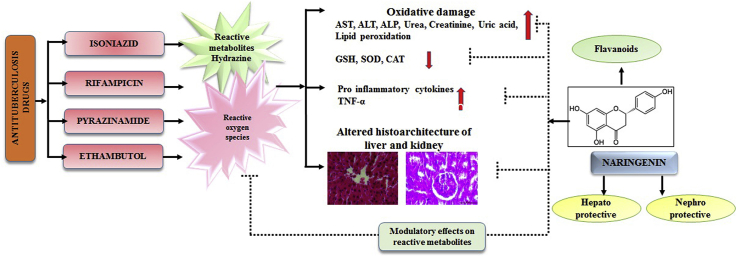
Abstract
Tuberculosis is one of the deadly diseases, which can be well treated by antituberculosis drugs (ATDs) i.e. isoniazid, rifampicin, pyrazinamide and ethambutol. These drugs also lead to severe hepatic and renal injury. The present study was designed to investigate efficacy of naringenin against ATDs induced hepato-renal injury. Rats were administered with ATDs for 8 weeks (3 day/week) followed by naringenin at three different doses (10, 20 and 40 mg/kg) conjointly for 8 weeks (3 days/week) orally. Silymarin (50 mg/kg) was used as positive control in the study. Hepatic and renal injury was measured by increased level of serological parameters such as aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, bilirubin, urea, uric acid and creatinine. The toxic effect of ATDs was also indicated by significant increase in lipid peroxidation along with decline in GSH, catalase and superoxide dismutase activity in liver and kidney tissues. Treatment with naringenin encountered ATDs induced injury as evident by significant reversal of biochemical indices towards their respective control in a dose dependent manner. Histopathological observations also supported biochemical findings. Assessment of TNF-α indicated therapeutic efficacy of naringenin at molecular level. Thus, results of this study clearly showed that naringenin possess protective role against ATDs induced hepato-renal injury and to take naringenin supplementation as food may be worthwhile to reduce ATDs induced hepato-renal injury.
Keywords: Antituberculosis drugs, Naringenin, Hepato-renal injury, Histopathology, Antioxidant activity, Oxidative stress
Graphical abstract
1. Introduction
Globally, tuberculosis has been declared as second leading cause of death from an infectious disease after the HIV.1 A multi-therapy of first-line anti tuberculosis drugs (ATDs) isoniazid, pyrazinamide, rifampicin, and ethambutol is used for treatment of tuberculosis for 2 months followed by a combination of isoniazid/rifampicin for 8 months. Administration of isoniazid alone for 9 months causes 1.6% of hepatic damage however that increases to 2.6% in combination with rifampicin.2 The ATDs cause various minor and major adverse reactions in body including skin reactions, gastrointestinal disorder, neurological disorder, vertigo and the most fatal one is hepatotoxicity.3,4 The ATDs are metabolized in liver and produce various toxic reactive metabolites such as hydrazine, which form highly reactive oxygen species that act as stimulators of lipid peroxidation and disturbance in antioxidant defense system resulting in cell death.5 The ATDs not only cause aberrations in liver as it is the main detoxifying site but also severely effect kidney because of its close association with liver as an excretory organ. Thus, kidneys get exposed to harmful effect of intermediate or finished toxic metabolites and get injured in many cases.
The efficacy and safety of herbal and natural product have turned the major pharmaceutical population towards medicinal research.6 Naringenin is a plant bioflavonoid, mainly found in grape fruits, tomatoes and citrus fruits and is considered to have beneficial effects on human health.7 Many studies have explored the role of naringenin in improving health. Naringenin exerts various biological properties that includes anti-inflammatory, antioxidant, immunomodulatory, hepatoprotective, nephroprotective and neuroprotective.8, 9, 10, 11, 12, 13 Management of tuberculosis treatment without any side effect is still a big challenge to the medical community. There is continuous search for alternative methods to lower the risk of liver diseases induced by anti-tuberculosis drugs. It was hypothesized that established lead compounds like naringenin may play excellent role in management of liver injury during tuberculosis treatment. To validate this hypothesis, study was undertaken to investigate whether treatment of naringenin mitigates anti-tuberculosis drugs induced hepatorenal injury by studying various markers of oxidative stress, enzymatic and non-enzymatic antioxidant status, inflammatory pathway and optical observations.
2. Materials and methods
2.1. Animals and chemicals
The study was carried out on female albino rats of Wistar strain (150 ± 10 g body weight). Animals were kept under standard husbandry conditions (25 ± 2 °C temp, 60–70% relative humidity, 14 h light and 10 h dark) and fed on standard pelleted diet and drinking water ad libitum. Experiments were conducted in accordance with the guidelines set by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) India with the approval of institutional animal ethics committee (994/Ere/Go/06/CPCSEA). The antituberculosis drugs isoniazid, rifampicin, ethambutol and pyrazinamide were generously obtained from the TB department of Chhattisgarh Institute of Medical Sciences (CIMS), Bilaspur (C.G.). Naringenin was purchased from Sigma-Aldrich Company.
