Abstract
Boerhavia diffusa is a perennial herb belonging to the Nyctaginaceae family. This plant has been widely used in Indian traditional medicinal system to cure several human ailments. However, traditional use of this plant in the treatment and management of wounds has not been validated by any comprehensive scientific study. The present study was aimed to explore the in vitro and in vivo wound healing potential of methanol extract (ME) and chloroform extract (CE) from B. diffusa leaf and subsequent identification of the bioactive metabolites, which might be responsible for enhancement of wound healing property of the extracts.
The study included in vitro cell viability and wound scratch assays as well as in vivo excision wound assays in rat models. Both ME and CE were analysed for their antioxidant properties and phenolics content. The gas chromatography-mass spectrometry analyses were performed for identification of bioactive metabolites present in the ME and CE. ME of B. diffusa leaf significantly enhanced viability and migration of human keratinocyte cells (HaCaT) as compared to the untreated and CE-treated groups. The topical application of ME of B. diffusa leaf in excision wound model significantly decreased the wound area by the 14th day (91%) as compared to control (22%) (p < 0.05). The GC-MS analysis revealed the presence of caffeic acid, ferulic acid and D-pinitol as the major bioactive metabolites in ME. These results suggest that ME of B. diffusa possesses significant wound healing potential, where D-pinitol and caffeic acid served as the lead constituent metabolites responsible for the healing.
Keywords: Topical ointment, HaCaT keratinocyte cell line, Albino Wistar rats, Antioxidant, D-pinitol
Graphical abstract
Highlights
-
•
Reporting wound healing potential of Boerhavia diffusa leaf extracts.
-
•
GC-MS analyses of B. diffusa leaf extracts to identify bioactive wound healing metabolites.
-
•
D-Pinitol and caffeic acid were identified as lead metabolites triggering wound healing.
List of abbreviations
- AAE
Ascorbic acid equivalents
- CE
Chloroform extract
- DMSO
Dimethyl sulfoxide
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- FRAP
Ferric reducing antioxidant power
- GAE
Gallic acid equivalents
- GC-MS
Gas chromatography-mass spectrometry
- HaCaT
Human keratinocyte cells
- KE
Kaempferol equivalents
- ME
Methanol extract
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5diphenyl-2H-tetrazoliumbromide
- TPTZ
2,4,6-tripyridyl-s-triazine
1. Introduction
The human skin acts as a semi-permeable barrier for the body. The integrity of the skin is critical for the protection, maintenance of body temperature, sensation, metabolism and communication.1 Skin disorders, especially wounds are quite common ailments. A wound is a break in the continuity of tissue. When there is a restoration of the wounded tissue to normal condition, the wound is considered to be healed.2
The process of wound healing is very complex and dynamic since it involves multiple cell types, chemical mediators and the extracellular matrices.3 Although wound healing is a natural process, by itself it may lack quality, promptness and aesthetics.4 Tissue repair begins with inflammation, followed by granular tissue formation, which leads to synthesis of new connective tissues and epithelial wound closure, and finally a scar-remodelling phase resulting in a functional physical barrier.5
Currently prescribed methods and medicines to treat wounds are largely ineffective and some even have adverse side effects.6 The principal constituents of many present day wound-healing medicines are polypeptide growth factors, which promote and control cell proliferation at the site of wound. However, these growth factors may also lead to the development of cancer due to their enhanced proliferation-inducing activities.7
Plant extracts have long been known for their effective wound healing properties.8 The ancient Ayurvedic literature presents about 164 medicinal plants indicating their use in wound healing.9 Among these, whole plant of B. diffusa has been routinely utilized for treating wounds and syphilitic ulcers.10 Various ethnopharmacological surveys have reported that roots and leaves of B. diffusa are used by several Indian tribes for the treatment of a number of ailments.11, 12, 13, 14
B. diffusa L. is commonly known as Punarnava in the Indian traditional medicinal system. It is an abundantly growing perennial creeping herb, found throughout India.15 Many traditional pharmacological uses of B. diffusa such as immunomodulatory, anticancer, antidiabetic, antifibrinolytic, anti-inflammatory, diuretic, hepatoprotective, antimicrobial, antifungal, anticonvulsant and antioxidant activities have been reported and scientifically validated.16, 17, 18, 19, 20, 21 Nevertheless, to the best of our knowledge, the wound healing potential of B. diffusa has not yet been explored scientifically. In addition, comprehensive metabolite profile of leaf extracts of B. diffusa has not been reported. Understanding the constituent metabolites present in the B. diffusa extracts and their modes-of-actions in wound healing would facilitate development of new herbal wound healing formulations with more safety and efficacy. In view of these facts, the present study was aimed to investigate the wound healing properties of B. diffusa leaf extracts with special reference to their bioactive metabolite profile and antioxidant potentials.
2. Materials and methods
2.1. Plant material collection
B. diffusa leaves were collected in the month of August 2017 from the local medicinal plant garden of Indian Institute of Technology Roorkee, India. B. diffusa plants were identified and authenticated by taxonomist Dr. Anup Chandra from the Forest Research Institute, Dehradun, India vide letter No. 172/Dis./2018/Syst.Bot./Rev.Gen./4–5.
2.2. Chemicals and reagents used
Methanol, chloroform, ethanol, sodium carbonate, Folin-Ciocalteau reagent, aluminum chloride, MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5diphenyl-2H-tetrazoliumbromide) and cell culture grade dimethyl sulfoxide (DMSO) were purchased from HiMedia (Mumbai, India). MSTFA and DPPH (1,1-diphenyl-2-picrylhydrazyl) were purchased from Sigma (Bangalore, India). All solvents used were of HPLC grade. Human keratinocyte (HaCaT) cell line was obtained from National Center for Cell Science (NCCS), India.
