Abstract
Objective:
Perinatal antibiotic exposure may be associated with changes in both early infancy gut microbiota and later childhood obesity. Our objective was to evaluate if Group B Streptococcus (GBS) antibiotic prophylaxis is associated with higher body mass index (BMI) in early childhood.
Methods:
Retrospective cohort study of mother/child dyads in a single hospital system over a 6-year period. All women with term, singleton, vertex, vaginal deliveries who received no antibiotics or received antibiotics only for GBS prophylaxis and whose children had BMIs available at 2–5 years of age were included. Children were divided into three groups for comparison: children born to GBS positive mothers that received antibiotics solely for GBS prophylaxis, children born to GBS negative women that received no antibiotics (healthy controls), and children born to GBS positive mothers who received no antibiotics. The primary outcome was the earliest available child BMI Z-score at 2–5 years of age. Multivariable linear regression was used to estimate differences in child BMI Z-scores between groups, adjusted for maternal BMI, age, race, parity, tobacco use, and child birthweight.
Results:
Of 4825 women, 786 (16.3%) were GBS positive and received prophylactic antibiotics, 3916 (81.2%) were GBS negative and received no antibiotics, and 123 (2.5%) were GBS positive but received no antibiotics. Childhood BMI Z-scores were similar between children exposed to intrapartum GBS prophylaxis and healthy controls who were unexposed in both unadjusted (mean (SE), 0.04 (0.04) versus −0.3 (0.02), p=0.11) and adjusted (0.01 (0.05) versus −0.04 (0.03), p=0.3) models.
Conclusions:
Exposure to intrapartum antibiotic prophylaxis for GBS was not associated with higher early childhood BMI Z-scores compared to healthy controls.
Keywords: microbiome, antibiotics, intrapartum, Group B Streptococcus, obesity
Introduction
Establishment of the intestinal microbiome in newborns starts in utero and can be impacted by antibiotic exposure.1,2 Ante- and postnatal antibiotics have been associated with changes in the gut microbiome.3,4 Some changes in composition are transient, while others persist long term.2–4
The microbiome is essential in determining energy extraction from food and plays a central role in metabolic pathways.2 Animal studies clearly show a direct effect of early antibiotic therapy on gene expression in key metabolic processes and overall adiposity.5 Human studies have demonstrated differential composition of intestinal microbiomes in over- versus normal-weight adult women6,7 and children.4,8,9 Further, in large cohort studies, early antibiotic use is associated with more frequent childhood obesity, especially in boys.4,10–16
Intrapartum antibiotic exposure is associated with changes in infant fecal microbiota, with decreased quantities and diversity of actinobacteria and bifidobacteria, and an overabundance of proteobacteria.17–20 While it is known that gut microbiome composition is affected by antepartum and early childhood antibiotics, and that microbiota have a significant effect on metabolism and obesity, it is not clear if intrapartum antibiotics are associated with childhood obesity.
We studied the impact of intrapartum antibiotic prophylaxis for GBS as it is not confounded by acute infection illness, is common, and because intrapartum antibiotic prophylaxis is the standard of care based on Centers for Disease Control and Prevention guidelines. The purpose of this study is to evaluate if intrapartum GBS prophylaxis is related to higher early childhood BMI Z-score, both overall and by neonatal sex.
Materials and Methods
This was a retrospective cohort study of all maternal/child dyads delivered at Denver Health Hospital (a large safety net hospital for the city and county of Denver) between January 1, 2008 and December 31, 2013. All women with vaginal deliveries of term (37 weeks 0 days to 41 weeks 6 days), liveborn, vertex, singletons were considered for inclusion. Dyads were excluded if there was no maternal GBS culture result available, or if the child did not have a BMI in the Denver Health system at 2–5 years of age, as this was the primary endpoint. This study was approved by the Colorado Multiple Institutional Review Board.
Women were categorized into groups based on GBS culture result and antibiotic exposure. Women were classified as GBS positive by a positive rectovaginal swab at greater than 35 weeks gestation, or a urine culture positive for GBS at any point during the pregnancy. GBS negative women had either a negative urine culture for GBS and a negative rectovaginal swab, or a negative rectovaginal swab with no urine culture available. Women receiving GBS antibiotic prophylaxis were identified by pharmacy administration records based on intrapartum administration of intravenous ampicillin, cefazolin, clindamycin, penicillin, or vancomycin and no other antibiotics during the delivery hospitalization.
