Abstract
Introduction
Previous studies have reported lower rates of coronary angiography and revascularization, and significantly higher mortality among patients infected with human immunodeficiency virus (HIV) presenting with acute myocardial infarction (AMI). This observational study was designed to evaluate characteristics and inpatient outcomes of patients with seropositive HIV infection presenting with AMI.
Methods
Using the National Inpatient Sample (NIS) database, we identified patients (admissions) with a primary diagnosis of myocardial infarction and a co-occurring HIV. We described baseline characteristics and outcomes. Our primary outcomes of interest were prevalence of coronary angiography, revascularization (percutaneous coronary intervention (PCI) or CABG), and mortality.
Results
From 2010 to 2014, of about 2,977,387 patients with a primary diagnosis of AMI, 10,907 (0.4%) were HIV seropositive. Patients with HIV were younger and more likely to be African American or Hispanic. Coronary angiography and revascularization were performed more frequently in the HIV population. The higher prevalence of revascularization was driven by a higher incidence of PCI. In a multivariable model, patients with HIV were no more likely to undergo revascularization than the general population. This was also the case for PCI. Unadjusted all-cause mortality was lower among patients with HIV. After controlling for confounders, this finding was not significant (OR 0.97, 95% CI 0.75–1.25, p = 0.79). The length of stay between both groups was comparable.
Conclusion
In this current analysis, we did not note any treatment bias or difference in the rate of in-hospital total mortality for HIV-seropositive patients presenting with AMI compared with the general population.
KEY WORDS: human immunodeficiency virus, acute myocardial infarction, revascularization
INTRODUCTION
In 2013, the United Nations (UN) reported an estimation of 35.3 (32.2–38.8) million people worldwide living with human immunodeficiency virus (HIV) with an annual incidence of 2.3 (1.9–2.7) million new HIV infection cases.1 While transmission of HIV encouragingly halved between 2001 and 2012, the infectious disease remains a significant morbidity and mortality burden to global health care.
Traditionally, the majority of deaths in HIV patients could be attributed to HIV-related diseases, with cardiovascular disease (CVD) accounting for only about 6.5–15% of mortality in HIV patients.2–4 However, with newer therapies to treat HIV infection, changing patterns of mortality etiologies for HIV patients are being recognized and reported.5 In fact, CVD-related deaths in HIV patients are increasing in incidence compared with HIV-related deaths, at least in part due to the longer life expectancy in patients taking anti-retroviral therapy (ART), approaching that of non-HIV individuals.6 In addition to a high preponderance of traditional risk factors for CVD, HIV patients also suffer from other pro-atherogenic HIV-specific factors, such as chronic inflammation and immune dysfunction attributable directly to HIV disease, as well as potential ART medication toxicity.7 Many studies have reported younger mean age (− 10 to 15 years) of acute myocardial infarction (AMI) in HIV patients compared with the general population.4 Some studies have noted a conservative treatment bias for such patients.8, 9 It is unclear, though, if AMI management bias persists for HIV patients using more recent data, or whether such bias translates into an appreciable in-hospital mortality difference. We conducted this observational study to evaluate practice patterns and inpatient outcomes of patients with seropositive HIV infection, presenting with AMI compared with the general non-HIV population.
METHOD
Data Source
We obtained and analyzed data from the National Inpatient Sample (NIS) database, the largest publicly available all-payer inpatient care database in US community hospitals, excluding rehabilitation and long-term acute care hospitals. The NIS contains information on all patients, regardless of payer, including individuals covered by Medicare, Medicaid, or private insurance, uninsured. This database contains over a hundred clinical and non-clinical data elements from approximately 7–8 million unweighted hospital stays of de-identified patients each year. Data from this database has been analyzed and used in different studies reporting trends and inpatient outcomes of disease.10–12 The institutional review boards at the University of Kentucky and Rochester General Hospital deemed this study exempt as it is a de-identified, publicly available database.
