Abstract
Electronic Nicotine Delivery Systems (ENDS), commonly referred to as “e-cigs,” were first introduced in the United States in 2007. Since then, their use has grown substantially, with the largest market among adolescents and young adults. ENDS are often perceived by the public as safe alternatives to traditional cigarettes and as aids in smoking cessation. Little is known about inhalational hazards of e-cigs. We describe the case of a 45-year-old man who developed acute respiratory symptoms associated with onset of severe fixed airways obstruction 9 months after he quit traditional cigarettes and began high-dose vaping. Lung biopsy showed respiratory bronchiolitis. Analysis of his heated e-cigarette solution identified a mixture containing vanillin, aldehydes, alcohols and other chemicals, the inhalation effects of which have not been well-studied. This case report adds to the growing literature describing potentially severe lung health effects of vaping and provides a framework for taking a clinical vaping history so that the health consequences of e-cigarettes may be better understood.
Key Points
• Long-term health consequences of vaping are unknown and claims that ENDS are a safer alternative to traditional cigarettes require further investigation.
• This clinical vignette raises concern that ENDS use may be linked to chronic irreversible obstructive lung disease.
• With the rise in ENDS use, particularly among adolescents and young adults, clinicians should elicit a brief vaping history to better understand potential exposures and lung health risks in their patients who vape.
INTRODUCTION
First introduced in 2007, the use of Electronic Nicotine Delivery Systems (ENDS) has grown substantially, with an estimated market worth in 2015 of $3.5 billion.1 From 2013 to 2014, the e-cigarette market expanded at a rate of 10.5 brands and 242 new flavors per month.2 ENDS are the most commonly used form of tobacco products among adolescents and young adults, with more than two million reporting use of ENDS products in 2015.3 Among adults, an estimated 3.2% are current e-cigarette users of which 59% are also regular cigarette smokers and 30% former smokers.4
The original e-cigarettes were often disposable and closely resembled traditional cigarettes in size and appearance. Current fourth-generation ENDS consist of a battery, atomizer, wicking material, heating element, and refillable cartridge. These systems allow users to add their choice of e-liquid and to determine a power setting in watts to control the amount of aerosolization and volatilization of the solution. Power setting intensity has a direct correlation with the nicotine concentration delivered to the user and with the amount of aldehyde generated during vaping.5, 6
E-liquids contain varying amounts of nicotine, flavorings, and carrier solutions such as propylene glycol (PG) and glycerin.7 Despite known pulmonary toxicity to workers from inhalation of artificial flavoring chemicals such as diacetyl and other alpha-diketones, these chemical compounds are used in many e-liquids, exposing users to levels above airborne levels recommended by the National Institute for Occupational Safety and Health (NIOSH).8, 9 Although PG and glycerin are generally recognized as safe for oral consumption, little is known about effects from inhalation. Studies from the entertainment industry describe respiratory symptoms and decreased lung function in those working in close proximity to theatrical smoke machines that aerosolize similar glycol compounds.10
We describe a middle-aged man who reported acute onset of marked dyspnea on exertion and cough associated with findings of severe fixed airways obstruction whose respiratory symptoms began 9 months after he quit smoking traditional cigarettes and transitioned solely to heavy vaping. His symptoms and severe obstructive lung disease persisted despite vaping cessation and aggressive medical treatment. While a number of acute inhalational syndromes have been linked to the use of ENDS, 11–16 this patient’s clinical presentation and course suggest that chronic lung injury may also occur.
CASE REPORT
A 45-year-old man with an unremarkable past medical history noted sudden onset of severe dyspnea on exertion and dry cough 9 months after he began vaping. He was a former heavy cigarette smoker (40 pack-years total) who had made several unsuccessful attempts to quit using both disposable and rechargeable e-cigarettes. He had continued to smoke traditional cigarettes throughout these previous quitting attempts before transitioning exclusively to vaping (using a later generation customizable e-cigarette device). He initially used a vaping liquid nicotine dose of 24 mg/ml, decreasing gradually to 3 mg/ml at the time of symptom onset. He typically vaped 5 puffs every 20 min, and his device was set to the second-highest vape setting (4.3 V) to increase nicotine concentration and delivery.