The primary comparison groups were women with a positive GBS culture result who received antibiotic prophylaxis alone (exposed) and women with a negative GBS culture result who received no antibiotics (unexposed – healthy controls). A third, secondary, comparison group included women who were GBS positive, but did not receive antibiotics. In our institution this usually is related to precipitous birth shortly after admission.21
The primary endpoint was the child’s first available BMI Z-score at 2–5 years of age. BMI Z-scores are measures of relative weight adjusted for a child’s age and sex. Childhood BMIs were normalized into Z-scores based on child sex and age at the time of BMI ascertainment.22
Demographic and clinical data were extracted from both Phillips OBTraceVue and the Denver Health Data Warehouse. Women were initially identified by querying OBTraceVue which is the electronic medical record used on Labor and Delivery. Women were then linked with their children using the Denver Health Data Warehouse, a validated administrative database with billing and clinical data for all encounters in the Denver Health system. There are multiple internal mechanisms for the assessment of the accuracy of data from the Denver Health Data Warehouse. Data validity checks occur at the level of the information technology management department as well as individually within each department on an ongoing basis.
Maternal and child BMIs, neonatal birthweight, race and ethnicity of both the mother and child, insurance status, maternal tobacco use, diagnostic codes for maternal diabetes, and antibiotic exposure were extracted from the Denver Health Data Warehouse. Maternal weight at delivery was used if available at or after 28 weeks gestation; it was otherwise documented as missing. Maternal height was used if available during pregnancy, or during adulthood if the pregnancy was also during adulthood for any visit in the Data Warehouse. Date of delivery, gestational age at delivery, parity and mode of delivery were extracted from OBTraceVue. Demographics and clinical characteristics were compared for the two primary comparison groups, as well as the 3-category study grouping using t-test or ANOVA for continuous and chi-square for categorical measures. Right skewed continuous measures were compared on the log scale and are reported as geometric mean and 95% confidence interval.
In a multivariable linear regression model, the effect of GBS prophylaxis on childhood BMI Z-scores was estimated while adjusting for clinically important covariates: maternal BMI, diabetes, age, tobacco use, insurance type, black race, Hispanic ethnicity, gestational age at delivery, birthweight, and season of birth. The child’s age at BMI measurement is accounted for in the calculation of the Z score and was therefore not considered as a separate covariate in modeling. A final model was selected using backwards stepwise elimination separately for all children and repeated for each sex (boys, girls). In addition, a sensitivity analysis was performed for children with the first BMI at 2–3 years of age.
For our sample size calculation, the anticipated effect size was estimated based on an existing study by Mueller et al who found a difference of 0.47 (0.19, 0.74) in Z-scores in early childhood among children with and without perinatal antibiotic exposure.16 Assuming a rate of exposure of 15% (85% unexposed), we estimated a need for 1,715 children to detect a difference in Z-score of 0.19 with 80% power, based on the lower bound of the confidence interval in the Mueller study.
Study data were collected and managed using Research Electronic Data Capture (REDCap) which is a secure, web-based application designed to support data capture and export for analysis for research studies.23 A P value <0.05 was considered statistically significant. All analyses were performed in SAS, and graphics were created with GraphPad Prism.
Results
Overall 13,874 vaginal deliveries of term, cephalic, singletons occurred during the study time period. Only the first delivery for each woman was included which resulted in 11,959 deliveries to unique women. Additional exclusions were made if child BMI Z-score at 2–5 years was unavailable (n=5694), GBS status was unavailable (n=635), or the neonate had other intrapartum antibiotic exposure (n=820), resulting in a final study population of 4825 dyads (some excluded dyads met more than one exclusion criteria). Women who were excluded for missing the primary endpoint did not differ significantly from those who were included by maternal BMI. However, excluded women were slightly older (25.7 versus 24.9 years, p<0.001) and more likely to be nulliparous (28.8% versus 19.3%, p<0.001) than those included.
