Abstract
Circulating tumor cells (CTCs) in blood can provide valuable information when detecting, diagnosing, and monitoring cancer. This paper describes a system that consists of a constriction-based microfluidic sensor with embedded electrodes that can detect and enumerate cancer cells in blood. The biosensor measures impedance in terms of magnitude and phase at multiple frequencies as cells transit through the constriction channel. Cancer cells deform as they move through while blood cells remain intact, thus generating differential impedance profiles that can be used for detecting and counting CTCs. Two versions of this device are reported, one where the electrodes are embedded into the disposable microfluidic channel, and the other in which the disposable chip is externally fixed to a reusable substrate housing the electrodes. Both configurations were tested by spiking breast or prostate cancer cells into murine blood, and both detected all tumor cells passing through the narrow channels while being able to differentiate between the two cell lines. The chip in its current format has a throughput of 1 μL/min. While the throughput is scalable by integrating more constriction channels in parallel, the presented assay is intended for post-enrichment label-free enumeration and characterization of CTCs.
Keywords: Circulating tumor cells, microfluidics, impedance, cancer, liquid biopsy
1. Introduction
Cancer is a global health concern and one of the leading causes of death worldwide (Bray et al. 2018; Torre et al. 2015; Torre et al. 2016). Mortality is dependent on how early one can detect the cancer and receive treatment. Current techniques for cancer detection and diagnosis are expensive, invasive, complex, and have slow response time, considering that they generally require specific equipment and trained experts to screen and diagnose cancer (Berry et al. 2006; Bleyer and Welch 2012; Siegel et al. 2012; Tabar et al. 2003; Weir et al. 2003). Therefore, there is a need for a simple, easy-to-use, and cost-effective techniques for early cancer diagnosis and monitoring treatment efficacy. Cancer progression is currently analyzed using tissue biopsies. Tissue biopsies are invasive and can be ineffective in terms of understanding the metastatic potential, disease progression, and treatment effectiveness. Additionally, biopsies require highly skilled personnel to conduct immunohistochemistry (IHC) studies, IHC staining, and readings. Recently, researchers have proven that analyzing circulating tumor cell (CTC) number is a viable substitute for tissue biopsies as a non-invasive diagnostic tool. CTCs are tumor cells that circulate throughout the body via the blood and lymphatic system and can potentially metastasize to form a secondary tumor at other distinct locations (Aceto et al. 2015; Alix-Panabières and Pantel 2016; Pantel and Speicher 2016; Wu et al. 2015). CTC count can be a good indicator of cancer prognosis, where decreasing CTC counts over time has shown to indicate a successful treatment or therapy (Aceto et al. 2015; Alix-Panabières and Pantel 2016; Pantel and Speicher 2016; Wu et al. 2015). The primary issue with CTC-related tests is their rarity as there are 1-100 CTCs in 1 mL of blood compared to about one billion blood cells in the same volume. Therefore, to count CTCs or to further analyze them, it is important to establish reliable enrichment and isolation methods.
Microfluidic technologies for cellular and bodily fluid analyses are advantageous in terms of cost, size, sample size requirement, and minimal reagent consumption (Crane et al. 2010; Jahn et al. 2008; Ohno et al. 2008; Situma et al. 2006; Yun et al. 2013). Therefore, various microfluidic devices have been designed and fabricated for the study of CTCs (Dong et al. 2013; Hajba and Guttman 2014; Li et al. 2013; Myung and Hong 2015). Research was done to enrich blood samples and isolate CTCs. CTC analysis can be divided into two main categories: label-based and label-free. Label-based methods rely on the binding of surface protein markers of CTCs and molecular markers such as antibodies, transferrin, and peptides. These techniques utilize nanoparticles to bind to CTCs for positive sorting/isolation and bind to leukocytes for negative sorting/isolation. The epithelial cell adhesion molecule (EpCAM) is the most commonly used biomarker but it cannot capture the entire CTC population in blood as some do not express EpCAM (Alunni-Fabbroni and Sandri 2010; Hajba and Guttman 2014; Myung and Hong 2015; Yu et al. 2011). CellSearch, which relies on the EpCAM biomarker to detect CTCs, is currently the only FDA-approved CTC isolation technique but has limitations because CTCs go through an epithelial-mesenchymal transition (EMT), which decreases the expression of EpCAM and other epithelial markers (Andree et al. 2016; Miller et al. 2010; Riethdorf et al. 2007).
Label-free technologies rely on biophysical properties of cells such as size, density, deformability, and dielectric properties. Some label-free isolation techniques include size/deformability-based micropores (Asghar et al. 2012), inertial microfluidics (Sollier et al. 2014), acoustophoresis (Antfolk et al. 2015; Augustsson et al. 2012), magnetophoresis (Karabacak et al. 2014), and dielectrophoresis (Alshareef et al. 2013; Gascoyne and Shim 2014). These techniques are advantageous because they do not rely on extensive pre-processing and incubation times, as well as being cost-effective. Label-free CTC isolation techniques have improved greatly over the last decade and typically are tested by spiking beads, murine blood, or human blood samples with cancer cells. Antfolk et al., for instance, has shown that acoustophoresis technology can capture ~95% of spiked cancer cells in RBC-lysed whole blood with a purity of >97% at a flow rate of 100 μL/min (Antfolk et al. 2015). Our group has also used a size-based microfluidic chip with sequential constriction channels separated by entrapment regions to capture ~96% of cancer cells spiked into whole murine blood at a flow rate of ~2.5 mL/hr (Ren et al. 2018a). Researchers have begun to create hybrid technologies too that include both label-free and label-based methods to improve efficiency. For example, Toner’s group created the CTC-iChip that first uses deterministic lateral displacement to remove most blood cells, followed by negative filtration of the remaining WBCs through magnetophoresis utilizing anti-CD45 and anti-CD66 beads (Karabacak et al. 2014).
