Abstract
Adherence to medications remains poor despite numerous efforts to identify and intervene upon non-adherence. One potential explanation is the limited focus of many interventions on one barrier. Little is known about the prevalence and impact of having multiple barriers in contemporary practice. Our objective was to quantify adherence barriers for patients with poorly-controlled cardiometabolic condition, identify patient characteristics associated with having multiple barriers, and determine its impact on adherence. We used a linked electronic health records and insurer claims dataset from a large health system from a recent pragmatic trial. Barriers to medication-taking before the start of the intervention were elicited by clinical pharmacists using structured interviews. We used multivariable modified Poisson regression models to examine the association between patient factors and multiple barriers and multivariable linear regression to evaluate the relationship between multiple barriers and claims-based adherence. Of the 1,069 patients (mean: 61 years of age) in this study, 25.1% had multiple barriers to adherence; the most common co-occurring barriers were forgetfulness and health beliefs (31%, n=268). Patients with multiple barriers were more likely to be non-white (Relative Risk [RR]:1.57, 95%CI: 1.21-1.74), be single/unpartnered (RR:1.36, 95%CI: 1.06-1.74), use tobacco (RR:1.54, 95%CI: 1.13-2.11), and have poor glycemic control (RR:1.77, 95%CI: 1.31-2.39) versus those with 0 or 1 barrier. Each additional barrier worsened average adherence by 3.1% (95%CI: −4.6%, −1.5%). In conclusion, >25% of non-adherent patients present with multiple barriers to optimal use, leading to meaningful differences in adherence. These findings should inform quality improvement interventions aimed at non-adherence.
Keywords: chronic disease, hypertension, hyperlipidemia, adherence
Poor adherence to medications for chronic cardiometabolic conditions is extremely common and costly.1 On average, half of patients do not take their medications as prescribed.2,3 Numerous interventions to improve adherence have been evaluated; unfortunately, those that do work have generally only had modest effects when subjected to rigorous evaluation.4,5 One reason for the limited effectiveness of prior interventions may be that they often attempt to address single barriers to adherence,4,5 such as costs or forgetfulness, or apply a one-size-fits-all approach.6,7 Yet, previous work has suggested that ≥30% of non-adherent patients have ≥2 reasons for non-adherence.8,9 Unfortunately, the existing data about the prevalence of barriers is limited; the small current studies have generally quantified barriers based only on patient self-report.9-11 Using data from a large pragmatic trial,12,13 we determined the prevalence of barriers to adherence, including multiple co-existing barriers and clustering patterns, in contemporary practice. We also identified the patient factors associated with having multiple barriers. These findings could inform the design of future interventions by improving their targeting and scope.
METHODS
The source population for this study included subjects from the Study of a Tele-pharmacy Intervention for Chronic diseases to (2) Improve Treatment adherence (STIC2IT) pragmatic trial [ClinicalTrials.gov ()].12,13 This trial evaluated a multi-component intervention delivered by licensed clinical pharmacists over the telephone and was conducted in clinics at a large multi-specialty group practice in eastern Massachusetts between August 2015 and August 2017. Subjects were included in the trial if they had suboptimal hyperlipidemia, hypertension or diabetes disease control and were non-adherent to prescribed medications for those conditions.12,13 Patients were excluded if they had <6 months of health plan enrollment before randomization or were <18 or >85 years of age. Of the 2,038 patients randomized to the intervention group, 94% of patients were approved for enrollment by their primary care providers, and 58% of those patients agreed to a phone consultation with a clinical pharmacist. For this present study, we included patients who received ≥1 clinical pharmacist consultation after randomization.
We used pharmacist consultation notes linked to electronic health record (EHR) and health insurance claims data.13 These sources contain patient-level data on inpatient and outpatient encounters and procedures, sociodemographic information, outpatient office visits, Emergency Room (ER) visits, and outpatient pharmacy prescription drug claims.
As part of the initial telephone consultation with study subjects, the clinical pharmacists elicited patients’ current barriers to medication adherence using a semi-structured interviewing guide based on the principles of brief negotiated interviewing, a technique with foundations in motivational interviewing.14 Prior to starting the trial, the clinical pharmacists underwent a 2-day training program that included script development, role-playing exercises, and training on how to elicit and classify barriers to adherence.13 Versus alternative approaches, we chose to classify barriers using structured pharmacist interviews to reduce patient burden and eliminate the need for a separate evaluation.