2.2. Study design
Animals were divided into seven groups of six animals in each. Group I served as control and served with vehicle only. Group II was administered with naringenin per se at the dose of 40 mg/kg orally. Group III to VII were administered orally with pyrazinamide, ethambutol, isoniazid and rifampicin at the doses of 210, 170, 85 and 65 mg/kg respectively for 8 weeks (3 alternative days in a week) and group III served as experimental control. Animals of groups IV, V and VI were given naringenin at the doses of 10, 20 and 40 mg/kg, p.o., respectively for 8 weeks (3 alternative days in a week considering every next day of ATD exposure). Group VII was treated with silymarin at dose of 50 mg/kg, p.o., same as naringenin as positive control. All the animals were euthanized after 24 h of the last treatment. Just before euthanasia of animals, blood was drawn from retro orbital venous sinus. Collected blood sample were centrifuged at 3000 rpm for 10 min to obtain serum and stored at −20 °C until analyzed. Various serological and biochemical parameters were assessed. Liver and kidney sample were fixed in Bouin's fixative for histopathology.
2.3. Assessment of hepatorenal markers in serum
The serum was used for determination of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), urea, uric acid, and creatinine using diagnostic kits according to the manufacturer's instructions (Erba diagnostics, Germany).
2.4. Assessment of oxidative stress markers and antioxidant status
Liver and kidney samples were processed to determine lipid peroxidation (LPO) which were estimated by measuring thiobarbituric acid reactive species (TBARS)14 and reduced glutathione (GSH) was measured by its reaction with 5-5’-dithiobis 2-nitrobenzoic acid (DTNB) to give a yellow colored product.15 Superoxide dismutase (SOD) was assayed by assessing inhibition of rate of adrenochrome formation,16 catalase (CAT) was examined by assessing decomposition of hydrogen peroxide/min.17
2.5. Histopathological observations
Tissue samples of liver and kidney were quickly excised and fixed in Bouin's fixative, dehydrated in graded series of alcohol, embedded in paraffin wax, sections were cut into 5 μm thickness, stained with hematoxylin and eosin (H&E) and observed under light microscope.
2.6. Assessment of tumor necrosis factor-alpha in serum
Serum tumor necrosis factor-α (TNF-α) was assessed using ELISA kits (Ray Biotech, INC, Norcross, GA 30092 USA) and the results were expressed as pg/ml. All the procedures of kit were performed following manufacturer's instruction manual.
2.7. Statistical analysis
Data were expressed as mean ± SE of six animals used in each group. The differences between mean values were calculated by one-way analysis of variance (ANOVA) which was considered significant at p ≤ 0.05 level followed by student's t-test.
3. Results
3.1. Serum biochemical parameters
Exposure to ATDs for 8 weeks significantly elevated (p ≤ 0.01) level of ALT, AST, ALP, urea, uric acid and creatinine as compared with control group (Table 1). Treatment of naringenin at three different doses (10, 20 and 40 mg/kg) restored serological variables towards control in a dose dependent manner. Doses of 20 and 40 mg/kg of naringenin were significantly (p ≤ 0.01) effective in restoring all the serological variables towards control. Efficacy of naringenin was well compared with silymarin positive control group and results clearly showed similar therapeutic potential of naringenin and silymarin.
Table 1.
Therapeutic effect of naringenin against antituberculosis drugs induced alterations in liver and kidney function tests toxicity.
| AST |
ALT |
ALP |
Urea |
Uric acid |
Creatinine |
|
|---|---|---|---|---|---|---|
| (IU/L) | (IU/L) | (IU/L) | (mg/dl) | (mg/dl) | (mg/dl) | |
| Control | 72.2 ± 3.8 | 29.7 ± 2.5 | 292.0 ± 25.9 | 15.6 ± 1.4 | 1.86 ± 0.1 | 0.72 ± 0.1 |
| Nar per se | 74.4 ± 4.0 | 29.3 ± 2.4 | 304.0 ± 23.3 | 15.7 ± 1.5 | 1.95 ± 0.1 | 0.74 ± 0.1 |
| ATD | 116 ± 6.1ф | 71.8 ± 4.9ф | 558.0 ± 35.8ф | 32.3 ± 2.6ф | 4.46 ± 0.3ф | 1.81 ± 0.2ф |
| ATD + Nar10 | 90.5 ± 4.9** | 38.5 ± 3.1** | 520.0 ± 45.3 | 20.3 ± 1.9** | 3.18 ± 0.3** | 0.91 ± 0.1** |
| ATD + Nar20 | 81.9 ± 5.5** | 32.5 ± 1.9** | 445.0 ± 35.1* | 18.62 ± 1.8** | 2.73 ± 0.1** | 0.88 ± 0.1** |
| ATD + Nar40 | 76.1 ± 4.2** | 31.2 ± 2.1** | 368.0 ± 25.6** | 17.1 ± 1.6** | 2.62 ± 0.2** | 0.82 ± 0.1** |
| ATD + Sily50 | 78.5 ± 5.1** | 37.1 ± 2.9** | 363.0 ± 29.1** | 19.5 ± 1.1** | 1.85 ± 0.1** | 0.87 ± 0.1** |
| ANOVA | 11.5@ | 30.1@ | 12.3@ | 12.7@ | 27.7@ | 28.5@ |
Data are mean ± S.E of n = 6; @ Significant at 5% for ANOVA.
фATD vs Control at P ≤ 0.01,*ATD + Therapy vs ATD at P ≤ 0.05, **ATD + Therapy vs ATD at P ≤ 0.01.
ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; ALP, Alkaline phosphatase; ATD, Antituberculosis drugs; Nar per se, Naringenin per se, Nar10, Naringenin 10 mg/kg; Nar20, Naringenin 20 mg/kg; Nar40, Naringenin 40 mg/kg; Sily50, Silymarin 50 mg/kg.