His past medical history was remarkable for cluster headaches and a positive purified protein derivative (PPD) in adolescence for which he was treated with isoniazid. Prior to onset of respiratory symptoms, he was on no medications. He worked full-time as a teacher, with no known hazardous exposures based on detailed occupational and environmental histories.
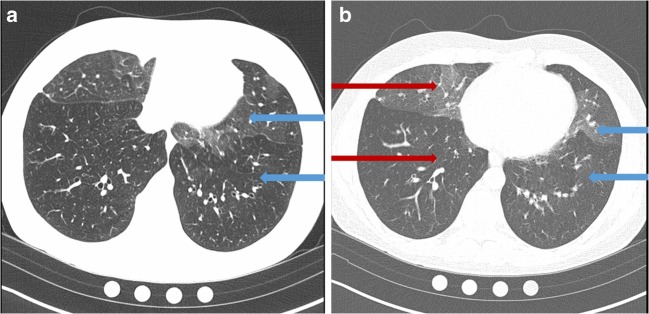
Several days after acute onset of severe dyspnea and cough, he sought evaluation from his primary care provider and was prescribed antibiotics for presumed pneumonia despite a normal chest x-ray. His symptoms worsened, and he stopped vaping 6 weeks later. Spirometry showed severe obstruction with a forced expiratory volume in 1 s (FEV1) of 1.0 L or 24 percent predicted (PP), a forced vital capacity (FVC) of 2.3 L (45 PP), and an FEV1/FVC ratio of 41%. There was no response to inhaled bronchodilator. Lung volumes showed marked air trapping (residual volume 239 PP), and diffusion capacity for carbon monoxide was 85 PP. Serum antinuclear antibody, rheumatoid factor, anti-neutrophil cytoplasmic antibodies, erythrocyte sedimentation rate, IgE and alpha-1-antitrypsin were normal. Chest high-resolution computed tomography (HRCT) showed mosaicism with severe air trapping and patchy ground glass opacities (Fig. 1). Exertional hypoxemia requiring supplemental oxygen was found on cardiopulmonary exercise testing. Surgical lung biopsy showed respiratory bronchiolitis with lymphoplasmacytic infiltrates and peribronchial aggregates of pigmented macrophages; there was no organizing pneumonia or obliterative bronchiolitis (Fig. 2).
Figure 1.
a Inspiratory image demonstrates mosaicism as shown in the difference in attenuation and vessel caliber between the regions marked by the blue arrows. The more lucent (black) region denoted by the lower arrow demonstrates vessel caliber attenuation compared to the region of higher attenuation. This regional difference signifies areas of air trapping in the lung due to small airways disease. The cause of mosaicism on inspiratory images can be confirmed on expiratory images. b Expiratory image is notable for marked air trapping in the regions with decreased attenuation denoted by the lower red and blue arrows. Increased attenuation due to normal air clearance is illustrated by the upper arrows.
Figure 2.
