Abstract
Ferroptosis is a form of regulated cell death that is triggered by iron accumulation and lipid peroxidation. Although plasma membrane injuries represent an important event in cell death, the impact of membrane repair mechanisms on ferroptosis remains unidentified. Here, we provide the first evidence that membrane repair dependent on endosomal sorting complexes required for transport (ESCRT)-III negatively regulates ferroptotic cancer cell death. The accumulation of ESCRT-III subunits (e.g., CHMP5 and CHMP6) in the plasma membrane are increased by classical ferroptosis activators (e.g., erastin and RSL3), which relies on endoplasmic reticulum stress and calcium influx. Importantly, the knockdown of CHMP5 or CHMP6 by RNAi sensitizes human cancer cells (e.g., PANC1 and HepG2) to lipid peroxidation-mediated ferroptosis in vitro and in vivo. These findings suggest that ESCRT-III confers resistance to ferroptotic cell death, allowing cell survival under stress conditions.
Keywords: ferroptosis, membrane repair, ESCRT, lipid peroxidation, DAMP
1. Introduction
Cell death is not only a physiological event for organism development, but also a pathological process involved in human disease, such as cancer and degenerative disease. Balancing cell death and survival is therefore important for maintaining homeostasis and adaptation to the environment stress. Cell death is generally divided into two categories: accidental cell death and regulated cell death (RCD) [1]. Accidental cell death is often considered as a form of passive cell death, whereas RCD is a form of active cell death that is characterized by specific morphological, biochemical, and genetic properties [1]. In recent years, types of RCD are increasingly being identified among various species, including mammals [2]. By understanding the components of the molecular machinery of RCD, new targeted therapies can be developed against them. Alternatively, by understanding how anti-injury mechanisms act to repair or remove dead or dying cells, researchers may be able to find new approaches to accelerate or delay cell death.
Membrane injury is a hallmark of RCD although it may exhibit different changes in morphology [3]. Phosphatidylserine is a major constituent of cell membranes. Phosphatidylserine exposure is a common feature of apoptotic cells [4], whereas plasma membrane disruption often occurs in regulated necrosis, such as necroptosis and pyroptosis [5]. As an important part of cellular stress responses, membrane repair can be modulated by various injury stimuli to reduce or delay cell death [6]. Thus, the activation of membrane repair machinery contributes to wound healing during tissue injury or infection [3]. In contrast, blocking membrane repair machinery may improve the activity of anticancer therapy [7].
The endosomal sorting complexes required for transport (ESCRT) assemble into multi-subunit machinery that plays a key role in membranes bending or budding away from the cytoplasm [8, 9]. The ESCRT complex is comprised of five sub-complexes (ESCRT-0, ESCRT-I, ESCRT-II, ESCRT-III, and VPS4) and plays a context-dependent role in membrane remodeling involved in multiple cellular processes. In particular, the ESCRT-III machinery has been recently recognized as an important membrane repair mechanism to limit necroptosis [10] and pyroptosis [11]. However, it remains unknown whether the ESCRT-III machinery also plays a similar role in protection against other types of RCD.
In this study, we provide the first evidence that the ESCRT-III-mediated membrane repair pathway reduces ferroptosis, an oxidative stress-dependent form of RCD driven by lipid peroxidation [12, 13]. The genetic inhibition of the components of ESCRT-III machinery (e.g., charged multivesicular body protein 5 and 6 [CHMP5 and CHMP6] strongly enhances ferroptotic cancer cell death in vitro or in vivo. These findings provide new insight into the cellular survival mechanisms during ferroptosis.
2. Methods
2.1. Reagents
The antibodies to CHMP3 (#sc-166361), CHMP4A (#sc-514869), CHMP5 (#sc-374337), and CHMP6 (#sc-398963) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibodies to SLC7A11 (#12691), EIF2AK3 (#3192), HSPA5 (#3177), and ACTB (#3700) were purchased from Cell Signaling Technology (Danvers, MA, USA). The antibody to GPX4 (#ab125066) was purchased from Abcam (Cambridge, MA, USA). Erastin (#S7242), RSL3 (#S8155), ferrostatin-1 (#S7243), liproxstatin-1 (#S7699), Z-VAD-FMK (#S7023), necrosulfonamide (#S8251), BAPTA-AM (#S7534), PD98059 (#S1177), and TUDCA (#S3654) were purchased from Selleck Chemicals (Houston, TX, USA). In addition, (1S-3R)-RSL3 (#1219810-16-8) was purchased from Cayman Chemicals (Houston, TX, USA).
