Abstract
Background
Poor sleep is common among adults with chronic low back pain (cLBP), but the influence of cLBP treatments, such as yoga and physical therapy (PT), on sleep quality is under studied.
Objective
Evaluate the effectiveness of yoga and PT for improving sleep quality in adults with cLBP.
Design
Secondary analysis of a randomized controlled trial.
Setting
Academic safety-net hospital and 7 affiliated community health centers.
Participants
A total of 320 adults with cLBP.
Intervention
Twelve weekly yoga classes, 1-on-1 PT sessions, or an educational book.
Main Measures
Sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI) global score (0–21) at baseline, 12 weeks, and 52 weeks. Additionally, we also evaluated how the proportion of participants who achieved a clinically meaningful improvement in sleep quality (> 3-point reduction in PSQI) at 12 weeks varied by changes in pain and physical function at 6 weeks.
Key Results
Among participants (mean age = 46.0, 64% female, 82% non-white), nearly all (92%) reported poor sleep quality (PSQI > 5) at baseline. At 12 weeks, modest improvements in sleep quality were observed among the yoga (PSQI mean difference [MD] = − 1.19, 95% confidence interval [CI] − 1.82, − 0.55) and PT (PSQI MD = − 0.91, 95% CI − 1.61, − 0.20) groups. Participants who reported a ≥ 30% improvement in pain or physical function at 6 weeks, compared with those who improved < 10%, were more likely to be a sleep quality responder at 12 weeks (odds ratio [OR] = 3.51, 95% CI 1.73, 7.11 and OR = 2.16, 95% CI 1.18, 3.95, respectively). Results were similar at 52 weeks.
Conclusion
In a sample of adults with cLBP, virtually all with poor sleep quality prior to intervention, modest but statistically significant improvements in sleep quality were observed with both yoga and PT. Irrespective of treatment, clinically important sleep improvements at the end of the intervention were associated with mid-intervention pain and physical function improvements.
Trial Registration
ClinicalTrials.gov Identifier: NCT01343927
KEY WORDS: back pain, chronic pain, yoga, physical therapy, education, sleep
BACKGROUND
Sleep disturbance is common among persons with chronic low back pain (cLBP), with 59% and 53% experiencing poor sleep quality and insomnia disorder, respectively.1–3 The relationship between back pain and sleep disturbance is thought to be bidirectional, and therefore, interventions that improve both back pain and sleep quality may be ideal.4,5 Given the known adverse effects of pharmacologic therapy for both back pain and sleep disturbance (e.g., sedation, drowsiness, and confusion), as well as increased risk of overdose and death with concomitant use of opioid and benzodiazepine medications,6 there is a growing need to evaluate non-pharmacologic therapies for these prevalent and highly comorbid conditions. While cognitive behavioral therapy for insomnia (CBT-I) is an effective treatment for improving sleep quality, uptake of this non-pharmacologic approach has been constrained by a limited number of trained clinicians.7,8 In contrast, physical therapy (PT) and yoga are widely available. However, while both PT9 and yoga10,11 have been shown to reduce pain and improve physical function in adults with cLBP, data on the impact of yoga and PT on sleep quality is sparse.
Maintaining or initiating a regular exercise routine is a common recommendation for both cLBP self-care12 and sleep hygiene.13 Physical therapists, who commonly receive referrals from primary care physicians for cLBP, provide 1-on-1 care emphasizing strength training, aerobic exercise, and flexibility.14 In a systematic review, aerobic and resistance exercises improved sleep quality in middle-aged and older adults.15 Previous studies evaluating the effectiveness of exercise therapy for improving sleep demonstrate modest16–19 to large20,21 improvements among older adults. Only one study of PT for sleep quality in older adults with cLBP found PT improved sleep quality to the same degree as a walking program and a group-based exercise course.22 However, that small study did not measure sleep quality immediately after the 8-week PT intervention—nor did they measure sleep quality over a longer follow-up period (e.g., 1 year). Thus, there is a need to further evaluate PT for sleep quality, particularly among young and middle-age adults with cLBP.
Yoga is a multi-factorial mind-body therapy that incorporates physical poses, rhythmic breathing, and meditation, and is growing in popularity in the USA.23 Previous studies have assessed the impact of yoga on sleep quality among diverse populations, including nurses,24 cancer patients,25–28 older adults,29–32 post or peri-menopausal women,17,33,34 and women with osteoarthritis.35,36 Furthermore, several studies have shown that yoga improves conditions related to poor sleep quality, such as anxiety and stress.37 A recent pooled analysis of clinical trials found yoga and exercise had similar small effects on sleep among menopausal women.17 We are unaware of any previous studies of yoga that assessed changes in sleep quality among adults with cLBP.
Recently, the Back to Health randomized controlled trial (RCT) found yoga to be non-inferior to PT for reducing pain and improving back-specific physical function among adults with cLBP.38,39 The goal of this paper was to perform a secondary analysis of Back to Health data to assess the effectiveness of yoga and PT, each compared with a back pain self-care educational book, for improving sleep quality. We hypothesized that participants receiving yoga or PT interventions would experience a greater improvement in sleep quality at 12 and 52 weeks compared with participants receiving only the educational book.
METHODS
Study Design
The original randomized controlled trial of yoga, PT, and education as interventions for cLBP has been described elsewhere.38,39 Briefly, 320 adults were randomized to a 12-week intervention phase and were subsequently followed over a 40-week maintenance phase. During this trial, measures of comorbidity and disability were collected, including information on sleep quality. Staff performing data collection, entry, and analysis were masked to treatment assignment. The Boston University Medical Campus Institutional Review Board approved the study prior to data collection. All participants provided written informed consent.
Population
English-speaking adults ages 18–64 who reported non-specific cLBP, defined as ≥ 12 weeks with an average pain intensity in the previous week of ≥ 4 on an 11-point (0–10) numerical rating scale, were enrolled. Our analytic sample included 320 participants enrolled from June 2012 to November 2014 at a large academic safety-net hospital and 7 affiliated federally qualified community health centers located in diverse neighborhoods. Participants were randomized 2:2:1 to yoga, PT, or education.
Interventions
Our yoga intervention was designed specifically to aid cLBP in patients naïve to yoga and is described previously.38 Participants attended up to 12 weekly 75-min classes which included yoga poses, breathing, relaxation, and meditation. Instructors helped adapt yoga poses to participants’ ability and we provided aids (e.g., chair, strap, blocks). Study staff and yoga instructors encouraged participants to practice yoga at home for 30 min daily using a DVD and home practice manual.
Our PT intervention included fifteen 60-min appointments over the course of 12 weeks.38 Each session included 1-on-1 work with the physical therapist and supervised aerobic exercise. Participants received written instructions and supplies for home practice and logged the number of exercises completed daily.
Education participants received The Back Pain Helpbook, including information on cLBP self-management, stretching, strengthening, and other self-care strategies, including a chapter on solutions for sleep problems.40 Every 3 weeks, participants received summary newsletters of assigned chapters and a 5- to 10-min check-in call from research staff.
