Abstract
Background:
Phenols and parabens are common additives to consumer products. There is evidence of adverse birth outcomes in association with prenatal exposure to these chemicals, in addition to psychosocial factors. We previously reported an increase in gestational length with bisphenol-A, methylparaben and propylparaben, and a decrease in gestational length with triclocarban.
Objectives:
We examined the modifying effect of psychosocial stress on the association between chemicals and gestational length in up to 752 women among a pregnancy cohort study.
Methods:
Urinary biomarkers were measured at up to three time points in pregnancy. Multiple linear regression models were conducted to investigate the association between gestational length and the interaction between average exposure biomarkers and LES. Multiple linear regression models regressing the exposure biomarkers in relation to gestational length were also stratified by LES, Negative LES, and Positive LES, based on the subjective ratings of events. Results were transformed into the change in gestational length for an inter-quartile-range difference in the exposure.
Results:
Of the four psychosocial stress measures, only the life events score (LES) was a significant modifier. Associations between triclocarban, bisphenol-S, methyl- and propylparaben in relation to gestational length were stronger among women with negative Total LES scores. Among women with negative Total LES scores, bisphenol-S and triclocarban were associated with a 3-5 day decrease in gestational length [(−3.15; 95% CI:−6.06, −0.24); (−4.68; 95% CI: −8.47, −0.89)], whereas methylparaben and propylparaben were associated with a 2-3 day increase in gestational length [(2.21; 95% CI: 0.02, 4.40); (2.92; 95% CI: 0.58, 5.26)]. Significant interactions were driven by negative life events, but the association with triclocarban was driven by few positive life events.
Conclusions:
Associations between exposure biomarkers and gestational length were stronger in the presence of negative life events. This provides evidence that stress makes the body more vulnerable to chemical exposure.
Keywords: endocrine disruptors, gestational age, birth outcomes, non-chemical stressor, interaction
Graphical Abstract
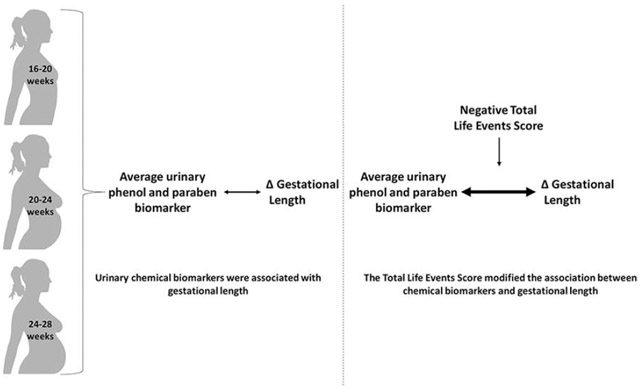
1. Introduction
Phenols and parabens are a group of chemicals commonly found in personal care products and household items, and exposure to these chemicals has been associated with gestational length, and a number of growth parameters at birth, including birth weight and birth length (Ferguson et al. 2018; Lassen et al. 2016; Philippat et al. 2014; Tang et al. 2013; Wolff et al. 2008). We previously reported on associations between bisphenols, parabens and triclocarban with various birth outcomes in this cohort of pregnant women in Puerto Rico (Aker et al. 2018). We found an increase in gestational length in association with benzophenone-3, bisphenol-A (BPA), and methyl- and propyl-paraben, and a decrease in gestational length in association with triclocarban. Bisphenol-S (BPS) was associated with changes in birth size, depending on the timing of exposure.
Maternal stress has also been associated with adverse birth outcomes. A population-based case control study reported a 60% increased odds of a very low birth weight infant among women who reported always feeling stressed as compared to women reporting no stress (Sable and Wilkinson 2000). Likewise, other studies have reported a shortened gestational length with maternal stress or a larger number of negative life events (Barbosa 2000; Dole et al. 2003; Dominguez et al. 2005; Rich-Edwards and Grizzard 2005). The effects of maternal stress on the fetus also extend beyond pregnancy, and potentially cause long-term adverse effects on the cognitive, behavioral and psychomotor development of the child (Kingston et al. 2012; Weinstock 2008).
There is a growing interest in looking at the combined effect of stressors in the environment on human health. In addition to exploring the effect of chemical mixtures on health, interactions between chemical and non-chemical stressors are important to consider in deepening our understanding of how the environment impacts humans. Although this area of research is relatively new and limited, evidence supports the notion of an increased risk of adverse effects with exposure to high concentrations of environmental chemicals and the modifying effect of high psychosocial stress levels. For example, a systematic review found the effect of chemical exposures, such as smoking and traffic pollution, in combination with high stress or socio-economic stressors was associated with worse fetal growth parameters than with any of the chemical or socio-economic stressors on their own (Vesterinen et al. 2017).
As a follow-up to our previous study, we examined the potential modifying effect of maternal stress on the associations we observed between biomarkers of several common chemical exposures and gestational age. We tested this question using the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) project, a multi-disciplinary research center in Northern Puerto Rico that established a pregnancy cohort to investigate the role of environmental contamination in adverse birth outcomes. The analysis explored relationships between gestational length and the interaction between urinary concentrations of phenols, parabens and triclocarban, and psychosocial factors (depression, perceived stress, social support and life events) using data from the same cohort described in Aker et al. (2018). This manuscript focuses on the exposure metabolites of interest based on our results from our previous analysis (benzophenone-3, BPA and BPS, triclocarban, methyl- and propyl-paraben). We hypothesized that increased psychosocial stress would exacerbate the decrease in gestational length in the case of triclocarban and BPS. BPA, benzophenone-3, methyl and propyl-paraben were associated with an increase in gestational age in our previous manuscript. We hypothesized that the combined effect of exposure to these chemicals and increased psychosocial effect would lead to a null association with gestational length.
2. Methods
2.1. Study Population:
The details of the study population were previously described in Aker et al., 2018. Briefly, participants for this study were from the PROTECT cohort, an ongoing prospective cohort of pregnant women recruited from two hospitals and five affiliated health clinics in Puerto Rico. The exclusion criteria included: women who lived outside the region, multiple gestations, use of oral contraceptives within three months prior to getting pregnant, pregnancy through in vitro fertilization, and any known medical health complications (including diabetes, hypertension, etc.). The present analysis includes 908 study participants recruited from 2011-2017 at 14 ± 2 weeks gestation. There were up to three study visits performed on each of the participants: visit 1 at 16-20 weeks gestation; visit 2 at 20-24 weeks gestation; and, visit at 24-28 weeks gestation. These study visits coincided with routine clinical visits and rapid fetal growth. All demographic data was collected at visit 1, and questionnaires on psychosocial status were administered at study visits 2 and 3 (further details found below). Spot urine samples for urinary exposure biomarker measurement were collected at each of the three study visits. This study was approved by the research and ethics committees of the University of Michigan School of Public Health, University of Puerto Rico, Northeastern University, and participating hospitals and clinics. All study participants provided full informed consent prior to participation.
