Abstract
CYP4B1 is an enigmatic mammalian cytochrome P450 monooxygenase acting at the interface between xenobiotic and endobiotic metabolism. A prominent CYP4B1 substrate is the furan pro-toxin 4-ipomeanol (IPO). Our recent investigation on metabolism of IPO related compounds that maintain the furan functionality of IPO while replacing its alcohol group with alkyl chains of varying structure and length revealed that, in addition to cytotoxic reactive metabolite formation (resulting from furan activation) non-cytotoxic ω-hydroxylation at the alkyl chain can also occur. We hypothesized that substrate reorientations may happen in the active site of CYP4B1. These findings prompted us to re-investigate oxidation of unsaturated fatty acids and fatty alcohols with C9-C16 carbon chain length by CYP4B1. Strikingly, we found that besides the previously reported ω- and ω−1-hydroxylations, CYP4B1 is also capable of α-, β-, γ-, and δ-fatty acid hydroxylation. In contrast, fatty alcohols of the same chain length are exclusively hydroxylated at ω, ω−1, and ω−2 positions. Docking results for the corresponding CYP4B1-substrate complexes revealed that fatty acids can adopt U-shaped bonding conformations, such that carbon atoms in both arms may approach the heme-iron. Quantum chemical estimates of activation energies of the hydrogen radical abstraction by the reactive compound 1 as well as electron densities of the substrate orbitals led to the conclusion that fatty acid and fatty alcohol oxidations by CYP4B1 are kinetically controlled reactions.
Keywords: CYP4B1, cytochrome P450 monooxygenase, fatty acid, fatty alcohol, biocatalysis
Introduction
Cytochrome P450 monooxygenases (P450 or CYP) are heme-thiolate containing enzymes present in all domains of life encompassing ~ 350,000 sequences in databases [1]. In the human genome, 57 different P450 genes encode related CYPs that can be clustered in 18 families and 45 subfamilies based on homology in sequence. CYP4B1 belongs to the cytochrome P450 family 4 that also includes the CYP4A, 4F, 4V, 4X, and 4Z subfamilies [2]. Typically, the majority of enzymes of the CYP4 family are involved in fatty acid and eicosanoid oxidation [3]. The predominant reaction is ω-hydroxylation, which serves to remove excess free fatty acids to prevent lipotoxicity, catabolize leukotrienes and prostanoids, and produces the bioactive metabolite, 20-HETE, from arachidonic acid [4].
CYP4B1 is unique, because it appears to act at the interface between xenobiotic and endobiotic metabolism [5–7]. In mammals, CYP4B1 is capable of ω- and ω−1-hydroxylation of unsaturated fatty acids, but in contrast to other CYP4 family members, this enzyme is also responsible for the metabolism of a range of chemically unrelated pro-toxic xenobiotics, including 4-ipomeanol (IPO) 13 [8] and perilla ketone (PK) 14 [9] (Figure 1). Strikingly, in humans this situation is very different, because no catalytic activity has been reported so far for native human CYP4B1. This intriguing observation can be explained by the fact that in the human enzyme an otherwise evolutionarily highly conserved proline residue of the meander region that should be located at position 427 of the so-called “ERR triad” and that is important for heme binding, has been replaced by a serine amino acid rendering it apparently inactive [10]. It has previously been reported that the exchange of serine 427 to proline can partially restore the catalytic activity of human CYP4B1 supporting fatty acid hydroxylation (compounds 1, 2, 4; preferentially at the ω-position) [10], as well as bioactivation of IPO 13 and PK 14 [11]. Moreover, by systematic screening against the native rabbit CYP4B1, Wiek et al. [12] identified and introduced an additional twelve amino acids into the human S427P variant resulting in an optimized variant that was as stable and active in metabolizing IPO 13 as the native rabbit CYP4B1.
Figure 1.
Compound library investigated within this study.
As mentioned above, CYP4 enzymes preferentially oxygenate primary (energetically less favored) C-H bonds over adjacent (energetically favored) secondary C-H bonds; e.g. the rabbit CYP4B1 hydroxylates lauric acid (C12) 4 at ω:ω−1 positions at a ratio of ~ 3:2 [13]. Recently the structure of native rabbit CYP4B1 in complex with octane (pdb 5T6Q) [14] was solved helping to identify structural features that explain this preference. In short, the overall topographical features of CYP4B1 mirror those of other P450s, but the crystal structure revealed that octane approaches the heme iron ‘end on’ meaning that the linear hydrocarbon is constrained to an extended conformation within a slot-like active site cavity. This cavity narrows nearer the heme so that the ω−1-hydrogens are more shielded from the active oxygen species than the ω-carbons, thus promoting the observed regioselectivity. An unusual feature that has evolved to contribute to this regioselectivity is an ester bond between the heme 5-methyl and a glutamic acid residue (E310 in rabbit CYP4B1 [15, 16], present also in some members of the CYP4 family [17]). The preference for octane ω-hydroxylation is retained in a mutated E310A variant albeit with a 4-fold loss in ω:ω−1 regioselectivity while maintaining a similar overall rate of metabolite formation [14]. In contrast, C12 4 is metabolized by E310A at ~ 60% of the rate of the native enzyme displaying a clear change in regioselectivity to predominantly ω−1 hydroxylation [16]. The recently solved structure of the CYP4B1 E310A variant in complex with octane (pdb 6C93) disclosed that noncovalent interactions maintain heme deformation in the absence of the ester linkage and that reduced rigidity probably underlies increased E310A catalyzed ω−1-hydroxylation [18].
In a previous study reporting on metabolism of PK 14 by CYP4B1, we noticed that besides cytotoxic reactive metabolite formation (resulting from furan activation) three non-cytotoxic hydroxylation products were also formed via hydroxylations on the alkyl chain [11]. In addition, in a very recent study, we examined a series of synthetically derived IPO 13 related N-alkyl-3-furancarboxamides maintaining the furan functionality while replacing the alcohol group with alkyl chains of varying structure [Kowalski et al., manuscript submitted]. In this case the amount of alkyl hydroxylation increased with increasing chain length, bolstering the hypothesis that substrate reorientations can occur in the active site of CYP4B1.
These findings prompted us in the present study to re-investigate oxidation of unsaturated fatty acids 1–8 and fatty alcohols 9–12 by rabbit and human S427P CYP4B1. Strikingly, we found that besides previously reported ω- and ω−1-hydroxylation, CYP4B1 is also capable of α-, β-, γ-, and δ-fatty acid hydroxylation of mid chain length fatty acids. In contrast, fatty alcohols of the same chain length are exclusively hydroxylated at ω, ω−1, and ω−2 positions. Docking studies of the corresponding CYP4B1-substrate complexes and quantum chemical calculations on the substrates lead to the conclusion that fatty acid and fatty alcohol oxidations by CYP4B1 are kinetically controlled reactions.
Materials and Methods
Chemicals and enzymes
The compounds investigated in this study are summarized in Figure 1. Unsaturated fatty acids of C9 to C16 carbon chain length 1–8 and the fatty alcohols decanol (C10-ol) 9, dodecanol (C12-ol) 10, tetradecanol (C14-ol) 11, and hexadecanol (C16-ol) 12 were purchased from Sigma-Aldrich (Germany).
Catalase from bovine liver and recombinant bovine superoxide dismutase were obtained from Sigma-Aldrich.
Glucose dehydrogenase (GDH) from Bacillus megaterium (gdhIV; GenBank D10626) was expressed via pET-22b(+) in E. coli BL21(DE3) as described previously [19]. Rat cytochrome P450 reductase (CPR) and human cytochrome b5 were expressed and purified as described previously [20].
CYP4B1 plasmid construction, enzyme expression and purification
Construction of the plasmids r-P422Δ1_pET (expressing the rabbit cyp4b1 cDNA, GenBank NM_001082103 isoform 1, now termed r-Δ1) and h-P427Δ3 (expressing the human cyp4b1 cDNA, GenBank NM_000779 transcript variant 2) mutant S427P (now termed h-Δ3P) has been reported previously [11]. The N- and C-terminal amino acid modifications introduced for expression in E. coli are summarized in the Supporting Information (Supplementary Table S1).
r-Δ1 was expressed from pET-22b(+) (Novagen, Germany), while pCWOri [21] was used to express h-Δ3P. Expression conditions in E. coli OverExpress C43(DE3) ([F− ompT hsdSB (rB− mB−) gal dcm (DE3)], Lucigen Corporation, USA) and purification by affinity chromatography utilizing a C-terminal His6-tag have also been reported previously [11].
