Abstract
Due to a series of systemic and intracellular obstacles in nucleic acid (NA) therapy, including fast degradation in blood, renal clearance, poor cellular uptake, and inefficient endosomal escape, NAs may need delivery methods to transport to the cell nucleus or cytosol to be effective. Advanced nanoscale biotechnology-associated strategies, such as controlling the particle size, charge, drug loading, response to environmental signals, or other physical/chemical properties of delivery carriers, have provided great help for the in vivo and in vitro delivery of NA therapeutics. In this review, we introduce the characteristics of different NA modalities and illustrate how advanced nanoscale biotechnology assists NA therapy. The specific features and challenges of various nanocarriers in clinical and preclinical studies are summarized and discussed. With the help of advanced nanoscale biotechnology, some of the major barriers to the development of NA therapy will eventually be overcome in the near future.
Keywords: nucleic acid therapy, RNAi, ASO, CRISPR/Cas, drug delivery, gene editing
Introduction
Nucleic acid (NA) therapy refers to the use of NA molecules as tools and drugs for disease intervention at the genetic level. Currently, therapeutic NAs can be roughly classified into two types according to whether there is Watson-Crick base pairing complementary to the target sequence: NAs complementary to the target sequence, such as plasmid DNA, CRISPR-Cas, and other gene-editing tools, antisense NAs, ribozymes, deoxyribozymes, small interfering RNAs (siRNAs), microRNA (miRNA) analogs, and inhibitors; and NAs that are not complementary to the target sequence, such as mRNAs, aptamers, CpG, and decoy NAs. Alternatively, according to their different structures, NA drugs can be divided into DNA and RNA drugs.1,2 The history of NA therapy can be dated back to the 1970s. In 1978, it was revealed by scientists that antisense oligonucleotides (ASOs) could combine with target mRNA to form a DNA/RNA duplex and subsequently degraded target mRNA.3 From then on, several ASO drugs were approved by the US Food and Drug Administration (FDA) and the European Commission (EC) for the next 40 years. In 2003, China’s Food and Drug Administration (CFDA) approved the world’s first gene therapeutic, Gendicine.4Most recently, two siRNA therapeutics, Onpattro® (patisiran) and Givlaari™ (givosiran), were approved by the FDA in 2018 and 2019, for the treatment of the polyneuropathy and acute hepatic porphyria, respectively.5 Meanwhile, more than 2,000 clinical trials of NA therapies are underway, and more NA drugs are in the pipeline of biopharmaceutical companies. These data show us that the NA pharmaceutical industry is booming today.
Different from traditional drugs such as small molecules and antibodies, NAs enter into cells and mainly rely on endocytosis mechanisms.6 They need delivery vectors to transport to the cell nucleus or cytosol to be effective. Currently, viruses are the most efficient NA delivery vectors used to develop human therapeutics. However, the immunogenicity and biosafety constraints are still huge limitations of virus vectors. Eligible NA delivery systems that balance tractability, transfection efficiency, and safety are key pharmaceutical technologies pursued by pharmaceutical companies. Optimistically, advanced nanoscale biotechnologies provide great chances to achieve this goal. Nanotechnology-based delivery systems can be designed to perform a variety of functions, including precisely targeting tissues or organs as well as protecting their payload NAs from rapid degradation. This review aims to highlight the nanoscale biotechnology-assisted NA therapies and summarize various nanocarriers in clinical and preclinical studies and their specific features.
Working Mechanisms and Clinical Advances of NA Therapies
Protein-Expression Modalities
The protein-expression modalities of NAs mainly include DNA and messenger RNA. DNA locates in the nucleus and plays an important role in storing genetic information. Messenger RNA is transcribed from the DNA template and serves as a bridge between DNA and protein. Protein expression was discovered in the 1990s by injecting DNA or messenger RNA directly into the target organ.7 This effect prompted the widespread application of NA vaccines. In the following decades, plasmid DNA and viral DNA became very hot research subjects, while messenger RNA was underappreciated due to its poor stability and high manufacturing costs. With the overcoming of technical obstacles such as the stability and immunogenicity of mRNA, mRNA-based therapy has gained new attention and has been widely studied and applied in the fields of protein replacement therapy, cancer immunotherapy, and infectious disease vaccines.8, 9, 10, 11 In addition, mRNA does not have the risk of genomic integration, does not need to cross the nucleus membrane to function, and is expressed instantaneously, which in the opinion of some researchers is an advantage over DNA therapy. With the surge in the number of mRNA clinical trials in the last decade, the renaissance for mRNA therapy has arrived.
RNAi-Based Therapeutics
RNA interference (RNAi) refers to the universal phenomenon of highly specific degradation of homologous mRNA induced by double-stranded RNA (dsRNA), which is conserved in eukaryotic cells.12 RNAi not only resists the invasion of viral NAs, but it also regulates gene expression through a post-transcriptional gene silencing mechanism.13 The constituent members of RNAi therapeutics mainly include siRNA, miRNA, and anti-miR. A typical siRNA molecule is composed of 21–23 nt, with 2 nt suspended at the 3′ and 5′ ends, respectively. It has two chains, that is, a sense and antisense chain, in which the antisense chain is completely complementary to the target mRNA and degrades it, thus preventing the mRNA from translating into protein.13 For miR, it originates in the nucleus and is processed and matured in the cytoplasm. Endogenous and natural miRs are single-stranded RNA molecules of ~22 bp in length.14 Synthetic miRs contain sense and antisense double-stranded structures to keep their stability. siRNA and miR are first loaded into an RNA-induced silencing complex (RISC) and then bind to their targeted mRNA molecules with the help of an activated RISC complex. Subsequently, siRNA degrades mRNA and prevents the mRNA translation process, whereas miR only inhibits the expression of targeted mRNA without degrading it.15,16
Anti-miR is an antagonist of miR and, as its name implies, it is complementary to the targeted miR and inhibits the latter’s activity.17 Anti-miR is a synthetic single-stranded structure that is mainly used to upregulate the restricted gene expression level. Such limited gene expression is often caused by the miR. Using anti-miRs to suppress the activity of tumor progression-associated miR is a promising direction for the development of anti-cancer drugs.
ASOs
An ASO is a kind of single-stranded deoxynucleotide analog with a length of 15–20 nt.18 It is the earliest and most mature species in the field of nucleotide drug development. Its antisense sequences (3′ to 5′) bind to target mRNA in a complementary manner, thereby inhibiting specific gene expression at the transcriptional and translational levels. ASO and siRNA have many similarities; for example, their biological distribution in vivo is similar, their target molecules are RNA, and both of them inhibit gene expressions.13,18 However, ASOs have a variety of mechanisms, including (1) binding to target mRNA to activate the activity of RNase H enzyme, thereby inducing target RNA degradation; (2) preventing ribosome from binding to mRNA through the steric hindrance and inhibiting the translation of target mRNA; and (3) selectively promoting the expression of a variable spliceosome by shutting off the splicing sites, thereby correcting erroneous splicing.18, 19, 20 The diverse action mechanisms have attracted more and more pharmaceutical companies to join the field of ASO drug development, and the indications for ASOs are gradually expanding, from rare diseases to cardiovascular diseases, inflammation, infectious diseases, and metabolic diseases.
Gene-Editing Modalities
Gene-editing technology was discovered more than 30 years ago. Before the emergence of the CRISPR-Cas system, zinc finger nuclease (ZFN) technology and transcription activator-like effector nuclease technology (TALEN) were predominant. It was not until the last 6 years that the CRISPR-Cas9 system emerged and quickly became the most popular gene-editing tool. The CRISPR-Cas system is based on an RNA-guided adaptive immune system that bacteria and archaea have evolved over time to defend against viruses and phage DNA.21,22 CRISPR-Cas, ZFN, and TALEN all have the ability to edit complex genomes. Compared to ZFN and TALEN, the CRISPR-Cas system is simpler, easier to operate, and more effective because it requires only a small piece of guide RNA (gRNA) to recognize a specific target sequence. To date, nucleases encoding plasmid vectors have been used for precise gene editing.23 However, the continuous expression of nucleases in plasmids also increases the chance of off-target gene editing. In recent years, successful cases of gene editing using mRNAs that instantaneously express ZFN, TALEN, and Cas protein have emerged.24, 25, 26 For example, precise gene editing was achieved in mice using zwitterionic amino lipids (ZALs) to deliver single-guide RNA (sgRNA)- and mRNA-encoding Cas9 protein.25 Cas9 mRNA and sgRNA delivered by red blood cell (RBC)-derived extracellular vesicles (EVs) also showed efficient gene editing in both human cells and xenograft mouse models.27 These nanoscale non-viral delivery systems provide powerful tools for in vivo gene editing.