Study Population and Data Extraction
Records of patients (admissions) with a diagnosis of myocardial infarction (ST- and non-ST elevation myocardial infarction) (International Classification of Diseases (ICD) 9-CM 410.XX) from 2010 to 2014 were extracted from the database. Patients that were managed with revascularization (PCI or CABG) were identified from the dataset. We excluded patients discharged alive on the same day (as well as patients with missing mortality and length of hospital stay. We compared clinical characteristics and inpatient outcomes between patients with versus without HIV (ICD 9-CM 042, 079.53, 795.71, and V08), using used ICD codes, Clinical Classification Software (CCS) codes, and reported Elixhauser comorbidities as reported by Healthcare Cost and Utilization Project (HCUP).13
Definition of Variables
We identified patients’ baseline characteristics and demographic variables such as admission age, gender, and race. We stratified age by decade and identified demographics, comorbidities, and clinical characteristics (inpatient outcomes, procedures, and disposition) using NIS documentation, ICD codes, or the Clinical Classification Software (CCS) coding. The CCS categories have been used to group medical conditions in previous studies.14, 15
Outcomes
The primary outcome was prevalence of coronary angiography (CAG) and revascularization. Other secondary outcomes of interest were all-cause inpatient mortality and length of stay (LOS).
Statistical Analysis
Our analyses were performed using the complex samples facility of SPSS to account for strata (NIS stratum), clustering (hospital ID), and weights (trend weights). We described continuous variables with means and standard deviation for normally distributed variables and median with interquartile range for variables that were not normally distributed and reported categorical variables as counts and proportions. We compared both groups using the chi-square test and Mann-Whitney U tests when applicable. Associations were considered significant if the p value was less than 0.05. We used the SPSS software (IBM SPSS Statistics for Windows, Version 24.0., Armonk, NY: IBM Corp Released 2016) for our statistical analysis.
RESULTS
Patient Population
From the NIS database, we identified 2,977,387 patients with a primary diagnosis of AMI. Of these, 10,907 patients (0.4%) had HIV. After excluding patients with missing outcome data (same day discharge, 64,675; missing, 80; missing length of stay, 10; missing mortality, 1440), we included 10,673 HIV patients in our final analysis.
Compared with AMI patients without HIV, HIV patients were younger and less likely to be female. Compared with AMI patients without HIV, a significantly higher number of those with HIV were Black or Hispanic (Table 1).
Table 1.
Baseline Characteristics of Patients With Acute Myocardial Infarction With Versus Without HIV
| HIV (n = 10,673) | No HIV (n = 2,911,495) | p value | |
|---|---|---|---|
| Age (years) | 54.1 ± 9.3 | 67.4 ± 14.1 | < 0.001 |
| Female gender | 2003 (18.8) | 1,136,308 (39) | < 0.001 |
| Ethnicity | |||
| White | 4518 (42.3) | 2,021,581 (69.4) | < 0.001 |
| Black | 4252 (39.8) | 282,791 (9.7) | < 0.001 |
| Hispanic | 1023 (9.6) | 205,311 (7.1) | < 0.001 |
| Asian/Pacific Islander | 49 (0.5) | 62,233 (2.1) | < 0.001 |
| Native American | 43 (0.4) | 15,682 (0.5) | 0.378 |
| Other | 412 (3.9) | 84,004 (2.9) | 0.064 |
| Comorbidity | |||
| Hypertension | 6983 (65.4) | 2,092,045 (71.9) | < 0.001 |
| Congestive heart failure | 78 (0.7) | 23,795 (0.8) | 0.659 |
| Diabetes mellitus | 2867 (26.9) | 1,079,916 (37.1) | < 0.001 |
| Obesity | 751 (7) | 433,277 (14.9) | < 0.001 |
| Peripheral vascular disorders | 774 (7.3) | 353,644 (12.1) | < 0.001 |
| Chronic renal failure | 2207 (20.7) | 586,964 (20.2) | 0.552 |
| Anemia | 2035 (19.1) | 512,055 (17.6) | 0.084 |
| Smoking | 5698 (53.4) | 1,158,457 (39.8) | < 0.001 |
| Dyslipidemia | 5813 (54.5) | 1,823,537 (62.6) | < 0.001 |
| Coronary artery disease | 8695 (81.5) | 2,392,181 (82.2) | 0.441 |
| Previous myocardial infarction | 1396 (13.1) | 347,757 (11.9) | 0.127 |
| History of PCI | 1694 (15.9) | 42,566(14.6) | 0.11 |
| History of CABG | 464 (4.4) | 238,978 (8.2) | < 0.001 |
| STEMI (current admission) | 3520 (33) | 895,721 (30.8) | 0.038 |
CAD coronary artery disease (old myocardial infarction, history of PCI or coronary artery bypass graft surgery, acute or chronic ischemic heart disease, coronary atherosclerosis, coronary calcification, or lipid-rich plaque); Obesity: BMI ≥ 30 kg/m2; PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, STEMI ST-elevation myocardial infarction
Hypertension, diabetes, obesity, dyslipidemia, and peripheral vascular disease comorbidities were less common in the HIV group. On the other hand, tobacco smoking was reported more often in this group of patients.