2.2. Urinary Biomarker Measurement:
Spot urine samples collected during the three study visits were divided into aliquots and frozen at −80°C. They were shipped overnight in dry ice to the Centers for Disease Control and Prevention for analysis. Samples were analyzed for seven phenols (2,4-dichlorophenol, and 2,5-dichlorophenol, BPA, BPS, BPF, benzophenone-3, triclosan), four parabens (ethylparaben, methylparaben, butylparaben and propylparaben) and triclocarban using online solid phase extraction-high-performance liquid chromatography-isotope dilution tandem mass spectrometry (Watkins et al. 2015; Ye et al. 2005, 2006).
The sample size for BPS and triclocarban was smaller than for the other urinary biomarkers because they were added to the analytical panel mid-way through the study, and thus, only available on a subset of the cohort (N: 540-544). Urinary specific gravity (SG) was measured using a digital handheld refractometer (AtagoCo., Ltd., Tokyo, Japan), and was used to account for urinary dilution. Samples below the limit of detection (LOD) were assigned a value of the LOD divided by √2 (Hornung and Reed 1990). Urinary biomarkers were corrected for SG in preliminary analyses. For the purpose of clarity, this manuscript focuses on the exposure biomarkers associated with changes in birth outcomes from our previous analysis (Aker et al., 2018), which are benzophenone-3, BPA, BPS, triclocarban, methyl- and propyl-paraben. Biomarker distributions can be found in our previous report, Aker et al. (2018). Methyl- and propyl-paraben were highly correlated (Spearman correlation: 0.78).
2.3. Psychosocial Scores:
The psychosocial status of study participants was determined via questionnaires. The questionnaire included questions from four instruments: 1) Center for Epidemiological Studies-Depression (CES-D) (Radloff 1977); 2) Perceived Stress Scale (PSS) (Cohen et al. 1983); 3) ENRICHD Social Support Instrument (ESSI) (ENRICHD Investigators 2001); and, 4) Life Experiences Survey (LES) (Sarason et al. 1978). The CES-D, PSS and ESSI instruments were administered at 28 weeks gestation in the clinic, whereas the LES questionnaire was administered during the in-home study visit (20-24 weeks gestation). All questions were translated and administered in Spanish. As described below, new categorical psychosocial scores were created for statistical analyses.
The CES-D is a 20-item score that asks participants how they felt in the past week to determine their depression status. A score of ≥16 for CES-D is typically used to determine depression (Lewinsohn et al. 1997); therefore CES-D was categorized into scores greater than or equal to 16 or below 16.
The PSS is a 10-item score that aims to determine the participants’ perceived stress levels during the previous month. There is no established cut-off for PSS. Therefore, we set the cut-off for PSS at the 75% percentile (score of 28, maximum=49), wherein participants with scores ≥28 were considered to have high perceived stress. A cut-off of 28 was also used previously used in the literature (Shah et al. 2010).
ESSI is a 7-item score that asks respondents how they currently feel with regards to the social support that they have. There is no established cut-off for ESSI; however, two recent studies used a cut-off of ≤18 for the ESSI score to define low social support (Aggarwal et al. 2010; Bucholz et al. 2014); therefore we used the same cut-off for our analyses. This population reported very high scores of social support, so a cut off of 18 also allowed us to establish a distinct reference group of women with lower social support.
LES is a 38-item score that asks participants whether they experienced a list of events since becoming pregnant, and allows participants to rate how positive or negative they perceived the event to be on a scale of −3 to 3. A score of zero is recorded if an event is not experienced. The questionnaire administered is included in Appendix II. Some questions from the original questionnaire were removed due to irrelevance to our study population; these included questions specific to students and men, and questions not relevant to women in our study, including being pregnant or having an abortion. The Total LES (TLES) score was categorized into three groups to take into account the overall negative and positive scores. The cut-off points were below −1, between −1 and 1, and above 1 (labelled “neagative”, “neutral” and “positive”). Two more scores were calculated from the LES score. A Positive LES score (PLES) was calculated as a sum of only life events scored > 0, and a Negative LES score (NLES) was calculated as a sum of only life events scored < 0. The categories for PLES and NLES scores were created to have a sufficient number of participants per group. The cut-offs were scores below 2 (−2), between 2 and 5 (−2, −5), and above 5 (−5) (labelled “low”, “medium”, and “high”).
2.4. Gestational length Calculation:
The American College of Gynecologists (ACOG) recommendations were used for the gestational length calculation of complete pregnancies (Committee on Obstetric Practice, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine 2017). In brief, ACOG recommends determining gestational length using either information from the first ultrasound or the last menstrual cycle date (LMP), depending on two cutoffs: 1) how far along the pregnancy the ultrasound was conducted, and 2) the difference in the estimated date of delivery (EDD) calculated from the LMP and from the ultrasound. Further details on this calculation can be found in the ACOG Committee Opinion document (Committee on Obstetric Practice, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine 2017).
2.5. Statistical Analyses:
A detailed description of the preliminary analyses conducted for exposure biomarkers, gestational length, and important covariates can be found in Aker et al. (2018). Briefly, after distributions of key demographic characteristics were calculated, multiple linear regressions (MLR) were conducted to examine associations between gestational length and each average exposure biomarker. Gestational length was right-skewed, but sensitivity analyses in our previous analysis revealed no differences in interpretation of models with the gestational length variable as is, and the transformed version of the variable. We re-ran the bivariate analyses on the potential covariates and urinary exposure biomarkers, stratified by TLES scores. To compare the differences in frequencies of categorical variables across the LES categories, we used the Chi square test. The continuous variables (age and urinary exposure biomarkers) were not normally distributed; therefore, to compare the continuous variables in the groups, we calculated the Mann–Whitney U test. Following this, we conducted univariate analysis on the four psychosocial scores we had available. The mean, median and select percentiles were calculated for each of the scores. In addition, the frequency of each life event was calculated in the case of LES.