Aptamers and Ribozymes
NA aptamers, which are capable of binding to their target molecules specifically, are considered to be one of the most promising directions in NA therapy.28 Both DNA and RNA molecules are able to form complex three-dimensional structures including aptamers and ribozymes. By using the systematic evolution of ligands by exponential enrichment (SELEX) method, promising candidate aptamers are selected.29,30 NA aptamers are mainly used to study the interactions between NA sequences and other molecules (mainly proteins). They can be easily produced and chemically modified with high stability, reproducibility, and minor immunogenicity and toxicity. Such features lay a good foundation for the development of aptamer-based biosensors, diagnostic systems, and medicinal drugs.
Ribozymes represent another kind of NA therapeutic, including naturally occurring RNA molecules and synthetic DNA molecules with catalytic capacity. They are capable of catalyzing the cleavage and connection of the phosphodiester bond of NAs.31 Since most ribozymes possess enzymatic self-cleavage of RNA backbone, they are used to cleave specific RNA transcripts and regulate gene expression. At present, ribozymes are widely studied in developing treatment for cancer and infectious diseases.32,33 They are attractive anti-cancer drugs that directly induce cleavage or replacement of target RNA, thereby retarding tumor cell growth. For viruses with an RNA genome such as HIV/AIDS, ribozymes are capable of degrading viral genomic RNA, inhibiting virus entry into cells, and preventing RNA replication and new virus production.34 In addition to the natural RNA ribozymes, DNAzymes, single DNA molecules with catalytic activity, are also obtained through in vitro screening technologies. Different from ribozymes, DNAzymes are capable of recruiting metal ions, small molecules, and bacterial pathogens for catalysis. Thus, DNAzymes are used for detecting metal ions, small molecules, and NAs related to disease diagnosis.35,36 Moreover, coupling DNAzymes with aptamers to make aptazymes enabled detection of a broad range of signals with highly specificity.37 Until now, ribozymes and DNAzymes still have a long way to go in treating human diseases. Their stability, delivery, expression, and structure design need to be further explored and solved.
ADME of Oligonucleotides
ADME refers to the process of absorption, distribution, metabolism, and excretion of new exogenous chemicals in the body. Although there are many kinds of NA therapeutics, only a few oligonucleotides (ASOs, siRNAs, and miRNAs) have relatively complete ADME data so far. Furthermore, their pharmacokinetic properties depend largely on the type of chemical modification on their backbone or ribose sugar, as the chemical modification strategy is directly related to the biostability of oligonucleotides and their binding performance to plasma proteins. It is reported that phosphorothioate (PS)-modified DNA showed beneficial effects on blood clearance, biodistribution, and cellular uptake of oligonucleotides through nonspecific combining with plasma proteins. However, 2′ modifications on the ribose sugar led to high resistance against enzyme degradation and prolonged the half-life of oligonucleotides in the body.20,38,39
Owing to the low absorption efficiency of oral oligonucleotides in the gastrointestinal tract, most unformulated or formulated oligonucleotide drugs are currently administered through parenteral routes, such as intravenous infusion (injection), subcutaneous injection, or local administration. For example, ASOs with PS modification combine with plasma proteins at >90% and exhibit a short distribution half-life of 1–2 h. Tissue accumulations occur mainly in the kidney, liver, spleen, lymph node, adipocyte, and bone marrow. However, unformulated siRNAs with a PS modification are eliminated in the blood in a few minutes after administration, with no detectable siRNA in the tissues.20,39,40 This is the most important reason why siRNA is considered to be of poor bioavailability in vivo, requiring delivery systems and optimizing modification strategies. Other studies reported that locked nucleic acid (LNA)-modified anti-miR-122 was efficiently up taken by the liver cells through intraperitoneal or intravenous injection.41, 42, 43 The biodistribution characteristics of ASOs in organs of different species are similar. However, under similar dosages, rodent tissues generally have lower drug concentrations (mass per gram of tissue) than do those of dogs and monkeys, while dogs, monkeys, and humans have similar pharmacokinetic characteristics.20 The main metabolites of unmodified ASOs are oligonucleotides with one base missing at the 3′ end, which will be further degraded into oligonucleotides with two or more bases missing. After introducing modification of ASOs with the second generation of chemical modification technology such as 2′-methoxyethyl, the metabolites of ASOs are oligonucleotides of much shorter endonuclease cleavage products.44,45 Most metabolites are ultimately excreted in urine and minor amounts are cleared through biliary elimination.46,47
Clinical Advances of NA Therapies
As an early developed NA therapeutic species, there are dozens of DNA-based gene therapeutics currently on the market. The first commercialized gene therapy, Gendicine, developed by Shenzhen Sibiono GeneTech, was approved by CFDA in 2003 for treatment of head and neck squamous cell carcinoma.4 It is a first-in-class DNA-based, adenoviral serotype-5 (Ad5) vector-mediated gene therapeutic. Data collected from the clinic in past years proved that Gendicine still produced 30%–40% complete response (CR) and 50%–60% partial response (PR), with a total response rate ranging from 90% to 96%.4 Inspired by the success of Gendicine, several other gene therapy products based on viral vectors had expedited development. In December 2017, Luxturna, also an adeno-associated virus (AAV)-based DNA therapeutic for patients with vision loss, was approved by the FDA.48 Until now, virus vectors have been widely used in gene therapeutics development. Among more than 2,500 gene therapy-associated clinical trials conducted since 1989, around 20% involved adenoviral vectors. Most DNA-based therapeutics are used in cancer therapy, infectious disease vaccines, and protein replacement therapy. The clinical application of mRNA therapy is very similar to DNA, and nearly 100 clinical trials are currently underway. However, these trials have not yet advanced to commercialization.
ASO is also an early developed and mature species in NA therapy. To date, several ASOs have been approved by the FDA or EC, and more than 100 are undergoing clinical trials. Another hot NA species that works by interfering with gene expression of disease-related target mRNAs is siRNA. The first siRNA clinical trial was initiated in 2004, and since then more than 30 siRNAs have been validated in clinical trials. In 2018, the world’s first siRNA drug Onpattro (patisiran) was approved by the FDA and EC for the treatment of the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults.5,49 This is a milestone event in the history of RNAi therapeutics development. With the approval of these NA therapeutics, the associated delivery and modification technologies have reached a mature stage, providing theoretical basis and practical experience for the development of more NA drugs. We summarize some typical NA therapeutics that have advanced to the clinical stages, including DNA, mRNA, ASO, siRNA, CRISPR-Cas, ribozyme, aptamer, and others. See Table 1 for details.
Table 1.
