Abstract
Immigrants arriving from high-incidence tuberculosis (TB) countries may pose a threat to TB control in low-incidence European host countries. Besides the immediate morbidity and mortality from any resurgence of TB, there would also be the increased economic cost of treatment of cases, tracing and preventive treatment of contacts, as well as concern over the potential emergence of drug-resistant forms of TB. This study analysed the 28 countries of the European Union, plus Iceland and Norway (EU+2). A Pearson correlation analysis of each country and all countries combined during the years 2011–2017 was conducted in order to detect any potential correlation between the number of immigrants annually and the TB notification rates per 100,000 total population. The overall data showed a significant negative correlation between the number of immigrants and TB rate. A negative correlation was also found for 22 of the 30 EU countries. In three countries (Germany, Italy, and Norway), a significant positive correlation between TB notification rates and immigration numbers was observed. Overall, the study did not show a clear pattern between TB transmission and immigration. Continued surveillance of migration and TB rates is essential, and there is a need for harmonization of case definitions and reporting standards to optimize TB control programs within Europe.
Keywords: Tuberculosis, European Union, Migrants, Migration
1. Introduction
Many countries in Europe have achieved high levels of economic growth, living standards, and human development, which have resulted in great strides in the improvement of health of their populations [1]. Life expectancy at birth in 2017 in the European Union (EU) was approximately 78.3 years for men and 83.5 years for women, which is better than the United States [2]. Not surprisingly, the spectre of traditional infectious diseases such as Tuberculosis (TB) has greatly receded across Europe. TB rates have shown the fastest decrease in Europe compared to any other World Health Organization (WHO) region, declining on average 5.4% per year during 2006–2015 to an estimated 35.5 incident (new and relapse) cases per 100,000 in 2015 [3].
TB is an infectious disease caused by Mycobacterium tuberculosis (Mtb) bacilli. It spreads via droplet transmission from person-to-person during close contact as well as by the airborne route. Factors like crowding and poor ventilation increase the risk of spread of TB within communities [4]. TB can thus be considered a “natural” biomarker of a country's state of development, as it declines with a higher human development index, lower childhood mortality, and access to improved sanitation [5].
In recent years there have been successive waves of migration into countries in the EU/EEA mainly from countries in Africa and the Middle East [6,7]. The most advanced economies naturally are also the most attractive destinations for migrants. In 2016, Germany received the highest number of immigrants (1029,900), of which 49.4% were from outside the EU or stateless. In the UK, the country with the second-highest immigrant population, these figures were 589,000 and 45.1% respectively [8]. The incidence of TB among these migrants to Europe is higher than among people born in Europe, and typically reflects the higher TB risk of their countries of origin [3]. Asylum seekers would tend to have higher rates still because of the breakdown of basic infrastructure, employment, education, and healthcare owing to civil war and unrest in countries such as Syria, Iraq, and Afghanistan.
Given the significant human influx from high TB risk countries, there is a possibility that rates of TB among native populations of European countries might rise through their acquisition of infection from recently arrived migrants. Beyond the immediate public health problem of human morbidity and mortality from resurgent TB, as well as the economic costs of running an effective TB control program including case detection, long-term treatment and follow-up, there is natural concern over the potential emergence of multidrug-resistant (MDR) and extremely drug-resistant (XDR) TB. In a recent review on TB as a re-emerging disease in Europe, the epidemic threat posed by immigration, as well as possible strategies to avert this danger, was discussed [9].
The European center for Disease Prevention and Control (ECDC) closely monitors TB epidemiology in Europe. Migrant TB cases as a proportion of all TB cases reported in the EU/EEA have continuously increased from 13.6% in 2007 to 21.8% in 2013 mainly due to a decrease in native TB cases (2007 to 2013) [10]. In an accompanying editorial, the potential impact of migration on TB epidemiology in Europe, the recent unprecedented volume of migration, and the predominant Syrian, Afghan, or Iraqi nationality of recent migrants (2016) were acknowledged [7]. Given the absence of any analytic epidemiology studies of migration and TB in Europe, we set out to study the potential association between migration and TB in Europe. An exploratory analysis was conducted using available surveillance data to examine for any potential correlation between population-level data on migration and TB in Europe.