3.2. Oxidative stress markers and antioxidant status
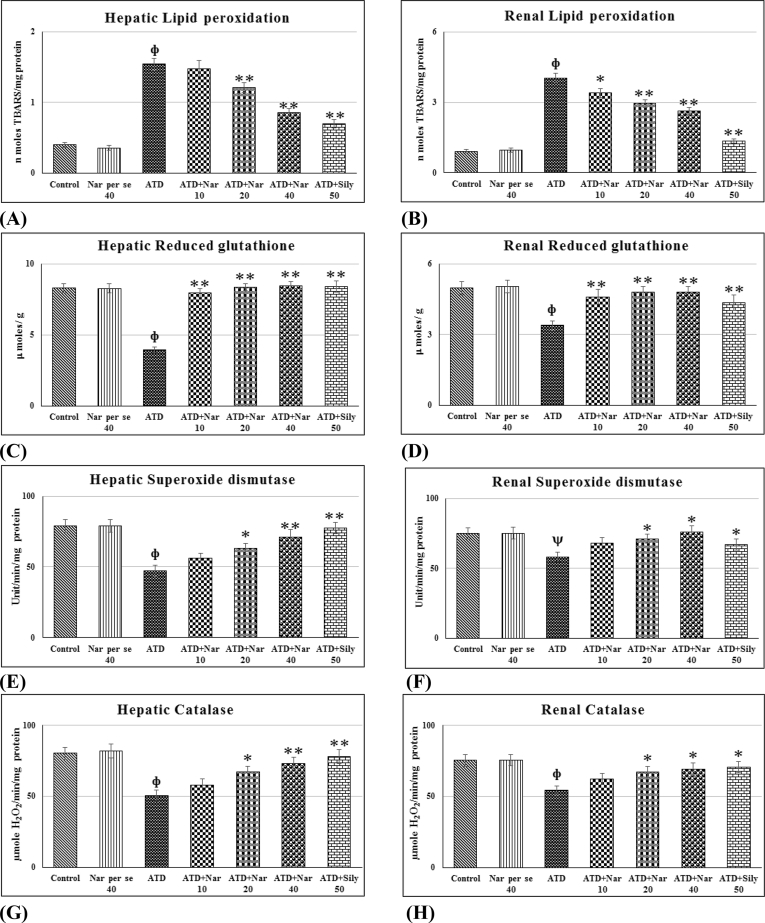
ATDs exposure for 8 weeks significantly (p ≤ 0.01) increased hepatic and renal LPO (Fig. 1A and B). It was observed that 10 mg/kg dose of naringenin was not effective for hepatic LPO but it significantly (p ≤ 0.05) effective for renal LPO. However, 20 and 40 mg/kg doses of naringenin significantly (p ≤ 0.01) reduced lipid peroxidation towards control. Level of GSH (Fig. 1C and D) was found significantly (p ≤ 0.01) decreased in liver and kidney after 8 weeks exposure to ATDs as compared to control group. All the three doses of naringenin significantly (p ≤ 0.01) reversed hepatorenal GSH towards control. SOD was found significantly (p ≤ 0.01) decreased in liver and kidney after 8 weeks of exposure to ATDs (Fig. 1E and F). The naringenin therapy at 10 mg/kg dose could not reverse SOD activity in significant manner; however, 20 and 40 mg/kg dose significantly reversed (p ≤ 0.01) hepatorenal SOD towards control. Administration of ATDs for 8 weeks significantly (p ≤ 0.01) lowers the CAT activity in liver and kidney (Fig. 1G and H). Doses of naringenin at 20 and 40 mg/kg significantly (p ≤ 0.01) reversed hepatorenal CAT activity towards control. Pharmacological action of naringenin was equally effective as positive control silymarin and showed no adverse effect in oxidative stress and antioxidant activity.
Fig. 1.
(A–H).Efficacy of naringenin on ATDs induced hepatorenal oxidative stress and antioxidant status. Values are mean ± SE of n = 6 in each group. p value ATD vs control at ψ ≤ 0.05; фATD vs Control at p ≤ 0.01, p value treatment vs ATD at *≤ 0.05, **≤ 0.01 for Student's t-test. @ Significant ANOVA at p ≤ 0.05, Lipid peroxidation (hepatic = 58.6@, renal = 89.8@), Reduced Glutathione (hepatic = 7.61@, renal = 4.71@), Superoxide dismutase (hepatic = 10.8@, renal = 3.93@), Catalase (hepatic = 9.35@, renal = 4.41@). Nar per se, Naringenin per se; ATD, Antituberculosis drugs; Nar10, Naringenin 10 mg/kg; Nar20, Naringenin 20 mg/kg; Nar40, Naringenin 40 mg/kg; Sily 50 mg/kg, Silymarin 50 mg/kg.
