Abstract
Objectives
The aim of this study was to develop and assess the effectiveness of a patient decision aid (PDA) to support treatment decision making in Spanish patients with moderate-to-severe rheumatoid arthritis (RA) who fail to achieve the therapeutic goal with the current disease-modifying antirheumatic treatment strategy.
Methods
The PDA was developed in accordance with the International Patient Decision Aids Standards recommendations. A steering group led the project. Three literature reviews and two focus groups were performed to develop the PDA prototype. To check its comprehensibility, acceptability, and feasibility, alpha-testing was performed using the Decision Support Acceptability Scale (DSAS). Beta-testing was conducted to assess preliminary evidence of PDA efficacy using the Decisional Conflict Scale (DCS) before and after PDA use. Readiness was evaluated using the Preparation for Decision Making Scale (PDMS).
Results
The PDA included (1) a brief description of RA, (2) treatment information, and (3) a values clarification section. Alpha-testing revealed that most patients considered that the information was presented in a good or excellent way and it could help clarify their values and facilitate treatment decision making. Most rheumatologists agreed that the PDA was easy to understand, to use, and allowed them to reach a shared decision. Beta-testing showed that PDA significantly reduced overall patients’ decisional conflict [33.2 (DE: 21.4) vs 24.6 (23.5); p < 0.001] and prepared the patient for decision making [PDMS: 67.5 (21.0)].
Conclusions
We developed a PDA for Spanish patients with moderate-to-severe RA that reduces patients’ decisional conflict and increases their readiness for decision making. The use of this PDA in routine clinical practice may improve the quality of the decision-making process and the quality of the choices made.
Electronic supplementary material
The online version of this article (10.1007/s40271-019-00381-y) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| Patient decision aids (PDAs) provide a useful tool to involve patients in the decision-making process, ultimately promoting shared patient–physician decision-making. |
| The use of a PDA in patients with moderate-to-severe rheumatoid arthritis (RA), who are unable to achieve their therapeutic goals with the current therapeutic strategy, reduces patients’ decisional conflict and should facilitate patient–physician communication by improving their knowledge of the disease and helping them clarify their values related to treatment. |
| Health professionals’ adoption of a PDA as part of RA patients’ care may facilitate shared decision making and help tailor treatment to patients’ needs. |
Introduction
Rheumatoid arthritis (RA) is amongst the most common chronic inflammatory diseases. It is characterized by inflammation and swelling of the joint synovium, leading to irreversible joint destruction and disability [1]. To date, RA is not a curable disease [2] and it may continue to manifest itself even after inflammatory activity is controlled [1]. Pain, disability, and work limitations associated with joint damage have a negative impact on patient health-related quality of life (HRQOL), spanning both the physical and mental domains of well-being [3].
To date, several efficacious agents are available for RA treatment, including conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs), biological (b) DMARDs and targeted synthetic (ts) DMARDs [4]. Among the csDMARDs, methotrexate has been adopted as the anchor drug [4]. However, when the therapeutic goal is not achieved with the initial csDMARDs strategy, the decision as to which treatment to choose is not straightforward, given the increasing number of therapeutic options and the lack of clear recommendations [4, 5].
According to current RA guidelines, treatment selection should be based on a shared decision between the patient and the rheumatologist [4, 6]. Shared decision making (SDM), defined as “an approach where clinicians and patients share the best available evidence when faced with the task of making decisions, and where patients are supported to consider options” [7], is essential in patient-centered care [8]. Achieving SDM depends on a good patient–physician relationship, which facilitates the exchange of information and allows patients to express their preferences and perspective throughout the decision-making process [9]. SDM involves three stages: (1) introduction of the disease and its treatment options to the patient, (2) detailed description of the options, and (3) exploration of patients’ preferences to facilitate patient decision making [9].
Several authors suggest that people with high decisional conflict (individual perception of uncertainty about which course of action to take) are more likely to delay the decision, to change their mind, or to express decisional regret [10, 11]. The decisional conflict related to treatment decision making arises from the inherent complexity of the choice involving trade-offs between benefits and risks, from scientific uncertainties, and from modifiable factors such as inadequate knowledge, unclear values, and insufficient support for decision making [12]. These modifiable factors can be addressed using patient decision aids (PDAs) [13]. PDAs are generally designed to provide detailed information related to the disease and its treatment options and to guide patients in the decision-making process [12]. They usually include a specific section that helps patients explore their own perspective and values regarding key aspects of the decision they face [14]. Nowadays, several PDAs for RA patients are available [15–23]; however, none of them were developed for the Spanish population and only one was available in Spanish.
In order to be effective, a PDA should improve the quality of the decision-making process and the quality of the choice made. Thus, to establish the effectiveness of a PDA, its impact on the following aspects should be measured: (1) awareness that a decision needs to be made, (2) knowledge of therapeutic options as well as the risks and benefits of each option, (3) clarity about what matters most for this decision, (4) involvement in the decision-making process, and (5) consistency between the choice made and the patient’s informed values [24].
The main objective of the study was to develop and assess the effectiveness of a PDA to support treatment decision making in Spanish patients with moderate-to-severe RA who fail to achieve the therapeutic goal with the current DMARD strategy.
Methods
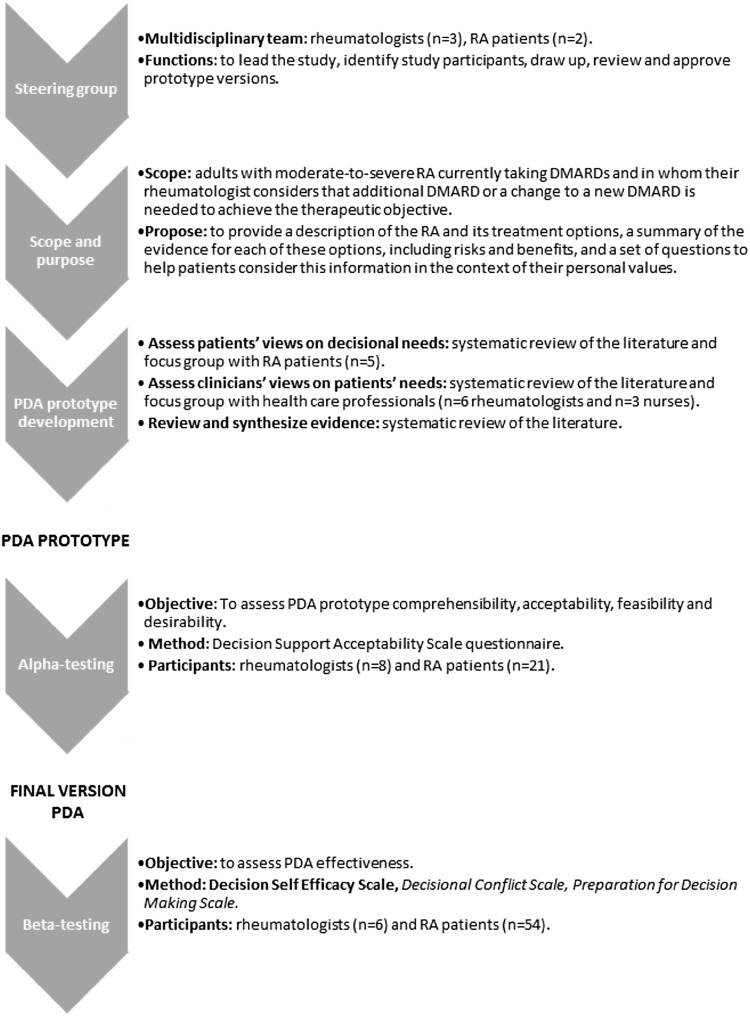
The PDA was developed in accordance with the systematic process proposed by the International Patient Decision Aids Standards (IPDAS) [25] (Fig. 1) and its quality criteria [26, 27].