There is a wealth of knowledge demonstrating that the CTC count can be used to diagnose cancer and evaluate the prognosis and progression of tumors (Budd et al. 2006; De Bono et al. 2008; Miller et al. 2010; Paterlini-Brechot and Benali 2007). For instance, Miller et al. evaluated CTC count by analyzing 7.5 mL of whole blood from breast, prostate, or colorectal cancer patients using CellSearch. They determined that for metastatic breast and prostate cancer, the cutoff value for favorable survival is 5 CTCs per mL, while in metastatic colorectal cancer patients it was 3 CTCs per 7.5 mL of blood (Miller et al. 2010). The current practice of CTC enumeration, regardless of the enrichment technique, relies primarily on fluorescent microscopy where CTC-specific (such as EpCAM+/CK+/CD45−) antibodies conjugated to fluorescent dyes attach to cells of interest (Lim et al. 2012; Lu et al. 2013; Sonn et al. 2017; Swennenhuis et al. 2009). As a result, even if the enrichment technique is label-free, postprocessing of CTCs is not. Microfluidic impedance cytometry, which relies on cellular bioelectric properties, can be an alternative approach to fluorescent microscopy for rapid enumeration of CTCs (Choi et al. 2013; Spencer et al. 2014). Furthermore, blood cells are in general smaller than CTCs, indicating that CTCs and blood cells when passing through channels with dimensions less than 8 μm will generate two distinct timing profiles. The deformability of cells through such constriction channels has been used by our group and others to distinguish tumor cells from non-tumorigenic cells (Babahosseini et al. 2017; Ren et al. 2017; Ren et al. 2018b) as well as control and drug-treated tumor cells (Babahosseini et al. 2016). Integration of microfluidic impedance spectroscopy with constriction channels provides information on comprehensive biophysical attributes of cells such as size and mechanical and electrical properties of cells (Babahosseini et al. 2016; Chen et al. 2011; Cheung et al. 2010; Song et al. 2013; Zhao et al. 2013). Here we, for the first time, demonstrate that such unique integrated platforms can be used to rapidly enumerate CTCs in blood samples and generate/process the data in less than one minute, thus eliminating fluorescent tagging and extensive video analysis. Both prostate and breast cancer cell lines were used in this study to see if the bioassay can be employed for more than one tumor type and if it can distinguish the type of tumor through biophysical properties. In this microfluidic CTC analyzer, postprocessing of the data is also automated to detect the presence of CTCs, their count, and their origin. Two different chips are presented. In one, the electrodes are part of the disposable chip providing more sensitivity. The other separates the electrodes from the disposable chip to reduce the fabrication cost while still allowing CTC enumeration.
2. Materials and Methods
2.1. Cell Culture and Sample Preparation
To test our microfluidic biosensor, we used single-cell suspensions of both breast and prostate cancer cell lines (MDA-MB-231 and LNCaP C4-2, respectively). Breast cancer cell line MDA-MB-231 (passage #8, American Type Culture Collection (ATCC), provided by Dr. Yasmine Kanaan, Howard University College of Medicine) was grown in F12:DMEM (Lonza, Basel, Switzerland) with 10% fetal bovine serum (FBS), 4 mM glutamine and penicillin-streptomycin (100 units per mL). Prostate cancer cell line LNCaP-C4-2 (passage #11, provided by Dr. Bethany Kerr, Wake Forest School of Medicine) expressing green fluorescence protein (GFP) by lentiviral transduction, was grown in RPMI-1640 (L-glutamine) with 10% FBS and 1% PenStrep (100 U/mL penicillin and 100 μg/mL streptomycin). Both cell lines were grown in T-25cm2 culture flasks at 37°C in a 5% CO2 in air atmosphere until cells were ready for subculture. The morphology of the cells was observed before trypsinization. The cells were then detached from the flask with a trypsin-EDTA solution (Sigma Aldrich). After trypsinization, cells were spun down in the centrifuge and rinsed with phosphate-buffered silane 1x (PBS). The cells were then spun down and resuspended in PBS for experimentation. Before experimentation, cell viability was observed at 100% via trypan blue exclusion test. For experimentation with spiked blood samples, murine whole blood was diluted with PBS (1:5 ratio), and the blood sample is spiked with cancer cells at ~104 cells/mL. Murine whole blood was collected under Wake Forest School of Medicine IACUC approval #A15-221 from the vena cava of anesthetized mice into 1M ethylenediaminetetraacetic acid.
2.2. Device Fabrication
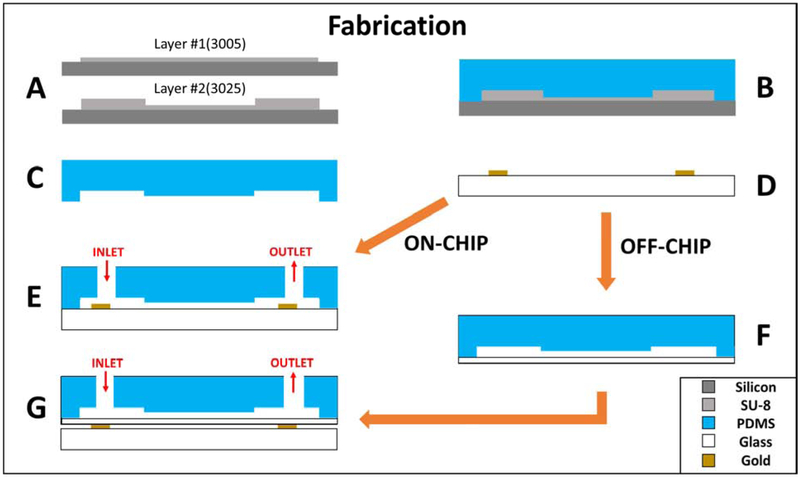
To fabricate the polydimethylsiloxane (PDMS) microfluidic channels, a master mold is fabricated onto a silicon wafer. Two layers of SU-8 (SU-8 3005 and SU-8 3025, MicroChem, Newton, MA) are patterned onto the wafer via photolithography (Figure 1A). SU-8 3005 was used to build the constriction channel with 8 μm height, while SU-8 3025 was used to build the remaining channel feature with a 25 μm height. The resulting mold is coated with tridecafluoro-1,1,2,2-tetrahydrooctyl-1-trichlorosilane (TFOCS, Fisher Scientific) to promote the easy release of PDMS. PDMS base and curing agent (10:1 weight ratio) are stirred generously and poured onto the master mold and cured at 65° C for 24 hours (Figure 1B and 1C). For the “on-chip” case, the resulting PDMS channels are then bonded directly onto the planar electrodes using plasma-activated bonding (Figure 1E). The alternative “off-chip” device involves bonding the channel onto a 100 μm thick glass slide (#0, Electron Microscopy Sciences, USA), which acts as a passivation layer between the electrodes and microfluidic channel (Figure 1F). Details of the fabrication process have been previously published by our lab (Soltanian-Zadeh et al. 2017; Zellner et al. 2013) and is illustrated in Figure 1. The cross-section of the constriction across the length of the channel for both cases is depicted in Figure 1E and Figure 1G. To fabricate the electrode sensors, a pyrex glass wafer is patterned with photoresist (S1827, MicroChem, Newton, MA) through photolithography. Metal deposition through electron beam evaporation of chrome (40 μm), an adhesion layer, and gold (100 μm) is done onto the patterned glass wafer. The glass wafer is then submerged into acetone for metal liftoff and diced to create individual electrodes (Figure 1D).