Coronary Angiography and Revascularization in HIV Patients
There was a higher proportion of ST-elevation myocardial infarction (STEMI) among patients that were HIV positive. Compared with the non-HIV group with AMI, more HIV patients underwent CAG and revascularization procedures. The higher prevalence of revascularization was driven primarily by a higher incidence of PCI. After adjusting for all variables that were significant in univariate analysis (age, gender, race, hypertension, obesity, peripheral vascular disease, tobacco use status, history of CABG, disease severity, and type of MI), there was no independent association between HIV infection and the prevalence of revascularization. This was also the case for isolated PCI (Fig. 1).]-->
Figure 1.
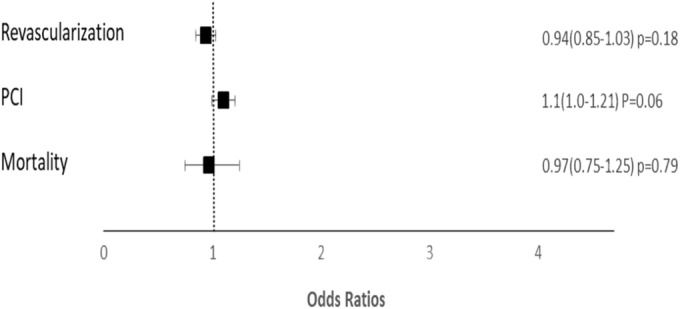
Association of HIV status with revascularization, PCA, and mortality. HIV, human immunodeficiency virus infection; PCI, percutaneous coronary intervention.
In univariate analysis, inpatient outcomes in HIV patients were comparable with that in the non-HIV cohort in terms of complications, including cardiac arrest, mechanical ventilation requirement, transient ischemic attack/stroke, and acute renal failure.
Unadjusted all-cause mortality was lower among patients with HIV. In multivariable analysis, controlling for the same variables listed above, there was no independent association between HIV and in-hospital mortality (Fig. 1). The length of stay between both groups was not significantly different (Table 2).
Table 2.
Clinical and Biological Events During Admission of Patients With Acute Myocardial Infarction With Versus Without HIV
| HIV (n = 10,673) | No HIV (n = 2,911,495) | p value | |
|---|---|---|---|
| Cardiac arrest | 459 (4.3) | 144,069 (4.9) | 0.18 |
| Cardiogenic shock | 489 (4.6) | 162,472 (5.6) | 0.046 |
| Balloon pump | 499 (4.7) | 136,002 (4.7) | 0.997 |
| TIA/stroke | 133 (1.2) | 51,088 (1.8) | 0.067 |
| Respiratory failure | 1063 (10) | 372,475 (12.8) | < 0.001 |
| Use of mechanical ventilation | 871 (8.2) | 246,544 (8.5) | 0.61 |
| Acute renal failure | 1627 (15.2) | 455,931 (15.7) | 0.608 |
| Dialysis for acute renal failure | 107 (1) | 25,915 (0.9) | 0.572 |
| Hemorrhage | 159 (1.5) | 37,948 (1.3) | 0.439 |
| Blood transfusion | 807 (7.6) | 224,416 (7.7) | 0.812 |
| Coronary angiography | 8164 (76.5) | 2,015,278 (69.2) | < 0.001 |
| All revascularization | 6454 (60.5) | 1,602,872 (55.1) | < 0.001 |
| Revascularization by PCI | 5737 (53.8) | 1,375,587 (47.2) | < 0.001 |
| Revascularization by CABG | 808 (7.6) | 251,147 (8.6) | 0.081 |
| In-hospital all-cause mortality | 358 (3.4) | 153,163 (5.3) | < 0.001 |
| Length of stay* | 3 (3) | 3 (4) | 0.061 |
TIA transient ischemic attack, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting
*Median with interquartile range
DISCUSSION
The main findings of this study are as follows: (1) HIV patients admitted for AMI in the NIS database are likely to be younger, male, black, or Hispanic; (2) in all comers with AMI, there was no treatment bias (with respect to HIV status) noted in terms of invasive management (revascularization); (3) mortality was comparable between patients with and without HIV. Our study noted AMI occurring at a younger age in HIV-seropositive patients compared with the control group (p < 0.001). The population with HIV are usually younger.7 HIV infection itself leads to chronic immune system and endothelial activation, as well as heightened inflammation and coagulation, all of which serve as independent etiological risk factors for the development of CVD associated with a preponderance of smoking in this specific population.7, 4, 16 Furthermore, exposure to some HIV ART, particularly protease inhibitors (both individual drugs and drug combinations), is reported to increase cardiovascular risk.17–19