2.2. Cell culture
PANC1 (#CRL-1469) and HepG2 (#HB-8065) cell lines were obtained from the American Type Culture Collection. These cells were grown in Dulbecco's Modified Eagle's Medium or Eagle's Minimum Essential Medium with 10% fetal bovine serum, 2 mM of L-glutamine, and 100 U/ml of penicillin and streptomycin. Cell line identity was validated by short tandem repeat profiling, and routine mycoplasma testing was negative for contamination.
2.3. RNAi
The human CHMP5-shRNA (5’-CCGGGAGTTGGATGCACTAGGTGATCTCGAGATCACCTAGTGCATCCAACTCTTTTTTG-3’), human CHMP6-shRNA (5’-CCGGGCGCAATCACTCAGGAACAAACTCGAGTTTGTTCCTGAGTGATTGCGCTTTTTTG-3’), and control empty shRNA (pLKO.1) were obtained from Sigma-Aldrich (St. Louis, MO, USA). RNAi was performed using Lipofectamine 3000 (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Stable knockdown cells were selected by adding puromycin.
2.4. Western blot
Western blot was performed as previously described [14]. In brief, proteins in the cell lysate or supernatants were resolved on 4%-12% Criterion XT Bis-Tris gels (#3450124, Bio-Rad, Hercules, CA, USA) and transferred to a nitrocellulose membrane. After blocking with 5% milk, the membrane was incubated for 2 h at 25°C or overnight at 4°C with various primary antibodies. After incubation with peroxidase-conjugated secondary antibodies for 1 h at routine temperature, the signals were visualized using enhanced or super chemiluminescence (Pierce, Rockford, IL, USA) and by exposure to X-ray films.
2.5. Cytotoxicity assays
The level of cell death was assayed using a LIVE/DEAD Cell Viability/Cytotoxicity Assay Kit (#L3224, Thermo Fisher Scientific) according to the manufacturer's protocol.
2.6. Iron assay
The level of ferrous iron in cell extract was assayed using an Iron Assay Kit (#ab83366, Abcam) according to the manufacturer's protocol. In the iron assay protocol, ferrous iron (Fe2+) reacted with Ferene S to produce a stable colored complex with absorbance at 593 nm.
2.7. Malondialdehyde assay
The relative malondialdehyde (MDA) concentration in cell lysates was assessed using a Lipid Peroxidation Assay Kit (#ab118970, Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions [15]. Briefly, the MDA in the sample reacted with thiobarbituric acid (TBA) to generate an MDA-TBA adduct. The MDA-TBA adducts were quantified colorimetrically (OD = 532 nm) or fluorometrically (Ex/Em = 532/553 nm).
2.8. HMGB1 analysis
ELISA assays were performed for the measurement of HMGB1 (#326054329, Sino-Test Corporation, Sagamihara, Japan) in cell culture supernatants or serum according to the manufacturer’s instructions.
2.9. Plasma membrane isolation
Plasma membrane isolation was performed using a Plasma Membrane Protein Extraction Kit (#ab65400, Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions.
2.10. Measurement of intracellular calcium
The cytosolic calcium signal was assayed using a Fura-2 Calcium Flux Assay Kit (#abl76766, Abcam) according to the manufacturer’s instructions. Cytosolic calcium increases were presented as the ratio of emitted fluorescence (510 nm) after excitation at 340 and 380 nm, relative to the ratio measured prior to cell stimulation. The relative concentrations of cytosolic calcium were normalized to cell numbers and expressed in arbitrary units based on the control group, which was assigned a value of 1.
2.11. Animals and treatments
All animal experiments were approved by institutional animal care and use committees. To generate murine subcutaneous tumors, 5 × 106 PANC1 or HepG2 cells in 100 μl PBS were injected subcutaneously to the right of the dorsal midline in 6- to 8-week-old athymic nude or B6 mice. Once the tumors reached 50–70 mm3 at day 7, mice were randomly allocated into groups and treated with (1S-3R)-RSL3 (30 mg/kg; i.p., once every other day) for 2 weeks. Tumors were measured twice weekly and volumes were calculated using the formula length × width2 × π/6.
2.12. Statistical analysis
Statistics were calculated with GraphPad Prism 7. A standard two-tailed unpaired Student’s t test or one-way ANOVA was used for statistical analysis. A P value of less than 0.05 was considered statistically significant.
