Abstract
Regenerative functions of exosomes rely on their contents which are influenced by pathological stimuli, including hypoxia, in rotator cuff tendon injuries (RCTI). The hypoxic environment triggers tenocytes and adjacent adipose-derived mesenchymal stem cells (ADMSCs) to release regenerative mediators to the ECM via the exosomes which elicit autocrine/paracrine responses to protect the tendon matrix from injury. We investigated the exosomal protein contents from tenocytes and subcutaneous ADMSCs from the shoulder of Yucatan microswine cultured under hypoxic conditions (2% O2). The exosomal proteins were detected using high-resolution mass-spectrometry nano-LC-MS/MS Tribrid system and were compiled using ‘Scaffold’ software. Hypoxic exosomes from tenocytes and ADMSCs carried 199 and 65 proteins, respectively. The key proteins identified by mass spectrometry and associated with ECM homeostasis from hypoxic ADMSCs included MMP2, COL6A, CTSD and TN-C and those from hypoxic tenocytes were THSB1, NSEP1, ITIH4 and TN-C. These findings were confirmed at the mRNA and protein level in the hypoxic ADMSCs and tenocytes. These proteins are involved in multiple signaling pathways of ECM repair/regeneration. This warrants further investigations for their translational significance in the management of RCTI.
Keywords: Exosomes, Rotator cuff tendon injury, Tendon regeneration, Tendon matrix, Tenocytes, ADMSCs
Introduction
Tissue repair, following an injury or insult, is orchestrated by the trans-differentiation of various resident and recruited cell phenotypes which includes both stem cells and somatic cells. The well-coordinated autocrine and paracrine signaling triggered by these cells facilitate the repair response and activate tissue healing [1]. Usually, the cells involved in tissue repair/regeneration act by releasing their contents to the injury site by the exocytosis of micro/nano-vesicular exosomes. The contents of these exosomes function as stimuli and/or signals for the adjacent cells [2]. Exosomes are vesicles of 20–100 nm diameter and are packed with signaling biomolecules including proteins, lipids and nucleic acids (mainly miRNAs). The exosomes are critical in tissue regeneration as their contents are potential regulators of cell-cell and cell-ECM (extracellular matrix) communication [3], [4]. The signaling molecules (microRNAs and proteins) released by native and/or manipulated exosomes possess significant therapeutic potential to address several pathological issues [3]. However, the identification of appropriate signals and proper stimuli for the cells to secrete exosomes of therapeutic interest is challenging [5].
Rotator cuff tendon injuries (RCTI) are common and represent a major cause for shoulder pain and weakness [6], [7]. The disorganization of ECM and persistent sterile inflammation are the classical pathological features of tendon injuries which delay the healing responses and impair the regenerative mechanisms [8], [9], [10]. Tendon is a highly collagenous and hypocellular tissue comprised primarily of tenocytes- the tendon cells responsible for collagen homeostasis and mechanical function [11]. A drastic increase in cell density and infiltration of fat tissues (in severe cases) in the tendon matrix have been observed in patients, both of which are considered to be the major hallmarks of severe RCTI [12]. However, the signaling mechanisms driving the increased cell density in RCTI tendon matrix have yet to be discovered. Interestingly, the heterogeneous phenotype of tenocytes have been reported in tendon tissue which is believed to play regulatory and functional roles in tendon regeneration [13] [14].
Stem cells have significant therapeutic value for augmenting tendon-to-bone healing, owing to their inherent regenerative potential [15]. Mesenchymal stem cells (MSCs) exhibit prime significance in tendon tissue engineering and regeneration due to their excellent paracrine, immunomodulatory and angiogenic potential [16] [17]. Evidence from clinical and animal studies using autologous MSCs exhibited improved healing effects with minimal side effects. However, the available information in the literature is inconclusive and the mechanism of action of MSCs in tendon healing is still largely unknown and warrants further research [18]. In addition, the risk of teratoma formation following stem cell administration raises concerns for stem cell-based regenerative strategies employed in the management of various diseases including RCTI [6]. Moreover, more than 90% of the injected stem cells are generally lost by extrusion within hours of administration [19].
Evidence from human biopsy and animal models has revealed that hypoxia is the primary trigger for the initiation and progression of RCTI pathology. Despite the hypovascular nature of tendon tissue, the extreme hypoxia triggers apoptosis and necrosis of tenocytes, aggravating RCTI [20], [21]. On the other hand, hypoxia modulates the inflammatory and repair responses mediated through HIF-1α [20]. The hypoxic environment promotes the migration and proliferation of MSCs as well as resident tendon stem cells to facilitate tendon healing [22], [23]. Therefore, the exosome-mediated communication between the cells of injured tissue and the resident/MSCs from surrounding tissues is a crucial event to initiate tissue regeneration [24], [25]. We hypothesize that, following a hypoxic insult, the RCTI tenocytes and the adjacent subcutaneous adipose tissue-derived stem cells release exosomes loaded with mediators that elicit autocrine and paracrine responses to protect the tendon tissue from injury and accelerate regenerative responses. Based on this background, the focus of this study is to screen and compare the protein mediators contained in the exosomes of hypoxia-challenged tenocytes and the adjacent subcutaneous adipose-derived mesenchymal stem cells (ADMSCs) isolated from the shoulder of Yucatan microswine.
Materials and methods
Tenocyte isolation
Tenocytes were isolated from the infraspinatus tendon of Yucatan microswine following previously published protocols. Yucatan microswine were recruited in the research project of DKAgrawal with prior approval from the IACUC of Creighton University, Omaha, NE, USA. The tendon tissues were harvested post-sacrifice for tenocyte isolation. Briefly, the skin around the shoulder region was disinfected using iodine and 70% isopropanol. The infraspinatus tendon tissue was harvested by dissecting the shoulder, washed with serum free DMEM containing antibiotics, finely chopped and digested with collagenase-1 at 37°C for 1–2 hours. The partially digested tendon tissue was collected by centrifugation and placed in culture flask for explant culture. The tendon cells migrated out from the tissue and adhered to the culture ware and became confluent within 12–14 days. The cells were pooled from six microswine and the cells in passage 2–4 were used for the studies. The isolated tenocytes were maintained in collagen-1 coated (Zen-Bio) culture wares using DMEM containing 20% FBS and antibiotics under standard cell culture conditions (37°C and 5% CO2).
Characterization of swine tenocytes
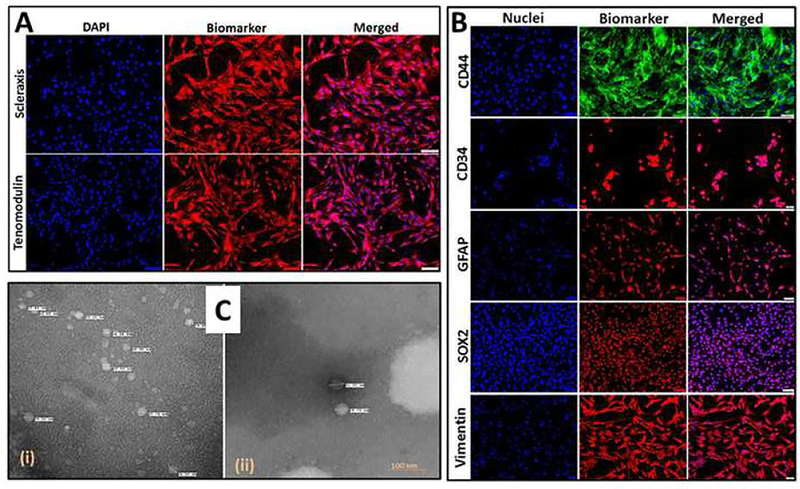
The cells were grown in a chamber slide, washed in sterile PBS, fixed with 5% formalin for 30 min and immunofluorescence of tenocyte biomarkers, tenomodulin and scleraxis were performed following our previously published protocols [8], [13]. The primary antibodies (1:100 dilution) used were anti-goat tenomodulin and anti-goat scleraxis and the secondary antibody was donkey-anti-goat-594 (1:300 dilution). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and imaged using a fluorescent microscope (VS120-S6-W, Olympus) at 20× magnification. A negative control with secondary antibody alone was maintained in a similar manner to detect background fluorescence and to set the exposure time.
Isolation of adipose-derived MSCs (ADMSCs)
The ADMSCs were isolated from the subcutaneous fat surrounding the shoulder joint of the Yucatan microswine following previously published protocols [26]. Briefly, the skin around the shoulder region was disinfected and an incision was made using a scalpel. The subcutaneous fat tissue surrounding the shoulder was aseptically harvested, washed with serum-free DMEM containing antibiotics, minced and digested with collagenase-1 (1mg/ml) at 37°C for 45–60 min. The digested adipose tissue was strained through 100μm pore size mesh and the cells were pelleted by centrifugation at 300g for 10 min. The cell pellets were suspended in DMEM containing 5% FBS and antibiotics and seeded in T25 culture flasks. The cells were confluent in 6–8 days and the cells from passage 2–4 were used for the studies.