Figure 1.
Fabrication steps(A-G) of both (E) ON-CHIP and (G) OFF-CHIP devices. Device perspective is the cross-section across the length of the constriction channel.
2.3. Channel Design and Experimental Setup
The microfluidic device consists of two main channels, delivery, and constriction, to prevent cell accumulation at the entrance of the constriction and prohibits clogging. The constriction channel has a 6 μm-width, 8 μm-height, and 500 μm-length. Both electrodes extend approximately 60 μm from the entrance and exit of the constriction channel.
The cell suspension is inserted in the delivery channel and flow is induced by applying a negative pressure, via a Harvard Apparatus syringe pump, at the other end of the delivery channel. A secondary negative pressure is applied using the syringe pump at the outlet of the constriction channel to create flow through the constriction. Once a cell has entered the constriction channel, the cell blocks the negative pressure the delivery channel experiences and prevents other cells from entering the channel until it has passed through the constriction. The device was able to collect impedance peaks of cells that can reach 100+ cells per minute.
As cells transit through the constriction channel, impedance information is obtained using a Zurich Instruments HF2IS Impedance Spectroscope. The impedance spectroscope inputs an AC voltage of 1V signal at eight different frequencies ranging from 500 Hz to 1 MHz simultaneously. To cross-verify the transit of cells in the constriction channel with the impedance data, we recorded high-speed videos at 100 frames/second.
3. Results
3.1. Data Collection
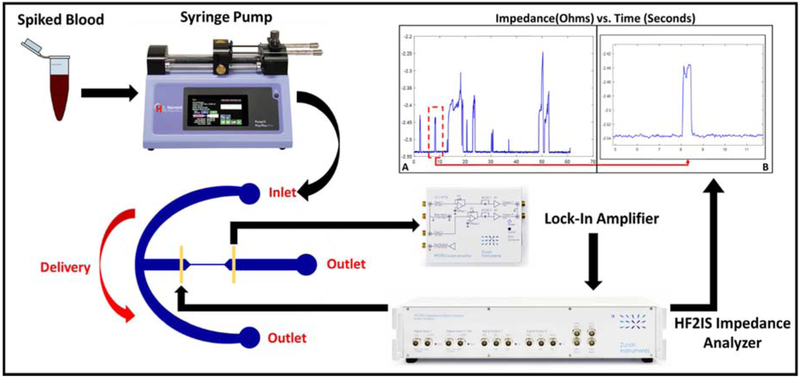
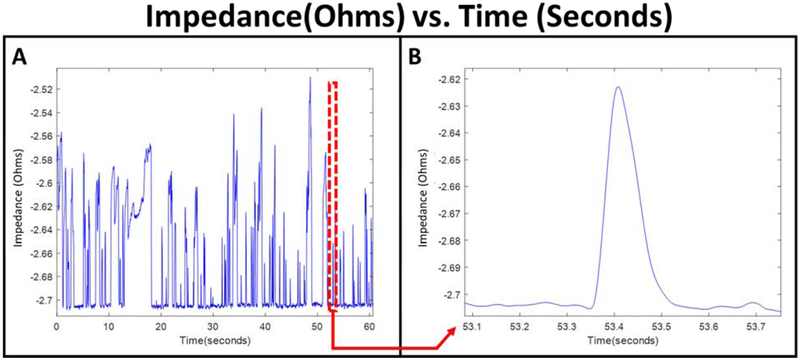
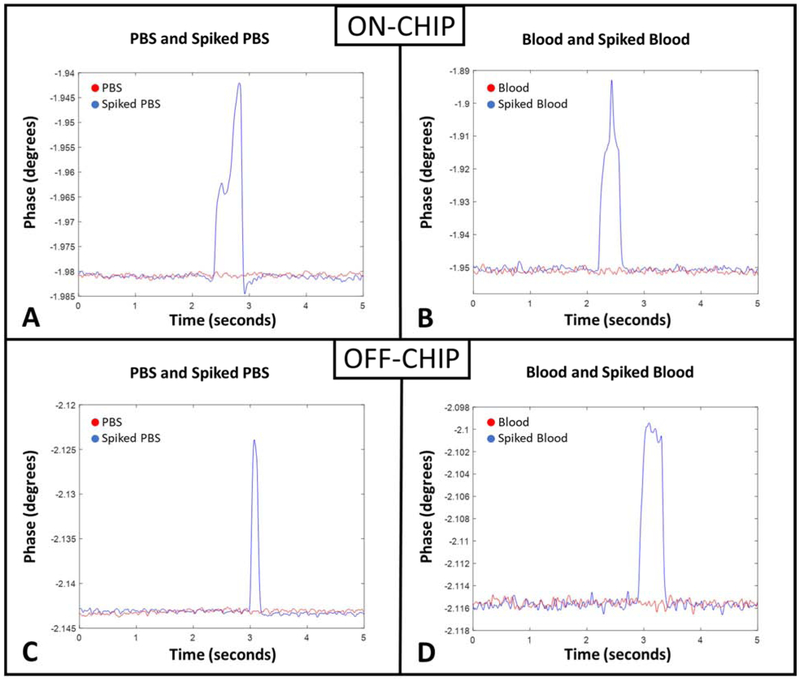
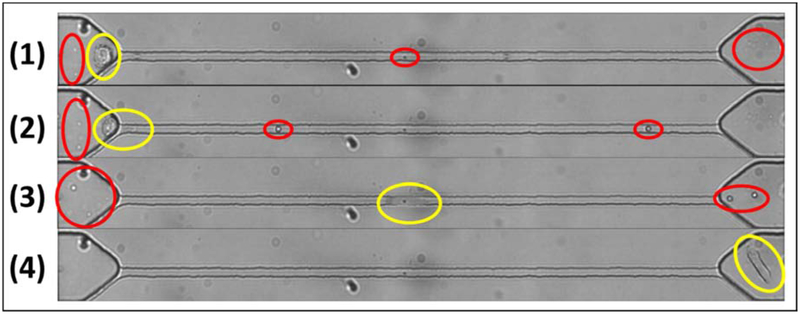
Figure 2 demonstrates the experimental setup where the sample enters the device and transits through the delivery, and consequently the constriction channel via a negative pressure applied at the outlet to initiate the flow. The device has an embedded pair of planar electrodes that extend outside the entrance and exit of the constriction channel. Therefore, impedance can be measured across the constriction channel, so when a cell enters the channel, we can obtain the impedance of the cell along with the surrounding medium (murine blood or PBS). CTCs within the constriction channel dominate the impedance in the channel compared to the surrounding medium, so we can obtain a clear peak that represents the CTC transiting through the channel. Figure 3A illustrates a sample run, where many (enriched) cells are observed within a minute. The transit of a single cell is shown in Figure 3B, where a cell is represented by a single peak. For the “on-chip” case, as PBS alone passes through the constriction, there is a constant baseline, which is shown by the red line in Figure 4A. However, for the sample of PBS spiked with cancer cells (see Supplementary Video S1), once a cell enters and transits through the constriction channel, the impedance deviates from the baseline towards a peak and returns to the baseline after transit. This is illustrated by the blue line in Figure 4A. In the case of blood and blood spiked with cancer cells (see Supplementary Video S2 and S3, respectively), the resulting impedance profile is the same, but the baseline is noisier (shown in Figure 4B) because of the passage of blood cells through the constriction. Figure 5 shows the transit of a cancer cell from a spiked blood sample, where blood cells (circled in red) surround the cancer cell (circled in yellow), as it transits through the constriction channel. Figure 5 begins with the cancer cell before deformation (1), then the cell beginning to deform into constriction channel (2), followed by the cell transiting through the constriction channel (3), and lastly the cell after exiting the constriction (4). The blood cells impact the baseline and noise level of the signal, however, the impedance profile of cancer cells transiting through the constriction channel is robust.