2. Methods
A dataset containing the numbers of inward migrants by countries of origin (source countries) outside Europe as well as by countries of destination (host countries) in Europe during 2011 to 2017 was retrieved from the Eurostat website for all 28 EU countries, along with Iceland and Norway [11]. Concurrently, the data on TB notification rates was accessed for the same 30 “EU+2″ countries and the same 7-year period from the European CDC website Surveillance Reports for the years 2017–2019 [3,12,13].
As a first step, to provide a concise overview of migration and TB rates, data from these two datasets were combined and tabulated to show: (i) the total numbers of inward migrants from outside EU+2 countries along with the mid-period TB rate for the five host countries with the highest numbers of inward migrants; and (ii) the total numbers of outward migrants to EU+2 countries along with the mid-period TB rate for the five source countries with the highest number of migrants to EU+2 countries [11,14].
In the next step, the combined dataset of annual migration numbers (independent variable) retrieved from the Eurostat website and TB notification rates (dependent variable) retrieved from the European CDC website for all 30 EU+2 countries was used for secondary data analysis. The analysis period was limited by the availability of data to the seven years from 2011 to 2017. However, this was deemed sufficient to observe any emergent trends in TB rates related to migration. It is also the relevant time period given the recentness of the increase in migration to Europe.
Pearson correlation analysis was conducted on the data for each country individually as well as all 30 EU+2 countries combined to determine any correlation between the numbers of immigrants annually and TB notification rates per 100 000 total population (using Excel's add-on tool Analyze-ItⓇ) [15].
3. Results
Table 1 juxtaposes the numbers of inward migrants for the period 2011–2017 with the mid-period (2014) TB rates for the five EU+2 countries with the highest numbers of inward migrants. Germany received the highest number of migrants, at 6.15 million, followed by the United Kingdom (UK), with 4.09 million [11]. Inward migrant numbers ranged from 2.25–2.55 million for the next three countries, Spain, France, and Italy [11]. National TB rates ranged from 5.6 per 100,000 in Germany to over 10 per 100,000 in the UK and Spain [11].
Table 1.
Total inward migrant numbers (2011–2017) and mid-period TB rates (2014) for five EU+2 countries receiving the highest numbers of migrants [11].
| EU+2 country | Total inward migrants (millions) | TB rate (per 100,000 population) |
|---|---|---|
| Germany | 6.15 | 5.6 |
| United Kingdom | 4.09 | 10.9 |
| Spain | 2.55 | 10.6 |
| France | 2.44 | 7.4 |
| Italy | 2.25 | 6.4 |
Table 2 displays the outward migrant numbers to EU+2 countries for the same period, 2011–2017, along with the mid-period (2014) national TB rates for the five countries experiencing the highest migration to EU+2. The dominant countries of birth of migrants to EU+2 were China (632,000) and India (547,000) [11]. Syria, Morocco, and Pakistan were the source of between 300,000–400,000 migrants each [11]. The TB incidence rates in Pakistan and India were in excess of 200 per 100,000, while Morocco had a rate of 101 per 100,000 [16].
Table 2.
Outward migrant numbers (2011–2017) and mid-period TB rates (2014) for the five source countries with the highest numbers of migrants to EU+2 [11,16].