Fig. 1.
International Patient Decision Aids Standards (IPDAS) model development process for decision aids
Steering Group
A multidisciplinary steering group of experts led the study and helped to define the scope and purpose of the PDA. The steering group participated in the identification of study participants, assisted in designing and reviewing the PDA prototype, and contributed to the interpretation of the results of the different phases of the study.
Patient Decision Aid (PDA) Prototype Development
A PDA prototype was developed based on the information identified in three literature reviews (two of them were systematic reviews of the literature) and two focus groups, one with RA patients and one with healthcare professional experts in RA management (rheumatologists and nurses). This first draft was reviewed by the steering group.
Since the PDA target was to support treatment decision making in Spanish patients with moderate-to-severe RA who fail to achieve the therapeutic goal with the current DMARD strategy, all study participants had previous experience with medications.
Literature Reviews
The objectives of the three literature reviews were as follows.
To identify available PDAs for patients with moderate-to-severe RA. The information from this literature review was used to confirm the need for a PDA in these patients and to define PDA purpose and scope.
To explore patients’ and physicians’ preferences for RA treatment characteristics. The information provided by this literature review was useful to identify the most important aspects related to RA treatment from patients’ and physicians’ perspectives, to be discussed during focus groups in order to define the content to be included in the PDA.
To summarize current evidence regarding the efficacy and safety of available RA treatments. This information was used to develop the content of the PDA.
The methods of these reviews are presented in the electronic supplementary material (S1).
Focus Groups
Two focus groups were conducted. RA patients were invited to participate in a patient focus group by the patient advocacy group ‘ConArtritis’. Healthcare professional experts in RA management were selected according to their professional experience and interest in the project and invited to participate in the expert focus group by the Steering Committee.
The focus group is a qualitative technique that aims to obtain data from a purposely selected group of individuals rather than from a statistically representative sample of a broader population. It is used to generate information on collective views and to understand participants’ experiences and beliefs [28]. The main objectives of the face-to-face focus groups were to explore the perceived need for decision support, including barriers and facilitators of decision making; to assess which information was perceived as important in decision making; and to investigate preferences related to treatment characteristics. The results of the literature reviews were presented during the focus groups and were used as inputs for discussion in both focus groups. The moderator probed participants’ experiences related to the management of the disease and the decision-making process, asked them to share and compare experiences, and discuss the extent to which they agree or disagree with each other. Information was gathered until data saturation, defined as when no additional information was provided by participants and whereby the collection of more data appeared to have no additional interpretive worth. Note-based analysis of focus group data, including analysis of notes from the focus group and any summary comments from the moderator or assistant moderator, was conducted. The audiotapes of the sessions were used to verify quotations or to glean more information. After reviewing the data as a whole, concepts, trends, and themes were identified to organize the data. The qualitative analysis of the data occurred along a continuum throughout the process and aimed to provide subjective insight into the perspectives of the participants and how their thoughts and perceptions could be applied to a larger population. The interpretation of the qualitative analysis allowed identification of the most important aspects of the data and the main points. Finally, data analysis of both focus groups was synthesized and summarized, in order to identify the main content to be included PDA.
PDA Prototype Alpha-Testing
The main objective of alpha-testing is to assess PDA prototype comprehensibility (degree to which the content of the PDA is understandable), acceptability (degree to which the PDA adds value to the consultation), feasibility (degree to which the PDA would fit into practice), and desirability (degree to which the PDA was presented in a visually appealing way). Alpha-testing was conducted using the Decision Support Acceptability Scale (DSAS) [29] in the context of the two discussion groups, one with RA patients and one with rheumatologists. In this phase, a minimum of ten participants was required [30]. DSAS includes questions regarding the length, graphics, understandability, balance of the PDA, and questions to assess participants’ opinions about the content of the PDA. The relative and absolute frequencies of each response were calculated. Moreover, participants were asked to provide recommendations for PDA improvement.
Based on the results obtained in the alpha-testing, the steering group discussed and proposed the adjustments to be implemented on the first PDA prototype.
PDA Prototype Beta-Testing
A pre-post test study was conducted in five hospitals of the public Spanish National Health System. The main objective of beta-testing was to assess the preliminary evidence of the PDA prototype efficacy. Study participants were recruited in clinical settings by rheumatologists. Patient recruitment was non-probabilistic. RA patients were invited to participated in the study if they met the following inclusion criteria: (1) older than 18 years, (2) currently receiving DMARD (cs or bDMARD), (3) require a change in treatment due to failure to achieve the therapeutic goal. For patient and rheumatologist recruitment, non-probability sampling was used.
During beta-testing, data from both patients and rheumatologists was gathered using a case report form (CRF). Both patients’ and rheumatologists’ CRFs were self-completed during the physician–patient encounter.