Yoga and PT participants who attended at least one session in the 12-week intervention phase were eligible to participate in the maintenance phase (weeks 13–52). After the 12-week assessment, yoga participants underwent a second randomization in a 1:1 ratio to weekly drop-in yoga classes or home practice only. Similarly, PT participants were randomized to receive five booster sessions in the maintenance phase or home practice only. Participation in yoga/PT maintenance and home practice groups was similar and did not appear to influence 52-week pain and function outcomes.39 For analyses of sleep outcomes, the two yoga (home practice, drop-in classes) and PT (home practice, booster sessions) maintenance phase groups were combined to optimize power.
Assessment of Sleep Quality
Sleep quality was measured with the Pittsburgh Sleep Quality Index (PSQI), a widely used, validated measure of sleep-related function.41–43 The PSQI is a 19-item questionnaire composed of 7 subscales, where scores range from 0 to 3, which correspond to distinct domains (habitual sleep efficiency, sleep disturbance, sleep duration, sleep latency, subjective sleep quality, sleep medication use, daytime dysfunction). Our primary outcome was the PSQI global score (0–21); higher scores indicate worse sleep quality. Prior studies define poor sleep quality with a score of ≥ 5 and a clinically meaningful improvement as a reduction of ≥ 3 points.41,44 In addition to the global score, we examined the PSQI 3-factor scoring system developed by Cole, which collapses the original 7 subscales into 3 domains: sleep efficacy (sleep duration and habitual sleep efficiency), perceived sleep quality (subjective sleep quality, sleep latency, and sleep medication use), and daily disturbances (sleep disturbances and daytime dysfunction).45,46
We calculated change scores for overall sleep quality by subtracting the baseline PSQI global and subscores from the 12- and 52-week scores. Thus, negative PSQI change-scores indicate improvements in overall sleep quality.
Predictor Variables
Baseline sociodemographic characteristics included age, sex, employment (currently employed, unemployed), and education (≥ high school, < high school). Additional clinical predictor variables included body mass index (BMI, kg/m2), smoking status (current smoker, not-current smoker), and self-report (yes or no) of depression or chronic obstructive pulmonary disorder (COPD).
Back-related pain and physical function were assessed using an 11-point numerical rating scale (NRS) and the Roland-Morris Disability Questionnaire (RMDQ), respectively.47 Changes in pain and physical function scores at 6 weeks were characterized as improvements of < 10%, 10–30%, or ≥ 30%. Improvements ≥ 30% for pain on 11-point NRS and physical function on RMDQ are thought to be clinically meaningful.48,49
Analysis
Our primary end point was at 12 weeks, reflecting change in PSQI immediately following the intervention period. A change in PSQI score from baseline to 12 weeks was analyzed using multiple linear regression, adjusting for age and RMDQ score. Secondary analysis similarly modeled change PSQI score from baseline to 52 weeks. We assessed whether the 12- and 52-week PSQI change scores differed by treatment group using the education intervention as the reference. These analyses were repeated to calculate change-scores for each of the seven individual domains of PSQI and the three factors previously described.45,46 We also stratified changes in the PSQI Global Score models by race and sex, to assess for potential effect modification, based on previous findings suggesting sleep quality may vary by race and sex.50,51
We performed univariate logistic regressions to explore whether treatment arm, baseline characteristics, and clinically meaningful improvements in pain or physical function at 6 weeks were associated with favorable sleep outcomes (≥ 3-point improvement on PSQI). The likelihood of response at 12 and 52 weeks, by participant characteristics, was presented as odds ratios (ORs) and 95% confidence intervals.
The analytic sample consisted of all participants with a response to at least one component of the PSQI question, for each time point. Thus, a complete case analysis was conducted (Appendix Table 4). To address missing data, we performed sensitivity analyses using two methods of multiple imputation, assuming that data on the covariates and outcome were missing at random. The first approach used multiple imputation for the entire sample, regardless of the treatment arm, while for the second, we employed multiple imputation separately by treatment arm. The latter method has been shown to reduce bias in the assessment of treatment effect, when there appears to be differential loss to follow-up between intervention groups.52
Table 4.
Pattern of Missing PSQI Values
| PSQI | Yoga (n = 127) | Education (n = 64) | PT (n = 129) | Total (n = 320) |
|---|---|---|---|---|
| Baseline | ||||
| Complete | 123 (97%) | 62 (97%) | 123 (95%) | 308 (96%) |
| Incomplete | 4 (3%) | 2 (3%) | 6 (5%) | 12 (4%) |
| Missing | 0 | 0 | 0 | 0 |
| 12 weeks | ||||
| Complete | 122 (96%) | 58 (91%) | 99 (77%) | 279 (87%) |
| Incomplete | 3 (2%) | 2 (3%) | 3 (2%) | 8 (3%) |
| Missing | 2 (2%) | 4 (6%) | 27 (21%) | 33 (10%) |
| 52 weeks | ||||
| Complete | 104 (82%) | 54 (84%) | 87 (67%) | 245 (77%) |
| Incomplete | 3 (3%) | 2 (3%) | 4 (3%) | 10 (3%) |
| Missing | 19 (15%) | 8 (13%) | 38 (29%) | 65 (20%) |
“Incomplete” indicates partial completion of survey
“Missing” indicates participant did not answer any of PSQI survey
All analyses were performed using SAS 9.4 (SAS Cary, NC).
RESULTS
Baseline Characteristics
Baseline characteristics are described in detail elsewhere.39 Table 1 presents the baseline characteristics. Among the 320 participants, most were female (64%), non-white (82%), and middle-aged (mean age = 46.0 [SD = 10.7] years). Participants had high sleep disturbance scores (mean PSQI score = 10.2 [SD = 3.9]) and nearly all (92%) met the threshold definition for poor sleep quality. Baseline covariates were similar between groups, apart from the RMDQ. Appendix Table 5 presents the PSQI subscores.
Table 1.