CYP4B1 concentrations of non-purified enzymes were determined by the CO-difference spectral assay with ε450–490 = 91 mM−1 cm−1 [22], while CO-difference spectra of purified CYP4B1 preparations were measured according to an optimized method by Zheng et al. [16].
Influence of cytochrome b5 on lauric acid conversion by CYP4B1
Coupling efficiency and NADPH consumption
The coupling efficiency and NADPH consumption in the absence and presence of cytochrome b5 were determined as follows: Reactions were carried out in 50 mM potassium phosphate buffer, pH 7.5 in a total reaction volume of 100 μL. The reaction mixtures contained 0.25 μM CYP4B1, 0.5 μM CPR, 100 U mL−1 superoxide dismutase, 1,000 U mL−1 catalase, 25 μg mL−1 1,2-didodecanoyl-sn-glycero-3-phosphocholine (DLPC). Optionally 0.25 μM cytochrome b5 were added. C12 4 (from a 10 mM stock solution dissolved in DMSO) and NADPH were added in final concentrations of 200 μM and the reaction mixtures were incubated at 30°C. The consumption of NADPH was followed by measuring the absorbance decrease at 340 nm. NADPH oxidation rates were calculated with ε340 = 6.22 mM−1 cm−1.
After complete consumption of NADPH, the samples were prepared for GC/MS analysis as described below. Since equimolar amounts of substrate and NADPH were applied, the coupling efficiency directly corresponds to the amount of C12 4 converted by CYP4B1.
Influence of cytochrome b5 and cofactor regeneration on lauric acid conversion
Reaction mixtures were prepared as described above. Optionally, 25 U mL−1 GDH and 20 mM glucose were added for cofactor recycling. Reactions with GDH contained 200 μM NADPH, whereas 1 mM NADPH was added if GDH was not present to ensure sufficient amounts of cofactor. Reaction mixtures were incubated at 30°C for 30 or 120 min. Thereafter, samples were prepared for GC/MS analysis as described below.
Fatty acid and fatty alcohol conversion by CYP4B1
Conversions of fatty acids 1–8 and fatty alcohols 9–12 were carried out in 50 mM potassium phosphate buffer, pH 7.5 and a total reaction volume of 100 μL. Reaction mixtures contained 0.25 μM CYP4B1, 0.5 μM CPR, 0.25 μM cytochrome b5, 100 U mL−1 superoxide dismutase, 1,000 U mL−1 catalase, 25 U mL−1 GDH, 20 mM glucose, 25 μg mL−1 DLPC, 200 μM substrate (from a 10 mM stock solution dissolved in DMSO) and 200 μM NADPH. Samples were incubated at 30°C for 90 min; this reaction time was chosen as an almost complete substrate conversion (as achieved for C12 4 after 120 min) was not desirable, so as to allow comparison of the conversion values for the individual substrates and also between the two CYP4B1 isoforms.
Sample preparation and gas chromatography / mass spectrometry (GC/MS) analysis
After the desired reaction time, 100 μM of an internal standard (Supplementary Table S2) and 2 μL of 37% HCl were added to each sample. Samples were extracted with 500 μL diethyl ether, the ether was dried with water free magnesium sulfate and evaporated completely. The residues were dissolved in 30 μL N,O-bis(trimethylsilyl)trifluoroacetamide containing 1% trimethylchlorosilane and incubated at 80°C for 30 min.
0.5 μL of a sample was injected to a GC/MS instrument (GC/MS-QP2010 plus, Shimadzu, Germany) equipped with an FS-Supreme-5 column (30 m × 0.25 mm × 0.25 μm, Chromatographie Service GmbH, Germany). Helium was used as carrier gas with a linear velocity of 30 cm min−1. The column oven temperature profiles for compounds 1–12 are provided in the Supplementary Table S2.
Identification of products from fatty acid 1–8 conversions was done as described previously (Supplementary Figure S1) [19]. Hydroxylation positions of fatty alcohols 9–12 conversion products were identified by their characteristic mass fragmentation patterns (Supplementary Figure S2).
Computational methods
The crystal structure of rabbit CYP4B1 (pdb 5T6Q) [14] was used for computational analysis of r-Δ1 (98.6% protein sequence identity; see Supplementary Table S1), and served as template to generate a homology model of the h-Δ3P variant (86.4% protein sequence identity; see Supplementary Material for pdb-file). The homology model of h-Δ3P was built using SWISS-MODEL [23, 24], whereas for r-Δ1 the coordinates of the corresponding crystal structure were used. Further preparation for docking was carried out by AutoDock Tools (Windows version 1.5.6r3) [25], assigning AMBER charges to the amino acids of the protein, whereas Gasteiger-Marsili charges were computed for the heme cofactor. The partial charge on the iron atom was set to 0.400e and ‒0.348e to each of its ligating nitrogen atoms, as in earlier docking studies [19]. Protonation states of the histidine residues were assigned manually by visual inspection in order to optimize their local hydrogen-bonding network. The rectangular grid box (80 × 48 × 56 points) was dimensioned large enough to comprise a linear alkane chain of length C16 in the binding pocket above the heme cofactor. The grid spacing was kept at the default value of 0.375 Å. Ligand molecules were prepared with HYPERCHEM on the basis of the octane molecule that was present is the crystal structure and energetically optimized using the MM+ force field parameters as implemented in HYPERCHEM version 6.02 (Hypercube Inc., Gainsville, FL, 1999). Atom types and Gasteiger-Marsili charges for use in AutoDock version 4.2 [26, 27] were computed with in-house PERL scripts. During docking all rotatable bonds of the ligands were treated as flexible, whereas the protein part was kept rigid. A total of 250 runs of the Lamarckian Genetic Algorithm for each ligand were carried out, otherwise default parameters of AutoDock were applied.
Activation energies for the P450-mediated hydrogen radical abstraction were computed according to the approach of Mayeno et al. [28] at AM1 level of theory, which was also used for obtaining the coefficients of the substrate orbitals.
Results
Influence of cytochrome b5 on CYP4B1 activity
Cytochrome b5 is a microsomal heme protein that has an electron transfer role in fatty acid desaturation and some other enzymatic reactions [29, 30]. The effects of cytochrome b5 on P450 catalysis are diverse; in a number of studies either no effect, stimulation, or inhibition with the addition of cytochrome b5 have been reported, often in a P450- and substrate-dependent manner that is not well understood (reviewed in [31]).
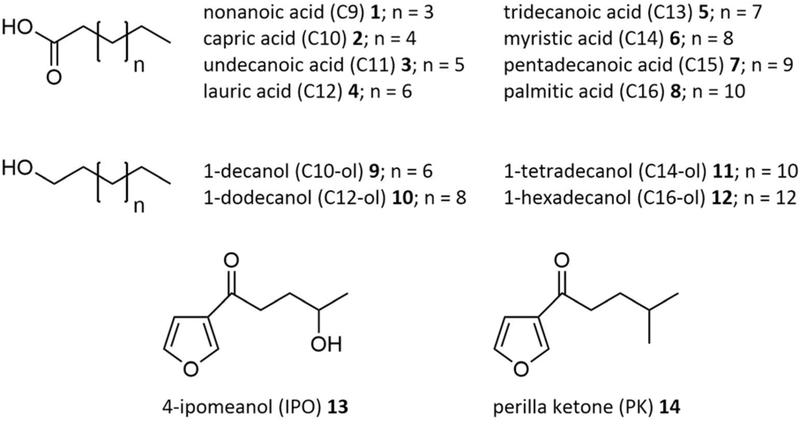
In order to investigate the influence of cytochrome b5 on CYP4B1 activity, reactions containing C12 4 and r-Δ1 with and without cytochrome b5 were performed. C12 4 was chosen, because this compound has traditionally been used as a model substrate to probe for ω-hydroxylase activity toward physiologically relevant substrates [5]. In addition, reactions with and without NADPH regeneration by GDH were investigated (Figure 2A). The reaction containing just r-Δ1 and CPR, but no cytochrome b5 or GDH, reached 24% substrate conversion after 30 min and almost twice as much (47%) after 120 min. The addition of cytochrome b5 to the reaction led to an increase of C12 4 conversion to 45% and 84% after 30 and 120 min, respectively. A similar effect was observed after the addition of GDH without cytochrome b5; here conversions of 59% and 95% were reached after 30 and 120 min, respectively. By combination of cytochrome b5 and GDH conversion further increased to 69 % after 30 min and 97 % after 120 min.