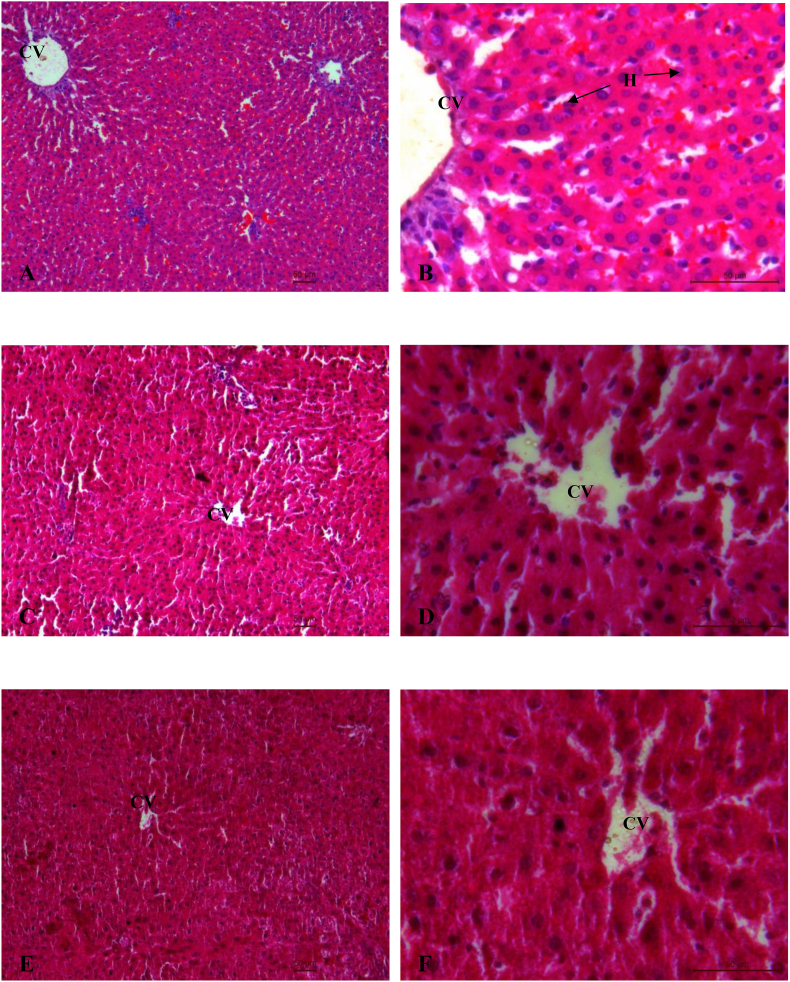
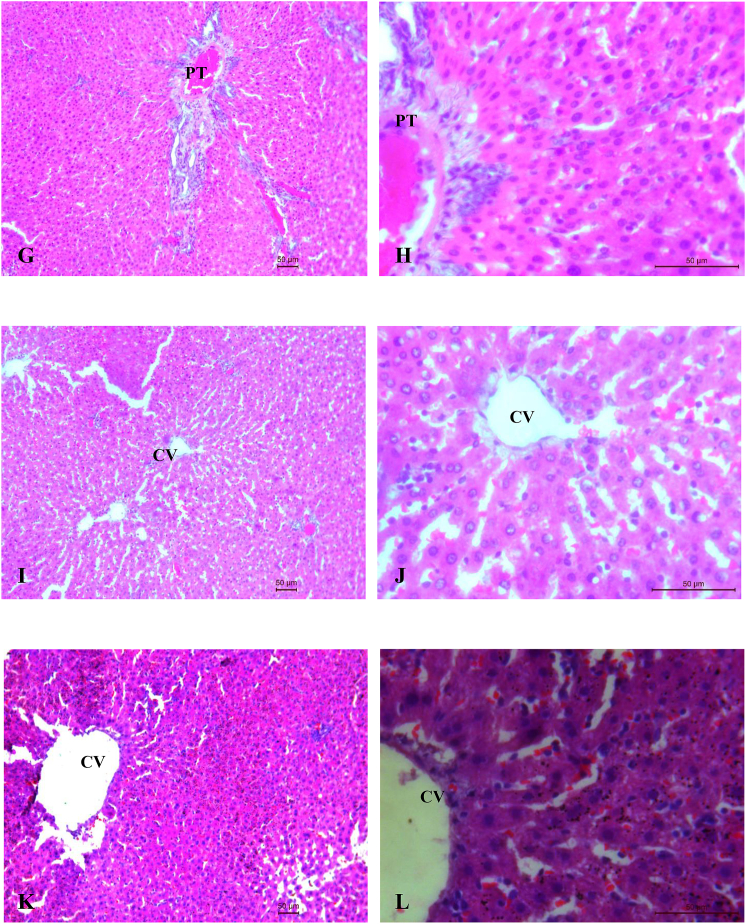
3.3. Histopathological observations
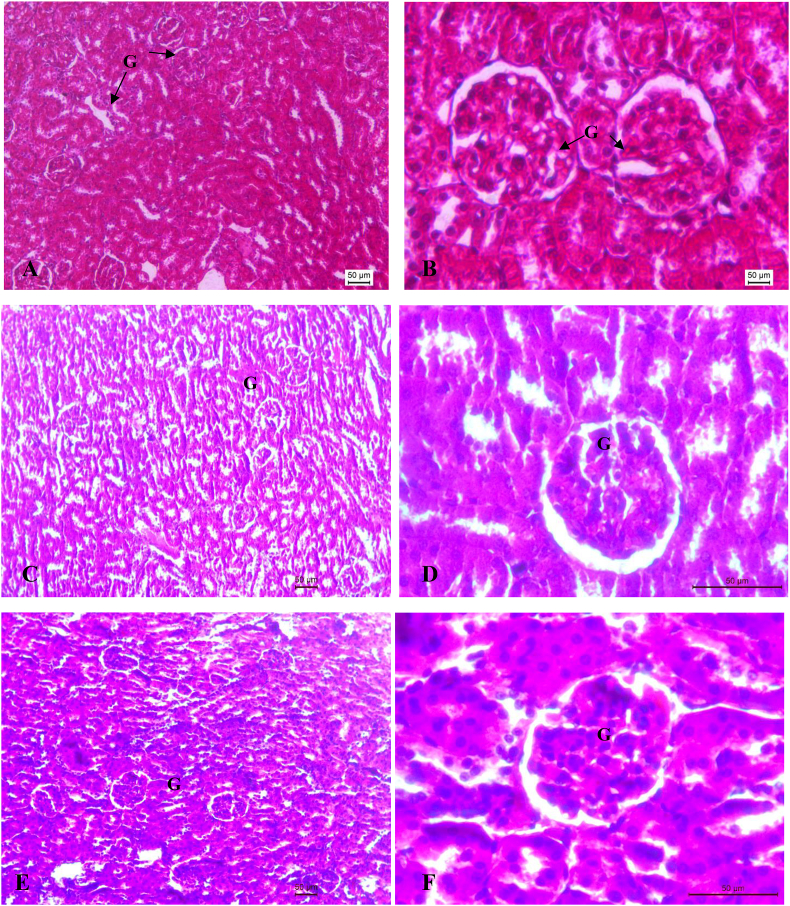
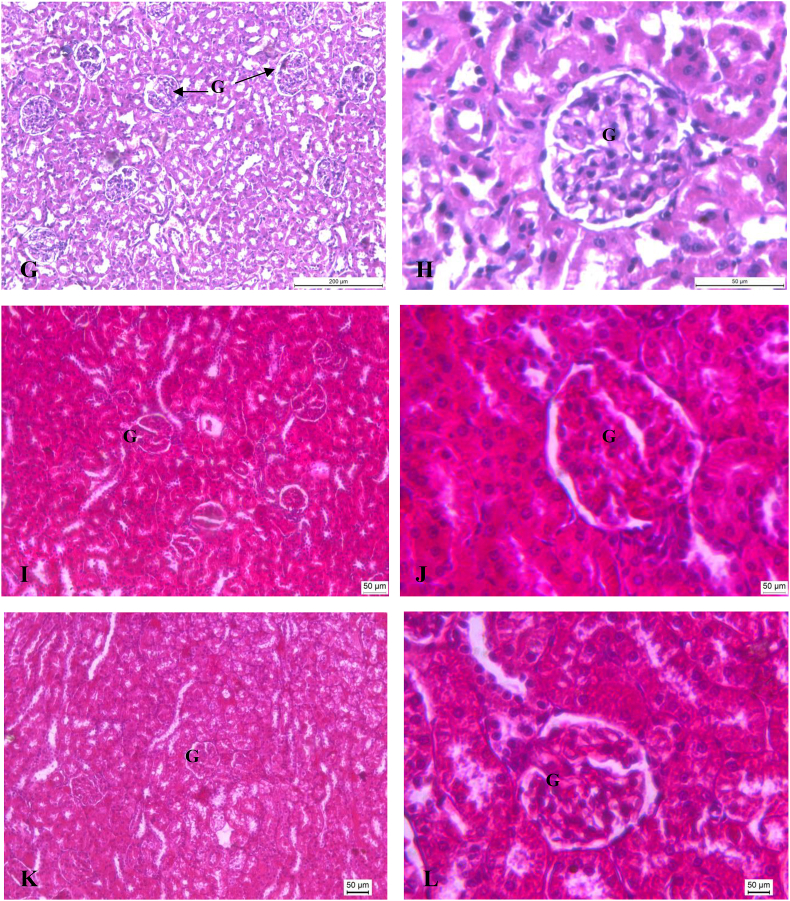
Histopathological profile of liver and kidney of control, ATDs and therapy groups are shown in Fig. 2 (A-L) and Fig. 3 (A-L) respectively. Normal cellular architecture with well differentiated hepatocytes, central veins and sinusoidal space were observed in control group of liver (Fig. 2A and B). Disturbed hepatic cord arrangement with prominent congestion in the central vein, vacuolation in cytoplasm, degenerated nuclei were observed in ATD intoxicated group (Fig. 2C and D). Treatment of naringenin at 10 and 20 mg/kg showed slight recovery in liver tissues (Fig. 2E–H). Naringenin treatment at 40 mg/kg showed maximum restoration in terms of less inflammatory cells, well distinguished hepatocytes with prominent nuclei and hepatocytes arranged in cord like fashion (Fig. 2I and J). The control group of kidney showed normal features of renal tubules and Bowman's capsules (Fig. 3A and B). Exposure to ATD caused damage in