Characterization of swine ADMSCs
The isolated ADMSCs were characterized by positive immunostaining to ADMSC biomarkers as determined by immunofluorescence using the above-mentioned protocol. The primary antibodies used were anti-goat CD44, anti-mouse GFAP, anti-mouse CD34, anti-mouse SOX2, and anti-mouse vimentin and the secondary antibodies were donkey-anti-goat-488 and donkey-anti-mouse-594. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and imaged using a fluorescent microscope (VS120-S6-W, Olympus) at 20× magnification.
Isolation of exosomes
The cells (tenocytes and ADMSCs) were cultured in T75 culture flasks under normoxia and hypoxia (2% O2) in DMEM containing 5% exosomes-depleted serum (Invitrogen) for 24h and the exosomes were isolated using commercially available kit following manufacturer’s instructions (Invitrogen). Briefly, the culture media was centrifuged at 2,000g for 30 mins at 4°C and the supernatant was incubated with exosomes isolation media for 24h at 4°C followed by centrifugation at 12,000g for 1h at 4°C. The supernatant was removed, and the exosome pellet was suspended in sterile PBS, stored at 4°C (temporarily) and used for further studies.
Transmission electron microscopy (TEM)
The freshly isolated exosomes were fixed overnight using ice-cold 2% glutaraldehyde in 0.1 M Sorenson’s buffer (pH 7.4) at 4°C. The samples were prepared for TEM analysis as reported previously [27]. The exosomes were then mounted onto formvar/silicon monoxide coated 200 mesh copper grids (Ted Pella Inc., Redding, CA). Grids were glow discharged for 60 seconds at 20μA with a GloQube glow discharge unit (Quorum Technologies, East Sussex, UK) prior to use. The sections were negatively stained with NanoVan (Nanoprobes, New York, NY) and examined using a Tecnai G2 Spirit TWIN (FEI, Hillsboro, OR) at a voltage of 80kV. Images were acquired digitally with an AMT (Woburn, MA) digital imaging system. NIH ImageJ software was used for determining the diameter of exosomes.
Screening of exosomal proteins using mass spectrometry
The exosomal contents were subjected to electrophoresis using SDS-PAGE. Then the protein fractions were excised from all bands of the SDS-PAGE gel, pooled, destained, reduced with tris-carboxyethylphosphine, alkylated with iodoacetamide, and digested overnight with sequencing-grade trypsin (Promega). Tryptic peptides were eluted from the gel and concentrated to 20μl by vacuum centrifugation and analyzed using a high-resolution mass spectrometry nano-LC-MS/MS Tribrid system, Orbitrap Fusion™ Lumos™ coupled with UltiMate 3000 HPLC system (Thermo Scientific). Approximately, 500ng peptides were run by the pre-column (Acclaim PepMap™ 100, 75μm × 2cm, nanoViper, Thermo Scientific) and the analytical column (Acclaim PepMap™ RSCL, 75 μm × 15 cm, nanoViper, Thermo Scientific). The samples were eluted using a 2h linear gradient of acetonitrile (4–45 %) in 0.1% formic acid. The peptide samples were analyzed using Mascot (Matrix Sciences, London, UK). Mascot was set up to search the SwissProt database (selected for Sus scrofa) based on the digestion enzyme trypsin. Parameters on MASCOT were set as follows: (1) Enzyme: trypsin, Max missed cleavage: (2) Peptide charge: 1+, 2+ and 3+, (3) Peptide tolerance: ± 0.8 Da, (4) Fixed modifications: carbamidomethyl (C), Variable modifications: oxidation (M), phospho (ST) and phospho (Y), MS/MS tolerance: ± 0.6 Da, (5) Instrument: ESI-TRAP. MASCOT results for different gel cuts of the same sample were combined and analyzed using Scaffold software (vs 4.7.5, Proteome Software Inc., Portland, OR), which allows multiple search results to be condensed into a single result scaffold file which was then converted to MS-excel sheets. The average of peptide counts from the control groups were compared with the hypoxic groups and the relative fold-change (RFC) was determined with respect to control (Av. Control – Av. Hypoxic/Av. Control).
qRT-PCR
Total cellular RNA was isolated by trizol method from hypoxic as well as normoxic tenocytes and ADMSCs, quantified using nanodrop, and 1μg RNA from each group was reverse transcribed to cDNA using cDNA synthesis kit (Promega) following the manufacturer’s protocol for 40μl reaction. The mRNA transcripts for the genes THBS1, NSEP1, TN-C, and ITIH4 from tenocytes and COL6A2, CTSD, MMP2 and TN-C from ADMSCs were amplified and quantified by real-time PCR (Applied Biosystems, CA, U.S.A.) using SYBR Green chemistry using corresponding forward and reverse primers for each gene (Table 1) according to previously published protocol [28]. The 18s rRNA for tenocytes and GAPDH for ADMSCs were used as housekeeping reference gene. The program set up was 95°C for 10 minutes; and 40 cycles of 95°C for 15 s, 60°C for 1 min. mRNA expression of the genes were normalized with the expression level of 18s rRNA. The fold-change of mRNA expression of the mitochondrial markers was determined by 2^-ΔΔCT method using the housekeeping gene and normoxic tenocytes and ADMSCs were used as control. The experiments were conducted in quadruplicates for ADMSCs and 7 times for tenocytes. The results are represented as fold-change with respect to normoxic control protocol [28].
Table 1:
Primer sequences used for PCR
| THBS1 | |
| Fw | AGATCCCGGCTACAACTGCC |
| Rev | ATCTGTGCAGGGGTTACGGG |
| NSEP1 | |
| Fw | GACAGAATGTGTACCGGGGCT |
| Rev | CTCTCGGGGTTGTCTTTGGC |
| TN-C | |
| Fw | TGCTGAACGAACTGCCCACAT |
| Rev | CCATCTGGGGTGGCATCTGAA |
| ITIH4 | |
| Fw | TTTGCCCCGGAGGTCTGGTC |
| Rev | TCCCCACTGAAGCTGACAAGG |
| COL6A2 | |
| Fw | CTCACGGAGTGCGACGTCAT |
| Rev | ACTTGGGGTCCTTGGCGATG |
| CTSD | |
| Fw | CCCCGCATCTCCGTGAACAA |
| Rev | TGCGCGTGACGTTGTGATAG |
| MMP2 | |
| Fw | CGCCCCCAAAACGGACAAAG |
| Rev | CTGGTCGAGTTCGCCTGTCT |
| 18s rRNA | |
| Fw | ACGTTGGCGAGAGCGTGG |
| Rev | AGGTGGAGGAGGCGAGAGAG |
| GAPDH | |
| Fw | CGGAGTGAACGGATTTGGCCG |
| Rev | GGAACTTGCCGTGGGTGGAA |
Expression of the major proteins identified from exosomes under hypoxic and normoxic tenocytes and ADMSCs
The tenocytes and ADMSCs were subjected to hypoxia and the immuno-positivity of tenocyte exosomes to thrombospondin-1(THBS1; ab-1823), nuclease-sensitive element-binding protein 1 (NSEP1; ab-114999), tenascin-C (TN-C; ab-226916), and inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4; ab-240221), and of ADMSCs exosomes to collagen type VI alpha 2 chain (COL6A2; ab-6588), cathepsin-D (CTSD; ab-72915), MMP2 (ab-110186) and tenascin-C (TN-C; ab-226916) were determined as mentioned in the previous section. The cells grown under normoxic conditions were used as control. Four-to-five images from different experiments were used for mean fluorescence intensity (MFI) determination using ImageJ software and the MFI values were normalized to 100 cells [28], [29].
Statistical analysis
The diameter of exosomes was represented as average ± standard deviation and the level of significance within the group was calculated by one sample ‘t’ test using Shapiro-Wilk normality test using GraphPad prism software. The level of significance was set at P values <0.05. For mass spectrometry analysis, the peptide identifications contained at least two identified peptides and were accepted with greater than 90% probability by the Peptide Prophet algorithm with Scaffold delta-mass correction [30], [31]. The proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony and the proteins sharing significant peptide evidence were grouped into clusters. Scaffold analysis was used to validate MS/MS-based peptide and protein identifications. The average of peptide counts from the control groups were compared with the hypoxic groups and the results were expressed as fold-change. The mRNA expression was expressed in fold-change relative to normoxic control and was represented as average ± standard error of mean. The statistical significance was calculated by Dunnett’s multiple comparison test using GraphPad prism software. The protein expression of biomarkers was represented as average ± standard error of mean with respect to MFI/100 cells and the statistical significance was calculated by one sample ‘t’ test using GraphPad prism software.
Results
Tenocytes and ADMSCs under hypoxia carried abundant exosomes
The tenocytes isolated from Yucatan microswine were immunopositive to tenomodulin and scleraxis (Fig. 1A). The ADMSCs were immunopositive to GFAP, CD44, CD34, SOX2 and vimentin (Fig. 1B). The exosomes were isolated from tenocytes and ADMSCs cultured under hypoxic conditions and the TEM imaging revealed their morphology and size (Fig. 1C). The exosomal contents in the tenocytes were more in quantity than in the ADMSCs and the average diameter of ADMSC exosomes was found to be 24.86 ± 5.67 nm (n=14, NS) and that of tenocyte exosomes were found to be 23.37 ± 5.58 nm (n=99, P<0.05).