We constructed MLR models to investigate associations between each categorical psychosocial score and gestational length. There was no evidence of non-linearity after the addition of penalized and smoothing splines, so we constructed MLR models to study the interaction between each mean-centered log average urinary exposure biomarker concentration and each categorical psychosocial score in relation to gestational length. For this analysis, the LES variable beta coefficients represented the trend in LES categories. The average urinary exposure biomarker concentration was calculated using the urinary exposure biomarker concentrations across the three study visits. In the case of a missing value at one of the study visits, the average was taken of the two remaining concentrations. In the case of two missing concentrations, the “average” phenol or paraben concentration was equal to the available concentration. Crude analyses included only the average specific gravity as a covariate in addition to the urinary biomarker and psychosocial score. Potential covariates were selected based on the literature. We maintained a covariate if it changed the main effect estimate by >10%, which were added in a step-wise fashion. Final models were controlled for the following confounders: specific gravity, maternal age, insurance type, alcohol use, and self-reported exposure to second-hand smoking. Other variables considered, but not included, were parity, smoking, maternal education, marital status, number of children, and BMI.
In MLR models with interaction terms exposure biomarker*psychosocial variable, only models with interactions with LES variables were statistically significant (p<0.05). Therefore, the remainder of the analyses focused on interactions between biomarkers of exposure and LES variables: TLES, PLES and NLES scores. Two analyses were conducted. First, to better understand the interaction effects, we studied the interaction between LES variables in categorical form and exposure biomarkers, in contrast to previous models that looked at the trend across the LES variables. Interaction p values less than 0.10 were considered of interest. Second, the MLR models were stratified by LES categories. To study the effect of infant sex, we further stratified the MLR models by infant sex. All effect estimates from MLR models were transformed to the change in gestational length in relation to the interquartile range (IQR) increase in urinary biomarker concentrations. The alpha level was set at 0.05 for stratified analysis. All statistical analyses were conducted in R Version 3.4.2.
As a sensitivity analysis, we included PLES scores as a covariate in models for NLES scores, and included NLES scores as a covariate in models for PLES scores. There were no differences in the results with the addition of these covariates. Second, we restricted our analysis to women with data from only one study visit, only two study visits, and all three study visits. The sample sizes in each group were too small to detect significant interactions, but the directions of the associations remained the same. Based on this, and the high intra-class correlation values (Supplementary Table 3), we proceeded with our calculation of the average exposure biomarker as described above. We also examined the interaction of depression, CES-D, and exposure biomarkers in more detail, because the interaction p value was 0.08, which indicated a suggestive association. However, no trends emerged after stratification of the results by CES-D scores.
3. Results
Of the 908 pregnant women included in the analysis, up to 752 women had exposure, outcome and covariate data. Table 1 presents demographic characteristics of the study population stratified by TLES scores. The study population was on average 26 years of age; over half had BMI levels <25; 3% were current smokers; and, approximately 20% were single. Women with positive TLES scores were generally younger (25 years versus 27 years) and less likely to have more than one more child. Women with overall negative TLES scores were more likely to be current smokers (4.9% versus 2%).
Table 1:
Summary demographics of the 908* pregnant women in the study population stratified by Total LES Scores (TLES)
| Total | Negative TLES Score |
Neutral TLES Score |
Positive TLES Score |
p | |
|---|---|---|---|---|---|
| N | 908 | 330 | 272 | 306 | |
| Maternal Age (median [IQR]) | 26 [22.00, 31.00] | 27 [23.00, 31.00] | 27 [22.00, 31.00] | 25 [21.00, 29.00] | <0.001 |
| BMI (%) | 0.16 | ||||
| 0-25 | 419 (56.5) | 151 (54.9) | 118 (53.6) | 150 (61) | |
| 25-29.9 | 200 (27.0) | 84 (30.5) | 57 (25.9) | 59 (24) | |
| >29.9 | 122 (16.5) | 40 (14.5) | 45 (20.5) | 37 (15) | |
| Insurance type (%) | 0.71 | ||||
| Mi Salud | 586 (63.3) | 216 (65.5) | 180 (66.2) | 190 (62.1) | |
| Private | 322 (36.7) | 114 (35.5) | 92 (36.1) | 116 (38.5) | |
| Household Income (%) | 0.91 | ||||
| <10,000 | 229 (29.5) | 89 (31) | 69 (29.4) | 71 (28) | |
| 10,000-30,000 | 244 (31.4) | 87 (30.3) | 70 (29.8) | 87 (34.3) | |
| 30,000-50,000 | 182 (23.5) | 68 (23.7) | 55 (23.4) | 59 (23.2) | |
| >50,000 | 121 (15.6) | 43 (15) | 41 (17.4) | 37 (14.6) | |
| Maternal Education (%) | 0.78 | ||||
| <High School/GED | 185 (20.6) | 68 (20.7) | 57 (21.4) | 60 (19.9) | |
| Some College | 324 (36.2) | 124 (37.8) | 88 (33.1) | 112 (37.1) | |
| College graduate | 387 (43.2) | 136 (41.5) | 121 (45.5) | 130 (43) | |
| Smoking (%) | 0.02 | ||||
| Never | 752 (83.7) | 269 (82) | 237 (88.4) | 246 (81.5) | |
| Ever | 118 (13.1) | 43 (13.1) | 25 (9.3) | 50 (16.6) | |
| Current | 28 ( 3.1) | 16 (4.9) | 6 (2.2) | 6 (2) | |
| Exposure to second hand smoking (%) | 0.86 | ||||
| None | 769 (88.4) | 278 (87.1) | 232 (89.9) | 259 (88.4) | |
| Up to 1 hour | 44 ( 5.1) | 19 (6) | 11 (4.3) | 14 (4.8) | |
| More than 1 hour | 57 ( 6.6) | 22 (6.9) | 15 (5.8) | 20 (6.8) | |
| Alcohol Consumption (%) | 0.15 | ||||
| None | 454 (50.8) | 165 (50.6) | 148 (55.4) | 141 (46.8) | |
| Before pregnancy | 380 (42.5) | 136 (41.7) | 108 (40.4) | 136 (45.2) | |
| Yes within the last few months | 60 ( 6.7) | 25 (7.7) | 11 (4.1) | 24 (8) | |
| Marital Status (%) | 0.09 | ||||
| Single | 178 (19.8) | 63 (19.2) | 48 (17.9) | 67 (22.1) | |
| Married | 510 (56.7) | 186 (56.7) | 168 (62.7) | 156 (51.5) | |
| Divorced | 11 ( 1.2) | 7 (2.1) | 1 (0.4) | 3 (1) | |
| Living together | 200 (22.2) | 72 (22) | 51 (19) | 77 (25.4) | |
| Number of previous children (%) | 0.002 | ||||
| 0 | 430 (47.9) | 142 (43.3) | 118 (44) | 170 (56.5) | |
| 1 | 358 (39.9) | 143 (43.6) | 108 (40.3) | 107 (35.5) | |
| >1 | 109 (12.2) | 43 (13.1) | 42 (15.7) | 24 (8) | |
P values calculated from Chi squared test for categorical variables and Kruskal test for continuous variables.