Baseline Characteristics of 320 Study Participants Randomized to Yoga, Physical Therapy, or Education
| Yoga (N = 127) | Physical therapy (N = 129) | Education (N = 64) | |
|---|---|---|---|
| Age, mean (SD) | 46.7 (10.2) | 46.0 (11.4) | 44.3 (10.3) |
| Female*, N (%) | 72 (56.7) | 90 (69.8) | 42 (65.6) |
| Race*, N (%) | |||
| White | 30 (23.6) | 17 (13.2) | 11 (17.2) |
| Non-white | 97 (76.4) | 112 (86.8) | 53 (82.8) |
| Ethnicity, N (%) | |||
| Hispanic | 20 (15.7) | 17 (13.2) | 6 (9.4) |
| Non-Hispanic | 107 (84.3) | 112 (86.8) | 58 (90.6) |
| BMI, mean (SD) | 30.5 (6.7) | 32.4 (7.3) | 31.8 (8.0) |
| Back pain intensity†, mean (SD) | 7.1 (1.5) | 7.2 (1.5) | 7.0 (1.4) |
| RMDQ score*, mean (SD) | 13.9 (5.6) | 15.6 (5.1) | 15.0 (5.0) |
| PSQI global score, mean (SD) | 10.0 (3.9) | 10.4 (3.8) | 10.0 (4.0) |
| Sleep efficiency, mean (SD) | 2.71 (2.02) | 2.72 (2.05) | 2.90 (2.07) |
| Perceived sleep quality, mean (SD) | 4.39 (1.93) | 4.70 (1.89) | 4.30 (2.10) |
| Daily disturbance, mean (SD) | 2.93 (1.23) | 3.00 (1.13) | 2.86 (1.04) |
| Poor sleep quality‡, N (%) | 117 (92.1) | 121 (93.8) | 57 (89.1) |
BMI, body mass index; RMDQ, Roland-Morris Disability Questionnaire; PSQI, Pittsburgh Sleep Quality Index; SD, standard deviation
*We observed baseline between-group differences for sex, race, and RMDQ (p value = 0.09, 0.09, and 0.03, respectively)
†Pain measured on 0–10 numerical rating scale
‡Poor sleep quality, participants with PSQI global score ≥ 5
Table 5.
Unadjusted PSQI Global and Subscores for Participants Randomized to Yoga, PT, or Education Interventions at Baseline, 12 weeks, and 52 weeks
| Baseline Mean (SE) |
12 weeks Mean (SE) |
52 weeks Mean (SE) |
12-week change Mean (95% CI) |
52-week change Mean (95% CI) |
|
|---|---|---|---|---|---|
| Yoga (n = 127) | |||||
| PSQI global score | 10.00 (0.35) | 8.74 (0.38) | 7.87 (0.44) | − 1.19 (− 0.59, − 1.79) | − 2.25 (− 3.08, − 1.42) |
| Subjective sleep quality | 1.79 (0.07) | 1.46 (0.07) | 1.41 (0.09) | − 0.31 (− 0.45, − 0.18) | − 0.41 (− 0.58, − 0.23) |
| Sleep latency | 1.84 (0.08) | 1.59 (0.09) | 1.37 (0.11) | − 0.26 (− 0.42, − 0.10) | − 0.52 (− 0.74, − 0.31) |
| Sleep duration | 1.46 (0.10) | 1.29 (0.10) | 1.10 (0.10) | − 0.15 (− 0.32, 0.03) | − 0.38 (− 0.58, − 0.19) |
| Habitual sleep efficiency | 1.26 (0.11) | 1.06 (0.11) | 0.92 (0.12) | − 0.17 (− 0.40, 0.05) | − 0.37 (− 0.63, − 0.11) |
| Sleep disturbance | 1.79 (0.06) | 1.66 (0.06) | 1.62 (0.07) | − 0.12 (− 0.24, 0.00) | − 0.19 (− 0.35, − 0.03) |
| Sleep medication use | 0.77 (0.10) | 0.78 (0.11) | 0.65 (0.11) | 0.02 (− 0.21, 0.24) | − 0.09 (− 0.30, 0.12) |
| Daytime dysfunction | 1.16 (0.08) | 0.95 (0.08) | 0.91 (0.08) | − 0.19 (− 0.35, − 0.03) | − 0.24 (− 0.42, − 0.06) |
| PT (n = 129) | |||||
| PSQI global score | 10.38 (0.34) | 9.35 (0.44) | 8.37 (0.52) | − 0.88 (− 0.12, − 1.64) | − 1.84 (− 0.84, − 2.83) |
| Subjective sleep quality | 1.78 (0.07) | 1.48 (0.09) | 1.31 (0.11) | − 0.27 (− 0.44, − 0.11) | − 0.42 (− 0.65, − 0.20) |
| Sleep latency | 2.10 (0.07) | 1.88 (0.09) | 1.66 (0.10) | − 0.20 (− 0.36, − 0.04) | − 0.45 (− 0.65, − 0.26) |
| Sleep duration | 1.49 (0.10) | 1.45 (0.11) | 1.14 (0.12) | − 0.02 (− 0.22, 0.18) | − 0.31 (− 0.53, − 0.10) |
| Habitual sleep efficiency | 1.24 (0.11) | 1.04 (0.11) | 0.80 (0.12) | − 0.16 (− 0.42, 0.09) | − 0.51 (− 0.83, − 0.19) |
| Sleep disturbance | 1.89 (0.05) | 1.73 (0.06) | 1.69 (0.08) | − 0.14 (− 0.27, − 0.01) | − 0.22 (− 0.38, − 0.07) |
| Sleep medication use | 0.89 (0.11) | 0.88 (0.12) | 1.03 (0.14) | 0.00 (− 0.25, 0.25) | 0.16 (− 0.14, 0.45) |
| Daytime dysfunction | 1.11 (0.07) | 0.92 (0.08) | 0.80 (0.08) | − 0.12 (− 0.29, 0.05) | − 0.19 (− 0.35, − 0.02) |
| Education (n = 64) | |||||
| PSQI global score | 10.02 (0.50) | 9.40 (0.54) | 9.13 (0.60) | − 0.58 (0.33, − 1.49) | − 1.04 (− 0.01, − 2.06) |
| Subjective sleep quality | 1.66 (0.11) | 1.60 (0.11) | 1.55 (0.13) | − 0.05 (− 0.26, 0.16) | − 0.14 (− 0.39, 0.10) |
| Sleep latency | 1.80 (0.13) | 1.58 (0.12) | 1.66 (0.14) | − 0.20 (− 0.46, 0.06) | − 0.18 (− 0.49, 0.13) |
| Sleep duration | 1.51 (0.14) | 1.34 (0.14) | 1.44 (0.17) | − 0.12 (− 0.36, 0.12) | − 0.19 (− 0.47, 0.10) |
| Habitual sleep efficiency | 1.42 (0.16) | 1.17 (0.16) | 1.07 (0.17) | − 0.18 (− 0.44, 0.09) | − 0.36 (− 0.76, 0.05) |
| Sleep disturbance | 1.84 (0.08) | 1.73 (0.09) | 1.60 (0.11) | − 0.10 (− 0.32, 0.12) | − 0.25 (− 0.48, − 0.03) |
| Sleep medication use | 0.84 (0.14) | 1.05 (0.17) | 0.80 (0.17) | 0.15 (− 0.17, 0.47) | 0.00 (− 0.31, 0.31) |
| Daytime dysfunction | 1.02 (0.08) | 1.02 (0.10) | 1.07 (0.08) | 0.00 (− 0.21, 0.21) | 0.07 (− 0.17, 0.31) |
Change in Sleep Quality at 12 and 52 Weeks
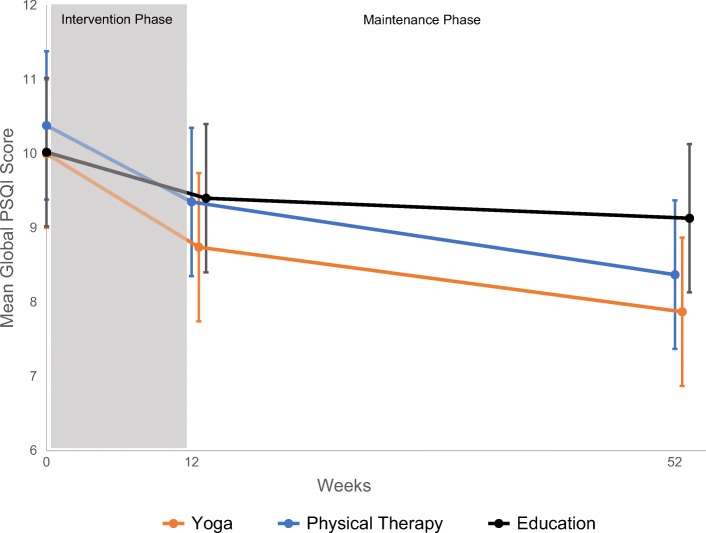
Table 2 displays within- and between-group treatment differences in PSQI change-scores for the 287 and 255 participants in the complete case analysis at 12 and 52 weeks, respectively. After adjustments for age and baseline RMDQ scores, all groups modestly improved by 12 weeks. The PSQI global scores in the yoga and PT groups tended to improve more than the education group (MD = − 0.63, 95% CI − 1.75, 0.49 and − 0.35, 95% CI − 1.51, 0.80, respectively), but these changes were not statistically significant. Changes in PSQI over 52 weeks are plotted in Figure 1. By 52 weeks, the improvements in sleep (as measured by PSQI scores) are significantly better in the yoga compared with the education group (MD = − 1.46, 95% CI − 2.90, − 0.03) and tended to be larger in PT compared with the education group (MD = − 1.00, 95% CI − 2.47, 0.47). Sleep outcomes were similar and not statistically different between yoga and PT at both 12 and 52 weeks. Imputation-based sensitivity analyses yielded similar results.