Following a successful screen for eligibility, RA patients were invited to participate in the study by rheumatologists. Once a patient agreed to participate in the study, they completed section A of the patient’s CRF. This section collected the patient’s sociodemographic characteristics (age, gender, level of education, time from diagnosis) and included the Decision Self-Efficacy Scale (DSES) and Decisional Conflict Scale (DCS). DSES measures self-confidence in one’s abilities in decision making, including shared decision making [31]. DCS assesses patients’ uncertainty about which treatment to choose, factors contributing to uncertainty, and perceived effectiveness of decision making. DCS measures personal perception of uncertainty in choosing options (‘uncertainty subscale’), assesses patients’ knowledge of therapeutic options, and the risks, benefits, and consequences of the options (‘informed subscale’), measures clarification about what matters most to patients for this decision (‘values clarity subscale’) determines support in decision making (‘support subscale’) and provides patients’ perception about the consistency between the choice made and their informed values (‘effective decision subscale’) [32]. DCS scores < 25 are associated with following through with decisions while scores > 37.5 are associated with decision delay or feeling uncertain about implementation [32]. This section formed the pre-test study and was completed before PDA use. At this point, patients proceeded to the PDA (administered by the rheumatologist during clinical encounter) and were asked to make a decision after reviewing the information provided by the PDA. Then, patients were asked to complete the CRF section B, which included the DCS and Preparation for Decision-Making Scale (PDMS). The PDMS assesses the patient’s perception of how useful the PDA was in helping patients to recognize that a decision needs to be made, in preparing the respondent for communicating with their practitioner, and in promoting patient involvement in the decision-making process [33]. This section formed the post-test and was completed after PDA use.
Lastly, rheumatologists described their experience and their perceptions in the rheumatologists’ CRF. It collected rheumatologists’ sociodemographic (age, gender) and job-related characteristics (time practicing specialty and number of patients seen per week) and included the PDMS, practitioner version, which assesses the physician’s perception of how useful the PDA was for patients.
All questionnaires used in the study have been psychometrically validated [10, 31, 34].
The primary outcome of the study was the change in decisional conflict assessed by the DCS 16-item statement format [32]. Accordingly, the sample size was calculated based on the expected percentage (30%) of variation in the DCS before and after use of the PDA, as reported in the literature [35], a power of 95% and a 95% confidence level. Thus, a minimum of 52 patients with RA was required.
Baseline characteristics of participants were analyzed descriptively. To assess the effectiveness of the PDA, paired comparisons were performed between the scores obtained by the patients in the DCS questionnaire before and after using the PDA. Paired t test and Wilcoxon test were used depending on the normality of data. Normality was checked by the Saphiro–Wilk test. All statistical analyses were completed using Stata v. 14.0.
Results
Steering Group
The steering group comprised three rheumatologists with extensive expertise in the management of RA patients and in patient-centered care, and two RA patients (ConArtritis Advocacy Group) with comprehensive knowledge of the disease and its treatment.
PDA Prototype Development
Available PDAs for Rheumatoid Arthritis (RA) Patients: Results of the Literature Review
The literature review identified a total of 15 PDAs for RA patients, mostly developed in Canada and in the USA. Most of them provide information related to a specific treatment [36–46] and their development process had not been published. There is only information available about the development and utility to reduce decisional conflict in three of the PDAs identified [20–22]. One of these PDAs is specific for motherhood [22]. None of the PDAs identified were developed for the Spanish population, and only one was translated to Spanish [23].
Scope and Purpose of the PDA
The purpose of the PDA was to improve decision making among adults with moderate-to-severe RA currently taking a DMARD and whose rheumatologist considers that an additional DMARD or a change to a new DMARD is needed to achieve the therapeutic goal.
Given the diversity in the patient profiles (computer experienced or computer inexperienced) and due to the heterogenicity detected between healthcare sites regarding Internet availability, both a paper-based and an online interactive multimedia PDA prototype were developed. The results presented are for both modalities.
Patient and Healthcare Professional Input: Results from the Focus Groups
Five patients with RA (60% female, age range: 38–67 years) and nine healthcare professionals (six rheumatologists and three nurses) participated in two focus groups.
The patients indicated that the disease-related information provided by the PDA should be clear, simple, and graphical in order to be easily understood. It should contain information related to RA risk factors, including genetic factors. According to patients, the PDA should also stress the concept that no cure is currently available for RA, and that it can lead to severe disability if the patient does not receive optimal treatment. Additionally, patients stated the importance of including all available information about treatment options, such as the potential benefits and risks associated with their use (including severe adverse events), and time to onset of treatment effects. Finally, they pointed out the importance of informing patients about the possibility of receiving psychological support to manage the emotional aspects of the disease.
Healthcare professionals pointed out that the main difficulty encountered, when presenting therapeutic options, was ensuring that patients understood the benefits and not only the risks associated with each treatment. They reported that some patients do not understand their disease and its treatment, do not know that RA has no cure, and think that most RA treatments may trigger severe adverse events. Moreover, they stated that, routinely, patients’ preferences are not considered during decision making. For this reason, they indicated the importance of including questions that allow them to evaluate patients’ perspective on their current situation (perceived treatment benefits), their main fears, and their willingness to change treatment.
Patients and rheumatologists agreed that one of the main challenges of the PDA is to explain the disease in a positive way, not just focusing on the risks of disease and treatments. However, they highlighted the need to emphasize that RA is a serious disease. Both groups of participants considered that the PDA would help to improve patients’ knowledge about the disease and its treatment, and would enhance the patient–rheumatologist relationship, which could contribute to improving treatment adherence. Despite recognizing the benefits of PDA use in clinical practice, patients and healthcare professionals considered that the time needed to complete PDAs might represent a barrier for their implementation.
PDA Prototype Alpha-Testing
A total of eight rheumatologists and 21 RA patients participated in the alpha-testing (Table 1).
Table 1.