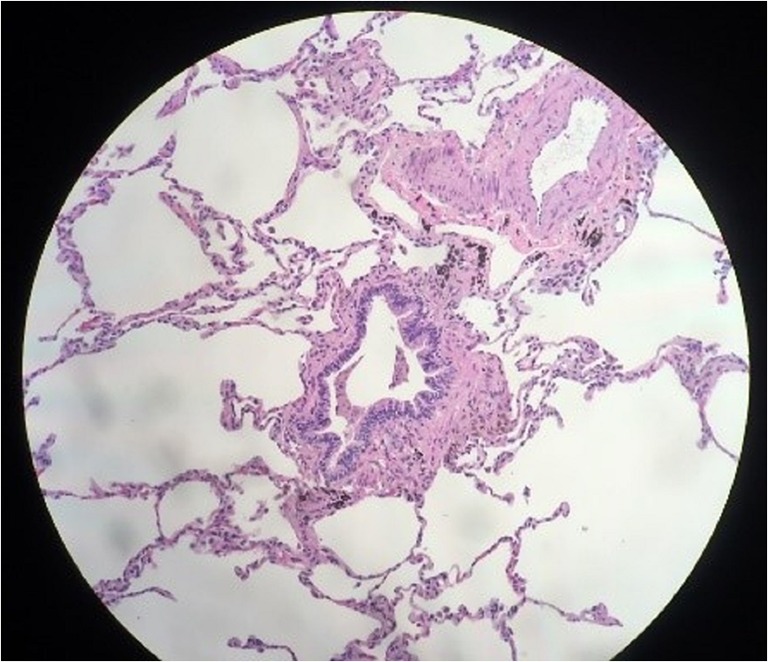
Lower power view showing respiratory bronchiolitis and peribronchiolar aggregates of pigmented macrophages on hematoxylin and eosin stain.
Because of his persistent disabling cough and dyspnea, the patient was started on albuterol and a 10-day prednisone burst. An inhaled corticosteroid and long-acting bronchodilator were later added to his treatment regimen, with no effect. Based on biopsy findings of bronchiolitis, treatment with azithromycin was added along with a more sustained course of prednisone. With no clinical improvement, the patient was treated with a course of mycophenolate mofetil. Despite these therapies, his symptoms persisted and his lung function did not improve. He was subsequently referred for lung transplant evaluation. Because his severe lung function abnormalities were stable, lung transplant was deferred. Counseling was provided on supplemental oxygen use, appropriate vaccinations, breathing techniques and the importance of regular exercise.
Three samples of vaping fluid used by the patient (unheated and heated) as well as remnant e-juice in the patient’s vape pen were analyzed using gas chromatography/mass spectrometry and Fourier transform infrared spectroscopy. Findings showed mixtures containing furfural, vanillin, nicotyrine, aldehydes, adamantanol, damascone, alcohols, propanediol, pyridines, glycerin, and propylene glycol. There were no detectable alpha-diketones including diacetyl.
DISCUSSION
To our knowledge, this is the first case report of an ex-smoker with a heavy e-cigarette habit who developed acute onset of respiratory symptoms associated with severe fixed airways obstruction that did not improve despite aggressive pharmacologic treatment and cessation of vaping. As in workers who developed severe fixed airways obstruction from exposure to diacetyl and other flavoring chemicals,17 this patient’s lung function did not continue to decline after he stopped vaping, though he remained severely obstructed and hypoxic. Unlike findings in workplace sampling of affected flavoring workers,18 there were no detectable alpha-diketones in analysis of his heated and unheated vaping liquids. There were aldehydes, vanillin and furfural, among other chemicals detected in the vaping liquid, some of which are used in flavor manufacturing facilities; their potential role in the risk for obstructive lung disease in these settings is unknown.
While the marked mosaic attenuation on chest HRCT scan was suggestive for obliterative bronchiolitis, surgical lung biopsy showed respiratory bronchiolitis. This histologic finding may have been linked to both his heavy vaping habit and his previous tobacco smoking.19 Notably, however, he had no respiratory symptoms or known functional limitation (having never undergone lung function testing) while he smoked cigarettes but developed acute symptom onset associated with severe lung function abnormalities only after 9 months of heavy vaping. While it is possible that his severe fixed airway obstruction was due to his heavy former smoking habit, this would not explain the onset of persistent respiratory symptoms after an extended period of complete smoking abstinence.