Representative Clinical Development Activities of NA Therapeutics
| Therapeutic Name | Condition(s) | Target Gene | Delivery System | Current Status | ClinicalTrials.gov | Reference |
|---|---|---|---|---|---|---|
| DNA Therapeutics | ||||||
| Gendicine (Ad-p53) | head and neck cancer | P53 | Ad5 | approved | NCT03544723 | 4 |
| NCT02842125 | ||||||
| Glybera (alipogene tiparvovec) | LPLD | LPLS447X | AAV | approved | NCT03293810 | 201 |
| Imlygic (talimogene laherparepvec) | melanoma | GM-CSF | rHSV-1 | approved | NCT02014441 | 202 |
| NCT02574260 | ||||||
| NCT00289016 | ||||||
| NCT01368276 | ||||||
| Luxturna (voretigene neparvovec) | inherited retinal dystrophy | RPE65 | AAV | approved | NCT01208389 | 48 |
| NCT00999609 | ||||||
| NCT00516477 | ||||||
| Zolgensma (AVXS-101) |
SMA |
SMN1, SMN2 |
AAV9 |
approved |
NCT03955679 |
203,204 |
| NCT02122952 | ||||||
| NCT03505099 | ||||||
| NCT03837184 | ||||||
| NCT03381729 | ||||||
| NCT03306277 | ||||||
| siRNA Therapeutics | ||||||
| Onpattro (patisiran, ALN-TTR02) | TTR-mediated amyloidosis | TTR | liposome | approved | NCT02939820 | 5,49 |
| NCT02510261 | ||||||
| NCT03759379 | ||||||
| NCT03862807 | ||||||
| NCT01960348 | ||||||
| NCT01961921 | ||||||
| NCT01617967 | ||||||
| Lumasiran (ALN-GO1) | PH1 | HAO1 | GalNAc-siRNA conjugate | III | NCT03681184 | 205 |
| NCT03350451 | ||||||
| NCT02706886 | ||||||
| Vutrisiran (ALN-TTRsc02) | TTR-mediated amyloidosis | TTR | GalNAc-siRNA conjugate | III | NCT03759379 | 206 |
| NCT02797847 | ||||||
| Givosiran (ALN-AS1) | acute hepatic porphyria | ALAS-1 | GalNAc-siRNA conjugate | III | NCT03338816 | 207 |
| NCT02949830 | ||||||
| NCT03505853 | ||||||
| NCT02452372 | ||||||
| Inclisiran (ALN-PCSsc) | hypercholesterolemia | PCSK9 | GalNAc-siRNA conjugate | III | NCT03814187 | 208,209 |
| NCT03400800 | ||||||
| NCT03397121 | ||||||
| NCT03705234 | ||||||
| NCT03851705 | ||||||
| NCT03399370 | ||||||
| ALN-AAT02 | alpha-1 liver disease | AAT | GalNAc-siRNA conjugate | II | NCT03767829 | 210 |
| AMG 890 | cardiovascular disease | Lp(a) | GalNAc-siRNA conjugate | I | NCT03626662 | 211 |
| DCR-PHXC | primary hyperoxaluria | LDHA | GalNAc-siRNA conjugate | I | NCT03392896 | 212 |
| QPI-1007 | nonarteritic anterior ischemic optic neuropathy (III), acute primary angle closure glaucoma (II) | caspase 2 | naked siRNA | III | NCT02341560 | 213 |
| Tivanisiran (SYL1001) | ocular pain, dry eye | TRPV1 | naked siRNA | III | NCT03108664 | 214 |
| MSC-derived exosomes with KrasG12D siRNA | pancreatic cancer | KrasG12D mutation | exosome | I | NCT03608631 | 184 |
| shRNA Therapeutics | ||||||
| TT-034 (PF-05095808) | hepatitis C | HCV | AAV | II | NCT01899092 | 215 |
| pbi-shRNA STMN1 LP | advanced and/or metastatic cancer | STMN1 | lipoplex | I | NCT01505153 | 216 |
| pbi-shRNA EWS/FLI1 type 1 LPX | Ewing’s sarcoma | EWS/FLI1 | lipoplex | I | NCT02736565 | 217,218 |
| shRNA | CLL | XPO1 | – | – | NCT02757586 | |
| ASO therapeutics | ||||||
| Inotersen | polyneuropathy | TTR | – | approved | NCT01737398 | 219,220 |
| Fomivirsen | CMV retinitis | IE2 | – | approved | NCT00002187 | 221 |
| Mipomersen | homozygous familial hypercholesterolemia (HoFH) | apolipoprotein B (apoB) | – | approved | NCT01598948 | 222,223 |
| NCT00477594 | ||||||
| NCT01414881 | ||||||
| NCT01475825 | ||||||
| NCT00607373 | ||||||
| NCT00770146 | ||||||
| NCT00694109 | ||||||
| NCT00794664 | ||||||
| NCT00706849 | ||||||
| NCT00707746 | ||||||
| NCT00362180 | ||||||
| Nusinersen | SMA | SMN1 | – | approved | NCT02052791 | 224 |
| NCT02386553 | ||||||
| NCT01703988 | ||||||
| NCT01780246 | ||||||
| NCT01839656 | ||||||
| NCT02292537 | ||||||
| NCT02462759 | ||||||
| NCT02594124 | ||||||
| Eteplirsen (AVI-4658) | DMD | exon 51 of the dystrophin pre-mRNA | – | approved | NCT00844597 | 225 |
| NCT00159250 | ||||||
| NCT01396239 | ||||||
| NCT03218995 | ||||||
| NCT02255552 | ||||||
| NCT02286947 | ||||||
| Alicaforsen | CD, UC | intracellular adhesion molecule-1 (ICAM-1) | – | III | NCT02525523 | 226 |
| miRNA Therapeutics | ||||||
| RG-101 | hepatitis C | miR-122 | GalNAc conjugation | I | NCT01646489 | 227 |
| RG-012 | Alport syndrome | miR-21 | – | I | NCT03373786 | 228 |
| NCT02136862 | ||||||
| Targomir (miR-16 mimic) | mesothelioma, NSCLS | EGFR | bacterial minicells | I | NCT02369198 | 187 |
| Remlarsen (MRG-201) |
keloid |
miR-29 |
cholesterol conjugation |
II |
NCT02603224 |
229 |
| NCT03601052 | ||||||
| mRNA Therapeutics | ||||||
| AGS-003 | renal cell carcinoma | – | DC | III | NCT01582672 | 230 |
| Ex vivo-transfected DCs | uveal melanoma | – | DC | III | NCT01983748 | |
| AZD8601 | type II diabetes, heart failure | VEGFA | citrate/saline vehicle | II | NCT02935712 | 10,231 |
| NCT03370887 | ||||||
| Ex vivo-transfected DCs | HIV-1 infection | – | DC | II | NCT00833781 | |
| VAL-506440 | influenza | H10N8 antigen | LNP | I | NCT03076385 | 9 |
| Aptamer Therapeutics | ||||||
| REG1 | acute coronary syndrome | factor IXa | – | II | NCT00113997 | 68 |
| NCT00715455 | ||||||
| NCT00932100 | ||||||
| Macugen (pegaptanib sodium) | AMD | VEGF | – | approved | NCT01487070 | 232,233 |
| NCT01573572 | ||||||
| NCT00549055 | ||||||
| NCT01486238 | ||||||
| NCT01189461 | ||||||
| NCT00324116 | ||||||
| NCT00858208 | ||||||
| NCT00239928 | ||||||
| AS1411 | AML | nucleolin | – | II | NCT01034410 | 234 |
| NCT00512083 | ||||||
| Zimura (ARC1905) |
AMD |
complement factor C5 |
– |
II |
NCT00709527 |
235 |
| NCT02397954 | ||||||
| NCT02686658 | ||||||
| NCT00950638 | ||||||
| Ribozyme Therapeutics | ||||||
| OZ1 | HIV | vpr/tat | retroviral vector LNL6 | II | NCT01177059 | 236 |
| NCT00074997 | ||||||
| RPI. 4610 (angiozyme) | kidney cancer | VEGFR-1 | – | II | NCT00021021 | 237 |
| DZ1 | nasopharyngeal carcinoma | EBV-LMP1 | – | II | NCT01449942 | 238 |
| SB012 | colitis, ulcerative | GATA-3 | – | II | NCT02129439 | 239 |
| Gene-editing therapeutics | ||||||
| NY-ESO-1 redirected autologous T cells with CRISPR-edited endogenous TCR and PD-1 | multiple myeloma, melanoma, synovial sarcoma, myxoid/round cell liposarcoma | NY-ESO-1, TCRα, TCRβ and PD-1 | lentiviral vector | I | NCT03399448 | |
| CTX001 | thalassemia, hemoglobinopathies | BCL11A | – | II | NCT03655678 | 240 |
| Mesothelin-directed CAR-T cells | solid tumor | PD-1 | – | I | NCT03747965 | 241 |
SMA, spinal muscular atrophy; SMN1, survival motor neuron 1; AAV9, adeno-associated virus serotype 9; LPLD, inherited metabolic disorder lipoprotein lipase deficiency; LPLS447X, human lipoprotein lipase (LPL) gene variant; GM-CSF, granulocyte-macrophage colony-stimulating factor; rHSV-1, recombinant herpes simplex virus type 1; TTR, transthyretin; PH1, primary hyperoxaluria type 1; HAO1, hydroxyacid oxidase 1; ALAS-1, 5′-aminolevulinate synthase 1; PCSK9, proprotein convertase subtilisin/kexin type 9; AAT, alpha-1 antitrypsin; Lp(a), lipoprotein (a); LDHA, lactate dehydrogenase A; TRPV1, transient receptor potential cation channel subfamily V member 1; HCV, hepatitis C virus; STMN1, stathmin 1; EWS/FLI1, a fusion gene containing the 5′ end of the EWSR1 gene (EWS RNA binding protein 1) and the 3′ end of the FLI1 gene (friend leukemia integration 1 transcription factor); CLL, chronic lymphocytic leukemia; XPO1, exportin 1; IE2, immediate-early protein 2; CMV, cytomegalovirus; HoFH, homozygous familial hypercholesterolemia; DMD, Duchenne muscular dystrophy; CD, Crohn’s disease; UC, ulcerative colitis; NSCLS, non-small-cell lung cancer; DC, dendritic cell; LNP, lipid nanoparticle; AMD, age-related macular degeneration; VEGF, vascular epithelial growth factor; AML, acute myeloid leukemia; vpr, virus protein regulatory; EBV-LMP1, Epstein-Barr virus-encoded latent membrane protein 1; TCR, T cell receptor; BCL11A, B cell lymphoma 11 A; PD-1, programmed cell death protein 1; NA, not available.