2.3. Sample preparation
B. diffusa leaves were separated from the stem, air-dried in shade for 15 days at room temperature. After drying, the leaves were crushed to a coarse powder and stored in air tight containers at 4 °C until further use. The leaf powder was subjected to successive solvent extraction. In the first solvent extraction, 4 g of leaf powder was macerated in 20 mL of chloroform followed by centrifugation at 10,000×g for 15 min. The resulting supernatant was collected, evaporated to dryness under reduced pressure to give chloroform extract (CE). The left over pellet from the first solvent extraction was then macerated in 20 mL of methanol, followed by centrifugation at 10,000×g for 15 min. The resulting supernatant was collected, dried under reduced pressure to yield methanol extract (ME). An aliquot of both CE and ME was dissolved in 50% methanol in a final concentration of 1 mg/mL and further used for the estimation of total phenolics, total flavonoids and antioxidant potential.
2.4. Assay of total phenolic content
The total phenolic content in CE and ME was measured by the Folin-Ciocalteau method using a multi-well plate reader.22 Briefly, 20 μL of CE or ME was added to 100 μL of Folin-Ciocalteu reagent (10% in distilled water) and incubated for 5 min at room temperature, followed by addition of 80 μL of 5% sodium carbonate. After 20 min incubation in dark, the absorbance was measured at 765 nm using a multi-well plate reader (BioTek Synergy HTX multi-mode plate reader, USA). A reagent blank was used as negative control. The total phenolic contents were determined from a standard curve prepared using gallic acid and results were expressed as milligrams of gallic acid equivalents per gram dry weight of leaves (mg GAE/g DW).
2.5. Assay of total flavonoid content
The total flavonoid content in CE and ME was determined as essentially described by Wang et al. with suitable modification.23 Extract (100 μL) was added to 100 μL of 2% aluminium chloride solution in ethanol and incubated at room temperature for 1 h. After incubation, absorbance was measured at 420 nm against a reagent blank in a multi-well plate reader (BioTek Synergy HTX multi-mode plate reader, USA). The total flavonoid content was expressed as milligrams of kaempferol equivalents per gram dry weight of leaves (mg KE/g DW).
2.6. DPPH antioxidant assay
The antioxidant activity of the leaf extracts was determined by analyzing their radical scavenging potential using DPPH method.24 DPPH radicals were prepared by dissolving DPPH in methanol. 40 μL of various concentrations of CE and ME was added to 160 μL of the 100 μM methanolic solution of DPPH in a microtitre plate. After incubation at room temperature for 30 min, absorbance was measured at 517 nm (BioTek Synergy HTX multi-mode plate reader, USA). All experiments were performed in triplicate. Control reaction consisted of all reagents except the leaf extract. Blank consisted only of methanol. Percentage of inhibition (%) of DPPH free radicals was calculated from the absorbance (A) using the following formula.
| Inhibition (%) = (1 - A Sample/A Control) x 100 |
The results were expressed as IC50 values. The IC50 value is the concentration of an antioxidant required to reduce the initial concentration of DPPH by 50%. Ascorbic acid was used as standard antioxidant.
2.7. Ferric reducing antioxidant power assay
Ferric reducing antioxidant power (FRAP) was calculated following the protocol of Benzie and Strain with minor modifications.25 First, a master mixture was prepared by adding 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) solution (1 mL) with 300 mM acetate buffer in 40 mM hydrochloric acid (10 mL) and 20 mM ferric chloride hexahydrate (1 mL). Thereafter, 40 μL of extract (CE or ME) and 60 μL of water was added to 1 mL of freshly prepared master mixture. The assay was incubated at 37 °C for 15 min and then absorbance was measured at 593 nm against a blank. In blank reaction, extract was replaced by 50% methanol. A calibration curve was prepared using different concentrations of standard ascorbic acid. Results were expressed as milligram of ascorbic acid equivalents per gram dry weight of leaves (mg AAE/g DW).
2.8. GC-MS analyses
The dried CE (1 mg) and ME (1 mg) were derivatized with 40 μL of N-Methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA). While preparing extracts for GC-MS analysis, 4-phenylphenol was added as internal standard. The derivatization mixtures were kept at 60 °C for 30 min, with occasional shaking. After centrifuging the derivatized extracts at 14,000 rpm for 5 min, the supernatant was carefully transferred to a 200 μL glass insert in a GC vial. GC-MS analysis was performed on Agilent 7890A gas chromatograph (Agilent Technologies, CA, USA) coupled with an Agilent 5975C mass detector (Agilent Technologies, CA, USA). One microliter of derivatized sample was injected into GC-MS by automatic sampler (7683B series, Agilent Technologies, CA, USA) with a split ratio of 10:1. DB-5 MS column (5% phenyl methyl polysiloxane: 30 m × 0.25 mm i.d. x 0.25 μm, Agilent Technologies, CA, USA) was used for metabolite separation. The temperature program was as follows: Initial temperature of 90 °C for 5 min, followed by temperature increase to 200 °C at the ramp rate of 12 °C/min, followed by temperature increase to 300 °C at the ramp rate of 6 °C/min and finally a 10 min hold at 300 °C. Total run time calculated was 45.8 min. As carrier gas helium was used at a flow rate of 1 mL/min. The inlet temperature and interface temperature was set at 280 °C. The MS unit was tuned to its maximum sensitivity and the mass range for total ion current was m/z 70–800. Samples were injected in triplicates. Scan was started after solvent delay of 5 min with scan frequency of 4 S−1 (2.0 HZ). Metabolites were identified by matching the mass spectra of target metabolite (3:1 signal to noise ratio) with the NIST-11 mass spectral library (National Institute of Standards and Technology), and our in-house mass spectral database. Metabolite identity was reported only when the matching value of the mass spectra comparison was more than 70%.
2.9. Cell viability assay
Cell viability assay was performed according to a previously described protocol.26 HaCaT cells were seeded at a density of 5 × 103 cells/well into a 96-well plate. Cells were incubated overnight in a 5% CO2 humidified atmosphere at 37 °C. Thereafter, cells were treated with different concentrations (0–40 μg/mL) of CE and ME dissolved in final DMSO concentration of 0.1%. After 48 h, 20 μL of MTT solution was added to each well in a final concentration of 0.5 mg/mL and further incubated for 4 h at 37 °C. The presence of viable mitochondria results in the formation of a purple formazan product. The media was replaced by 200 μL of DMSO. The formazan formation was estimated by taking absorbance at 570 nm in a microplate reader. The experiments were performed in triplicate. Cell viability was calculated as a percentage compared to the experimental control.