Although our HIV group had fewer females (18.8%) than the non-HIV group, this is still a considerably higher proportion of females than has been reported in other studies of HIV patients with myocardial infarction.20–22 The impact of gender on CVD outcomes for HIV patients is complicated. Compared with that in males, the incidence of AMI in females was significantly lower.23 However, females with AMI have higher hospital mortality than males, which may be at least partially attributable to a lower prevalence of PCI in females.24 Consistent with other studies of HIV patients with myocardial infarction, we observed a higher proportion of African Americans and Hispanics in our HIV group.20–22
Our study did not find a significant difference in baseline comorbidities commonly associated with the development of CAD (hypertension, diabetes, obesity, dyslipidemia, and peripheral vascular disease) between the HIV and control groups. This finding contrasts a cohort study including nearly 4000 HIV patients conducted in 2007, which reported a higher prevalence of hypertension, diabetes, and hyperlipidemia in the HIV group.25 On the other hand, the prevalence of smoking in our study was higher in the HIV group, which is consistent with previous reports.26 The study published in 2007 analyzed data from patient populations admitted from 1996 to 2004, whereas our study analyzed data from patients admitted between 2010 and 2014. The reason for this shift is unclear. This may be related to changes in the risk profile of patients with HIV. This could be the goal of future research in the field.
Whether HIV-seropositive patients receive similar AMI care compared with non-HIV patients has been a topic of concern and debate. A previous study from the 1997–2006 NIS database by Pearce et al. raised a concern that typical invasive procedures occurred at a significantly lower prevalence for HIV-seropositive patients presenting with AMI.27 The authors posited that this lower procedure prevalence might potentiate the additional mortality burden in HIV patients. Another study by Smilowitz et al. that analyzed admissions from the early 2000s reported similar findings.8 Singh et al. also found a lower likelihood of PCI, and use of DES among patients with HIV. This study did not include all comers, which suggests that a lot of patients may have been excluded, skewing the results of the study. Interestingly, the studies by Pearce and Smilowitz noted increasing use of invasive strategies in patients with HIV compared with a relatively flat trend among patients without HIV. These results support the findings in our study, inclusive data from more recent admissions 2010–2014. Another prospective multicenter observational study (2003 to 2006) also suggested that the inpatient management of ACS and in-hospital mortality in HIV-infected patients were as good as those of HIV-uninfected patients.22 This evolution in practice among this high-risk population may be the results of dedicated physicians working hard to provide appropriate care, or at least an increase in comfort level, dealing with such high-risk patients. It is not possible to ascertain from our study if the lack of treatment difference represents appropriateness of intervention or a higher quality of treatment than in the past. It is also possible that the improvement in mortality of patients with AMI and HIV is related to improvement of HIV treatment rather than a lack of difference in prevalence of revascularization or change in care compared with the non-HIV group. These hypotheses need to be further studied. For now, however, in the contemporary era of ACS therapy, as pertains to appropriate CAG and revascularization, it appears that patients with HIV infection are no longer at a treatment disadvantage.