Sociodemographic and clinical characteristics of the participants in PDA alpha- and beta-testing
| Alpha-testing | Beta-testing | |||
|---|---|---|---|---|
| Rheumatologists (n = 8) | RA patients (n = 21) | Rheumatologists (n = 6) | RA patients (n = 54) | |
| Age [mean (SD)] (years) | 44.75 (8.55) | 48.95 (12.34) | 37.67 (11.52) | 58.82 (12.85) |
| Women [% (n)] | 50 (4) | 90.5 (19) | 66.67 (4) | 90.38 (47) |
| Time practicing specialty, years (SD) | 17.13 (8.32) | – | 11.5 (9.4) | – |
| Number of patients seen per week (SD) | 33.13 (21.58) | – | 28.33 (28.23) | – |
| Marital status [% (n)] | ||||
| Single | – | 28.6 (6) | – | – |
| Partnership/married | 47.6 (10) | |||
| Separated/divorced | 19 (4) | |||
| Widowed | 0 (0) | |||
| No response | 4.8 (1) | |||
| Level of education [% (n)] | ||||
| Primary school | – | 14.3 (2) | – | 38.46 (20) |
| High School | 23.8 (5) | 34.62 (18) | ||
| University or higher | 57.1 (12) | 21.15 (11) | ||
| Others | 4.8 (1) | 5.77 (3) | ||
| Time from diagnosis [mean (SD)] (years) | – | 15.71 (12.08) | – | 11.72 (12.48) |
| Comorbidities (% yes) | – | 57 (12) | – | 44.44 (24) |
PDA patient decision aids, RA rheumatoid arthritis, SD standard deviation
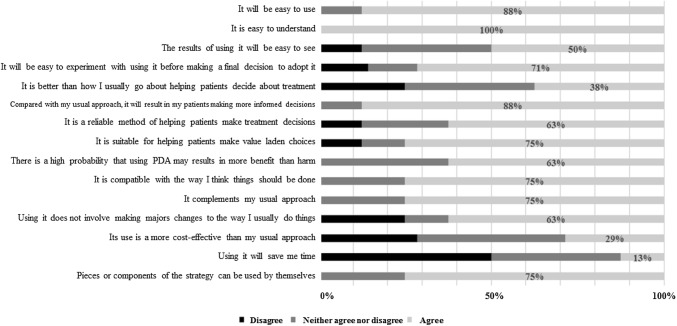
Most of the rheumatologists agreed that the PDA was easy to use and to understand. Moreover, they considered that, compared with their usual approach, the PDA was a better or equally efficient strategy and it would result in their patients making a more informed and value-based decision (Fig. 2).
Fig. 2.
Results of PDA alpha-testing (initial evaluation of the first draft PDA prototype). Rheumatologists’ perspective (N = 8)
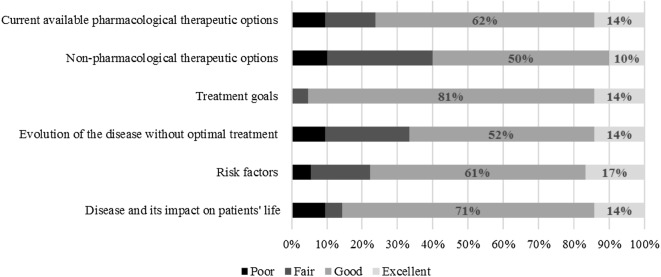
As regard to the patients’ perspective, most of them reported that the information provided by PDA was presented in a good or excellent way (Fig. 3). More than 60% of patients agreed that the length of the presentation and the amount of information provided was just right. Nearly 40% considered the presentation of the information to be balanced. Most of them believed that PDA may be useful during treatment decision making (76%), would allow an easy decision making (95%), and would help clarify patients’ values (71%).
Fig. 3.
Results of PDA alpha-testing (initial evaluation of the first draft PDA prototype). Patients’ perspective regarding the information provided by the PDA (N = 21)
Nearly 60% of patients requested more information about the treatment, mainly related to adverse events, and 18% asked for more information about non-pharmacological treatment.
Related to value clarification sections, patients reported their preference for answering a Likert scale with more than three options.
Final PDA Prototype
The feedback received from the participants of alpha-testing and from the steering group was used to refine the PDA prototype. The main proposals for PDA improvements were related to its content, the language used, and the questions asked to assess patients’ values and preferences.
The wording of the document was reviewed in order to make it more positive and more understandable for patients. Following patients’ suggestions, more specific information was included related to adverse events, pharmacological and non-pharmacological treatment, and psychological affectation of the disease. Furthermore, to meet the needs expressed by patients and healthcare professionals during the alpha-test, the questions included in the values clarification section were reformulated, a question related to pregnancy was included, and a 3-point Likert scale was changed to a 10-point Likert scale.
The final PDA prototype included the following main sections.
General introduction to the PDA A description of PDA scope and purpose, brief instructions for use, members of the steering group and disclosure of interest (source of funding).
RA information Description of the disease, including its main symptoms (articular and extra-articular manifestations), treatment goals and therapeutic options (including main adverse events), evolution of RA in the absence of optimal treatment, and other information of interest (smoking cessation, pregnancy, vaccines, travel, psychological assistance, and links of interest to patient association websites, or the Spanish Rheumatology Society website).
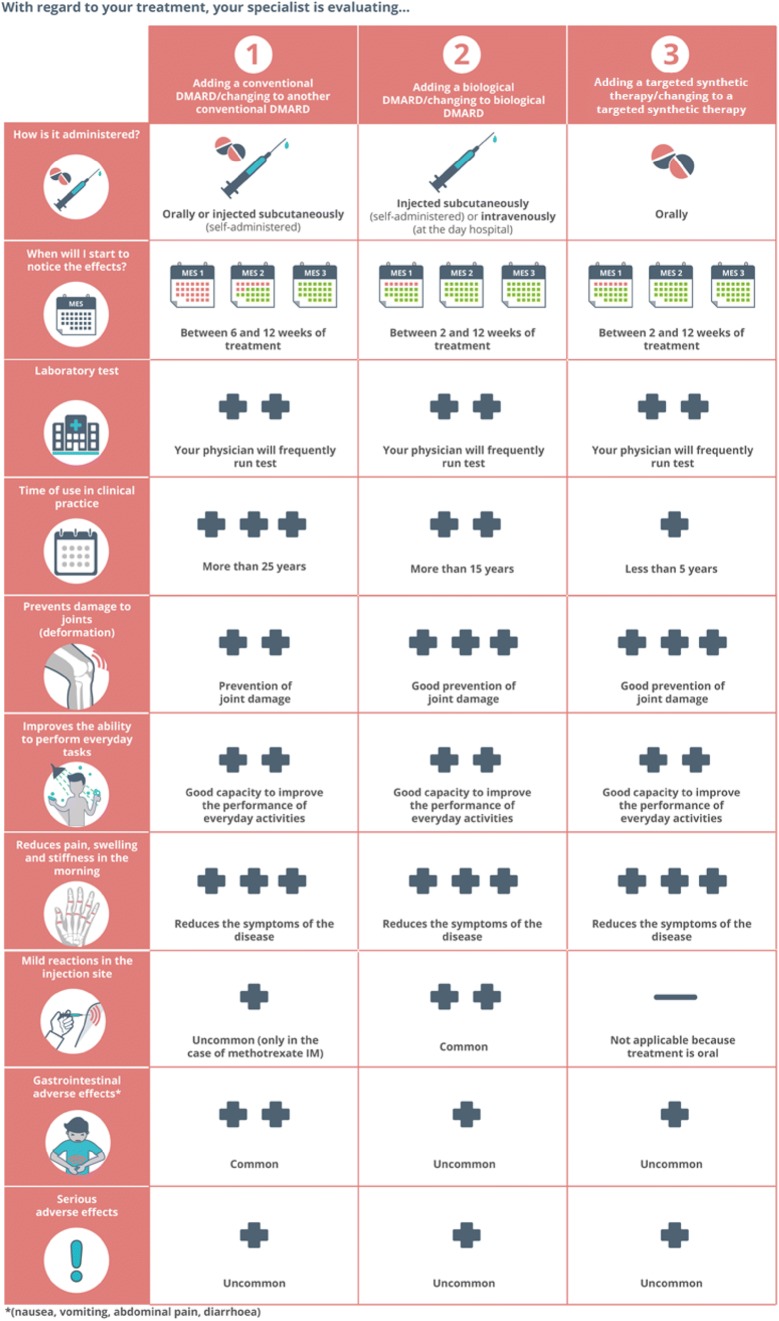
Treatment options comparison A matrix including verbal and graphical information allows easy comparisons between the three options: add or change to another csDMARD; add or change to bDMARD; add or change to tsDMARD. Elements that are compared included administration route, approximate time to benefit, follow-up process, time of use in clinical practice, clinical benefits (prevention of joint damage, improvement in the ability to perform daily activities, reduction of pain, swelling, and morning stiffness), and frequency of side effects (injection-site reaction, gastrointestinal side effects, severe side effects). To improve comprehensibility and desirability, some of the information in this section is presented using pictographs (Fig. 4).