2.10. In vitro scratch wound-healing assay
The scratch wound healing assay was carried out according to a previously reported protocol.27 The keratinocytes HaCaT (1 × 105 cells/well) were seeded in 12-well cell culture plate. After reaching confluency, a scratch was made in the monolayer using a sterile p200 tip. Cell debris were washed out using phosphate buffer saline. Fresh medium containing 1% fetal bovine serum and CE or ME (5 μg/mL) was added to the cells. Cells with medium without extract were used as control. The cells were incubated for up to 60 h at 37 °C in 5% CO2. Images of the scratch were taken at 0 h, 12 h, 24 h, 48 h, and 60 h using a phase contrast inverted microscope (Axiovert 25, Zeiss, Germany). Three images were photographed for each well under 10x magnifications after incubation to estimate the proliferation and/or migration of cells. The area enclosed between the scratch edges was measured using ImageJ software. The wound closure percentage was calculated compared to the initial scratch area at 0 h.
2.11. Excision wound healing activity
Albino Wistar rats (weight 200–250 g)28 of either sex were selected for this experiment because these experimental animals are readily available, docile, easy to handle and routinely used as animal model29,30 for testing wound healing activity. The rats were allowed to acclimatize to the laboratory conditions for 5 days before initiation of the experiment. Rats were maintained under standard laboratory conditions (12 h light/darkness at 25 ± 3 °C) with standard animal diet and water available ad libitum. These animal experiments were carried out at the M. M. College of Pharmacy, Maharishi Markandeshwar (Deemed to be University), Mullana, Ambala, Haryana-133203, India. The Institutional Animal Ethical Committee (1355/PO/Re/L/10/CPCSEA) permitted the studies under the certification (Ref. No. IAEC 18/02 of dated 07.02.2018).
The animals were randomly divided into five groups, each group having six rats. The following five experimental groups were framed:
Group I: Wounded control (untreated) group
Group II: Standard group (5.0% w/w povidone-iodine ointment)
Group III: Base group (Only base of ointment)
Group IV: CE ointment group (10% w/v)
Group V: ME ointment group (10% w/v)
10% (w/v) ointment was prepared for the extracts in saline containing 0.1% propylene glycol. Animals of all groups were anaesthetized by ketamine/xylazine anesthesia (1 mg of ketamine and 0.1 mg of xylazine per 10 g of body weight i.p.) prior to and during creation of the wounds. A full thickness of excision wound of circular area of 8 mm was created using biopsy punches. The surgical procedures were performed entirely in an aseptic area. The wound was left completely undressed during the study. CE, ME, base and the standard ointment (500 mg each) were applied on the wounds of animals in their respective groups (groups II to V) once a day for 14 days. Wound areas were recorded and measured by millimeter scale on day 1, day 5, day 9 and day 14.
2.12. Evaluating in vitro wound healing activity of major bioactive metabolites
From the results of the in vitro and in vivo wound healing assays as well as phytochemical and GC-MS analyses of the extracts, three compounds present in ME were chosen to be tested for their potential wound-healing activities on the HaCaT keratinocyte cell line. First, the cytotoxicity of each pure compound, namely D-pinitol, caffeic acid and ferulic acid, was separately evaluated via the MTT assay by following the same procedure as that carried out in section 2.9. HaCaT cells were treated with different concentrations (0–50 μM) of each compound dissolved in DMSO (final concentration of 0.1%).
Based on their effect on the viability of the HaCaT cells, the following concentrations were chosen to evaluate the in vitro wound healing ability of the pure compounds: 5 μM of caffeic acid, 3 μM of ferulic acid, and 5 μM of D-pinitol. The protocol for scratch assay was the same as that presented in section 2.10.
2.13. Statistical analysis
Each experiment was performed at least three times. Statistical analyses were performed using Microsoft Office Excel 2007 data analysis. Data are expressed as the mean ± SD. Significant differences between the control and test sample were determined by one way analysis of variance in Microsoft Office Excel 2007 data analysis with the significance factor p < 0.05.
3. Results
3.1. Total phenolic and flavonoid contents
The total phenolic and total flavonoid contents in the B. diffusa leaf extracts are shown in Table 1. Of the two leaf extracts analysed, ME showed significantly higher total phenolics as well as total flavonoid content as compared to CE. In general, in both CE and ME, total flavonoid content was significantly lower than that of total phenolic content, suggesting that leaf extracts are rich in phenolic compounds.
Table 1.
Quantitative estimation of total phenolics, total flavonoids and antioxidant activities (DPPH radical scavenging and FRAP) from B. diffusa leaf extracts. Results are expressed as mean ± SD (n = 3).
| Leaf extract | Total phenolic content (mg GAE/g DW) | Total flavonoid content (mg KE/g DW) | DPPH radical scavenging activity (IC50 mg/mL) | FRAP value (mg AAE/g DW) |
|---|---|---|---|---|
| ME | 17.60 ± 0.65 | 3.67 ± 0.54 | 0.50 ± 0.04 | 48.09 ± 1.22 |
| CE | 0.11 ± 0.01 | 0.44 ± 0.01 | 17.10 ± 0.68 | 6.88 ± 0.64 |
3.2. Antioxidant capacity
DPPH produces radicals in a methanolic solution. Radical scavenging molecules can sequester DPPH radicals and subsequently decrease the visible color from purple to straw-colored. Antioxidant potential of ME was found to be significantly higher than that of CE (Table 1). Ascorbic acid was used as standard antioxidant with IC50 value of 0.16 μg/mL. Furthermore, ME showed high antioxidant activity as revealed from FRAP assays as well (Table 1). FRAP activity was significantly higher for ME as compared to CE.