3. Results
3.1. ESCRT-III accumulation in plasma membrane during ferroptosis
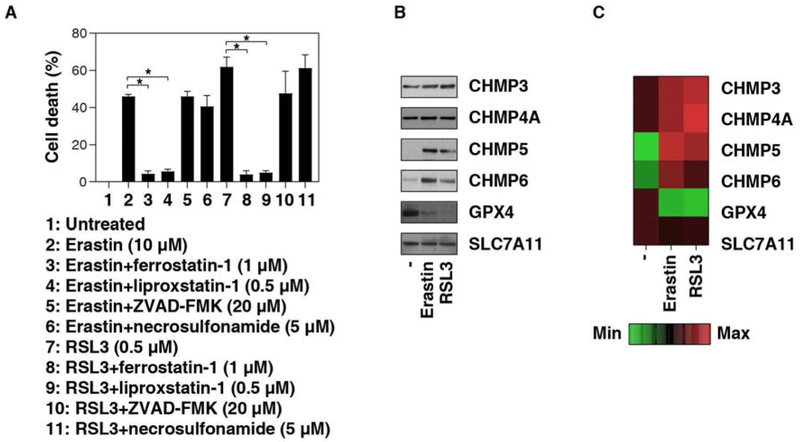
There are two classical types of ferroptosis activators [12, 13]. Type I ferroptosis activators (e.g., erastin) act through the inhibition of system xc−, an amino acid antiporter that mainly mediates the exchange of extracellular cystine and intracellular glutamate across the plasma membrane [16]. Type II ferroptosis activators (e.g., RSL3) function through the inhibition of glutathione peroxidase 4 (GPX4), a selenocysteine-containing phospholipid hydroperoxidase that can remove lipid peroxides [17]. As expected, erastin- or RSL3-induced cell death in human PANC1 cell lines was blocked by the ferroptosis inhibitors (e.g., ferrostatin-1 or liproxstatin-1), but not by an apoptosis inhibitor (e.g., Z-VAD-FMK) or a necroptosis inhibitor (e.g., necrosulfonamide) (Fig. 1A).
Fig. 1. ESCRT-III accumulation in plasma membrane during ferroptosis.
(A) PANC1 cells were treated with erastin or RSL3 in the absence or presence of indicated cell death inhibitor for 12 h and cell death was assayed (n = 3, *P < 0.05). (B) Western blot analysis of indicated protein expression in isolated plasma membrane in PANC cells following treatment with erastin (10 μM) or RSL3 (0.5 μM) for 12 h. (C) Heat map of protein expression in isolated plasma membrane in PANC cells following treatment with erastin (10 μM) or RSL3 (0.5 μM) for 12 h.
To determine the effects of ESCRT-III machinery on erastin- or RSL3-induced ferroptosis, we first measured the protein levels of the components of ESCRT-III machinery, such as CHMP3, CHMP4A, CHMP5, and CHMP6, in isolated plasma membranes from the PANC1 cell line. Compared to CHMP3 and CHMP4A, the plasma membrane levels of CHMP5 and CHMP6 were significantly increased in PANC1 cells following treatment with erastin or RSL3 (Fig. 1B and 1C). Interestingly, the plasma membrane level of solute carrier family 7 member 11 (SLC7A11), a core component of system xc−, was not changed by erastin or RSL3 (Fig. 1B and 1C). In contrast, the plasma membrane level of GPX4 disappeared in PANC1 cells following treatment with erastin or RSL3 (Fig. 1B and 1C). These findings indicate that ferroptosis activators can induce ESCRT-III accumulation in the plasma membrane of PANC1 cells.
3.2. Calcium influx triggers ESCRT-III accumulation in plasma membrane during ferroptosis
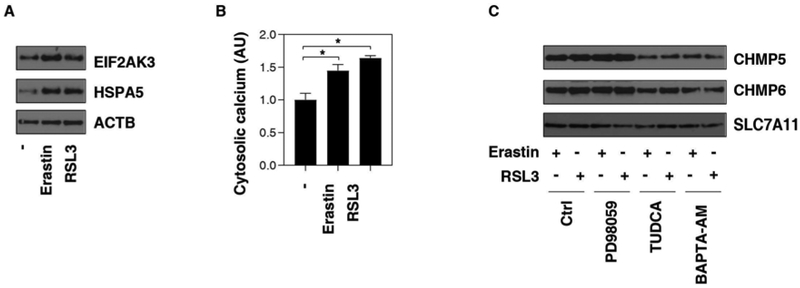
The accumulation of unfolded proteins in the endoplasmic reticulum (ER) represents a cellular stress induced by multiple cell death stimuli, including various ferroptosis activators [18-23]. Consistent with previous studies [18], the levels of ER stress markers, such as heat shock protein family A (Hsp70) member 5 (HSPA5, also known as BIP or GRP78) and eukaryotic translation initiation factor 2 alpha kinase 3 (EIF2AK3, also known as PERK), were upregulated by erastin or RSL3 (Fig. 2A). In addition to protein folding and secretion, ER is critical for calcium homeostasis. Indeed, the intracellular calcium level was upregulated in PANC1 cells in response to erastin or RSL3 (Fig. 2B). Like pretreatment with tauroursodeoxycholic acid (TUDCA, a known ER stress inhibitor), pretreatment with the calcium chelator BAPTA-AM also prevented erastin- or RSL3-induced CHMP5 and CHMP6 accumulation in the plasma membrane (Fig. 2C). Ferroptosis was recognized as a RAS-RAF-MEK-ERK pathway-dependent cell death in cancer cells [24]. However, pretreatment with the MEK inhibitor PD98059 failed to affect erastin- or RSL3-induced CHMP5 and CHMP6 accumulation in the plasma membrane (Fig. 2C). These findings suggest that ER stress-associated calcium influx, but not activation of the MEK pathway, triggers ESCRT-III accumulation in plasma membranes during ferroptosis.