Figure 2.
The experimental setup for CTC analysis by deformability impedance spectroscopy. The impedance results reflect the ON-CHIP device where we evaluate MDA-MB-231 cells spiked in murine blood.
Figure 3.
Sample impedance plot of the ON-CHIP configuration evaluating MDA-MB-231 cells spiked in murine blood during a single run (A) and an example of the impedance of a single cancer cell (B).
Figure 4.
Representation of impedance measured across the constriction channel. (A) ON-CHIP and (C) OFF-CHIP sample of impedance (Phase at frequency = 50 kHz) with only PBS (red) and PBS spiked with MDA-MB-231 (blue). (B) ON-CHIP and (D) OFF-CHIP sample (Phase at frequency = 50 kHz) with only murine blood (red) and murine blood spiked with MDA-MB-231 (blue).
Figure 5.
Transit of a MDA-MB-231 cell (circled in yellow) through the constriction region. (1) Cancer cell before deformation, (2) cell beginning to deform, (3) cell in constriction channel, and (4) cell after the constriction. The surrounding white and red blood cells indicated by the red circles.
The impedance profiles for the “off-chip” device are shown in Figure 4C and 4D, where the baseline is clearly noisier compared to the “on-chip” device. Despite the loss of sensitivity due to the passivation layer, the cells of interest are still easily detected for both PBS (Figure 4C) and blood (Figure 4D) spiked with cancer cells. Although the noise level has increased in the spiked blood sample, the biosensors were sensitive enough to detect 100% of the cancer cells that transit through the constriction channel in both “on-chip” and “off-chip” scenarios.
3.2. Data Analysis
The impedance peak is a representation of the electrical properties of the cell and can be used to count, evaluate, and distinguish these cells of interest. We obtained the impedance results of breast and prostate cancer cells individually spiked into PBS and whole murine blood for both the “on-chip” and “off-chip” cases. Each experiment was repeated on multiple devices to reduce the effects of device-to-device variation. Impedance data were collected in an automated fashion utilizing a MATLAB script to collect peaks with respect to the baseline. The threshold for determining peaks was any peak greater than two times the maximum noise variation of the sample without spiked cancer cells.
To evaluate the impedance data of the cell populations, we plotted histograms to show the distribution of magnitude and phase. Histograms were used to evaluate the cell population rather than the average/standard deviation since histograms illustrate the distribution of cells and reveal outliers that can influence the data. We have collected magnitude and phase data for different frequencies for every cell with redundant results, so we present data from one single frequency for each scenario for the purpose of brevity.
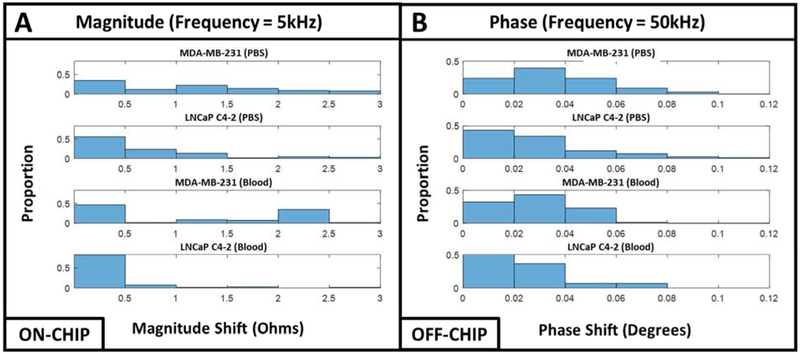
For the on-chip experiments, we evaluated the magnitude at a frequency of 5 kHz, which typically represents a combination of cell membrane properties and other biophysical properties such as cell size (Cheung et al. 2005; Heileman et al. 2013; Morgan et al. 2006). Figure 6A illustrates the histogram profile of magnitude for both MDA-MB-231 and LNCaP C4-2 spiked in PBS and murine blood. From these histogram plots, there is a clear difference between the two cell populations spiked in PBS and murine blood. For example, the cell magnitude of MDA-MB-231 and LNCaP C4-2 cells spiked into PBS at 5 kHz shows how the MDA-MB-231 cells have a bimodal distribution, where the LNCaP C4-2 cells have a unimodal distribution. This is also consistent with both cell lines spiked into the murine blood.