3.3. GC-MS analysis of bioactive metabolites from B. diffusa leaf extracts
To assess the physiological role of metabolites in wound healing mechanism, metabolic profiles of the methanol and chloroform extracts of the B. diffusa leaves were acquired using GC-MS. The representative GC-MS (total ion current: TIC) chromatograms of ME and CE are shown in Supplementary Figs. S1A–B. The corresponding mass spectral fragmentation patterns are shown in supplementary Fig S2 (ME) and supplementary Fig S3 (CE). The GC-MS analyses of ME showed significantly higher number of metabolites as compared to CE. In ME, 33 metabolites were identified (Table 2), whereas in CE, only 4 metabolites were identified (Table 3). ME was found to be rich in phenolics, sugars and amino acids. Based on the relative abundance, the top three major metabolites present in ME were D-pinitol (9.4%), fructofuranose (7.1%) and β-d-glucopyranose (4.9%). CE contained palmitic acid (21.6%) followed by stearic acid (14.0%) and glycerol (0.5%). Metabolites were characterized based on their retention time and specific fragmentation pattern.
Table 2.
List of 33 identified metabolites from ME of B. diffusa leaves after GC-MS analyses. The relative amounts of the individual metabolites are expressed as a percentage relative to the total area (RPA,%).
| S.No. | Compound | TMS derivative | RT (min) | Area (RPA%) | PubChem CID | Qualification Ions |
|---|---|---|---|---|---|---|
| 1 | Ethylene glycol | 2 | 7.80 | 0.0128 | 81858 | 191, 103 |
| 2 | l-Valine | 1 | 9.91 | 0.2018 | 22211754 | 174, 156 |
| 3 | l-Alanine | 2 | 10.07 | 0.5709 | 11424808 | 218, 116 |
| 4 | 2-Pyrrolidinone | 1 | 10.94 | 0.0067 | 84461 | 157, 142 |
| 5 | l-Proline | 1 | 11.40 | 1.9422 | 13514636 | 187, 172 |
| 6 | l-Valine | 2 | 11.85 | 0.2201 | 11108121 | 246, 144 |
| 7 | l-Isoleucine | 2 | 12.93 | 0.0736 | 21632765 | 260, 158 |
| 8 | l-Threonine | 2 | 12.96 | 0.1475 | 91696554 | 248, 130 |
| 9 | Succinic acid | 2 | 13.19 | 0.2194 | 520988 | 262, 247 |
| 10 | Uracil | 2 | 13.54 | 0.028 | 552702 | 256, 241 |
| 11 | Fumaric acid | 2 | 13.63 | 0.0193 | 5353016 | 245, 83 |
| 12 | l-Serine | 3 | 13.71 | 0.0507 | 90474444 | 306, 204 |
| 13 | l-Threonine | 3 | 14.03 | 0.0767 | 21632766 | 320, 291 |
| 14 | D-(−)-Citramalic acid | 3 | 15.16 | 0.0392 | 526005 | 349, 247 |
| 15 | Malic acid | 3 | 15.26 | 1.0752 | 522155 | 350, 233 |
| 16 | l-Threonic acid | 4 | 16.11 | 0.1114 | 528672 | 409, 292 |
| 17 | l-Asparagine | 2 | 16.88 | 0.0675 | 276, 159 | |
| 18 | l-Glutamic acid | 3 | 17.02 | 0.0513 | 12451984 | 363, 246 |
| 19 | dl-Phenylalanine | 2 | 17.36 | 0.0705 | 12451977 | 294, 192 |
| 20 | 3,4-Dihydroxy-benzyl alcohol | 3 | 18.18 | 0.0065 | 101663520 | 356, 267 |
| 21 | 4-Methylcatechol | 2 | 19.37 | 0.0275 | 530364 | 268, 253 |
| 22 | d-Fructofuranose | 5 | 19.61 | 7.1974 | 528401 | 437, 257 |
| 23 | D-Pinitol | 5 | 20.07 | 9.4039 | 91750479 | 507, 318 |
| 24 | l-Tyrosine | 3 | 21.76 | 0.1753 | 14189425 | 397, 218 |
| 25 | β-d-Glucopyranose | 5 | 22.00 | 4.9769 | 13587619 | 435, 204 |
| 26 | d-Gluconic acid | 6 | 22.57 | 0.0486 | 523310 | 435, 333 |
| 27 | Oxaloacetic acid | 3 | 22.80 | 0.007 | 553054 | 333, 231 |
| 28 | d-Glucuronic acid | 5 | 23.29 | 0.0149 | 22211710 | 539, 305 |
| 29 | Ferulic acid | 2 | 24.08 | 0.0837 | 5379186 | 338, 323 |
| 30 | Caffeic acid | 3 | 24.67 | 0.1278 | 5376254 | 396, 219 |
| 31 | L-Tryptophan | 2 | 25.91 | 0.1436 | 521975 | 348, 202 |
| 32 | Sucrose | 8 | 30.70 | 2.0906 | 10931011 | 451, 361 |
| 33 | 1-Monopalmitin | 2 | 30.80 | 0.0976 | 552033 | 459, 371 |
Table 3.
Four identified metabolites from CE of B. diffusa leaves after GC-MS analyses. The relative amounts of the individual metabolites are expressed as percentage relative to the total area (RPA, %).
| S.No. | Compound | TMS derivative | RT (min) | Area (RPA%) | PubChem CID | Qualification Ions |
|---|---|---|---|---|---|---|
| 1 | Glycerol | 3 | 12.54 | 0.5493 | 522285 | 293, 205 |
| 2 | 2-(Methylthio) benzothiazole | NA | 17.82 | 0.0343 | 11989 | 181, 148 |
| 3 | Palmitic acid | 1 | 23.36 | 21.6938 | 521638 | 328, 313 |
| 4 | Stearic acid | 1 | 26.23 | 14.0422 | 87777 | 356, 341 |
3.4. Effect of B. diffusa leaf extracts on keratinocyte viability
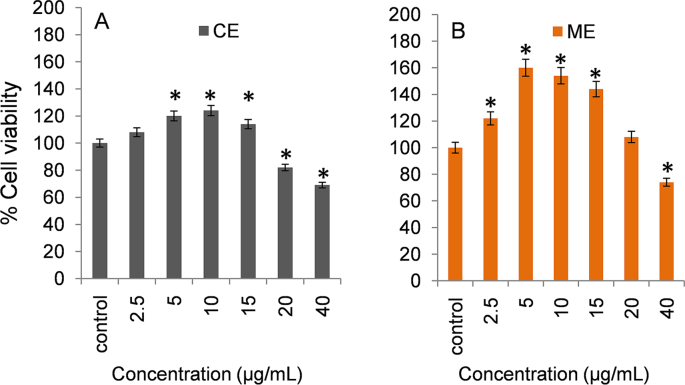
The effect of ME and CE on the cell viability of keratinocytes (HaCaT cell line) was studied using the scratch assay. The effect of a range of concentrations of ME and CE are shown in Fig. 1. HaCaT cells are immortalized human skin keratinocytes that mimic many properties of normal epidermal keratinocytes. They are non-invasive and can differentiate under appropriate experimental conditions. A significant enhancement in the viability of keratinocytes, as shown by the MTT method, was evident as early as day 2 in concentration ranging from 2.5 to 20 μg/mL for ME and from 2.5 to 15 μg/mL for CE. Higher dose of ME (≥40 μg/mL) and CE (≥20 μg/mL) was found to be detrimental for cell viability.