Totals may not add up to 100% due to missing values.
908 women had data on LES and gestational length.
Of the 38 life events included in the LES questionnaire, 14 life events were experienced by at least 10% of the study participants (Table 2). Half of the participants experienced changes in eating and sleeping habits. Approximately 25% experienced mean negative changes in social and recreational activities, a mean negative change in financial status, or a mean positive change in personal achievements. Ten percent experienced an illness and/or death of a close family member. No main effects in models regressing gestational length against psychosocial variables were statistically significant, and all p-values exceeded 0.50 (Supplementary Table 4). Additionally, none of the interaction terms between any of the urinary biomarkers and CES-D, PSS and ESSI were statistically significant (Supplementary Table 5). In turn, the remainder of this manuscript focuses on the results of the interaction between phenol, paraben and triclocarban concentrations with TLES, PLES and NLES on gestational length.
Table 2:
Life events that occurred in at least 10% of the study population and their mean scores. N=908.
| Life Event | N (%) | Mean score |
|---|---|---|
| Drastic change in eating habits | 463 (51.9) | 0.21 |
| Drastic change in sleeping habits | 462 (51.7) | −0.52 |
| Major change in social activities | 246 (27.6) | −0.29 |
| Change in amount/type recreation | 231 (25.9) | −0.35 |
| Change in financial status | 215 (24.1) | −0.39 |
| Successful personal achievement | 211 (23.7) | 1.16 |
| Change in number of discussions with spouse | 192 (21.5) | −0.70 |
| Change in church activities | 150 (16.8) | 0.37 |
| Change in proximity to family member | 149 (16.7) | 0.65 |
| Moved to a new location | 163 (15.6) | 1.55 |
| Changes in the workplace | 127 (14.2) | −0.14 |
| Changes in spouse's workplace | 127 (14.2) | 0.22 |
| Death of a close family member | 95 (10.7) | −1.70 |
| Illness in a close family member | 91 (10.2) | −1.56 |
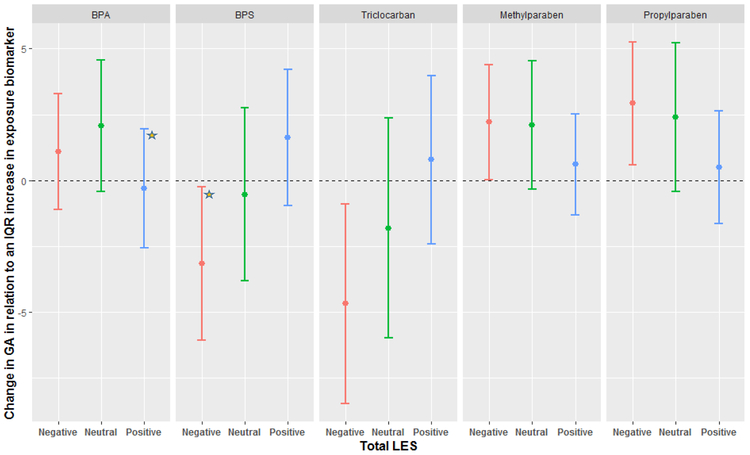
In a previous study, we observed 1-2 day increases in gestational length in relation to an IQR increase in urinary benzophenone-3, BPA, methylparaben and propylparaben, as well as a 2 day decrease in gestational length in relation to urinary triclocarban (Supplementary Table 1) (Aker et al. 2018). Figure 1 shows the results of the MLR models regressing these urinary exposure biomarkers against gestational length when stratified by TLES categories. There was a general linear trend across the TLES categories such that the strongest associations between the urinary biomarker and gestational length were among women with negative TLES scores, with the exception of BPA where no trends emerged. Among women with positive TLES scores, associations between the urinary biomarkers and gestational length were largely not statistically significant (Supplementary Table 6). When treated as an ordinal variable, the interaction between TLES and BPS, triclocarban and propylparaben was statistically significant (Supplementary Table 3). BPS and triclocarban were associated with a 3-5 day decrease in gestational length among women with negative TLES scores [(−3.15 change in gestational length days per IQR increase in exposure biomarker (Δ); 95% CI-6.06, −0.24); (Δ −4.68; 95% CI: −8.47, −0.89), respectively], whereas methylparaben and propylparaben were associated with a 2-3 day increase in gestational length among women with negative TLES scores [(Δ 2.21; 95% CI: 0.02, 4.40); (Δ 2.92; 95% CI: 0.58, 5.26), respectively]. Of these associations, the interaction p value was suggestive in the case of BPS and PPB (p value 0.06, 0.10). BPS was not independently associated with gestational length, the strength of the interaction between BPS and TLES (ordinal and categorical), as well as the linear trend observed across the TLES categories lend confidence to these results. Nevertheless, these associations should be interpreted with caution. Associations between benzophenone-3 and gestational length remained unchanged across all the LES categories.
Figure 1:
Adjusted change in gestational length in relation to average mean-centered exposure biomarker concentration across three time points during pregnancy, stratified by TLES. Beta coefficients were transformed to change in gestational days for an IQR increase in exposure biomarker concentration. The star represents a statistically significant interaction as compared to the Neutral category.
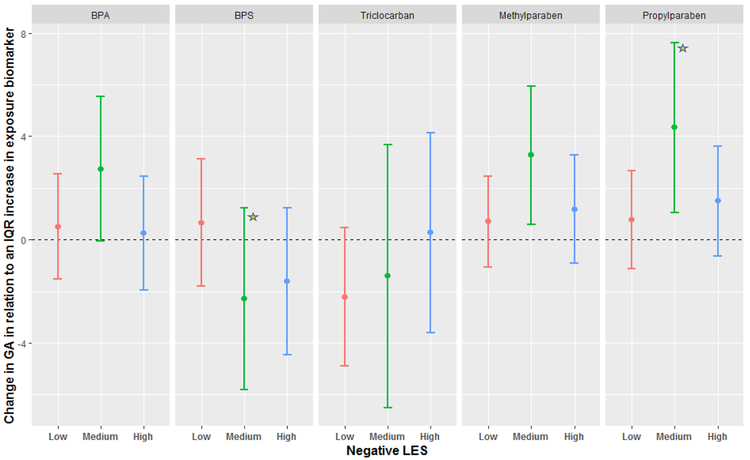
Figure 2 shows the MLR results stratified by NLES scores. No clear patterns emerged, although associations between BPA, methylparaben and propylparaben and gestational length were strongest among women with NLES scores in the medium category. The association between BPS and gestational length was not statistically significant in any of the NLES categories; however, the 2 day decrease in gestational length among women with medium NLES was statistically different compared to the change in gestational length among women in the low NLES category (p value = 0.08).