| Source country | Total outward migrants (x 100,000) | TB rate (per 100,000 population) |
|---|---|---|
| China | 6.32 | 67 |
| India | 5.47 | 223 |
| Syria | 3.87 | 23 |
| Morocco | 3.85 | 101 |
| Pakistan | 3.00 | 270 |
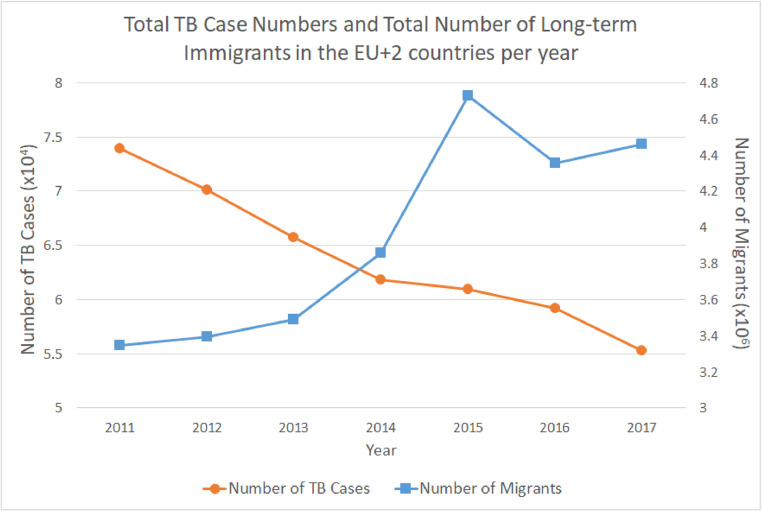
Fig. 1 provides a simultaneous overview of total inward migration and overall TB case numbers for EU+2 countries between the years 2011 and 2017. There is a clear downtrend in TB case numbers in the 30 countries over this period, from almost 7.4 per 10,000 to about 5.5 per 10,000. Superimposed on this is a corresponding dramatic surge in migrant numbers from just over 3.3 million in 2011 to a peak of over 4.7 million in 2015, followed by a drop to approximately 4.5 million.
Fig. 1.
Trends in total TB case numbers (yellow circles) and total immigrant numbers (blue squares) in EU+2 countries, 2011–2017. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3 presents the results of the analysis of correlation between migrant numbers and TB rates by country and overall for the 30 EU+2 countries during the entire period from 2011 to 2017.
Table 3.
Correlation analysis between migrant numbers and TB rates per country and overall for 30 EU+2 countries (all of DF = 4).
| Country | Pearson's r | Fisher 95% CI | p-value |
|---|---|---|---|
| Combined | −0.857 | −0.979 to −0.294 | 0.0137* |
| Austria | −0.673 | −0.946 to 0.163 | 0.0976 |
| Belgium | 0.428 | −0.479 to 0.893 | 0.3377 |
| Bulgaria | −0.765 | −0.973 to 0.122 | 0.0763 |
| Croatia | −0.944 | −0.992 to −0.659 | 0.0014** |
| Cyprus | 0.485 | −0.422 to 0.907 | 0.2699 |
| Czech Republic | −0.322 | −0.865 to 0.569 | 0.4818 |
| Denmark | −0.597 | −0.931 to 0.283 | 0.1568 |
| Estonia | −0.885 | −0.983 to −0.395 | 0.0081** |
| Finland | −0.678 | −0.947 to 0.153 | 0.0942 |
| France | −0.469 | −0.903 to 0.439 | 0.2885 |
| Germany | 0.846 | 0.257 to 0.977 | 0.0163* |
| Greece | −0.757 | −0.962 to −0.009 | 0.0489* |
| Hungary | −0.943 | −0.992 to −0.657 | 0.0014** |
| Iceland | 0.340 | −0.555 to 0.870 | 0.4556 |
| Ireland | −0.908 | −0.986 to −0.489 | 0.0047** |
| Italy | 0.878 | 0.368 to 0.982 | 0.0094** |
| Latvia | 0.482 | −0.426 to 0.906 | 0.2735 |
| Lithuania | −0.478 | −0.905 to 0.429 | 0.2776 |
| Luxembourg | −0.511 | −0.913 to 0.394 | 0.2416 |
| Malta | −0.020 | −0.762 to 0.744 | 0.9656 |
| The Netherlands | −0.575 | −0.927 to 0.314 | 0.1767 |
| Norway | 0.910 | 0.498 to 0.987 | 0.0045** |
| Poland | −0.656 | −0.943 to 0.191 | 0.1094 |
| Portugal | −0.909 | −0.987 to −0.495 | 0.0046** |
| Romania | −0.011 | −0.758 to 0.748 | 0.9814 |
| Slovakia | −0.857 | −0.978 to −0.292 | 0.0138* |
| Slovenia | −0.692 | −0.950 to 0.127 | 0.0849 |
| Spain | −0.423 | −0.892 to 0.485 | 0.3447 |
| Sweden | 0.161 | −0.674 to 0.815 | 0.7309 |
| The United Kingdom | −0.792 | −0.968 to −0.096 | 0.0338* |
Note: p-values < 0.05*, p-values < 0.01 **.