Fig. 1.
MTT assay performed to evaluate the effect of various doses ME (A) and CE (B) of B. diffusa leaf extracts on the viability of human keratinocytes. The viability of control treatment (without extract) was considered as 100% and results are expressed as % cell viability with respect to untreated control cells. Results are expressed as mean ± SD (n = 3). Statistical significance is denoted by *p < 0.05 compared to control.
3.5. Migration of keratinocytes (HaCaT) into in vitro wounds
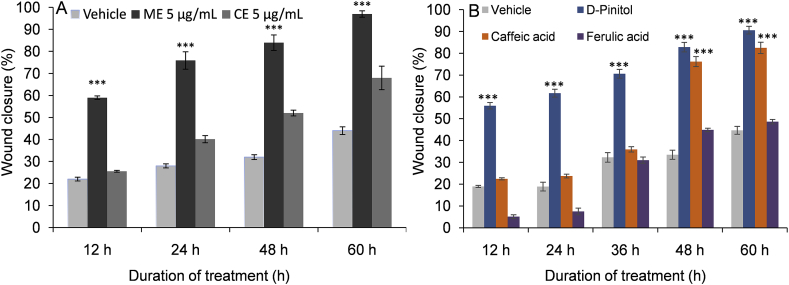
Scratches were made in confluent HaCaT monolayers. The scratches were treated with either ME (5 μg/mL) or CE (5 μg/mL). The progress of the closure of the scratches was recorded at intervals of 12 h for a total duration of 60 h. Movement of the cells could be detected as early as 12 h. Both ME and CE stimulated the migration of keratinocytes into the denuded area. ME resulted in faster and more complete closure of wound area than did CE (Fig. 2A). Treating scratched HaCaT cells with 5 μg/mL of ME closed the scratch wound by 59% within the first 12 h of treatment, and after 60 h, almost 97% closure was observed. In comparison to ME, CE (5 μg/mL) showed slow wound closure efficacy with 68% closure after 60 h. In vehicle treated cells, 44% closure was observed after 60 h. The migration of keratinocytes was seen to be more significant in the ME-treated cells compared to vehicle-treated and CE-treated cells (see Fig. 3). The pure D-pinitol and caffeic acid was tested for in vitro wound closure efficiency, a significant wound closure was observed at 60 h (Fig. 2B). Ferulic acid showed very poor wound closure efficiency (Fig. 2B).
Fig. 2.
Effect of B. diffusa leaf extracts and some pure metabolites on scratch wound healing in HaCaT cell line. (A) Effect of ME and CE on scratch wound healing in HaCaT cell line. (B) Effect of three pure metabolites, D-pinitol (5 μM), caffeic acid (5 μM), and ferulic acid (3 μM) on scratch wound healing in HaCaT cell line. Results are mean ± SD. Statistical significance is denoted by *p < 0.001 compared to control (vehicle).
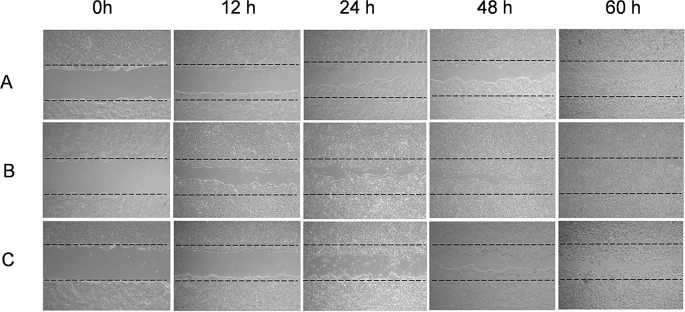
Fig. 3.
Digital image showing the effect of B. diffusa leaf extracts on human keratinocyte cell migration in a scratch assay: (A) control cells treated with vehicle; (B) cells treated with ME; (C) cells treated with CE. A confluent monolayer of human keratinocytes (HaCaT) was scratched using a sterile p200 tip. ME and CE were applied as treatments to the wound (open gap) and keratinocyte media served as control. The dashed lines mark the boundaries of the scratch wound at 0 h. Migrations of keratinocytes were photographed using light microscope fitted with digital camera with 10x magnification.
