Abstract
Post-exertional malaise (PEM) is a potentially debilitating aspect of Gulf War Illness (GWI) that has received limited research attention. The purpose of the present investigation was to determine symptom severity changes following exercise in Veterans with GWI compared to control Veterans without GWI (CO). Sixty-seven Veterans (n=39 GWI; n=28 CO) underwent a 30-minute submaximal exercise challenge at 70% of heart rate reserve. Symptom measurements (e.g. fatigue, pain) occurred pre-, immediately post-, and 24-hours post-exercise. Self-reported physical and mental health, and physiological and perceptual responses to exercise were compared between groups using descriptive statistics, independent samples t-tests and repeated measures Analysis of Variance (RM-ANOVA). Post-exertional malaise was modeled using Group by Time (2 × 3) doubly-multivariate, RM-MANOVAs for (1) mood, (2) pain and (3) GWI-related symptoms, respectively (α=0.05). Data were analyzed for the full sample of Veterans with GWI (n=39) compared to CO (n=28) and a subsample of Veterans (n=18) who endorsed “feeling unwell after physical exercise or exertion” (“PEM endorsers”) during screening. Veterans with GWI reported significantly lower physical and mental health. Groups exercised at similar relative exercise intensities, but GWI perceived exercise as more painful and fatiguing. Group-by-Time interactions were not significant for the entire sample for the three PEM models, however limiting the GWI sample to “PEM endorsers” resulted in significant interactions for Pain- and GWI-related PEM models. These results indicate that not all GVs with GWI experience PEM 24 hr after exercise, and that more research is needed to determine the extent that exercise worsens symptoms in GWI.
Keywords: Chronic Disease, Cognition, Exercise, Fatigue, Pain, Persian Gulf War
1. Introduction
Shortly after returning home from the Persian Gulf War, Veterans began experiencing a myriad of symptoms with no clearly discernable cause or pathophysiological explanation.1–3 Based on estimates by the Gulf War Research Advisory Committee and the 2010 National Academy of Medicine report, between 175,000 to 250,000 of American Gulf War Veterans (GVs), approximately 25–35% of the 1990–1991 Gulf War Veteran population, report symptoms consistent with Gulf War Illness (GWI) - a chronic, multi-symptom illness principally characterized by fatigue, pain, and problems with cognitive function.1–3 It has been nearly 30 years since the conclusion of the Persian Gulf War and yet GWI remains a disabling problem for a substantial percentage of GVs,4–7 with longitudinal data indicating declining physical and mental health in symptomatic GVs, despite significant health care utilization.5 One condition that is reported by GVs with GWI,8 but has received scant research attention in controlled laboratory studies, is post-exertional malaise (PEM).
PEM is hallmark feature of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) – another chronic multisymptom illness sharing substantial symptom overlap with GWI.2,9,10 Clinically, PEM is defined as an increase in pain, fatigue, cognitive problems, and other associated symptoms that is sustained for an inordinately long period of time following mental and/or physical exertion.11,12 ME/CFS patients report that even minimal exertion dramatically worsens their entire symptom complex.12 Given the central role of physical exertion in precipitating PEM, exercise challenges are frequently used in ME/CFS research and these studies have described changes for several symptoms following exercise including increased fatigue, sore throat, headaches, muscle pain, joint pain, confusion, and decreased energy.13–18
To our knowledge, the PEM response has not been systematically studied under controlled laboratory settings in GWI. Although several studies have used exercise challenges to describe differences in immune parameters,19,20 lactate production,21 pain sensitivity,22 and perceived exertion23 in GVs with GWI, these studies have not tested whether symptom exacerbation occurs following a single bout of exertion. Our goal was to explore whether GVs with GWI demonstrate a similar symptom exacerbation response to that seen in the literature involving ME/CFS. Therefore, the purpose of the present investigation was to determine the extent to which symptoms associated with GWI change following an acute sub-maximal aerobic exercise challenge in GVs with GWI compared to an otherwise healthy GV control group (CO). Our primary hypothesis was that GVs with GWI would report a significant worsening of symptoms from pre- to post-exercise. We further expected that this worsening of symptoms in GV with GWI would be significantly greater than any changes exhibited by the CO. Finally, we expected that the largest changes in symptom severity would be observed in GVs with GWI who reported PEM during the screening phase of this study.
2. Methods
The methods and data reported here are part of an ongoing large-scale multisite study aimed toward determining brain, autonomic, and immune system deficits in GWI (Department of Veterans Affairs Merit Review Award # I01CX001329: Cook and Falvo: PIs). This collaboration included the William S. Middleton Memorial Veterans Hospital & the University of Wisconsin – Madison, Madison, WI; the War Related Illness and Injury Study Center within the Department of Veterans Affairs, New Jersey Health Care System, East Orange, NJ; and the Kessler Foundation (Rocco Ortenzio Neuroimaging Center), East West Orange, NJ. The scope of the present report is focused on drawing comparisons between GWI and CO Veterans in terms of (i) physical and mental health symptoms at baseline, (ii) perceptual, cardiorespiratory, and metabolic responses to sub-maximal exercise, and (iii) potential exacerbation of symptoms that would be indicative of a PEM response immediately and 24hr following exercise.
2.1. Participants
Sixty-seven GVs who were deployed to the Persian Gulf during Operations Desert Shield or Desert Storm (n=39 GWI and n=28 CO) were recruited and volunteered for the study. Participants were recruited from both the Madison and NJ VA Hospitals through letters sent to Veterans within Veteran Integrated Service Networks (VISN) ‘2’ (NJ) and ‘12’ (WI). As our primary method of identifying potentially eligible Veterans, we used the VA Informatics and Computing Infrastructure Corporate Data Warehouse to identify names and mailing addresses of Veterans in the VISN 2 and 12 areas who were between the ages of 45–65 and had been deployed to the Persian Gulf War. Additional recruitment methods included the Defense Manpower Data Center, outpatient referral clinics, and distribution of study fliers to the VA Office of Public Health Gulf War News Letter service and local Veteran Service Organizations, hospitals, and clinics.
2.1.1. Inclusion/Exclusion Criteria
To be included in the study, participants had to be deployed to the Persian Gulf during Operations Desert Shield and/or Desert Storm and meet criteria for our GWI and CO groups. A GWI diagnosis was based on the Kansas Case Definition (71) which was derived from a population-based survey of over 2,000 Veterans who served during the 1990–1991 Gulf War and whose symptoms began during or following deployment. To meet criteria, Veterans must endorse at least one moderately severe symptom or multiple mildly severe symptoms which has/have persisted for the previous 6 months in at least three of the following domains; pain, fatigue neurological/cognitive/mood, skin, gastrointestinal, and respiratory – with symptoms first becoming a problem during or after the Gulf War. In order to be eligible for the present study, Veterans with GWI were required to endorse moderately severe symptoms in either the pain or fatigue domain.
Screening for inclusion/exclusion criteria took place during an initial 20–25 min phone call followed by a 1-hr in-person interview. The initial study visit included a health records check in the VA Computerized Patient Record System, physical health exam, and the administration of the Mini-International Neuropsychiatric Interview (MINI). Veterans were excluded from participation if they: 1) screened for current or lifetime bipolar I disorder, psychotic disorders, or mood disorder with psychotic features (from MINI); 2) screened for evidence of active illicit substance use or fit criteria for active substance dependence and substance dependence in partial remission for less than 1 year (from MINI); 3) reported or had a medical record of using select medications including beta & calcium channel blockers, anti-convulsants, non-steroidal anti-inflammatory drugs within 48-hr of testing; 4) had a diagnosis of a chronic conditions (e.g., cancer, rheumatoid arthritis) not associated with Gulf War service which could explain their symptoms (self-report and medical record review); 5) had absolute contraindications to exercise testing according to American College of Cardiology/American Heart Association guidelines24; or 6) had contraindications to MRI testing (e.g. ferrous material in the body, claustrophobia). Stable use (≥3 months if taking) of psychotropic medications (i.e., antidepressants, antipsychotics, mood stabilizers – except when used to treat exclusionary psychiatric conditions), and select analgesics and sedatives was permitted, however, concurrent use of multiple (>1) sedatives was exclusionary.
2.2. Experimental Procedures
Participants reported to the Exercise Psychology Laboratory at the University of Wisconsin – Madison or the Cardiorespiratory Physiology Laboratory of the WRIISC for symptom assessment and exercise testing procedures. Participants completed 3 days of testing for this study. Visit 1 involved confirmation of the inclusion and exclusion criteria and baseline characterization of demographics and self-reported mental and physical health. Visit 2 occurred approximately 1-week following baseline data collection and consisted of symptom measurement and exercise challenge. Visit 3 occurred 24-hr post-exercise and consisted of symptom measurement.
Prior to study initiation, personnel from both sites received in-depth training on the methods that were employed (e.g. standardized assessments of GWI diagnostic criteria, behavioral symptom and cardiorespiratory data collection methods). This included both in-person site visits and tele- and video-conference training. Regular communication between sites and periodic data quality checks were also performed to check for methodological consistency, minimize between-site variability, and ensure data integrity.