Fig. 2. Calcium influx triggers ESCRT-III accumulation in plasma membrane during ferroptosis.
(A) Western blot analysis of EIF2AK3 and HSPA5 expression in whole cell extracts in PANC1 cells following treatment with erastin (10 μM) or RSL3 (0.5 μM) for 12 h. (B) Analysis of cytosolic calcium level in PANC1 cells following treatment with erastin (10 μM) or RSL3 (0.5 μM) for 12 h (n = 3, *P < 0.05). (C) Western blot analysis of CHMP5, CHMP6, and SLC7A11 expression in isolated plasma membrane in PANC1 cells following treatment with erastin (10 μM) or RSL3 (0.5 μM) in the absence or presence of PD98059 (10 μM), TUDCA (50 μM), or BAPTA-AM (10 μM) for 12 h.
3.3. Inhibition of ESCRT-III promotes ferroptosis
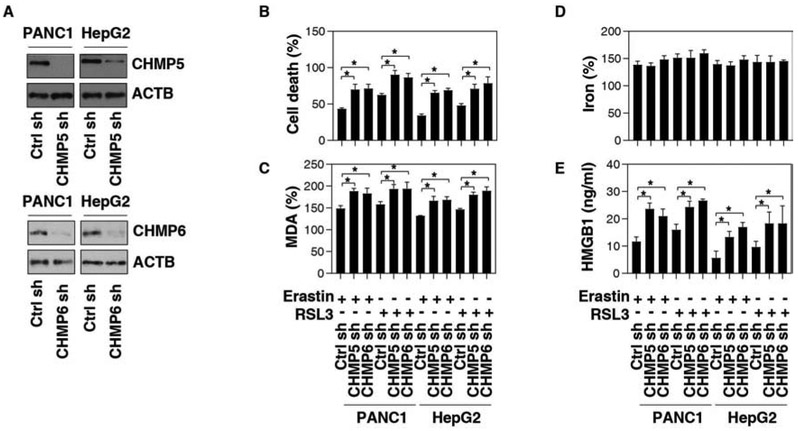
To investigate the functional consequence on ferroptosis of ESCRT-III subunit accumulation in plasma membranes, CHMP5 or CHMP6 was knocked down by shRNAs in PANC1 or HepG2 cell lines (Fig. 3A). Compared to the control shRNA group, suppressing CHMP5 or CHMP6 expression by shRNA increased erastin- or RSL3-induced cell death (Fig. 3B), indicating that ESCRT-III plays a pro-survival role in ferroptosis.
Fig. 3. Inhibition of ESCRT-III promotes ferroptosis.
(A) Western blot analysis of CHMP5 and CHMP6 expression in indicated gene knockdown PANC1 or HepG2 cells. (B-E) Analysis of cell death (B), intracellular MDA (C), intracellular iron (D), and HMGB1 release (E) in indicated PANC1 or HepG2 cells in response to erastin (10 μM) or RSL3 (0.5 μM) for 12 h (n = 3, *P < 0.05).
We next assayed the impact of genetic inhibition of ESCRT-III on iron accumulation and lipid peroxidation, two important events for ferroptosis induction. The knockdown of CHMP5 or CHMP6 blocked erastin- or RSL3-induced production of MDA, one of the final products of polyunsaturated fatty acid peroxidation in ferroptosis (Fig. 3C). In contrast, erastin- or RSL3-induced iron accumulation was not affected by the suppression of CHMP5 or CHMP6 expression (Fig. 3D). High-mobility group box 1 (HMGB1), a representative damage-associated molecular pattern (DAMP), is involved in ferroptosis-mediated inflammation responses [25]. Furthermore, the knockdown of CHMP5 or CHMP6 by shRNAs increased erastin- or RSL3-induced HMGB1 release (Fig. 3E). These findings further suggest that ESCRT-III-mediated membrane repair can reduce lipid peroxide production and DAMP release during ferroptosis.