Fig. 1:
(A) Characterization of tenocytes: Immunofluorescence analysis for the expression of Scleraxis and Tenomodulin showing their expression. Images were acquired at 20× magnification using CCD camera attached to the Olympus microscope. (B) Characterization of ADMSCs: Immunofluorescence analysis for the expression of CD44, CD34, GFAP, SOX2 and Vimentin showing their expression. Images were acquired at 20× magnification using CCD camera attached to the Olympus microscope. (C) The examination of the exosome morphology using TEM analysis in tenocytes (i) and ADMSCs (ii) cultured under hypoxic conditions. The tenocytes under hypoxia exhibited more exosomes but smaller size than compared with hypoxic ADMSCs.
Screening of exosomal proteins using mass spectrometry identified several regenerative mediators
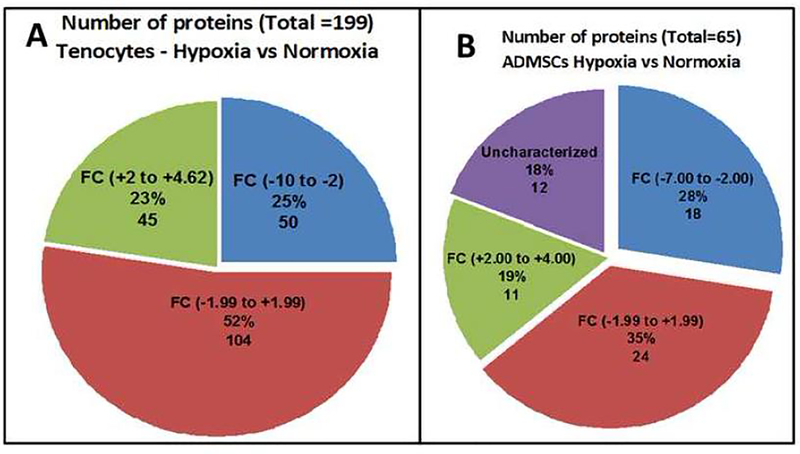
The expression of 199 proteins were detected in hypoxic tenocytes in comparison with the normoxic controls (Table S1) with an overall relative fold-change (RFC) ranging from −10 to +4.62 (Fig. 2A). Among these, the RFC of 104 proteins ranged from −2 to +2, 50 proteins were in the range of −10 to −2 (Table S1) and 45 of them were within the range of +2 to +5 (Table S1). The proteins with RFC > 2 (16 Nos., Table S2A) and RFC < 2 (15 Nos., Table S2B) were considered to be relevant and their overall functions were depicted in Table S2A and Table S2B. Similarly, 65 proteins were detected in hypoxic ADMSCs in comparison with the normoxic controls (Table S3). The overall RFC value ranged from −7 to +4 (Fig. 2B). Among these, the RFC of 24 proteins ranged between −2 to +2, 18 proteins were in the range of −7 to −2 (Table S3) and 11 of them were in the range of +2 to +4 (Table S3) and 12 proteins were found to be uncharacterized (Table S3). The proteins with RFC > 2 (5 Nos., Table S2C) and RFC < 2 (8 Nos., Table S2C) were considered to be relevant and their overall functions are depicted in Table S2C.
Fig. 2:
The pie diagram showing the distribution of proteins based on the relative fold change. (A) Proteins detected from the exosomes of tenocytes and (B) proteins detected from the exosomes of ADMSCs.
The upregulated proteins from hypoxic ADMSCs which are associated with ECM homeostasis were MMP2, COL6A, CTSD and TN-C and those from hypoxic tenocytes were THSB1, NSEP1, ITIH4 and TN-C. Among the highly expressed proteins, the ECM glycoprotein tenascin (TN-C) was upregulated in both tenocytes and ADMSCs. These proteins were further examined for their expression on their respective cell types under hypoxic conditions.
Major ECM associated genes screened from exosomes were upregulated at mRNA level
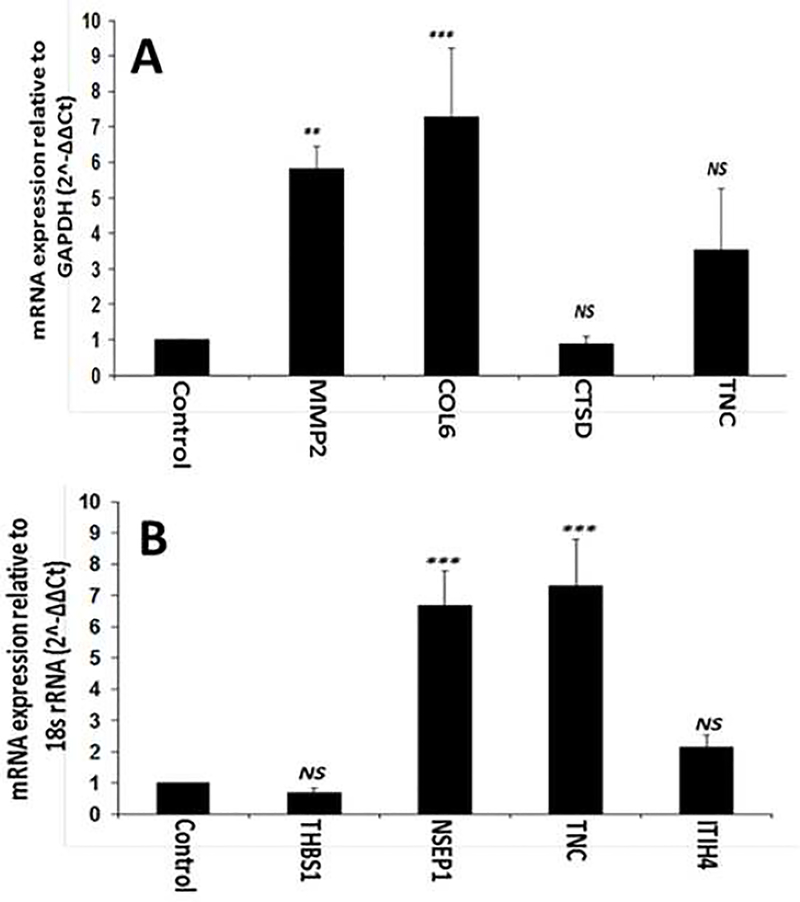
The ECM associated proteins that were upregulated in the exosomes of hypoxic ADMSCs included MMP2, COL6A, CTSD and TN-C and the mRNA transcript analysis of these genes revealed similar alterations as in exosomes (Fig. 3A). More than 5-fold upregulation of MMP2 (P<0.01) transcripts were observed in hypoxic ADMSCs when compared with normoxic cells. Similarly, COL6A exhibited a significant increase (~7-fold, P<0.001) in hypoxic cells when compared to normoxic ADMSCs. The expression of CTSD transcripts from hypoxic ADMSCs was found to be similar to that of control cells (P>0.05). In addition, the expression of TN-C was increased in hypoxic cells, however, was statistically not significant (P>0.05). Similarly, THSB1, NSEP1, ITIH4 and TN-C were the major exosomal proteins contained in the exosomes of hypoxic tenocytes and the mRNA transcript analysis of these genes revealed similar trend (Fig. 3B). The miRNA transcripts for NSEP1 (~7-fold, P<0.001) and TN-C (~7-fold, P<0.001) were significantly upregulated in hypoxic tenocytes when compared with the normoxic controls. ITIH4 transcripts were also increased (~2-fold, P>0.05) however, was not statistically significant. And, no considerable alteration in THSB1 (P>0.05) transcripts was observed in hypoxic tenocytes when compared with normoxic cells. Thus, MMP2, COL6A, NSEP1 and TN-C were significantly upregulated and there was a trend of upregulation of CTSD, THSB1 and ITIH4 transcripts, however, were not statistically significant.