Nanotechnology-Associated Strategies Used for Improving NA Therapy
Barriers of NA Therapy
When administered intravenously, the therapeutic NAs are easily degraded by endonucleases in physiological fluids, resulting in rapid renal clearance and short circulation time in the blood. It has been shown that the half-life of plasmid DNA following intravenous infusion in mice is only 10 min.50 Intracellular transport of NAs begins in the early endosomes and then transfers to lysosomes containing various nucleases. NAs are negatively charged molecules and large in size, so they cannot diffuse across cell membranes.51 Even if a small amount of NAs is taken up by cells, they may remain in the lysosome, resulting in poor therapeutic efficiency. In addition, the bioavailability of NAs may be impeded by endothelial barriers, liver retention, renal clearance, and unexpected tissue accumulation.51 These barriers significantly limit the development of NA therapy.
Inspiringly, the continuous improvements and innovations in nanotechnology-associated strategies will assist in breaking through these barriers. Owing to their specific size, high drug-loading efficiency, unique physical and chemical stability, and other factors, nanocarriers have attracted extensive interest in NA delivery.52 They have great potential to improve NA stability, delivery, and drug-loading efficiency, avoiding their degradation and ensuring controlled drug release.
Improve Stability and Avoid Degradation
Nucleases are vital for organisms to avoid virus invasion and missense RNA/DNA accumulation.53 However, they are also responsible for the poor stability of NAs. Several chemical modification strategies have been used to improve the stability of therapeutic NAs, such as the introduction of bases, sugar, backbone, phosphodiester linkage modifications, or synthetic nucleotides, thus significantly improving the therapeutic efficiency. Most chemical modification strategies of NAs have been extensively reviewed.54
Entrapping NAs in a suitable nanocarrier is another strategy to avoid endonuclease degradation and prolong the half-life of NAs. After interaction with nanocarriers to form nanoparticles (NPs), the dense packing of NAs inside or on the surface of NPs increases the steric hindrance of the entire system, leading to steric inhibition of nuclease degradation. In a typical example, the degradation rate of the gold NP (Au NP)/oligonucleotide complex was much slower than that of free oligonucleotides due to the increased steric hindrances.55 The condensed structure of NP further protected NAs from exposure to nuclease or other destructive enzymes, thereby improving NA tolerance and pharmacokinetics in blood.49,56 Another benefit of nanocarriers is that they can significantly increase the amount of NAs at the target site. At the same dose, free NAs can hardly reach an effective therapeutic concentration, while NA carried by nanocarriers can. Our previous study found that ultra-small 2-nm Au NPs were able to maintain the stability of oligonucleotides, and Au NP/oligonucleotide nanocomplexes were more likely to achieve effective drug concentrations than were free oligonucleotides.57 A similar effect was also proven using in vivo research.58
Improve Delivery and Promote Lysosome Escape
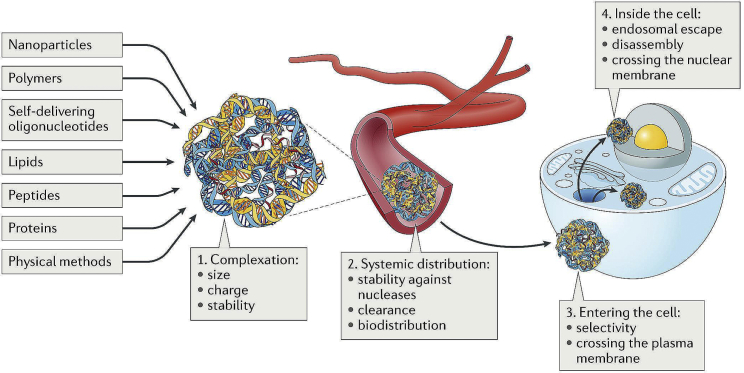
How to deliver NA molecules to the target site safely and efficiently is the bottleneck problem of most NA molecules. Both viral and non-viral carriers are used for NA delivery. A few modified viral carriers have been approved for treatment of human disease or tested in clinical trials, including retroviruses, lentiviruses, adenoviruses, and AAVs.59 However, drawbacks such as immunogenicity and carcinogenesis are still associated with viral carriers.50 Non-viral carriers have great potential to break these limitations, especially from a safety standpoint (Figure 1).
Figure 1.
Challenges and Strategies for Efficient Intracellular NA Delivery
Reproduced from Lostalé-Seijo et al.257 with permission.
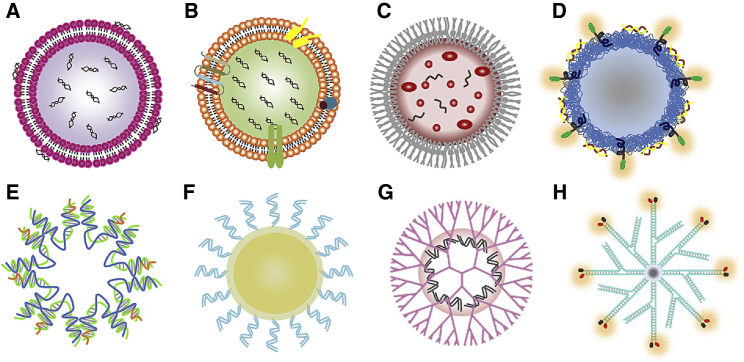
Nanoscale non-viral vectors have aroused great interest since their emergence. Nanomaterials are small in size (a few to a hundred nanometers) and have a large surface area-to-volume ratio. These unique properties allow them to efficiently carry and absorb other substances, and become a source of materials for constructing new drug delivery systems.60 Various types of nanocarriers have been used for NA delivery, including liposomes, polymeric NPs, inorganic NPs, dendrimers, DNA/RNA nanostructures, and others. (Figure 2)
Figure 2.
Scheme of Representative Nanocarriers Used for NA Therapy
(A) Liposome; (B) exosome; (C) bacterial-derived nanocell; (D) polymeric NP; (E) DNA nanostructure; (F) inorganic NP; (G) dendrimer-based NP; and (H) RNA nanostructure.