Figure 2.
Lauric acid 4 conversion by rabbit CYP4B1 (r-Δ1). All reactions contained 100 U mL−1 superoxide dismutase and 1,000 U mL−1 catalase. All reactions (except No. 1) also contained 0.25 μM r-Δ1 and 0.5 μM CPR. Where it applies (+), 0.25 μM cytochrome b5 and/or 25 U mL−1 GDH and 20 mM glucose were added. Data represent the average ± standard deviation of three experiments. (A) Influence of cytochrome b5 and cofactor regeneration (GDH) on r-Δ1 activity. NADPH concentration was 200 μM in reactions containing GDH and 1 mM in reactions without GDH. The negative control (reaction 1) did not contain r-Δ1 and CPR. (B) Determination of the NADPH coupling efficiencies and (C) NADPH consumption rates in the presence (+) and absence (−) of cytochrome b5. Reactions 6 and 7 contained equimolar amounts of C12 4 and NADPH (200 μM each).
To further investigate the effect of cytochrome b5 on C12 4 conversion, the NADPH coupling efficiencies and conversion rates were determined under the chosen reaction conditions. In general, the NADPH coupling efficiency of a P450 enzyme is dependent on the substrate: If substrate binding is weak the enzyme-substrate-complex can fall apart and intramolecular uncoupling reactions within the catalytic cycle of the P450 can occur. In addition, the coupling efficiency depends on the interaction between P450 and CPR, inefficient interaction can lead to intermolecular uncoupling during electron transport between both enzymes. In both cases NADPH is consumed, but instead of oxidizing the substrate, reactive oxygen species like H2O2 are formed [32, 33].
The efficiency of NADPH consumption and substrate conversion can be tested by adding both compounds in equimolar concentrations to the reaction. After complete NADPH oxidation the percentage of converted substrate is equal to the coupling for the P450 with this specific substrate under the given reaction conditions. In the case of C12 4, addition of cytochrome b5 boosted the coupling efficiency from 26% to 54% (Figure 2B). In contrast, monitoring of the NADPH consumption revealed that the addition of cytochrome b5 led to a reduction from 8.4 pmol s−1 to 6.6 pmol s−1 (Figure 2C).
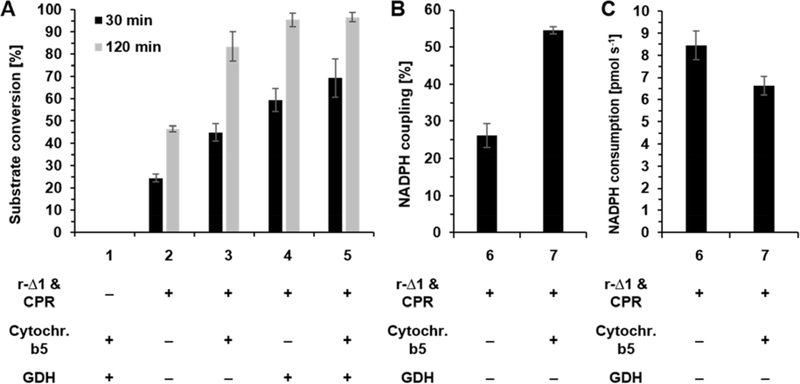
While analyzing the product distributions of the C12 4 conversions by r-Δ1 in the absence/presence of cytochrome b5 and/or GDH, we noticed to our surprise that in addition to previously reported ω- and ω−1-hydroxylation metabolites [13], additional NADPH-dependent chromatography peaks occurred in comparison to the no-P450 negative control (Figure 3A). These new products could clearly be attributed to hydroxylations at the ω−10 (α), ω−9 (β), ω−8 (γ), and ω−7 (δ) carbon atoms of C12 4 (Supplementary Figure S1). In-depth analysis of the GC/MS data revealed that these metabolites were present in all reactions at every time points and that their relative distribution among total reaction products remained almost unaltered over the reaction time of 120 min (Supplementary Table S3). Moreover, it was noted that the addition of cytochrome b5 and GDH while enhancing reaction rates, had only marginal effects on the product distributions (Supplementary Table S3).
Figure 3.
GC/MS chromatograms of (A) lauric acid 4 and (B) dodecanol 11 conversions by rabbit and human CYB4B1. Black dashed-dotted line: negative control without CYP4B1 and CPR (NC). Green solid line: r-Δ1. Blue dashed line: h-Δ3P. Mass spectra of the individual reaction products (ω, ω−1, ω−2, α, β, γ, δ) and lactones (15 and 16) are shown in the Supplementary Material (Supplementary Figures S1 and S2). IS: internal standard, S: substrate (C12 4 or C12-ol 11), γ-dodecalactone 15, δ-dodecalactone 16.
Conversion of fatty acids by CYP4B1
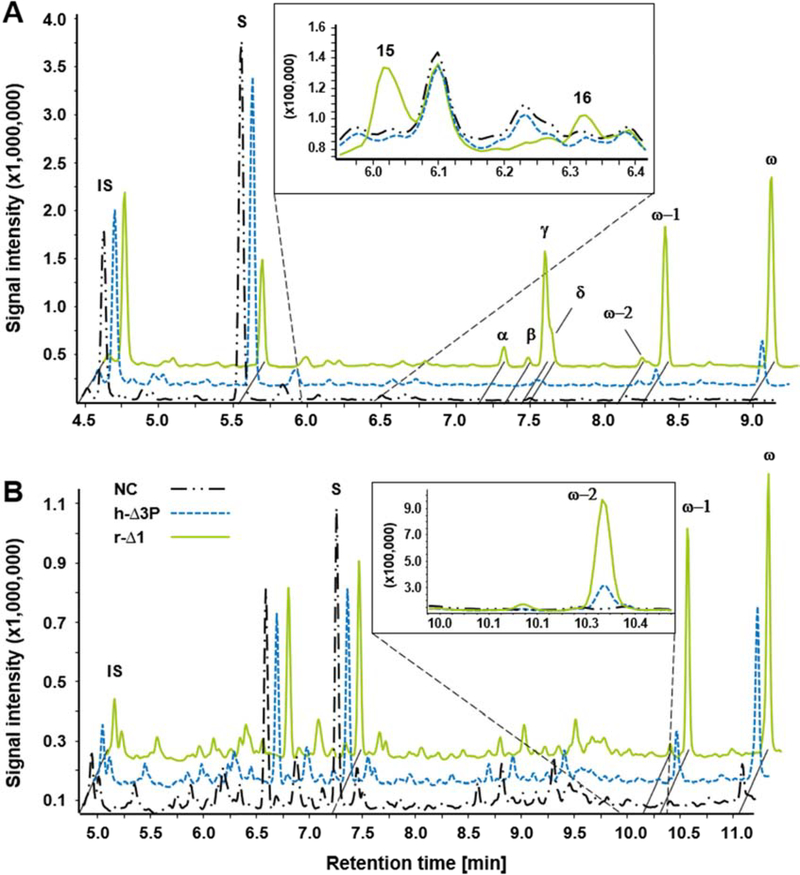
Since, to the best of our knowledge, hydroxylations at positions other than ω/ω−1 had not been reported for any CYP4B1 isoform so far, this prompted us to evaluate conversion of fatty acids 1–8 with C9 to C16 carbon chain lengths by both, r-Δ1 and h-Δ3P, in reaction systems containing cytochrome b5 and GDH.