For the trial, we had created 6 a priori categories for barriers based on peer-reviewed literature:6,7,15 (1) cost, (2) treatment complexity/forgetfulness barriers (hereafter referred to as “forgetfulness”), (3) health beliefs (e.g., low perceived need for therapy), (4) lack of medication knowledge/poor health literacy (hereafter referred to as “knowledge”), (5) side effects (e.g., experiencing an adverse drug effect), and (6) cognitive impairment.13 The pharmacists selected from these 6 pre-defined categories when they documented their consultation in the EHR. Because patients could have multiple barriers, we also summed the total number of identified barriers for each patient and created “barrier clusters” to classify and depict the groupings of barriers typically experienced by patients. For instance, individuals who were identified as having cost and forgetfulness barriers, but no other barriers, were classified in the “cost-forgetfulness” cluster.
Of note, patients could also be documented as not having an adherence barrier if the clinical pharmacists felt that the pattern of medication filling in the prescription claims data (as presented in the EHR system) did not actually represent current non-adherence and if the patients had no active barriers. For example, situations in which the pharmacists classified patients with no current adherence barriers included if the patient’s treatment regimen had changed, they had used an alternative insurance plan or paid cash for their medications (and thus, their medication fills were not recorded in the claims data), or if they had recently become adherent (defined at the clinical pharmacist’s discretion).
We used insurance claims and structured EHR data to measure patient factors in the 365 days before the initial pharmacist consultation. If patients had fewer than 365 days of insurance eligibility, then characteristics were measured beginning from the date of insurance enrollment. Demographic characteristics were measured from structured fields in EHR data, including age, sex, race/ethnicity (classified as “white” or “non-white”), and martial/partner status. We also measured clinical factors such as patients’ Body Mass Index (BMI), their latest glycated hemoglobin A1c (HbA1c), their latest systolic blood pressure (SBP), their latest low-density lipoprotein (LDL) and current tobacco use from structured EHR fields.13 While the overall proportion of patients missing any of these variables was low (<10%), for patients who did not have any available measurements of BMI, HbA1c, SBP, and LDL, we imputed the patient’s value using mean values for that condition.16
Other clinical characteristics were measured in claims data using International Classification of Disease 9th edition (ICD-9) or 10th edition (ICD-10) codes, including clinical comorbidities, a combined comorbidity score (a measure of overall health status based on comorbidities),17 number of outpatient physician office visits, hospitalizations, and ER visits. Using pharmacy claims data, we measured the number of unique medications that each patient had filled and their baseline adherence to chronic medications (described in detail below). By definition, none of the characteristics measured from claims data were missing because insurance coverage was an inclusion criterion for the parent trial.12
To calculate baseline adherence (i.e., prior to the intervention) for each medication used to treat any of the 3 chronic conditions that were the focus of the intervention, we created a drug supply diary from prescription claims data beginning on the date of their first fill in the year prior to their randomization date.18 Chemically-related medications were considered to be interchangeable (e.g., 2 beta-blockers). From these supply diaries, we measured the average proportion of days that patients had medications available, or the proportion of days covered (PDC).19 We then averaged the PDC for all medications used to treat a single condition (e.g., all antihypertensives) and calculated an overall average adherence across each patient’s eligible conditions.19,20 Of note, patients were included in the parent STIC2IT trial if their overall average baseline adherence for their eligible chronic conditions was poor (PDC<0.80); a PDC of ≥0.80 corresponds to the minimum level of adherence associated with reductions in outcomes like hospitalization or stroke.2 This PDC adherence calculation captures the implementation phase of the adherence taxonomy.21
First, we examined the prevalence of barriers to optimal adherence, regardless of whether patients had other barriers and determined how often patients had multiple barriers to adherence (defined by having ≥2 recorded barriers). We also described the characteristics of patients by their individual barriers to adherence, by their number of barriers to adherence, and within the most common multiple adherence barrier clusters.
Second, we used multivariable modified Poisson regression analysis to estimate the independent patient factors associated with having multiple barriers to adherence (compared with those having 0 or 1 barrier) among all patients with pharmacist consultations.22 In brief, modified Poisson models generate the estimated relative risks (RRs) with robust standard errors and are appropriate when outcomes are common (e.g., incidences of ≥5%).22 In secondary analyses, we modeled the association of patient factors with having multiple barriers to adherence compared with having just 1 barrier and no barriers separately. For clinical interpretability and based on prior research17, for the modeling, we stratified age into 3 categories (<55, 55-64, and ≥65 years), BMI as ≥30 or <30, HbA1c as ≥8% or <8%, SBP as ≥130 or <130 mmHg, LDL as ≥190 or <190 mg/dL, combined patient comorbidity score as ≥1 or <1, number of office visits as ≥2 or <2, and number of unique medications as <5, 5-10 and ≥10.