Many solid tumors have leaky vasculatures and incomplete lymphatic drainage systems, and nanocarriers are more preferential to accumulate in such circumstance.61 This characteristic, known as the enhanced permeability and retention (EPR) effect, has been extensively explored in nanocarrier-assisted cancer therapy.62, 63, 64 The EPR effect along with prolonged circulation time of NA nanoparticles (NANPs) greatly improve the NA therapy efficiency.65, 66, 67 Additionally, NPs are able to achieve active targeted delivery through functionalization with specific cellular molecules.68 In this case, therapeutic NAs accumulate at the intended sites, which not only improves the treatment effect but also avoids unexpected side effects in other tissues.69, 70, 71, 72 Sometimes nucleus is considered as the final barrier for effective NA therapy, specifically for the delivery of DNA vaccines and genome-editing nucleases.73 Small molecules have the ability to transport into or out of nucleus by free diffusion, but macromolecules larger than 9 nm cannot pass through the nucleus freely.74 However, ultra-small NPs delivered NAs efficiently into the nucleus, yielding excellent anticancer results.57
As we mentioned above, NAs are easily trapped in endosomes and lysosomes after cellular uptake. Specially designed nanocarriers allow more NAs to escape from endosomes and lysosomes, resulting in effective NA therapy.75,76 It was reported that bioreducible and cationic polymer poly(beta-amino esters) (PBAEs) promoted miRNA escape from endosome to cytoplasm.77 Fluorinated dendrimers and polymeric NPs exhibited better NA delivery in serum due to potent endosomal disruption caused by serum protein adsorption resistance.78, 79, 80
Achieve High Loading Efficiency and Controlled Drug Release
Many nanocarriers have a large surface area and cavity, such as porous silica, micelles, dendritic macromolecules, liposomes, and others. Drugs or NAs are absorbed on their surface or encapsulated in their cavity. The NA loading efficiency will be greatly improved by selecting a proper nanocarrier and adjusting its physical and chemical properties. Compared with silica NPs, bioactive glass (BG) NPs show 45-fold improvement and 7-fold enhancement in diclofenac sodium and miRNA loading, respectively.81 In addition to BG, nanomicelles, polymeric core-shell NPs, and others also showed high loading capacity for exogenous therapeutic NAs.82,83
Controlled drug release is beneficial to improve drug efficacy and reduce side effects as the release behavior of the drug is limited, and thereby the amount and release rate of drugs can be adjusted selectively. In order to minimize side effects of drug on normal tissues and cells, novel strategies are needed to control drug release, especially if the drug is toxic. Nanocarriers with porous, multiple layers and hollow structures are easily engineered into a controlled drug release system. Liposomes,84 shell-like structure nano-robots,85 as well as some stimuli-sensitive smart nanosystems86 have gained interests to achieve controlled release of NAs.
Nanocarriers Used for NA Delivery
Lipid-Based Nanocarriers
Lipid-based nanocarriers, including liposomes, lipid NPs (LNPs), lipid emulsions, lipid implants, and others, are one of the most widely used non-viral vectors for NA therapy. Phospholipids containing liposomes were first testified in the 1980s to deliver SV40 DNA to monkey kidney cells.87 Such spherical, self-assembled closed structures consist of an inner aqueous phase encircled by one or several concentric lipid bilayers (Figure 2A). Liposomes composed of classical cationic lipids, such as N-[1-(2, 3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA), 1,2-dioleoyloxy-3-trimethylammonium propane chloride (DOTAP), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), were previously used for both in vitro and in vivo delivery of DNA, siRNA, and mRNA. In recent years, they also showed promise in delivering mRNA to dendritic cells with high efficiency for cancer immunotherapy. The excipient DLin-MC3-DMA ((6Z,9Z,28Z,31Z-heptatriaconta-6,9,28,31-tetraen-19-yl-4-(dimethylamino)butanoate) used in patisiran (a liposome formulation) is an ionizable, pH-sensitive lipid. A typical ionizable lipid is composed of hydrocarbon chains, linker, and headgroup. Liposomes composed of ionizable lipids are neutral at a physiologic pH environment, while under acidic pH conditions, such as endosomes and lysosomes, they are ionized and protonated, which further triggers osmotic lysosome and endosome rupture. As a result, siRNAs successfully escape from the endosome/lysosome and trigger RNAi in cytoplasm for hATTR amyloidosis therapy. Besides DLin-MC3-DMA, dozens of other ionizable lipids were investigated preclinically and clinically, including cKK-E12,88 DLin-KC2-DMA (1,2-dilinoleyl-4-(2-dimethylaminoethyl)-[1,3]-dioxolane),89 L319 (di((Z)-non-2-en-1-yl) 9-((4-(dimethylamino)butanoyl)oxy)heptadecanedioate),90 C12-200,91 and others. Some representative nanocarriers used for NA therapy are shown in Figure 2.
The liposome/NA complexes that are formed mostly rely on the electrostatic interactions between positively charged lipids and negatively charged NAs. Liposomes and siRNAs are condensed into slightly positively charged nanocomplexes, which are subsequently delivered to negatively charged cells for uptake, internalization, escape, release, and expression. Although this process is efficient and straightforward, major issues such as the cationic lipid-associated cytotoxicity, off-target effects, and limitation of transfected cell types still need to be addressed. Meanwhile, advanced technologies such as bio-orthogonal liposome fusion,92 click chemistry,93 and surface engineering94 developed in recent years are also expected to combine with lipid nanocarriers to develop more effective, precise, and safe NA delivery systems.
Polymer-Based Nanocarriers
Polymers and their derivatives are another major material source for drug delivery systems. Various polysomes formed by polymers, e.g., NP, nanomicelle, dendrimer, hydrogel, and nanoemulsion, are widely used NA nanocarriers. The first clinical application of nanoscale biotechnology in targeted siRNA therapy is the use of natural polymer cyclodextrin as the carrier for siRNA delivery.50 Cyclodextrin is a natural macrocyclic oligosaccharide with internal hydrophobic and external hydrophilic structures, which can interact with NAs at the external structure and improve their stability. Although cyclodextrin is capable of promoting cell membrane permeability, its toxicity prevents it from being applied in the clinic.95, 96, 97 Other natural polymers such as chitosan,98, 99, 100 hyaluronic acid,101 dextran,102 and gelatin,54 are also widely studied biocompatible carriers for NAs.
In addition to natural polymers, a large number of synthetic polymers are excellent NA carriers, e.g., the classic and cationic polymer of polyethylenimine (PEI), poly-l-lysine) (PLL), poly[(2-dimethylamino)ethyl methacrylate] (pDMAEMA), and polyamidoamine (PAMAM), newly developed polymers of hDD90-118,103 N5,104 and PAA8k-(2-3-2),105 and others. Since 1995, PEI has been used as an ideal cationic delivery carrier for NAs.106 To improve its performance, block co-polymers of PEG-PEI were used instead of PEI to decrease toxicity.107 As one of the most important pH-sensitive cationic polymers, pDMAEMA is widely used for DNA, siRNA, mRNA, and miRNA delivery with acceptable cytotoxicity and combined transfection efficiency.108, 109, 110, 111, 112 Notably, some polymers have been found to have a dual function, providing therapeutic benefits while also serving as delivery vehicles. For example, PolyMetformin, a polymer derivative of drug metformin with anti-cancer and anti-diabetic effects, was capable of delivering siRNA for RNAi therapy and did not lose its anti-cancer properties.75 Another polymer, a near-infrared absorbing dendronized semiconducting polymer, delivered DNA efficiently as well as controlled gene expression spatiotemporally together with a heat-inducible promoter.113
Among the various types of polysomes, a nanoemulsion is the colloidal dispersion of two immiscible liquids (usually oil and water) with diameters of 10 to several hundred nanometers.114, 115, 116 It overcomes the major problem of liposome or particle aggregation.117 Besides intravenous injection, local administration of nanoemulsions, such as oral and intranasal administration, also showed improved gene transfection efficiency.118,119 Dendrimer is another popular, highly branched, radiation-like functional polymer-based nanocarrier. With an inner core, an amidoamine backbone, and multiple terminal amine groups, dendrimers have additional compartment space for loading NAs.8,120 In some cases, cationic amphiphilic dendrimer composed of PEG-PLA polymer and G0-C14 dendrimer showed synergistic anti-cancer effects by encapsulating chemotherapeutic drugs in their hydrophobic interlayer and NAs in their hydrophilic cavity.121,122 Polymeric micelle has been actively studied in the field of polymer chemistry. It is self-assembled from synthetic block copolymers or graft copolymers and possesses an inner hydrophobic core and an outer hydrophilic shell. Compared with other charged macromolecules such as proteins, NAs more favorably incorporate into the inner core of micelles formed from positively charged polymers through ionic interaction. Therefore, NAs such as CRISPR-Cas incorporated into polymeric micelles are very stable.123,124 These advantages make polymeric micelles a very suitable nanocarrier for in vivo and in vitro NA delivery.