renal tubules and glomerulus (Fig. 3C and D). Naringenin treatment at 10 and 20 mg/kg dose, showed slight recovery pattern (Fig. 3E–H). Most of the tubules were observed with wider lumen and glomerulus appeared to be normal in most of the areas after treatment of naringenin at 40 mg/kg of dose (Fig. 3I and J).
Fig. 2.
(A–L). Photo micrographs of liver; (A:100X and B:400X) of control group; (C:100X and D: 400X) of ATD administered group for 8 weeks; (E:100X and F:400X) of ATDs + 10 mg/kg dose of naringenin; (G:100X and H:400X) of ATDs + 20 mg/kg dose of naringenin; (I:100X and J:400X): ATDs + 40 mg/kg dose of naringenin; (K:100X and L:400X) of ATDs + 50 mg/kg dose of silymarin. H, Hepatocytes; CV, Central vein; PT, Portal triad.
Fig. 3.
(A–L). Photo micrographs of Kidney; (A:100X and B:400X) of control group; (C:100X and D: 400X) of ATD administered group for 8 weeks; (E:100X and F:400X) of ATDs + 10 mg/kg dose of naringenin; (G:100X and H:400X) of ATDs + 20 mg/kg dose of naringenin; (I:100X and J:400X): ATDs + 40 mg/kg dose of naringenin; (K:100X and L:400X) of ATDs + 50 mg/kg dose of silymarin. G, Glomerulus.