Long-term health consequences of vaping are unknown, and claims that ENDS are a safer alternative to traditional cigarettes await further investigation.20 Research using in vitro cell and animal models exposed to e-liquid aerosols have demonstrated injury to conducting epithelial cells, loss of epithelial barrier function, pro-inflammatory effects through induction of IL-8, and reduced levels of glutathione, an important antioxidant for controlling oxidative stress and inflammation.21–23 A recent study compared inflammatory markers in induced sputum from e-cigarette users, cigarette smokers, and never smokers. E-cigarette users showed a unique pattern of heightened neutrophil activation as well as changes in mucin secretion , MUC5AC/MUC5B ratios (reflecting mucin barrier and clearance functions in distal airways), and elevated protein markers of inhalational smoke exposure and lung inflammation (aldehyde detoxifying enzyme ALDH3A1, thioredoxin, and matrix metalloproteinases) similar to that of traditional cigarette smokers.24 Alterations in airway mucin and neutrophil activation have been implicated in the development of chronic lung disease.25, 26 The authors concluded that their findings challenge the notion that e-cigarettes are a healthier alternative than tobacco smoking.
E-cigarette use has also been shown to have short-term deleterious effects in smokers with no underlying lung disease based on increased fractional exhaled nitric oxide (a marker of small airways inflammation) and respiratory flow resistance and impedance.27 Among users with known underlying lung disease, there was an increased prevalence of chronic bronchitis among e-cigarette users.28
There are a number of potential sources of lung injury inherent to electronic nicotine delivery systems. These include metal particulates from heated coils as well as flavoring chemicals such as alpha-diketones, furfural, vanillin, aldehydes, and aerosolized propylene glycol and glycerin.29 Additionally, those who switch from traditional cigarettes to e-cigarettes often change their puffing pattern to longer and slower puffs.30 This pattern is referred to as “vaping topography” and encompasses the number of puffs, puff volume, intervals between puffs, and average puff flow rate. High power settings featured on later generation ENDS as well as nicotine concentration can affect vaping topography. As in this case with severe fixed obstruction, users often prefer high vape settings and may compensate for lower nicotine concentrations through a more intense puffing regimen. These practices can lead to increased generation of formaldehyde, acetaldehyde and higher concentrations of other toxicants in e-cigarette smoke.31, 32 Our patient used the second-highest power setting as he decreased his e-liquid nicotine concentration, likely increasing his inhalation of aldehydes and other chemical constituents.
We found 15 published case reports of inhalational injury associated with ENDS, including cases with acute eosinophilic pneumonia, hypersensitivity pneumonitis, respiratory bronchiolitis, bronchiolitis obliterans organizing pneumonia, diffuse alveolar hemorrhage, and acute inhalational lung injury.11–16 Timing from e-cigarette exposure to onset of symptoms ranged from a few days to several months. The cases ranged in age from 18–70, and the most common presenting symptoms were dyspnea and cough. All except one case with fatal diffuse alveolar hemorrhage recovered with medical treatment and vaping cessation. Our patient’s severe fixed airways obstruction persisted despite vaping cessation, suggesting that lung injury linked to vaping may not always be reversible.
The variable lung injuries that have been associated with vaping are likely related in part to differences in exposure, including chemical constituents and dose.33 Based on findings in this case report, we developed an abbreviated vaping history questionnaire (Table 1) for use by clinicians that elicits information on both the ENDS device itself and patterns of use (known as vaping topography).30–32 This information should be helpful in quantifying vaping intensity and in better understanding links between a patient’s use of electronic cigarettes and their risk for lung disease.
Table 1.