Indeed, hydroxylations at positions other than ω and ω−1 were found for compounds C10 - C14 2–6 and for both r-Δ1 and h-Δ3P (Table 1). The conversion of C12 4 resulted in the most diverse product pattern amongst the tested substrates; seven hydroxylation products could be detected via GC/MS. Most abundant — not only for C12 4 but for most fatty acids 1–7 — was the ω-hydroxylation product. Unexpectedly, r-Δ1 generated large amounts (9 – 35%) of previously unknown ω−6 to ω−10 hydroxy metabolites from compounds C10 - C13 2–5. Interestingly, no ω−3 (except of trace amounts in case of C14 6), nor ω−4, nor ω−5 hydroxylations were observed for any compound. In addition, hydroxylation did not occur at positions ≥ ω−11.
Table 1.
Substrate oxidation rates (SOXR) and product distributions of the fatty acids 1–7 and fatty alcohols 9–11 conversion by r-Δ1 and h-Δ3P.[a]
| Compound | CYP4B1 | SOXR [nmol Min−1 nmolP450−1] | Product distribution [%] [b] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ω | ω-1 | ω-2 | ω-3 | ω-6 | ω-7 | ω-8 | ω-9 | ω-10 | |||
| C9 (1) | r-Δ1 | 1.3 | 59 | 41 | – | – | - | - * | x | x | x |
| h-Δ3P | 0.2 | 79 | 21 | – | – | – | – * | x | x | x | |
| C10 (2) | r-Δ1 | 3.2 | 22 | 24 | 1 | – | 27 | 3 | 23 * | x | x |
| h-Δ3P | 0.5 | 56 | 30 | 9 | – | 3 | < 1 | 1 * | x | x | |
| C11 (3) | r-Δ1 | 5.0 | 27 | 20 | 2 | – | < 1 | 35 | 1 | 12 * | x |
| h-Δ3P | 0.9 | 66 | 25 | – | – | – | 5 | – | 4 * | x | |
| C12 (4) | r-Δ1 | 6.7 | 40 | 28 | 2 | – | – | 5 | 18 | 1 | 4 * |
| h-Δ3P | 3.9 | 80 | 14 | < 1 | – | – | 1 | 2 | << 1 | 1 * | |
| C13 (5) | r-Δ1 | 1.2 | 34 | 45 | 2 | – | – | 5 | 5 | 9 | 1 |
| h-Δ3P | 1.7 | 53 | 41 | – | – | – | – | – | 6 | << 1 | |
| C14 (6) | r-Δ1 | 1.0 | 42 | 43 | 4 | 1 | – | – | – | 6 | 4 |
| h-Δ3P | 1.2 | 41 | 51 | 7 | – | – | – | – | 1 | – | |
| C15 (7) | r-Δ1 | 0.7 | 50 | 50 | – | – | – | – | – | – | – |
| h-Δ3P | 0.6 | 51 | 43 | – | – | – | – | – | – | – | |
| C10-ol (9) | r-Δ1 | 3.4 | 48 | 49 | 3 | – | – | – | – | x | x |
| h-Δ3P | 3.5 | 75 | 25 | – | – | – | – | – | x | x | |
| C12-ol (10) | r-Δ1 | 5.5 | 56 | 42 | 2 | – | – | – | – | – | – |
| h-Δ3P | 3.6 | 78 | 21 | 1 | – | – | – | – | – | – | |
| C14-ol (11) | r-Δ1 | 1.3 | 35 | 53 | 12 | – | – | – | – | – | – |
| h-Δ3P | 1.5 | 54 | 42 | 4 | – | – | – | – | – | – | |
Data represent mean values of three experiments; standard deviations were within ≤ 14%.
ω-4, ω-5 and ≥ ω-11 were never detected; where the sum of products for an individual compound is < 100%, low amounts of the corresponding lactones were observed (see Figure 3A).
x: Formation of this product is not possible for this compound.
–: Product was not detected.
Indicates the α-carbon atom of the respective compound.
Notably, as a general trend, it was observed that independent of the carbon chain length and CYP4B1 isoform, the positions α and γ were preferred over β and δ positions, e.g. the α:β:γ:δ-distribution for C11 3 is 12:1:35:1 in the case of r-Δ1 and 4:0:5:0 from h-Δ3P.
Additionally, small amounts of two C12 4 hydroxy fatty acid derivatives could be identified by their characteristic m/z ratios during MS analysis, namely γ-dodecalactone 15 and δ-dodecalactone 16; these products result from non-enzymatic intramolecular esterification of γ- and δ-hydroxy C12 (Supplementary Figure S1). Noteworthy, γ- and δ-lactones have a variety of commercial uses as flavorings and fragrances, e.g. δ-decalactone is a common fragrance compound with a creamy peach note [34].
Detailed information about fatty acid conversion is given in Figure 4A, substrate oxidation rates (SOXR) and product patterns observed for both CYP4B1 isoforms are summarized in Table 1. Briefly, a comparison of the conversions by r-Δ1 and h-Δ3P reveals an increased acceptance of medium- over shorter- and longer-chained fatty acids. For r-Δ1 an increase in substrate conversion from C9 1 (14%) to C12 4 (79%) was observed, whereas conversions for C13 5 and C14 6 dropped to 13% and 11%, respectively. Notably, although not tremendously pronounced, the acceptance of h-Δ3P of fatty acids with chain length higher than twelve carbon atoms seems to be slightly better than r-Δ1. Here, SOXR for C13 5 and C14 6 of 1.7 and 1.2 nmol min−1 nmolP450−1 were reached, respectively, as compared to 1.2 and 1.0 by r-Δ1. Furthermore, fatty acids with chain length ≤ 12 carbon atoms were generally less well accepted as substrates by h-Δ3P compared to r-Δ1. Conversion of C15 7 was low, whereas C16 8 was not converted by either enzyme.
Figure 4.
Conversion of (A) fatty acids 1-8 and (B) fatty alcohols 9-12 by r-Δ1 and h-Δ3P. The product distributions of these reactions are summarized in Table 1.
Conversion of fatty alcohols by CYP4B1
Regarding the product distribution, in contrast to fatty acids of the same chain length GC/MS analysis revealed only two major (namely ω and ω−1) and one minor (namely ω−2) hydroxylation products in case of all tested fatty alcohols. By way of example, the gas chromatogram of C12-ol 10 (Figure 3B), as well as m/z fragmentation patterns of detected products are shown (Supplementary Figure S2). Detailed information on SOXR and product distributions are summarized in Table 1.
Analogous to the fatty acids, the highest conversion was observed for C12-ol 10 in the case of r-Δ1 (62%), while h-Δ3P converted C12-ol 10 and C10-ol 9 to nearly the same extent (~40%) (Figure 4B).
Interestingly, the SOXR of h-Δ3P for C10-ol 9 is 7-fold higher than that of C10 (3.5 vs. 0.5), whereas the SOXR of r-Δ1 for C10-ol 9 (as well as C12-ol 10) are lower than those of C10 2 and C12 4. (Table 1). Both, r-Δ1 and h-Δ3P were not capable to turn over C16-ol 12.
As a general trend it can be stated that independent of the carbon chain length, the ω−1 and ω−2 positions are more preferred for hydroxylation by r-Δ1, whereas h-Δ3P is more selective for ω-hydroxylation.
Computational analyses of fatty acid and fatty alcohol conversions
As described in the Material and methods section, the crystal structure of the rabbit CYP4B1 (pdb 5T6Q) [14] was used for computational analyses of r-Δ1, and served as template to generate a homology model of the h-Δ3P variant. The most pronounced difference between the crystal structure and the homology model is that the unresolved residues, aspartic acid 196 to asparagine 200 and isoleucine 272 to arginine 276 in the crystal structure, were reconstructed. Due to the high sequence similarity between rabbit and human enzyme, no notable deviations of the backbone atoms were observed. Likewise, all side chain rotamers in the binding site were conserved. Since the crystal structure shows a bound n-octanol molecule in the active site, we expect no substantial change upon docking of similar ligands, e.g. fatty alcohols and fatty acids that also possess linear carbon chains. The reported QMEAN4 score [23] of SWISS-Model was −1.27, which is very similar to that of the original crystal structure (−1.17) with a resolution of 2.7 Å. The MolProbity score [35] after adding hydrogens, but without possible flips of corresponding side chains, was 1.60, residing in the 93rd percentile of structures with similar resolution.