Pearson's correlation analysis showed a statistically significant positive linear correlation between TB notification rates and immigration numbers for three countries: Germany, Italy, and Norway. A statistically significant negative correlation was shown for Croatia, Estonia, Greece, Hungary, Ireland, Portugal, Slovakia, and the UK. No significant correlation was demonstrated between these two variables for the other 19 countries analysed.
The overall data for all thirty EU+2 countries demonstrated a statistically significant negative correlation between migrant numbers and incidence of tuberculosis.
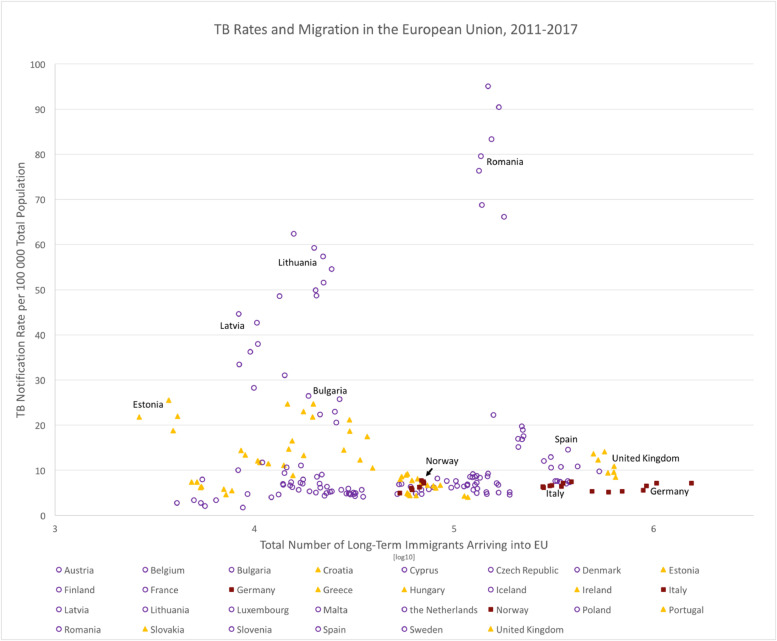
Fig. 2, a scatterplot of cumulative inward migration and TB rates for individual EU+2 countries over the entire period 2011–2017, offers a visual representation of the data. There is a large variation (over 500-fold) between the smallest and largest total number of immigrants for any year, any EU+2 country. Nevertheless, the TB rate lies below 20 per 100,000 for the majority of the EU countries. Romania has a relative high TB incidence irrespective of a comparatively lower immigration number. There was no evidence of any non-linear correlation between annual migrant numbers and annual TB rates, per country or overall. As can be observed in Fig. 2, low rates of TB incidence persist for countries even with a large influx of migrants.
Fig. 2.
TB Notification Rates per 100 000 Total population and Total Number of Immigrants in the 30 EU countries and a combined analysis, 2011–2017. Whilst the TB rate shows the percentage of TB incidence in the countries, the total number of immigrants is presented as a logarithmic scale in order to be able to discern the data points of the countries in the six years. Countries with a significant positive correlation are represented as red squares, whereas countries with a significant negative correlation are represented with yellow triangles. All other data points are represented as open purple circles. Note that the immigration number is not available for Bulgaria in 2011.
4. Discussion
Pearson Analysis showed a strong negative correlation for incidence of TB and number of immigrants overall for EU+2 between the years 2011 and 2017. Indeed, TB incidence decreased by 25% even while the total number of immigrants increased by 33% during the 7-year period (Fig. 1.).
Notwithstanding the overall statistically significant negative correlation, it must be remarked that Pearson's correlation coefficients for Italy, Germany, and Norway were statistically significant and positive, strongly so for Norway. Positive correlations observed for Belgium, Cyprus, Czech Republic, Iceland, Latvia, Sweden did not achieve significance.
Had a significant positive correlation between TB rates in Europe and immigration numbers been detected overall in this study, such a provisional finding would warrant further studies employing an analytic study design to confirm any such worrying association, identify the affected sub-populations, and elucidate the underlying risk factors. The negative correlation found overall in this study is therefore reassuring, and indicates rather that there is not yet any cause for undue concern.