All study procedures were approved by the institutional review boards and the Research and Development Committees of the University of Wisconsin – Madison, VA Madison and the Department of Veterans Affairs, New Jersey Health Care System. All participants provided informed consent according to the Declaration of Helsinki prior to testing.
2.3. Participant characteristics
Several questionnaires were completed at baseline in order to characterize participant demographics and mental/physical health symptoms. These included: 1) demographic and medical history questionnaires; 2) the Veterans Rand 36-item Medical Health Survey (VR-36)25; 3) the Pittsburgh Sleep Quality Index26; 4) the Centers for Disease Control (CDC) Symptom Inventory27; 5) the short-form McGill Pain Questionnaire-2 (SF-MPQ-2)28; 6) the Widespread Pain Index29; 7) the Multidimensional Fatigue Inventory30; 8) the Fatigue Severity Scale31; 9) the brief version of the Profile of Mood States (POMS-BF)32; and 10) the International Physical Activity Questionnaire – Short Form33.
2.4. Endorsement of Post-exertional Malaise
One item of the Kansas Case Definition questionnaire asks whether GVs experience “feeling unwell after physical exercise or exertion”. These responses were used to determine how many GVs in our sample reported PEM as a problem prior to characterization of the PEM response under controlled laboratory conditions. We also used this information to determine whether the magnitude of the difference between GWI and CO changed when we restricted the GWI group only to those who endorsed that exertion worsened their symptoms (GWI+PEM). Thus, comparisons between GWI and CO with respect to illness severity, perceptual, cardiorespiratory and metabolic responses during exercise, and symptom responses following exercise were followed up with comparisons between the GWI+PEM sub group and CO group (See Data Processing & Analysis section).
2.5. Characterization of the Post-exertional Malaise Response
Importantly, there are no universally agreed-upon definitions of PEM. Various case definitions for ME/CFS describe PEM as including pain, cognitive problems, and other associated symptoms present for an “inordinately long amount of time” or as a period of extreme, prolonged exhaustion following mental or physical activity.11,12,34 These descriptions are generally inadequate for research purposes, and thus for the present study we operationally define PEM as a greater increase in symptom severity as measured by the SFMPQ-2, POMS-BF, and CDC Symptom Inventory VAS ratings from baseline to post-exercise for the GWI group relative to the CO group. Because of the potential heterogeneity for symptom responding,35 PEM may present as an increase in one symptom (e.g., the patient’s primary symptom of fatigue) or as a cluster of symptoms (e.g., pain, fatigue, and confusion). Thus, our approach was to descriptively examine symptoms at the pre-exercise time-point (e.g. whether a symptom is endorsed and how severe the symptom is rated) and then compare the magnitude of change in these symptoms from pre- to post-exercise between GWI and CO. We therefore modeled the PEM response based on “Mood”, “Pain” and “GWI-related” symptoms (See section 2.7 Data Processing & Analysis section).
We used the SFMPQ-2, POMS-BF, and a 0–100 visual analogue scale version of the CDC Symptom Inventory to characterize the PEM response because they measure symptoms that are associated with GWI (e.g., pain, fatigue, mood disturbance) and have been shown to be sensitive to acute exercise in our previous studies involving ME/CFS patients.18,35,36 These questionnaires were delivered at pre-exercise, immediately and 24-hr post-exercise using instructional sets that advised participants to rate how they were feeling “right now” or “at this moment. For the CDC Symptom Inventory, 0 “none” to 100 “worst imaginable” visual analogue scale ratings were obtained for 16 of 19 symptoms (e.g. fatigue, muscle pain, joint pain, memory/concentration problems, headaches, muscle weakness and swollen or tender lymph nodes). Two items pertaining to sleep (i.e., unrefreshing sleep, sleeping problems) and one pertaining to gastrointestinal (i.e. diarrhea) were removed because they did not apply to changes that might occur immediately following exercise. To minimize the possibility of introducing response bias that might artificially inflate Veteran reports of symptom exacerbation37, participants were reminded throughout testing (e.g., study advertisements, informed consent, and interactions with study personnel) that the purpose of the study was to investigate potential pathophysiological mechanisms of GWI. Test administrators were also instructed to avoid communicating any expected directional effects of exercise on symptom severity to participants.
2.6. Submaximal Exercise Challenge
Participants completed 30 min of exercise on an electronically-braked cycle ergometer (Lode Corival, Lode B.V., Groningen, The Netherlands or Ergoline 100, Windhagen, Germany). Exercise intensity was set at 70% of the participant’s age-predicted heart rate reserve (HRR). Following supine monitoring of resting heart rate (HR) for 5 min, age-predicted maximum HR was calculated via the following formula: 209 – 0.70 × Age.38 Next, target HR was calculated via American College of Sports Medicine recommendations: Target HR = (HRmax – HRrest) × %intensity + HRrest.39 Following a 2-minute period of resting data collection, exercise began at 50 Watts and the intensity of exercise was gradually increased until participants reached their target HR. Once the target HR was reached (~5 min), participants completed 30 min of steady-state exercise at the target intensity. Exercise intensity (70±5%) of HRR) was maintained by making minor Watt adjustments throughout the exercise session. Exercise ended with a 3-min active recovery period at 0 Watts. Following the delivery of standardized instructional sets, perceptual ratings were taken every 5 minutes during exercise using validated scales for ratings of perceived exertion (RPE),40 leg muscle pain,41 and a 0–10 visual analog scale for feelings of overall fatigue.42
During exercise electrocardiography and a HR rate monitor (POET II; Criticare Systems, Waukesha, WI; T12×; Cosmed, Rome, Italy; Polar, Lake Success, NY) were used to monitor heart rhythm and rate during exercise and recovery. Blood pressure was monitored every five min during steady-state exercise via manual auscultation. Oxygen consumption (VO2), carbon dioxide production (VCO2), ventilation (VE), HR, and work rate measures were obtained breath-by-breath during exercise using a metabolic cart (Parvo Medics TrueOne; Parvo Medics, Sandy, Utah; Quark CPET; Cosmed, Rome, Italy) and a bidirectional non-rebreathing valve attached to an oronasal mask (Hans-Rudolph, Kansas City, MO). The flowmeter was calibrated prior to each exercise test by making multiple comparisons to a 3-liter piston syringe. Oxygen and carbon dioxide sensors were calibrated by the presentation of known gas concentrations. Prior to exercise testing, room environment (temperature, humidity, barometric pressure) and participant height and weight were recorded. Blood lactate (Nova Lactate Pro, Nova Biomedical, Waltham, MA) was measured via finger stick at baseline, the end of the 30-min steady state exercise period, and 2-min after active recovery.
2.7. Data Processing & Analysis
Participant characteristics and self-report data for GVs were examined and compared using descriptive statistics, including the Hedges’ g effect size metric and 95% confidence intervals.43 Cardiorespiratory, perceptual, and lactate data during exercise were compared between groups using independent samples t-tests and repeated measures Analysis of Variance (RM-ANOVA).
To test the primary hypothesis, we conducted three separate Group-by-Time (2 × 3) doubly-multivariate, repeated-measures MANOVAs (α = 0.05) with partial η2 effect sizes. We chose this analytic approach as an efficient and statistically justifiable means for exploring PEM in GVs. The overall MANOVA compared the groups on a linear combination of the dependent variables within a given model and across the repeated measures collected, thereby limiting the number of analyses and reducing the likelihood of type I errors. We tested three models representing mood, pain and disease-relevant symptom domains each with an overall level of significance (α) equal to 0.05. Assuming a large effect size f(V) of 0.65 and α = 0.05, a total sample of 67 participants provided a power (error probability) of 0.80, 0.85, 0.94 to detect a group-by-time interaction for MANOVA models 1, 2, and 3, respectively. Given the lack of prior studies of symptom responses to exercise challenge in Veterans with GWI, the assumption of a large effect size for this power analysis may be considered theoretical.44
Model 1 included the six POMS-BF subscale scores (Tension, Depression, Anger, Vigor, Fatigue, Confusion) as dependent variables to examine mood related PEM. Model 2 used the four SF-MPQ-2 subscale scores (Continuous, Intermittent, Neuropathic, Affective) as dependent variables to reflect pain related PEM. Model 3 included select symptoms from the CDC VAS measures to represent a more disease-relevant PEM model (i.e. GWI-related PEM). We used a two-step approach to make an a priori decision about which of the 16 CDC VAS items best aligned with the Kansas definition of GWI. First, we examined the face validity of the items by determining whether each CDC-VAS could be considered representative of one of the six categories comprising the GWI case definition (i.e., Fatigue, Pain, Neurological/Cognitive/Mood, Skin, Respiratory, Gastrointestinal). For instance, a CDC-VAS item that asks about fatigue was assigned to the fatigue GWI category. Alternatively, a CDC-VAS item that asks about tender lymph nodes and swollen glands did not fit with any GWI categories and was eliminated from further consideration. After assigning items to GWI categories, we used a statistical approach to further optimize our model by eliminating items that were highly correlated and thus would not be expected to explain unique variance. This also served to improve the ratio between sample size and number of variables in the model. This step was achieved by exploring bivariate correlations between items from different GWI categories. For instance, two CDC VAS items, (1) joint pain and (2) muscle aches and pain, were assigned to the pain GWI category, but were found to be highly correlated (r ≥ 0.80). After exploring correlations between those two items and the lone item that was assigned to the fatigue GWI category, it was determined that joint pain would be included in the final model because it had a lower correlation with fatigue. Thus, the CDC VAS muscle aches and pain item was eliminated from the model. The final GWI PEM model included fatigue, joint pain, headache, memory problems, depressed mood, shortness of breath, and nausea.