Figure 2:
Adjusted change in gestational length in relation to average mean-centered exposure biomarker concentration across three time points during pregnancy, stratified by NLES. Beta coefficients were transformed to change in gestational days for an IQR increase in exposure biomarker concentration. The star represents a statistically significant interaction as compared to the Low category.
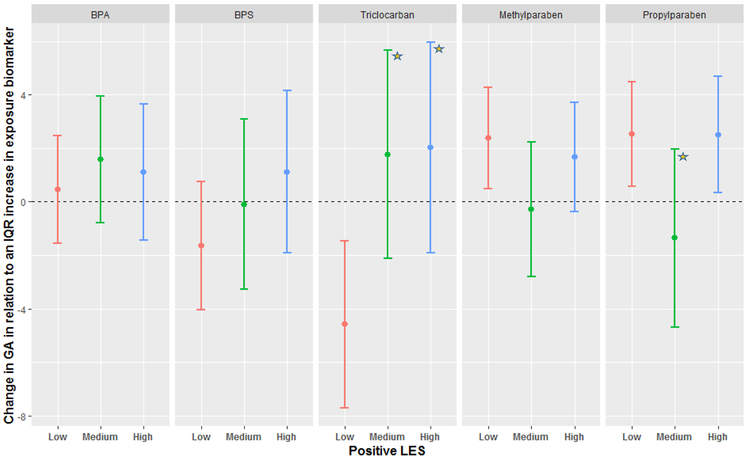
Figure 3 shows the MLR results stratified by PLES scores. There was a strong association between triclocarban and reduced gestational length among women with low PLES scores (Δ −4.58; 95% CI: −7.70, −1.46). Methylparaben and propylparaben (Spearman correlation = 0.78; Aker et al, 2018) were significantly associated with gestational length among women with low PLES scores; however, these associations were also observed among women with high PLES scores.
Figure 3:
Adjusted change in gestational length in relation to average mean-centered exposure biomarker concentration across three time points during pregnancy, stratified by PLES. Beta coefficients were transformed to change in gestational days for an IQR increase in exposure biomarker concentration. The star represents a statistically significant interaction as compared to the Low category.
After stratification by fetal sex, the associations with parabens discussed above were only observed in girls, and had larger magnitudes (Supplementary Table 7). On the other hand, associations with BPA and BPS were only observed among males. However, the sample sizes for each group in this analysis were relatively small (60-90 women), so caution must be taken when interpreting these results.
4. Discussion
Women with negative TLES scores had strong associations between some exposure biomarkers and gestational length, especially in the case of BPS, triclocarban, methylparaben and propylparaben. The inverse association between triclocarban and gestational length was strongest among women with low PLES scores, suggesting a protective effect of positive life events against the impact of triclocarban on pregnancy duration. Fetal sex may also add another layer of complexity.
Life events, a proxy for measuring maternal stress, have been linked to adverse birth outcomes (Barbosa 2000; Dole et al. 2003; Dominguez et al. 2005; Rondó et al. 2003; Ruiz et al. 2002; Talge et al. 2007). However, in our population, LES scores were not associated with gestational length in adjusted models. This indicates that experiencing negative life events, and thus experiencing stress, may not be sufficient to illicit an adverse effect in our cohort. It was only the combination of overall negative TLES scores and higher concentrations of exposure biomarkers that we observe changes in gestational length of up to five days.
Olson et al. prescribe to a “two-hit” theory under an allostatic freedom which could help describe the potential biological mechanisms at play (Olson et al. 2015). The two-hit theory postulates that each “hit” or stressor to the body cumulatively leads to changes in the complex mechanisms involved in parturition, potentially leading to a tertiary outcome with an increased number of “hits”. Rat dams were exposed to both stress and the pro-inflammatory cytokine, IL-1β. Even though neither stressor elicited a response on their own, the combined effect led to preterm birth in the rats. Thus, our results could be described under this framework, where the first “hit” may be maternal stress, and the second “hit” is the chemical exposure. When only one of our stressors was present in the model (LES or exposure biomarker), a small or no effect was observed on gestational length, but the interaction of the two led to changes in parturition and gestational length, consistent with a “two-hit” mechanism.
The prevailing theory on the mechanism between maternal stress and gestational length is hypothesized to occur via the maternal-placental-fetal hypothalamic-pituitary-adrenal (HPA) axis or inflammatory responses (de Weerth and Buitelaar 2005; Kingston et al. 2012; Kramer and Hogue 2009; Rich-Edwards and Grizzard 2005). Maternal stress’ influence on the maternal HPA axis stimulates cortisol production via corticotropin-releasing hormone (CRH) (de Weerth and Buitelaar 2005). Cortisol concentration is an important physiological marker because higher levels of maternal cortisol are linked to increased intrauterine constriction and preterm birth (Carpenter et al. 2017; Cherak et al. 2018; Smith 2007).
CRH also stimulates the release of prostaglandins and inflammatory responses in the maternal-placental-fetal systems, thereby preparing women for labor and stimulating myometrial contractions (Smith and Nicholson 2007; Thomson 2013; Wadhwa et al. 2001). In fact, women in preterm labor have higher levels of CRH compared to gestational age-matched women, as well as accelerated rates of CRH over the course of their gestation (Wadhwa et al. 2001, 2004). So it is possible that through this mechanism, an increase in CRH levels from maternal stress could lead to an earlier parturition. Elevated levels of circulating CRH in the early third trimester were reported among women with high perceived stress levels (Wadhwa et al. 1996; Weinstock 2005). In addition, our team previously reported an increase in CRH in association with methylparaben and propylparaben, and a decrease in CRH in association with BPS among a smaller subset of the PROTECT cohort (Aker et al. 2019). However, the direction of these associations are in the opposite direction of what one would expect to cause an increase or decrease in gestational length, respectively.