Vaping History Questions for Clinicians
| Vaping device history: | |
| Does your ENDS device have an adjustable power setting? | |
| If yes, what power setting do you use to vape? | |
| What flavors of e-liquid do you use? | |
| What is the concentration of nicotine in your e-liquid? | |
| Vaping topography history: | |
| For how many months or years have you vaped? | |
| On average, how many times an hour do you use your vaping device? | |
| How many puffs do you take with each use? | |
| Would you characterize your usual puff duration as less than or greater than 3 seconds? |
In a recent consensus report, the National Academies of Sciences, Engineering, and Medicine (NAS) noted that substituting e-cigarettes for combustible cigarettes can diminish exposure to harmful carcinogens. The NAS report also acknowledged the high degree of exposure variability based on the type of ENDS device and e-liquid used as well as the current uncertainties regarding health risks.20 As recently as November 2018, the Food and Drug Administration released results from the National Youth Tobacco Survey which found that e-cigarette use among middle school and high school students within the past year rose dramatically by 48% and 78% respectively.34 With the expanding use of and market for e-cigarettes, as well as high prevalence of use among adolescents and young adults, this case of persistent severe fixed airways obstruction linked to heavy vaping raises public health concerns and points to the need both for expanded clinical awareness and further research on potential hazards of electronic nicotine delivery systems.
Acknowledgments
The authors would like to acknowledge Brent Moore for providing results of mass spectrometry analysis of the vaping liquid. J. Caleb Richards, M.D., Assistant Professor of Radiology, National Jewish Health, provided images and image interpretation for this case report. We would also acknowledge Mr. Thomas Lowrance and Randall L. Rosenblatt, M.D., for their assistance in providing clinical details for this case report.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Prior Presentations: In poster format at the American Thoracic Society Conference. May 21, 2018. San Diego, CA.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robehmed N. E-Cigarette sales surpass 1 billion as big tobacco moves in. Forbes. September 17, 2013. Available at: https://www.forbes.com/sites/natalierobehmed/2013/09/17/e-cigarette-sales-surpass-1-billion-as-big-tobacco-moves-in/#6a2e4df03d6d. Accessed August 30, 2019.
- 2.Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23 Suppl 3:iii3–9. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jamal A, Gentzke A, Hu SS, et al. Tobacco use among middle and high school Students—United States, 2011-2016. MMWR Morb Mortal Wkly Rep. 2017;66:597–603. doi: 10.15585/mmwr.mm6623a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quickstats: Cigarette smoking status among current adult e-cigarette users, by age group—national health interview survey, United States 2015. MMWR Morb Mortal Wkly Report 2016; 65:1177. [DOI] [PubMed]
- 5.Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine & Tobacco Research. 2014;16(10):1319–1326. doi: 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farsalinos K, Poulas K, Voudris V. Changes in puffing topography and nicotine consumption depending on the power setting of electronic cigarettes. Nicotine Tob Res. 2017. [DOI] [PubMed]
- 7.Clapp PW, Jaspers I. Electronic Cigarettes: Their Constituents and Potential Links to Asthma. Curr Allergy Asthma Rep. 2017;17(11):79. doi: 10.1007/s11882-017-0747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen JG, Flanigan SS, LeBlanc M, et al. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ Health Perspect. 2016;124(6):733–739. doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farsalinos KE, Kistler KA, Gillman G, Voudris V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob Res. 2015;17(2):168–174. doi: 10.1093/ntr/ntu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varughese S, Teschke K, Brauer M, Chow Y, van Netten C, Kennedy SM. Effects of theatrical smokes and fogs on respiratory health in the entertainment industry. Am J Ind Med. 2005;47(5):411–418. doi: 10.1002/ajim.20151. [DOI] [PubMed] [Google Scholar]