We also performed secondary analyses based on whether our participants endorsed that exertion worsened their symptoms (section 2.4). Thus, analyses conducted for the full sample were repeated for the subset of patients who endorsed feeling unwell following physical exercise or exertion on the Kansas questionnaire (GWI+PEM).
Because our primary (GWI vs. CO) and secondary (GWI+PEM vs. CO) analyses consisted of 6 total MANOVA tests, the threshold for a significant interaction effect was set to p<0.008 using the Bonferroni correction for familywise error.
3. Results
3.1. Missing data during exercise
Data for perceptual and cardiorespiratory responses during exercise were partially missing for 13 participants either because of early test termination or issues related to instrumentation/technical difficulties. Five participants asked to stop exercising early because of gastrointestinal symptoms (n=1), lightheadedness (n=1), physical fatigue (n=2), or low back pain (n=1). Instrumentation/technical issues included participant discomfort with wearing the oronasal mask during exercise (n=1), difficulty with equipment interfacing that affected communication between the metabolic cart software and ECG/Heart Rate Monitor (n=4) or cycle ergometer (n=1), and corrupted (n=1) or inadvertently deleted (n=1) files.
3.2. Participant characteristics
We first compared whether our groups (GWI & CO) differed between the two sites (Wisconsin and New Jersey) by examining demographic and baseline characteristics (Tables 1–3) with independent samples t-tests. Veterans with GWI were significantly (p=0.02; Hedges’ g=0.78, 95% CI: 0.13, 1.43) older at the New Jersey site (M= 54.06; SD=4.84) compared to Wisconsin (M=50.71; 3.59). Otherwise, none of the measured variables were significantly different between sites (p<0.05). Thus, data from both sites were combined for the statistical analyses of interest.
Table 1.
Baseline group characteristics for in GWI (n=39), GWI subgroup with PEM endorsement (GWI+PEM; n=18), and healthy controls (n=28)
| GWI (n=39) | GWI+PEM (n=18) | CO (n=28) | GWI vs. CO | GWI+PEM vs. CO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ES | 95% Cl | ES | 95% Cl | |
| Age (years) | 52.26 | 4.49 | 52.61 | 4.91 | 52.61 | 5.20 | −0.07 | −0.56, 0.41 | 0.00 | −0.59, 0.59 |
| Body Mass Index (kg/m2) | 31.55 | 5.72 | 30.32 | 5.64 | 30.33 | 4.58 | 0.23 | −0.26, 0.72 | 0.00 | −0.59, 0.59 |
| Sex (Male/Female, %) | 89.7/10.3 | 88.9/11.1 | 89.3/10.7 | n/a | n/a | |||||
| Met criteria for Fibromyalgia (Yes/No, %) | 51.3/48.7 | 66.7/33.3 | 0/100 | n/a | n/a | |||||
| VR-36 Vitality | 30.90 | 21.82 | 28.33 | 17.24 | 72.50 | 15.06 | −2.13 | −2.74, −1.53 | −2.73 | −3.54, −1.91 |
| VR-36 Pain | 55.38 | 24.42 | 48.19 | 21.40 | 81.52 | 12.90 | −1.26 | −1.80, −0.73 | −1.96 | −2.68, −1.25 |
| VR-36 Physical Composite Score | 60.02 | 19.88 | 53.04 | 18.90 | 87.37 | 8.80 | −1.67 | −2.23, −1.11 | −2.48 | −3.26, −1.70 |
| VR-36 Mental Composite Score | 54.15 | 20.86 | 49.47 | 18.35 | 86.10 | 10.81 | −1.82 | −2.39, −1.24 | −2.54 | −3.32, −1.75 |
| MDFI Total | 66.69 | 14.38 | 69.56 | 10.85 | 37.68 | 13.15 | 2.07 | 1.47, 2.66 | 2.55 | 1.76, 3.34 |
| Fatigue Severity Scale score | 42.69 | 14.06 | 47.89 | 12.11 | 20.00 | 9.48 | 1.81 | 1.24, 2.39 | 2.59 | 1.80, 3.39 |
| Pittsburgh Sleep Quality score | 11.61 | 4.45 | 12.06 | 4.68 | 7.25 | 3.64 | 1.05 | 0.53, 1.56 | 1.16 | 0.52, 1.80 |
| IPAQ - Total activity (MET mins/week) | 1693.82 | 2328.10 | 1181.65 | 1060.82 | 2902.87 | 2761.20 | −0.48 | −0.98, 0.03 | −0.75 | −1.38, −0.12 |
| IPAQ- Sitting (mins/week) | 5487.10 | 11671.34 | 5071.11 | 6858.29 | 3489.50 | 4049.59 | 0.21 | −0.27, 0.70 | 0.29 | −0.30, 0.89 |
Note. Values for GWI, GWI+PEM and CO groups are listed as means (SD) unless specified otherwise. Criteria for fibromyalgia are based on American College of Rheumatology 2011 criteria27. VR-36 = Veterans RAND 36-item Health Survey; MDFI = Multidimensional Fatigue Inventory; IPAQ = International Physical Activity Questionnaire.
Table 3.
Baseline group characteristics for in GWI (n=39), GWI subgroup with PEM endorsement (GWI+PEM; n=18), and healthy controls (n=28) - CDC Symptom Inventory
| CDC Symptom InventoryA | GWI (n=39) | GWI+PEM (n=18) | CO (n=28) | GWI vs. CO | GWI+PEM vs. CO | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ES | 95% Cl | ES | 95% Cl | |
| 1. Sore throat | 1.00 | 1.81 | 1.50 | 2.33 | 0.36 | 0.73 | 0.43 | −0.06, 0.92 | 0.72 | 0.11, 1.33 |
| 2. Tender lymph nodes or swollen glands | 0.62 | 1.44 | 0.78 | 1.52 | 0.11 | 0.32 | 0.45 | −0.04, 0.94 | 0.68 | 0.07, 1.28 |
| 3. Diarrhea | 2.95 | 3.27 | 2.67 | 2.66 | 0.75 | 1.51 | 0.81 | 0.31, 1.32 | 0.93 | 0.31, 1.55 |
| 4. Unusually fatigued or unwell for at least one day after exerting yourself in any way | 4.64 | 3.64 | 5.56 | 3.42 | 0.61 | 1.50 | 1.35 | 0.82, 1.89 | 2.01 | 1.29, 2.73 |
| 5. Muscle aches or pains | 4.82 | 3.63 | 5.17 | 3.26 | 1.93 | 2.42 | 0.90 | 0.39, 1.41 | 1.15 | 0.51, 1.79 |
| 6. Pain in several joints | 5.03 | 3.54 | 5.72 | 3.16 | 1.50 | 2.53 | 1.10 | 0.58, 1.62 | 1.49 | 0.82, 2.15 |
| 7. Fever | 0.08 | 0.27 | 0.11 | 0.32 | 0.00 | 0.00 | 0.38 | −0.11, 0.87 | 0.54 | −0.06, 1.14 |
| 8. Chills | 1.33 | 1.74 | 1.78 | 1.83 | 0.32 | 0.86 | 0.69 | 0.19, 1.19 | 1.08 | 0.45, 1.72 |
| 9. Unrefreshing sleep | 6.46 | 3.38 | 6.78 | 3.08 | 1.18 | 2.06 | 1.80 | 1.23, 2.37 | 2.20 | 1.46, 2.95 |
| 10. Sleep problems | 7.13 | 3.50 | 7.67 | 2.91 | 2.39 | 2.89 | 1.44 | 0.89, 1.98 | 1.79 | 1.10, 2.49 |
| 11. Headaches | 2.87 | 2.92 | 3.11 | 3.22 | 0.86 | 1.41 | 0.82 | 0.32, 1.33 | 0.97 | 0.35, 1.59 |
| 12. Forgetfulness or memory problems | 3.72 | 3.98 | 3.89 | 3.63 | 0.68 | 1.89 | 0.92 | 0.41, 1.43 | 1.17 | 0.53, 1.81 |
| 13. Difficulty thinking or concentrating | 4.26 | 4.29 | 4.50 | 4.41 | 1.14 | 2.75 | 0.83 | 0.32, 1.33 | 0.95 | 0.33, 1.57 |
| 14. Nausea | 0.85 | 1.46 | 1.06 | 1.70 | 0.11 | 0.42 | 0.64 | 0.14, 1.13 | 0.85 | 0.23, 1.46 |
| 15. Stomach or abdominal pain | 2.21 | 3.25 | 3.28 | 3.23 | 0.39 | 0.69 | 0.71 | 0.21, 1.21 | 1.37 | 0.71, 2.02 |
| 16. Sinus or nasal problems | 2.46 | 3.14 | 2.61 | 3.18 | 1.18 | 2.80 | 0.42 | −0.07, 0.91 | 0.48 | −0.12, 1.08 |
| 17. Shortness of breath | 1.49 | 1.93 | 2.00 | 1.88 | 0.07 | 0.38 | 0.94 | 0.43, 1.45 | 1.58 | 0.90, 2.25 |
| 18. Eyes been sensitive to light | 2.10 | 3.09 | 2.39 | 3.62 | 0.64 | 1.97 | 0.54 | 0.05, 1.03 | 0.63 | 0.03, 1.24 |
| 19. Depressed | 3.23 | 3.84 | 3.78 | 3.90 | 0.85 | 1.92 | 0.74 | 0.23, 1.24 | 1.00 | 0.37, 1.63 |
The instructional set for the CDC Symptom inventory asked respondents to report symptoms that had experienced “over the past month”
Participant demographics and self-reported mental and physical health for the full GWI sample, GWI+PEM subgroup, and CO are presented in Tables 1–3. GVs with GWI reported lower mental and physical health including severe fatigue, poor sleep quality, depressed mood, widespread pain, limited function and a variety of additional disease-related symptoms – with large differences compared to CO (Effect size range = 0.82 – 2.13). Eighteen of the 39 Veterans with GWI (46%) endorsed “feeling unwell after physical exercise or exertion” (GWI+PEM) during eligibility screening with the Kansas questionnaire. These Veterans reported greater symptom severity (i.e. higher symptom scores) and had larger differences than the entire GWI sample when compared to CO (Effect Size range = 1.15 – 2.73).