Finally, we evaluated the relative importance of having multiple barriers on patient adherence. To do so, we used separate linear regression models to estimate the relationship between overall average adherence and 1) having multiple barriers and 2) each additional barrier (modeled as a continuous term beginning with 0). We estimated each of these models as unadjusted models and adjusting for other patient baseline factors. We also conducted exploratory subgroup analyses by study condition (e.g., hypertension).
Statistical significance was determined using 2-sided tests with alpha=0.05. All analyses were conducted using SAS 9.4 (Cary, NC). The Institutional Review Board of the Brigham and Women’s Hospital approved this study. Dr. Lauffenburger had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
RESULTS
Of the 2,038 intervention patients in the parent STIC2IT trial, 1,069 (52%) received an initial clinical pharmacist consultation in which the barriers to adherence were collected.12 Among the 1,069 patients included in the present study, their mean age was 61.1 years (Standard Deviation [SD]: 11.0), and 43% were female.
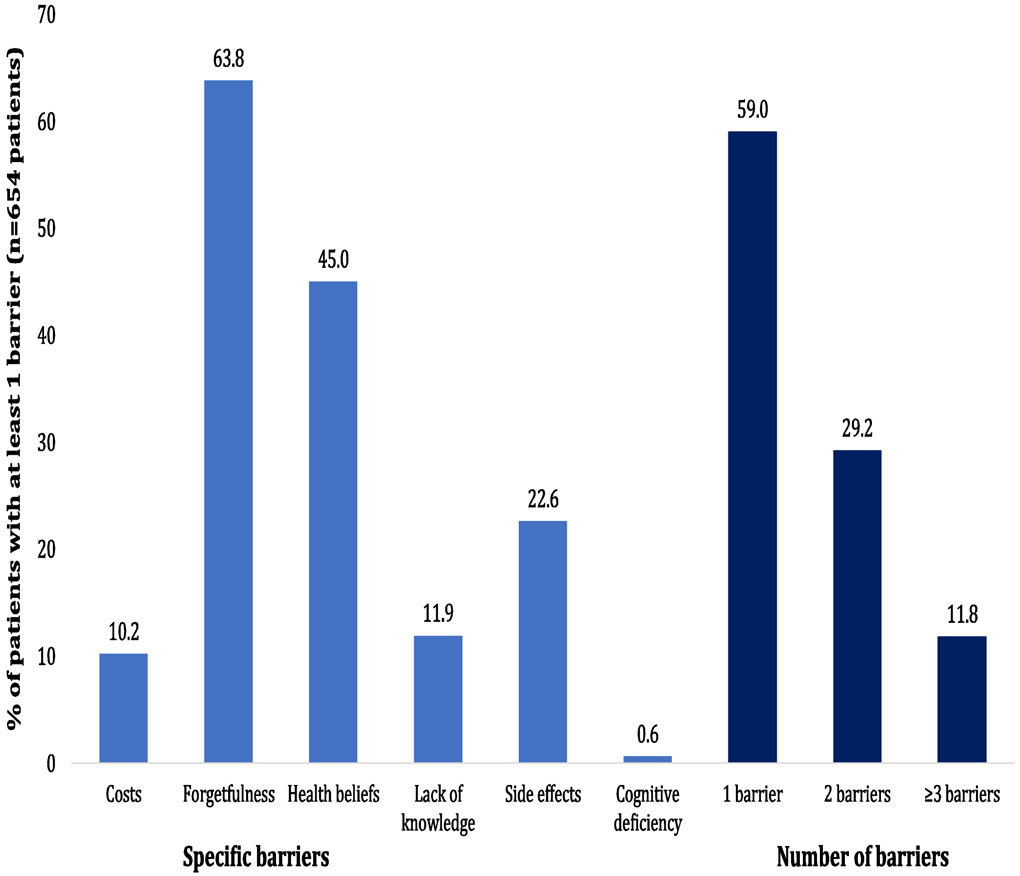
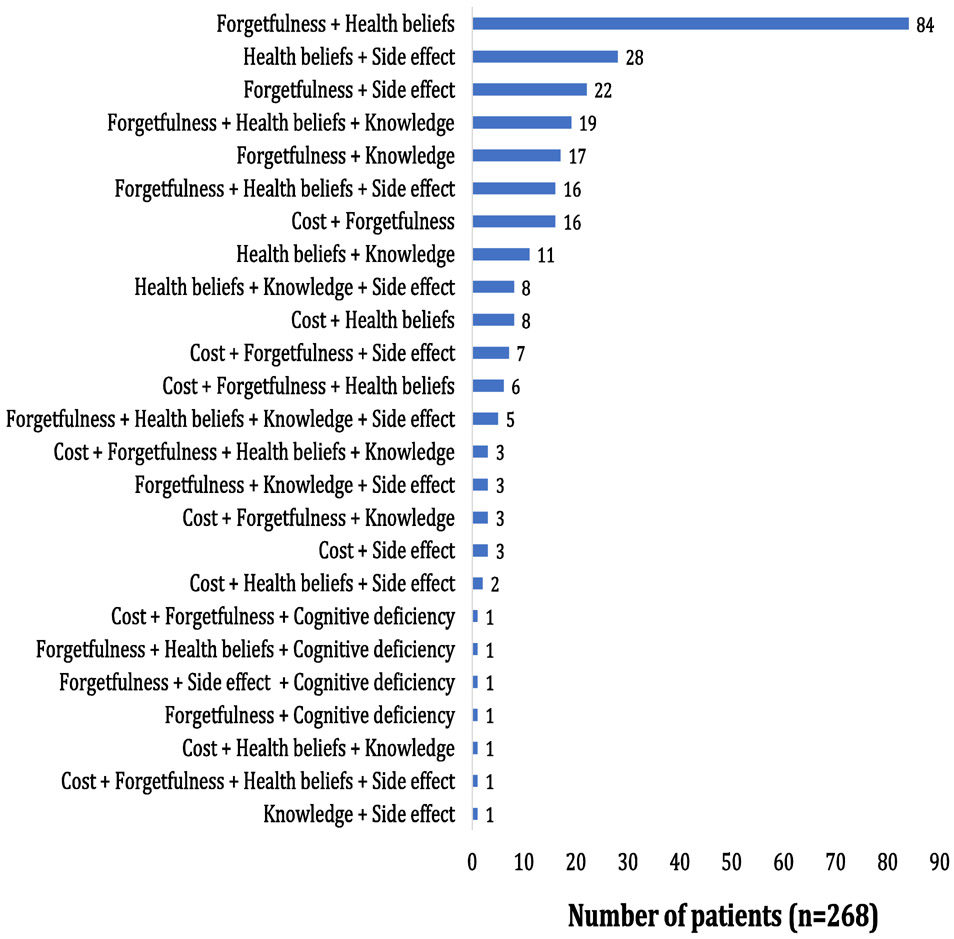