Integrated care for CVD in HIV patients by utilizing multidisciplinary collaboration, shared protocols, and incorporating screening activities is now strongly promoted.28 The above focused efforts and resultant positive changes appear to be evident in the steady decline in mortality due to cardiovascular causes among HIV-seropositive individuals, despite the immunometabolic perturbations caused by the disease and the widespread use of ART, both of which independently increase the risk of CAD and cardiovascular mortality.4, 6, 29–31 The steady decline in cardiovascular morbidity and mortality was also reported in a study by Smith et al. that noted a significant downward trend in the incidence of mortality from cardiovascular causes among HIV patients.3
One of the strengths of our study is our sample number in the contemporary era. We were able to sample patients that are representative of all admissions to community hospitals in the USA. A difference between this study and other studies addressing this subject is that we addressed all comers with AMI. We expected differences in baseline characteristics based on the population of patients with HIV being youngers and more likely to have other comorbidities. Our decision to include all comers was because this gives a real-world analysis of AMI among this high-risk population. Limitations of this study include those inherent to a retrospective analysis of data from administrative database. We used ICD codes to define our comorbidities; hence, our analysis is limited to the accuracy of the database. The time of diagnosis and duration of HIV are not recorded in the database. The use of ART or other medications in these patients is also not known. Vital signs as well as other clinical indices that may have influenced decision to pursue invasive versus non-invasive management are also not reported. Due to the nature of the database, we are unable to know the rationale for revascularization or otherwise in these patients. We are also not able to know how many vessels were revascularized. Finally, outcomes reported in this study are limited to inpatient admissions and long-term outcomes of these patients (admissions) are not reported.
In conclusion, our study did not find any treatment bias and difference in treatment (revascularization) or all-cause in-hospital mortality between HIV patients presenting with AMI and the control group. This highlights an encouraging improvement in cardiovascular care for HIV patients over time.
Funding Information
Penny Warren Research Award, University of Kentucky; Dr. Abdel-Latif and Dr. Smyth are supported by the University of Kentucky Clinical and Translational Science Pilot Award (UL1TR001998 and R56 HL124266).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Gbolahan O. Ogunbayo and Le Dung Ha are co-first authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UNAIDS. GLOBAL REPORT: UNAIDS report on the global AIDS epidemic 2013. 2013. Available at: www.unaids.org/.../unaids/.../2013/gr2013/UNAIDS_Global_Report_2013. Accessed 26 April 2018.
- 2.Collaboration TATC Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996-2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, Kowalska JD, De Wit S, Law M, Sadr W, Kirk O, Friis-Moller N, D’Arminio Monforte A, Phillips AN, Sabin CA, Lundgren JD. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet. 2014;384:241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 4.Boccara F, Lang S, Meuleman C, Ederhy S, Mary-Krause M, Costagliola D, Capeau J, Cohen A. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol. 2013;61:511–523. doi: 10.1016/j.jacc.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 5.Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, Cavassini M, Calmy A, Bernasconi E, Schmid P, Flepp M, Kowalska J, Ledergerber B, Barth J, Battegay M, Bernasconi E, Böni J, Bucher HC, Burton-Jeangros C, Calmy A, Cavassini M, Cellerai C, Egger M, Elzi L, Fehr J, Fellay J, Flepp M, Francioli P, Furrer H, Fux CA, Gorgievski M, Günthard H, Haerry D, Hasse B, Hirsch HH, Hirschel B, Hösli I, Kahlert C, Kaiser L, Keiser O, Kind C, Klimkait T, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Metzner K, Müller N, Nadal D, Pantaleo G, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013;14:195–207. doi: 10.1111/j.1468-1293.2012.01051.x. [DOI] [PubMed] [Google Scholar]