3.3. Perceptual, lactate and cardio-respiratory responses during exercise
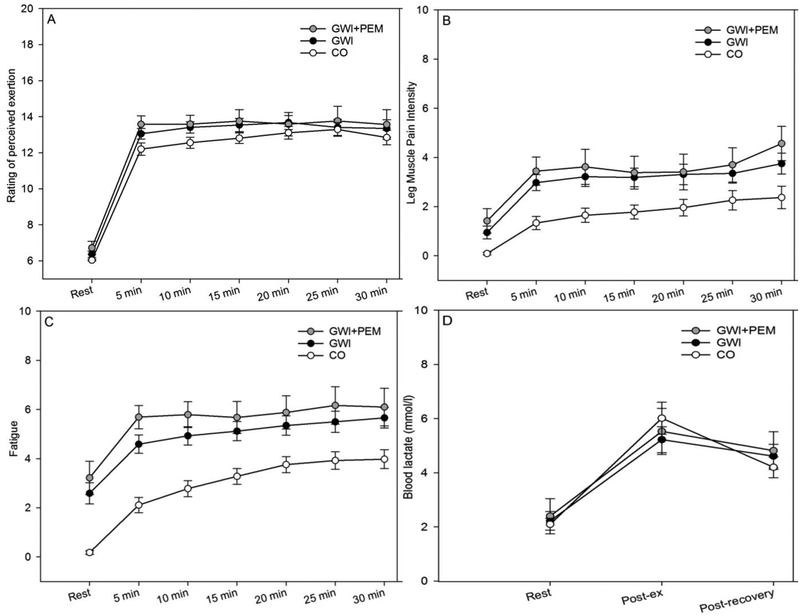
Perceptual and blood lactate responses to exercise for the full GWI sample, GWI+PEM subgroup, and CO are shown in Figure 1. Significant increases from baseline were observed for leg muscle pain intensity (F(6,342) = 35.54, p<0.001, partial η2 = 0.38), feelings of fatigue (F(6,348)= 60.26, p<0.001, partial η2 = 0.51), RPE (F(6,342) = 238.57, p<0.001, partial η2 = 0.81), and blood lactate (F(2,106) = 45.79, p<0.001, partial η2 = 0.46). Compared to CO, GWI participants reported higher leg muscle pain intensity (F(1,57)= 8.6, p=0.005, partial η2 = 0.13) and feelings of fatigue (F(1,58)= 17.32, p<0.001, partial η2 = 0.23), but a between-group difference was not observed for RPE (F(1,57)= 1.3, p=0.26, partial η2 = 0.02) or blood lactate (F(1,53) = 0.24, p=0.63, partial η2 = 0.004). Time and group effects for perceptual responses were similar when the GWI+PEM subgroup was compared to CO.
Figure 1.
Mean (SE) perceptual and lactate responses to exercise challenge in Veterans with Gulf War Illness (GWI), the GWI subgroup with PEM endorsement (GWI+PEM) subgroup, and control Veterans without GWI (CO)
Note. Total participants with complete listwise data across all time-points for each outcome are listed as follows: Ratings of Perceived Exertion (GWI=32; GWI+PEM=15; CO=27); Leg Muscle Pain Intensity (GWI=33; GWI+PEM=15; CO=26); Fatigue (GWI=33; GWI+PEM=15; CO=27); Blood Lactate (GWI=32; GWI+PEM=16; CO=23)
Average cardio-respiratory responses during steady-state exercise in the full GWI sample, GWI+PEM subgroup, and CO are shown are shown in Table 4. Effect sizes and 95% CI indicated that GWI (GWI and GWI+PEM) and CO were not significantly different across several different measures of relative exercise intensity (e.g., HR, RER). However, moderate-large between-groups differences were observed for VE/ VCO2 (g = 0.64; 95% CI: 0.09, 1.19), VO2 (−1.0; 95% CI: −1.56, −0.43), and power (Watts)(g = −1.36; 95% CI: −1.95, −0.76), respectively.
Table 4.
Average steady-state cardiorespiratory responses to exercise challenge in GWI (n=31), GWI subgroup with PEM endorsement (GWI+PEM; n=15), and healthy controls (n=24).
| GWI (n=31) | GWI+PEM (n=15) | CO (n=24) | GWI vs. CO | GWI+PEM vs. CO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ES | 95% Cl | ES | 95% Cl | |
| Heart rate (beats per min) | 134.72 | 11.45 | 131.43 | 14.42 | 134.87 | 8.25 | −0.01 | −0.55, 0.52 | −0.30 | −0.96, 0.35 |
| (mL·kg·min−1) | 16.05 | 3.32 | 16.12 | 3.10 | 20.12 | 4.78 | −1.00 | −1.56, −0.43 | −0.93 | −1.60, −0.25 |
| (L·min−1) | 43.29 | 11.83 | 44.55 | 12.53 | 47.16 | 10.77 | −0.34 | −0.87, 0.20 | −0.22 | −0.87, 0.42 |
| RER | 0.92 | 0.05 | 0.92 | 0.05 | 0.92 | 0.04 | −0.03 | −0.56, 0.51 | −0.09 | −0.74, 0.55 |
| Power (Watts) | 74.04 | 18.76 | 72.39 | 18.92 | 103.87 | 24.08 | −1.39 | −1.98, −0.79 | −1.39 | −2.10, −0.67 |
| 29.82 | 4.56 | 31.39 | 4.73 | 27.09 | 3.40 | 0.66 | 0.11.1.20 | 1.07 | 0.38, 1.75 | |
Note. RER = respiratory exchange ratio; = minute ventilation; = ventilatory equivalent for carbon dioxide; = oxygen consumption
3.4. Post-Exertional Malaise Models
Symptom responses from pre-, immediately-post, and 24-hr post -exercise challenge are illustrated in Figures 2–4. The doubly multivariate repeated-measures comparisons presented below describe the linear composite of our dependent variables of interest from pre- to immediately- and 24-hr post-exercise challenge as a function of group. Analyses are presented for both the full sample and for a sub sample of GVs with GWI that endorsed PEM (GWI+PEM).
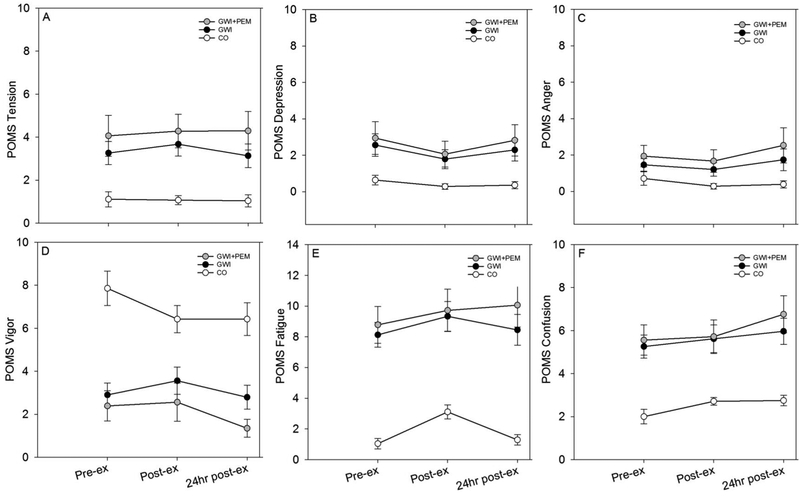
Figure 2.