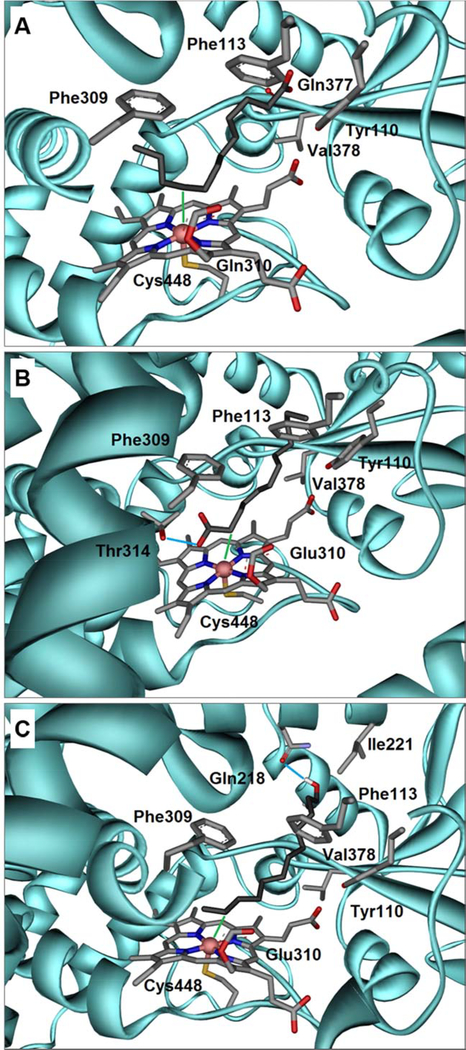
Docking studies suggested that fatty acids can bind in two different bonding conformations, such that carbon atoms in both arms may approach the heme-Fe (Figures 5A & 5B). With increasing carbon chain length, the hydrophobic hydrocarbon chain is increasingly twisted and bent inside the binding pocket. Likewise, the loss of entropy upon binding longer chains also increases as more rotatable bonds are frozen. Eventually, the binding pocket cannot accommodate chain lengths that exceed C15 7 anymore. As a consequence of the chain bending near the heme the hydroxylation pattern is shifted to the ω/ω−1 positions (Figure 5A). Hydrogen-bond formation with the COOH-group of threonine 314, which is very close to the heme and therefore strongly conserved, was observed for part of the fatty acids (Figure 5B).
Figure 5.
(A) Docking of lauric acid 4, into the crystal structure of rabbit CYP4B1 demonstrates a U-shaped folding of the carbon chain inside the binding pocket. Here, the ω−3 position is closest to the Heme-Fe (3.04 Å) denoted as green line. The Heme moiety is covalently linked via the OE2 atom of glutamine 310 and the sulfur atom of cysteine 448 furthermore ligates the iron atom. The carboxylate group of C12 4 forms a hydrogen-bond with the backbone oxygen atom of glutamine 377. Also shown are the side chains of the residues forming close hydrophobic contacts; parts of the helical secondary structure were omitted for clarity. (B) A docked conformation of C12 4 showing an alternative binding pose in which the front positions (α, β, γ, δ) get close to the Heme-Fe, whereby the α-position is within 3.31 Å (green line). The carboxylate group of C12 4 forms a hydrogen-bond with the side chain of threonine 314 denoted as light blue line. (C) The obtained docking conformation of 1-dodecanol 10 shows that the terminal positions are preferably hydroxylated. Among those the ω−1 position is closest to the Heme-Fe (2.94 Å), indicated as green line. The hydroxyl group of C12-ol 10 forms a hydrogen-bond with the side chain of glutamine 218 denoted as light blue line. Color code for all Figures: grey = carbon; red = oxygen; blue = nitrogen; yellow = sulfur; coral pink = iron; cyan = CYP4B1 amino acid backbone. The respective pdb-files of the docked ligands in the corresponding reference frame are available as Supplementary Material.
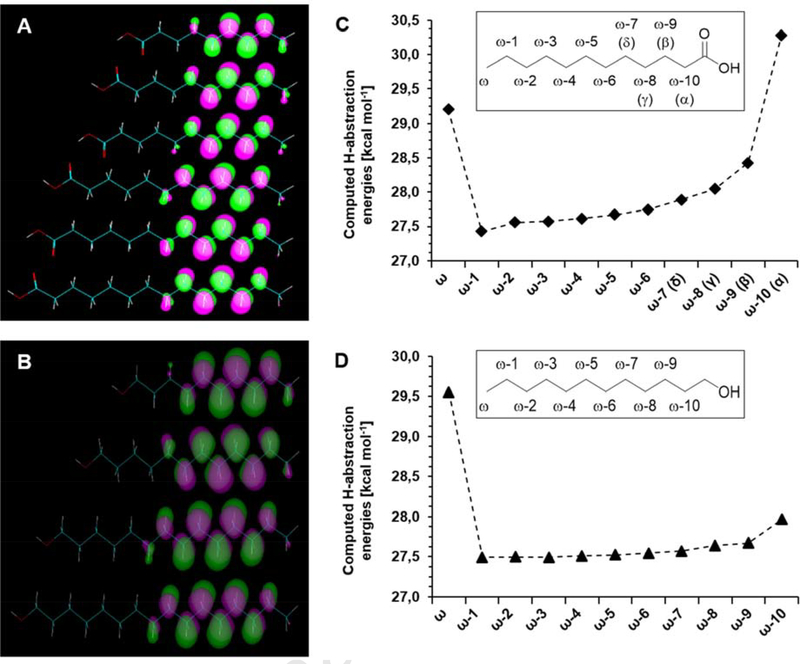
To reveal further factors that influence the hydroxylation of fatty acids, the electron densities of the Highest Occupied Molecular Orbitals (HOMOs) of fatty acids C9 – C14 1–6 and the activation energies for the associated hydrogen abstraction at various carbon atoms of C12 4 were computed. The coefficients of the HOMOs indicate that sufficient electron density is present far into the chains of fatty acid molecules meaning that, in principle, all these positions would be available for hydroxylation (Figure 6A). In contrast, whereas there is a high barrier for hydrogen abstraction from the ω as well as ω−10 (α) carbon atom, the least energy is required at position ω−1, which then slowly increases towards the carboxy group (Figure 6B). Since all of the energies are within 3 kcal mol−1 no thermodynamic preference of a particular position is apparent. Instead docking reveals that a U-shaped folding of the carbon chain allows the front positions (α, β, γ, δ) to get close to the heme-Fe (Figure 5A), suggesting a kinetic control of the hydroxylation.
Figure 6.
(A) Localization of the Highest Occupied Molecular Orbitals (HOMOs) in the completely linear chains of fatty acids C9 – C14 1-6. (B) Computed activation energies for hydrogen abstraction at carbon atoms of lauric acid 4. (C) Localization of the HOMOs in the completely linear chains of fatty alcohols C10-ol – C16-ol 9-12. (D) Computed activation energies for hydrogen abstraction at carbon atoms of 1-dodecanol 10.
The fatty alcohols appear to be less reactive, although alternative binding conformations were also found. According to analysis of the orbitals of the fatty alcohols, the following picture emerges: The presence of substantial electron density throughout the chain is even more pronounced in comparison to the fatty acids, so again all these positions could come into play for hydroxylation (Figure 6C). Moreover, hydrogen abstraction of C12-ol 10 (Figure 6D) was computed to require less activation energy for most of the positions (especially α and β) in comparison to C12 4. However, docking reveals that in contrast to the corresponding fatty acids for which two major binding modes were found, the fatty alcohols preferably bind with the end of the chain facing the heme-Fe, thereby forming a hydrogen-bond to either the side chain of glutamine 218 (Figure 5C) or to the carbonyl oyxgen atom of glutamine 377. Consequently, since only ω to ω−2 positions are actually hydroxylated, this indicates that these positions are the closest to the heme-Fe, again suggesting kinetic control.