Of the 4825 included women, 3916 (81%) were GBS negative and received no antibiotics. A total of 909 women were GBS positive; 786 (16%) were GBS positive and received intrapartum antibiotic prophylaxis, 123 (3%) were GBS positive but did not receive antibiotics (e.g. delivered too quickly). Among women who were GBS positive and received antibiotics, most (97%) received penicillin. Other antibiotics used for GBS prophylaxis included cefazolin (2.0%) and clindamycin (0.9%). Of the 762 women who received penicillin for intrapartum prophylaxis, 36 (4.7%) also received another antibiotic used for GBS prophylaxis (cefazolin, clindamycin or ampicillin).
When categorized into three groups based on GBS culture result and antibiotic exposure, differences were noted between groups for maternal and neonatal baseline characteristics (Table 1) in both 3-way comparisons, and in comparisons of our two primary groups of interest (GBS positive with antibiotic prophylaxis and GBS negative with no antibiotic exposure). Mean age for the 2–5 year child BMI was 2.40 years (95 % CI 2.38–2.42). Mean child age at BMI measurement was similar between exposure groups: GBS positive with antibiotic, GBS negative, and GBS positive without antibiotic exposure (mean (SD): 2.38 (0.6), 2.40 (0.6), 2.49 (0.7), respectively p=0.25).
Table 1.
Maternal and Neonatal Characteristics Categorized by Group B Streptococcal Culture Result and Intrapartum Prophylactic Antibiotic Exposure
| Characteristic | Group 1: GBS Positive, Received Prophylactic Antibiotics n = 786 | Group 2: GBS Negative, No Antibiotics Received n = 3916 | Group 3: GBS Positive, No Prophylactic Antibiotics Received n = 123 | Overall (3-way) P-value | Group 1 versus Group 2 P-value |
|---|---|---|---|---|---|
| Maternal Age (years), geomean (95% CI) | 25.2 (24.8,25.6) | 24.8 (24.6,25.0) | 25.1 (24.1,26.2) | 0.32 | 0.15 |
| Spontaneous | 757 (96.3) | 3814 (97.4) | 120 (97.6) | ||
| Gestational Age at Delivery (weeks), geomean (95% CI) | 39.6 (39.5,39.6) | 39.6 (39.5,39.6) | 39.2 (39.0,39.4) | 0.003 | 0.89 |
| Birthweight (gms), geomean (95% CI) | 3283 (421.6) | 3272 (432.7) | 3176 (459.3) | 0.047 | 0.52 |
| Male | 385 (49.0) | 1962 (50.1) | 52 (42.3) | ||
| Maternal BMI (kg/m2), geomean (95% CI) | 32.1 (31.7,32.6) | 30.6 (30.4,30.8) | 31.1 (30.0,32.2) | <.001 | <.001 |
| Black Race | 126 (16.7) | 315 (8.4) | 25 (21.7) | <.001 | <.001 |
| Hispanic Ethnicity | 534 (70.8) | 2999 (79.9) | 79 (68.7) | <.001 | <.001 |
| Public Insurance | 726 (92.4) | 3746 (95.7) | 120 (97.6) | <.001 | <.001 |
| Tobacco Use | 191 (24.3) | 657 (16.8) | 36 (29.3) | <.001 | <.001 |
| Pregestational | 22 (3.0) | 77 (2.1) | 2 (1.7) | ||
| Nulliparous | 169 (21.6) | 747 (19.1) | 12 (9.8) | 0.007 | 0.12 |
Numbers are n (%) unless otherwise specified. Geomean is geometric mean. CI is confidence interval. BMI is body mass index. Missing data among groups 1, 2 and 3 respectively is as follows: maternal BMI (n=39, n=283, n=21), parity (n=2, n=8, n=0), race/ethnicity (n=32, n=161, n=8), and diabetes (n=46, n=161, n=6), and birthweight (n=42, n=254, n=8).
In unadjusted pairwise comparisons between GBS positive women with antibiotic prophylaxis and GBS negative women without antibiotics, there were no significant differences in the primary endpoint (mean difference and 95% CI 0.07, −0.02 to 0.17) (Table 2).
Table 2.