Mean (SE) POMS-BF symptoms before, after, and 24 hours after exercise in Veterans with Gulf War Illness (GWI; n=39), GWI subgroup with PEM endorsement (GWI+PEM; n=18), and control Veterans without GWI (CO; n=28). Note. Total participants with complete listwise data across all time-points was: GWI=38; GWI+PEM=17; CO=28.
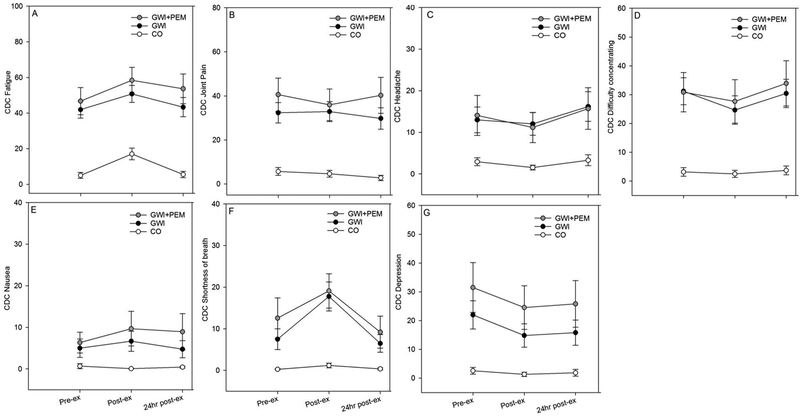
Figure 4.
Mean (SE) CDC VAS symptoms before, after, and 24 hours after exercise in Veterans with Gulf War Illness GWI (n=39), GWI subgroup with PEM endorsement (GWI+PEM; n=18), and control Veterans without GWI (CO; n=28). Note. Total participants with complete listwise data across all time-points was: GWI=36; GWI+PEM=16; CO=28.
3.4.1. Model 1: Mood-related PEM Response
3.4.1.1. Full sample (GWI vs. CO)
Group (Wilks’ Λ = 0.55; F (6,59) = 8.01; p < 001; partial η2 = 0.45) and Time (Wilks’ Λ = 0.50; F (12,53) = 4.35; p < 0.001, partial η2 = 0.50) effects were significant, but the Group ×Time interaction was not (Wilks’ Λ = 0.80; F (12,53) = 1.12; p =0.37, partial η2 = 0.20).
3.4.1.2. PEM endorsers only (GWI+PEM vs. CO)
Group (Wilks’ Λ =0.32; F (6,38) = 13.23; p < 0.001; partial η2 =0.68) and Time (Wilks’ Λ =0.43; F (12,32) = 3.52; p =0.002, partial η2 = 0.57) effects were significant, but the Group × Time interaction was not (Wilks’ Λ =0.60; F (12,32) = 1.79; p =0.09, partial η2 = 0.40).
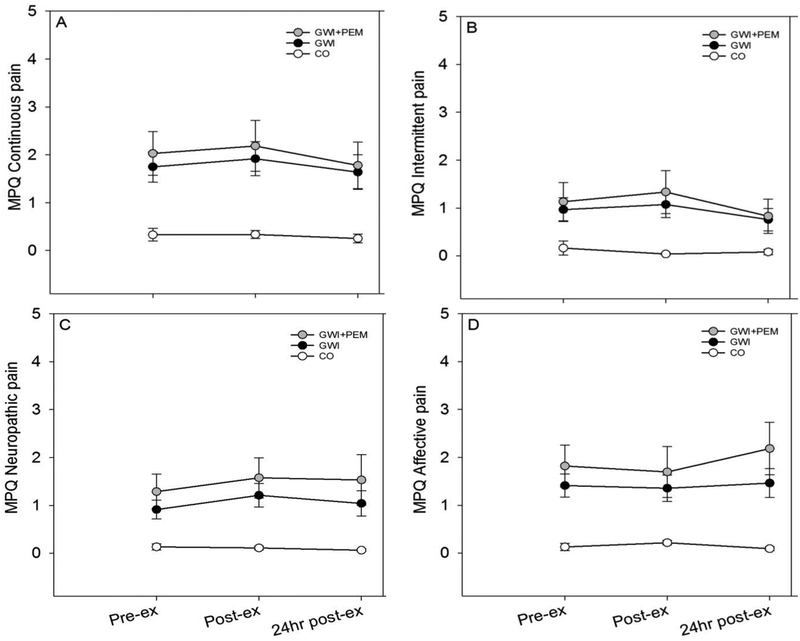
3.4.2. Model 2: Pain-related PEM Response
3.4.2.1. Full sample (GWI vs. CO)
The Group (Wilks’ Λ = 0.76; F (4,61) = 4.74; p = 0.002, partial η2 =0.24) effect was significant, but the main effect of Time (Wilks’ Λ = 0.90; F (8,57) = 0.83; p = 0.58, partial η2 =0.10) and Group × Time interaction effects were not (Wilks’ Λ = 0.82; F (8,57) = 1.52; p = 0.17, partial η2 =0.18).
3.4.2.2. PEM endorsers only (GWI+PEM vs. CO)
Main effects for Group (Wilks’ Λ = 0.63; F (4,40) = 5.97; p = 0.001; partial η2 =0.37) and Time (Wilks’ Λ = 0.59; F (8,36) = 3.16; p=0.008, partial η2 =0.41) were significant, as was the Group × Time interaction effect (Wilks’ Λ =0.53; F (8,36) = 4.05; p =0.002, partial η2 =0.47).
3.4.3. Model 3: GWI-related PEM Response
3.4.3.1. Full sample (GWI vs. CO)
Group (Wilks’ Λ = 0.53; F (7,56) = 7.16; p < 0.001; partial η2 =0.47) and Time (Wilks’ Λ = 0.41; F (14,49) = 4.99; p < 0.001, partial η2 =0.59) effects were significant, but the Group × Time interaction was not (Wilks’ Λ =0.71; F (14,49) = 1.42; p = 0.18, partial η2 =0.29).
3.4.3.2. PEM endorsers only (GWI+PEM vs. CO)
Main effects for Group (Wilks’ Λ =0.36; F (7,36) = 9.28; p <0.001; partial η2 =0.64) and Time (Wilks’ Λ = 0.26; F (14,29) = 5.98; p < 0.001, partial η2 =0.74) were significant, as was the Group × Time interaction (Wilks’ Λ =0.36; F (14,29) = 3.66; p = 0.002, partial η2 =0.64).
4. Discussion
We sought to determine the extent that an acute exercise challenge affected symptoms in GVs with GWI compared to a similarly deployed but otherwise healthy group of GVs. Veterans with GWI reported a multitude of mental and physical health symptoms that are emblematic of the disease and clearly distinguished them from CO Veterans. Despite similar ratings of perceived effort during exercise, exercise was rated as more painful and fatiguing for Veterans with GWI. Cardiorespiratory and metabolic data indicated that both groups exercised at the same relative intensity with respect to HR, VE, RER, and blood lactate, but that GVs with GWI displayed a lower power output, had less efficient ventilation, and consumed less oxygen at 70% of their HRR. Although symptom data demonstrated clear group differences at pre-, immediately post-, and 24 hr post-exercise, the evidence for PEM responses to the exercise challenge was less definitive and somewhat dependent on whether GVs with GWI met our criteria for PEM endorsement.
The phenomenon of PEM is considered common in GWI. Symptom worsening following physical exertion is a component of the diagnostic criteria, although it is not required for diagnosis.1,9,45 However, to our knowledge, experimental evidence of symptom worsening following exercise or physical exertion is lacking in GWI research. In the National Health Survey of Gulf War Era Veterans and Their Families,45 GVs were twice as likely to report a “chronic fatigue syndrome-like” illness in part due to their report of fatigue lasting greater than 24 hr after exertion. In a subsample (n=2,189) from the same National Health Survey cohort, the prevalence of chronic multisymptom illness was determined based on meeting two out of three symptom clusters including “Fatiguability”, “Mood and Cognition” and “Musculoskeletal” – with the “Fatiguability” cluster represented as “persistent fatigue 24 hr or more following exertion”. Chronic multisymptom illness was found to be two times more prevalent (28.9% vs. 15.8%; OR=2.16) among GVs, however the number of Veterans endorsing fatigue following exertion was not reported. A recent meta-analysis of 129,000 GVs across four different countries did not list PEM or “fatigue with exertion” among the 56 most commonly reported symptoms and did not mention symptom exacerbation or worsening as characteristic of GWI.46 Given the paucity of data, we attempted to model symptom responses immediately and 24 hr following a controlled exercise challenge.
