INTRODUCTION
Familial hypercholesterolemia (FH) is an autosomal dominant or recessive disorder characterized by abnormally high levels of low-density lipoprotein (LDL).1 LDL elevations in FH occur in early childhood and are often caused by mutations affecting the LDL receptor (LDLR), its adaptor, apolipoprotein B, or proprotein convertase subtilisin/nexin type 9 genes.1 FH is the most common genetic cause of premature coronary heart disease (> 50% of men are affected by age 50, and > 30% of women by age 602) and early mortality.1 In the USA, only an estimated 10% of > 1 million FH cases have been diagnosed.2
The American Heart Association recommends cascade molecular (genetic) screening among relatives of those diagnosed with FH.1 The UK and Netherlands’ integrated healthcare systems have successfully deployed cascade screening through “family member letters” mailed to relatives, augmented with telephone calls to primary care providers as needed.3 Here, we report the results of a pilot study to assess feasibility and uptake of an electronic pathway to identify high-risk individuals and initiate cascade molecular testing. Testing was done for the three most prevalent causes of FH recognized at study initiation in US primary care clinics.
METHODS
Primary care patients at Duke University and Medical College of Wisconsin (MCW) were identified as potentially eligible (Table 1) based on cholesterol results from a web-based risk assessment service (MeTree), linked to EHR information,4 or an EHR query alone. After mailed invitation letters, follow-up calls to nonresponders were made at 10 days. Participants who confirmed a personal or family history of early CAD and no previous genetic testing were eligible. After telephone consent, subjects returned saliva samples for DNA extraction. Samples were enriched for FH-variant-associated exons and splice junctions using Illumina Truseq Custom Amplicon Kits and next-generation sequencing. Variant pathogenicity was determined using the American College of Medical Genetics standards.5 All pathogenic variants were confirmed by Sanger sequencing and communicated to participants by phone by a board-certified genetic counselor. Variant-positive participants and their primary care providers were mailed their results with a cascade screening (family) letter for participants to share with adult biologic relatives. The family letter outlined benefits of genetic screening, the specific variant, and procedures for free testing at MCW. Samples received within 6 months were sequenced for the relevant variant.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion: | |
| • LDL cholesterol ≥ 190 mg/dL OR total cholesterol ≥ 310 mg/dL (≥ 8 mmol/L). | |
| AND one of the following: | |
|
• A family history of FH by clinical criteria or hypercholesterolemia OR • A personal history of early heart disease or a family history of early heart disease in a first-degree relative (early heart disease defined as onset < 55 for males and < 65 for females) | |
| Exclusion: | |
| • Previously had molecular genetic testing | |
| • A family member with previous molecular genetic testing | |
| • No phone available or non-English speaking | |
| • PCP was not a MeTree study participant | |
| • No qualifying PCP appointment during the MeTree study time frame |
RESULTS
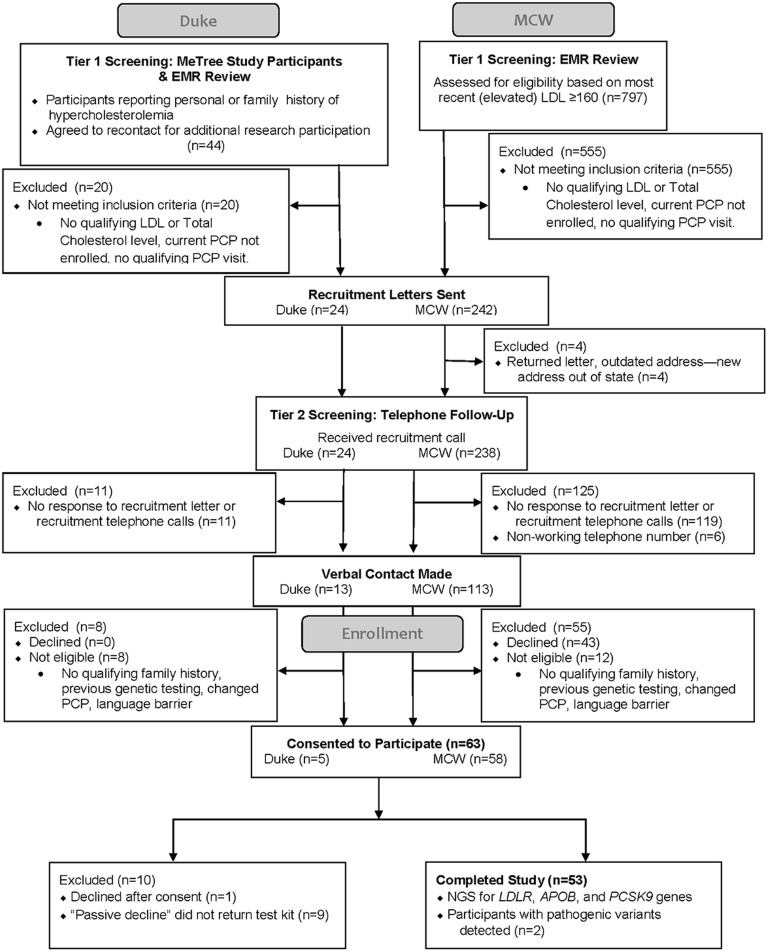
We identified 246 potentially eligible patients; 126 responded to the invitation and 106 met eligibility criteria (Fig. 1). Only one was excluded due to prior genetic testing. Of the 53 (50% of eligible) participants who completed testing (30% male, 11.3% < 50), 4% had pathogenic LDLR variant gene and were diagnosed with FH. These two were given family letters. MCW received samples from 4 family members (1 from one family, 3 from the other). Two (50%) were positive for the relevant variant (one from each family).