In total, 654 patients had ≥1 recorded barrier to optimal adherence. In these 654 patients presenting with ≥1 barrier (Figure 1), the most commonly recorded barrier was forgetfulness (64%), followed by health beliefs (45%) and experiencing side effects (23%). In total, 268 and 77 patients presented with ≥2 and ≥3 barriers to adherence, respectively. The distribution of the barrier clusters among the 268 patients with multiple (≥2) barriers to adherence is shown in Figure 2. The 3 most common clusters were combinations of 1) forgetfulness and health beliefs (31%), 2) health beliefs and side effects (10%), and 3) forgetfulness and side effects (8%). The prevalence of clusters among all 654 patients with ≥1 barrier is shown in Supplementary Figure 1, which mirror the key results.
Figure 1.
Prevalence of barriers to optimal adherence
Figure 2.
Prevalence of medication adherence barrier clusters
Patients’ baseline characteristics are shown in Table 1, stratified by experiencing 0, 1, 2, and ≥3 barriers to optimal adherence. More than 60% of patients with ≥3 adherence barriers were men and of non-white race/ethnicity. Those patients experiencing ≥3 barriers also used more medications on average than those with fewer barriers. Recorded current use of tobacco was also higher in patients with ≥3 barriers. Patient characteristics are shown in Supplementary Table 1 by individual barrier (e.g., forgetfulness) and Supplementary Table 2 for the 3 most common multiple adherence barrier clusters.
Table 1.