In addition to the effects CRH has on prostaglandins and inflammatory mediators, chronic stress leads to inhibition of the hypothalamic-pituitary-adrenal axis. During chronic stress, prolonged periods of elevated levels of circulating cortisol inhibit hypothalamic production of thyroid stimulating hormone (TSH), leading to a decreased release of TSH from the pituitary gland, and subsequent decreased release of thyroxine (T4) from the thyroid. Furthermore, CRH inhibits conversion of thyroxine (T4) to the bioactive triiodothyronine (T3) (Helmreich et al. 2005; Heyma and Larkins 1982). However, these effects depend on the duration and intensity of the stressor (Helmreich et al. 2005). In one study, decreased levels of FT4 was associated with higher rates of perinatal syndromal depression (Pedersen et al. 2016), which may be a result of decreased release of T4. There is evidence that circulating levels of thyroid hormones, particularly decreased FT4 and increased T3, are associated with preterm birth (Johns et al. 2017). Our previous analysis found associations between urinary concentrations of BPA and triclocarban and an increase in T3 (Aker et al. 2019). Thus, decreased FT4 levels from depression or high stress may be the first hit, and the increase in T3 from triclocarban could be the second hit, leading to a shortened gestational length. BPA was also associated with an increase in FT4, which may explain why we do not observe a shortened gestational length with negative TLES.
Besides the neuroendocrine pathway, a potential mechanism of action could be via inflammatory responses. Some stressful life events during pregnancy were suggestively associated with elevated levels of 8-iso-PGF2α (an oxidative stress marker) measured at a median of 32 weeks (Eick et al. 2018). Additionally, chronic stress is related to immunosuppression and changes in the response to antigens (Elenkov and Chrousos 1999), which is related to a decreased gestational age at birth. Exposure to some phenol and parabens have also been associated with oxidative stress and inflammatory biomarkers, including BPA and propylparaben (Neier et al. 2015; Watkins et al. 2015). In summary, we suggest several pathways that could lead to an increase in hits with the combined effect of chemical exposure and maternal stress, including: 1) a direct effect on cortisol; 2) a direct effect on CRH; 3) an indirect effect on thyroid hormones via CRH and cortisol; and 4) a direct effect on inflammatory responses.
The associations with parabens were especially difficult to interpret. Parabens were associated with an increase in gestational length; however, maternal stress is generally associated with a decrease in gestational length. Parabens were associated with a decrease in estriol (Aker et al. 2019), while stress is associated with an increase in CRH (Weinstock 2005), and subsequent increase in estriol (Smith et al. 2012). Additionally, parabens have antimicrobial properties which could decrease inflammation (Turakka et al. 1988), while stress is associated with an increase in inflammation (Bronson and Bale 2014). Because of these opposite biological effects, no effect on gestational length with high levels of stress and paraben exposure would have been expected. This unexpected finding could indicate that parabens affect gestational length through a different mechanism than the one proposed, or it could indicate that interaction between stressors act differently than in the presence of each stressor alone. This was also observed in an animal study that exposed rats to various stressors across two generations, and also observed different effects on birth outcomes with different combinations of stressors (Verstraeten et al. 2019).
Given the lack of significant associations between triclocarban and gestational length when the results were stratified by NLES scores, this association could indicate that the relationship between triclocarban and gestational length among women with negative TLES scores is driven by the lack of positive life events, rather than the presence of negative life events. This is consistent with the literature that suggests positive life events and perceived social support may act as a buffer to stress and negative events (Cohen et al. 1984). For example, positive life events were correlated with lower salivary cortisol levels among a group of 60 pregnant women, while negative life events were not (Pluess et al. 2012).
We did not observe any interactions between exposure biomarkers and other psychosocial scores. One reason may be due to the different timing the scores attempt to capture. The ESSI score asks participants how they currently feel; the CES-D score asks participants how they felt in the past week, the PSS score focuses on the previous month, and LES pertains to events since becoming pregnant. While the scores are dependent on each other given they include similar questions, it is interesting to note that the only significant interactions we observed included the psychosocial score that has the widest time frame and would presumably include the least subjective questions, LES.
We did not identify other studies that have investigated the same associations as this study. We did, however, identify a study that looked at the combined effect of phthalate exposure and stressful life events on the anogenital distance (AGD) among over 700 women in a well-educated cohort based in four medical centers across the U.S. (Barrett et al. 2016). The study found that among boys, prenatal exposure to stressful life events modified the association between first trimester di(2- ethylhexyl) phthalate (DEHP) metabolite concentrations and altered genital measurements, such that the association between DEHP and altered genital measurements was only significant among mothers with low stress (and not high stress). While our results are not directly comparable, both studies support further exploration of the effect of chemical and non-chemical stressors on perinatal health.
While our study is among the first to explore the effects of endocrine disruptors in addition to psychosocial variables, there were a few limitations. The timing of maternal stress could influence the effect on the fetus (Weinstock 2005); however, we do not have precise data on when the life event took place apart from happening sometime between the beginning of pregnancy and the second study visit (20-24 weeks gestation). Similarly, we have no data on the participants’ LES profiles past the second study visit. Evidence also suggests that the type of life event is an important factor to consider (i.e. a family death versus changes in diet), rather than combining all life events as equal in magnitude and/or importance (Eick et al. 2018; Talge et al. 2007). However, because our LES scores allowed the participant to rate how positive or negative the event was, this limitation is unlikely to have greatly affected our results. While our analysis used urinary exposure biomarker data from three time points, our analyses still resembles a cross-sectional study in this context. In addition, we constructed multiple models in this exploratory study, which increases the risk of Type 1 error.
Our study also had many strengths. Our large sample size allowed us to explore the modifying effects of both psychosocial variables and fetal sex. We also had data on a total of four psychosocial scores, which allowed us to capture different methods of exploring stress during pregnancy. Most studies that examine the effect of life events focus solely on negative life events. However, our study shows the importance of both negative and positive life events, and our most consistent results were based on the TLES scores rather than the Negative and Positive LES scores separately. Furthermore, our urinary exposure biomarker panel included BPS and triclocarban which have not been studied in detail to date.
Our cohort also has higher levels of triclocarban as compared to other populations (Ashrap et al. 2018) allowing us to study the association between this biomarker, maternal stress, and gestational length in a vulnerable population.
5. Conclusion
This study is one of the first to examine the effect of chemical exposure in the presence of maternal stress. Associations were stronger in the presence of negative life events, and positive events had some protective effect on the association between exposure biomarkers and gestational length. This indicates that stress makes the body more vulnerable to the effects of chemical exposure. Pregnancy is a stressful period in a woman’s life, and the increased vulnerability to chemical exposure could have adverse effects on her and the fetus. More research is required to verify these associations.
Supplementary Material
Highlights.