Computational analysis of 4-ipomeanol and perilla ketone conversions
We also performed docking for the two previously studied pro-toxins IPO 13 and PK 14. For IPO 13 the energetically most favorable docking position in both enzymes is the one with the furan functionality in close proximity to the heme-Fe leading to furan activation via epoxidation (Supplementary Figure S3A). In contrast, for PK 14, two different conformations in r-Δ1 as well as in h-Δ3P were found for which the predicted docking scores are practically equal in energy: The first leads to furan activation by epoxidation (Supplementary Figure S3B), while the second allows hydroxylations at the carbon atoms of the isopropyl group (Supplementary Figure S3C). The respective pdb-files of the docked ligands in the corresponding reference frame are available as Supplementary Material. In summary, these in silico data correspond very well with the experimentally obtained results of our previous study (Supplementary Figure S4) [11].
Discussion
Fatty acids are endogenous substrates of many P450s [36, 37]). Fatty acid hydroxylation is of particular interest from a mechanistic point of view, since members falling within certain P450 subfamilies exhibit preference for thermodynamically disfavored oxygen insertion at positions remote from activating functional groups resulting in the synthesis of products of high biological activity [38]. Hydroxylations may occur close to the carboxyl group giving rise to α- or β-hydroxylated fatty acids (e.g. bacterial CYP152 peroxygenases [39]), in-chain (e.g. plant CYP703 [40] or bacterial CYP116 [34]), at the subterminal ω−1 to ω−3 positions (e.g. bacterial self-sufficient CYP102 [41]), or at the terminal ω-position (e.g. bacterial CYP153 [42], yeast CYP52 [43], or plant CYP86 [44]). Similar to bacteria and plants, mammalian species comprise a set of P450s covering fatty acid hydroxylation at various positions: CYP1 and CYP2 concentrate on subterminal, bis-allylic and olefinic oxidations, whereas the CYP4 family members generally favor ω- over ω−1-hydroxylations [38].
Rabbit CYP4B1 was previously shown to metabolize C12 4 as well as C7 - C10 fatty acids with emphasis on terminal ω-hydroxylation; the positional selectivity increased with decreasing chain length [5]. Within our study this observation was confirmed; whereas for C9 - C12 1–4 the ω:ω−1 ratio was ~ 3:2, in case of C14 6 and C15 7 the ω:ω−1 the ratio decreased to 1:1. In addition, under the given reaction conditions we frequently observed the formation of α-, β-, γ-, and δ-hydroxy fatty acids for carbon chain length C10 – C13 2–5 with clear preference for α- and γ- over β- and δ-positions. It has previously been reported that induction of CYP4B1 under hypoxic conditions was correlated with ω−9-oxidations of arachidonic acid, albeit this type of reaction appeared at odds with the prototypic oxygen insertion at the ω-position [45], and hydroxylations at α- to δ-positions for unsaturated fatty acids have to the best of our knowledge not been reported for CYP4B1 so far.
We aimed to explain formation of the new metabolites by in silico analysis based on the recently solved crystal structure of rabbit CYP4B1 (pdb 5T6Q) [14] and the homology model of h-3ΔP. In silico fatty acid docking revealed that there were no fundamental differences between the observed conformations of rabbit CYP4B1 (r-Δ1) and the “activated” human CYP4B1 S427P variant (h-Δ3P). Nevertheless, our experimental data revealed that h-Δ3P in comparison to r-Δ1 showed generally a stronger selectivity for ω- over ω−1 hydroxylation, accompanied by generally lower formation of α- to δ-hydroxy fatty acids, albeit the preference for α- and γ- over β- and δ-positions was retained. We hypothesize that these differences in product distribution are attributed to varying residence times of the substrates in the active sites of the P450s; the longer the substrate stays within the active site, the more conformations can possibly be obtained leading to hydroxylations at all positions that are able to get into close proximity to the heme-Fe and consequently to more diverse hydroxylation patterns.
Consistent with our observation are the previously calculated KD values determined from Type I spin state shifts of r-Δ1 [11]. The KD values for C10 - C12 2–4 that were hydroxylated with the lowest selectivity are in range of 30 – 33 μM, whereas those of longer (C14 – C16) and shorter (C8 -C9) fatty acids are in range of 7 – 25 μM. The tighter binding of short and long fatty acids may lead to less flexible arrangements within the active site and consequently to higher ω/ω−1-selectivity.
Calculations of the localization of the HOMOs in the orbital analysis of fatty alcohols, as well as fatty acids C9 - C14 1–6 [19] revealed that the electron densities are concentrated towards the end of the chains with the exception of the ω-position. Together with the calculated hydrogen abstraction energies these data suggest that fatty acid and fatty alcohol hydroxylations by CYP4B1 are kinetically rather than thermodynamically controlled reactions. This is also reflected by the preferred docking conformations, which differ between fatty acids and fatty alcohols.
Similar observations of shifted regioselectivities have been made previously: Jóźwig et al. [46] reported that for CYP267B1 from Sorangium cellulosum the regioselectivity of hydroxylation was shifted from positions located closer to the methyl terminal carbon (ω−1, ω−2, ω−3) towards the in-chain positions (ω−3 and ω−4) with increasing chain length of the fatty acid. The authors also found evidence for C14 6 to be bound U-shaped and in two different orientations [46]. Khatri et al. [19] investigated fatty acid hydroxylation by CYP267A1 from the same organism and noticed that the wild-type enzyme contains a natural heme-signature variant (a conserved phenylalanine at position 366 exchanged by a leucine residue) that led to a shift in regioselectivity from subterminal (ω−1 to ω−3) positions to in-chain (ω−4 to ω−9) positions. The “back-mutation” L366F completely revoked this behavior resulting in exclusive subterminal hydroxylation [19]. Fiorentini et al. [47] recently screened CYP153 family enzymes that also exhibit very high ω-selectivity. The authors reported that they detected trace amounts of α-, β-, and γ-hydroxy fatty acid products from conversion of C12 4 by a CYP153 from Pseudomonas sp. and also explained this by the presence of a leucine residue instead of a bulkier phenylalanine residue (conserved in CYP153) that provides a higher flexibility to the substrate posing within the active site to allow inverted orientations of fatty acid substrates [47].
The crystal structure of CYP4B1 solved by Hsu et al. [14] suggests a very narrow substrate binding site directing the ω-position of octane close to the heme iron. Nevertheless, the substrate binding site is at least big enough to accept the furan compounds IPO 13 and PK 14 [11] as well as the even bulkier 2-aminoanthracene [48] as substrates.
External factors such as the addition of cytochrome b5 can influence fatty acid hydroxylation activity as well: Loughran et al. [49] reported that the turnover of C12 4 by CYP4A7 increased from 28 to 130 min−1 through the addition of cytochrome b5; based on their data the authors suggested that cytochrome b5 in this particular case did not serve as an electron donor for CYP4A7, but rather took a conformational role in such that it docks at the proximal surface of the heme pocket to open the substrate channel for greater access to substrates of varying flexibility, bulk, and chain length [49].
Regarding the role of cytochrome b5 in CYP4B1 fatty acid hydroxylation, we observed that under the given experimental conditions, addition of cytochrome b5 led to an increase of C12 4 conversion from 24 to 45% within 30 min. The coupling efficiency was improved from 26 to 54%, which may be caused by the ability of cytochrome b5 to act as an electron buffer between P450 and CPR. This might also be the reason for the observed decrease of the NADPH consumption rate from 8.4 to 6.6 pmol s−1 (corresponding to 10.1 and 7.9 nmol min−1 nmolCPR−1, respectively). Since the product distribution was not affected by the addition of cytochrome b5 and assuming that the intramolecular uncoupling reactions within the catalytic cycle of CYP4B1 remain unaltered, our results imply that cytochrome b5 directly influences electron transfer. However, it must be noted that the roles of cytochrome b5 in P450 catalysis are generally not only substrate dependent, but can also vary considerably with regard to the chosen experimental conditions [31]. It also remains to be elucidated whether the first, the second, or both electrons are transferred via cytochrome b5 to CYP4B1, e.g. by stopped-flow spectroscopy of CPR flavin reduction and P450 heme reduction.