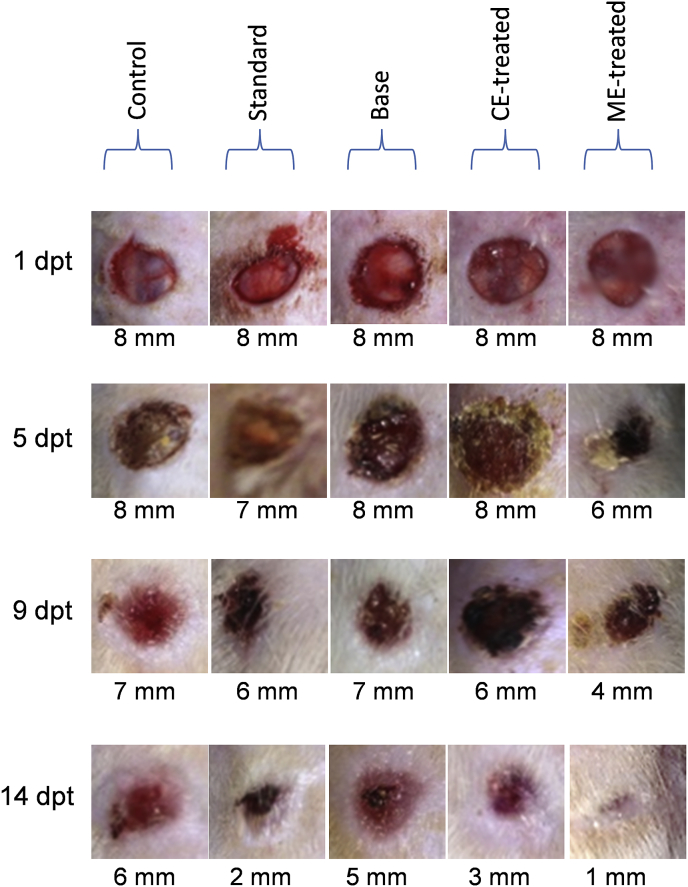
3.6. Wound contraction
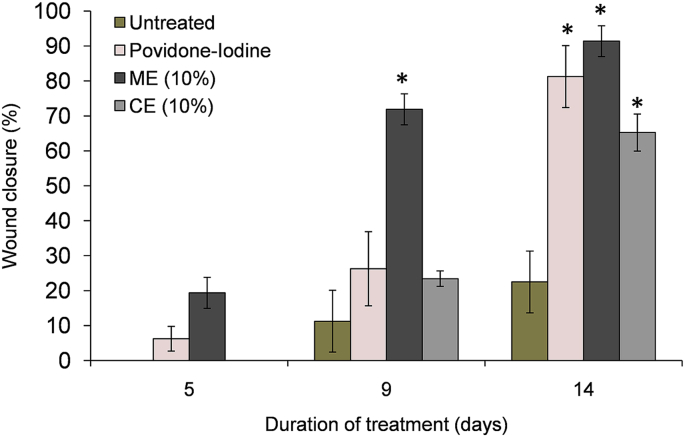
The effect of ME and CE from B. diffusa leaf on excision wound model in albino Wistar rats is shown in Fig. 4. A significant healing pattern with almost complete wound closure was observed in ME-treated rats within 14 days (91% wound closure; Fig. 4), whereas CE-treated and untreated animal groups lagged far behind (Fig. 5). Wounds treated with povidone-iodine showed healing behavior comparable to wounds treated with ME. ME left no prominent scar at the wound sites while CE-treated and povidone-iodine ointment-treated groups had prominent scars.
Fig. 4.
Effect of ME and CE from B. diffusa leaf and standard ointment (povidone-iodine) on percentage (%) wound contraction of excision wound models in rats. Results are mean ± SD. Statistical significance is denoted by *p < 0.05 compared to control.
Fig. 5.
Changes seen in wound contraction area of excision wounds of albino Wistar rats when treated with standard (povidone-iodine), ointment base, CE and ME. CE and ME extracts were prepared from B. diffusa leaf. Control group remained untreated.
4. Discussion
Wound healing is a mechanism-based complex re-programming process of restoring cellular structures and tissue layers in damaged tissue to enable it to attain normal physiological state and commencing the fibroblastic stage in which the wounded area shrinks.31 In general, the healing process involves three phases viz. Inflammatory, proliferative and remodelling phases, which are characterized by haemostasis and inflammation, cell proliferation and migration, and epithelialization, angiogenesis and collagen deposition, respectively. The wounds start to shrink during remodelling phase.32 The mechanism of wound healing involves activation of a series of cells such as keratinocytes, fibroblasts, endothelial cells and macrophages.33 For successful wound closure, the cross-talk between healthy keratinocytes and other cell types participating in wound healing is required.34 Therefore, therapeutic plant metabolites that are able to stimulate keratinocyte/fibroblast growth and proliferation may be able to accelerate the wound healing process, as observed in the present study. Our study demonstrated the effect of methanol and chloroform extracts from B. diffusa leaf in enhancing the viability of human keratinocyte (HaCaT) cells in vitro. As evident from the in vitro scratch assay, ME caused significantly higher stimulation of keratinocytes as compared to CE. The total phenolics, flavonoids and antioxidant properties were also significantly higher in ME as compared to CE.
Phenolics and flavonoids reduce lipid peroxidation, which, in turn, enhances the viability of collagen fibrils by increasing the strength of collagen fibres, preventing cell damage and accelerating DNA synthesis.35 Phenolics, flavonoids and terpenoids are known to promote the wound healing process because of their antioxidant and antimicrobial properties, which seem to be responsible for wound contraction and an enhanced rate of epithelialization.36
It has been previously shown that the presence of reactive oxygen species delays the wound healing process.35 While antioxidant enhances the healing process by reducing the damage caused by free oxygen radicals.37 Plant-derived antioxidants such as phenolics, flavonoids and tannins act as potent scavengers of free radicals, and thereby prevent the damage due to free radicals during wound healing process. In our study, higher wound healing properties as exhibited by ME could be due to its enhanced antioxidant potential in comparison to CE. Potent antioxidant capacity in ME can be directly linked to high content of phenolics and flavonoids in ME as compared to CE. Moreover, the process of wound healing was also enhanced in the animals treated with ME, indicating a clear role of these phenolics and flavonoids in the process of wound healing both in vitro and in vivo. The GC-MS analyses of CE and ME provide a complete investigation for all the major metabolites present in those extracts. The ME was found to be rich in phenolics, sugars, and amino acids. On the contrary, only four metabolites were identified in CE. This may therefore in part explain the enhanced efficacy of ME as compared to CE in wound healing process in vitro and in vivo. ME in particular was found to be rich in D-pinitol content, which is an insulinomimetic.38 Sivakumar et al. demonstrated the beneficial effect of D-pinitol against oxidative stress, which could be attributed to its free radical scavenging capacity.39 Yu et al. reported that topically applied insulin improved diabetic wound healing by regulating wound inflammatory cells and repairing cellular functions.40 Bioactive insulin induces activation of the IR/IRS/PI3K/AKT pathways involved in cutaneous wound healing,41 which leads to tissue regeneration and growth, proliferation and migration of keratinocytes and fibroblasts.42 These studies show a strong correlation between the insulinomimetic D-pinitol and the PI3K/AKT pathway in wound healing. Furthermore, caffeic and ferulic acids were present in ME which were known to promote wound healing process mainly because of their potent antioxidant and anti-inflammatory properties.43
In order to determine the wound-healing potential of the plant extracts, two major parameters were chosen: (i) wound closure time and (ii) wound contraction. It was observed that the efficacy of ME to heal wounds was comparable with that of the standard drug (povidone-iodine).