- 6.Cerrato E, Calcagno A, D’Ascenzo F, Biondi-Zoccai G, Mancone M, Grosso Marra W, Demarie D, Omedè P, Abbate A, Bonora S, DiNicolantonio JJ, Estrada V, Escaned J, Moretti C, Gaita F. Cardiovascular disease in HIV patients: from bench to bedside and backwards. Open Hear. 2015;2:e000174. doi: 10.1136/openhrt-2014-000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escarcega RO, Franco JJ, Mani BC, Vyas A, Tedaldi EM, Bove AA. Cardiovascular disease in patients with chronic human immunodeficiency virus infection. Int J Cardiol. 2014;175:1–7. doi: 10.1016/j.ijcard.2014.04.155. [DOI] [PubMed] [Google Scholar]
- 8.Smilowitz NR, Gupta N, Guo Y, Coppola JT, Bangalore S. Influence of Human Immunodeficiency Virus Seropositive Status on the In-Hospital Management and Outcomes of Patients Presenting With Acute Myocardial Infarction. J Invasive Cardiol. 2016;28:403–409. [PubMed] [Google Scholar]
- 9.Singh V, Mendirichaga R, Savani GT, Rodriguez AP, Dabas N, Munagala A, Alfonso CE, Cohen MG, Elmariah S, Palacios IF. Coronary revascularization for acute myocardial infarction in the HIV population. J Interv Cardiol. 2017;30:405–414. doi: 10.1111/joic.12433. [DOI] [PubMed] [Google Scholar]
- 10.Ha LD, Ogunbayo G, Elbadawi A, Olorunfemi O, Messerli A. Early versus delayed coronary artery bypass graft surgery for patients with non-ST elevation myocardial infarction. Coron Artery Dis. 2017;28:670–674. doi: 10.1097/MCA.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 11.Elbadawi Ayman, Ogunbayo Gbolahan O., Elgendy Islam Y., Olorunfemi Odunayo, Saad Marwan, Ha Le Dung, Alotaki Erfan, Baig Basarat, Abuzaid A.S., Shahin Hend I., Shah Abrar, Rao Mohan. Impact of Left Atrial Appendage Exclusion on Cardiovascular Outcomes in Patients With Atrial Fibrillation Undergoing Coronary Artery Bypass Grafting (From the National Inpatient Sample Database) The American Journal of Cardiology. 2017;120(6):953–958. doi: 10.1016/j.amjcard.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Ogunbayo GO, Misumida N, Olorunfemi O, Elbadawi A, Saheed D, Messerli A, Elayi CS, Smyth SS. Comparison of Outcomes in Patients Having Acute Myocardial Infarction With Versus Without Sickle-Cell Anemia. Am J Cardiol. 2017;120:953–958. doi: 10.1016/j.amjcard.2017.07.108. [DOI] [PubMed] [Google Scholar]
- 13.Elixhauser A. Clinical classifications for health policy research, version 2: Software and user’s guide. Healthcare Cost and Utilization Project (HCUP-3) Research Note 2. Rockville, MD: Agency for Health Care Policy and Research. AHCPR Publication No. 96-0046; 1996. [Google Scholar]
- 14.Cook CB, Tsui C, Ziemer DC, Naylor DB, Miller WJ. Common reasons for hospitalization among adult patients with diabetes. Endocr Pract. 2006;12:363–370. doi: 10.4158/EP.12.4.363. [DOI] [PubMed] [Google Scholar]
- 15.Chi M, Lee C, Wu S. The prevalence of chronic conditions and medical expenditures of the elderly by chronic condition indicator (CCI) Arch Gerontol Geriatr. 2011;52:284–289. doi: 10.1016/j.archger.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Nordell AD, McKenna M, Borges AH, Duprez D, Neuhaus J, Neaton JD. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc. 2014;3:1–10. doi: 10.1161/JAHA.114.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai M, Joyce V, Bendavid E, Olshen RA, Hlatky M, Chow A, Holodniy M, Barnett P, Owens DK. Risk of Cardiovascular Events Associated with Current Exposure to HIV Antiretroviral Therapies in a US Veteran Population. Clin Infect Dis. 2015;61:445–452. doi: 10.1093/cid/civ316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durand M, Sheehy O, Baril J-G, Lelorier J, Tremblay CL. Association Between HIV Infection, Antiretroviral Therapy, and Risk of Acute Myocardial Infarction: A Cohort and Nested Case-Control Study Using Québec’s Public Health Insurance Database. J Acquir Immune Defic Syndr. 