Fig. 3:
qRT-PCR analysis for the expression of COL6A2, CTSD, TN-C and MMP2 by ADMSCs (A) and THBS1, NSEP1, TN-C, and ITIH4 by tenocytes (B) cultured under hypoxic condition with respect to normoxic controls: The results were expressed as fold change of expression relative to control. (NS – non-significant, and * P<0.05, ** P<0.01 and *** P<0.001)
Major ECM associated genes screened from exosomes were upregulated at the protein level
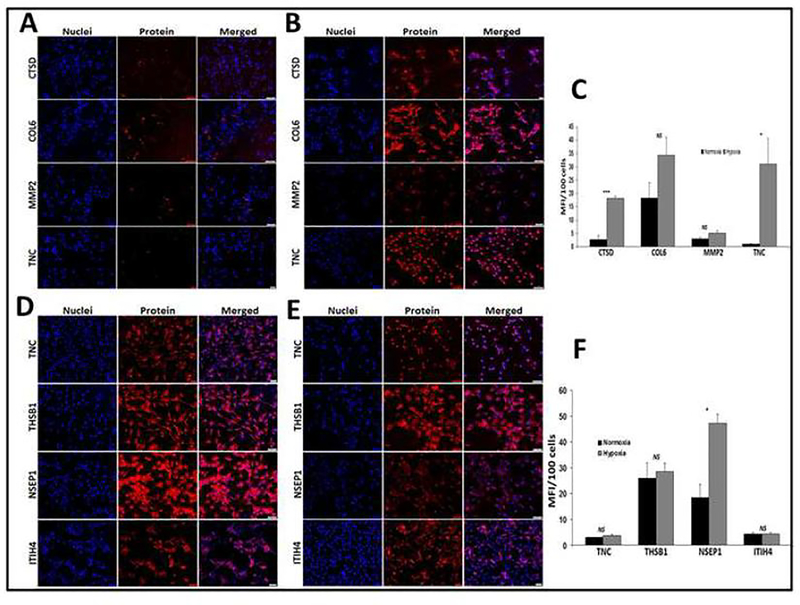
The mean fluorescence intensity (MFI) for the protein expression of CTSD (P<0.001) and TN-C (P<0.05) was significantly greater in hypoxic ADMSCs when compared to the corresponding normoxic cells (Figs. 4A, 4B and 4C). The MFI corresponding to the expression of MMP2 and COL6A were also increased in hypoxic ADMSCs, however, were not statistically significant (Figs. 4D, 4E and 4F). In hypoxic tenocytes, the MFI for the protein expression of NSEP1 (P<0.05) was significantly higher when compared with normoxic tenocytes (Figs. 4D, 4E and 4F). The protein expression of TN-C and THSB1 displayed upregulation in hypoxic tenocytes, however, was statistically not significant (Figs. 4D, 4E and 4F). The expression of ITIH4 was similar between normoxic and hypoxic tenocytes. Thus, the protein expression studies revealed significant upregulation of CTSD, TN-C and NSEP1 among the seven upregulated proteins obtained from mass spectrometry. MMP2, COL6A and THSB1 were also increased, however, was not statistically significant (Figs. 4A–4F).
Fig. 4:
Immunofluorescence analysis for the expression of COL6A2, CTSD, TN-C and MMP2 by ADMSCs cultured under (A) hypoxia, (B) normoxia and (C) quantification of the expression proteins normalized to 100 cells and for the expression of THBS1, NSEP1, TN-C, and ITIH4 by tenocytes cultured under (D) hypoxia, (E) normoxia and (F) quantification of the expression proteins normalized to 100 cells: Images in the left columns show nuclear staining with DAPI; the images in the middle column show expression of proteins while the images in the right column show overlay of proteins with DAPI. Images were acquired at 20× magnification using CCD camera attached to the Olympus slide scanner. The graphs (C and F) represent MFI mean values with standard error. (NS – non-significant, * P<0.05, and *** P<0.001).
Discussion
The findings in this study revealed changes in the protein contents of exosomes in ADMSCs and tenocytes under the influence of hypoxia. Generally, the tendon cells are adapted to withstand the hypoxic insult by upregulating the expression of the mediators for cell survival and energy conservation [32], [33], [34]. For example, the hypoxic stimulation of epithelial cells releases exosomes, which in turn triggers the adjacent fibroblast population to upregulate type I collagen synthesis and facilitate ECM remodeling and local tissue repair [35]. Also, the hypoxia activates the differentiation of ADMSCs to tenocytes via HIF-1α signaling, and the hypoxia-driven genes, especially VEGF, accelerate the hypoxic adaptations/responses to various cell phenotypes [36], [37], [38]. These findings led to the hypothesis that the hypoxic insult in the shoulder tenocytes and adjacent ADMSCs results in the release of survival/regenerative mediators through the exocytosis of exosomes to the ECM. Screening of the exosome contents paves the way to understand their regenerative/repair mechanisms and opens new translational avenues for exosomes as cell-free therapeutics in the management of RCTI.
Interestingly, the tissue remodeling protein, tenascin, was upregulated in the exosomes of both tenocytes and ADMSCs. Tenascin is associated with collagen fibers and concentrated mainly in the tendon belly and is pivotal for proper organization and orientation of collagen fibers [39]. Also, tenascin upregulation has been associated with most tendon disorders (especially at the sites where excessive mechanical force is required) implying its role in maintaining the mechano-elastic properties of tendon tissue [39], [40]. Based on these results, it is reasonable to speculate that the coordination of surviving cells increases the local concentration of tenascin to compromise/defend the loss of mechanical integrity following RCTI.
Thrombospondin-1 (TSP-1), the most upregulated protein of tenocyte exosomes, is a matricellular protein that plays a significant role in wound healing and ECM remodeling [41]. Even though members of TSP family (TSP-3 and TSP-4) were reported in regenerative tendon tissues, the exact function of TSP-1 has not been studied in rotator cuff tendon tissues [42]. However, our result revealed the possible involvement of TSP-1 in tendon regeneration following hypoxic insult which warrants further investigation. In addition, the nuclease-sensitive element-binding protein 1 (NSEP-1) or Y-box binding protein-1 (YB-1), the suppressor of type I collagen gene, [43] were also upregulated in the exosomes of tenocytes. Interestingly, there was a concomitant decrease in observed collagen type I content in the exosomes of tenocytes. This suggests that the hypoxia-mediated activation of YB-1 results in the downregulation of type I collagen, resulting in the concomitant upregulation of type III collagen, the typical hallmark of RCTI [9], [45], [100], [101].
Inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4), a glycoprotein and protease inhibitor, stabilizes the ECM by counteracting the fibrotic changes mediated by MMPs and proinflammatory cytokines [46], [47]. Similarly, alpha 2-HS glycoprotein (AHSG) or fetuin-A, which was found to be upregulated in the exosomes of hypoxic tenocytes, exhibits multiple functions including insulin signaling, prevention of ECM calcification, immunomodulation, TGF-β signaling and acts as an endogenous ligand for TLR-4 [48], [49], [50]. Even though the role of AHSG in RCTI has not been well defined, the AHSG-inhibitory effects of MMPs suggest its possible role in ECM disorganization and inflammation associated with RCTI [51]. Interestingly, our recent findings revealed MMP hyperactivity (especially MMP-9) and TLR-4-mediated activation of inflammasomes in the pathophysiology of ECM disorganization following RCTI [8], [52]. Hence, the ratios of MMPs to ITIH4 and AHSG determine the pathological/regenerative fate of tendon tissues following RCTI which warrants further investigation.
Apart from the glycolytic function, the hydrolytic enzyme α-enolase (ENO1) participates in multiple biological functions such as a surface marker for hematopoietic cells, plasminogen receptors on endothelial cells and as a hypoxic stress protein [53]. The surface expression of ENO1 accumulates plasminogen and activates the degradation of ECM [53]. However, ENO1-mediated degradation of collagens or other matrix components have not been reported. The upregulation of ENO1 in the cytosol of hypoxic cells contribute to tolerating the oxidative/ischemic stress by increasing anaerobic metabolism and reducing the survival rate of fibroblasts- resulting in insufficient ECM deposition [54], [55]. These findings suggest that the increased content of ENO1 promotes tenocyte survival via energy conservation and prevention of hypoxic damage. However, the extracellular function of ENO1 in hypoxic tenocytes requires further attention.
Fibronectin is a normal constituent of the tendon matrix and facilitates the attachment, spreading and adhesion of cells in addition to the prevention of cell detachment from the friction of gliding [56]. In addition, fibronectin acts as binding sites for collagen and hyaluronic acid and functions as tissue lubricant [56]. Fibronectin promotes ECM formation through the RGD mediated interaction with integrins, association with cytoskeletal actin and by providing vascularization for collagen deposition [57]. Following RCTI, the level of fibronectin increases along with collagen type III and downregulates to a/the basal level after the healing process is completed [58], [59]. The upregulation and increased secretion of fibronectin in the exosomes of hypoxic tenocytes suggests that tenocytes respond to hypoxia by secreting matrix components such as tenascin, fibronectin and thrombospondins in order to compromise for the ECM disorganization characterized by the decrease in collagen type I to III ratio following RCTI.
Apart from the ECM component proteins and ECM regulatory/repair proteins, the hypoxic tenocytes carried increased levels of cell survival proteins and anti-oxidant mediators in the exosomes. For example, the 14–3-3 family of proteins protects the cells from apoptosis via Akt signaling by sequestering several pro-apoptotic mediators including Bad and FOXO [60]. Furthermore, the 14–3-3 proteins shield the cells from oxidative injury through the activation of Mst family kinase (MST1) and by triggering several pro-survival proteins [61]. Therefore, 14–3-3 proteins can be considered as hypoxia responsive signals for cell survival [62]. Apart from cell survival proteins, several antioxidant signaling proteins including alpha-2-HS-glycoprotein, hemoglobin fetal subunit-beta, and transthyretin [50], [63], [64] were upregulated in the exosomes of hypoxic tenocytes. Glutaredoxin-1 (Grx1), the anti-oxidant sulfhydryl disulfide oxidoreductase enzyme, protects the cells from oxidative stress, metabolic/glucose stress and apoptosis through the activation of selenoproteins [65], [66]. However, Grx1 was downregulated in the exosomes of hypoxic tenocytes, suggesting the possibility for alternate routes of antioxidant signaling responses in tenocytes without the involvement of selenoprotein, which requires further investigation.