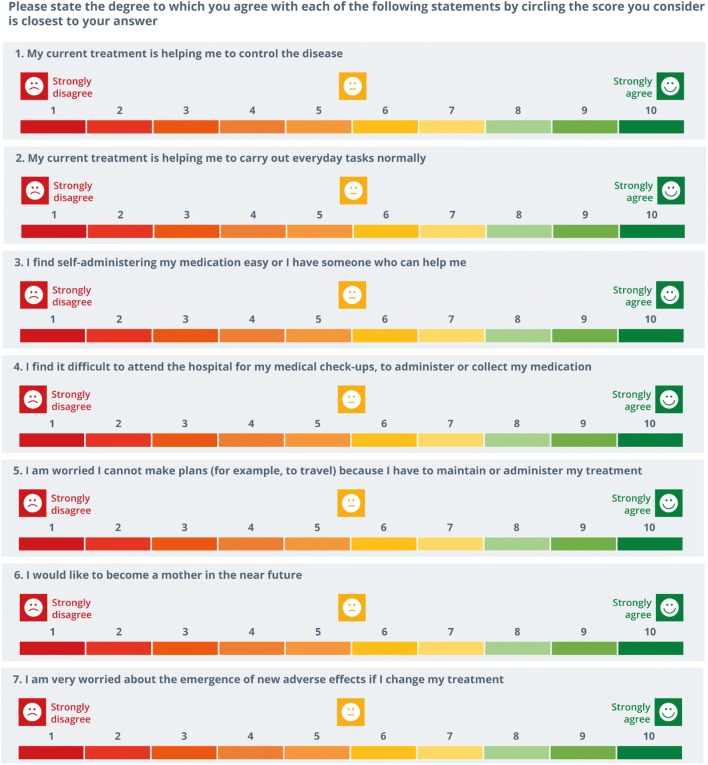
Values clarifications A set of questions was formulated to help patients to reflect and be aware regarding their current situation and to clarify and communicate their personal values. These questions included patients’ perception related to current treatment efficacy, patients’ ability to self-inject treatment, patients’ concern about treatment storage, patients’ difficulties to get to or spending hours at the hospital, patients’ desire to become a mother, and patients’ worries about adverse events (Fig. 5). Patients should indicate the degree to which they agree or disagree with each question on a 10-point Likert scale. The format of this section (die cut—paper format or printed separately—online format) allows patients to share their results with their rheumatologist.
References The main bibliographic references used to develop PDA content.
Fig. 4.
Matrix that allows patients to compare treatment options
Fig. 5.
Set of questions that allows patients to clarify their values
Final PDA Prototype Beta-Testing
A total of 54 patients and six rheumatologists participated in the beta-testing (Table 1).
The mean (SD) DSES score obtained [72.3 (19.9)] indicated that patients’ confidence in their abilities in decision making was moderate to high (Table 2).
Table 2.
Results of beta-testing
| Before PDA use [mean (SD)] | After PDA use [mean (SD)] | p value | |
|---|---|---|---|
| Patients (N = 54) | |||
| DSES total score | 72.3 (19.9) | – | |
| DCS total score | 33.2 (21.4) | 24.6 (23.5) | < 0.001‡ |
| Informed subscale | 39.0 (27.9) | 29.3 (27.0) | 0.004† |
| Values clarity subscale | 35.6 (29.6) | 25.0 (27.1) | 0.005‡ |
| Support subscale | 24.1 (22.8) | 22.8 (26.8) | 0.024† |
| Uncertainly subscale | 41.1 (29.4) | 30.2 (28.2) | < 0.001‡ |
| Effective decision subscale | 27.7 (26.6) | 18.1 (22.9) | < 0.001† |
| DCS score % patients (n) | 0.008 | ||
| < 25 | 40.7 (22) | 57.4 (31) | |
| 25–37.5 | 25.9 (14) | 20.4 (11) | |
| > 37.5 | 33.3 (18) | 22.2 (12) | |
| Patients’ PDMS total score | 67.5 (21.0) | ||
| Rheumatologists (N = 6) | |||
| Rheumatologists’ PDMS total score | 49.4 (18.5) | ||
DCS Decisional Conflict Scale, DSES Decision Self-Efficacy Scale, PDA patient decision aids, PDMS Preparation for Decision Making Scale, SD standard deviation
†Wilcoxon test
‡Test t
Before PDA use, 33.33% of participants presented a high decisional conflict (DCS > 37.5) as compared with 22.22% after PDA use (p = 0.008). Decisional conflict scores indicated a statistically significant reduction in overall decisional conflict [33.2 (21.4) vs 24.6 (23.5); p < 0.001] and in all decisional conflict subscales including feeling uncertain [41.1 (29.4) vs 30.2 (28.2); p < 0.001], feeling unclear about values related to the treatment election [35.6 (29.6) vs 25.0 (27.1); p = 0.005], feeling uninformed [39.0 (27.9) vs 29.3 (27.0); p = 0.004], feeling that the decision made it is not effective or good [27.7 (26.6) vs 18.1 (22.9); p < 0.001], and feeling unsupported [24.1 (22.8) vs 22.8 (26.8); p = 0.024] after PDA use (Table 2).