Finally, for IPO 13 and PK 14, we previously observed major differences in the ligand binding and metabolic conversion [11]. These two substrates differ only in the hydrophobicity of the terminal portion of their alkyl chains, where the hydroxyl group of IPO 13 is replaced by a methyl group in PK 14 (Figure 1). This results in very substantially increased metabolic conversion of PK 14 by r-Δ1 with hydroxylations at the alkyl chain in three different positions making up the majority of oxidative metabolites of PK 14, but not of IPO 13 (Supplementary Figure S4). Our hypothesis that PK 14, in contrast to IPO 13, can enter the active site of CYP4B1 in two different binding orientations (one resulting in alkyl chain hydroxylation, the other in furan ring activation through epoxidation) is now confirmed by the in silico analysis carried out within this study.
Supplementary Material
Highlights.
CYP4B1 fatty acid hydroxylation patterns are more diverse than previously reported
The presence of cytochrome b5 reduces uncoupling and increases CYP4B1 activity
Computational docking allows interpretation of the observed hydroxylation patterns
Activation energy calculations imply that the reactions are kinetically controlled
Acknowledgments
Funding information
This work was initially supported by the Strategic Research Fund of the Heinrich-Heine University Düsseldorf, Germany [to C.W. and M.G.], the National Institutes of Health [grant number R01 GM49054 to A.E.R.], and subsequently by the UW School of Pharmacy Brady Fund for Natural Products Research [to A.E.R.] as well as the National Institutes of Health [training grant number T32 GM07750 to J.P.K.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nelson DR, Cytochrome P450 diversity in the tree of life, Biochimica et Biophysica Acta: Proteins and Proteomics 1866 (2018) 141–154 10.1016/j.bbapap.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jarrar BY, Lee S-J, Molecular functionality of cytochrome P450 4 (CYP4) genetic polymorphisms and their clinical implications, International Journal of Molecular Sciences 20 (2019) 10.3390/ijms20174274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guengerich FP, Cytochrome P450 enzymes, in: McQueen C(Ed.), Comprehensive Toxicology, Elsevier, Oxford, 2018, pp. 54–86 10.1016/B978-0-12-801238-3.01960-7. [DOI] [Google Scholar]
- [4].Hsu M-H, Savas Ü, Griffin KJ, Johnson EF, Human cytochrome P450 family 4 enzymes: Function, genetic variation and regulation, Drug Metabolism Reviews 39 (2007) 515–538 10.1080/03602530701468573. [DOI] [PubMed] [Google Scholar]
- [5].Baer BR, Rettie AE, CYP4B1: an enigmatic P450 at the interface between xenobiotic and endobiotic metabolism, Drug Metabolism Reviews 38 (2006) 451–76 10.1080/03602530600688503. [DOI] [PubMed] [Google Scholar]
- [6].Edson KZ, Rettie AE, CYP4 enzymes as potential drug targets: focus on enzyme multiplicity, inducers and inhibitors, and therapeutic modulation of 20-hydroxyeicosatetraenoic acid (20-HETE) synthase and fatty acid omega-hydroxylase activities, Current topics in medicinal chemistry 13 (2013) 1429–40 10.2174/15680266113139990110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang JY, Wang Y, Prakash C, Xenobiotic-metabolizing enzymes in human lung, Curr Drug Metab 7 (2006) 939–48 10.2174/138920006779010575. [DOI] [PubMed] [Google Scholar]
- [8].Baer BR, Rettie AE, Henne KR, Bioactivation of 4-ipomeanol by CYP4B1: adduct characterization and evidence for an enedial intermediate, Chemical Research in Toxicology 18 (2005) 855–64 10.1021/tx0496993. [DOI] [PubMed] [Google Scholar]
- [9].Roellecke K, Virts EL, Einholz R, Edson KZ, Altvater B, Rossig C, von Laer D, Scheckenbach K, Wagenmann M, Reinhardt D, Kramm CM, Rettie AE, Wiek C, Hanenberg H, Optimized human CYP4B1 in combination with the alkylator prodrug 4-ipomeanol serves as a novel suicide gene system for adoptive T-cell therapies, Gene Therapy (2016) 10.1038/gt.2016.38. [DOI] [PubMed] [Google Scholar]
- [10].Zheng YM, Fisher MB, Yokotani N, Fujii-Kuriyama Y, Rettie AE, Identification of a meander region proline residue critical for heme binding to cytochrome P450: implications for the catalytic function of human CYP4B1, Biochemistry 37 (1998) 12847–12851 10.1021/bi981280m. [DOI] [PubMed] [Google Scholar]
- [11].Roellecke K, Jäger VD, Gyurov VH, Kowalski JP, Mielke S, Rettie AE, Hanenberg H, Wiek C, Girhard M, Ligand characterization of CYP4B1 isoforms modified for high-level expression in Escherichia coli and HepG2 cells, Protein Engineering, Design and Selection 30 (2017) 207–218 10.1093/protein/gzw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wiek C, Schmidt EM, Roellecke K, Freund M, Nakano M, Kelly EJ, Kaisers W, Yarov-Yarovoy V, Kramm CM, Rettie AE, Hanenberg H, Identification of amino acid determinants in CYP4B1 for optimal catalytic processing of 4-ipomeanol, Biochemical Journal 465 (2015) 103–114 10.1002/jcc.20084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cheesman MJ, Baer BR, Zheng YM, Gillam EM, Rettie AE, Rabbit CYP4B1 engineered for high-level expression in Escherichia coli: Ligand stabilization and processing of the N-terminus and heme prosthetic group, Archives of Biochemistry and Biophysics 416 (2003) 17–24 10.1016/S0003-9861(03)00278-9. [DOI] [PubMed] [Google Scholar]
- [14].Hsu MH, Baer BR, Rettie AE, Johnson EF, The crystal structure of cytochrome P450 4B1 (CYP4B1) monooxygenase complexed with octane discloses several structural adaptations for omega-hydroxylation, J Biol Chem 292 (2017) 5610–5621 10.1074/jbc.M117.775494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Henne KR, Kunze KL, Zheng YM, Christmas P, Soberman RJ, Rettie AE, Covalent linkage of prosthetic heme to CYP4 family P450 enzymes, Biochemistry 40 (2001) 12925–12931 10.1021/bi011171z. [DOI] [PubMed] [Google Scholar]
- [16].Zheng YM, Baer BR, Kneller MB, Henne KR, Kunze KL, Rettie AE, Covalent heme binding to CYP4B1 via Glu310 and a carbocation porphyrin intermediate, Biochemistry 42 (2003) 4601–4606 10.1021/bi020667t. [DOI] [PubMed] [Google Scholar]
- [17].Ortiz De Montellano PR, Mechanism and role of covalent heme binding in the CYP4 family of P450 enzymes and the mammalian peroxidases, Drug Metabolism Reviews 40 (2008) 405–426 10.1080/03602530802186439. [DOI] [PubMed] [Google Scholar]
- [18].Jennings GK, Hsu M-H, Shock LS, Johnson EF, Hackett JC, Noncovalent interactions dominate dynamic heme distortion in cytochrome P450 4B1, Journal of Biological Chemistry 293 (2018) 11433–11446 10.1074/jbc.RA118.004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Khatri Y, Hannemann F, Girhard M, Kappl R, Hutter M, Urlacher VB, Bernhardt R, A natural heme-signature variant of CYP267A1 from Sorangium cellulosum So ce56 executes diverse omega-hydroxylation, FEBS J 282 (2015) 74–88 10.1111/febs.13104. [DOI] [PubMed] [Google Scholar]
- [20].Chen W, Koenigs LL, Thompson SJ, Peter RM, Rettie AE, Trager WF, Nelson SD, Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6, Chemical Research in Toxicology 11 (1998) 295–301 10.1021/tx9701687. [DOI] [PubMed] [Google Scholar]