Seven out of fifteen downregulated proteins in the exosomes of hypoxic tenocytes belonged to the keratin family of cytoskeletal proteins. Cytoskeletal keratins are a family of intermediate filaments with diverse cellular functions such as cell motility, differentiation, signaling and survival. The plasticity and dynamicity of keratins are essential to retain the cell phenotype and the maintenance of cell-cell and cell-ECM interactions [67], [68]. Interestingly, it has been reported that hypoxia triggers the disassembly of keratin filaments via complex III-generated mitochondrial ROS. The hypoxia responsiveness of keratin disassembly offers a feedback inhibition to ROS by altering the intracellular organization and location of mitochondria in addition to stimulating cell migration across the oxygen gradient [69]. This suggests another probable cellular event associated with tissue regeneration- the alteration of plasticity and dynamicity of keratin filaments in tendon tissue, additionally warranting further research.
Dolichyl-diphospho oligosaccharide-protein glycosyl transferase subunit (also called advanced glycation end product receptor-1 or AGER1) functions as a cell surface receptor which protects the cells from AGE-mediated oxidative stress [70]. The cytoprotective effects of AGER1 have been attributed to its ability to facilitate the removal of AGEs by endocytosis, inhibition of RAGE (the conventional receptor for AGE which triggers inflammation), MAPK, and NF-κB [70], [71]. Transient hypoxia and subsequent AGE mediated oxidative stress has been considered to be the major trigger for AGER1 expression, however, persistent oxidative stress reduces the AGER1 activity [70]. This suggests a possible reason for the decreased level of AGER1 in hypoxic tenocytes, as observed in our findings. In addition, the comorbidity of diabetes has been proven to be associated with RCTI. In diabetes, the increase in AGEs results in non-enzymatic crosslinking of the collagen fibrils in the tendon matrix, preventing the hydrolytic turnover of ECM and further affecting the mechanical integrity of the tissue [72], [73], [74]. Our recent finding demonstrated an increased expression of RAGEs in the tendon tissues of both RCTI patients and rat models, revealing the impact of HMGB1-RAGE axis-mediated sterile inflammation and ECM disorganization in RCTI pathology [8], [75]. Therefore, the assessment of the ratio of AGER1-to-RAGE and their competition for AGE binding may reveal the health status of tendon ECM [71].
Four (two ECM components – type VI collagen and tenascin and two ECM remodeling enzymes – MMP2 and cathepsin-D) out of five upregulated proteins in the exosomes of hypoxic ADMSCs were directly associated with ECM biology. Collagen type VI and tenascin (described above) have been upregulated in tendon tissues following RCTI [76]. The interaction of type VI collagen with type I and III subtypes is prerequisite for ECM homeostasis. Specifically, collagen VI triggers the activation of various MMPs, promotes fibroblast activation and mobilization for ECM synthesis and acts as a reservoir for various growth mediators [77], [78]. Even though gelatinase-A (MMP2) is an ECM remodeling enzyme (which degrades type I collagen the major component of tendon ECM), its hyperactivity is associated with the tendon ECM disorganization in RCTI patients [52]. Interestingly, the release of MMP-2 from ADMSCs is crucial for their subsequent differentiation into cells of the musculoskeletal lineage [79]. Similarly, the upregulation of aspartate protease cathepsin-D (predominantly intracellular) in the exosomes of hypoxic ADMSCs suggests its ECM remodeling function. Both the association of cathepsin-D expression with MMPs and its potential role in the inactivation of TIMP2 have already been established under physiological and pathological conditions [80], [81]. Interestingly, we observed a concomitant increase of MMP2 along with cathepsin-D, signifying the potential role of ADMSCs in ECM regeneration. Moreover, the findings such as the type VI collagen as a substrate for MMP2, tenascin as an inducer for MMP2 and association of cathepsin-D with both MMP2 and tenascin [82], [83], [84] suggest the exosomal content of ADMSCs under hypoxic conditions drives the ECM repair, warranting further investigation.
Three (Hsp70, endoplasmin, and PDI) out of eight downregulated proteins in the hypoxic exosomes exhibited the function of molecular chaperons. The expression of these molecular chaperons maintains the proteostasis and fidelity of stem cell signaling and also helps to retain the self-renewal capacity of stem cells [85]. In addition, these chaperons are necessary for stem cell differentiation, protection of the cells from various stresses, maintenance of ECM integrity, and immunomodulation [86], [87], [88]. Interestingly, we observed the concomitant downregulation of two proteins (Pigment epithelium-derived factor and annexin) which have an anti-inflammatory function, suggesting the association of the chaperone proteins (mentioned above) in preventing inflammation. Therefore, it is reasonable to speculate that under normoxic conditions, ADMSCs function by releasing exosomal contents for maintaining ECM integrity and modulating the immune responses. However, under hypoxia, the exosomes carry ECM components, ECM remodeling enzymes and inflammatory mediators, suggesting that the extent of hypoxia drives the ECM regeneration via an alteration in the exosomal contents.
Vimentin is an MSC marker which belongs to type III intermediate filaments that is responsible for cellular resistance to various types of stress. However, depleted level of cellular vimentin represents an increased differentiation state of stem cells characterized by the upregulation of differentiation markers [89]. Vimentin facilitates wound healing via the activation of keratin filaments, however, excessive scarring is produced [90]. Interestingly, extracellular vimentin has been identified to be an activating ligand for superoxide production by interacting with the non-toll pattern recognition receptor (nontoll PRR), Dectin-1. The Vimentin-Dectin-1 axis is pro-inflammatory and is a potent inducer of oxidative damage to membrane lipids [91]. This suggests that the decreased level of vimentin in the exosomes of hypoxic ADMSCs represents tissue regeneration by defending oxidative stress and inflammation.
The major focus of this study was to screen and identify the proteins contained in the exosomes of tenocytes and ADMSCs isolated from the swine shoulder in response to hypoxia. Even though most of the detected proteins were associated with ECM repair/remodeling, there existed several proteins that functions in gene expression, cell biology and exosome integrity which are also related either directly or indirectly to ECM homeostasis. Furthermore, several intracellular proteins were present in the exosomes of both tenocytes and ADMSCs and their extracellular functions are still largely unknown. In the current study, the exosomal proteins were screened based on their relative fold change. The majority of the detected proteins were excluded since they did not meet the inclusion criteria of the fold change difference of two. The excluded proteins may also have an impact on tendon tissue regeneration, which warrants further investigations. In addition, the mechanism of action and signaling cascade of the detected proteins requires further examination at the cellular and molecular level in regard to RCTI.
Among the screened proteins, MMP2, COL6A, CTSD and TN-C from hypoxic ADMSCs and THSB1, NSEP1, ITIH4 and TN-C from hypoxic tenocytes were directly associated with ECM homeostasis. The expression status of these proteins (discussed above in regard to their ECM function) at the protein and mRNA level revealed a similar trend to the respective exosomes. Similarly, these seven proteins were upregulated in the cells of shoulder tissues under the influence of hypoxia at the mRNA and protein levels. These findings confirmed the association of these protein mediators in the regulation of ECM regeneration following a hypoxic insult. Hypoxia being a significant contributing factor for the initiation of RCTI, the upregulation of these seven proteins in shoulder tenocytes and surrounding ADMSCs reflects the alterations in the tendon ECM regenerative machinery following hypoxia and the subsequent initiation of pathology. At the same time, the effect and interactions of other significantly altered exosomal proteins should not be neglected.