Based on the results of our doubly-multivariate MANOVA models that involved the full sample of GWI and CO, we did not observe any significant Group-by-Time interactions. This indicates that changes in symptom responses over time did not differ between groups. Interestingly, in a secondary analysis where we restricted the GWI group only to those participants who endorsed feeling unwell after exercise or physical exertion (GWI+PEM), significant interactions were observed for both the pain-related and the GWI-related PEM models, but not for the mood-related PEM model.
4.1. Model 1: Mood-related PEM Response
In MANOVA models for both the full GWI sample and the secondary analysis involving the GWI+PEM sample, we did not detect a significant Group-by-Time Interaction, indicating that mood related changes were not a primary feature of PEM in our sample of Veterans with GWI (Figure 2). These results are in agreement with our prior work involving ME/CFS and symptom responses to a submaximal exercise challenge,18 but differ from those where we employed a maximal exercise challenge.36 Similar to the present study, changes in POMS subscale scores were not significantly different between ME/CFS patients and healthy controls 24 hr after sub-maximal exercise.35 However, in the maximal exercise study, we showed large and significant differences between groups for POMS fatigue (ES=0.90; 95% CI: 0.06, 1.74), confusion (ES=0.93; 95% CI: 0.09, 1.78), and total mood disturbance (ES=0.90; 95% CI: 0.06, 1.75) at 72 hr after exercise. Differences between our previous results and results from the present study may be due to the groups studied (GWI vs. ME/CFS), features of the exercise stimulus (sub-max vs. max), or post-exercise measurement time-points (immediately after, 24-hr, 48-hr, 72-hr).
4.2. Model 2: Pain-related PEM Response
We observed a significant group-by-time interaction for the GWI+PEM vs. CO comparison that was not observed in the full sample. Visual inspection of Figure 3 indicated that individual symptom changes in the GWI group were larger than those reported by the CO group. Specifically, immediately after exercise we observed small increases in continuous, intermittent, and neuropathic pain for GV with GWI. Conversely, affective symptoms slightly decreased immediately after exercise, but then increased at 24hr post-exercise, especially in the GWI+PEM sub-group. An additional finding that was not reflective of PEM was that continuous and intermittent pain dropped below pre-exercise values at 24-hr post-exercise, indicating an improvement in these types of pain symptoms. These findings highlight the importance of taking the multi-dimensional nature of pain into account when studying PEM and suggest that pain dimension responses to exercise differ in terms of direction and time-course.
Figure 3.
Mean (SE) SF-MPQ-2 symptoms before, after, and 24 hours after exercise in Veterans with Gulf War Illness (GWI; n=39), GWI subgroup with PEM endorsement (GWI+PEM; n=18), and control Veterans without GWI (CO; n=28). Note. Total participants with complete listwise data across all time-points was: GWI=38; GWI+PEM=17; CO=28.
4.3. Model 3: GWI-related PEM Response
Symptoms in the GWI-related model differed between groups and changed over time. Similar to model 2, in the GWI+PEM sub-group, we also observed a significant group-by-time interaction that was not observed in the full sample. Visual inspection of mean changes for individual symptoms revealed larger changes in the GWI-PEM group rather than the CO group (Figure 4). For instance, although fatigue symptoms increased for the GWI+PEM subgroup and CO immediately after exercise, the CO group appeared to show a larger recovery at 24-hr post-exercise. For nausea symptoms, both full GWI and the GWI+PEM sub-group increased from pre- to immediately post-exercise, but GWI+PEM Veterans displayed a smaller recovery response at 24-hr post-exercise. In the case of shortness of breath, the GWI+PEM sub-group showed a large increase immediately after exercise whereas the CO group did not. Thus, we observed symptom responses that would be consistent with PEM for certain CDC VAS symptoms, but changes in other CDC VAS were suggestive of exercise-related improvements. For instance, depression severity decreased by approximately 7 points (~22%) immediately after exercise. This illustrates that not all symptoms in our GWI-related model were worsened by exercise which again highlights the complex nature of this disease and the need for systematic studies into the nature of PEM in GWI.
4.4. Acute Exercise Challenge in GWI
In general, exercise challenge studies of GWI have focused on determining perceptual and physiological responses during exercise or the physiological consequences of acute exercise challenge. Our group has reported greater perceived exertion and pain during exercise,22,23 similar aerobic capacities among GVs with CFS,47 and greater pain sensitivity post submaximal exercise.22 Results for the current study extend these finding by showing that both feelings of fatigue and leg muscle pain during exercise are elevated in GVs with GWI despite rating similar levels of effort. Perceptual responses during exercise are an important, yet often overlooked variable that help provide important contextual information when exercise is used as a stressor. Although beyond the scope of this initial investigation, testing the interplay between pain, effort, and fatigue during exercise and the experience of PEM will be an important consideration for future studies.
Rayhan and colleagues have reported that acute exercise challenge resulted in differential physiological responses from pre- to 1-hr post-exercise in GVs with CFS.48 One group demonstrated orthostatic intolerance and the other enhanced pain sensitivity as a function of tender point counts. Additionally, these subgroups exhibited different structural and functional brain outcomes. In a follow-up study these same subgroups were found to have differential micro-RNA expression in cerebral spinal fluid post-exercise challenge.49 Other research has reported delayed phosphocreatine recovery following ankle flexion suggestive of reduced muscle oxidative capacity and/or mitochondrial dysfunction.50 Recently, investigators in our group reported direct evidence for mitochondrial DNA (mtDNA) damage, expressed as increased lesion frequency and copy number, in GVs with GWI.51 None of these studies tested whether symptom exacerbation occurred as a result of the exercise challenge or whether symptom exacerbation was related to the primary outcome of interest (e.g. pain psychophysics, brain, muscle oxidative function). Studies that measure both the behavioral and physiological consequences of exercise are necessary for determining when the pathophysiological mechanisms that are being tested are a phenomenon of GWI or an epiphenomenon of some other factor. For example, Li et al., examined autonomic nervous system (ANS) measures in a sample of GVs who were stratified based on “self-reported post-exertional fatigue” and reported greater ANS disturbance indicated by greater heart rate responses to 70 degree head-up tilt and greater Composite Autonomic Scale Scoring total averages in the post-exertional fatigue group.10 Although the study by Li and colleagues did not employ an exercise challenge or measure symptom exacerbation directly, it highlights the potential impact of PEM on the pathophysiology of disease.
The phenomenon of PEM has received greater research attention in ME/CFS, including a recommendation by the National Academy of Medicine to change the name of the disease to systemic exertion intolerance disease (SEID) and requiring the presence of PEM for diagnosis.12 These studies have also focused on determining the physiological consequences of acute exercise challenge and have reported alterations in cardiorespiratory responses to exercise,52,53 pain regulation,54 immune and adrenergic system function,16,36,55,56 and gut microbiome interactions.57 However, symptom exacerbation has also been a focus of many of these investigations. We have previously reported symptom exacerbation following acute exercise challenge both immediately post- and up to 72-hr post-exercise for mood, pain, fatigue and cognition.18,36 Moreover, these studies have demonstrated significant relationships between changes in symptoms and changes in both gene expression36 and brain function.18 The symptom worsening effects of exercise in ME/CFS have been supported by several other labs,58–62 however the exact nature of PEM for a given person with ME/CFS appears to be highly variable.35 A recent meta-analysis of exercise in ME/CFS reported moderate to large effects of acute exercise on ratings of fatigue (Effect Size = 0.73; 95% CI: 0.24, 1.23), but concluded that the results were heterogeneous and that better controlled studies were needed.63 In the present study, PEM responses were clearly not as robust as seen in the ME/CFS literature. Potential explanations include that, compared to ME/CFS, PEM in GWI may (i) follow a different time course, (ii) require a more robust physical stimulus, or (iii) be less prevalent and/or severe. Our results should be viewed as hypothesis generating and the need for replication and extension of this research is clear.
4.5. Limitations
Notable limitations for the current study include a single dose of exercise, lack of a validated instrument for determining PEM endorsement, restricting the time-course of symptom measurement to 24 hr, and limited generalizability. It is possible that our dose of exercise was insufficient to induce PEM in GVs with GWI. We selected a dose of 70% of peak HRR for 30 min because this was similar to exercise intensities and durations that have been employed in previous ME/CFS PEM research and because it was a dose that we determined could be completed by the majority of this largely inactive population. As mentioned, there is a need for future studies that directly examine the dose-response issue of PEM severity and exercise intensity in both GWI and ME/CFS. It is also possible that our psychometric instruments were not sensitive enough, or representative of PEM, in GWI. However, there is currently no validated instrument for measuring PEM responses to exercise. Recent Common Data Elements Workgroups for both ME/CFS (https://www.commondataelements.ninds.nih.gov/MECFS.aspx#tab=Data_Standards) and GWI (https://cdmrp.army.mil/gwirp/research_highlights/19gwi_cde_initiative_highlight.aspx) have recommended the development of such instruments. Developing a PEM specific instrument will be challenging given the heterogeneity of these diseases and of the PEM response but would make a valuable contribution if achieved. Our choice to measure symptoms immediately- and 24-hr post-exercise was based primarily from research involving ME/CFS patients showing robust symptom responses for these time-points and diminishing symptoms further out.64 In ME/CFS, clinical case definitions and survey data indicate that over 80% of patients endure PEM for ≥24 hr, which can last anywhere from days to weeks.65,66 GVs with GWI may have a different temporal response and future research that tracks symptoms over a longer period of time will help to determine whether there is a pattern of PEM in GWI that is distinct from ME/CFS. We had strict inclusion and exclusion criteria for this study and thus we may not have captured the most severely affected GVs. Further, the more severely affected persons with GWI may not be inclined to volunteer for an acute exercise study for fear of symptom worsening. We also tested few female Veterans and thus may not represent the proportion of female Veterans with GWI.