Thereby it is worth to conclude that ME has the potential to satisfy all requirements of an ideal dressing material in that it provides a micro-environment at the surface of the wound in which healing occurs at an accelerated rate. Further, in the ME-treated animals, no prominent scar at the wound sites was observed while povidone-iodine ointment-treated groups exhibited scars. This property of ME, in general, is of cosmetic value especially to patients who are prone to scar formation. As a result, this study provides a scientific validation of wound healing potential of B. diffusa leaf ME and rationale of its use in the discovery and preparation of novel wound healing formulations and/or dressings.
5. Conclusion
This study has evidently demonstrated the in vitro and in vivo wound healing activity of ME of B. diffusa leaves. Future research warrants identification of the actual bioactive metabolites present in the B. diffusa leaves responsible for its wound healing property and subsequent understanding of their mechanisms of action at cellular level.
Author contribution statement
DS conceived and designed the study. KJ and RM carried out the experiments, data processing and statistical analyses. SC and SG carried out in vivo animal experiments. KJ and DS prepared the figures and tables. DS and PR interpreted the results. DS, PR and KJ wrote the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
KJ is grateful to UGC for providing with Junior/Senior Research Fellowship.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2019.02.002.
Funding
This work was partially supported by an initiation research grant from the Indian Institute of Technology Roorkee (FIG 100624 to D. Sircar).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Langøen A., Bianchi J. Maintaining skin integrity. In: Flanagan M., editor. Wound Healing and Skin Integrity: Principles and Practice. John Wiley and Sons; Vol UK: 2013. pp. 18–32. [Google Scholar]
- 2.Attama A.A., Uzor P.F., Nnadi C.O., Okafor C.G. Evaluation of the wound healing activity of gel formulations of leaf extract of Aspila africana. Fam. Compositae. J Chem Pharm Res. 2011;3:718–724. [Google Scholar]
- 3.Dorjsembe B., Lee H.J., Kim M., Dulamjav B., Jigjid T., Nho C.W. Achillea asiatica extract and its active compounds induce cutaneous wound healing. J Ethnopharmacol. 2017;206:306–314. doi: 10.1016/j.jep.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Biswas T.K., Pandit S., Chakrabarti S., Banerjee S., Poyra N., Seal T. Evaluation of Cynodon dactylon for wound healing activity. J Ethnopharmacol. 2017;197:128–137. doi: 10.1016/j.jep.2016.07.065. [DOI] [PubMed] [Google Scholar]
- 5.Werner S., Krieg T., Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 6.Pinto N. de CC., Cassini-Vieira P., Souza-Fagundes EM de, Barcelos L.S., Castañon M.C.M.N., Scio E. Pereskia aculeata Miller leaves accelerate excisional wound healing in mice. J Ethnopharmacol. 2016;194:131–136. doi: 10.1016/j.jep.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Ellis L.M., Staley C.A., Liu W. Down-regulation of vascular endothelial growth factor in a human colon carcinoma cell line transfected with an antisense expression vector specific for c-src. J Biol Chem. 1998;273:1052–1057. doi: 10.1074/jbc.273.2.1052. [DOI] [PubMed] [Google Scholar]
- 8.Gupta N., Jain U.K. An update-prominent wound healing property of indigenous medicines. Res J Pharm Technol. 2011;4:203–213. [Google Scholar]
- 9.Biswas T., Mukherjee B. Plant medicines of Indian origin for wound healing activity: a review. Int J Low Extrem Wounds. 2003;2:25–39. doi: 10.1177/1534734603002001006. [DOI] [PubMed] [Google Scholar]
- 10.Wajid M., Jabeen S., Aslam Muhammad Shahzad, Ahmad M.S. An update review on ethnomedicinal, phytochemical and pharmacological profile of genus Boerhavia. Int J Complement Altern Med. 2017;6:189–195. [Google Scholar]
- 11.Mishra S., Aeri V., Gaur P.K., Jachak S.M. Phytochemical, therapeutic, and ethnopharmacological overview for a traditionally important herb: Boerhavia diffusa linn. BioMed Res Int. 2014;2014 doi: 10.1155/2014/808302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uniyal B., Shiva V. Traditional knowledge on medicinal plants among rural women of the Garhwal Himalaya , Uttaranchal. Indian J Tradit Knowl. 2005;4:259–266. [Google Scholar]
- 13.Kadel C., Jain A.K. Folklore claims on snakebite among some tribal communities of Central India. Indian J Tradit Knowl. 2008;7:296–299. [Google Scholar]
- 14.Senthilkumar M., Gurumoorthi P., Janardhanan K. Some medicinal plants used by Irular , the tribal people of Marudhamalai hills, Coimbatore. Nat Product Radiance. 2006;5:382–388. [Google Scholar]
- 15.Mahesh A., Kumar H., Mk R., Devkar R.A. Detail study on boerhaavia diffusa plant for its medicinal importance-A review. Res J Pharm Sci J Pharm Sci. 2012;1:28–36. [Google Scholar]
- 16.Srivastava R., Saluja D., Dwarakanath B.S., Chopra M. Inhibition of human cervical cancer cell growth by ethanolic extract of boerhaavia diffusa linn. (Punarnava) root. Evidence-Based Complement Altern Med. 2011;2011:1–13. doi: 10.1093/ecam/nep223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pari L., Amarnath Satheesh M. Antidiabetic effect of Boerhavia diffusa: effect on serum and tissue lipids in experimental diabetes. J Med Food. 2004;7:472–476. doi: 10.1089/jmf.2004.7.472. [DOI] [PubMed] [Google Scholar]