Above mentioned observations drawn a conclusion that 40 mg/kg dose maximally retrieved ATD induced damage. Thus, group of 40 mg/kg dose of naringenin was further carried forward for specific parameters such as TNF-α.
3.4. TNF-α
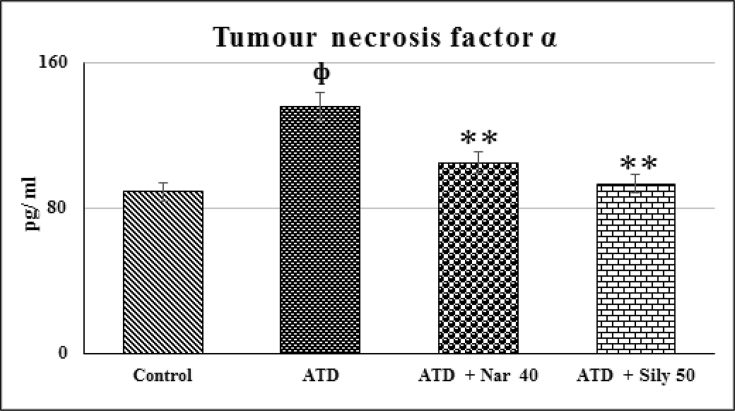
There was significant (p ≤ 0.01) elevation in TNF-α after administration of ATDs for 8 weeks (Fig. 4A). Dose of naringenin at 40 mg/kg significantly (p ≤ 0.01) restored molecular deviation towards control that was well compared with silymarin treated positive control.
Fig. 4.
Efficacy of naringenin on ATD induced alterations in serum TNF-α level. Values are mean ± SE of n = 6 in each group. p value ATD vs control at ψ ≤ 0.05; фATD vs Control at p ≤ 0.01, p value treatment vs ATD at *≤ 0.05, **≤ 0.01 for Student's t-test. @ Significant ANOVA at P ≤ 0.05, TNF-α (18.7@). ATD, Antituberculosis drugs; Nar40, Naringenin 40 mg/kg; Sily 50 mg/kg, Silymarin 50 mg/kg.
4. Discussion
Hepatic and renal injury has been severe concern during treatment of tuberculosis. Administration of isoniazid, pyrazinamide, rifampicin and ethambutol causes aberrations and dysfunction in liver and kidney as these drugs are bio transformed in liver, whereas kidney are involved in excretion of metabolic waste and active metabolites of these drugs. Thus, these organs are affected when substance is metabolized and generates more toxic products like free radicals which leads to induction of oxidative stress, inhibition of antioxidant defense system, inflammation of cells give rise to condition like necrosis.18 Involvement of oxidative stress in hepatotoxicity caused by combination of isoniazid and rifampicin has already been established. Previous researchers reported the role of oxidative stress in mechanism of isoniazid and rifampicin-induced hepatic inflammation.19 Thus, main focus of the present study was to explore a novel and effective therapeutic agent to reverse antituberculosis drug-induced hepato-renal injury. Naringenin is a flavonoid that has attracted attention in the last few decades due to its ability to curb oxidative stress under various pathological conditions. Silymarin was used as positive control in respect to naringenin as it has been used for centuries for treatment of different diseases including liver and kidney disorders.20 This study was conducted to investigate protective effects of naringenin against antituberculosis drugs induced hepato-renal injury in rats considering serological variables, oxidative stress, antioxidant status, cytokine profiling and histopathology of liver and kidney.
An important class of enzymes that is amino transferases such as ALT and AST are well-known diagnostic indicators of hepatic injury and also link to carbohydrate and amino acid metabolism.21 The ALP is a reliable marker of liver function. It is found histochemically in microvilli of bile canaliculi and on sinusoidal surface of hepatocytes.22 These enzymes are released into bloodstream during liver injury. Significant elevation in AST, ALT and ALP was occurred after administration of ATD due to cellular impairment. Elevated levels of ALT and AST lead to hamper in various biological metabolisms and ALP cause disruption in bile flow.23 The same findings have also been reported previously.24,25 Serum levels of urea, uric acid and creatinine were used as renal prognostic factor. Uric acid is a potent scavenger of peroxynitrite so, hyperuricemia condition increased production of endogenous reactive oxygen species.26 Creatinine level in serum has been used to predict glomerular filtration rate. Renal function test indicated significantly increased level of urea, uric acid and creatinine in ATD administered groups as compared to control group, which indicated increased conversion of ammonia to urea, increased production of free radicals and low glomerular filtration rate due to dystrophic changes in kidney. These findings corroborated other studies.27,28 Treatment with different doses of naringenin effectively protected liver and kidney in dose dependent manner from ATD induced injury as indicated by significant restoration of serum ALT, AST, ALP, urea, uric acid and creatinine. These results were in accordance with previous findings in which naringenin has been reported to protect cadmium-induced hepatotoxicity11 and nephrotoxicity12 through its antioxidant capacity.