3.4. ESCRT-III inhibits ferroptosis in vivo
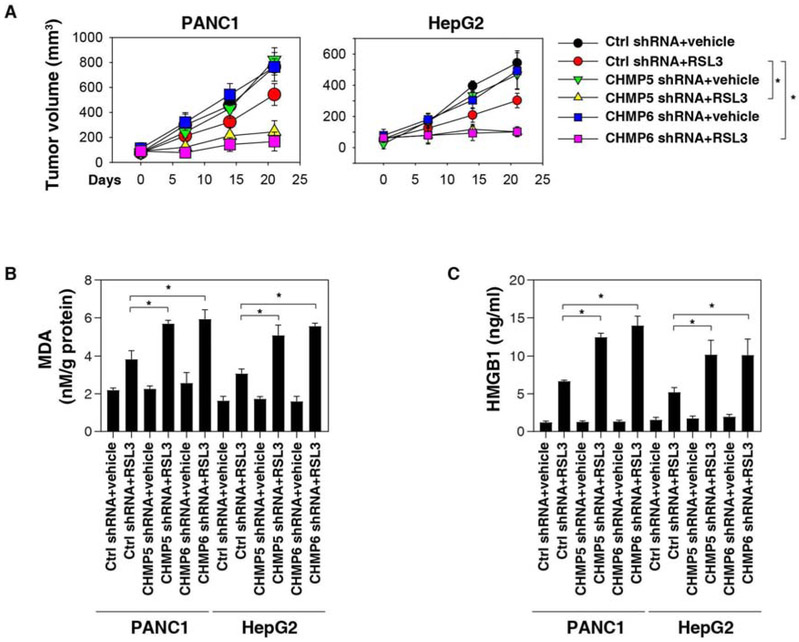
To further assess whether ESCRT-III regulates ferroptosis in vivo, CHMP5- or CHMP6-knockdown PANC1 or HepG2 cells were implanted subcutaneously into the right flank of immunodeficient mice. One week later, tumor-bearing mice were treated with (1S-3R)-RSL3, a form of RSL3 with increased plasma and metabolic stability. Compared to control shRNA cells, RSL3 effectively reduced the growth of tumors formed (Fig. 4A) by CHMP5- or CHMP6-knockdown cells as it locally increased MDA levels (Fig. 4B) and serum HMGB1 (Fig. 4C). In contrast, the ferroptosis inhibitor liproxstatin-1 reversed the suppression of CHMP5- or CHMP6-induced tumor suppression, MDA production, and HMGB1 release (Fig. 4A-4C). Together, these findings demonstrate that the genetic inhibition of ESCRT-III increased the anticancer activity of RSL3 via the induction of ferroptosis in vivo.
Fig. 4. Effects of genetic inhibition of CHMP5 and CHMP6 on ferroptosis in vivo.
(A) Athymic nude mice were injected subcutaneously with the indicated PANC1 or HepG2 cells for 7 days and then treated with RSL3 (30 mg/kg; i.p., once every other day) at day 7 for 2 weeks. Tumor volumes were calculated weekly (n = 5 mice/group, *P < 0.05). (B-C) In parallel, MDA levels (B) in isolated tumors or serum HMGB1 at day 14 after treatment were assayed (n = 3 mice/group, *P < 0.05).
4. Discussion
Cell death events must be precisely regulated via an integrated mechanism, including positive and negative feedback loops, as well as extensive cross talk between parallel pathways. In this study, we demonstrated that the ESCRT-III machinery functions as a novel negative feedback regulator of ferroptotic cancer death. Consequently, the inhibition of the ESCRT-III machinery enhanced anticancer activity of ferroptosis inducers. These findings increase our understanding of the molecular network for the regulation of ferroptosis and shed new light on mechanisms of plasma membrane repair during cell death.
Ferroptosis was originally studied by screening small-molecule compounds for selectively killing RAS mutation cells [26]. This screen identified erastin as an RAS-selective lethal compound that triggers a caspase-independent cell death [26]. Later, ferroptosis was used to define an iron-dependent form of nonapoptotic cell death that is morphologically, biochemically, and genetically distinct from apoptosis, necrosis and autophagy [16]. However, these observations were challenged by recent studies. First, ferroptosis can occur in both a RAS-dependent and -independent manner [15, 16, 27, 28]. Oxidative stress-mediated ferroptosis mediates inflammation and tissue injury in brain, liver, kidney, and heart [29-32]. Second, ferroptosis may be an autophagy-dependent cell death under some circumstances [33]. In particular, certain selective kinds of autophagy, such as ferritinophagy [34], lipophagy [35], clockophagy [36], and chaperone-mediated autophagy [37], promote ferroptotic cancer cell death through promoting iron overload or lipid peroxidation. Third, ferroptosis is generally categorized as a type of regulated necrosis associated with plasma membrane rupture and DAMP release [13]. Our current study indicates that ESCRT III-mediated plasma membrane repair can reduce lipid peroxidation and DAMPs (e.g., HMGB1) during ferroptosis, providing a new mechanism for the regulation of anticancer activity of ferroptosis activators.