Considering that the Pearson correlation coefficient is very sensitive to extreme data values, the strongly positive correlation for Germany was likely influenced by the extreme changes from 2014 to 2015 in both migration numbers (884,893 to 1543,848) and TB rates (5.6 to 7.2). The juxtaposition of large-scale migration and rising TB rates in Germany has already triggered several recent epidemiological publications on the topic.
Kuehne et al. underlined migration as an important factor impacting infectious disease epidemiology [17]. In reviewing the indicators of migration background utilized in German infectious disease surveillance, they found that a foreign origin was more frequent for TB than for syphilis [17]. While this is consistent with the known higher risk of TB among migrants into Europe from source countries with high TB rates, we cannot from this alone conclude that migration has been a factor in the observed increase in TB rates in Germany. The authors suggested further research to map the complex relationship between migration and infectious diseases [17].
Fiebig et al. provide a valuable framework for the interpretation of the findings in Germany from the present study [18]. In a commentary on the recent uptrend in TB notifications in Germany, the authors begin with the caveat that increased TB notification rates could be artefactual, i.e., arising from improved screening, case detection and reporting rather than reflecting any real increase in TB incidence [18]. Despite its plausibility given such global initiatives as the World Health Organization (WHO) “End TB Strategy” whose initiation coincided with the increasing TB incidence numbers in Germany, and which aims to eradicate TB in low incidence countries such as Germany, the authors dismiss this explanation, noting the absence of any significant changes to the diagnosis or reporting of TB in Germany temporally corresponding to this reversal in the decline of TB. Assuming therefore that the alternative must be true, i.e. a real increase in TB rates, Fiebig et al. acknowledge that this could be due to a changing demographic context, i.e., migration, but hasten to point out that the available data do not permit distinction among three possible scenarios, i.e., reactivation of latent TB infection (LTBI) among the native population, reactivation of LTBI among the population of foreign origin, and progression of recent infection to disease among recent migrants [18].
Pending more comprehensive data and definitive conclusions about migration and TB, an effective public health response for host countries is already available in the form of targeted screening for TB among recent migrants. Medical screening of recent migrants is an essential element of TB control programs in such countries [19]. In Germany, all asylum seekers are required to undergo screening upon entry for TB. Bozorgmehr et al., noting that comprehensive evidence on the yield from such TB screening in Germany was lacking, conducted a meta-analysis of German data which estimated the yield of TB screening in asylum seekers to be 3.47 (95% CI: 1.78–5.73) per 1000, consistent with the yield internationally of such active TB screening programs for asylum seekers upon entry. It was recommended that further research into developing more targeted screening programs is needed [19].
Representing one end of the spectrum in TB control in Europe are countries like Cyprus where effective TB programs which are inclusive of migrants have existed since the late 1900s, typified by a 100% case detection rate in 2012 and a treatment success rate of >85% since 2006 [20]. With TB approaching elimination, >80% of TB in Cyprus now occurs in the migrant population [20]. Unsurprisingly, the present study found a positive, albeit non-significant, correlation between the increase in TB rates and the influx of immigrants in Cyprus.
A standardized national TB elimination program in Italy is not yet in place; however, collaborative efforts are underway to establish one [21]. In an attempt to understand the transmission dynamics of TB in Sardinia, a molecular epidemiological study was conducted using Mycobacterial Interspersed Repetitive Units-Variable Number Tandem Repeats (MIRU-VNTR) genotyping [22]. All isolates from both natives and migrants were unique, with high allelic diversity [22]. Though limited by its small size, this study showed no TB transmission between migrants and native population, indicating that the presence of immigrants was not a risk factor for contracting TB in the community [22]. Previous DNA fingerprinting studies in other European countries using IS6110 restriction fragment length polymorphism (RFLP) had arrived at different conclusions: A study on transmission of TB in Denmark, where two-thirds of all TB patients are immigrants, with half from Somalia, found transmission between Somalis and Danes to be almost non-existent [23]. On the other hand, a study in the Netherlands found that 17% of 623 Dutch TB cases were attributable to recent transmission from a non-Dutch source, while another study of immigrants in Madrid found that, of 183 cases in 59 clusters, 53% of the clusters involving immigrants also included autochthonous cases, demonstrating marked transmission permeability between immigrant and autochthonous populations [24,25].