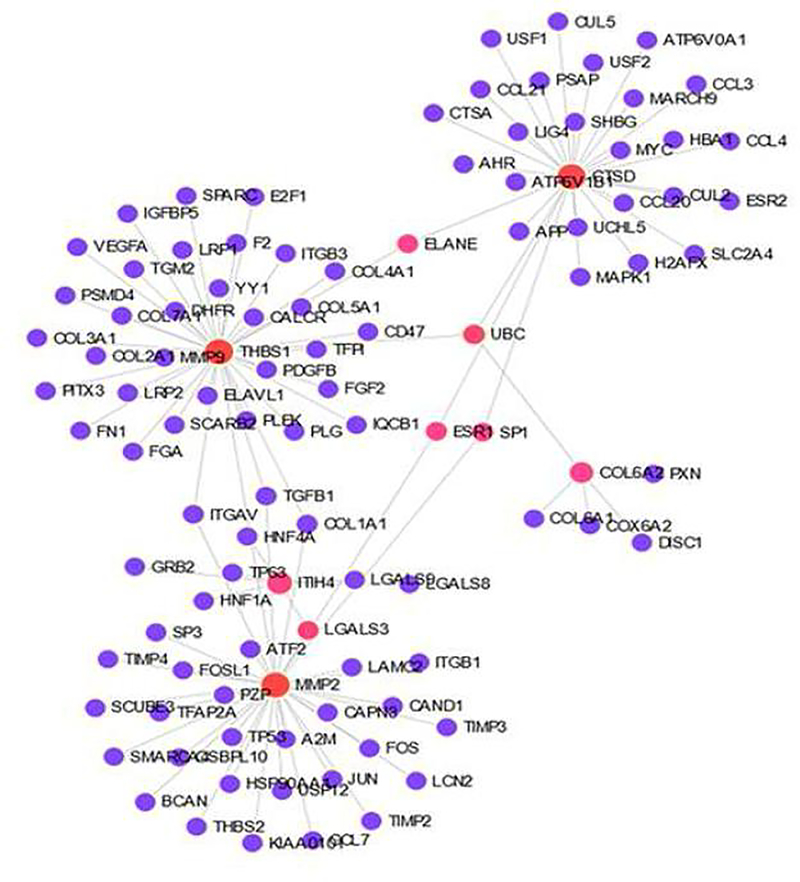
Moreover, the cumulative signaling effects, interconnecting networks and the possible combination of exosomal proteins released from tenocytes and ADMSCs requires further attention. For instance, the NetworkAnalyst program [9], [29] revealed the interaction of the seven upregulated proteins from both tenocytes and ADMSCs that are directly associated with ECM function (input genes given were THBS1, NSEP1, TN-C, ITIH4, COL6A2, CTSD, MMP2) which involve 97 genes (excluding the input genes) and constitutes 40 pathways (Fig. 5, Tables S4 and S5). In fact, this reveals the complexity of protein-protein interactions and the network of downstream signaling of exosomal proteins in response to hypoxia. In addition, several uncharacterized proteins were contained in the studied exosomes, which requires further characterization and functional analyses. Furthermore, the non-protein mediators carried in the exosomes, such as miRNAs, non-coding RNAs and lipids play functional and/or regulatory roles in the tissue regeneration [92]. Also, the study examined primarily the highly altered exosomal proteins. However, potential role of less altered proteins to influence the RCTI pathology cannot be ruled out and warrants further investigation. To the best of our knowledge, this is the first report on the exosome biology of rotator cuff tenocytes and shoulder subcutaneous adipose tissue in regard to RCTI pathology. However, the elucidation of the molecular interplay between tenocytes and adjacent ADMSCs under hypoxic conditions and the downstream ECM regenerative pathways mediated by the exosomal proteins warrants extensive in silico, in vitro, and in vivo investigations. Nonetheless, the expression of regenerative protein mediators in the exosomes released by the tenocytes and ADMSCs in response to hypoxia opens new translational opportunities for the management of RCTI.
Fig. 5:
Genes interconnecting with THBS1, NSEP1, TN-C, ITIH4, COL6A2, CTSD, and MMP2 as examined by NetworkAnalyst indicating the interplay of exosome proteins between ADMSCs and tenocytes.
Conclusions
The proteins contained in the exosomes of hypoxia-challenged tenocytes and the subcutaneous ADMSCs isolated from the shoulder of Yucatan microswine revealed the expression of protein mediators associated with ECM regeneration. The exosomes of tenocytes and ADMSCs cultured under hypoxic conditions carried 199 and 65 proteins respectively, when compared with respective normoxic cells. The difference in fold-change was the screening criteria. Sixteen upregulated proteins and 15 downregulated proteins from tenocytes and 5 upregulated proteins and 8 downregulated proteins from ADMSC were considered to be relevant. Most of these proteins were associated with ECM homeostasis, cell integrity, antioxidant response and inflammation, suggesting the impact of exosome signaling in ECM regeneration. Among the screened proteins, MMP2, COL6A, CTSD and TN-C from hypoxic ADMSCs and THSB1, NSEP1, ITIH4 and TN-C from hypoxic tenocytes were directly associated with ECM homeostasis which exhibited similar expression at the mRNA and protein levels. Moreover, these proteins are involved in multiple signaling pathways dealing with ECM repair/regeneration which warrants further investigation and may open translational significance of exosome-based management strategies for RCTI.
Supplementary Material
Acknowledgement
This work was supported by LB506 grant to DKA and LB606 grant to MFD from the State of Nebraska. The research work of DKA is also supported by grants R01HL120659 and R01HL144125 from the National Institutes of Health. The contents of this original research article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the State of Nebraska.
Footnotes
Financial and competing interests’ disclosure: All authors have read the journal’s policy on disclosure of potential conflicts of interest and all authors declare that there is no conflict. No writing assistance was utilized in the production of this manuscript.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Anversa P (2003) Primitive Cells and Tissue Regeneration. Circ Res 92:579–582. 10.1161/01.RES.0000066879.66293.87 [DOI] [PubMed] [Google Scholar]
- 2.Aminzadeh MA, Rogers RG, Fournier M, et al. (2018) Exosome-Mediated Benefits of Cell Therapy in Mouse and Human Models of Duchenne Muscular Dystrophy. Stem Cell Rep 10:942–955. 10.1016/j.stemcr.2018.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy C, Withrow J, Hunter M, et al. (2018) Emerging role of extracellular vesicles in musculoskeletal diseases. Mol Aspects Med 60:123–128. 10.1016/j.mam.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke J, Kolhe R, Hunter M, et al. (2016) Stem Cell-Derived Exosomes: A Potential Alternative Therapeutic Agent in Orthopaedics. Stem Cells Int 2016:1–6. 10.1155/2016/5802529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlan RS, Pisano S, Oliveira MI, et al. (2017) Exosomes as Reconfigurable Therapeutic Systems. Trends Mol Med 23:636–650. 10.1016/j.molmed.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imam MA, Holton J, Horriat S, et al. (2017) A systematic review of the concept and clinical applications of bone marrow aspirate concentrate in tendon pathology. SICOT-J 3:58 10.1051/sicotj/2017039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raney EB, Thankam FG, Dilisio MF, Agrawal DK (2017) Pain and the pathogenesis of biceps tendinopathy. Am J Transl Res 9:2668–2683 [PMC free article] [PubMed] [Google Scholar]
- 8.Thankam FG, Roesch ZK, Dilisio MF, et al. (2018) Association of Inflammatory Responses and ECM Disorganization with HMGB1 Upregulation and NLRP3 Inflammasome Activation in the Injured Rotator Cuff Tendon. Sci Rep 8:. 10.1038/s41598-018-27250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thankam FG, Boosani CS, Dilisio MF, et al. (2016) MicroRNAs Associated with Shoulder Tendon Matrisome Disorganization in Glenohumeral Arthritis. PLOS ONE 11:e0168077 10.1371/journal.pone.0168077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thankam FG, Boosani CS, Dilisio MF, Agrawal DK (2017) MicroRNAs associated with inflammation in shoulder tendinopathy and glenohumeral arthritis. Mol Cell Biochem. 10.1007/s11010-017-3097-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouda MB, Thankam FG, Dilisio MF, Agrawal DK (2017) Alterations in tendon microenvironment in response to mechanical load: potential molecular targets for treatment strategies. Am J Transl Res 9:4341–4360 [PMC free article] [PubMed] [Google Scholar]
- 12.Thankam FG, Dilisio MF, Agrawal DK (2016) Immunobiological factors aggravating the fatty infiltration on tendons and muscles in rotator cuff lesions. Mol Cell Biochem. 10.1007/s11010-016-2710-5 [DOI] [PubMed] [Google Scholar]
- 13.Thankam FG, Dilisio MF, Dietz NE, Agrawal DK (2016) TREM-1, HMGB1 and RAGE in the Shoulder Tendon: Dual Mechanisms for Inflammation Based on the Coincidence of Glenohumeral Arthritis . PLOS ONE 11:e0165492 10.1371/journal.pone.0165492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bi Y, Ehirchiou D, Kilts TM, et al. (2007) Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13:1219–1227. 10.1038/nm1630 [DOI] [PubMed] [Google Scholar]
- 15.Mora MV (2015) Stem cell therapy in the management of shoulder rotator cuff disorders. World J Stem Cells 7:691 10.4252/wjsc.v7.i4.691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peach MS, Ramos DM, James R, et al. (2017) Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering. PLOS ONE 12:e0174789 10.1371/journal.pone.0174789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Hindieh J, Leong DJ, Sun HB (2017) Advances of stem cell based-therapeutic approaches for tendon repair. J Orthop Transl 9:69–75. 10.1016/j.jot.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pas HIMFL Moen MH, Haisma HJ, Winters M (2017) No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med 51:996–1002. 10.1136/bjsports-2016-096794 [DOI] [PubMed] [Google Scholar]
- 19.Thankam FG, Muthu J (2015) Alginate–polyester comacromer based hydrogels as physiochemically and biologically favorable entities for cardiac tissue engineering. J Colloid Interface Sci 457:52–61. 10.1016/j.jcis.2015.06.034 [DOI] [PubMed] [Google Scholar]
- 20.Millar NL, Reilly JH, Kerr SC, et al. (2012) Hypoxia: a critical regulator of early human tendinopathy. Ann Rheum Dis 71:302–310. 10.1136/ard.2011.154229 [DOI] [PubMed] [Google Scholar]