Table 2.
Sleep Outcomes at 12 and 52 weeks
| Mean Within-group difference (95% confidence interval) | Mean Between-group difference (95% confidence interval) | |||||
|---|---|---|---|---|---|---|
| PSQI* | Yoga | PT | Education | Yoga vs Education | PT vs Education | Yoga vs PT |
| Global score | ||||||
| 12 weeks† | − 1.19 (− 1.82, − 0.55) | − 0.91 (− 1.61, − 0.20) | − 0.55 (− 1.47, 0.37) | − 0.63 (− 1.75, 0.49) | − 0.35 (− 1.51, 0.80) | − 0.28 (− 1.24, 0.68) |
| 52 weeks† | − 2.32 (− 3.16, − 1.48) | − 1.86 (− 2.77, − 0.95) | − 0.86 (− 2.02, 0.30) | − 1.46 (− 2.90, − 0.03) | − 1.00 (− 2.47, 0.47) | − 0.46 (− 1.71, 0.78) |
| Sleep efficiency‡ | ||||||
| 12 weeks | − 0.32 (− 0.64, 0.01) | − 0.22 (− 0.58, 0.13) | − 0.33 (− 0.80, 0.14) | 0.02 (− 0.55, 0.59) | 0.11 (− 0.48, 0.70) | − 0.09 (− 0.58, 0.39) |
| 52 weeks | − 0.78 (− 1.20, − 0.36) | − 0.80 (− 1.26, − 0.34) | − 0.46 (− 1.05, 0.13) | − 0.33 (− 1.05, 0.40) | − 0.35 (− 1.09, 0.40) | 0.02 (− 0.61, 0.65) |
| Perceived sleep quality§ | ||||||
| 12 weeks | − 0.56 (− 0.92, − 0.21) | − 0.43 (− 0.81, − 0.04) | − 0.10 (− 0.61, 0.41) | − 0.47 (− 1.08, 0.15) | − 0.33 (− 0.96, 0.31) | − 0.14 (− 0.66, 0.39) |
| 52 weeks | − 1.05 (− 1.48, − 0.62) | − 0.71 (− 1.17, − 0.24) | − 0.26 (− 0.85, 0.34) | − 0.79 (− 1.53, − 0.05) | − 0.45 (− 1.20, 0.31) | − 0.34 (− 0.99, 0.30) |
| Daily disturbances‖ | ||||||
| 12 weeks | − 0.31 (− 0.53, − 0.09) | − 0.26 (− 0.50, − 0.02) | − 0.08 (− 0.39, 0.23) | − 0.23 (− 0.61, 0.15) | − 0.18 (− 0.57, 0.21) | − 0.05 (− 0.37, 0.28) |
| 52 weeks | − 0.44 (− 0.70, − 0.18) | − 0.42 (− 0.70, − 0.14) | − 0.16 (− 0.52, 0.20) | − 0.28 (− 0.73, 0.16) | − 0.26 (− 0.72, 0.19) | − 0.02 (− 0.40, 0.36) |
*PSQI global score and 3-factor scoring system. Complete case analysis for those with PSQI data available at 12 (n = 287) and 52 weeks (n = 255)
†Adjusting for baseline age and Roland-Morris Disability Questionnaire score
‡Sleep efficiency: sleep duration and habitual sleep efficiency
§Perceived sleep quality: subjective sleep quality, sleep latency, and sleep medication use
‖Daily disturbances: sleep disturbances, daytime dysfunction
Change in PSQI global score over 52 weeks. In this complete case analysis, we used all PSQI data available at baseline (n= 320), 12 weeks (n= 287), and 52 weeks (n= 255).
In the 3-factor analysis, perceived sleep quality (factor 2) was the domain most improved in the yoga and PT groups (Table 2). In Appendix Table 6, we present 12- and 52-week changes in global PSQI by sex and race. We did not observe statistically significant effect modification by race or sex at either time point.
Table 6.