Lipid peroxidation is the crucial index of oxidative stress. The process involves free radical chain reaction mechanism resulting in cell damage, as free radicals steal electrons from lipids in cell membranes. Elevated level of lipid peroxides in the liver and kidney reflected the cellular damage. Although ATD itself does not generate free radicals directly but generates free radicals via metabolic pathway by producing reactive metabolites such as hydrazine and diacetylhydrazine; both of these metabolites lead to formation of free radicals and cause severe cellular injury.29 Naringenin could prevent formation of free radicals by reducing production of reactive metabolites and exerted protective and antioxidant effects as reported in previous findings.30,31 It may quench free radicals due to its contain 4-hydroxyl group in β-ring that possess electron donating properties and inhibits lipid peroxidation.32 The GSH is a sulfhydryl peptide which plays essential role in cellular defense against toxicity. Exposure to ATD caused decline in GSH level which might increase the susceptibility of the liver and kidney to free radical damage.21 SOD is a metalloprotein that catalyze breakdown of superoxide anion into oxygen and hydrogen peroxide, whereas CAT is a hemeprotein which catalyze conversion of hydrogen peroxide to water and oxygen protects cell from oxidative damage by H2O2 and OH. Lower level of SOD and CAT indicated impairment of antioxidant defense system due to administration of ATD.33,34 Naringenin restored antioxidant enzymes by scavenging free radical due to presence of its hydroxyl group and reduced hepato-renal damage. These observations were similar to other reports.35
The extent of hepatic and renal damage was also corroborated by histopathological evaluation. ATD induced hepato-renal injury comprising of centrilobular necrosis, cell augmentation, cellular degeneration, degenerated nuclei, damage in renal tubules, glomerulus and heavy lymphocytic infiltration in agreement with previous findings.36 Naringenin showed healing effects on cells or lessened inflammatory cells, absence of necrosis, regeneration of hepatocytes and wider lumen and glomerulus.
Pro-inflammatory cytokines such as TNF-α production was increased due to oxidative stress, depletion of antioxidant system and injury.37 In the present study, ATD administration to rats for eight weeks caused an increase in the level of TNF-α compared with control group. Administration of naringenin reduced production of inflammatory cytokines and chemokines38 suggesting its anti-inflammatory effect and a subsequent recovery towards normalization.
5. Conclusion
Present findings demonstrated that naringenin at 20 and 40 mg/kg dose potentially effective in reversing all the serological, tissue, biochemical and histological indices in the liver and kidney. It can be suggested that naringenin may be useful to lessen or cure hepato-renal injury caused during ATD treatment regimen. However, further study on many other aspects of pharmacological action of naringenin needs to be performed.
Conflicts of interest
The authors have no conflict of interest with the findings from this study.
Acknowledgments
Authors are thankful to Chhattisgarh Institute of Medical Sciences (CIMS), Bilaspur, India for providing antituberculosis drugs and All India Institute of Medical Sciences (AIIMS), New Delhi, India for transmission electron microscope facility. The work was financially supported by UGC Major Research Project [(F42-520/2013(SR)] dated 23-03-2013.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.World Health Organization . World Health Organization; Geneva: 2016. Global Tuberculosis Report. [Google Scholar]
- 2.Luangchosiri C., Thakkinstian A., Chitphuk S., Stitchantrakul W., Petraksa S., Sobhonslidsuk A. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. BMC Complement Altern Med. 2015;15(1):334. doi: 10.1186/s12906-015-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbex M.A., Varella M.D., Siqueira H.R., Mello F.A. Antituberculosis drugs: drug interactions, adverse effects, and use in special situations-part 1: first-line drugs. J Bras Pneumol. 2010;36(5):626–640. doi: 10.1590/s1806-37132010000500016. [DOI] [PubMed] [Google Scholar]
- 4.Ramappa V., Aithal G.P. Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. J Clin Exp Hepatol. 2013;3(1):37–49. doi: 10.1016/j.jceh.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sodhi C.P., Rana S.V., Mehta S.K. Study of oxidative stress in isoniazid-induced hepatic injury in young rats with and without protein-energy malnutrition. J Biochem Toxicol. 1996;11(3):139–146. doi: 10.1002/(SICI)1522-7146(1996)11:3<139::AID-JBT6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Del Prete A., Scalera A., Iadevaia M.D. Herbal products: benefits, limits, and applications in chronic liver disease. Evid Based Complement Alternat Med. 2012;2012:837939. doi: 10.1155/2012/837939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao V., Kiran S.D., Rohini P., Bhagyasree P. Flavonoid: a review on naringenin. J Pharmacogn Phytochem. 2017;6(5):2778–2783. [Google Scholar]
- 8.Jayaraman J., Jesudoss V.A., Menon V.P., Namasivayam N. Anti-inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicol Mech Methods. 2012;22(7):568–576. doi: 10.3109/15376516.2012.707255. [DOI] [PubMed] [Google Scholar]
- 9.Mershiba S.D., Dassprakash M.V., Saraswathy S.D. Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol Biol Rep. 2013;40(5):3681–3691. doi: 10.1007/s11033-012-2444-8. [DOI] [PubMed] [Google Scholar]