4.6. Conclusion
Cross-sectional, epidemiological, and clinical trial data make clear that GVs with GWI suffer numerous symptoms and conditions that defy medical explanation and result in profound reduction in quality of life. It is also clear from these studies that a significant percentage of GVs report feeling unwell following exercise or physical exertion. However, the exact nature, severity, and time-course of PEM in GWI is unknown and to our knowledge has not been tested in controlled laboratory settings. We report here that symptom exacerbation in response to an acute submaximal aerobic exercise challenge did not occur in all GVs with GWI either immediately- or 24-hr post-exercise. This appears to be due, in part, to the majority of our sample not endorsing symptom worsening with exertion. More PEM research that systematically determines the mode, intensity, and duration of exercise that are tolerable (i.e. do not produce PEM) and necessary (i.e. produce clear PEM) is needed to better understand this phenomenon in this population. Future investigations should also be aimed toward further exploring the difference between those GWV who do and do not exhibit PEM. We show here that acute exercise does not bring about a significant worsening of symptom severity for the majority of GVs that were tested.
Table 2.
Baseline group characteristics in GWI (n=39), GWI subgroup with PEM endorsement (GWI+PEM; n=18), and healthy controls (n=28) – Mood and Pain
| GWI (n=39) | GWI+PEM (n=18) | CO (n=28) | GWI vs. CO | GWI+PEM vs. CO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ES | 95% Cl | ES | 95% Cl | |
| Profile of Mood States Brief FormA | ||||||||||
| Tension | 5.92 | 4.62 | 7.44 | 5.36 | 2.00 | 2.47 | 1.00 | 0.49, 1.52 | 1.39 | 0.73, 2.05 |
| Depression | 4.95 | 4.75 | 5.72 | 4.92 | 1.07 | 1.76 | 1.01 | 0.49, 1.52 | 1.36 | 0.71, 2.02 |
| Anger | 5.67 | 4.82 | 6.78 | 5.19 | 2.32 | 2.58 | 0.82 | 0.31, 1.32 | 1.15 | 0.52, 1.79 |
| Vigor | 4.44 | 3.57 | 3.56 | 3.01 | 9.00 | 3.30 | −1.30 | −1.84, −0.77 | −1.68 | −2.36, −0.99 |
| Fatigue | 11.31 | 5.21 | 11.67 | 5.03 | 2.79 | 2.87 | 1.92 | 1.33, 2.50 | 2.27 | 1.52, 3.02 |
| Confusion | 7.97 | 4.29 | 8.67 | 3.93 | 3.25 | 1.94 | 1.33 | 0.79, 1.87 | 1.86 | 1.15, 2.56 |
| Total Mood Disturbance | 31.90 | 19.55 | 37.83 | 19.88 | 2.43 | 11.16 | 1.76 | 1.19, 2.33 | 2.30 | 1.54, 3.06 |
| Short Form McGill Pain QuestionnaireB | ||||||||||
| Continuous Pain | 2.80 | 2.10 | 3.53 | 2.19 | 0.69 | 0.84 | 1.23 | 0.70, 1.76 | 1.85 | 1.15, 2.55 |
| Intermittent Pain | 2.35 | 2.58 | 2.84 | 2.73 | 0.38 | 0.63 | 0.97 | 0.46, 1.48 | 1.37 | 0.72, 2.03 |
| Neuropathic Pain | 2.26 | 2.07 | 2.85 | 2.64 | 0.21 | 0.31 | 1.27 | 0.74, 1.80 | 1.57 | 0.89, 2.24 |
| Affective Pain | 2.46 | 2.46 | 3.50 | 2.69 | 0.35 | 0.43 | 1.10 | 0.58, 1.62 | 1.82 | 1.12, 2.52 |
| Total Pain | 2.48 | 2.05 | 3.22 | 2.34 | 0.43 | 0.43 | 1.27 | 0.74, 1.81 | 1.85 | 1.13, 2.57 |
Note:
The instructional set for the Profile of Mood States Brief Form asked respondents to indicate how they were feeling “during the past week, including today”
The instructional set for the Short Form McGill Pain Questionnaire-2 asked respondents to indicate how they were feeling “during the past week”
Highlights:
Studies of post-exertional malaise that involve Veterans with Gulf War (GWI) Illness rarely measure potential changes in symptoms
We examined the effect of acute aerobic exercise on mood, fatigue, and other GWI related symptoms in 39 Veterans with GWI and 28 health control Veterans
In the full sample, we did not observe differences between groups in terms of post-exertional exacerbation of symptoms
When the GWI group was restricted only to Veterans who endorsed feeling unwell following exercise or physical exertion during baseline testing, Veterans with GWI displayed a larger exacerbation of symptoms than healthy controls
Acknowledgements and funding sources
This work was supported by a multi-site grant from the United States (U.S.) Department of Veterans Affairs Clinical Science Research and Development (CSR&D) service (I01CX001329; Dane B. Cook and Michael J. Falvo, Co-PIs). Jacob B. Lindheimer was supported by Career Development Award number IK2 CX001679 from the U.S. Department of Veterans Affairs CSR&D service. Ryan J. Dougherty was supported by a National Research Service Award from the National Institute on Aging of the National Institutes of Health under Award Number F31AG062009.
The contents do not represent the views of the Department of Veterans Affairs or the United States Government. The authors would like to thank the participants for volunteering for the study. We would also like to thank Nicholas Gretzon and Susen Schroeder for assistance with study coordination, data collection, and preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Kang HK, Mahan CM, Lee KY, Magee CA & Murphy FM Illnesses among united states veterans of the gulf war: a population-based survey of 30,000 veterans. Journal of Occupational and Environmental Medicine 42, 491–501 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Research Advisory Committee on Gulf War Veterans’ Illnesses. Gulf war illness and the health of gulf war veterans: scientific findings and recommendations. (U.S. Government Printing Office, 2008). [Google Scholar]
- 3.Committee on Gulf War and Health, Volume 10: Update of Health Effects of Serving in the Gulf War, Board on the Health of Select Populations, Institute of Medicine & National Academies of Sciences, Engineering, and Medicine Gulf War and Health: Volume 10: Update of Health Effects of Serving in the Gulf War, 2016. (National Academies Press, 2016). [PubMed] [Google Scholar]
- 4.Forman-Hoffman VL et al. Chronic Widespread Pain in Veterans of the First Gulf War: Impact of Deployment Status and Associated Health Effects. The Journal of Pain 8, 954–961 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Li B, Mahan CM, Kang HK, Eisen SA & Engel CC Longitudinal health study of US 1991 Gulf War Veterans: changes in health status at 10-year follow-up. American Journal of Epidemiology 174, 761–768 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Ozakinci G, Hallman WK & Kipen HM Persistence of symptoms in Veterans of the first Gulf War: 5-year follow-up. Environmental Health Perspectives 114, 1553–1557 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas HV, Stimpson NJ, Weightman AL, Dunstan F & Lewis G Systematic review of multisymptom conditions in Gulf War Veterans. Psychological Medicine 36, 735 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Khan F, Kennedy G, Spence VA, Newton DJ & Belch JJF Peripheral cholinergic function in humans with chronic fatigue syndrome, Gulf War syndrome and with illness following organophosphate exposure. Clin. Sci 106, 183–189 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Steele L Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. American Journal of Epidemiology 152, 992–1002 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Li M et al. Self-reported post-exertional fatigue in gulf war veterans: Roles of autonomic testing. Frontiers in Neuroscience (2014). doi: 10.3389/fnins.2013.00269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carruthers BM et al. Myalgic encephalomyelitis: International Consensus Criteria: Review: ME: Intl. Consensus Criteria. Journal of Internal Medicine 270, 327–338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clayton EW Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA 313, 1101–1102 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Cook DB, Nagelkirk PR, Poluri A, Mores J & Natelson BH The influence of aerobic fitness and fibromyalgia on cardiorespiratory and perceptual responses to exercise in patients with chronic fatigue syndrome. Arthritis and rheumatism 54, 3351–62 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Togo F et al. Sleep Is Not Disrupted by Exercise in Patients with Chronic Fatigue Syndromes: Medicine & Science in Sports & Exercise 42, 16–22 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshiuchi K et al. A real-time assessment of the effect of exercise in Chronic Fatigue Syndrome. Physiology & Behavior 92, 963–968 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Light AR et al. Gene expression alterations at baseline and following moderate exercise in patients with chronic fatigue syndrome and fibromyalgia syndrome. Journal of Internal Medicine 271, 64–81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snell CR, Vanness JM, Strayer DR & Stevens SR Exercise capacity and immune function in male and female patients with chronic fatigue syndrome (CFS). In vivo (Athens, Greece) 19, 387–90 (2005). [PubMed] [Google Scholar]
- 18.Cook DB et al. Neural consequences of Post-Exertion Malaise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Brain, Behavior, and Immunity 62, 87–99 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Broderick G et al. Altered immune pathway activity under exercise challenge in Gulf War Illness: an exploratory analysis. Brain, Behavior, and Immunity 28, 159–169 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Fletcher MA, Maher KJ & Klimas NG Natural killer cell function in chronic fatigue syndrome. Clinical and Applied Immunology Reviews 2, 129–139 (2002). [Google Scholar]