PSQI Global Score Stratified by Sex and Race
| Mean Within-group difference (95% confidence interval) | Mean Between-group difference (95% confidence interval) | ||||
|---|---|---|---|---|---|
| Global PSQI* | Yoga | PT | Education | Yoga vs education | PT vs education |
| Full sample | (n = 127) | (n = 129) | (n = 64) | ||
| 12 weeks | − 1.19 (− 1.82, − 0.55) | − 0.91 (− 1.61, − 0.20) | − 0.55 (− 1.47, 0.37) | − 0.63 (− 1.75, 0.49) | − 0.35 (− 1.51, 0.80) |
| 52 weeks | − 2.32 (− 3.16, − 1.48) | − 1.86 (− 2.77, − 0.95) | − 0.86 (− 2.02, 0.30) | − 1.46 (− 2.90, − 0.03) | − 1.00 (− 2.47, 0.47) |
| Male | (n = 55) | (n = 39) | (n = 22) | ||
| 12 weeks | − 1.03 (− 1.87, − 0.19) | − 1.82 (− 2.99, − 0.64) | − 0.24 (− 1.68, 1.20) | − 0.79 (− 2.44, 0.87) | − 1.58 (− 3.41, 0.25) |
| 52 weeks | − 1.22 (− 2.53, 0.10) | − 2.43 (− 4.34, − 0.53) | 0.91 (− 1.38, 3.20) | − 2.12 (− 4.74, 0.50) | − 3.34 (− 6.26, − 0.42) |
| Female | (n = 72) | (n = 90) | (n = 42) | ||
| 12 weeks | − 1.32 (− 2.23, − 0.42) | − 0.58 (− 1.46, 0.30) | − 0.65 (− 1.83, 0.53) | − 0.67 (− 2.16, 0.81) | 0.07 (− 1.40, 1.54) |
| 52 weeks | − 3.01 (− 4.09, − 1.94) | − 1.69 (− 2.73, − 0.66) | − 1.48 (− 2.82, − 0.13) | − 1.53 (− 3.25, 0.19) | − 0.22 (− 1.91, 1.47) |
| White | (n = 30) | (n = 17) | (n = 11) | ||
| 12 weeks | − 1.38 (− 2.68, − 0.08) | − 1.72 (− 3.51, 0.08) | 0.07 (− 2.13, 2.28) | − 1.45 (− 3.98, 1.08) | − 1.79 (− 4.54, 0.96) |
| 52 weeks | − 2.49 (− 4.02, − 0.97) | − 1.43 (− 3.50, 0.63) | 1.28 (− 1.55, 4.12) | − 3.78 (− 6.98, − 0.57) | − 2.72 (− 6.09, 0.65) |
| Non-white | (n = 97) | (n = 112) | (n = 53) | ||
| 12 weeks | − 1.14 (− 1.88, − 0.40) | − 0.76 (− 1.54, 0.01) | − 0.66 (− 1.69, 0.36) | − 0.47 (− 1.74, 0.79) | − 0.10 (− 1.38, 1.18) |
| 52 weeks | − 2.30 (− 3.30, − 1.30) | − 1.93 (− 2.95, − 0.90) | − 1.18 (− 2.48, 0.11) | − 1.11 (− 2.75, 0.52) | − 0.74 (− 2.39, 0.90) |
*Adjusting for baseline age and Roland-Morris Disability Questionnaire score
Responder Analysis
At 12 weeks, 10% more yoga (44/125, 35%) and PT (36/102, 35%) participants experienced a clinically meaningful improvement in overall sleep quality (3-point PSQI change) than the education group (15/60, 25%), though this difference was not statistically significant (Table 3). By 52 weeks, the proportion of sleep responders were similar in all three groups (yoga, 44/108, 41%; PT, 37/91, 41%; education, 20/56, 36%).
Table 3.
Predictors of a 3-Point Improvement in PSQI from Baseline to 12 or 52 weeks
| Total | 12-week responders | 52-week responders | |||
|---|---|---|---|---|---|
| Predictor | N | N (%) | OR (95% CI) | N (%) | OR (95% CI) |
| Treatment | |||||
| Yoga | 127 | 44/125 (35.2) | 1.63 (0.82, 3.25) | 44/108 (40.7) | 1.24 (0.64, 2.41) |
| PT | 129 | 36/102 (35.3) | 1.64 (0.80, 3.33) | 37/91 (40.7) | 1.23 (0.62, 2.46) |
| Education | 64 | 15/60 (25.0) | Ref | 20/56 (35.7) | Ref |
| Sex | |||||
| Female | 204 | 60/189 (31.8) | 0.84 (0.50, 1.40) | 77/179 (43.0) | 1.64 (0.93, 2.88) |
| Male | 116 | 35/98 (35.7) | Ref | 24/76 (31.6) | Ref |
| Race | |||||
| White | 58 | 18/54 (33.3) | 1.01 (0.54, 1.90) | 14/51 (27.5) | 0.51 (0.26, 1.00) |
| Non-white | 262 | 77/233 (33.1) | Ref | 87/204 (42.7) | Ref |
| Age group | |||||
| ≥ 34 | 58 | 17/50 (34.0) | Ref | 22/44 (50.0) | Ref |
| 35–44 | 67 | 23/59 (39.0) | 1.24 (0.57, 2.72) | 24/51 (47.1) | 0.89 (0.40, 1.99) |
| 45–54 | 122 | 34/110 (30.9) | 0.87 (0.43, 1.77) | 30/98 (30.6) | 0.44 (0.21, 0.92) |
| ≥ 55 | 73 | 21/68 (30.9) | 0.87 (0.40, 1.89) | 25/62 (40.3) | 0.68 (0.31, 1.47) |
| Education level | |||||
| < High school | 126 | 36/111 (32.4) | Ref | 60/100 (39.2) | Ref |
| ≥ High school | 191 | 57/173 (33.0) | 1.02 (0.62, 1.70) | 40/153 (40.0) | 0.97 (0.58, 1.62) |
| Current smoker | |||||
| No | 223 | 69/202 (34.2) | Ref | 76/183 (41.5) | Ref |
| Yes | 97 | 26/85 (30.6) | 0.85 (0.49, 1.47) | 25/72 (34.7) | 0.75 (0.43, 1.32) |
| Employed | |||||
| No | 176 | 49/157 (31.2) | Ref | 48/144 (33.3) | Ref |
| Yes | 144 | 46/130 (35.4) | 1.21 (0.74, 1.98) | 53/111 (47.8) | 1.83 (1.10, 3.04) |
| Depression | |||||
| No | 242 | 70/219 (32.0) | Ref | 84/195 (43.1) | Ref |
| Yes | 66 | 22/57 (38.6) | 1.34 (0.73, 2.45) | 14/51 (27.5) | 0.50 (0.25, 0.98) |
| COPD | |||||
| No | 239 | 73/218 (33.5) | Ref | 83/186 (44.6) | Ref |
| Yes | 79 | 20/67 (29.9) | 0.85 (0.47, 1.53) | 17/67 (25.4) | 0.42 (0.23, 0.79) |
| Pain improvement, 6 weeks | |||||
| < 10% | 77 | 20/75 (26.7) | Ref | 23/69 (33.3) | Ref |
| 10–30% | 136 | 31/129 (24.0) | 0.87 (0.45, 1.67) | 46/112 (41.1) | 1.39 (0.75, 2.61) |
| > 30% | 67 | 37/66 (56.1) | 3.51 (1.73, 7.11) | 26/59 (44.1) | 1.58 (0.77, 3.23) |
| RMDQ improvement, 6 weeks | |||||
| < 10% | 121 | 28/116 (24.1) | Ref | 33/103 (32.0) | Ref |
| 10–30% | 73 | 26/70 (37.1) | 1.86 (0.97, 3.54) | 23/60 (38.3) | 1.32 (0.68, 2.56) |
| > 30% | 88 | 35/86 (40.7) | 2.16 (1.18, 3.95) | 40/79 (50.6) | 2.18 (1.19, 3.98) |
OR, odds ratio; COPD, chronic obstructive pulmonary disease; RMDQ, Roland Morris Disability Questionnaire
Irrespective of treatment, participants who reported a 30% improvement in pain or physical function at 6 weeks, compared with non-improvers, were more likely to report an improvement in sleep quality at both 12 (OR = 3.51, 95% CI 1.73, 7.11 and OR = 2.16, 95% CI 1.18, 3.95, respectively) and 52 weeks (OR = 1.58, 95% CI 0.77, 3.23 and OR = 2.18, 95% CI 1.19, 3.98, respectively). Additionally, female, non-white, and employed participants were among those likely to have improvements in sleep quality by 52 weeks. Participants who self-reported depression or COPD were less likely to improve their sleep, compared with their unaffected counterparts, at 52 weeks (OR = 0.50, 95% CI 0.25, 0.98 and OR = 0.42, 95% CI 0.23, 0.79, respectively).