- 10.Yilma A.N., Singh S.R., Morici L., Dennis V.A. Flavonoid naringenin: a potential immunomodulator for Chlamydia trachomatis inflammation. Mediat Inflamm. 2013;2013:102457. doi: 10.1155/2013/102457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renugadevi J., Prabu S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp Toxicol Pathol. 2010;62(2):171–181. doi: 10.1016/j.etp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Renugadevi J., Prabu S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology. 2009;256(1-2):128–134. doi: 10.1016/j.tox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Raza S.S., Khan M.M., Ahmad A. Neuroprotective effect of naringenin is mediated through suppression of NF-κB signaling pathway in experimental stroke. Neuroscience. 2013;230:157–171. doi: 10.1016/j.neuroscience.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S.K., KrishnaMurti C. Production of lipid peroxides by brain. J Neurochem. 1968;15(2):147–149. doi: 10.1111/j.1471-4159.1968.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 15.Roberts J.C., Francetic D.J. The importance of sample preparation and storage in glutathione analysis. Anal Biochem. 1993;211(2):183–187. doi: 10.1006/abio.1993.1254. [DOI] [PubMed] [Google Scholar]
- 16.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 17.Aebi H. Methods in Enzymology. Academic Press; 1984. Catalase in vitro; pp. 121–126. [Google Scholar]
- 18.Ali Z.Y. Biochemical evaluation of some natural products against toxicity induced by anti-tubercular drugs in rats. Nucl Sci J. 2012;5(10):69–80. [Google Scholar]
- 19.Attri S., Rana S.V., Vaiphei K. Isoniazid–and rifampicin–induced oxidative hepatic injury–protection by N–acetylcysteine. Hum Exp Toxicol. 2000;19(9):517–522. doi: 10.1191/096032700674230830. [DOI] [PubMed] [Google Scholar]
- 20.Das S.K., Mukherjee S., Vasudevan D.M. Medicinal properties of milk thistle with special reference to silymarinn: an overview. Nat Product Radiance. 2008;7(2):182–192. [Google Scholar]
- 21.Dong Y., Huang J., Lin X. Hepatoprotective effects of Yulangsan polysaccharide against isoniazid and rifampicin-induced liver injury in mice. J Ethnopharmacol. 2014;152(1):201–206. doi: 10.1016/j.jep.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Bais B., Saiju P. Ameliorative effect of Leucas cephalotes extract on isoniazid and rifampicin induced hepatotoxicity. Asian Pac J Trop Biomed. 2014;4:633–648. [Google Scholar]
- 23.Thapa B.R., Walia A. Liver function tests and their interpretation. Indian J Pediatr. 2007;74(7):663–671. doi: 10.1007/s12098-007-0118-7. [DOI] [PubMed] [Google Scholar]
- 24.Saad E.I., El-Gowilly S.M., Sherhaa M.O., Bistawroos A.E. Role of oxidative stress and nitric oxide in the protective effects of α-lipoic acid and aminoguanidine against isoniazid–rifampicin-induced hepatotoxicity in rats. Food Chem Toxicol. 2010;48(7):1869–1875. doi: 10.1016/j.fct.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Miglani S., Patyar R.R., Patyar S., Reshi M.R. Effect of goat milk on hepatotoxicity induced by antitubercular drugs in rats. J Food Drug Anal. 2016;24(4):716–721. doi: 10.1016/j.jfda.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooper D.C., Morimoto K., Bette M., Weihe E., Koprowski H., Dietzschold B. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol. 1998;72(5):3711–3719. doi: 10.1128/jvi.72.5.3711-3719.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaswal A., Sinha N., Bhadauria M., Shrivastava S., Shukla S. Therapeutic potential of thymoquinone against anti-tuberculosis drugs induced liver damage. Environ Toxicol Pharmacol. 2013;36(3):779–786. doi: 10.1016/j.etap.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Shukla S., Sinha N., Jaswal A. Pharmacology and Nutritional Intervention in the Treatment of Disease. InTech; 2014. Anti oxidative, anti peroxidative and hepatoprotective potential of Phyllanthus amarus against anti Tb drugs; pp. 283–294. [Google Scholar]
- 29.Lynch T., Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007;76:391–396. [PubMed] [Google Scholar]
- 30.Hermenean A., Ardelean A., Stan M. Protective effects of naringenin on carbon tetrachloride-induced acute nephrotoxicity in mouse kidney. Chem Biol Interact. 2013;205(2):138–147. doi: 10.1016/j.cbi.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Wang J., Yang Z., Lin L., Zhao Z., Liu Z., Liu X. Protective effect of naringenin against lead-induced oxidative stress in rats. Biol Trace Elem Res. 2012;146(3):354–359. doi: 10.1007/s12011-011-9268-6. [DOI] [PubMed] [Google Scholar]
- 32.Amić D., Davidović-Amić D., Bešlo D., Trinajstić N. Structure-radical scavenging activity relationships of flavonoids. Croat Chem Acta. 2003;76(1):55–61. [Google Scholar]
- 33.Wali A.F., Avula B., Ali Z. Antioxidant, hepatoprotective potential and chemical profiling of propolis ethanolic extract from Kashmir Himalaya region using UHPLC-DAD-QToF-MS. BioMed Res Int. 2015;2015:393462. doi: 10.1155/2015/393462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain T., Gupta R.K., Sweety K. Evaluation of antihepatotoxic potential of Solanum xanthocarpum fruit extract against antitubercular drugs induced hepatopathy in experimental rodents. Asian Pac J Trop Biomed. 2012;2(6):454–460. doi: 10.1016/S2221-1691(12)60075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozkaya A., Sahin Z., Dag U., Ozkaraca M. Effects of naringenin on oxidative stress and histopathological changes in the liver of lead acetate administered rats. J Biochem Mol Toxicol. 2016;30(5):243–248. doi: 10.1002/jbt.21785. [DOI] [PubMed] [Google Scholar]
- 36.Jahromi H.K., Pourahmad M., Abedi H.A., Jahromi Z.K. Protective effects of salep against isoniazid liver toxicity in wistar rats. J Tradit Complement Med. 2018;8(1):239–243. doi: 10.1016/j.jtcme.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avitsur R., Hunzeker J., Sheridan J.F. Role of early stress in the individual differences in host response to viral infection. Brain Behav Immun. 2006;20(4):339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Lim R., Barker G., Wall C.A., Lappas M. Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium. Mol Hum Reprod. 2013;19(7):451–462. doi: 10.1093/molehr/gat015. [DOI] [PubMed] [Google Scholar]