A limitation of an ecological study such as this is that it can only demonstrate correlation and not causation. Although likely, with the present method it was not possible to demonstrate that the overall the increase in migration appears not to have had a significant impact on TB rates. In countries such as Germany, Italy, and Norway, a positive correlation was observed. With the present method, it was neither possible to conclude that these observed increases in national TB rates in Germany, Italy, and Norway were caused by recent migration flows, nor to further answer the question of whether there was any migrant-to-native transmission. This study, using readily available surveillance data, sought to provide a preliminary answer on the public health risk of the emergence of TB in Europe related to migration from high TB risk countries. There is clearly a need for continued national and regional surveillance in Europe to confirm if there is any real trend. However, given that the TB risk is higher among the foreign-born population, active surveillance among recent migrants should be prioritized to allow earlier detection and treatment of TB [3]. A stratified analysis of the influence of migration on TB rates among the foreign-born population could reveal whether a trend in the TB rate among (non)-native born population would be correlated with immigration. A separate analysis of the effect of immigration from high burden countries such as Eritrea could lead to different conclusions. The chosen approach is that different types of immigrants are pooled together: regular (labor) immigrants, who are generally considered to be more healthy than the general population in their country of origin could lead to a healthy migrant effect which may be different from asylum seekers with a higher risk of exposure. Since in the recent years the influx of both migrant populations have increased this could warrant further analysis.
Another limitation of this study is that, because it analyses migration numbers and TB rates in the same time period, it could not detect increases in TB rates that might follow with a certain lag time some years after the increase in migrant numbers. Such a time lag is biologically plausible given TB's natural history of prolonged latency. Migrants from countries with a high TB rate, who have a higher risk of developing disease than the European-born population, could still develop TB years after entry, which could then result in a rise in TB rates.
Transmission of TB requires exposure of close contacts to an infectious case for several hours, usually in an enclosed environment in the household or workplace settings. The prevention and control of TB spread in the community is thus based on timely tracing of close contacts and prompt preventive treatment of LTBI, as well as effective treatment of active TB disease. Whether the recent slowdown in the decline of TB rates in countries like Germany is due to the reactivation of TB among migrants from high TB incidence countries, progression to active TB following migrant-to-migrant transmission after arrival in the host country, progression to active TB among the native population following transmission from recent migrants, or some combination of the above, cannot be concluded. Success in TB control can therefore be achieved by the same tried and tested means, i.e., high-quality contact tracing, chest X-Ray (CXR) screening, preventive treatment of LTBI and treatment of active TB disease. The addition of targeted active screening among recent migrants should be considered by all countries with significant inward migration from high TB burden countries.
Given that an enlightened and rational public health approach in a globalized world should entail particular focus on migrants who might suffer from TB, it is important to note that, in Europe, the consequences of being diagnosed with active TB for asylum seekers, the most vulnerable subgroup of migrants, are far from negative. Based on National Contact Point responses to surveys by the European Migration Network, in European countries the decision to grant refuge is independent of any diagnosis of active TB [26]. There is variation across countries in whether TB screening is mandatory, encouraged or optional [26]. In countries where it is mandatory, there has been no experience of refusals [26,27].
CXR screening for TB is generally accepted by migrants and has higher cost-effectiveness if targeted at individuals from countries with higher TB incidence [28,29]. LTBI screening with sequential tuberculin skin testing (TST) or interferon-gamma release assay (IGRA) is likewise more cost-effective if targeted at migrants at high risk for progression to active TB, e.g., those with immunosuppressive conditions (like HIV), recently infected, from high endemic countries, or from crowded living conditions [28,30]. CXR screening programs for TB exist in most EU countries, including Belgium, Finland, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the UK [31]. Only a few countries in Europe have an LTBI screening program, including Belgium, Greece, the Netherlands, Norway, Spain, Sweden, and the UK [31].