Age 2–5 Years Childhood Body Mass Index Z-Score by GBS Antibiotic Prophylaxis Exposure, Unadjusted
| Early Childhood BMI Z-Scores | Group 1 GBS Positive, Received Prophylactic Antibiotics | Group 2 GBS Negative, No Antibiotics Received | Group 3 GBS Positive, No Prophylactic Antibiotics Received | Overall (3-way) P-value | Group 1 versus Group 2 P-value |
|---|---|---|---|---|---|
| OVERALL | N = 786 | N = 3916 | N = 123 | ||
| Z-score, (mean(SD)) | 0.04 (1.1) | −0.03 (1.2) | −0.12 (1.2) | 0.18 | 0.10 |
| Obese (>95%ile) | 69 (8.8) | 305 (7.8) | 11 (8.9) | ||
| MALES | n = 385 | n =1962 | n =52 | ||
| Z-score, (mean(SD)) | 0.10 (1.1) | 0.00 (1.2) | −0.52 (1.1) | 0.002 | 0.85 |
| FEMALES | n =401 | n =1954 | n =71 | ||
| Z-score, (mean(SD)) | −0.01 (1.2) | −0.07 (1.2) | 0.17 (1.3) | 0.20 | 0.23 |
Since other investigators have noted an effect of antibiotic exposure in males but not females, we next analyzed male and female children separately. For females, differences in BMI- Z-scores remained non-significant (mean difference and 95% CI 0.05, −0.07 to 0.18). However, significant differences were seen in comparisons among male neonates. Z-scores at 2–5 years for male neonates of GBS positive women who did not receive prophylaxis were significantly lower than male neonates of women who were GBS positive who received prophylaxis (mean (SD), −0.52 (1.1) versus 0.10 (1.1), respectively, p<0.001). Despite this driving the significant overall p-value for the 3-group comparison (p=0.002) in Table 2, the primary pairwise comparison between children of women GBS positive who received antibiotics and GBS negative who received no antibiotics, was not significant (mean difference and 95% CI: 0.10 −0.03 to 0.23) (Table 2).
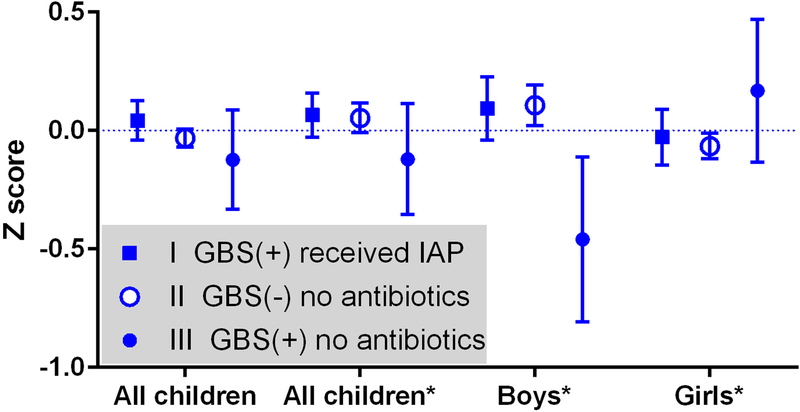
Linear regression modeling was utilized to estimate the effect of GBS and intrapartum GBS antibiotic prophylaxis on childhood BMI Z-scores between all three comparison groups adjusted for maternal and neonatal characteristics. The final adjusted multivariable model for all children included maternal BMI, child weight at birth, black race and gestational age at delivery. Adjusted sex-specific models included the same covariates as the model of all children except boys further excluded gestational age at delivery, and girls further excluded black race. Childhood BMI Z-scores at 2–5 years of age were similar between children exposed to intrapartum GBS prophylaxis and those who were unexposed in the adjusted models overall (mean (SE) 0.07 (0.05) versus 0.05 (0.03), p=0.19) and among girls (−0.03 (0.06) versus −0.07 (0.03), p=0.30). BMI Z-score in boys of GBS positive women that did not receive intrapartum antibiotic prophylaxis remained low in the adjusted model (−0.46 (−0.11, −0.81)) driving a significantly different three-way comparison (p=0.007); however the primary comparison among boys remained non-significant (0.09 (0.07) versus 0.11 (0.04), p=0.83) (Figure 1).