DISCUSSION
In this secondary analysis of a RCT of 320 adults with cLBP treated with yoga, PT, or back pain education, we found over 90% of participants had poor sleep quality prior to intervention, i.e., a global PSQI score of 5 or higher. Modest improvements in sleep quality were reported in all groups at 12 weeks and 52 weeks, but there were no statistically different benefits for yoga or PT compared with education other than improved sleep quality with yoga compared with education at 52 weeks. However, participants with clinically important improvements in back-related pain or physical function at 6 weeks, compared with others, were much more likely to have a clinically important improvement in sleep quality by 12 weeks.
While the association of cLBP and poor sleep quality is well established,1 previous studies of adults with cLBP reported a lower prevalence (e.g. 59%) of sleep disturbance. The greater prevalence of sleep disturbance in our population may be partially explained by our predominately low-income minority sample.50,51 Factors associated with poor sleep quality that aggregate in disadvantaged communities, such as neighborhood noise, inopportune light exposure, air pollution, and irritants (e.g., tobacco smoke); perceived discrimination; and poor access to treatment, may explain the higher prevalence.53,54 Additionally, two-thirds of our participants were obese, and roughly a third were current smokers; each of these factors is associated with poor sleep.39,53
Our findings of modest improvement in sleep quality are consistent with a range of smaller studies of yoga for sleep in older adults,29–32 including those with knee osteoarthritis.35,36 Likewise, our finding of a modest improvement in sleep quality with PT is consistent with a study of 60 older adults with cLBP who received 8 weeks of PT and experienced a 1.5-point improvement in PSQI.22 However, four additional studies of mixed aerobic and resistance exercise interventions for sleep quality found larger improvements in sleep quality, i.e., 2.7 to 5.5 points.18–21 Our interventions were developed to address cLBP, and focus less on aerobic exercise compared with improving flexibility and strength. Thus, interventions focused on more vigorous exercise may explain the larger treatment effect in these studies.18–21
We found that improvements in pain or physical function mid-intervention were associated with clinically meaningful improvements in sleep quality at the end of the intervention period. There are several mechanisms supporting bi-directional associations between pain/physical function and sleep and prior research has examined the role of sleep interventions on pain.1,55,56 It is unclear if improvements in pain or physical function caused improved sleep, or improved sleep from yoga caused improvements in pain or function. To establish directionality of these potential therapeutic mechanisms, a prospective study would be needed in adults with co-occurring cLBP and sleep dysfunction, including formal mediation analyses and measures of both pain and sleep quality more frequently (e.g., weekly) over treatment and follow-up periods.”
Few studies of yoga and PT have measured long-term sleep outcomes. We found reductions in global PSQI persisted over 52 weeks in participants in the yoga and PT but not the education group. While these outcomes need to be cautiously interpreted as they were the results of secondary analyses and were not accompanied by significant changes in the proportion of individuals with a clinically meaningful improvement in PSQI, they suggest the potential for long-term improvements in sleep quality, particularly with yoga. Other trials of yoga26,30 and exercise20 have observed similar gradual improvement over longer follow-up periods, i.e., 6 months to 2 years and support the need for long-term evaluations of effects of behavioral interventions on sleep quality.
Yoga and PT are resource and time intensive, and additional analyses of cost-effectiveness are needed. Preliminary evidence suggests yoga57,58 and PT59 are cost-effective for managing cLBP, and a cost-effectiveness analysis of the Back to Health Study is currently underway. Our yoga and PT interventions are relatively low-cost when compared with invasive procedures, such as epidural injections and surgery. In our study, the overall annual costs were $650 and $1400 per yoga and PT participant (unpublished), respectively. Additionally, yoga and PT interventions teach self-care strategies, which can be practiced at home at no cost. Insurance coverage for yoga and incorporation of these services in community health centers may reduce the financial burden to patients.
Strengths of our study include a large sample size, a well-validated sleep outcome measure, abundant information on key covariates, and a 1-year follow-up period. Our sample can be considered representative with regard to describing sleep quality in low-income primarily minority adults with cLBP.
The principal limitation of our study is that this is a secondary analysis of a RCT designed to evaluate the effectiveness of yoga and PT for improving pain and back-specific function in adults with cLBP. Nonetheless, over 90% of Back to Health Study participants had poor sleep quality at baseline. Although we did not use an objective measure of sleep quality (e.g., polysomnography) and could not identify participants with insomnia disorder, the PSQI is a commonly used, well-validated, reliable measure of sleep for patients with primary insomnia.43
Moderate loss to follow-up at 12 and 52 weeks (10% and 20%, respectively) with disproportionately greater loss to follow-up in PT is an additional limitation. Fortunately, our sensitivity analyses using multiple imputation, to evaluate bias, provided similar results. Additionally, it seems unlikely that the disproportionate loss to follow-up in the PT group was due to sleep outcomes.
Although the yoga and PT interventions were designed to improve back pain rather than sleep quality, our interventions included components thought to be important for improving sleep quality, i.e., modest exercise, meditation, relaxation.13,60 More significant improvements in sleep may potentially be achieved by tailoring yoga practices for bedtime use, or by combining yoga or PT with sleep hygiene or CBT-I. Further research is needed in this area, particularly among racial/ethnic minorities who have a high proportion of sleep disturbance, yet are underrepresented in studies on behavioral and yoga interventions for sleep.
Our findings suggest that effective treatment of cLBP may improve sleep quality. Future research on clinical interventions should further explore the interaction of improvements in back pain and sleep quality. Developing optimal interventions may hinge on improving our understanding of the associations between cLBP and poor sleep quality.
CONCLUSIONS
In a sample of low-income racially diverse adults with cLBP, over 90% reported poor sleep quality at baseline. It is important for primary care providers to be aware of the common coexistence of sleep problems with cLBP and to identify and address these co-occurring conditions. Overall, yoga and PT provided modest improvements in sleep quality. However, participants who had a clinically meaningful reduction in pain mid-intervention were three-and-a-half times as likely to have a clinically significant improvement in sleep quality at the end of the 12-week intervention period. Yoga and PT may be helpful in co-managing back pain symptoms and poor sleep quality. Future research is needed to develop and evaluate yoga and PT interventions that may maximize benefit to both of these symptomatic domains.