Figure 6.
(A) ON-CHIP histogram representation of MDA-MB-231 and LNCaP C4-2 cells spiked in PBS and blood for magnitude measured at a frequency of 5kHz. (B) OFF-CHIP histogram representation of MDA-MB-231 and LNCaP C4-2 cells spiked in PBS and blood for phase measured at a frequency of 50kHz.
For the off-chip experiments, we evaluated the phase data at a frequency of 50 kHz for both cell lines spiked in PBS and murine blood, which is depicted in Figure 6B. Just as described for the on-chip experiments, the histograms reveal differences in the cell populations for this scenario too. For example, the phase values at 50 kHz show how the MDA-MB-231 cells and LNCaP C4-2 cells both have varying distributions. For the case of the MDA-MB-231 cells in PBS, there is a chi-squared distribution, however, the LNCaP C4-2 cells in the same scenario have a right-skewed distribution. Using the same frequency but spiked in blood, the MDA-MB-231 and LNCaP C4-2 cells maintain their normal and right-skewed distribution, respectively.
4. Discussion
CTC detection and enumeration are clinically important as it can be used to detect early cancer metastasis, predict prognosis, and monitor response to treatment. Typically, methods for enumerating CTCs rely on surface markers, such as the epithelial cell adhesion molecule (EpCAM), however, a sub-population of CTCs go through epithelial-mesenchymal transition (EMT) that changes the expression of cell surface markers (Budd et al. 2006; De Bono et al. 2008; Miller et al. 2010; Paterlini-Brechot and Benali 2007). Thus, label-free methods are advantageous due to their reliance on biophysical characteristics rather than surface markers. Current label-free methods of enumeration cancer cells rely on fluorescent microscopy, but this method requires expensive pre-processing for fluorescent tagging and extensive post-processing of videos to observe and enumerate the cells (Lim et al. 2012; Lu et al. 2013; Sonn et al. 2017; Swennenhuis et al. 2009). Impedance flow cytometry methods have been developed in research (Chen et al. 2015; Cheung et al. 2010; Emaminejad et al. 2012; Gawad et al. 2001; Sun and Morgan 2010) and commercially by companies such as Amphasys (Kaufmann et al.). Despite these current methods, no technologies have attempted to detect cancer cells of different origins within blood samples. We report a system that can not only enumerate cells in blood samples but can also identify different types of cancer based on their bioelectrical properties.
Biophysical characteristics of cells extracted through label-free impedance analysis can be used to study cells in suspension for biological research and in blood for clinical applications (Chen et al. 2015; Cheung et al. 2010; Choi et al. 2013; Spencer et al. 2014; Sun and Morgan 2010). Electrical properties of cells can be used as a powerful biomarker to identify cells, including CTCs because they provide a complex representation of cells by probing them at different frequencies. Higher frequencies provide information about the contents of the cells, while lower frequencies provide information about the cell membrane and surface properties. This technique has been widely used to identify and distinguish a variety of different cells, including healthy, diseased, and drug-treated cells (Cheung et al. 2005; Heileman et al. 2013; Morgan et al. 2006).
Giaever and Keese first introduced electric cell-substrate impedance sensing (ECIS) and studied adherent mammalian fibroblasts by measuring their response to an AC signal (Giaever and Keese 1984). ECIS techniques were then used as a label-free method to measure cell electrical properties, growth and proliferation, motility, and response to chemical compounds (Campbell et al. 2007; Giaever and Keese 1984; Szulcek et al. 2014; Tran et al. 2016b; Zudaire et al. 2008). Throughput of ECIS sensing of cells was improved using microelectrode arrays, where an array of electrode pairs can measure impedance in up to hundreds of individual locations simultaneously (Anh-Nguyen et al. 2016; Liu et al. 2009; Robilliard et al. 2018; Tran et al. 2016a). Our lab has utilized ECIS to study the effects of anti-cancer drug SAHA on two breast cell lines (Srinivasaraghavan et al. 2012), to study various nano-scale coating of electrodes (Srinivasaraghavan et al. 2014), and to distinguish basal and claudin-low subtypes of triple negative breast cancer cells (Srinivasaraghavan et al. 2015). Impedance spectroscopy has not only been used in research but also in commercialized settings to monitor cellular behavior as a replacement to more conventional fluorescence microscopy. For instance, commercial ECIS-based techniques developed by companies such as xCELLigence and Applied Biophysics have developed label-free assays to continuously monitor live cell proliferation, morphology, and viability (Bird and Kirstein 2009; Daza et al. 2013; Ke et al. 2011; Shih et al. 2013). Limitations of ECIS render this method irrelevant for some clinical applications because ECIS requires the cells to be adherent for its electrical properties to be probed for their impedance values. Lengthy experimentation time is another issue, but even more limiting is the fact that ECIS is generally used for studying cell populations (thousands of cells) and CTCs that are isolated range from a few cells to up to one hundred, in rare cases, cells per mL of blood. To alleviate these issues, electrodes have been integrated into microfluidics to study the electrical properties of particles flowing through microchannels (Ayliffe et al. 1999; Chen et al. 2015; Cheung et al. 2010; Emaminejad et al. 2012; Gawad et al. 2001; Kaufmann et al. ; Sun and Morgan 2010). Ayliffe et al. introduced the first microfluidic device with embedded electrodes to obtain bioelectrical information on single cells (Ayliffe et al. 1999).
Constriction-based microfluidics for studying cell samples can provide mechanical properties of cells by studying transit times through the deformation channel. Cell mechanical proprieties are evaluated by observing the time it takes for a cell to enter (entry time) and transit (transit time) through the constriction channel (Adamo et al. 2012; Bow et al. 2011). Our group has used constriction-based microfluidics to distinguish cancer and normal cells through repetitive deformation of cells (Babahosseini et al. 2016; Babahosseini et al. 2017; Ren et al. 2017; Ren et al. 2018b). The approach was then extended to trap and enrich CTCs in whole blood (Ren et al. 2018a). Utilizing constriction-based microfluidics is advantageous compared to alternative label-free methods such as atomic force microscopy (Kirmizis and Logothetidis 2010) and optical stretching (Guck et al. 2005; Van Vliet et al. 2003).