Patient characteristics and the number of barriers to optimal adherence
| Number of barriers | ||||
|---|---|---|---|---|
| Variable | 0 (N=415) |
1 (N=386) |
2 (N=191) |
≥3 (N=77) |
| Men | 59.5% | 55.7% | 55.5% | 61.0% |
| Age, mean ± SD (years) | 63.2 ± 10.6 | 60.7 ± 11.1 | 58.7 ± 10.9 | 57.9 ± 11.1 |
| Black | 21.5% | 36.3% | 43.5% | 47.1% |
| Hispanic/Latino | 4.3% | 6.7% | 3.1% | 10.3% |
| Single/unpartnered | 38.3% | 44.3% | 57.6% | 46.7% |
| Coronary artery disease | 8.9% | 4.7% | 2.1% | 5.2% |
| Congestive heart failure | 3.6% | 2.6% | 4.2% | 3.9% |
| Chronic kidney disease | 7.7% | 4.9% | 8.4% | 2.6% |
| Chronic obstructive pulmonary disease/Asthma | 5.1% | 3.9% | 5.2% | 5.2% |
| Depression | 7.5% | 6.0% | 5.2% | 5.2% |
| Diabetes mellitus | 19.5% | 15.8% | 18.9% | 16.9% |
| Hyperlipidemia* | 35.9% | 23.8% | 29.3% | 24.7% |
| Hypertension* | 34.7% | 25.1% | 25.7% | 23.4% |
| Tobacco use | 9.4% | 13.2% | 19.4% | 23.4% |
| Body mass index, mean ± SD (kg/m2) | 29.4 ± 5.6 | 31.0 ± 6.5 | 31.9 ± 6.4 | 31.8 ± 7.3 |
| Latest glycated hemoglobin A1c, mean ± SD (%) | 6.5 ± 1.2 | 6.7 ± 1.6 | 7.0 ± 1.7 | 8.1 ± 2.6 |
| Latest systolic blood pressure, mean ± SD (mmHg) | 127.7 ± 14.5 | 129.9 ± 15.9 | 130.9 ± 15.4 | 129.9 ± 18.0 |
| Latest low-density lipoprotein, mean ± SD (mg/dL) | 167.6 ± 6.3 | 179.0 ± 42.4 | 172.2 ± 42.6 | 176.5 ± 44.3 |
| Comorbidity score, mean ± SD | 0.9 ± 1.6 | 0.7 ± 1.4 | 0.8 ± 1.5 | 0.7 ± 1.7 |
| Emergency room visit | 6.5% | 3.1% | 5.2% | 3.9% |
| No. office visits, mean ± SD | 3.4 ± 5.5 | 2.6 ± 5.2 | 3.1 ± 6.1 | 2.1 ± 4.4 |
| Hospitalization | 9.6% | 4.4% | 5.2% | 2.6% |
| No. unique drugs, mean ± SD | 9.4 ± 6.3 | 8.5 ± 5.8 | 8.3 ± 5.6 | 10.2 ± 6.2 |
| Adherence (proportion of days covered), mean ± SD | 62.6 ± 23.2 | 55.6 ± 22.4 | 54.0 ± 23.8 | 52.4 ± 21.6 |
Abbreviations: SD, Standard Deviation
Defined as having hyperlipidemia or hypertension per diagnoses in administrative claims data
As shown in Table 2, patients with multiple (≥2) pharmacist-classified barriers to optimal adherence were more likely to be non-white (RR: 1.57, 95%CI: 1.21-1.74) and single (RR: 1.36, 95%CI: 1.06-1.74) compared with patients with <2 (i.e., 1 or 0) barriers. Patients with multiple barriers were also more likely to use tobacco (RR: 1.54, 95%CI: 1.13-2.11) and have poor glycemic control (RR: 1.77, 95%CI: 1.31-2.39) than patients with <2 barriers. Secondary analyses comparing characteristics of patients with multiple barriers stratified by the referent group (i.e., versus 1 barrier and versus no barriers) are shown in Supplementary Table 3. These results were similar to the overall findings but had wider confidence intervals due to smaller sample sizes for the referent groups.
Table 2.
Association between patient factors and having multiple barriers to optimal adherence compared with having fewer barriers
| Factors (ref: no unless
otherwise specified) |
Relative risk of multiple barriers (95% Confidence Interval) |
|---|---|
| Men (ref: women) | 1.06 (0.81-1.37) |
| Age (ref: <55 years) | |
| 55-64 years | 1.05 (0.78-1.41) |
| ≥65 years | 0.87 (0.60-1.26) |
| Non-white race/ethnicity | 1.57 (1.20-2.04) |
| Single/unpartnered | 1.36 (1.06-1.74) |
| Coronary artery disease | 0.46 (0.21-1.00) |
| Congestive heart failure | 2.00 (0.99-4.04) |
| Chronic kidney disease | 1.47 (0.83-2.62) |
| Chronic obstructive pulmonary disease/Asthma | 1.42 (0.77-2.62) |
| Depression | 1.07 (0.60-1.91) |
| Diabetes | 1.03 (0.61-1.73) |
| Hyperlipidemia | 1.55 (0.98-2.45) |
| Hypertension | 0.75 (0.49-1.14) |
| Tobacco use | 1.55 (1.13-2.11) |
| Body mass index ≥30 (ref: <30) | 1.23 (0.95-1.59) |
| Latest glycated hemoglobin A1C ≥8% (ref: <8%) | 1.76 (1.31-2.38) |
| Latest systolic blood pressure ≥130 mmHg (ref: <130 mmHg) | 1.17 (0.91-1.50) |
| Latest low-density lipoprotein ≥190 mg/dL (ref: <190 mg/dL) | 0.95 (0.72-1.24) |
| Comorbidity score: ≥1 (ref: <1) | 0.88 (0.50-1.54) |
| Emergency room visit | 1.43 (0.76-2.68) |
| ≥2 office visits (ref: <2) | 0.96 (0.62-1.49) |
| Hospitalization | 0.68 (0.35-1.31) |
| No. unique drugs filled (ref: <5) | |
| 5-10 | 0.94 (0.69-1.29) |
| ≥10 | 0.88 (0.63-1.23) |
Note: The study sample consisted of all 1,069 patients with recorded barriers to adherence, comparing those with ≥2 barriers to adherence with those with 0 or 1 barriers.