Life events modified associations between chemical exposure and gestational length
Relationships were stronger in the presence of overall negative life event scores
There was evidence of buffering with positive life events
Acknowledgements:
We gratefully acknowledge Antonia Calafat and Xiaoyun Ye at the Centers for Disease Control and Prevention for analysis of urinary phenol, paraben and triclocarban concentrations. This work was supported by the National Institute of Environmental Health Sciences, National Institutes of Health (Grants P42ES017198, P50ES026049, P30ES017885, UG3OD023251, and UH3OD023251). The authors also thank the nurses and research staff who participated in cohort recruitment and follow up, as well as the Federally Qualified Health Centers (FQHC) in Puerto Rico that facilitated participant recruitment, including Morovis Community Health Center, Prymed in Ciales, Camuy Health Services, Inc. and the_Delta OBGyn Group in Manati, as well as the Manati Medical Center and the Metro Pavia Hospital in Arecibo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests: The authors declare they have no actual or potential competing financial interests.
8. References:
- Aggarwal B, Liao M, Allegrante JP, Mosca L. 2010. Low Social Support Level is Associated with Non-Adherence to Diet at 1-Year in the Family Intervention Trial for Heart Health (FIT Heart). J Nutr Educ Behav 42:380–388; doi: 10.1016/j.jneb.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker AM, Ferguson KK, Rosario ZY, Mukherjee B, Alshawabkeh AN, Cordero JF, et al. 2018. The associations between prenatal exposure to triclocarban, phenols and parabens with gestational age and birth weight in northern Puerto Rico. Environ Res 169:41–51; doi: 10.1016/j.envres.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrap P, Watkins DJ, Calafat AM, Ye X, Rosario Z, Brown P, et al. 2018. Elevated concentrations of urinary triclocarban, phenol and paraben among pregnant women in Northern Puerto Rico: Predictors and trends. Environ Int; doi: 10.1016/j.envint.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa GA. 2000. The association of life events to gestational age at delivery among low-income, urban, African American women. J Perinatol Off J Calif Perinat Assoc 20: 438–442. [DOI] [PubMed] [Google Scholar]
- Barrett ES, Parlett LE, Sathyanarayana S, Redmon JB, Nguyen RHN, Swan SH. 2016. Prenatal stress as a modifier of associations between phthalate exposure and reproductive development: results from a multicenter pregnancy cohort study. Paediatr Perinat Epidemiol 30:105–114; doi: 10.1111/ppe.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz EM, Strait KM, Dreyer RP, Geda M, Spatz ES, Bueno H, et al. 2014. Effect of Low Perceived Social Support on Health Outcomes in Young Patients With Acute Myocardial Infarction: Results From the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) Study. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 3; doi: 10.1161/JAHA.114.001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter T, Grecian SM, Reynolds RM. 2017. Sex differences in early-life programming of the hypothalamic-pituitary-adrenal axis in humans suggest increased vulnerability in females: a systematic review. J Dev Orig Health Dis 8:244–255; doi: 10.1017/S204017441600074X. [DOI] [PubMed] [Google Scholar]
- Cherak SJ, Giesbrecht GF, Metcalfe A, Ronksley PE, Malebranche ME. 2018. The effect of gestational period on the association between maternal prenatal salivary cortisol and birth weight: A systematic review and meta-analysis. Psychoneuroendocrinology 94:49–62; doi: 10.1016/j.psyneuen.2018.04.023. [DOI] [PubMed] [Google Scholar]
- Cohen LH, McGowan J, Fooskas S, Rose S. 1984. Positive life events and social support and the relationship between life stress and psychological disorder. Am J Community Psychol 12: 567–587. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. 1983. A global measure of perceived stress. J Health Soc Behav 24: 385–396. [PubMed] [Google Scholar]
- Committee on Obstetric Practice, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine. 2017. Committee Opinion No 700: Methods for Estimating the Due Date. Obstet Gynecol 129:e150–e154; doi: 10.1097/AOG.0000000000002046. [DOI] [PubMed] [Google Scholar]
- de Weerth C, Buitelaar JK. 2005. Physiological stress reactivity in human pregnancy--a review. Neurosci Biobehav Rev 29:295–312; doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. 2003. Maternal stress and preterm birth. Am J Epidemiol 157: 14–24. [DOI] [PubMed] [Google Scholar]
- Dominguez TP, Schetter CD, Mancuso R, Rini CM, Hobel C. 2005. Stress in African American pregnancies: testing the roles of various stress concepts in prediction of birth outcomes. Ann Behav Med Publ Soc Behav Med 29:12–21; doi: 10.1207/s15324796abm2901_3. [DOI] [PubMed] [Google Scholar]
- Eick SM, Barrett ES, van’t Erve TJ, Nguyen RHN, Bush NR, Milne G, et al. 2018. Association between prenatal psychological stress and oxidative stress during pregnancy. Paediatr Perinat Epidemiol 32:318–326; doi: 10.1111/ppe.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov null, Chrousos null. 1999. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab TEM 10:359–368. [DOI] [PubMed] [Google Scholar]
- ENRICHD Investigators. 2001. Enhancing Recovery in Coronary Heart Disease (ENRICHD) study intervention: rationale and design. Psychosom Med 63: 747–755. [PubMed] [Google Scholar]
- Ferguson KK, Meeker JD, Cantonwine DE, Mukherjee B, Pace GG, Weller D, et al. 2018. Environmental phenol associations with ultrasound and delivery measures of fetal growth. Environ Int 112:243–250; doi: 10.1016/j.envint.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmreich DL, Parfitt DB, Lu X-Y, Akil H, Watson SJ. 2005. Relation between the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during repeated stress. Neuroendocrinology 81:183–192; doi: 10.1159/000087001. [DOI] [PubMed] [Google Scholar]
- Heyma P, Larkins RG. 1982. Glucocorticoids decrease in conversion of thyroxine into 3, 5, 3’-triiodothyronine by isolated rat renal tubules. Clin Sci Lond Engl 1979 62: 215–220. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl Occup Environ Hyg 5:46–51; doi: 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Seely EW, Meeker JD. 2017. Longitudinal Profiles of Thyroid Hormone Parameters in Pregnancy and Associations with Preterm Birth. PLOS ONE 12:e0169542; doi: 10.1371/journal.pone.0169542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston D, Tough S, Whitfield H. 2012. Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child Psychiatry Hum Dev 43:683–714; doi: 10.1007/s10578-012-0291-4. [DOI] [PubMed] [Google Scholar]