Figure 1.
Average effect of prophylactic antibiotics for Group B Streptococcus (GBS) on 2–5 year old childhood body mass index Z-score measured in number of standard deviations from the mean. The y-axis is mean calculated Z-score and the error bars represent 95% confidence intervals. *Adjusted multivariable model of all children includes maternal BMI, child weight at birth, black race and gestational age at delivery. Adjusted sex-specific models include same covariates as model of all children except boys further excluded gestational age at delivery, and girls further excluded black race. GBS (+) is GBS positive. GBS (−) is GBS negative. IAP is intrapartum antibiotic prophylaxis.
A sensitivity analysis of children with a first available BMI Z-score at 2–3 years of age (95.8% of the cohort) revealed findings similar to the whole cohort (data not shown).
Discussion
We did not find a significant association between intrapartum GBS antibiotic prophylaxis and early childhood BMI Z-scores when compared to healthy controls (GBS negative with no antibiotic exposure). These findings may provide reassurance to clinicians who are concerned about the effect of fetal exposure to antibiotics for GBS prophylaxis. Importantly however, this study does not address the potential impact of antibiotic exposure at other times in pregnancy or for other indications.
Our primary findings do not support several existing studies that have shown associations between early antibiotic exposure in the first six12,14, twelve10, and twenty-four months of life15 and childhood obesity, especially in boys.10,11 However our study is unique in that we are isolating a specific peripartum antibiotic exposure. As a result of focusing only on GBS antibiotic prophylaxis in otherwise healthy neonates and mothers, this study design may have removed important unmeasured confounding present in other studies related to inflammatory response in the setting of known infection, and risk factors for infection.
As part of a secondary comparison, we found that Z-scores for male neonates of GBS positive women who did not receive prophylaxis were significantly lower than male neonates of women who were GBS positive who received prophylaxis. However, in the primary comparison between male neonates of GBS positive women who received antibiotics and GBS negative who received no antibiotics, there was not a significant difference in unadjusted or adjusted comparisons. Other studies have demonstrated sex-specific differences in the association between antibiotic exposure and childhood obesity.10,11 Our sample of male neonates (n=2399) was large enough to detect a difference of 0.19 in Z-score based on our primary sample size calculation; our observed difference was smaller and its 95% confidence interval did not contain what we deemed a clinically important difference of 0.19 among boys in the primary adjusted comparison. Using data from our cohort, we calculated the average difference in weight associated with a 0.19 Z-score difference, which was 268 grams for children ages 2–2.5 years and 512 grams for children ages 4.5–5.5 years. Differences in weight smaller than these are not likely to be clinically relevant. Nonetheless, future studies should continue to analyze results by neonatal sex.
The primary strength of our study is that many children delivered at Denver Health continue to seek care within our community-based outpatient clinics enabling linkage of mothers and babies for childhood outcome studies such as this one. In this study, 53% of our cohort had childhood BMI Z-scores available. Women who were excluded for missing the primary endpoint had similar characteristics to the included women, with the exception of the proportion in each group who were nulliparous and a slight difference in mean maternal age. In addition, we were able to ascertain antibiotic exposure from pharmacy administration records rather than by parental recall, or reliance on infection diagnoses, as has been done in other studies.11,12,14 We also limited our population to neonates born vaginally to eliminate confounding by mode of delivery. Our study design also allowed direct capture of important potential confounders from the maternal medical record, as opposed to obtaining this information from maternal questionnaires subject to recall bias, as was done in some studies.16
The study is limited by our available population. Our sample size was adequate to detect a difference in the primary endpoint of 0.19. Detection of smaller differences would have required a larger sample size which would not have been feasible as a single-center study in a reasonable timeline. In addition, this is predominantly a low-income, Hispanic population; results may not be generalizable to other populations. We relied on electronic data from a validated administrative database which did not provide reliable data regarding the impact of breastfeeding, a factor that has been reported to influence the neonatal gut microbiome.24
This study demonstrated that exposure to intrapartum antibiotic prophylaxis for GBS was not significantly associated with higher early childhood BMI Z-scores. It may be that the previously demonstrated associations between early antibiotic exposure and childhood obesity are also dependent on the indication for antibiotics and resultant inflammatory milieu. In addition, the effect may depend on the trimester or timing of exposure.25 Further study is warranted to better elucidate the relationship between intrapartum antibiotic exposure and childhood obesity, as well as investigate if there are particular antibiotics that have a greater effect on the neonatal microbiome than others.