The mean (SD) score obtained in PDMS [67.5 (21.0)] indicated that patients perceived that PDA helped them to communicate with their practitioner and to make the decision. Conversely, rheumatologists showed a more neutral perception, reporting a mean (SD) score for this questionnaire of 49.4 (18.5) (Table 2).
Discussion
The proportion of patients willing and asking to be involved in clinical decisions during encounters with their physicians is constantly growing [47]. This changing attitude implies the need for the physician to consider the patient’s preferences and to provide them with more information regarding their disease and treatments. Thus, the patient needs to develop a knowledge base that is relevant to the decision-making problem and then establish their knowledge-based preferences for treatment options [48].
SDM has been shown to increase patient knowledge, reduce anxiety over the care process, and improve health outcomes [49] by ensuring that medical care better aligns with patients’ preferences and values. However, the benefits of SDM are not limited to patients. Indeed, it has the potential to provide benefits to clinicians and the healthcare system, reducing unwarranted variation in care and costs [49]. In RA, where there are no clear treatment recommendations following DMARD failure, involving patients and integrating their preferences in the decision-making process may contribute to patients’ engagement and improve treatment adherence. This process might be facilitated by the use of a PDA, providing unbiased information related to the disease and treatment options and contributing to the clarification of patients’ values [50].
The results of the study suggest that the use of a PDA in Spanish patients with moderate-to-severe RA improves the quality of the decision making by helping patients increase their awareness that a decision needs to be made, reducing the perception that they have inadequate understanding of the benefits/risks associated with each option, helping them to clarify their values and fostering patient–rheumatologist communication. Additionally, the use of the PDA reduced the number of patients who perceived that a poor quality or ineffective decision had been made.
It has been suggested that people with high decisional conflict (DCS score ≥ 37.5) are more likely to delay the decision, to change their mind, or to express decisional regret [32]. Our results show that the proportion of patients with high DCS scores significantly decreased after the use of the PDA.
Our findings are in line with previous studies performed in Canadian and Dutch RA patients [15–17, 35]. A web-based PDA for initiating DMARDs in Dutch patients with rheumatic diseases [15], including RA, ankylosing spondylitis, and psoriatic arthritis, was perceived as a helpful tool for the decision-making process and increased the number of patients who played an active role in medical decision making [16]. Similarly, an online PDA to support Canadian patients making decisions about taking methotrexate for RA, showed a reduction in patients’ decisional conflict and an improvement in their knowledge [35]. Finally, a web-based PDA for Canadian RA patients, whose physicians had recommended they begin using or switch to a new bDMARD or tsDMARD, decreased patients’ decisional conflict and improved perceived self-management capacity [17]. Differently from the aforementioned PDA, the PDA developed in our study is based on a Spanish RA population and it encompasses all treatment options after initial DMARD failure, grouped in terms of class of DMARDs.
We developed a PDA following the recommendations of IPDAS [25] and its best practices [26, 27]. The use of the structured development process proposed by IPDAS ensures that the comprehensibility, acceptability, and feasibility of the PDA meets the needs of the individuals for whom the PDA is designed. The main strength of the present work is that it was an interactive process and, therefore, the needs of the different stakeholders involved in RA management (including nurses, rheumatologists, and patients) were considered and adopted during development. Additionally, the development of both a paper-based and online interactive PDA minimized potential barriers for patient access.
The study has several limitations, inherent to the PDA development. During prototype development (focus groups), feedback was obtained from a small number of healthcare professionals and RA patients. Some authors have reported that focus group communication was positively influenced by small group size (between four and six participants) [51]. Despite the small size of the focus group, it is important to keep in mind that data saturation occurred with the iterative qualitative process, which indicated that further feedback may not have necessarily generated new data [52, 53]. Most patients who participated in the alpha- and beta-testing had a higher level of education, and therefore might not represent the entire target audience. Beta-testing is limited by a lack of randomization, the absence of a comparator arm, and the lack of a gold standard measure to assess improvement in the decision-making process and decision quality. Moreover, patients’ disease severity was not collected during the project. The main strengths of the beta-testing is the inclusion of patients at the time when an actual treatment decision was needed and that the questionnaire employed during beta-testing (DCS) is the most commonly used to assess effectiveness of a PDA in the literature [11, 54]. Future multi-center randomized trials should be conducted to further study the impact of this PDA and to compare it with other interventions.
Since the regional health authorities have not encouraged the use of PDAs among professionals or patients, the complexity and difficulty of implementing SDM in the Spanish healthcare setting may be high [55]. Use of a PDA may ensure patients are adequately informed before making medical decisions and promote the physician’s role as facilitator of patient participation in decision making. The PDA developed in this study may help to change the attitudes of the stakeholders involved in the decision-making process, resulting in the adaptation of SDM into routine clinical practice.
Conclusion
This is the first PDA systematically developed and validated for Spanish patients with moderate-to-severe RA following the recommendations of IPDAS. The developed PDA reduced patients’ decisional conflict and increased patients’ preparation for decision making. It may enhance the involvement of RA patients in decision making regarding their treatment options and will help educate patients about asking the right questions. In the same way, it will help healthcare professionals recognize the patient’s role in the decision-making process, contributing to improving the quality of healthcare. The use of this PDA in routine clinical practice may improve the quality of the decision-making process and the quality of the choices that are made.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the Spanish Patient Advocacy Group Coordinadora Nacional de Artritis (ConArtritis), its president Antonio I. Torralba, and its director Laly Alcaide for their support. Also, they are very grateful to all the professionals and patients who participated in the study, substantially contributing to the development of the PDA.
Author contributions
JAS, TD, LL, and MC contributed to the concept and design of the study. JLP, JAJ, and JARI led the project, participated in data collection, and assisted in the identification of study participants. MC drafted the manuscript. TD, LL, JAS, and JIM critically reviewed the manuscript for important intellectual content and contributed to its final version. All authors participated in the PDA elaboration and discussed and contributed to the interpretation of the results. They all reviewed and gave their final approval to the version submitted.
Compliance with Ethical Standards
Conflict of interest
This research was supported by Eli Lilly & Co, Madrid (Spain). JIM, TD, and JAS are employees of Eli Lilly & Co. MC and LL work for an independent research entity that received funding from Eli Lilly & Co to coordinate and conduct the study, and to write up the manuscript. JAJ, JARI, and JLI have nothing to disclose.