Finally, having multiple pharmacist-classified barriers to optimal adherence had a strong relationship with worse overall average claims-based adherence to medication (Table 3). For example, the presence of each additional pharmacist-classified barrier worsened average claims-based adherence to medications by −3.1% (95%CI: −4.6%, −1.5%) after adjusting for all other patient characteristics. Exploratory subgroup analyses by the three chronic conditions suggested that the relationship could be stronger, on average, for patients with diabetes and hyperlipidemia (Supplemental Table 4)
Table 3.
Relative contribution of having multiple barriers on adherence to medication
| Unadjusted difference in adherence (95% Confidence Interval) |
Difference in adherence adjusted for patient factors (95% Confidence Interval) |
|
|---|---|---|
| All patients: Proportion of days covered, mean ± standard deviation | 57.8 ± 23.2 | |
| Number of barriers: | ||
| Multiple barriers (≥2) | −5.7 (−8.9,−2.5) | −4.0 (−7.3, −0.8) |
| Each additional barrier | −3.8 (−5.3, −2.4) | −3.1 (−4.6, −1.5) |
Note: The study sample consisted of all 1,069 patients with recorded barriers to adherence.
DISCUSSION
In this study of non-adherent patients with poorly-controlled cardiometabolic disease, >25% of patients experienced ≥2 barriers to optimal medication adherence. Forgetfulness and low perceived need for therapy (i.e., health beliefs) were the most common co-occurring barriers. Patients with multiple barriers were also more likely to be of minority race/ethnicity and have worse disease control than those with fewer barriers. We also observed that patients who experience more barriers to adherence have worse claims-based adherence to medications, with differences that persist after accounting for other patient factors. Even these small gaps in adherence are thought to be potentially clinically meaningful, resulting in higher risks of stroke and cardiovascular-related death.2
To our knowledge, this is the largest known study to quantify the prevalence of barriers to optimal adherence in patients with cardiometabolic disease. It also uniquely links data from EHR and insurer claims to allow for wider assessment of patient factors. Moreover, it is also by far the largest of other known studies in which pharmacists elicited barriers.8,11,23 For example, a study of 179 non-adherent patients receiving pharmacist consultations found that 58% had ≥2 reasons for non-adherence.24 Like in our study, previous research has also suggested that forgetfulness is the most common barrier.6,10,23,25 For instance, >25% of patients have previously reported forgetfulness as a barrier to adherence to clinical pharmacists at their insurance plan.23
The common co-occurrence of forgetfulness and health beliefs barriers in patients warrants some further consideration. One possible explanation is that having difficulty remembering to take medications could be related to not believing that they are important to take and therefore purposefully not integrating medication-taking into daily routines. Behavioral research suggests that memory recall is worse when insufficient attention is given towards specific tasks or that people may voluntarily suppress recall for activities that make them uncomfortable.26,27 Our study may provide further empirical support for the link between memory and perceived need. Either way, the fact that these barriers commonly exist together suggests future interventions should try to address both barriers.
These findings suggest that existing adherence interventions could be improved by being more multi-faceted. Adherence is known to be a complex multifactorial behavior, and the strongest interventions appear to be those that are multicomponent interventions.4 The parent STIC2IT trial was, in fact, designed on this basis.12 By contrast, even eliminating prescription medication copayments, arguably one of the most successful single-barrier interventions to improve adherence4, increases average adherence by only 5% (though the intervention did not identify or target patients specifically with cost barriers).28 Our results also identified the most common co-occurring barriers and the patient groups who are most likely to have multiple barriers. Therefore, even in the absence of efforts to elicit barriers, our results could guide the design of dual or multicomponent interventions. Because a non-trivial number of patients are struggling with multiple barriers to adherence, interventions should be designed to be multifaceted enough to address them.