- 21.Liang M, Cornell HR, Zargar Baboldashti N, et al. (2012) Regulation of Hypoxia-Induced Cell Death in Human Tenocytes. Adv Orthop 2012:1–12. 10.1155/2012/984950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang T-F, Yew T-L, Chiang E-R, et al. (2013) Mesenchymal Stem Cells From a Hypoxic Culture Improve and Engraft Achilles Tendon Repair. Am J Sports Med 41:1117–1125. 10.1177/0363546513480786 [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Wang JH-C (2013) Human Tendon Stem Cells Better Maintain Their Stemness in Hypoxic Culture Conditions. PLoS ONE 8:e61424 10.1371/journal.pone.0061424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xin T, Greco V, Myung P (2016) Hardwiring Stem Cell Communication through Tissue Structure. Cell 164:1212–1225. 10.1016/j.cell.2016.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G, Yang P (2018) A novel cell-cell communication mechanism in the nervous system: exosomes. J Neurosci Res 96:45–52. 10.1002/jnr.24113 [DOI] [PubMed] [Google Scholar]
- 26.Meyer J, Salamon A, Herzmann N, et al. (2015) Isolation and Differentiation Potential of Human Mesenchymal Stem Cells From Adipose Tissue Harvested by Water Jet-Assisted Liposuction. Aesthet Surg J 35:1030–1039. 10.1093/asj/sjv075 [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Deng W, Klinke II DJ (2015) Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. The Analyst 140:6631–6642. 10.1039/C5AN00688K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thankam FG, Chandra IS, Kovilam AN, et al. (2018) Amplification of Mitochondrial Activity in the Healing Response Following Rotator Cuff Tendon Injury. Sci Rep 8:. 10.1038/s41598-018-35391-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thankam FG, Boosani CS, Dilisio MF, et al. (2018) Genes interconnecting AMPK and TREM-1 and associated microRNAs in rotator cuff tendon injury. Mol Cell Biochem. 10.1007/s11010-018-3456-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keller A, Nesvizhskii AI, Kolker E, Aebersold R (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74:5383–5392 [DOI] [PubMed] [Google Scholar]
- 31.Nesvizhskii AI, Keller A, Kolker E, Aebersold R (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75:4646–4658 [DOI] [PubMed] [Google Scholar]
- 32.Alvarez-Tejado M, Naranjo-Suárez S, Jiménez C, et al. (2001) Hypoxia Induces the Activation of the Phosphatidylinositol 3-Kinase/Akt Cell Survival Pathway in PC12 Cells: PROTECTIVE ROLE IN APOPTOSIS. J Biol Chem 276:22368–22374. 10.1074/jbc.M011688200 [DOI] [PubMed] [Google Scholar]
- 33.Kumar H, Choi D-K (2015) Hypoxia Inducible Factor Pathway and Physiological Adaptation: A Cell Survival Pathway? Mediators Inflamm 2015:1–11. 10.1155/2015/584758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nauta T, van Hinsbergh V, Koolwijk P (2014) Hypoxic Signaling During Tissue Repair and Regenerative Medicine. Int J Mol Sci 15:19791–19815. 10.3390/ijms151119791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Jong OG, Van Balkom BWM, Schiffelers RM, et al. (2014) Extracellular vesicles: potential roles in regenerative medicine. Front Immunol 5:608 10.3389/fimmu.2014.00608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Y, Zhou Y, Cheng T, et al. (2016) Hypoxia enhances tenocyte differentiation of adipose-derived mesenchymal stem cells by inducing hypoxia-inducible factor-1α in a co-culture system. Cell Prolif 49:173–184. 10.1111/cpr.12250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malila Y, Thanatsang K, Arayamethakorn S, et al. (2019) Absolute expressions of hypoxia-inducible factor-1 alpha (HIF1A) transcript and the associated genes in chicken skeletal muscle with white striping and wooden breast myopathies. PLOS ONE 14:e0220904 10.1371/journal.pone.0220904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palazon A, Tyrakis PA, Macias D, et al. (2017) An HIF-1α/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell 32:669–683.e5. 10.1016/j.ccell.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarvinen TAH, Kannus P, Jarvinen TLN, et al. (2000) Tenascin-C in the pathobiology and healing process of musculoskeletal tissue injury. Scand J Med Sci Sports 10:376–382. 10.1034/j.1600-0838.2000.010006376.x [DOI] [PubMed] [Google Scholar]
- 40.Riley GP, Harrall RL, Cawston TE, et al. (1996) Tenascin-C and human tendon degeneration. Am J Pathol 149:933–943 [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S-A, Kang J-H, Cho I, et al. (2001) Cell-type specific regulation of thrombospondin-1 expression and its promoter activity by regulatory agents. Exp Mol Med 33:117–123. 10.1038/emm.2001.21 [DOI] [PubMed] [Google Scholar]
- 42.Stenina-Adognravi O (2014) Invoking the power of thrombospondins: Regulation of thrombospondins expression. Matrix Biol 37:69–82. 10.1016/j.matbio.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norman JT, Lindahl GE, Shakib K, et al. (2001) The Y-box Binding Protein YB-1 Suppresses Collagen α1(I) Gene Transcription via an Evolutionarily Conserved Regulatory Element in the Proximal Promoter. J Biol Chem 276:29880–29890. 10.1074/jbc.M103145200 [DOI] [PubMed] [Google Scholar]
- 44.El-Naggar AM, Veinotte CJ, Cheng H, et al. (2015) Translational Activation of HIF1α by YB-1 Promotes Sarcoma Metastasis. Cancer Cell 27:682–697. 10.1016/j.ccell.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 45.Thankam FG, Dilisio MF, Dougherty KA, et al. (2016) Triggering receptor expressed on myeloid cells and 5’adenosine monophosphate-activated protein kinase in the inflammatory response: a potential therapeutic target. Expert Rev Clin Immunol 12:1239–1249. 10.1080/1744666X.2016.1196138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sira MM, Behairy BE, Abd-Elaziz AM, et al. (2014) Serum Inter-Alpha-Trypsin Inhibitor Heavy Chain 4 (ITIH4) in Children with Chronic Hepatitis C: Relation to Liver Fibrosis and Viremia. Hepat Res Treat 2014:1–7. 10.1155/2014/307942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chandler KB, Brnakova Z, Sanda M, et al. (2014) Site-Specific Glycan Microheterogeneity of Inter-Alpha-Trypsin Inhibitor Heavy Chain H4. J Proteome Res 13:3314–3329. 10.1021/pr500394z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanno T, Yasutake K, Tanaka K, et al. (2017) A novel function of N-linked glycoproteins, alpha-2-HS-glycoprotein and hemopexin: Implications for small molecule compound-mediated neuroprotection. PloS One 12:e0186227 10.1371/journal.pone.0186227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inaoka Y, Osawa M, Mukasa N, et al. (2015) Allelic Imbalance of mRNA Associated with α2-HS Glycoprotein (Fetuin-A) Polymorphism. Dis Markers 2015:865053 10.1155/2015/865053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnaud P, Kalabay L (2002) Alpha2-HS glycoprotein: a protein in search of a function. Diabetes Metab Res Rev 18:311–314. 10.1002/dmrr.315 [DOI] [PubMed] [Google Scholar]
- 51.Schure R, Costa KD, Rezaei R, et al. (2013) Impact of matrix metalloproteinases on inhibition of mineralization by fetuin. J Periodontal Res 48:357–366. 10.1111/jre.12015 [DOI] [PubMed] [Google Scholar]
- 52.Thankam FG, Evan DK, Agrawal DK, Dilisio MF (2018) Collagen type III content of the long head of the biceps tendon as an indicator of glenohumeral arthritis. Mol Cell Biochem. 10.1007/s11010-018-3449-y [DOI] [PubMed] [Google Scholar]
- 53.Hsiao K-C, Shih N-Y, Fang H-L, et al. (2013) Surface α-Enolase Promotes Extracellular Matrix Degradation and Tumor Metastasis and Represents a New Therapeutic Target. PLoS ONE 8:e69354 10.1371/journal.pone.0069354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sedoris KC, Thomas SD, Miller DM (2010) Hypoxia induces differential translation of enolase/MBP-1. BMC Cancer 10:. 10.1186/1471-2407-10-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan SS, Zong M, Zhang H, et al. (2015) Decreased expression of alpha-enolase inhibits the proliferation of hypoxia-induced rheumatoid arthritis fibroblasts-like synoviocytes. Mod Rheumatol 25:701–707. 10.3109/14397595.2015.1014141 [DOI] [PubMed] [Google Scholar]
- 56.Banes AJ, Link GW, Bevin AG, et al. (1988) Tendon synovial cells secrete fibronectin in vivo and in vitro. J Orthop Res 6:73–82. 10.1002/jor.1100060110 [DOI] [PubMed] [Google Scholar]
- 57.Kular JK, Basu S, Sharma RI (2014) The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng 5:204173141455711. 10.1177/2041731414557112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams IF, McCullagh KG, Silver IA (1984) The distribution of types I and III collagen and fibronectin in the healing equine tendon. Connect Tissue Res 12:211–227 [DOI] [PubMed] [Google Scholar]