Inorganic and Hybrid NPs
Inorganic NPs are emerging as a research hotspot of NA carriers, as they have the advantages of precise size control, tunable surface properties, and high drug loading efficiency. Frequently used inorganic NPs for drug delivery include metal NPs, quantum dots, and calcium-based NPs such as calcium phosphate,125, 126, 127 Au NPs are one of the preferred carriers for NAs. Au NPs are easily fabricated with an ultra-small size (smaller than nuclear pore complex), which is crucial for nuclear targeting via nuclear pore complex.128 Besides, the functional diversity of Au NPs is achieved easily by modifying different bioactive ligands e.g., peptides, polymers, NAs, and antibodies on their surface.129 It is reported that actively targeted Au NPs with cyclic RGD peptide decoration showed excellent anti-cancer effects after systemic administration.130 Spherical NA (SNA) Au NP conjugates (13-nm Au NPs functionalized with densely packed and highly oriented NAs through thiol groups) naturally penetrated the skin and entered keratinocytes without the need for transfection agents.131 The strong interaction between thiol and Au NPs is a reliable way to decorate and functionalize Au NPs. In addition, the calcium-based NPs are new choice for pH-responsive delivery of NAs. When the environmental pH decreases, the solubility of calcium phosphates trended to increase and dissolve into ions, thus releasing the loaded NAs in the acidic environment such as lysosome.132
Hybrid nanosystems, or nanocomposites, combine the benefits of different nanomaterials to provide an attractive platform for NA therapy. Although some inorganic NPs such as Au NPs exhibit great potential for targeted delivery of therapeutic NPs, their application may be hindered by their user-unfriendly nature. The combination of polymers and inorganic NPs to form polymer nanocomposites often performs better than when each is used alone. For example, a hybrid nanocarrier composed of Au NPs and poly(dimethylaminoethyl acrylate) increased the DNA therapeutic efficiency as well as reduced adverse effects.133 Another hybrid nanogel composed of Au NPs, tumor-targeting endosomolytic peptides, and siRNA achieved a triple combination of chemotherapy, phototherapy, and RNAi therapy.134 Table 2 includes some typical types of nanocarriers used for NA therapy and their clinical stages.
Table 2.
Typical Types of Nanocarriers Used for NA Therapy
| Nanocarriers | Materials | Therapeutic NAs | Condition(s) | Clinical Stage | References |
|---|---|---|---|---|---|
| Liposome | DOTAP, cholesterol | IL-22 mRNA | colon cancer | clinical | 11 |
| DDA, MPLA, TDB | pCMFO | Mycobacterium tuberculosis infection | preclinical | 84 | |
| LNP | DLin-MC3-DMA, cholesterol, PEG, DSPC | siRNA against TTR mRNA | hATTR amyloidosis | clinical | 49 |
| DOTMA, DOPE | tumor antigen encoding mRNA | melanoma | clinical | 242 | |
| lipid, phospholipid, cholesterol, PEG | tumor antigen encoding mRNA | melanoma | preclinical | 243 | |
| Polymeric NP | PEI, PEG, TAT peptide | TRAIL | hepatoma | preclinical | 107 |
| polypeptide, cholesterol | PLK1 siRNA | lung cancer | preclinical | 244 | |
| PLGA | MAPK1 siRNA | – | preclinical | 245 | |
| Exosome | mesenchymal cells | Kras siRNA | pancreatic cancer | clinical | 184 |
| Minicell/nanocell | bacterial | miRNA | mesothelioma, NSCLC | clinical | 187 |
| Inorganic NP | Au NP | siRNA against Bcl2L12 mRNA | glioblastoma | preclinical | 67 |
| silver NP | antisense oligonucleotide against ICAM-1 | – | preclinical | 246 | |
| Dendrimer NP | poly(amidoamine) dendrimer, PEG | virus antigen encoding mRNA | pathogen infection | preclinical | 247 |
| poly(amidoamine) dendrimer, lipid | Tie2 siRNA | lung diseases | preclinical | 248 | |
| Nanomicelle | PEG-PAA block copolymer | BDNF expressing mRNA | sensory nerve disorders | preclinical | 249 |
| polyethyleneimine-stearic acid co-polymer | HIV-1 gag encoding mRNA | HIV infection | preclinical | 250 | |
| Nanoemulsion | MCT, DOPE, DOTAP, DSPE-PEG | plasmid containing alpha-l-iduronidase gene | mucopolysaccharidosis type I | preclinical | 251 |
| DNA nanostructure | self-assembled DNA tetrahedron | anti-luciferase siRNA | luciferase-KB tumor | preclinical | 252 |
| RNA nanostructure | RNA transcripts | anti-red fluorescent protein siRNA | ovarian cancer | preclinical | 253 |
| Hybrid NP | PEG, PEI, SPION | plasmid DNA encoding luciferase and red fluorescence protein | MSC transplantation | preclinical | 254 |
| PAMAM dendrimer, Au NP | plasmid DNA | – | preclinical | 255 | |
| PEI, Fe2O3 | siRNA | – | preclinical | 256 |
PEG, polyethylene glycol; DLin-MC3-DMA, (6Z,9Z,28Z,31Z-heptatriaconta-6,9,28,31-tetraen-19-yl-4-(dimethylamino)butanoate; TTR, transthyretin; DDA, dimethyldioctadecylammonium; MPLA, monophosphoryl lipid A; TDB, trehalose 6,6′-dibehenate; pCMFO, a plasmid DNA that secretes the fusion of four multistage antigens (Rv2875, Rv3044, Rv2073c, and Rv0577) of Mycobacterium tuberculosis; TRAIL, the human tumor necrosis factor-related apoptosis-inducing ligand-encoding plasmid gene; SPION, superparamagnetic iron oxide nanoparticles; ICAM-1, intracellular adhesion molecule-1; NSCLC, non-small-cell lung cancer; BDNF, brain-derived neurotrophic factor; PAA, polyamino acid; HIV, human immunodeficiency virus; MCT, medium chain triglycerides; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000]; MAPK1, mitogen-activated protein kinase.
Self-Assembled NA Nanostructures
In recent years, the discovery that DNA is capable of self-assembling into nanostructures has extended its biological function far beyond carrying genetic information. Because its complementary sequences can be accurately predicted, DNA may be engineered into various advanced morphologies and multi-dimensional architectures. Some self-assembled DNA nanostructures could even be taken up by cells without the aid of other transfection reagents.135 It was reported that rectangular DNA origami nanostructures exhibited preferential renal uptake in mice, and had excellent kidney-protection performance, which was comparable to clinical renal protection drugs.136 Through hybridization, DNA nanostructures were used for multiple therapeutic NAs delivery, including siRNAs,137 ASOs,138 gRNA of the CRISPR-Cas9 system,139 and aptamers.140 Recently, an autonomous DNA nanorobot engineered by DNA origami technology was programmed to transport thrombin and present it specifically in tumors. In this system, thrombin was encapsulated in the inner cavity of the nanorobot to induce intravascular thrombosis, and DNA aptamer was functionalized on the outside of the nanorobot to provide a nucleolin targeting domain and a molecular trigger for the mechanical opening of the DNA nanorobot. The novel DNA nanorobot integrated tumor-targeting and controlled drug release, which showed a broad prospect of DNA nanotechnology in precise cancer therapy.141 Afonin and Shapiro142 developed a split-functionality approach using programmable R/DNA hybrids to trigger the release of siRNA. They first split siRNA into two R/DNA hybrids. When the hybrids existed in the same circumstance, they recognized each other through toehold interaction, re-associated, and released siRNA in the cell. This split-functionality approach can also be used to introduce other functionalities such as aptamer, fluorescent dye, targeting agents, and others.