Interventions could also be more generally improved by being targeted towards patients’ specific barriers rather than providing one-size-fits-all support, which is what is often done.5,12 Evidence from recent trials suggest that targeting adherence interventions to patients based on clinical need, predicted benefit, or adherence barriers may improve outcomes.12,29 In practice, clinicians, support staff, or clinical pharmacists could potentially use this approach or other such tools to identify specific barriers to optimal adherence, whether by telephone or in-person.8,11 These tools could potentially even be delivered as part of other screening tools, such as social needs screenings, or integrated as data into EHRs, which could then be used to deploy barrier-targeted interventions.30 Regardless of approach, we identified specific barriers and clusters of barriers which commonly present and in which patients, to allow for better intervention deployment.
Several limitations should be acknowledged. Patients had to have a pharmacist consultation to be included in the study, so the overall prevalence of barriers may differ in clinical practice and also change over time (i.e., explaining our low observed rates of cognitive impairment). While the adherence barriers were identified based on literature and expertise, patients could have had other barriers (e.g., provider or system-level barriers, beyond the scope of the trial).6 Not all patients were classified as having an adherence barrier, as sometimes the pharmacists felt that their poor disease control was not caused by an adherence issue. However, this study mimics how a quality improvement intervention would identify poorly-adherent patients in practice, and therefore could actually be considered a study strength.23 The study size also precluded us from estimating associations with individual multiple barrier clusters. We also could not measure and control for all potential patient factors, such as their healthcare plan. Finally, this study was conducted in practices in Massachusetts and may not be fully generalizable to other places.
In conclusion, a large fraction of patients with cardiometabolic disease experience multiple barriers to optimal adherence to chronic medications in contemporary practice, leading to noticeable gaps in adherence compared with those with fewer barriers. Forgetfulness and low perceived need for therapy commonly occur together. These findings could inform the design and target of interventions to improve management.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank the clinical pharmacists at Atrius for their support with study.
Funding: This research was supported by a grant from the NIH National Heart, Lung, and Blood Institute (NHLBI) to BWH (R01 HL 117918). Dr. Lauffenburger was supported by a career development grant (K01 HL 141538) from the NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Choudhry NK, Setoguchi S, Levin R, Winkelmayer WC, Shrank WH. Trends in adherence to secondary prevention medications in elderly post-myocardial infarction patients. Pharmacoepidemiol Drug Saf 2008;17:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–497. [DOI] [PubMed] [Google Scholar]
- 3.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 2009;15:728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viswanathan M, Golin CE, Jones CD, Ashok M, Blalock SJ, Wines RC, Coker-Schwimmer EJ, Rosen DL, Sista P, Lohr KN. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med 2012;157:785–795. [DOI] [PubMed] [Google Scholar]
- 5.Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: Systematic review and meta-analysis. Prev Med 2017;99:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gellad WF, Grenard JL, Marcum ZA. A systematic review of barriers to medication adherence in the elderly: looking beyond cost and regimen complexity. Am J Geriatr Pharmacother 2011;9:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhry NK, Denberg TD, Qaseem A, Clinical Guidelines Committee of American College of P. Improving Adherence to Therapy and Clinical Outcomes While Containing Costs: Opportunities From the Greater Use of Generic Medications: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 2016;164:41–49. [DOI] [PubMed] [Google Scholar]
- 8.Doucette WR, Farris KB, Youland KM, Newland BA, Egerton SJ, Barnes JM. Development of the Drug Adherence Work-up (DRAW) tool. J Am Pharm Assoc 2012;52:e199–204. [DOI] [PubMed] [Google Scholar]