- 21.Rose MR et al. Evaluation of neuromuscular symptoms in UK Gulf War veterans: A controlled study. Neurology 63, 1681–1687 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Cook DB, Stegner AJ & Ellingson LD Exercise Alters Pain Sensitivity in Gulf War Veterans With Chronic Musculoskeletal Pain. The Journal of Pain 11, 764–772 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Cook DB et al. Perceived exertion in fatiguing illness: Gulf War Veterans with Chronic Fatigue Syndrome: Medicine & Science in Sports & Exercise 35, 569–574 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Gibbons RJ A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). 57 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Kazis LE The Veterans SF-36 Health Status Questionnaire: Development and Application in the Veterans Health Administration. 5, 18 (2000). [Google Scholar]
- 26.Buysse DJ, Reynolds CF, Monk TH, Berman SR & Kupfer DJ The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Research 28, 193–213 (1989). [DOI] [PubMed] [Google Scholar]
- 27.Wagner D et al. Psychometric properties of the CDC Symptom Inventory for assessment of Chronic Fatigue Syndrome. Population Health Metrics 3, (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dworkin RH et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain 144, 35–42 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Wolfe F, Clauw D & Fitzcharles M-A The American College of Rheumatology preliminary diagnostic criteria for Fibromyalgia and measurement of symptom severity. Arthritis Care & Research 62, 600–610 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Smets EMA, Garssen B, Bonke B & De Haes JCJM The Multidimensional Fatigue Inventory (MFI): Psychometric qualities of an instrument to assess fatigue. Journal of Psychosomatic Research 39, 315–325 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Krupp LB, LaRocca NG, Muir-Nash J & Steinberg AD The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 46, 1121–1123 (1989). [DOI] [PubMed] [Google Scholar]
- 32.McNair DM & Heuchert JWP Profile of Mood States Technical Updates. (Multi-Health Systems Inc., 2005). [Google Scholar]
- 33.Craig CL et al. International physical activity questionnaire: 12-country reliability and validity. Medicine & Science in Sports & Exercise 35, 1381–1395 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Fukuda K et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Annals of internal medicine 121, 953–959 (1994). [DOI] [PubMed] [Google Scholar]
- 35.Lindheimer JB et al. Symptom variability following acute exercise in myalgic encephalomyelitis/chronic fatigue syndrome: a perspective on measuring post-exertion malaise. Fatigue: Biomedicine, Health & Behavior 5, 69–88 (2017). [Google Scholar]
- 36.Meyer JD et al. Post-exertion malaise in chronic fatigue syndrome: symptoms and gene expression. Fatigue: Biomedicine, Health & Behavior 1, 190–209 (2013). [Google Scholar]
- 37.Lindheimer JB et al. Influence of pain anticipation on brain activity and pain perception in Gulf War Veterans with chronic musculoskeletal pain. Psychophysiology (2019). doi: 10.1111/psyp.13452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka H, Monahan KD & Seals DR Age-predicted maximal heart rate revisited. Journal of the American College of Cardiology 37, 153–156 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Medicine, A. C. of S. ACSM’s guidelines for exercise testing and prescription. 9th Edition. (Wolters Kluwer/Lippincott Williams & Wilkins Health, 2014). [Google Scholar]
- 40.Borg GAV Psychophysical bases of perceived exertion. Medicine & Science in Sports & Exercise 14, 377–381 (1982). [PubMed] [Google Scholar]
- 41.Cook DB, O’Connor PJ, Eubanks SA, Smith JC & Lee M Naturally occurring muscle pain during exercise: assessment and experimental evidence. Medicine & Science in Sports & Exercise 29, 999–1012 (1997). [DOI] [PubMed] [Google Scholar]
- 42.Gift AG Visual analogue scales: Measurement of subjective phenomena. Nursing Research 38, 286–288 (1989). [PubMed] [Google Scholar]
- 43.Fritz CO, Morris PE & Richler JJ Effect size estimates: Current use, calculations, and interpretation. Journal of Experimental Psychology: General 141, 2–18 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Brysbaert M How Many Participants Do We Have to Include in Properly Powered Experiments? A Tutorial of Power Analysis with Reference Tables. Journal of Cognition 2, 16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang HK, Li B, Mahan CM, Eisen SA & Engel CC Health of US Veterans of 1991 Gulf War: A Follow-Up Survey in 10 Years: Journal of Occupational and Environmental Medicine 51, 401–410 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Maule AL et al. Meta-analysis of self-reported health symptoms in 1990–1991 Gulf War and Gulf War-era veterans. BMJ Open 8, e016086 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagelkirk PR et al. Aerobic capacity of Gulf War Veterans with Chronic Fatigue Syndrome. Military Medicine 168, 750–55 (2003). [PubMed] [Google Scholar]
- 48.Rayhan RU et al. Exercise Challenge in Gulf War Illness Reveals Two Subgroups with Altered Brain Structure and Function. PLoS ONE 8, e63903 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baraniuk JN & Shivapurkar N Exercise – induced changes in cerebrospinal fluid miRNAs in Gulf War Illness, Chronic Fatigue Syndrome and sedentary control subjects. Scientific Reports 7, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koslik HJ, Hamilton G & Golomb BA Mitochondrial Dysfunction in Gulf War Illness Revealed by 31Phosphorus Magnetic Resonance Spectroscopy: A Case-Control Study. PLoS ONE 9, e92887 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y et al. Role of mitochondrial DNA damage and dysfunction in veterans with Gulf War Illness. PLoS ONE 12, e0184832 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cook DB et al. Responses to Exercise Differ for Chronic Fatigue Syndrome Patients with Fibromyalgia: Medicine & Science in Sports & Exercise 44, 1186–1193 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snell CR, Stevens SR, Davenport TE & Van Ness JM Discriminative validity of metabolic and workload measurements for identifying people with Chronic Fatigue Syndrome. Physical Therapy 93, 1484–1492 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Van Oosterwijck J et al. Pain inhibition and postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: An experimental study: Pain inhibition and postexertional malaise in ME/CFS. Journal of Internal Medicine 268, 265–278 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Broderick G et al. A pilot study of immune network remodeling under challenge in Gulf War Illness. Brain, Behavior, and Immunity 25, 302–313 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Moneghetti KJ et al. Value of Circulating Cytokine Profiling During Submaximal Exercise Testing in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Scientific reports 8, 2779 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shukla SK et al. Changes in Gut and Plasma Microbiome following Exercise Challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). PLOS ONE 10, e0145453 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nijs J, Almond F, De Becker P, Truijen S & Paul L Can exercise limits prevent post-exertional malaise in chronic fatigue syndrome? An uncontrolled clinical trial. Clinical rehabilitation 22, 426–35 (2008). [DOI] [PubMed] [Google Scholar]
- 59.VanNess JM, Stevens SR, Bateman L, Stiles TL & Snell CR Postexertional malaise in women with chronic fatigue syndrome. Journal of women’s health (2002) 19, 239–44 (2010). [DOI] [PubMed] [Google Scholar]
- 60.White AT et al. Severity of symptom flare after moderate exercise is linked to cytokine activity in chronic fatigue syndrome. Psychophysiology 47, 615–624 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meeus M et al. Symptom fluctuations and daily physical activity in patients with chronic fatigue syndrome: a case-control study. Archives of physical medicine and rehabilitation 92, 1820–6 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Keech A et al. Capturing the post-exertional exacerbation of fatigue following physical and cognitive challenge in patients with chronic fatigue syndrome. Journal of psychosomatic research 79, 537–49 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Loy BD, O’Connor PJ & Dishman RK Effect of Acute Exercise on Fatigue in People with ME/CFS/SEID: A Meta-analysis. Medicine & Science in Sports & Exercise 48, 2003–2012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Light AR, White AT, Hughen RW & Light KC Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. The journal of pain : official journal of the American Pain Society 10, 1099–112 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chu L, Valencia IJ, Garvert DW & Montoya JG Deconstructing post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: A patient-centered, cross-sectional survey. PloS one 13, e0197811 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carruthers BM et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. Journal of Chronic Fatigue Syndrome 11, 7–115 (2003). [Google Scholar]