- 11.Thota D, Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med. 2014;47(1):15–17. doi: 10.1016/j.jemermed.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Flower M, Nandakumar L, Singh M, Wyld D, Windsor M, Fielding D. Respiratory bronchiolitis-associated interstitial lung disease secondary to electronic nicotine delivery system use confirmed with open lung biopsy. Respirol Case Rep. 2017;5(3):e00230. doi: 10.1002/rcr2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He T, Oks M, Esposito M, Steinberg H, Makaryus M. “Tree-in-Bloom”: Severe Acute Lung Injury Induced by Vaping Cannabis Oil. Ann Am Thorac Soc. 2017;14(3):468–470. doi: 10.1513/AnnalsATS.201612-974LE. [DOI] [PubMed] [Google Scholar]
- 14.Hureaux J, Drouet M, Urban T. A case report of subacute bronchial toxicity induced by an electronic cigarette. Thorax. 2014;69(6):596–597. doi: 10.1136/thoraxjnl-2013-204767. [DOI] [PubMed] [Google Scholar]
- 15.Atkins G, Drescher F. Acute inhalational lung injury related to the use of electronic nicotine delivery system (ENDS) Chest. 2015;148(4):83A. doi: 10.1378/chest.2281610. [DOI] [Google Scholar]
- 16.Khan MS, Khateeb F, Akhtar J, et al. Organizing pneumonia related to electronic cigarette use: A case report and review of literature. Clin Respir J. 2018;12(3):1295–1299. doi: 10.1111/crj.12775. [DOI] [PubMed] [Google Scholar]
- 17.Akpinar-Elci M, Travis WD, Lynch DA, Kreiss K. Bronchiolitis obliterans syndrome in popcorn production plant workers. Eur Respir J. 2004;24(2):298–302. doi: 10.1183/09031936.04.00013903. [DOI] [PubMed] [Google Scholar]
- 18.Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347(5):330–338. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- 19.Fraig M, Shreesha U, Savici D, Katzenstein AL. Respiratory bronchiolitis: a clinicopathologic study in current smokers, ex-smokers, and never-smokers. Am J Surg Pathol. 2002;26(5):647–653. doi: 10.1097/00000478-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Stratton Kathleen, Kwan Leslie Y., Eaton David L., editors. Public Health Consequences of E-Cigarettes. Washington, D.C.: National Academies Press; 2018. [PubMed] [Google Scholar]
- 21.Sherwood CL, Boitano S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir Res. 2016;17(1):57. doi: 10.1186/s12931-016-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerloff J, Sundar IK, Freter R, et al. Inflammatory Response and Barrier Dysfunction by Different e-Cigarette Flavoring Chemicals Identified by Gas Chromatography-Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. Appl In Vitro Toxicol. 2017;3(1):28–40. doi: 10.1089/aivt.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reidel B, Radicioni G, Clapp PW, et al. E-Cigarette Use Causes a Unique Innate Immune Response in the Lung, Involving Increased Neutrophilic Activation and Altered Mucin Secretion. Am J Respir Crit Care Med. 2018;197(4):492–501. doi: 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kesimer M, Ford AA, Ceppe A, et al. Airway Mucin Concentration as a Marker of Chronic Bronchitis. N Engl J Med. 2017;377(10):911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caughey GH. Serine proteinases of mast cell and leukocyte granules. A league of their own. Am J of Respir Crit Care Med. 1994;150:S138–142. doi: 10.1164/ajrccm/150.6_Pt_2.S138. [DOI] [PubMed] [Google Scholar]
- 27.Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400–1406. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- 28.Bowler RP, Hansel NN, Jacobson S, et al. Electronic Cigarette Use in US Adults at Risk for or with COPD: Analysis from Two Observational Cohorts. J Gen Intern Med. 2017;32:1315–1322. doi: 10.1007/s11606-017-4150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol. 2017;313(2):L193–L206. doi: 10.1152/ajplung.00071.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YH, Gawron M, Goniewicz ML. Changes in puffing behavior among smokers who switched from tobacco to electronic cigarettes. Addict Behav. 2015;48:1–4. doi: 10.1016/j.addbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosmider L, Kimber CF, Kurek J, Corcoran O, Dawkins LE. Compensatory Puffing With Lower Nicotine Concentration E-liquids Increases Carbonyl Exposure in E-cigarette Aerosols. Nicotine Tob Res. 2017. [DOI] [PubMed]
- 32.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–394. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- 33.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities' regulation. Int J Environ Res Public Health. 2013;10(6):2500–2514. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FDA News Release. Results from 2018 National Youth Tobacco show dramatic increase in e-cigarette use among youth over past year. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm625917.htm. Accessed August 30, 2019.