Embedding electrodes in constriction-based devices provides impedance data that can be used to both obtain and evaluate electrical and mechanical properties simultaneously. Impedance peaks represent cell electrical properties, and peak widths represent cell transit time, which is dependent on cell deformability, and both parameters are influenced by cell size (Babahosseini et al. 2016; Chen et al. 2011; Cheung et al. 2010; Song et al. 2013; Zhao et al. 2013). Using impedance data to obtain cell timing information alleviates issues experienced when obtaining cell transit times through video analysis. Typically, extensive post processing is needed to study high-speed videos frame-by-frame to capture timing data, whereas transit time can be simply obtained through peak widths. Obtaining this timing information from impedance can also be automated, which exponentially decreases post-processing times. In our control experiment where only blood sample was moving through the channel, the cells moving very fast without deformation and hence no considerable peak was generated beyond the noise level. We simply used a signal-to-noise ratio of 2-to-1, and we were able to detect all cancer cells without incorrectly identifying any blood cell as a tumor cell. Because we can detect all cancer cells regardless of origin in a blood sample, our biosensor can be used subsequently to established CTC isolation technologies that have a higher capture rate but lower purity. Also, an expanded higher throughput version of this device multiple constriction channels in parallel can be employed that further approaches a clinically-ready device.
This is the first system to use constriction-based impedance data to evaluate cancer cells in blood. Spencer et al. has previously conducted microfluidic impedance cytometry on tumor cells in blood, however, the samples they compared were blood spiked with beads, and breast cancer cells (MCF-7) spiked in varying counts of white blood cells yielded from lysed whole blood (Spencer et al. 2014). Although they were able to detect the cancer cells within a blood sample, their results have some false positives of MCF-7 cells, and they do not indicate the identification of different types of cancer cells. The ability to identify different types of cancer is valuable in clinical applications because it can potentially be used to replace the more invasive and potentially dangerous biopsies, and imaging tests, such as a computerized tomography scans, magnetic resonance imaging, positron emission tomography scans, or X-rays (Chappy 2004; Harnden et al. 2008; Loeb et al. 2013; Smith et al. 2012). Our system identified cancer cells from two different origins spiked in murine blood samples; however, its clinical applicability needs to be verified by processing blood samples from patients with different tumor types. Additionally, there is a potential for false positives when testing patient samples due to non-blood and non-tumor cells that circulate throughout the blood, such as circulating epithelial cells (Paterlini-Brechot and Benali 2007). There may be cases that produce impedance peaks and phase shifts comparable to those of CTCs. If that occurs, we may need to combine the approach presented in this paper with our previous work in which we employed microfluidic chips with multiple constrictions. Our work has shown that we can distinguish between normal and cancer cells at the single cell level with more than 90% accuracy when using sectional cell transit velocities and bioimpedance information when cells move through repetitive deformations (Babahosseini et al. 2016; Babahosseini et al. 2017; Ren et al. 2017; Ren et al. 2018b; Ren et al. 2019). Nevertheless, the results presented here establishes the foundation that microfluidic deformability assay equipped with embedded electrodes can be used to monitor the presence of CTCs in blood samples. The measured sensitivity is high enough that allows the electrodes to be positioned off-chip and be reusable to lower the cost of the assay.
5. Conclusions
To conclude, we developed a label-free constriction-based CTC detection and enumeration assay coupled with sensors to conduct impedance spectroscopy on murine blood samples spiked with breast and prostate cancer cells. From the impedance data and the corresponding video analysis, we were able to determine that readily distinguishable electronic signals generated by our chips correlate with the passage of cancer cells through the constriction channel. Tumor cell transitioning through the constriction channel are represented by peaks that were collected using an automated peak detection script using MATLAB. In addition, we were able to distinguish breast and prostate cancer cells in murine blood samples, demonstrating that this system can potentially be used for a variety of cancer cell types. The current design dimensions can easily be modified to allow our system to be used on human blood samples. Throughput of this device will be improved in future generations by adding multiple channels in parallel. If needed for more heterogeneous cell populations, information regarding the mechanical properties of cells, such as transit times, can also be extracted and employed for more accurate determination of CTCs, their clusters, or their origins. To conclude, the biosensors mentioned in this paper show promise for label-free detection and enumeration of CTCs after currently established CTC enrichment techniques.
Supplementary Material
Highlights.
Circulating tumor cells detected from whole murine at a rate of 100%
Impedance can distinguish breast & prostate cancer cells in blood samples
Low-cost OFF-CHIP device maintains sensitivity to detect all cancer cells in blood
Acknowledgments:
The authors acknowledge the Micro & Nano Fabrication Laboratory at Virginia Tech for the equipment support.
Funding: This research was funded by the National Institute of Health (NIH) National Cancer Institute, grant number R21CA210216.