Acknowledgments
We thank David Felson, MD, MPH, and the Boston University Clinical Epidemiology Research Unit for their constructive review of this paper.
Appendix
Author Contributions
Drs. Roseen, Gerlovin, and Saper had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Roseen, Sherman, Saper.
Acquisition, analysis, or interpretation of data: Roseen, Gerlovin, Femia, Cho, Bertisch, Redline, Sherman, Saper.
Drafting of the manuscript: Roseen.
Critical revision of the manuscript for important intellectual content: Roseen, Gerlovin, Femia, Cho, Bertisch, Redline, Sherman, Saper.
Statistical analysis: Roseen, Gerlovin.
Obtained funding: n/a.
Administrative, technical, or material support: Roseen, Femia, Saper.
Study supervision: Roseen, Saper.
Funding Information
The Back to Health Study (5R01-AT005956) was funded by the National Center for Complementary and Integrative Health (NCCIH). Dr. Roseen is supported by a Ruth L. Kirschstein National Research Service Award (1F32AT009272) from the NCCIH and by the Boston University Clinical and Translational Science Institute (CTSI) Clinical Research Training Program (1UL1TR001430). Drs. Bertisch and Redline are supported in part by NCCIH (R34 AT008923).
Compliance with Ethical Standards
The Boston University Medical Campus Institutional Review Board approved the study prior to data collection. All participants provided written informed consent.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Disclaimer
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NCCIH.
Footnotes
Prior Presentations This work was presented, in part, at the International Research Congress on Integrative Medicine & Health (May 2018) in Baltimore, MD, USA, and the International Back and Neck Pain Forum (July 2019) in Quebec City, Quebec, Canada.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eric J. Roseen, Email: Eric.Roseen@bmc.org.
Hanna Gerlovin, Email: Gerlovin@bu.edu.
Alexandra Femia, Email: Alexandra.Femia@gmail.com.
Jae Cho, Email: JCho1415@bu.edu.
Suzanne Bertisch, Email: SBertisch@partners.org.
Susan Redline, Email: SRedline@bwh.harvard.edu.
Karen J. Sherman, Email: Karen.J.Sherman@kp.org.
Robert Saper, Email: Robert.Saper@bmc.org.
References
- 1.Kelly GA, Blake C, Power CK, O’Keeffe D, Fullen BM. The association between chronic low back pain and sleep: a systematic review. Clin J Pain. 2011;27(2):169–181. doi: 10.1097/AJP.0b013e3181f3bdd5. [DOI] [PubMed] [Google Scholar]
- 2.Alsaadi SM, McAuley JH, Hush JM, Maher CG. Prevalence of sleep disturbance in patients with low back pain. Eur Spine J. 2011;20(5):737–743. doi: 10.1007/s00586-010-1661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang NK, Wright KJ, Salkovskis PM. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res. 2007;16(1):85–95. doi: 10.1111/j.1365-2869.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- 4.Alsaadi SM, McAuley JH, Hush JM, et al. The bidirectional relationship between pain intensity and sleep disturbance/quality in patients with low back pain. Clin J Pain. 2014;30(9):755–765. doi: 10.1097/AJP.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 5.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones JD, Mogali S, Comer SD. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 2012;125(1–2):8–18. doi: 10.1016/j.drugalcdep.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho KK FP, Pinheiro MB, Silva DA, Miller CB, Grunstein R, Simic. Sleep interventions for osteoarthritis and spinal pain: a systematic review and meta-analysis of randomized controlled trials. Osteoarthritis and Cartilage. 2018. [DOI] [PubMed]
- 8.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163(3):191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- 9.Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142(9):776–785. doi: 10.7326/0003-4819-142-9-200505030-00014. [DOI] [PubMed] [Google Scholar]
- 10.Cramer H, Lauche R, Haller H, Dobos G. A systematic review and meta-analysis of yoga for low back pain. Clin J Pain. 2013;29(5):450–460. doi: 10.1097/AJP.0b013e31825e1492. [DOI] [PubMed] [Google Scholar]
- 11.Wieland LS, Skoetz N, Pilkington K, Vempati R, D’Adamo CR, Berman BM. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev. 2017;1:CD010671. [DOI] [PMC free article] [PubMed]
- 12.Qaseem Amir, Wilt Timothy J., McLean Robert M., Forciea Mary Ann. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Annals of Internal Medicine. 2017;166(7):514. doi: 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- 13.Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH. The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep Med Rev. 2015;22:23–36. doi: 10.1016/j.smrv.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freburger JK, Carey TS, Holmes GM, et al. Exercise prescription for chronic back or neck pain: who prescribes it? who gets it? What is prescribed? Arthritis Rheum. 2009;61(2):192–200. doi: 10.1002/art.24234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang P-Y, Ho K-H, Chen H-C, Chien M-Y. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. Journal of Physiotherapy. 2012;58(3):157–163. doi: 10.1016/S1836-9553(12)70106-6. [DOI] [PubMed] [Google Scholar]
- 16.Rogers LQ, Courneya KS, Oster RA, et al. Physical Activity and Sleep Quality in Breast Cancer Survivors: A Randomized Trial. Med Sci Sports Exerc. 2017;49(10):2009–2015. doi: 10.1249/MSS.0000000000001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guthrie KA, Larson JC, Ensrud KE, et al. Effects of Pharmacologic and Nonpharmacologic Interventions on Insomnia Symptoms and Self-reported Sleep Quality in Women With Hot Flashes: A Pooled Analysis of Individual Participant Data From Four MsFLASH Trials. Sleep. 2018;41(1). [DOI] [PMC free article] [PubMed]
- 18.King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA. 1997;277(1):32–37. [PubMed] [Google Scholar]
- 19.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of the effect of exercise on sleep. Sleep. 1997;20(2):95–101. doi: 10.1093/sleep/20.2.95. [DOI] [PubMed] [Google Scholar]
- 20.Vaz Fragoso CA, Miller ME, King AC, et al. Effect of Structured Physical Activity on Sleep-Wake Behaviors in Sedentary Elderly Adults with Mobility Limitations. J Am Geriatr Soc. 2015;63(7):1381–1390. doi: 10.1111/jgs.13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, Zee PC. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010;11(9):934–940. doi: 10.1016/j.sleep.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eadie J, van de Water AT, Lonsdale C, et al. Physiotherapy for sleep disturbance in people with chronic low back pain: results of a feasibility randomized controlled trial. Arch Phys Med Rehabil. 2013;94(11):2083–2092. doi: 10.1016/j.apmr.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1–16. [PMC free article] [PubMed]
- 24.Fang R, Li X. A regular yoga intervention for staff nurse sleep quality and work stress: a randomised controlled trial. J Clin Nurs. 2015;24(23–24):3374–3379. doi: 10.1111/jocn.12983. [DOI] [PubMed] [Google Scholar]