- 18.Hiruma-Lima C.A., Gracioso J.S., Bighetti E.J.B., Germonsén Robineou L., Souza Brito A.R.M. The juice of fresh leaves of Boerhaavia diffusa L. (Nyctaginaceae) markedly reduces pain in mice. J Ethnopharmacol. 2000;71:267–274. doi: 10.1016/s0378-8741(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 19.Olaleye M.T., Akinmoladun A.C., Ogunboye A.A., Akindahunsi A.A. Antioxidant activity and hepatoprotective property of leaf extracts of Boerhaavia diffusa Linn against acetaminophen-induced liver damage in rats. Food Chem Toxicol. 2010;48:2200–2205. doi: 10.1016/j.fct.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 20.Umamaheswari A., Nuni A., Shreevidya R. Evaluation of antibacterial activity of Boerhaavia diffusa L. leaves. Int J Green Pharm. 2010;4:75. [Google Scholar]
- 21.Kaur M., Goel R.K. Anti-convulsant activity of boerhaavia diffusa: plausible role of calcium channel antagonism. Evid Based Complement Altern Med. 2011;2011 doi: 10.1093/ecam/nep192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998;299:152–178. [Google Scholar]
- 23.Wang H., Gao X.D., Zhou G.C., Cai L., Yao W.B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 2008;106:888–895. [Google Scholar]
- 24.Turkoglu A., Duru M.E., Mercan N., Kivrak I., Gezer K. Antioxidant and antimicrobial activities of Laetiporus sulphureus. Bull.) Murrill. Food Chem. 2006;101:267–273. [Google Scholar]
- 25.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 26.Varshney R., Gupta S., Roy P. Cytoprotective effect of kaempferol against palmitic acid-induced pancreatic β-cell death through modulation of autophagy via AMPK/mTOR signaling pathway. Mol Cell Endocrinol. 2017;448:1–20. doi: 10.1016/j.mce.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 27.Su L., Fu L., Li X. Loss of CAR promotes migration and proliferation of HaCaT cells, and accelerates wound healing in rats via Src-p38 MAPK pathway. Sci Rep. 2016;6:19735. doi: 10.1038/srep19735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sengupta P. The laboratory rat: relating its age with human's. Int J Prev Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 29.Dorsett-Martin W.A. Rat models of skin wound healing: a review. Wound Repair Regen. 2004;12:591–599. doi: 10.1111/j.1067-1927.2004.12601.x. [DOI] [PubMed] [Google Scholar]
- 30.Nagar H.K., Srivastava A.K., Srivastava R., Kurmi M.L., Chandel H.S., Ranawat M.S. Pharmacological investigation of the wound healing activity of Cestrum nocturnum (l.) ointment in Wistar albino rats. Journal of Pharmaceutics. 2016;2016 doi: 10.1155/2016/9249040. Article ID 9249040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Henhena N., Mahmood A.A., Al-magrami A. Histological study of wound healing potential by ethanol leaf extract of Strobilanthes crispus in rats. J Med Plants Res. 2011;5:3660–3666. [Google Scholar]
- 32.Phillips G.D., Whitehead R.A., Knighton D.R. Initiation and pattern of angiogenesis in wound healing in the rat. Am J Anat. 1991;192:257–262. doi: 10.1002/aja.1001920305. [DOI] [PubMed] [Google Scholar]
- 33.Muhammad A.A., Pauzi N.A.S., Arulselvan P., Abas F., Fakurazi S. In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. BioMed Res Int. 2013;2013 doi: 10.1155/2013/974580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pastar I., Stojadinovic O., Tomic-Canic M. Role of keratinocytes in healing of chronic wounds. Surg Technol Int. 2008;17:105–112. [PubMed] [Google Scholar]
- 35.Geethalakshmi R., Sakravarthi C., Kritika T., Arul Kirubakaran M., Sarada D.V.L. Evaluation of antioxidant and wound healing potentials of Sphaeranthus amaranthoides. Burm.f. Biomed Res Int. 2013;2013 doi: 10.1155/2013/607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuchiya H., Sato M., Miyazaki T. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol. 1996;50:27–34. doi: 10.1016/0378-8741(96)85514-0. [DOI] [PubMed] [Google Scholar]
- 37.Martin A. The use of antioxidants in healing. Dermatol Surg. 1996;22:156–160. doi: 10.1111/j.1524-4725.1996.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 38.Bates S.H., Jones R.B., Bailey C.J. Insulin-like e € ect of pinitol. Br J Pharmacol. 2000;130:1944–1948. doi: 10.1038/sj.bjp.0703523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivakumar S., Subramanian S.P. Pancreatic tissue protective nature of D-Pinitol studied in streptozotocin-mediated oxidative stress in experimental diabetic rats. Eur J Pharmacol. 2009;622:65–70. doi: 10.1016/j.ejphar.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 40.Yu T., Gao M., Yang P. Topical insulin accelerates cutaneous wound healing in insulin-resistant diabetic rats. Am J Transl Res. 2017;9:4682–4693. [PMC free article] [PubMed] [Google Scholar]
- 41.Li G., Li Y.Y., Sun J.E., Lin W.H., Zhou R.X. ILK-PI3K/AKT pathway participates in cutaneous wound contraction by regulating fibroblast migration and differentiation to myofibroblast. Lab Invest. 2016;96:741–751. doi: 10.1038/labinvest.2016.48. [DOI] [PubMed] [Google Scholar]
- 42.Abdelkader D.H., Osamn M.A., Elgizawy S.A., Faheem A.M., McCarron P.A. The role of insulin in wound healing Process : mechanism of action and pharmaceutical applications. J Anal Pharm Res. 2016;2:1–6. [Google Scholar]
- 43.Song H.S., Park T.W., Sohn U.D. The effect of caffeic acid on wound healing in skin-incised mice. KOREAN J PHYSIOL PHARMACOL. 2008;12:343–347. doi: 10.4196/kjpp.2008.12.6.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.