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Materials: Video S1: MDA-MB-231 spiked in PBS, Video S2: Murine blood, Video S3: MDA-MB-231 spiked in murine blood.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aceto N, Toner M, Maheswaran S, Haber DA, 2015. Trends in cancer 1(1), 44–52. [DOI] [PubMed] [Google Scholar]
- Adamo A, Sharei A, Adamo L, Lee B, Mao S, Jensen KF, 2012. Analytical chemistry 84(15), 6438–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alix-Panabières C, Pantel K, 2016. Cancer discovery 6(5), 479–491. [DOI] [PubMed] [Google Scholar]
- Alshareef M, Metrakos N, Juarez Perez E, Azer F, Yang F, Yang X, Wang G, 2013. Biomicrofluidics 7(1), 011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alunni-Fabbroni M, Sandri MT, 2010. Methods 50(4), 289–297. [DOI] [PubMed] [Google Scholar]
- Andree KC, van Dalum G, Terstappen LW, 2016. Molecular oncology 10(3), 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anh-Nguyen T, Tiberius B, Pliquett U, Urban GA, 2016. Sensors and Actuators A: Physical 241, 231–237. [Google Scholar]
- Antfolk M, Antfolk C, Lilja H, Laurell T, Augustsson P, 2015. Lab on a chip 15(9), 2102–2109. [DOI] [PubMed] [Google Scholar]
- Asghar W, Wan Y, Ilyas A, Bachoo R, Kim Y.-t., Iqbal SM, 2012. Lab on a Chip 12(13), 2345–2352. [DOI] [PubMed] [Google Scholar]
- Augustsson P, Magnusson C, Nordin M, Lilja H, Laurell T, 2012. Analytical chemistry 84(18), 7954–7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayliffe HE, Frazier AB, Rabbitt R, 1999. Journal of Microelectromechanical systems 8(1), 50–57. [Google Scholar]
- Babahosseini H, Srinivasaraghavan V, Zhao Z, Gillam F, Childress E, Strobl JS, Santos WL, Zhang C, Agah M, 2016. Lab on a Chip 16(1), 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babahosseini H, Strobl JS, Agah M, 2017. Analytical Methods 9(5), 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DA, Inoue L, Shen Y, Venier J, Cohen D, Bondy M, Theriault R, Munsell MF, 2006. JNCI Monographs 2006(36), 30–36. [DOI] [PubMed] [Google Scholar]
- Bird C, Kirstein S, 2009. Nature methods 6(8), 622. [Google Scholar]
- Bleyer A, Welch HG, 2012. New England Journal of Medicine 367(21), 1998–2005. [DOI] [PubMed] [Google Scholar]
- Bow H, Pivkin IV, Diez-Silva M, Goldfless SJ, Dao M, Niles JC, Suresh S, Han J, 2011. Lab on a Chip 11(6), 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, 2018. CA: a cancer journal for clinicians 68(6), 394–424. [DOI] [PubMed] [Google Scholar]
- Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen LW, 2006. Clinical Cancer Research 12(21), 6403–6409. [DOI] [PubMed] [Google Scholar]
- Campbell CE, Laane MM, Haugarvoll E, Giaever I, 2007. Biosensors and Bioelectronics 23(4), 536–542. [DOI] [PubMed] [Google Scholar]
- Chappy SL, 2004. AORN journal 80(5), 885–901. [DOI] [PubMed] [Google Scholar]
- Chen J, Xue C, Zhao Y, Chen D, Wu M-H, Wang J, 2015. International journal of molecular sciences 16(5), 9804–9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zheng Y, Tan Q, Shojaei-Baghini E, Zhang YL, Li J, Prasad P, You L, Wu Y, Sun Y, 2011. Lab on a Chip 11(18), 3174–3181. [DOI] [PubMed] [Google Scholar]
- Cheung K, Gawad S, Renaud P, 2005. Cytometry Part A 65(2), 124–132. [DOI] [PubMed] [Google Scholar]
- Cheung KC, Di Berardino M, Schade-Kampmann G, Hebeisen M, Pierzchalski A, Bocsi J, Mittag A, Tárnok A, 2010. Cytometry Part A 77(7), 648–666. [DOI] [PubMed] [Google Scholar]
- Choi H, Kim KB, Jeon CS, Hwang I, Lee S, Kim HK, Kim HC, Chung TD, 2013. Lab on a Chip 13(5), 970–977. [DOI] [PubMed] [Google Scholar]
- Crane MM, Chung K, Stirman J, Lu H, 2010. Lab on a Chip 10(12), 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daza P, Olmo A, Canete D, Yufera A, 2013. Sensors and Actuators B: Chemical 176, 605–610. [Google Scholar]
- De Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D, 2008. Clinical cancer research 14(19), 6302–6309. [DOI] [PubMed] [Google Scholar]
- Dong Y, Skelley AM, Merdek KD, Sprott KM, Jiang C, Pierceall WE, Lin J, Stocum M, Carney WP, Smirnov DA, 2013. The Journal of Molecular Diagnostics 15(2), 149–157. [DOI] [PubMed] [Google Scholar]
- Emaminejad S, Javanmard M, Dutton RW, Davis RW, 2012. Lab on a Chip 12(21), 4499–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoyne P, Shim S, 2014. Cancers 6(1), 545–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawad S, Schild L, Renaud P, 2001. Lab on a Chip 1(1), 76–82. [DOI] [PubMed] [Google Scholar]
- Giaever I, Keese CR, 1984. Proceedings of the National Academy of Sciences 81(12), 3761–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R, Mitchell D, 2005. Biophysical journal 88(5), 3689–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajba L, Guttman A, 2014. TrAC trends in analytical chemistry 59, 9–16. [Google Scholar]
- Harnden P, Naylor B, Shelley MD, Clements H, Coles B, Mason MD, 2008. Cancer: Interdisciplinary International Journal of the American Cancer Society 112(5), 971–981. [DOI] [PubMed] [Google Scholar]
- Heileman K, Daoud J, Tabrizian M, 2013. Biosensors and Bioelectronics 49, 348–359. [DOI] [PubMed] [Google Scholar]
- Jahn A, Reiner JE, Vreeland WN, DeVoe DL, Locascio LE, Gaitan M, 2008. Journal of Nanoparticle Research 10(6), 925–934. [Google Scholar]
- Karabacak NM, Spuhler PS, Fachin F, Lim EJ, Pai V, Ozkumur E, Martel JM, Kojic N, Smith K, Chen P.-i., 2014. Nature protocols 9(3), 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S, Schade G, Di Berardino M, AG A
- Ke N, Wang X, Xu X, Abassi YA, 2011. Mammalian Cell Viability, pp. 33–43. Springer. [DOI] [PubMed] [Google Scholar]
- Kirmizis D, Logothetidis S, 2010. International journal of nanomedicine 5, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Stratton ZS, Dao M, Ritz J, Huang TJ, 2013. Lab on a Chip 13(4), 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LS, Hu M, Huang MC, Cheong WC, Gan ATL, Looi XL, Leong SM, Koay ES-C, Li M-H, 2012. Lab on a Chip 12(21), 4388–4396. [DOI] [PubMed] [Google Scholar]