- 59.Kjær M (2004) Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol Rev 84:649–698. 10.1152/physrev.00031.2003 [DOI] [PubMed] [Google Scholar]
- 60.Neal CL, Xu J, Li P, et al. (2012) Overexpression of 14–3-3ζ in cancer cells activates PI3K via binding the p85 regulatory subunit. Oncogene 31:897–906. 10.1038/onc.2011.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pennington K, Chan T, Torres M, Andersen J (2018) The dynamic and stress-adaptive signaling hub of 14–3-3: emerging mechanisms of regulation and context-dependent protein–protein interactions. Oncogene 37:5587–5604. 10.1038/s41388-018-0348-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang Y, Lv P, Sun Z, et al. (2015) 14–3-3ζ up-regulates hypoxia-inducible factor-1α in hepatocellular carcinoma via activation of PI3K/Akt/NF-κB signal transduction pathway. Int J Clin Exp Pathol 8:15845–15853 [PMC free article] [PubMed] [Google Scholar]
- 63.Newton DA, Rao KMK, Dluhy RA, Baatz JE (2006) Hemoglobin Is Expressed by Alveolar Epithelial Cells. J Biol Chem 281:5668–5676. 10.1074/jbc.M509314200 [DOI] [PubMed] [Google Scholar]
- 64.Sousa MM, do Amaral JB, Guimarães A, Saraiva MJ (2005) Up-regulation of the extracellular matrix remodeling genes, biglycan, neutrophil gelatinase-associated lipocalin, and matrix metalloproteinase-9 in familial amyloid polyneuropathy. FASEB J 19:124–126. 10.1096/fj.04-2022fje [DOI] [PubMed] [Google Scholar]
- 65.Sun J, Wei X, Lu Y, et al. (2017) Glutaredoxin 1 (GRX1) inhibits oxidative stress and apoptosis of chondrocytes by regulating CREB/HO-1 in osteoarthritis. Mol Immunol 90:211–218. 10.1016/j.molimm.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 66.Lu J, Holmgren A (2009) Selenoproteins. J Biol Chem 284:723–727. 10.1074/jbc.R800045200 [DOI] [PubMed] [Google Scholar]
- 67.Windoffer R, Beil M, Magin TM, Leube RE (2011) Cytoskeleton in motion: the dynamics of keratin intermediate filaments in epithelia. J Cell Biol 194:669–678. 10.1083/jcb.201008095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karantza V (2011) Keratins in health and cancer: more than mere epithelial cell markers. Oncogene 30:127–138. 10.1038/onc.2010.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Na N, Chandel NS, Litvan J, Ridge KM (2010) Mitochondrial reactive oxygen species are required for hypoxia-induced degradation of keratin intermediate filaments. FASEB J Off Publ Fed Am Soc Exp Biol 24:799–809. 10.1096/fj.08-128967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai W, He JC, Zhu L, et al. (2006) Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc Natl Acad Sci 103:13801–13806. 10.1073/pnas.0600362103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Torreggiani M, Liu H, Wu J, et al. (2009) Advanced glycation end product receptor-1 transgenic mice are resistant to inflammation, oxidative stress, and post-injury intimal hyperplasia. Am J Pathol 175:1722–1732. 10.2353/ajpath.2009.090138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang S-W, Wang W-T, Chou L-C, et al. (2016) Diabetes mellitus increases the risk of rotator cuff tear repair surgery: A population-based cohort study. J Diabetes Complications 30:1473–1477. 10.1016/j.jdiacomp.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 73.Collier TA, Nash A, Birch HL, de Leeuw NH (2016) Intra-molecular lysine-arginine derived advanced glycation end-product cross-linking in Type I collagen: A molecular dynamics simulation study. Biophys Chem 218:42–46. 10.1016/j.bpc.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zieman SJ, Kass DA (2004) Advanced Glycation End Product Cross-Linking: Pathophysiologic Role and Therapeutic Target in Cardiovascular Disease . Congest Heart Fail 10:144–151. 10.1111/j.1527-5299.2004.03223.x [DOI] [PubMed] [Google Scholar]
- 75.Thankam FG, Dilisio MF, Dietz NE, Agrawal DK (2016) TREM-1, HMGB1 and RAGE in the Shoulder Tendon: Dual Mechanisms for Inflammation Based on the Coincidence of Glenohumeral Arthritis. PLOS ONE 11:e0165492 10.1371/journal.pone.0165492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thakkar D, Grant TM, Hakimi O, Carr AJ (2014) Distribution and expression of type VI collagen and elastic fibers in human rotator cuff tendon tears. Connect Tissue Res 55:397–402. 10.3109/03008207.2014.959119 [DOI] [PubMed] [Google Scholar]
- 77.Theocharidis G, Drymoussi Z, Kao AP, et al. (2016) Type VI Collagen Regulates Dermal Matrix Assembly and Fibroblast Motility. J Invest Dermatol 136:74–83. 10.1038/JID.2015.352 [DOI] [PubMed] [Google Scholar]
- 78.Urciuolo A, Quarta M, Morbidoni V, et al. (2013) Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun 4:1964 10.1038/ncomms2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arai Y, Park S, Choi B, et al. (2016) Enhancement of Matrix Metalloproteinase-2 (MMP-2) as a Potential Chondrogenic Marker during Chondrogenic Differentiation of Human Adipose-Derived Stem Cells. Int J Mol Sci 17:. 10.3390/ijms17060963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bowe EA, Murray RC, Jeffcott LB, Davies ME (2007) Do the matrix degrading enzymes cathepsins B and D increase following a high intensity exercise regime? Osteoarthritis Cartilage 15:343–349. 10.1016/j.joca.2006.08.014 [DOI] [PubMed] [Google Scholar]
- 81.Pranjol MZI, Gutowski N, Hannemann M, Whatmore J (2015) The Potential Role of the Proteases Cathepsin D and Cathepsin L in the Progression and Metastasis of Epithelial Ovarian Cancer. Biomolecules 5:3260–3279. 10.3390/biom5043260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sis B, Saǧol Ö, Küpelioǧlu A, et al. (2004) Prognostic significance of matrix metalloproteinase-2, cathepsin D, and tenascin-C expression in colorectal carcinoma. Pathol - Res Pract 200:379–387. 10.1016/j.prp.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 83.Sis B, Tuna B, Yorukoglu K, Kargi A (2004) Tenascin C and Cathepsin D Expression in Adipocytic Tumors: An Immunohistochemical Investigation of 43 Cases. Int J Surg Pathol 12:11–15. 10.1177/106689690401200102 [DOI] [PubMed] [Google Scholar]
- 84.Veidal SS, Karsdal MA, Vassiliadis E, et al. (2011) MMP mediated degradation of type VI collagen is highly associated with liver fibrosis--identification and validation of a novel biochemical marker assay. PloS One 6:e24753 10.1371/journal.pone.0024753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bradley E, Bieberich E, Mivechi NF, et al. (2012) Regulation of embryonic stem cell pluripotency by heat shock protein 90. Stem Cells Dayt Ohio 30:1624–1633. 10.1002/stem.1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Di Rocco G, Baldari S, Gentile A, et al. (2018) Protein disulfide isomerase as a prosurvival factor in cell therapy for muscular and vascular diseases. Stem Cell Res Ther 9:. 10.1186/s13287-018-0986-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bakthisaran R, Tangirala R, ChM Rao (2015) Small heat shock proteins: Role in cellular functions and pathology. Biochim Biophys Acta BBA - Proteins Proteomics 1854:291–319. 10.1016/j.bbapap.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 88.Li H, Song H, Luo J, et al. (2012) Knockdown of glucose-regulated protein 78 decreases the invasion, metalloproteinase expression and ECM degradation in hepatocellular carcinoma cells. J Exp Clin Cancer Res CR 31:39 10.1186/1756-9966-31-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dmello C, Sawant S, Alam H, et al. (2017) Vimentin regulates differentiation switch via modulation of keratin 14 levels and their expression together correlates with poor prognosis in oral cancer patients. PloS One 12:e0172559 10.1371/journal.pone.0172559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pérez-Sala D, Oeste CL, Martínez AE, et al. (2015) Vimentin filament organization and stress sensing depend on its single cysteine residue and zinc binding. Nat Commun 6:. 10.1038/ncomms8287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thiagarajan PS, Yakubenko VP, Elsori DH, et al. (2013) Vimentin is an endogenous ligand for the pattern recognition receptor Dectin-1. Cardiovasc Res 99:494–504. 10.1093/cvr/cvt117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davis ME (2016) Exosomes: What Do We Love So Much About Them? Circ Res 119:1280–1282. 10.1161/CIRCRESAHA.116.309942 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.