Ethical standards
This study was performed in accordance with the Helsinki Declaration of 1964, as revised in 2013, and was approved by the Clinical Research Ethics Committee of Hospital Universitario 12 de Octubre (Madrid). All study participants agreed to participate in the study and signed informed consent before enrollment.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rhematoid arthritis: a synopsis. Am J Manag Care. 2014;20:S128–S135. [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11(5):276–289. doi: 10.1038/nrrheum.2015.8. [DOI] [PubMed] [Google Scholar]
- 3.Matcham F, Scott IC, Rayner L, Hotopf M, Kingsley GH, Norton S, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum [Internet]. 2014;44(2):123–130. doi: 10.1016/j.semarthrit.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 5.Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68(1):1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 6.Stoffer MA, Smolen JS, Woolf A, Ambrozic A, Berghea F, Boonen A, et al. Development of patient-centred standards of care for osteoarthritis in Europe: the eumusc . net-project. Clin Epidemiol Res. 2014;73:902–905. [Google Scholar]
- 7.Elwyn G, Laitner S, Coulter A, Walker E, Watson P, Thomson R. Implementing shared decision making in the NHS. BMJ. 2010;341(7780):971–972. doi: 10.1136/bmj.c5146. [DOI] [PubMed] [Google Scholar]
- 8.Weston WW. Informed and shared decision-making: the crux of patient-centered care. Can Med Assoc J [Internet]. 2001;165(4):438–9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC81370/. Accessed Feb 2018. [PMC free article] [PubMed]
- 9.Elwyn G, Frosch D, Thomson R, Joseph-williams N, Lloyd A, Kinnersley P, et al. Shared decision making : a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’connor AM. Validation of a decisional conflict scale. Med Decis Mak. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 11.Knops AM, Goossens A, Ubbink DT, Legemate DA, Stalpers LJ, Bossuyt PM. Interpreting patient decisional conflict scores: behavior and emotions in decisions about treatment. Med Decis Mak. 2013;33(1):78–84. doi: 10.1177/0272989X12453500. [DOI] [PubMed] [Google Scholar]
- 12.Connor AMO, Légaré F, Stacey D. Risk communication in practice: the contribution of decision aids. BMJ. 2003;327:736–740. doi: 10.1136/bmj.327.7417.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connor AMO, Wennberg JE, Legare F, Hilary A, Moulton BW, Sepucha KR, et al. Toward the “Tipping Point”: decision aids and informed patient choice. Health Aff. 2007;26(3):716–725. doi: 10.1377/hlthaff.26.3.716. [DOI] [PubMed] [Google Scholar]
- 14.Fagerlin A, Pignone M, Abhyankar P, Col N, Feldman-stewart D, Gavaruzzi T, et al. Clarifying values: an updated review. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S8. doi: 10.1186/1472-6947-13-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nota I, Drossaert CHC, Melissant HC, Taal E, Vonkeman HE, Haagsma CJ, et al. Development of a web-based patient decision aid for initiating disease modifying anti-rheumatic drugs using user-centred design methods. BMC Med Inform Decis Mak [Internet]. 2017;17(1):51. http://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-017-0433-5. Accessed Feb 2018. [DOI] [PMC free article] [PubMed]
- 16.Nota I, Drossaert CHC, Taal E, Vonkeman HE, Haagsma CJ, Van De Laar MAFJ. Evaluation of a patient decision aid for initiating disease modifying anti-rheumatic drugs. Arthritis Res Ther [Internet]. 2016;18:252. doi: 10.1186/s13075-016-1138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li LC, Shaw C, Lacaille D, Elaine Y, Jones AC, Koehn C, et al. Effects of a web-based patient decision aid on biologic and small molecule agents for rheumatoid arthritis, ANSWER-2: a Proff-o-concept study. Arthritis Care Res. 2018;70(3):343–352. doi: 10.1002/acr.23287. [DOI] [PubMed] [Google Scholar]
- 18.Barton JL, Koenig CJ, Evans-young G, Trupin L, Anderson J, Ragouzeos D, et al. The design of a low literacy decision aid about rheumatoid arthritis medications developed in three languages for use during the clinical encounter. BMC Med Inform Decis Mak. 2014;14:104. doi: 10.1186/s12911-014-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barton JL, Trupin L, Schillinger D, Evans-Young G, Imboden J, Montori VM, et al. Low literacy decision aid enhances knowledge and reduces decisional conflict among diverse population of adults with rheumatoid arthritis: results of a pilot study. Arthritis Care Res. 2016;68(7):889–898. doi: 10.1002/acr.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li LC, Adam P, Townsend AF, Stacey D, Lacaille D, Cox S, et al. Improving healthcare consumer effectiveness: an Animated, Self-serve, Web-based Research Tool (ANSWER) for people with early rheumatoid arthritis. BMC Med Inform Decis Mak [Internet]. 2009;9:40. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2733893&tool=pmcentrez&rendertype=abstract. Accessed Feb 2018. [DOI] [PMC free article] [PubMed]
- 21.Fraenke L, Peters E, Charpenier P, Olsen B, Errante L, Schoen R, et al. A decision tool to improve the quality of care in rhematoid arthritis. Arthritis Care Res. 2012;64(7):977–985. doi: 10.1002/acr.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meade T, Dowswell E, Manolios N, Sharpe L. The motherhood choices decision aid for women with rheumatoid arthritis increases knowledge and reduces decisional conflict: a randomized controlled trial. BMC Musculoskelet Disord [Internet]. 2015;16(1):260. http://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/s12891-015-0713-0. Accessed Feb 2018. [DOI] [PMC free article] [PubMed]
- 23.Agency for Healthcare Research and Quality (AHRQ). Medicines for RA: a review of the research for adults. 2012. http://effectivehealthcare.ahrq.gov/ehc/products/203/1314/rheum_arth_cons_fin_to_post.pdf. Accessed Feb 2018.