Acknowledgment:
The authors thank Renee Starr, Josh Durfee, and Bram Vanhout for data extraction for this manuscript.
Dr. Metz was supported by the National Institute on Child Health and Human Development under award number 5K12HD001271-18 during the completion of this project. This project was also supported by Colorado Clinical and Translational Sciences Institute (CCTSI) under award number UL1 RR025780. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Presented as a poster presentation at the Infectious Diseases Society for Obstetrics and Gynecology Annual Meeting, Park City, UT, August 10–12, 2017.
Disclosure Statement: The authors report no conflicts of interest. Each author has indicated that he/she has met the journal’s requirements for authorship.
References
- 1.Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nat Rev Endocrinol 2015;11:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeissig S, Blumberg RS. Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol 2014;15:307–10. [DOI] [PubMed] [Google Scholar]
- 3.Fouhy F, Guinane CM, Hussey S, et al. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob Agents Chemother 2012;56:5811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korpela K, Salonen A, Virta LJ, et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nature communications 2016;7:10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012;488:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr 2008;88:894–9. [DOI] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 2009;457:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr 2008;87:534–8. [DOI] [PubMed] [Google Scholar]
- 9.Vael C, Verhulst SL, Nelen V, Goossens H, Desager KN. Intestinal microflora and body mass index during the first three years of life: an observational study. Gut Pathog 2011;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azad MB, Bridgman SL, Becker AB, Kozyrskyj AL. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes (Lond) 2014;38:1290–8. [DOI] [PubMed] [Google Scholar]
- 11.Murphy R, Stewart AW, Braithwaite I, et al. Antibiotic treatment during infancy and increased body mass index in boys: an international cross-sectional study. Int J Obes (Lond) 2014;38:1115–9. [DOI] [PubMed] [Google Scholar]
- 12.Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond) 2011;35:522–9. [DOI] [PubMed] [Google Scholar]
- 13.Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics 2015;135:617–26. [DOI] [PubMed] [Google Scholar]
- 14.Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2013;37:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr 2014;168:1063–9. [DOI] [PubMed] [Google Scholar]
- 16.Mueller NT, Whyatt R, Hoepner L, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond) 2015;39:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azad MB, Konya T, Persaud RR, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG 2016;123:983–93. [DOI] [PubMed] [Google Scholar]
- 18.Corvaglia L, Tonti G, Martini S, et al. Influence of Intrapartum Antibiotic Prophylaxis for Group B Streptococcus on Gut Microbiota in the First Month of Life. J Pediatr Gastroenterol Nutr 2016;62:304–8. [DOI] [PubMed] [Google Scholar]
- 19.Aloisio I, Mazzola G, Corvaglia LT, et al. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl Microbiol Biotechnol 2014;98:6051–60. [DOI] [PubMed] [Google Scholar]
- 20.Aloisio I, Quagliariello A, De Fanti S, et al. Evaluation of the effects of intrapartum antibiotic prophylaxis on newborn intestinal microbiota using a sequencing approach targeted to multi hypervariable 16S rDNA regions. Appl Microbiol Biotechnol 2016;100:5537–46. [DOI] [PubMed] [Google Scholar]
- 21.Bienenfeld S, Rodriguez-Riesco LG, Heyborne KD. Avoiding Inadequate Intrapartum Antibiotic Prophylaxis for Group B Streptococci. Obstet Gynecol 2016;128:598–603. [DOI] [PubMed] [Google Scholar]
- 22.2000 CDC Growth Charts for the United States: Methods and Development. 2002. (Accessed October 25, 2017, at https://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf.) [PubMed]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health 2014; 14:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassidy-Bushrow AE, Burmeister C, Havstad S, Levin AM, Lynch SV, Ownby DR et al. Prenatal antimicrobial use and early-childhood body mass index. International Journal of Obesity. 2018. January 1;42(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]