In mammalian cells, ESCRT-III is composed of 12 subunits, namely CHMP1A, CHMP1B, CHMP2A, CHMP2B, CHMP3, CHMP4A, CHMP4B, CHMP4C, CHMP5, CHMP6, CHMP7, and IST1 [8, 38, 39]. They cycle between an inactive monomeric state in different sub-cellular distributions and an activated state in which they polymerize into filaments on the membrane [8, 38, 39]. We found that certain ESCRT-III subunits, especially CHMP5 and CHMP6, accumulated in plasma membrane during ferroptosis. Importantly, the silencing of CHMP5 or CHMP6 expression sensitized cells to erastin- or RSL3-induced ferroptosis in vitro or in vivo. Other studies demonstrate that the knockdown of CHMP2A or CHMP4B promotes mixed-lineage kinase domain-like pseudokinase (MLKL)-dependent necroptosis [10], whereas the knockdown of CHMP3 enhances gasdermin D-mediated pyroptosis [11]. Notably, calcium signaling is especially important in the activation of ESCRT-III machinery in the inhibition of necroptosis [10], pyroptosis [11], as well as ferroptosis (current study). Thus, calcium-dependent ESCRT-III machinery may play a wider role in modulating various types of RCD by delaying cell membrane rupture.
Lipid peroxidation seems to play a central role in the initiation of ferroptosis, although the downstream effector remains unidentified [12]. However, the cell could use several different pathways for limiting lipid peroxidation during ferroptosis. For example, the induction of ferroptosis is involved in controlling the expression, activity, and degradation of GPX4, a glutathione-dependent enzyme for catalyzing the reduction of lipid peroxides [17]. GPX4 is found in the mitochondria, microsomes, cytosol, nuclei, and membrane. Interestingly, the plasma membrane level of GPX4 was decreased in response to erastin and RSL3, indicating that GPX4 in the membrane is not essential for the repair of damaged lipids during ferroptosis. The conditional depletion of GPX4 also can cause apoptosis, necroptosis, or pyroptosis in mice [40-45]. Alternatively, ESCRT-III machinery could interact simultaneously with different membrane proteins and thereby generate a sorting domain to seal and repair membrane damage. In addition, the adaptive pro-survival responses to ferroptosis are also involved in the activation of the nuclear factor, erythroid 2-like 2 (NFE2L2, also known as NRF2) that regulates antioxidant gene expression with the antioxidant responsive element [46-49].
In summary, we demonstrated that ESCRT-III is an important negative regulator of ferroptotic cancer cell death, which modulates lipid peroxidation and DAMP release. Further studies are needed to assess the benefits of ESCRT-III activation in the protection against ferroptosis induced in tissue injury and inflammation response in diseases.
Supplementary Material
ESCRT-III accumulation in plasma membrane during ferroptosis
Calcium influx triggers ESCRT-III accumulation in plasma membrane
Inhibition of ESCRT-III promotes ferroptosis in vitro
Inhibition of ESCRT-III promotes ferroptosis in vivo
Acknowledgments
We thank Dave Primm (Department of Surgery, University of Texas Southwestern Medical Center) for his critical reading of the manuscript. D.T. was supported by grants from the US National Institutes of Health (R01CA229275) and the American Cancer Society (Research Scholar Grant RSG-16-014-01-CDD [D.T.]). E.D. was supported by the Natural Science Foundation of Jilin Province of China (20160101062JC), the Health Foundation of the Finance Department of Jilin Province (sczsy201516), and the National Natural Science Foundation of China (30870355 and 81370497).
Abbreviations
- CHMP
charged multivesicular body protein
- DAMPs
damage-associated molecular patterns
- EIF2AK3
eukaryotic translation initiation factor 2 alpha kinase 3
- ESCRT
endosomal sorting complexes required for transport
- GPX4
glutathione peroxidase 4
- HMGB1
high-mobility group box 1
- HSPA5
heat shock protein family A (Hsp70) member 5
- MDA
malondialdehyde
- MLKL
mixed-lineage kinase domain-like pseudokinase
- NFE2L2/NRF2
nuclear factor, erythroid 2-like 2
- SLC7A11
solute carrier family 7 member 11
- RCD
regulated cell death
- TUDCA
tauroursodeoxycholic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflicts of interest or financial interests.