- Liu Q, Yu J, Xiao L, Tang JCO, Zhang Y, Wang P, Yang M, 2009. Biosensors and Bioelectronics 24(5), 1305–1310. [DOI] [PubMed] [Google Scholar]
- Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, Rosario DJ, Scattoni V, Lotan Y, 2013. European urology 64(6), 876–892. [DOI] [PubMed] [Google Scholar]
- Lu Y-T, Zhao L, Shen Q, Garcia MA, Wu D, Hou S, Song M, Xu X, OuYang W-H, OuYang WW-L, 2013. Methods 64(2), 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MC, Doyle GV, Terstappen LW, 2010. Journal of oncology 2010, 617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan H, Sun T, Holmes D, Gawad S, Green NG, 2006. Journal of Physics D: Applied Physics 40(1), 61. [Google Scholar]
- Myung J, Hong S, 2015. Lab on a Chip 15(24), 4500–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K.i., Tachikawa K, Manz A, 2008. Electrophoresis 29(22), 4443–4453. [DOI] [PubMed] [Google Scholar]
- Pantel K, Speicher M, 2016. Oncogene 35(10), 1216. [DOI] [PubMed] [Google Scholar]
- Paterlini-Brechot P, Benali NL, 2007. Cancer letters 253(2), 180–204. [DOI] [PubMed] [Google Scholar]
- Ren X, Foster BM, Ghassemi P, Strobl JS, Kerr BA, Agah M, 2018a. Analytical chemistry 90(12), 7526–7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Ghassemi P, Babahosseini H, Strobl JS, Agah M, 2017. ACS sensors 2(2), 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Ghassemi P, Kanaan YM, Naab T, Copeland RL, Dewitty RL, Kim I, Strobl JS, Agah M, 2018b. ACS sensors 3(8), 1510–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Ghassemi P, Strobl JS, Agah M, 2019. Biomicrofluidics 13(4), 044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethdorf S, Fritsche H, Müller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, 2007. Clinical cancer research 13(3), 920–928. [DOI] [PubMed] [Google Scholar]
- Robilliard L, Kho D, Johnson R, Anchan A, O’Carroll S, Graham E, 2018. Biosensors 8(3), 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih SC, Barbulovic-Nad I, Yang X, Fobel R, Wheeler AR, 2013. Biosensors and Bioelectronics 42, 314–320. [DOI] [PubMed] [Google Scholar]
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, 2012. CA: a cancer journal for clinicians 62(4), 220–241. [DOI] [PubMed] [Google Scholar]
- Situma C, Hashimoto M, Soper SA, 2006. Biomolecular engineering 23(5), 213–231. [DOI] [PubMed] [Google Scholar]
- Smith RA, Cokkinides V, Brawley OW, 2012. CA: a cancer journal for clinicians 62(2), 129–142. [DOI] [PubMed] [Google Scholar]
- Sollier E, Go DE, Che J, Gossett DR, O'Byrne S, Weaver WM, Kummer N, Rettig M, Goldman J, Nickols N, 2014. Lab on a Chip 14(1), 63–77. [DOI] [PubMed] [Google Scholar]
- Soltanian-Zadeh S, Kikkeri K, Shajahan-Haq AN, Strobl J, Clarke R, Agah M, 2017. Electrophoresis 38(16), 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Wang Y, Rosano JM, Prabhakarpandian B, Garson C, Pant K, Lai E, 2013. Lab on a Chip 13(12), 2300–2310. [DOI] [PubMed] [Google Scholar]
- Sonn CH, Cho JH, Kim JW, Kang MS, Lee J, Kim J, 2017. Oncology letters 13(4), 2717–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D, Hollis V, Morgan H, 2014. Biomicrofluidics 8(6), 064124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasaraghavan V, Strobl J, Agah M, 2012. Lab on a Chip 12(24), 5168–5179. [DOI] [PubMed] [Google Scholar]
- Srinivasaraghavan V, Strobl J, Agah M, 2015. Biomedical microdevices 17(4), 80. [DOI] [PubMed] [Google Scholar]
- Srinivasaraghavan V, Strobl J, Wang D, Heflin JR, Agah M, 2014. Biomedical microdevices 16(5), 689–696. [DOI] [PubMed] [Google Scholar]
- Sun T, Morgan H, 2010. Microfluidics and Nanofluidics 8(4), 423–443. [Google Scholar]
- Swennenhuis JF, Tibbe AG, Levink R, Sipkema RC, Terstappen LW, 2009. Cytometry Part A: The Journal of the International Society for Advancement of Cytometry 75(6), 520–527. [DOI] [PubMed] [Google Scholar]
- Szulcek R, Bogaard HJ, van Nieuw Amerongen GP, 2014. JoVE (Journal of Visualized Experiments)(85), e51300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabar L, Yen M-F, Vitak B, Chen H-HT, Smith RA, Duffy SW, 2003. The Lancet 361(9367), 1405–1410. [DOI] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A, 2015. CA: a cancer journal for clinicians 65(2), 87–108. [DOI] [PubMed] [Google Scholar]
- Torre LA, Siegel RL, Ward EM, Jemal A, 2016. Cancer Epidemiology and Prevention Biomarkers 25(1), 16–27. [DOI] [PubMed] [Google Scholar]
- Tran TB, Baek C, Min J, 2016a. PloS one 11(4), e0153813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TB, Nguyen PD, Baek C, Min J, 2016b. Biosensors and Bioelectronics 77, 631–637. [DOI] [PubMed] [Google Scholar]
- Van Vliet K, Bao G, Suresh S, 2003. Acta materialia 51(19), 5881–5905. [Google Scholar]
- Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK, 2003. Journal of the National Cancer Institute 95(17), 1276–1299. [DOI] [PubMed] [Google Scholar]
- Wu S, Liu S, Liu Z, Huang J, Pu X, Li J, Yang D, Deng H, Yang N, Xu J, 2015. PLoS One 10(4), e0123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Stott S, Toner M, Maheswaran S, Haber DA, 2011. The Journal of cell biology 192(3), 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H, Kim K, Lee WG, 2013. Biofabrication 5(2), 022001. [DOI] [PubMed] [Google Scholar]
- Zellner P, Shake T, Sahari A, Behkam B, Agah M, 2013. Analytical and bioanalytical chemistry 405(21), 6657–6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Chen D, Li H, Luo Y, Deng B, Huang S-B, Chiu T-K, Wu M-H, Long R, Hu H, 2013. Biosensors and Bioelectronics 43, 304–307. [DOI] [PubMed] [Google Scholar]
- Zudaire E, Cuesta N, Murty V, Woodson K, Adams L, Gonzalez N, Martínez A, Narayan G, Kirsch I, Franklin W, 2008. The Journal of clinical investigation 118(2), 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.