While continued surveillance of both migration and TB rates by European agencies is essential, there is a need for harmonization of case definitions and reporting standards to allow systematic study of screening programs, to achieve optimization of TB control programs among migrants to Europe, and to further advance toward TB elimination targets [31] Attention must continue to be devoted to enhancing existing TB prevention and control measures both among residents and recent migrants in Germany and other European countries where the decline in TB is losing momentum to preserve the significant gains made thus far toward the elimination of TB in Europe. However, the most cost-effective strategy for control of TB among migrants in any particular country depends on its epidemiology in the local migrant population, and whether for LTBI or active TB, is likely to involve targeting high-risk groups for screening [29,30].
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgments
We would like to thank Professor Monica Teleman (Department of Epidemiology, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania) for her aid in the writing process for the initial draft of this manuscript.
Funding
This research did not receive external funding from agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Not required.
References
- 1.UNDP . UN; New York: 2017. Human development report 2016: human development for everyone. [Google Scholar]
- 2.Eurostat. Life expectancy at birth by sex 2017 [updated 15 Nov 2019; cited 5 Dec 2019]. Available from:https://ec.europa.eu/eurostat/web/products-datasets/product?code=sdg_03_10.
- 3.European Centre for Disease Prevention and Control/WHO Regional Office for Europe . European Centre for Disease Prevention and Control; Stockholm: 2017. Tuberculosis surveillance and monitoring in Europe 2017. [Google Scholar]
- 4.Narasimhan P., Wood J., Macintyre C.R., Mathai D. Risk factors for tuberculosis. Pulm Med. 2013;2013 doi: 10.1155/2013/828939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dye C., Lonnroth K., Jaramillo E., Williams B.G., Raviglione M. Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ. 2009;87(9):683–691. doi: 10.2471/BLT.08.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pareek M., Greenaway C., Noori T., Munoz J., Zenner D. The impact of migration on tuberculosis epidemiology and control in high-income countries: a review. BMC Med. 2016;14:48. doi: 10.1186/s12916-016-0595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Werf M.J., Zellweger J.P. Impact of migration on tuberculosis epidemiology and control in the EU/EEA. Euro Surveill. 2016;21(12) doi: 10.2807/1560-7917.ES.2016.21.12.30174. [DOI] [PubMed] [Google Scholar]
- 8.Eurostat . EU; Luxembourg: 2016. The EU in the world. [Google Scholar]
- 9.Baglio G. [Tuberculosis and immigration: the answers that epidemiology can provide (and society is waiting for)] Epidemiol Prev. 2015;39(2):73–74. [PubMed] [Google Scholar]
- 10.Kodmon C., Zucs P., van der Werf M.J. Migration-related tuberculosis: epidemiology and characteristics of tuberculosis cases originating outside the European Union and European Economic Area, 2007 to 2013. Euro Surveill. 2016;21(12) doi: 10.2807/1560-7917.ES.2016.21.12.30164. [DOI] [PubMed] [Google Scholar]
- 11.Eurostat. Migration and migrant population statistics 2019 [updated Mar 2019; cited 16 Apr 2019]. Available from:https://ec.europa.eu/eurostat/statistics-explained/index.php/Migration_and_migrant_population_statistics.
- 12.European centre for disease prevention and control/WHO regional office for Europe. Tuberculosis surveillance and monitoring in Europe 2018 - 2016 data. Stockholm: European Centre for Disease Prevention and Control; 2018. [Google Scholar]
- 13.WHO . WHO Regional Office for Europe; Copenhagen: 2019. Regional office for Europe/European Centre for disease prevention and control. Tuberculosis surveillance and monitoring in Europe 2019 - 2017 data. [Google Scholar]
- 14.World Health Organization . World Health Organization; 2015. Global tuberculosis report 2015. [Google Scholar]
- 15.Analyse-it Software, Ltd.; 2018. Analyse-it for Microsoft Excel (version 5.11) [Google Scholar]