References
- [1].Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, Garcia-Saez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jaattela M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, Lopez-Otin C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Munoz-Pinedo C, Nagata S, Nunez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G, Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018, Cell death and differentiation, 25 (2018) 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G, The molecular machinery of regulated cell death, Cell Res, 29 (2019) 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cooper ST, McNeil PL, Membrane Repair: Mechanisms and Pathophysiology, Physiol Rev, 95 (2015) 1205–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Segawa K, Nagata S, An Apoptotic 'Eat Me' Signal: Phosphatidylserine Exposure, Trends Cell Biol, 25 (2015) 639–650. [DOI] [PubMed] [Google Scholar]
- [5].Zhang Y, Chen X, Gueydan C, Han J, Plasma membrane changes during programmed cell deaths, Cell Res, 28 (2018) 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McNeil PL, Steinhardt RA, Plasma membrane disruption: repair, prevention, adaptation, Annu Rev Cell Dev Biol, 19 (2003) 697–731. [DOI] [PubMed] [Google Scholar]
- [7].Lauritzen SP, Boye TL, Nylandsted J, Annexins are instrumental for efficient plasma membrane repair in cancer cells, Semin Cell Dev Biol, 45 (2015) 32–38. [DOI] [PubMed] [Google Scholar]
- [8].McCullough J, Frost A, Sundquist WI, Structures, Functions, and Dynamics of ESCRT-III/Vps4 Membrane Remodeling and Fission Complexes, Annu Rev Cell Dev Biol, 34 (2018) 85–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stoten CL, Carlton JG, ESCRT-dependent control of membrane remodelling during cell division, Semin Cell Dev Biol, 74 (2018) 50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gong YN, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, Linkermann A, Green DR, ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences, Cell, 169 (2017) 286–300 e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ruhl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P, ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation, Science, 362 (2018) 956–960. [DOI] [PubMed] [Google Scholar]
- [12].Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D, Ferroptosis: process and function, Cell Death Differ, 23 (2016) 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD, Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease, Cell, 171 (2017) 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ 3rd, Lotze MT, Endogenous HMGB1 regulates autophagy, J Cell Biol, 190 (2010) 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, Zhong M, Yuan H, Zhang L, Billiar TR, Lotze MT, Zeh HJ 3rd, Kang R, Kroemer G, Tang D, The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity, Cell Rep, 20 (2017) 1692–1704. [DOI] [PubMed] [Google Scholar]
- [16].Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR, Ferroptosis: an iron-dependent form of nonapoptotic cell death, Cell, 149 (2012) 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR, Regulation of ferroptotic cancer cell death by GPX4, Cell, 156 (2014) 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhu S, Zhang Q, Sun X, Zeh HJ 3rd, Lotze MT, Kang R, Tang D, HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells, Cancer Res, 77 (2017) 2064–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sauzay C, Louandre C, Bodeau S, Anglade F, Godin C, Saidak Z, Fontaine JX, Usureau C, Martin N, Molinie R, Pascal J, Mesnard F, Pluquet O, Galmiche A, Protein biosynthesis, a target of sorafenib, interferes with the unfolded protein response (UPR) and ferroptosis in hepatocellular carcinoma cells, Oncotarget, 9 (2018) 8400–8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang N, Zeng GZ, Yin JL, Bian ZX, Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in Burkitt's Lymphoma, Biochem Biophys Res Commun, 519 (2019) 533–539. [DOI] [PubMed] [Google Scholar]
- [21].Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR, Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis, Elife, 3 (2014)e02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee YS, Lee DH, Choudry HA, Bartlett DL, Lee YJ, Ferroptosis-Induced Endoplasmic Reticulum Stress: Cross-talk between Ferroptosis and Apoptosis, Mol Cancer Res, 16 (2018) 1073–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang Z, Zhang L, Zhou L, Lei Y, Zhang Y, Huang C, Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress, Redox Biol, (2018) 101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR, RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels, Nature, 447 (2007) 864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wen Q, Liu J, Kang R, Zhou B, Tang D, The release and activity of HMGB1 in ferroptosis, Biochem Biophys Res Commun, 510 (2019) 278–283. [DOI] [PubMed] [Google Scholar]
- [26].Dolma S, Lessnick SL, Hahn WC, Stockwell BR, Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells, Cancer Cell, 3 (2003) 285–296. [DOI] [PubMed] [Google Scholar]
