. Mutation in the cartilage-derived morphogenetic protein-1 (CDMP1) gene in a kindred affected with fibular hypoplasia and complex brachydactyly (DuPan syndrome) Clin. Genet. 2002;61:454–458. doi: 10.1034/j.1399-0004.2002.610610.x. [DOI] [PubMed] [Google Scholar]
- 18.Harada M, et al. Developmental failure of the intra-articular ligaments in mice with absence of growth differentiation factor 5. Osteoarthritis Cartilage. 2007;15:468–474. doi: 10.1016/j.joca.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Pregizer SK, et al. Impact of broad regulatory regions on Gdf5 expression and function in knee development and susceptibility to osteoarthritis. Ann. Rheum. Dis. 2018;77:450. doi: 10.1136/annrheumdis-2017-212475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grüneberg H, Lee AJ. The anatomy and development of brachypodism in the mouse. J. Embryol. Exp. Morphol. 1973;30:119–141. [PubMed] [Google Scholar]
- 21.Daans M, Luyten FP, Lories RJ. GDF5 deficiency in mice is associated with instability-driven joint damage, gait and subchondral bone changes. Ann. Rheum. Dis. 2011;70:208–213. doi: 10.1136/ard.2010.134619. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, et al. Heads, Shoulders, Elbows, Knees, and Toes: Modular Gdf5 Enhancers Control Different Joints in the Vertebrate Skeleton. PLoS Genet. 2016;12:e1006454. doi: 10.1371/journal.pgen.1006454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capellini TD, et al. Ancient selection for derived alleles at a GDF5 enhancer influencing human growth and osteoarthritis risk. Nat. Genet. 2017;49:1202–1210. doi: 10.1038/ng.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiapour AM, Cao J, Young M, Capellini TD. The role of Gdf5 regulatory regions in development of hip morphology. PLoS One. 2018;13:e0202785. doi: 10.1371/journal.pone.0202785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 27.DiLeone RJ, Russell LB, Kingsley DM. An extensive 3′ regulatory region controls expression of Bmp5 in specific anatomical structures of the mouse embryo. Genetics. 1998;148:401–8. doi: 10.1093/genetics/148.1.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurth TB, et al. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63:1289–1300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- 29.Roelofs AJ, De Bari C. Immunostaining of Skeletal Tissues. Methods Mol. Biol. 2019;1914:437–450. doi: 10.1007/978-1-4939-8997-3_25. [DOI] [PubMed] [Google Scholar]
- 30.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001;23:291–299. [PubMed] [Google Scholar]
- 32.Karystinou A, et al. Yes-associated protein (YAP) is a negative regulator of chondrogenesis in mesenchymal stem cells. Arthritis Res. Ther. 2015;17:147. doi: 10.1186/s13075-015-0639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ijiri K, et al. Differential expression of GADD45β in normal and osteoarthritic cartilage: Potential role in homeostasis of articular chondrocytes. Arthritis Rheum. 2008;58:2075–2087. doi: 10.1002/art.23504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kan A, et al. SOX11 contributes to the regulation of GDF5 in joint maintenance. BMC Dev. Biol. 2013;13:4. doi: 10.1186/1471-213X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartmann C, Tabin CJ. Wnt-14 Plays a Pivotal Role in Inducing Synovial Joint Formation in the Developing Appendicular Skeleton. Cell. 2001;104:341–351. doi: 10.1016/S0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 36.Guo X, et al. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Decker RS, et al. Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Dev. Biol. 2017;426:56–68. doi: 10.1016/j.ydbio.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sergijenko A, Roelofs AJ, Riemen AHK, De Bari C. Bone marrow contribution to synovial hyperplasia following joint surface injury. Arthritis Res. Ther. 2016;18:166. doi: 10.1186/s13075-016-1060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 40.Bobacz K, et al. Differentially regulated expression of growth differentiation factor 5 and bone morphogenetic protein 7 in articular cartilage and synovium in murine chronic arthritis: potential importance for cartilage breakdown and synovial hypertrophy. Arthritis Rheum. 2008;58:109–118. doi: 10.1002/art.23145. [DOI] [PubMed] [Google Scholar]
- 41.Reynard LN, Bui C, Syddall CM, Loughlin J. CpG methylation regulates allelic expression of GDF5 by modulating binding of SP1 and SP3 repressor proteins to the osteoarthritis susceptibility SNP rs143383. Hum. Genet. 2014;133:1059–1073. doi: 10.1007/s00439-014-1447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dell’Accio F, Vincent TL. Joint surface defects: clinical course and cellular response in spontaneous and experimental lesions. Eur. Cell. Mater. 2010;20:210–217. doi: 10.22203/eCM.v020a17. [DOI] [PubMed] [Google Scholar]
- 43.Tsumaki N, et al. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J. Cell. Biol. 1999;144:161–173. doi: 10.1083/jcb.144.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parrish WR, et al. Intra-articular therapy with recombinant human GDF5 arrests disease progression and stimulates cartilage repair in the rat medial meniscus transection (MMT) model of osteoarthritis. Osteoarthritis Cartilage. 2017;25:554–560. doi: 10.1016/j.joca.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Lehmann W, et al. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat. Commun. 2016;7:10498. doi: 10.1038/ncomms10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.