- 16.Geneva: World Health Organization; 2019. Global tuberculosis report 2019. [Google Scholar]
- 17.Kuehne A., Fiebig L., Jansen K., Koschollek C., Santos-Hovener C. [Migration and infectious disease surveillance in Germany: Analyses of Tuberculosis, HIV and Syphilis surveillance data] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58(6):560–568. doi: 10.1007/s00103-015-2157-y. [DOI] [PubMed] [Google Scholar]
- 18.Fiebig L., Hauer B., Brodhun B., Altmann D., Haas W. Tuberculosis in Germany: a declining trend coming to an end? Eur Respir J. 2016;47(2):667–670. doi: 10.1183/13993003.01410-2015. [DOI] [PubMed] [Google Scholar]
- 19.Bozorgmehr K., Razum O., Saure D., Joggerst B., Szecsenyi J., Stock C. Yield of active screening for tuberculosis among asylum seekers in Germany: a systematic review and meta-analysis. Euro Surveill. 2017;22(12) doi: 10.2807/1560-7917.ES.2017.22.12.30491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voniatis C., Migliori G.B., Voniatis M., Georgiou A., D'Ambrosio L., Centis R. Tuberculosis elimination: dream or reality? The case of Cyprus. Eur Respir J. 2014;44(2):543–546. doi: 10.1183/09031936.00044314. [DOI] [PubMed] [Google Scholar]
- 21.Blasi F., Matteelli A., Sotgiu G., Cirillo D.M., Palmieri F., Fattorini L. Moving towards tuberculosis elimination: a call for action from Italy and a possible model for other low tuberculosis incidence countries. Eur Respir J. 2017;49(2) doi: 10.1183/13993003.02242-2016. [DOI] [PubMed] [Google Scholar]
- 22.Ruggeri M., Molicotti P., Cubeddu M., Cannas S., Bua A., Zanetti S. Tuberculosis in Sardinia: An investigation into the relationship between natives and immigrants. Int J Mycobacteriol. 2016;5(3):280–287. doi: 10.1016/j.ijmyco.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Lillebaek T., Andersen A.B., Bauer J., Dirksen A., Glismann S., de Haas P. Risk of Mycobacterium tuberculosis transmission in a low-incidence country due to immigration from high-incidence areas. J Clin Microbiol. 2001;39(3):855–861. doi: 10.1128/JCM.39.3.855-861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borgdorff M.W., Nagelkerke N., van Soolingen D., de Haas P.E.W., Veen J., van Embden J.D.A. Analysis of tuberculosis transmission between nationalities in the Netherlands in the period 1993–1995 using DNA fingerprinting. American Journal of Epidemiology. 1998;147(2):187–195. doi: 10.1093/oxfordjournals.aje.a009433. [DOI] [PubMed] [Google Scholar]
- 25.Alonso Rodriguez N., Chaves F., Inigo J., Bouza E., Garcia de Viedma D., Andres S. Transmission permeability of tuberculosis involving immigrants, revealed by a multicentre analysis of clusters. Clin Microbiol Infect. 2009;15(5):435–442. doi: 10.1111/j.1469-0691.2008.02670.x. [DOI] [PubMed] [Google Scholar]
- 26.European Migration Network Ad-Hoc Query on Tuberculosis screening of foreigners. Eur Migrat Netw. 2012 [Google Scholar]
- 27.European Migration Network Ad-hoc query on infectious diseases during the international protection procedure. Eur Migrat Netw. 2017 [Google Scholar]
- 28.Panagiotopoulos T. Screening for infectious diseases in newly arrived migrants in Europe: the context matters. Euro Surveill. 2018;23(28) doi: 10.2807/1560-7917.ES.2018.23.28.1800283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenaway C., Pareek M., Abou Chakra C.N., Walji M., Makarenko I., Alabdulkarim B. The effectiveness and cost-effectiveness of screening for active tuberculosis among migrants in the EU/EEA: a systematic review. Euro Surveill. 2018;23(14) doi: 10.2807/1560-7917.ES.2018.23.14.17-00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenaway C., Pareek M., Abou Chakra C.N., Walji M., Makarenko I., Alabdulkarim B. The effectiveness and cost-effectiveness of screening for latent tuberculosis among migrants in the EU/EEA: a systematic review. Euro Surveill. 2018;23(14) doi: 10.2807/1560-7917.ES.2018.23.14.17-00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunst H., Burman M., Arnesen T.M., Fiebig L., Hergens M.P., Kalkouni O. Tuberculosis and latent tuberculous infection screening of migrants in Europe: comparative analysis of policies, surveillance systems and results. Int J Tuberc Lung Dis. 2017;21(8):840–851. doi: 10.5588/ijtld.17.0036. [DOI] [PubMed] [Google Scholar]