Similar to DNA nanostructures, a variety of programmable RNA nanostructures with defined size, shape, and stoichiometry have been developed for diverse applications. RNA has a flexible structure and contains a large variety of loops and ligands, allowing for folding into various complicated structures. As early as the 1980s, Guo et al.143 found that the bacteriophage phi29-encoded small RNA had the ability to package DNA into procapsids. This small RNA was called packaging RNA (pRNA). Taking advantages of pRNA, they engineered therapeutic siRNAs and receptor-binding RNA aptamers into RNA NPs and delivered various therapeutic molecules to cancer cells.144 They then demonstrated that the three-way junction (3WJ) region extracted from pRNA could serve as a platform for constructing trivalent RNA NPs capable of delivering therapeutics.145,146 In addition to pRNA, numerous naturally occurring RNA motifs, for example, kissing hairpin loops, paranemic motifs, kink-turns, C-loops, multi-helix junctions, protein binding motifs, and loop/loop-receptor pairs, can be employed toward generating RNA triangles, squares, nanorings, nanocubes, nanocages, and other various 3D nano-scaffolds.144,147, 148, 149, 150, 151 Some of these nanostructures have been used for cellular applications through the attachment of functional molecules on the RNA structures. Afonin and colleagues152 designed a series of interdependent complementary NANPs. By developing computational algorithms, NP interactions were accurately predicted and their re-association process was traced. Taking advantages of the dynamic interaction and conformational changes of NANPs, the system was able to activate multiple functionalities including transcription, Förster resonance energy transfer (FRET), aptamers, and specific gene silencing. For prediction of RNA structures and hybrid interactions of RNA with other molecules, computational tools such as Nano-Tiler, RNA2D3D, HyperFold, NUPACK, and so on have been widely used,153, 154, 155, 156, 157, 158 which speed up the applications of RNA nanotechnology for biomedical purpose, tissue engineering, biosensing, resistive biomemory, and potential computer logic gate modules.
NA-Based Logic Gate Nanomachine and Molecular Programming
NA-based logic devices were first reported in 1994 by Adleman159 to address a computational problem related to the famous “traveling salesman” problem with DNA and enzymes. In recent years, by introducing input modules (e.g., NAs, ions, proteins) and computational modules (readable signals), many DNA/RNA-based logic systems were created.152,155,160,161 Promising applications for autonomous logic gates rested in the development of smart biosensors that could be used for diagnosing disease and mimicking neural networks and memory systems.162,163 For example, Saito and colleagues160 designed several triangle RNA-protein complex nanostructures in vitro using dsRNA motifs. In a remarkable study, they designed protein-responsive RNA nanodevices that include RNA and a small RNA binding protein (RNP). The specific RNA and RNP interactions induced both structural and functional changes in the RNA nanodevices, which further produced a signal conversation between input protein signal and output RNA (aptamer) and protein signals. Such RNA nanodevices could serve as platforms to construct molecular robots that regulate their structures and produce desired functions by sensing cellular signals. Although there has been much successful evidence about NA-based logic devices during the last 10 years, many challenges must be overcome before the practical applications can be realized. The incompatibility of input (for example, small molecules) and output signals (for example, fluorescence),164 the lack of signal amplification and restoration modules,165,166 and slow reaction kinetics between NA167,168 and other molecules are critical issues in the future development of next-generation NA-based logic nanodevices.
Stimuli-Responsive Nanocarriers
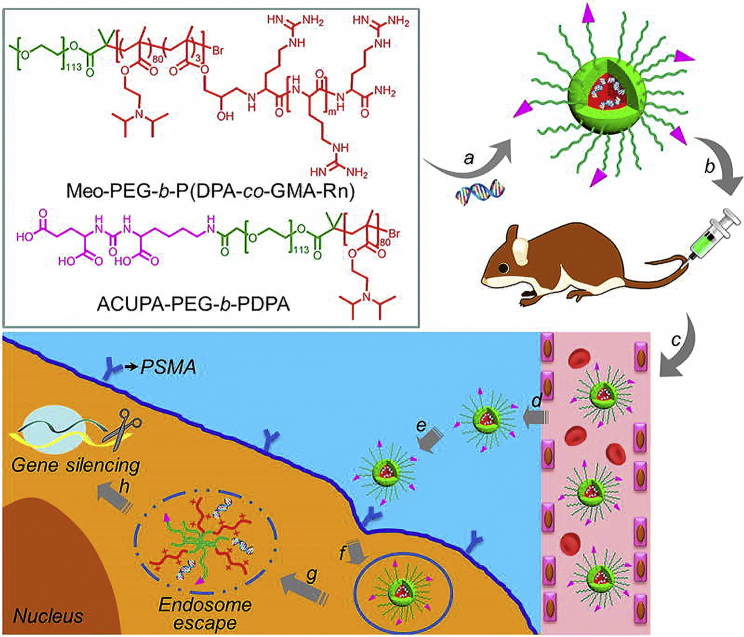
A stimuli-responsive carrier is referred to a delivery system that recognizes and responds dynamically to the microenvironment in various ways, including protonation, hydrolysis, and conformational change.169 The concept of stimuli-responsive drug delivery first appeared in the late 1970s.170 Since then, much attention has been focused on stimuli-responsive materials for drug/gene delivery. By taking advantages of various endogenous stimuli, including variations in pH, redox potential, and the concentrations of enzymes, various pH-, redox- and enzyme-responsive NA delivery systems were developed. Consider the pH-responsive system as an example. The pH variation is one of the most common exploited stimuli to design stimuli-sensitive nanocarriers for NA delivery. The pH values of healthy tissues (~7.4) and the extracellular environment of solid tumors (6.5–7.2) are slightly different, and the pH values of lysosomes (4–5) or endosomes (5–6) are much lower than those of cytoplasm.169 For pH-sensitive nanocarriers, they are constructed to store and stabilize their payloads at physiological pH (~7.4) and rapidly release their payloads when the environmental pH trigger point is reached. Based on this mechanism, researchers applied polymer materials containing ionized groups or acid-labile chemical bonds, as well as pH-sensitive fusogenic peptides to build pH-sensitive nanocarriers, for targeted delivery of NAs in lysosome or tumor sites (Figure 3).171, 172, 173 In addition to pH changes, the microenvironment of some pathological tissues (e.g., tumor) is accompanied by changes in glutathione, enzyme (such as metalloproteinase) concentration, and others, making it possible to design a novel redox-sensitive system174 and enzyme-sensitive system175 for controlled delivery of NAs.
Figure 3.
Structure of a pH-Sensitive Nanoformulation and the Schematic Illustration of In Vivo Prostate Cancer-Targeted RNAi Therapy
Reproduced from Xu et al.173 with permission.
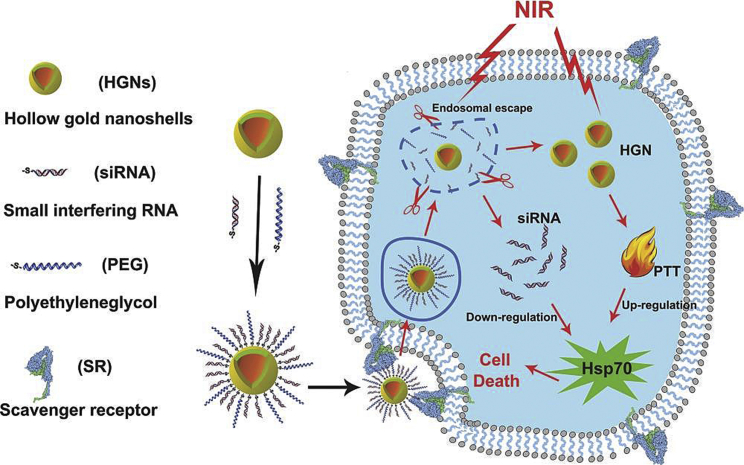
The most widely applied exogenous stimuli include temperature changes, light, and magnetic fields. Exogenous stimuli-responsive systems are designed according to one or more of those exogenous stimuli.176, 177, 178 Wang et al.179 prepared a versatile photothermal gold nanoshell densely packed with heat shock protein 70 (Hsp70) siRNA for cancer therapy. Upon near-infrared light irradiation, siRNAs were released from the gold nanoshell and successfully escaped from endosomes. Meanwhile, due to the high photothermal efficiency of gold, the tumor site temperature increases, which results in hyperthermia therapy in mice under mild temperature. The schematic illustration of this nanoshell is shown in Figure 4.
Figure 4.
A Light-Responsive Nanostructure for Efficient siRNA Delivery and Sensitizing Photothermal Therapy
Reproduced from Wang et al.179 with permission.