- 9.Newman-Casey PA, Robin AL, Blachley T, Farris K, Heisler M, Resnicow K, Lee PP. The Most Common Barriers to Glaucoma Medication Adherence: A Cross-Sectional Survey. Ophthalmology 2015;122:1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vawter L, Tong X, Gemilyan M, Yoon PW. Barriers to antihypertensive medication adherence among adults--United States, 2005. J Clin Hypertens (Greenwich) 2008;10:922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Bae YH, Worley M, Law A. Validating the Modified Drug Adherence Work-Up (M-DRAW) Tool to Identify and Address Barriers to Medication Adherence. Pharmacy (Basel) 2017;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhry NK, Isaac T, Lauffenburger JC, Gopalakrishnan C, Lee M, Vachon A, Iliadis TL, Hollands W, Elman S, Kraft JM, Naseem S, Doheny S, Lee J, Barberio J, Patel L, Khan NF, Gagne JJ, Jackevicius CA, Fischer MA, Solomon DH, Sequist TD. Effect of a Remotely Delivered Tailored Multicomponent Approach to Enhance Medication Taking for Patients With Hyperlipidemia, Hypertension, and Diabetes: The STIC2IT Cluster Randomized Clinical Trial. JAMA Intern Med 2018;178:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhry NK, Isaac T, Lauffenburger JC, Gopalakrishnan C, Khan NF, Lee M, Vachon A, Iliadis TL, Hollands W, Doheny S, Elman S, Kraft JM, Naseem S, Gagne JJ, Jackevicius CA, Fischer MA, Solomon DH, Sequist TD. Rationale and design of the Study of a Tele-pharmacy Intervention for Chronic diseases to Improve Treatment adherence (STIC2IT): A cluster-randomized pragmatic trial. Am Heart J 2016;180:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, Meli SM, Chaisson CE, Samet JH. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA 2014;312:502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan DC, Shrank WH, Cutler D, Jan S, Fischer MA, Liu J, Avorn J, Solomon D, Brookhart MA, Choudhry NK. Patient, physician, and payment predictors of statin adherence. Med Care 2010;48:196–202. [DOI] [PubMed] [Google Scholar]
- 16.Franklin JM, Gopalakrishnan C, Krumme AA, Singh K, Rogers JR, Kimura J, McKay C, McElwee NE, Choudhry NK. The relative benefits of claims and electronic health record data for predicting medication adherence trajectory. Am Heart J 2018;197:153–162. [DOI] [PubMed] [Google Scholar]
- 17.Sun JW, Rogers JR, Her Q, Welch EC, Panozzo CA, Toh S, Gagne JJ. Adaptation and Validation of the Combined Comorbidity Score for ICD-10-CM. Med Care 2017;55:1046–1051. [DOI] [PubMed] [Google Scholar]
- 18.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44–47. [DOI] [PubMed] [Google Scholar]
- 19.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288:455–461. [DOI] [PubMed] [Google Scholar]
- 20.Choudhry NK, Shrank WH, Levin RL, Lee JL, Jan SA, Brookhart MA, Solomon DH. Measuring concurrent adherence to multiple related medications. Am J Manag Care 2009;15:457–464. [PMC free article] [PubMed] [Google Scholar]
- 21.Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, Lewek P, Matyjaszczyk M, Mshelia C, Clyne W, Aronson JK, Urquhart J, ABC Project Team. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmcol 2012;73:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 2003;157:940–943. [DOI] [PubMed] [Google Scholar]
- 23.Abughosh SM, Wang X, Serna O, Henges C, Masilamani S, Essien EJ, Chung N, Fleming M. A Pharmacist Telephone Intervention to Identify Adherence Barriers and Improve Adherence Among Nonadherent Patients with Comorbid Hypertension and Diabetes in a Medicare Advantage Plan. J Manag Care Spec Pharm 2016;22:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witry MJ, Doucette WR, Zhang Y, Farris KB. Multiple Adherence Tool Evaluation Study (MATES). J Manag Care Spec Pharm 2014;20:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoehr GP, Lu SY, Lavery L, Bilt JV, Saxton JA, Chang CC, Ganguli M. Factors associated with adherence to medication regimens in older primary care patients: the Steel Valley Seniors Survey. Am J Geriatr Pharmacother 2008;6:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calabrese EJ. Converging concepts: adaptive response, preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res Rev 2008;7:8–20. [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi H, Fujii T, Abe N, Suzuki M, Takagi M, Mugikura S, Takahashi S, Mori E. Memory repression: brain mechanisms underlying dissociative amnesia. J Cogn Neurosci 2010;22:602–613. [DOI] [PubMed] [Google Scholar]
- 28.Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, Reisman L, Fernandes J, Spettell C, Lee JL, Levin R, Brennan T, Shrank WH, Post-Myocardial Infarction Free Rx E, Economic Evaluation T. Full coverage for preventive medications after myocardial infarction. N Engl J Med 2011;365:2088–2097. [DOI] [PubMed] [Google Scholar]
- 29.Lauffenburger JC, Lewey J, Jan S, Makanji S, Ferro CA, Krumme AA, Lee J, Ghazinouri R, Haff N, Choudhry NK. Effectiveness of Targeted Insulin-Adherence Interventions for Glycemic Control Using Predictive Analytics Among Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA Netw Open 2019;2:e190657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundar KR. Universal Screening for Social Needs in a Primary Care Clinic: A Quality Improvement Approach Using the Your Current Life Situation Survey. Perm J 2018;22:18–089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.