2011;57:245–253. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 19.Holmberg SD, Moorman AC, Williamson JM, Tong TC, Ward DJ, Wood KC, Greenberg AE. Protease inhibitors and cardiovascular outcomes in patients with HIV-1 For personal use . Only reproduce with permission from The Lancet Publishing Group. Lancet. 2002;360:1747–1748. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 20.Freiberg MS, Chang C-CH, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern Med. 2013;173:614. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, Boccara F, Bingham A, Costagliola D. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–1230. doi: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 22.Boccara F, Mary-Krause M, Teiger E, Lang S, Lim P, Wahbi K, Beygui F, Milleron O, Gabriel Steg P, Funck-Brentano C, Slama M, Girard PM, Costagliola D, Cohen A. Acute coronary syndrome in human immunodeficiency virus-infected patients: Characteristics and 1 year prognosis. Eur Heart J. 2011;32:41–50. doi: 10.1093/eurheartj/ehq372. [DOI] [PubMed] [Google Scholar]
- 23.Kappert K, Böhm M, Schmieder R, Schumacher H, Teo K, Yusuf S, Sleight P, Unger T. Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: Analysis of the telmisartan randomized assessment study in ACE-intolerant subjects with cardiovascular disease (TRANSCEND) and the ongoing telmisartan alone and in combination. Circulation. 2012;126:934–941. doi: 10.1161/CIRCULATIONAHA.111.086660. [DOI] [PubMed] [Google Scholar]
- 24.Milcent C, Dormont B, Durand-Zaleski I, Steg PG. Gender differences in hospital mortality and use of percutaneous coronary intervention in acute myocardial infarction: Microsimulation analysis of the 1999 nationwide French hospitals database. Circulation. 2007;115:833–839. doi: 10.1161/CIRCULATIONAHA.106.664979. [DOI] [PubMed] [Google Scholar]
- 25.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased Acute Myocardial Infarction Rates and Cardiovascular Risk Factors among Patients with Human Immunodeficiency Virus Disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh JY, Greene K, He H, Schafer S, Hedberg K. Population-based study of risk factors for coronary heart disease among HIV-infected persons. Open AIDS J. 2012;6:177–80. doi: 10.2174/1874613601206010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce D, Ani C, Espinosa-Silva Y, Clark R, Fatima K, Rahman M, Diebolt E, Ovbiagele B. Comparison of in-hospital mortality from acute myocardial infarction in HIV sero-positive versus sero-negative individuals. Am J Cardiol. 2012;110:1078–1084. doi: 10.1016/j.amjcard.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 28.Haldane Victoria, Legido-Quigley Helena, Chuah Fiona Leh Hoon, Sigfrid Louise, Murphy Georgina, Ong Suan Ee, Cervero-Liceras Francisco, Watt Nicola, Balabanova Dina, Hogarth Sue, Maimaris Will, Buse Kent, McKee Martin, Piot Peter, Perel Pablo. Integrating cardiovascular diseases, hypertension, and diabetes with HIV services: a systematic review. AIDS Care. 2017;30(1):103–115. doi: 10.1080/09540121.2017.1344350. [DOI] [PubMed] [Google Scholar]
- 29.Khunnawat C, Mukerji S, Havlichek D, Touma R, Abela GS. Cardiovascular Manifestations in Human Immunodeficiency Virus-Infected Patients. Am J Cardiol. 2008;102:635–642. doi: 10.1016/j.amjcard.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, Gerstoft J. Ischemic Heart Disease in HIV-Infected and HIV-Uninfected Individuals: A Population-Based Cohort Study. Clin Infect Dis. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 31.Silverberg MJ, Leyden WA, Xu L, Horberg MA, Chao CR, Towner WJ, Hurley LB, Quesenberry CP, Klein DB. Immunodeficiency and Risk of Myocardial Infarction Among HIV-Positive Individuals With Access to Care. JAIDS J Acquir Immune Defic Syndr. 2014;65:160–166. doi: 10.1097/QAI.0000000000000009. [DOI] [PubMed] [Google Scholar]