- Kramer MR, Hogue CR. 2009. What Causes Racial Disparities in Very Preterm Birth? A Biosocial Perspective. Epidemiol Rev 31:84–98; doi: 10.1093/ajerev/mxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen TH, Frederiksen H, Kyhl HB, Swan SH, Main KM, Andersson A-M, et al. 2016. Prenatal Triclosan Exposure and Anthropometric Measures including Anogenital Distance in Danish Infants. Environ Health Perspect; doi: 10.1289/ehp.1409637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. 1997. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 12: 277–287. [DOI] [PubMed] [Google Scholar]
- Neier K, Marchlewicz EH, Dolinoy DC, Padmanabhan V. 2015. Assessing Human Health Risk to Endocrine Disrupting Chemicals: a Focus on Prenatal Exposures and Oxidative Stress. Endocr Disruptors Austin Tex 3; doi: 10.1080/23273747.2015.1069916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Hou Y, King BR, Tang X, Read MA, Smith R, et al. 2004. Estrogen receptor-mediated down-regulation of corticotropin-releasing hormone gene expression is dependent on a cyclic adenosine 3’,5’-monophosphate regulatory element in human placental syncytiotrophoblast cells. J Clin Endocrinol Metab 89:2312–2318; doi: 10.1210/jc.2003-030948. [DOI] [PubMed] [Google Scholar]
- Olson DM, Severson EM, Verstraeten BSE, Ng JWY, McCreary JK, Metz GAS. 2015. Allostatic Load and Preterm Birth. Int J Mol Sci 16:29856–29874; doi: 10.3390/ijms161226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen C, Leserman J, Garcia N, Stansbury M, Meltzer-Brody S, Johnson J. 2016. Late pregnancy thyroid-binding globulin predicts perinatal depression. Psychoneuroendocrinology 65:84–93; doi: 10.1016/j.psyneuen.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia F, Imperatore A, Challis JRG. 2010. Neuroendocrine Mechanisms in Pregnancy and Parturition. Endocr Rev 31:783–816; doi: 10.1210/er.2009-0019. [DOI] [PubMed] [Google Scholar]
- Philippat C, Botton J, Calafat AM, Ye X, Charles M-A, Slama R, et al. 2014. Prenatal exposure to phenols and growth in boys. Epidemiol Camb Mass 25:625–635; doi: 10.1097/EDE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M, Wurmser H, Buske-Kirschbaum A, Papousek M, Pirke K-M, Hellhammer D, et al. 2012. Positive life events predict salivary cortisol in pregnant women. Psychoneuroendocrinology 37:1336–1340; doi: 10.1016/j.psyneuen.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Radloff LS. 1977. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas 1:385–401; doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Rich-Edwards JW, Grizzard TA. 2005. Psychosocial stress and neuroendocrine mechanisms in preterm delivery. Am J Obstet Gynecol 192:S30–35; doi: 10.1016/j.ajog.2005.01.072. [DOI] [PubMed] [Google Scholar]
- Rondó PHC, Ferreira RF, Nogueira F, Ribeiro MCN, Lobert H, Artes R. 2003. Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. Eur J Clin Nutr 57:266–272; doi: 10.1038/sj.ejcn.1601526. [DOI] [PubMed] [Google Scholar]
- Ruiz RJ, Fullerton J, Brown CEL, Dudley DJ. 2002. Predicting Risk of Preterm Birth: The Roles of Stress, Clinical Risk Factors, and Corticotropin-Releasing Hormone. Biol Res Nurs 4:54–64; doi: 10.1177/1099800402004001007. [DOI] [PubMed] [Google Scholar]
- Sable MR, Wilkinson DS. 2000. Impact of perceived stress, major life events and pregnancy attitudes on low birth weight. Fam Plann Perspect 32: 288–294. [PubMed] [Google Scholar]
- Sarason IG, Johnson JH, Siegel JM. 1978. Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol 46: 932–946. [DOI] [PubMed] [Google Scholar]
- Shah M, Hasan S, Malik S, Sreeramareddy CT. 2010. Perceived Stress, Sources and Severity of Stress among medical undergraduates in a Pakistani Medical School. BMC Med Educ 10:2; doi: 10.1186/1472-6920-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon M, King TL, Kennedy HP. 2007. Allostasis: a theoretical framework for understanding and evaluating perinatal health outcomes. J Obstet Gynecol Neonatal Nurs JOGNN 36:125–134; doi: 10.1111/j.1552-6909.2007.00126.x. [DOI] [PubMed] [Google Scholar]
- Smith R 2007. Parturition. N Engl J Med 356:271–283; doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- Smith R, Nicholson RC. 2007. Corticotrophin releasing hormone and the timing of birth. Front Biosci J Virtual Libr 12: 912–918. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. 1988. Allostasis: A new paradigm to explain arousal pathology In: Handbook of life stress, cognition and health. John Wiley & Sons:Oxford, England: 629–649. [Google Scholar]
- Talge NM, Neal C, Glover V, Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. 2007. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry 48:245–261; doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang R, Chen M-J, Ding G-D, Chen X-J, Han X-M, Zhou K, et al. 2013. Associations of prenatal exposure to phenols with birth outcomes. Environ Pollut Barking Essex 1987 178:115–120; doi: 10.1016/j.envpol.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Thomson M 2013. The physiological roles of placental corticotropin releasing hormone in pregnancy and childbirth. J Physiol Biochem 69:559–573; doi: 10.1007/s13105-012-0227-2. [DOI] [PubMed] [Google Scholar]
- Vesterinen HM, Morello-Frosch R, Sen S, Zeise L, Woodruff TJ. 2017. Cumulative effects of prenatal-exposure to exogenous chemicals and psychosocial stress on fetal growth: Systematic-review of the human and animal evidence. PLoS ONE 12; doi: 10.1371/journal.pone.0176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Porto M, Sandman CA. 1996. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosom Med 58:432–446; doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, et al. 2004. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol 191:1063–1069; doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Garite TJ. 2001. Chapter 9 The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system In: Progress in Brain Research. Vol. 133 of The Maternal Brain. Elsevier; 131–142. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, Ferguson KK, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF, Meeker JD. 2015. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int J Hyg Environ Health 218:212–219; doi: 10.1016/j.ijheh.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M 2008. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 32:1073–1086; doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Weinstock M 2005. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun 19:296–308; doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. 2008. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect 116:1092–1097; doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Bishop AM, Reidy JA, Needham LL, Calafat AM. 2006. Parabens as Urinary Biomarkers of Exposure in Humans. Environ Health Perspect 114:1843–1846; doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. 2005. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 383:638–644; doi: 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.