- 24.Sepucha KR, Borkhoff CM, Lally J, Levin CA, Matlock DD, Ng CJ, et al. Establishing the effectiveness of patient decision aids: key constructs and measurement instruments. BMC Med Inform Decis Mak [Internet]. 2013;13(SUPPL. 2):S12. http://www.biomedcentral.com/1472-6947/13/S2/S12. Accessed Feb 2018. [DOI] [PMC free article] [PubMed]
- 25.Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ. A systematic development process for patient decision aids. BMC Med Inform Decis Mak [Internet]. 2013;13(Suppl 2):S2. http://www.biomedcentral.com/1472-6947/13/S2/S2. Accessed Feb 2018. [DOI] [PMC free article] [PubMed]
- 26.Elwyn G, Connor AMO, Bennett C, Newcombe RG, Politi M, Drake E, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument ( IPDASi ) PLoS One. 2009;4(3):e4705. doi: 10.1371/journal.pone.0004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elwyn G, Connor AO, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan D. The focus group guide book. London: Sage Publications; 1998. [Google Scholar]
- 29.O’Connor A, Cranney A. User manual—acceptability [document on the Internet]. Decis Support Accept Scale. 2002;1–5. http://decisionaid.ohri.ca/docs. Accessed Feb 2018.
- 30.Connor AO, Jacobsen MJ. Workbook on developing and evaluating patient decision aids. 2003. https://decisionaid.ohri.ca/docs/develop/develop_da.pdf. Accessed Feb 2018.
- 31.O’connor AM. User manual—decision self-efficacy scale. 1995. https://decisionaid.ohri.ca/docs/develop/user_manuals/UM_decision_selfefficacy.pdf. Accessed Feb 2018.
- 32.O’Connor AM. User manual—decisional conflict scale (16 item statment format). 1993;1–16. https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_decisional_conflict.pdf. Accessed Feb 2018.
- 33.Graham I, O’connor AM. User manual—preparation for decision making scale. 1995. https://decisionaid.ohri.ca/docs/develop/user_manuals/UM_prepdm.pdf. Accessed Feb 2018.
- 34.Bennett C, Graham ID, Kristjansson E, Kearing SA, Clay KF, O’Connor AM. Validation of a preparation for decision making scale. Patient Educ Couns. 2010;78(1):130–133. doi: 10.1016/j.pec.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Li LC, Adam PM, Backman CL, Lineker S, Jones CA, Lacaille D, et al. Proof-of-concept study of a web-based methotrexate decision aid for patients with rheumatoid arthritis. Arthritis Care Res. 2014;66(10):1472–1481. doi: 10.1002/acr.22319. [DOI] [PubMed] [Google Scholar]
- 36.West Michigan Rheumatology P. Should I take methotrexate for my rheumatoid arthritis? 2013. http://www.mi-arthritis.com/patient-decision-aids/methotrexate.html. Accessed Feb 2018.
- 37.West Michigan Rheumatology P. Should I take sulfasalazine for my rheumatoid arthritis? 2013. http://www.mi-arthritis.com/patient-decision-aids/sulfasazine.html. Accessed Feb 2018.
- 38.West Michigan Rheumatology P. Should I take leflunomide for my rheumatoid arthritis? 2013. http://www.mi-arthritis.com/patient-decision-aids/leflunomide.html. Accessed Feb 2018.
- 39.West Michigan Rheumatology P. Should I take Abatacept/Orencia for my rheumatoid arthritis? 2013. http://www.mi-arthritis.com/patient-decision-aids/anti-t-cell-antibody-abatac.html.
- 40.West Michigan Rheumatology P. Should I take TNF blocker for my rheumatoid arthritis? 2013. http://www.mi-arthritis.com/patient-decision-aids/anti-tumor-necrosis-factor.html. Accessed Feb 2018.
- 41.West Michigan Rheumatology P. Should I take Ritiximab/Rituxan for my rheumatoid arthritis? 2013. http://www.mi-arthritis.com/patient-decision-aids/anti-b-cell-antibody-rituxi.html. Accessed Feb 2018.
- 42.Rader T. I have never taken medication for rheumatoid arthritis before. Should I take methotrexate (Rheumatrex) alone or with other disease modifying anti-rheumatic drugs for RA? 2011. http://musculoskeletal.cochrane.org/decision-aids. Accessed Feb 2018.
- 43.Rader T. Should i take tocilizumab (actemra) for RA? 2011. http://musculoskeletal.cochrane.org/decision-aids. Accessed Feb 2018.
- 44.Rader T. should i take abatacept (orencia) for RA? 2011. http://musculoskeletal.cochrane.org/decision-aids. Accessed Feb 2018.
- 45.Rader T. should i take etanercept (enbrel) for RA? 2011. http://musculoskeletal.cochrane.org/decision-aids. Accessed Feb 2018.
- 46.Rader T. should i take methotrexate (rheumatrex) for RA? 2011. http://musculoskeletal.cochrane.org/decision-aids. Accessed Feb 2018.
- 47.Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns [Internet]. 2012;86(1):9–18. doi: 10.1016/j.pec.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldman-stewart D, Brien MAO, Clayman ML, Davison BJ, Jimbo M, Labrecque M, et al. Providing information about options in patient decision aids. BMC Med Inform Decis Mak [Internet]. 2013;13(Suppl 2):S4. http://www.biomedcentral.com/1472-6947/13/S2/S4. Accessed Feb 2018. [DOI] [PMC free article] [PubMed]
- 49.Emily Oshima Lee, M.A., and Ezekiel J. Emanuel, M.D. P. Shared Decision Making to Improve Care and Reduce Costs. N Engl J Med [Internet]. 2013;368(1):4–6. http://www.nejm.org/doi/10.1056/NEJMp1214605. Accessed Feb 2018. [DOI] [PubMed]
- 50.Stacey D, Légaré F, Lewis K, Mj B, Cl B, Kb E, et al. Decision aids for people facing health treatment or screening decisions (review) Cochrane Database Syst Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tausch AP, Menold N. Methodological aspects of focus groups in health research: results of qualitative interviews with focus group moderators. Glob Qual Nurs Res. 2016;3:2333393616630466. doi: 10.1177/2333393616630466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandelowski M. Focus on qualitative methods sample size in qualitative. Res Nurs Heal. 1995;18:179–183. doi: 10.1002/nur.4770180211. [DOI] [PubMed] [Google Scholar]
- 53.Guest G, Bunce A, Johnson L. How many interviews are enough? Field methods [Internet]. 2006;18(1):59–82. http://journals.sagepub.com/doi/10.1177/1525822X05279903. Accessed Feb 2018.
- 54.Kennedy ADM. On what basis should the effectiveness of decision aids be judged? Heal Expect. 2003;6(3):255–268. doi: 10.1046/j.1369-6513.2003.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perestelo-Perez L, Perez-Ramos J, Gonzalez-Lorenzo M, Rivero-Santana A, Serrano-Aguilar P. Decision aids for patients facing health treatment decisions in Spain: preliminary results. Patient Educ Couns [Internet]. 2010;80(3):364–371. doi: 10.1016/j.pec.2010.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.