Figure 1.
Study participants.
DISCUSSION
This first US study to test FH cascade screening demonstrates feasibility of systematic identification of high-risk patients with follow-on cascade screening in US clinics. The high uptake of screening by 50% of eligible patients suggests a demand for DNA-based testing for FH in primary care. Diagnosis is particularly important since statins are often inadequate and access to PCSK-9 inhibitors frequently requires a diagnosis. Our 4% variant-positive rate was lower than that in several studies in lipid clinics,2 but similar to that of a Scottish primary care study.6 Because of institutional review board (IRB) limitations on collection of the number of family letters given to relatives and their demographics, we do not know the denominator or demographic information for our cascade screening results; however, reaching 4 individuals, 50% of whom were diagnosed with FH, is an excellent start.
Current practice guidelines recommend FH cascade testing be widely clinically implemented, and our pilot supports the feasibility of this approach; however, logistics (including the length of time needed to fulfill true cascade screening) and IRB delays and barriers to DNA testing including inability to enroll children were troublesome. To facilitate larger studies, further efforts should be made to reduce the burden of genetic “exceptionalism”1 by IRBs. Our study suggests that with accessibility to genetic counselors and free testing, cascade screening is feasible in US primary care practices. Implementation research should address optimal information delivery, particularly family member contact and tracking logistics, costs, and insurance implications.
Funding Information
This study was funded by NIH-NHGRI under grant U01-HG007282.
Compliance with Ethical Standards
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
Dr. David Dimmock is a consultant for Pegvaliase trials at Biomarin; on the Scientific Advisory Board at Audentes Therapeutics; and pending consultant for mitochondrial disease drugs Ichorion Therapeutics. Dr. Lori Orlando has ownership in MeTree. The goal for MeTree&You is to eventually allow entities to use MeTree for clinical purposes. All remaining authors declare that they do not have a conflict of interest.
Footnotes
Joan Neuner and David Dimmock are shared first author.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gidding SS, Champagne MA, de Ferranti SD, et al. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132(22):2167–2192. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 2.Sturm AC, Knowles JW, Gidding SS, et al. Clinical genetic testing for familial hypercholesterolemia: JACC Scientific Expert Panel. J Am Coll Cardiol. 2018;72(6):662–680. doi: 10.1016/j.jacc.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 3.Louter L, Defesche J. Roeters van Lennep J. Cascade screening for familial hypercholesterolemia: practical consequences. Atheroscler Suppl. 2017;30:77–85. doi: 10.1016/j.atherosclerosissup.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Orlando LA, Buchanan AH, Hahn SE, et al. Development and validation of a primary care-based family health history and decision support program (MeTree©) NCMJ. 2013;74(4):287–296. [PMC free article] [PubMed] [Google Scholar]
- 5.Ademi Z, Watts GF, Pang J, et al. Cascade screening based on genetic testing is cost-effective: evidence for the implementation of models of care for familial hypercholesterolemia. J Clin Lipidol. 2014;8(4):390–400. doi: 10.1016/j.jacl.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Norsworthy PJ, Vandrovcova J, Thomas ER, et al. Targeted genetic testing for familial hypercholesterolaemia using next generation sequencing: a population-based study. BMC Med Genet. 2014;15:70. doi: 10.1186/1471-2350-15-70. [DOI] [PMC free article] [PubMed] [Google Scholar]