- [27].Yu Y, Xie Y, Cao L, Yang L, Yang M, Lotze MT, Zeh HJ, Kang R, Tang D, The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents, Mol Cell Oncol, 2 (2015) e1054549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schott C, Graab U, Cuvelier N, Hahn H, Fulda S, Oncogenic RAS Mutants Confer Resistance of RMS13 Rhabdomyosarcoma Cells to Oxidative Stress-Induced Ferroptotic Cell Death, Front Oncol, 5 (2015) 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Forster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O'Donnell VB, Kagan VE, Schick JA, Conrad M, Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice, Nature cell biology, 16 (2014) 1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kain HS, Glennon EKK, Vijayan K, Arang N, Douglass AN, Fortin CL, Zuck M, Lewis AJ, Whiteside SL, Dudgeon DR, Johnson JS, Aderem A, Stevens KR, Kaushansky A, Liver stage malaria infection is controlled by host regulators of lipid peroxidation, Cell Death Differ, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Brasen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR, Krautwald S, Synchronized renal tubular cell death involves ferroptosis, Proc Natl Acad Sci U S A, 111 (2014) 16836–16841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li W, Feng G, Gauthier JM, Lokshina I, Higashikubo R, Evans S, Liu X, Hassan A, Tanaka S, Cicka M, Hsiao HM, Ruiz-Perez D, Bredemeyer A, Gross RW, Mann DL, Y.Y. Tyurina, Gelman AE, Kagan VE, Linkermann A, Lavine KJ, Kreisel D, Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation, J Clin Invest, 129 (2019) 2293–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D, Ferroptosis is a type of autophagy-dependent cell death, Semin Cancer Biol, (2019). [DOI] [PubMed] [Google Scholar]
- [34].Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, Kang R, Tang D, Autophagy promotes ferroptosis by degradation of ferritin, Autophagy, 12 (2016) 1425–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao H, Kang R, Wang X, Tang D, Dai E, Lipid storage and lipophagy regulates ferroptosis, Biochem Biophys Res Commun, (2018). [DOI] [PubMed] [Google Scholar]
- [36].Yang M, Chen P, Liu J, Zhu S, Kroemer G, Klionsky DJ, Lotze MT, Zeh HJ, Kang R, Tang D, Clockophagy is a novel selective autophagy process favoring ferroptosis, Sci Adv, 5 (2019) eaaw2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M, Shan B, Pan H, Yuan J, Chaperone-mediated autophagy is involved in the execution of ferroptosis, Proc Natl Acad Sci U S A, 116 (2019) 2996–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lata S, Schoehn G, Solomons J, Pires R, Gottlinger HG, Weissenhorn W Structure and function of ESCRT-III, Biochem Soc Trans, 37 (2009) 156–160. [DOI] [PubMed] [Google Scholar]
- [39].Chiaruttini N, Roux A, Dynamic and elastic shape transitions in curved ESCRT-III filaments, Curr Opin Cell Biol, 47 (2017) 126–135. [DOI] [PubMed] [Google Scholar]
- [40].Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Radmark O, Wurst W, Bornkamm GW, Schweizer U, Conrad M, Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death, Cell metabolism, 8 (2008) 237–248. [DOI] [PubMed] [Google Scholar]
- [41].Ran Q, Van Remmen H, Gu M, Qi W, Roberts LJ 2nd, Prolla T, Richardson A, Embryonic fibroblasts from Gpx4+/− mice: a novel model for studying the role of membrane peroxidation in biological processes, Free Radic Biol Med, 35 (2003) 1101–1109. [DOI] [PubMed] [Google Scholar]
- [42].Cole-Ezea P, Swan D, Shanley D, Hesketh J, Glutathione peroxidase 4 has a major role in protecting mitochondria from oxidative damage and maintaining oxidative phosphorylation complexes in gut epithelial cells, Free Radic Biol Med, 53 (2012) 488–497. [DOI] [PubMed] [Google Scholar]
- [43].Ran Q, Liang H, Gu M, Qi W, Walter CA, Roberts LJ 2nd, Herman B, Richardson A, Van Remmen H, Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis, J Biol Chem, 279 (2004) 55137–55146. [DOI] [PubMed] [Google Scholar]
- [44].Canli O, Alankus YB, Grootjans S, Vegi N, Hultner L, Hoppe PS, Schroeder T, Vandenabeele P, Bornkamm GW, Greten FR, Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors, Blood, 127 (2016) 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kang R, Zeng L, Zhu S, Xie Y, Liu J, Wen Q, Cao L, Xie M, Ran Q, Kroemer G, Wang H, Billiar TR, Jiang J, Tang D, Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis, Cell Host Microbe, 24 (2018) 97–108 e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D, Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells, Hepatology, 63 (2016) 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chen D, Tavana O, Chu B, Erber L, Chen Y, Baer R, Gu W, NRF2 Is a Major Target of ARF in p53-Independent Tumor Suppression, Mol Cell, 68 (2017) 224–232 e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wu KC, Cui JY, Klaassen CD, Beneficial role of Nrf2 in regulating NADPH generation and consumption, Toxicol Sci, 123 (2011) 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sun X, Niu X, Chen R, He W, Chen D, Kang R, Tang D, Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis, Hepatology, 64 (2016) 488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.