- [21].Barnes HJ, Maximizing expression of eukaryotic cytochrome P450s in Escherichia coli, Methods Enzymol 272 (1996) 3–14 10.1016/S0076-6879(96)72003-7. [DOI] [PubMed] [Google Scholar]
- [22].Omura T, Sato R, The Carbon Monoxide-Binding Pigment of Liver Microsomes. II. Solubilization, Purification, and Properties, Journal of Biological Chemistry 239 (1964) 2379–85. [PubMed] [Google Scholar]
- [23].Benkert P, Biasini M, Schwede T, Toward the estimation of the absolute quality of individual protein structure models, Bioinformatics 27 (2010) 343–350 10.1093/bioinformatics/btq662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, A P T. de Beer, C. Rempfer, L. Bordoli, R. Lepore, T. Schwede, SWISS-MODEL: homology modelling of protein structures and complexes, Nucleic Acids Research 46 (2018) W296–W303 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sanner MF, Python: a programming language for software integration and development, Journal of molecular graphics & modelling 17 (1999) 57–61 [PubMed] [Google Scholar]
- [26].Huey R, Morris GM, Olson AJ, Goodsell DS, A semiempirical free energy force field with charge-based desolvation, Journal of Computational Chemistry 28 (2007) 1145–1152 10.1002/jcc.20634. [DOI] [PubMed] [Google Scholar]
- [27].Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ, Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function, Journal of Computational Chemistry 19 (1998) 1639–1662 . [DOI] [Google Scholar]
- [28].Mayeno AN, Robinson JL, Yang RSH, Reisfeld B, Predicting activation enthalpies of cytochrome-P450-mediated hydrogen abstractions. 2. Comparison of semiempirical PM3, SAM1, and AM1 with a density functional theory method, Journal of Chemical Information and Modeling 49 (2009) 1692–1703 10.1021/ci8003946. [DOI] [PubMed] [Google Scholar]
- [29].Guengerich FP, Mechanisms of cytochrome P450-catalyzed oxidations, ACS Catalysis 8 (2018) 10964–10976 10.1021/acscatal.8b03401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Storbeck K-H, Swart AC, Fox CL, Swart P, Cytochrome b5 modulates multiple reactions in steroidogenesis by diverse mechanisms, The Journal of Steroid Biochemistry and Molecular Biology 151 (2015) 66–73 [DOI] [PubMed] [Google Scholar]
- [31].Bart AG, Scott EE, Structural and functional effects of cytochrome b5 interactions with human cytochrome P450 enzymes, Journal of Biological Chemistry 292 (2017) 20818–20833 10.1074/jbc.RA117.000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Denisov IG, Makris TM, Sligar SG, Schlichting I, Structure and chemistry of cytochrome P450, Chemical Reviews 105 (2005) 2253–2278 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- [33].Krest CM, Onderko EL, Yosca TH, Calixto JC, Karp RF, Livada J, Rittle J, Green MT, Reactive intermediates in cytochrome P450 catalysis, Journal of Biological Chemistry 288 (2013) 17074–17081 10.1074/jbc.R113.473108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Manning J, Tavanti M, Porter JL, Kress N, De Visser SP, Turner NJ, Flitsch SL, Regio- and enantio-selective chemo-enzymatic C−H-lactonization of decanoic acid to (S)-δ-decalactone, Angewandte Chemie 131 (2019) 5724–5727 10.1002/ange.201901242. [DOI] [PubMed] [Google Scholar]
- [35].Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC, MolProbity: all-atom structure validation for macromolecular crystallography, Acta Crystallographica Section D 66 (2010) 12–21 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hammerer L, Winkler CK, Kroutil W, Regioselective biocatalytic hydroxylation of fatty acids by cytochrome P450s, Catalysis Letters 148 (2018) 787–812 10.1007/s10562-017-2273-4. [DOI] [Google Scholar]
- [37].Van Bogaert INA, Groeneboer S, Saerens K, Soetaert W, The role of cytochrome P450 monooxygenases in microbial fatty acid metabolism, The FEBS Journal 278 (2011) 206–221 10.1111/j.1742-4658.2010.07949.x. [DOI] [PubMed] [Google Scholar]
- [38].Hlavica P, Lehnerer M, Oxidative biotransformation of fatty acids by cytochromes P450: Predicted key structural elements orchestrating substrate specificity, regioselectivity and catalytic efficiency, Current Drug Metabolism 11 (2010) 85–104 10.2174/138920010791110881. [DOI] [PubMed] [Google Scholar]
- [39].Shoji O, Watanabe Y, Peroxygenase reactions catalyzed by cytochromes P450, JBIC Journal of Biological Inorganic Chemistry 19 (2014) 529–539 10.1007/s00775-014-1106-9. [DOI] [PubMed] [Google Scholar]
- [40].Morant M, Jørgensen K, Schaller H, Pinot F, Møller BL, Werck-Reichhart D, Bak S, CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen, The Plant Cell 19 (2007) 1473–1487 10.1105/tpc.106.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Whitehouse CJC, Bell SG, Wong L-L, P450BM3 (CYP102A1): Connecting the dots, Chemical Society Reviews 41 (2012) 1218–1260 10.1039/C1CS15192D. [DOI] [PubMed] [Google Scholar]
- [42].Scheps D, Honda Malca S, Hoffmann H, Nestl BM, Hauer B, Regioselective ω-hydroxylation of medium-chain n-alkanes and primary alcohols by CYP153 enzymes from Mycobacterium marinum and Polaromonas sp. strain JS666, Organic & Biomolecular Chemistry 9 (2011) 6727–6733 10.1039/C1OB05565H. [DOI] [PubMed] [Google Scholar]
- [43].Park H-G, Kim V, Kim H, Lee R, Cho M-A, Park S-W, Chun Y-J, Kim D, CYP52A23 from Candida albicans and its substrate preference for fatty acid hydroxylation, Archives of Biochemistry and Biophysics 671 (2019) 27–34 10.1016/j.abb.2019.06.002. [DOI] [PubMed] [Google Scholar]
- [44].Pinot F, Beisson F, Cytochrome P450 metabolizing fatty acids in plants: characterization and physiological roles, The FEBS Journal 278 (2011) 195–205 10.1111/j.1742-4658.2010.07948.x. [DOI] [PubMed] [Google Scholar]
- [45].Mastyugin V, Aversa E, Bonazzi A, Vafaes C, Mieyal P, Schwartzman ML, Hypoxia-induced production of 12-Hydroxyeicosanoids in the corneal epithelium: Involvement of a cytochrome P-4504B1 isoform, Journal of Pharmacology and Experimental Therapeutics 289 (1999) 1611–1619 [PubMed] [Google Scholar]
- [46].Jóźwik IK, Litzenburger M, Khatri Y, Schifrin A, Girhard M, Urlacher V, Thunnissen A-MWH, Bernhardt R, Structural insights into oxidation of medium-chain fatty acids and flavanone by myxobacterial cytochrome P450 CYP267B1, Biochemical Journal 475 (2018) 2801–2817 10.1042/bcj20180402. [DOI] [PubMed] [Google Scholar]
- [47].Fiorentini F, Hatzl A-M, Schmidt S, Savino S, Glieder A, Mattevi A, The extreme structural plasticity in the CYP153 subfamily of P450s directs development of designer hydroxylases, Biochemistry 57 (2018) 6701–6714 10.1021/acs.biochem.8b01052. [DOI] [PubMed] [Google Scholar]
- [48].Smith PB, Tiano HF, Nesnow S, Boyd MR, Philpot RM, Langenbach R, 4-Ipomeanol and 2-aminoanthracene cytotoxicity in C3H/10T1/2 cells expressing rabbit cytochrome P450 4B1, Biochem Pharmacol 50 (1995) 1567–75 10.1016/0006-2952(95)02029-2. [DOI] [PubMed] [Google Scholar]
- [49].Loughran PA, Roman LJ, Miller RT, Masters BSS, The kinetic and spectral characterization of the E. coli-expressed mammalian CYP4A7: Cytochrome b5 effects vary with substrate, Archives of Biochemistry and Biophysics 385 (2001) 311–321 10.1006/abbi.2000.2136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.