- 25.Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100(10):2253–2260. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 26.Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118(15):3766–3775. doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233–3241. doi: 10.1200/JCO.2012.43.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cramer H, Pokhrel B, Fester C, et al. A randomized controlled bicenter trial of yoga for patients with colorectal cancer. Psychooncology. 2016;25(4):412–420. doi: 10.1002/pon.3927. [DOI] [PubMed] [Google Scholar]
- 29.Chen KM, Chen MH, Chao HC, Hung HM, Lin HS, Li CH. Sleep quality, depression state, and health status of older adults after silver yoga exercises: cluster randomized trial. Int J Nurs Stud. 2009;46(2):154–163. doi: 10.1016/j.ijnurstu.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Chen KM, Chen MH, Lin MH, Fan JT, Lin HS, Li CH. Effects of yoga on sleep quality and depression in elders in assisted living facilities. J Nurs Res. 2010;18(1):53–61. doi: 10.1097/JNR.0b013e3181ce5189. [DOI] [PubMed] [Google Scholar]
- 31.Hariprasad VR, Sivakumar PT, Koparde V, et al. Effects of yoga intervention on sleep and quality-of-life in elderly: A randomized controlled trial. Indian J Psychiatry. 2013;55(Suppl 3):S364–368. doi: 10.4103/0019-5545.116310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halpern J, Cohen M, Kennedy G, Reece J, Cahan C, Baharav A. Yoga for improving sleep quality and quality of life for older adults. Altern Ther Health Med. 2014;20(3):37–46. [PubMed] [Google Scholar]
- 33.Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause. 2014;21(4):339–346. doi: 10.1097/GME.0b013e31829e4baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchanan DT, Landis CA, Hohensee C, et al. Effects of Yoga and Aerobic Exercise on Actigraphic Sleep Parameters in Menopausal Women with Hot Flashes. J Clin Sleep Med. 2017;13(1):11–18. doi: 10.5664/jcsm.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taibi DM, Vitiello MV. A pilot study of gentle yoga for sleep disturbance in women with osteoarthritis. Sleep Med. 2011;12(5):512–517. doi: 10.1016/j.sleep.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung C, Wyman JF, Resnick B, Savik K. Yoga for managing knee osteoarthritis in older women: a pilot randomized controlled trial. BMC Complement Altern Med. 2014;14:160. doi: 10.1186/1472-6882-14-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirkwood G, Rampes H, Tuffrey V, Richardson J, Pilkington K. Yoga for anxiety: a systematic review of the research evidence. Br J Sports Med. 2005;39(12):884–891. doi: 10.1136/bjsm.2005.018069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saper RB, Sherman KJ, Delitto A, et al. Yoga vs. physical therapy vs. education for chronic low back pain in predominantly minority populations: study protocol for a randomized controlled trial. Trials. 2014;15:67. doi: 10.1186/1745-6215-15-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saper RB, Lemaster C, Delitto A, et al. Yoga, Physical Therapy, or Education for Chronic Low Back Pain: A Randomized Noninferiority Trial. Ann Intern Med. 2017;167(2):85–94. doi: 10.7326/M16-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore JE LK, Von Korff M, Gonzalez VM, Laurent DD. The Back Pain Helpbook. New York: Perseus Books; 1999.
- 41.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 42.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 43.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 44.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29(1):112–116. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- 46.Hughes JM, Song Y, Fung CH, et al. Measuring Sleep in Vulnerable Older Adults: A Comparison of Subjective and Objective Sleep Measures. Clin Gerontol. 2018;41(2):145–157. doi: 10.1080/07317115.2017.1408734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB. Assessing health-related quality of life in patients with sciatica. Spine (Phila Pa 1976). 1995;20(17):1899–1908. doi: 10.1097/00007632-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976). 2008;33(1):90–94. doi: 10.1097/BRS.0b013e31815e3a10. [DOI] [PubMed] [Google Scholar]
- 49.Jordan K, Dunn KM, Lewis M, Croft P. A minimal clinically important difference was derived for the Roland-Morris Disability Questionnaire for low back pain. J Clin Epidemiol. 2006;59(1):45–52. doi: 10.1016/j.jclinepi.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Wang R, Zee P, et al. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38(6):877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: Analysis of the 2002 national health interview survey data. Arch Intern Med. 2006;166(16):1775–1782. doi: 10.1001/archinte.166.16.1775. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan TR, White IR, Salter AB, Ryan P, Lee KJ. Should multiple imputation be the method of choice for handling missing data in randomized trials? Stat Methods Med Res. 2018;27(9):2610–2626. doi: 10.1177/0962280216683570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kingsbury JH, Buxton OM, Emmons KM. Sleep and its Relationship to Racial and Ethnic Disparities in Cardiovascular Disease. Curr Cardiovasc Risk Rep. 2013;7(5). [DOI] [PMC free article] [PubMed]
- 54.Hicken MT, Lee H, Ailshire J, Burgard SA, Williams DR. “Every shut eye, ain’t sleep”: The role of racism-related vigilance in racial/ethnic disparities in sleep difficulty. Race Soc Probl. 2013;5(2):100–112. doi: 10.1007/s12552-013-9095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vitiello MV, McCurry SM, Shortreed SM, et al. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain. 2014;155(8):1547–1554. doi: 10.1016/j.pain.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salwen JK, Smith MT, Finan PH. Mid-Treatment Sleep Duration Predicts Clinically Significant Knee Osteoarthritis Pain reduction at 6 months: Effects From a Behavioral Sleep Medicine Clinical Trial. Sleep. 2017;40(2). [DOI] [PMC free article] [PubMed]
- 57.Chuang LH, Soares MO, Tilbrook H, et al. A pragmatic multicentered randomized controlled trial of yoga for chronic low back pain: economic evaluation. Spine (Phila Pa 1976). 2012;37(18):1593–1601. doi: 10.1097/BRS.0b013e3182545937. [DOI] [PubMed] [Google Scholar]
- 58.Aboagye E, Karlsson ML, Hagberg J, Jensen I. Cost-effectiveness of early interventions for non-specific low back pain: a randomized controlled study investigating medical yoga, exercise therapy and self-care advice. J Rehabil Med. 2015;47(2):167–173. doi: 10.2340/16501977-1910. [DOI] [PubMed] [Google Scholar]
- 59.Torstensen TA, Ljunggren AE, Meen HD, Odland E, Mowinckel P, Geijerstam S. Efficiency and costs of medical exercise therapy, conventional physiotherapy, and self-exercise in patients with chronic low back pain. A pragmatic, randomized, single-blinded, controlled trial with 1-year follow-up. Spine (Phila Pa 1976) 1998;23(23):2616–2624. doi: 10.1097/00007632-199812010-00017. [DOI] [PubMed] [Google Scholar]
- 60.Black DS, O’Reilly GA, Olmstead R, Breen EC, Irwin MR. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: a randomized clinical trial. JAMA Intern Med. 2015;175(4):494–501. doi: 10.1001/jamainternmed.2014.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]