Because of the complexity of the biological environment, sometimes it is necessary to design a nanosystem that responds to both internal and external stimuli, e.g., a pH- and light-responsive system and a pH- and magnetic-responsive system. The combination of internal and external stimuli responses will integrate multiple factors to achieve synergistic therapy. Yang et al.180 developed dual-stimuli-responsive NPs containing light- and pH-responsive polypeptides. Under near-infrared light irradiation, the NPs responded to both laser irradiation and the acidic environment of the tumor site, thus selectively accumulating in the tumor site, and they were taken up by tumor cells. With the extensive exploration of this field, there will be more optimized stimuli-responsive nanosystems with high sensitivity and rapid response to both endogenous and exogenous stimuli for NA delivery in the future.
Exosomes and Nanocells
Other notable examples of advanced nanoscale biotechnology application in the pharmaceutical industry are exosomes and bacterial membrane-like nanocells. Exosomes are a kind of endogenous NPs with a diameter of 30–150 nm that are released by most cells in the body or isolated from extracellular fluids, such as blood, urine, saliva, cerebrospinal fluid, and others.181 Exosomes contain a large amount of proteins and other active substances found in the parent cell, which play an important role in cell communications. Differing from liposomes and other nanomaterials, the parent cell-derived transmembrane proteins and membrane-anchored proteins inside exosomes promote cellular uptake and achieve directed release of their internal contents.182 As vehicles derived from organisms, exosomes are safe, avoiding the fate of some synthetic nanomaterials being cleared soon.183 Hence, using exosomes to deliver NAs, proteins, and other molecules for therapeutic purposes is experiencing an explosion of research, particularly for cancer therapy. In recent research, exosomes derived from normal fibroblast-like mesenchymal cells were engineered to carry short hairpin RNA or siRNA against the mutation KrasG12D of pancreatic cancer. Researchers found that these engineered exosomes suppressed cancer growth in multiple mouse models of pancreatic cancer.184 In addition, exosomes also show great potential in delivering larger NAs. Endogenous exosomes or exosome-liposome hybrid NPs could even encapsulate plasmid CRISPR-Cas9 and Cas proteins, and they showed positive results in in vivo gene editing.185,186 Beyond the above advantages, several issues remain to be addressed with exosomes. Some of the exosome physiological functions and their relationship with pathological outcomes are still unclear. The isolation of exosomes is still time-consuming and non-replicable, making it difficult to get duplicate results from different laboratories.
EnGeneIC Ltd. (Sydney, NSW, Australia) developed a nanocell/minicell platform called EnGeneIC Dream Vector (EDV).187,188 It is bacterially derived nanocells/minicells formed by genetically modified bacteria when they divide at their pole and center. EDV contains nonviable nanocells/minicells with a diameter around 400 nm, and it is used to load chemotherapeutic drugs, siRNA, or miRNA. Recently, a clinical trial evaluated the safety, dosage, and activity of minicell-loaded mimic miRNA. Results showed that minicells were safe and could support further studies on minicells combined with chemotherapy or immune checkpoint inhibitors.187
Challenges
Nanotoxicity
A main challenge in the development of nanoscale biotechnology-assisted NAs therapy is nonspecific toxicity of nanocarriers. Although nanocarriers enhance the stability of NAs and improve their transfection efficiency, the toxicity caused by excipient nanomaterials still exists and remains the primary issue. On the one hand, due to the exposure of nanomaterials and the dramatic increase of surface area, nanomaterials are more reactive, which may lead to extensive interactions between biological systems and cause damage to cells.189 Some nanomaterials used for NA delivery may exist in the human body for a long time and induce chronic toxicity. On the other hand, some nanocarriers with complex structures may bring unexpected toxicity. To maximize the NA delivery efficiency, nanocarriers are often designed as multi-modified, smart systems. Each additional modification means an extra risk of toxicity. For example, the cationic functional groups obtain better encapsulation of negatively charged NAs; however, their positive charges also cause cell damage due to electrostatic interaction with the negatively charged cell surface.190 Although nanocarriers may be associated with toxicity, many strategies are used to eliminate such unfavorable factors. For example, chemical modification191 and pretreatment with anti-allergic drugs before dosing nanoformulation192 are beneficial.
In addition, since many NANPs are administered intravenously, their immunomodulatory effects are largely unknown and must be defined before translating into the clinic. Recent studies revealed that the immunological recognition of NANPs relies on multiple characteristics, including size, shape, sequence, 3D structure, composition, and connectivity.193,194 The unmethylated cytosine-phosphate-guanosine (CpG) oligodeoxynucleotides (ODNs) is known as an immunostimulant.195, 196, 197 The size and shape have been reported to play vital roles in pharmacokinetic profile and biodistribution of NPs. Compared with RNA monomers, locally administered compact RNA globular structures with intramolecular connectivity induced more interferon (IFN) production.194 Larger size RNA square, 3D RNA tetrahedron showed a stronger immune response than did smaller, planar RNA NPs.198 The surface characteristics of NPs, for example, charge, and hydrophobicity influence protein absorption of NPs. When immunoglobulin G (IgG), laminin, or complement proteins are adsorbed on the surface of NPs, specific uptake by immune cells is triggered, which may lead to immune stimulation response.199 Studies showed that NPs first entered plasmacytoid-like dendritic cells through scavenger receptor-mediated endocytosis, and then the endosomal Toll-like receptors initiated IFN production, thereby generating an immunostimulatory response.193,200 In addition to the intrinsic characters of NANPs, delivery systems may play an important role in immunological activity. One study showed that nanocomplexes formed by NAs and delivery systems exhibited immunological activity regardless of their shape, size, composition, and connectivity.193 This means that by adjusting the assembling of NAs and introducing nanoscaffolds, the desirable immunostimulatory effects or undesirable immunostimulation could be fine-tuned.196,197 Taken together, since NANPs can be designed and programmed to exhibit no, low, or high immunostimulation, their application should not be limited by immunotoxicity.
Manufacture and Quality Control
Other problems that may be addressed are related to the manufacture and quality control of NA nanoformulations. Although a large number of nanocarriers have been obtained in the laboratory, which have the functions of endosomal escape, cell targeting, and controlled drug release and are stimuli-responsive, their clinical translation is not so welcome. Sophisticated designs often complicate the potential manufacturing processes, let alone scale-up production and precise quality control. The key strategy for the development of multifunctional nanoformulations is to consider operability, scalability, as well as controllability. In addition, further efforts are needed to develop standardized manufacturing processes to yield nanoformulations of consistent quality and quantity, suitable for stabilized loading of NAs.
Conclusions and Prospects
Nonviral delivery systems have kept the attention of researchers for many years. Numerous nonviral delivery systems have been exploited to deliver NAs by altering their physicochemical properties or using their own nanobiology properties. Some have even shown ideal delivery efficiency clinically, e.g., liposomes, stable NA lipid particles, cyclodextrin-based RONEDL technology, EDV nanocell platform, and others. Besides nanomaterials, particles of biological origin such as exosomes derived from various cells also possess the potential to develop optimally efficient and biocompatible NA delivery systems. Despite all of the advantages, nanoscale biotechnology-based nanocarriers also face nanotoxicity from the materials and manufacturing issues. Such crucial issues need urgently to be addressed in NA therapy. The mechanisms of NPs that interact with biological circumstances (e.g., proteins, immune cells) are important subjects for future study. Furthermore, additional efforts also need to be made to develop specific quality control processes to yield uniform NPs with consistent NA loading efficiency and quantity.
In summary, this review illustrates various types of nanoscale biotechnology-based delivery systems, and it demonstrates the developments and challenges in delivery of NAs.
Diverse techniques currently provide a large number of options, more or less in line with the requirements of broad application. Nevertheless, further efforts are needed to better understand the nanoscale properties and nanoformulations.
Author Contributions
Y.W. and Q.H. wrote the paper. C.L. and X.W. contributed to summarize clinical advances of nucleic acid therapeutics. Y.Y. and J.Y. provided insightful discussions and comments on the manuscript. X.-J.L. and Y.H. revised the manuscript and led the project.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31871003, 31901053); the Fundamental Research Funds for the Central Universities, China; Beijing Institute of Technology Research Fund Program for Young Scholars, China; the Hunan Provincial Natural Science Foundation of China (2018JJ1019, 2019JJ50196); the Hu-Xiang Young Talent Program (2018RS3094), and the Postdoctoral Science Foundation of China (2018M630085).
Contributor Information
Yuanyu Huang, Email: yyhuang@bit.edu.cn.
Xing-Jie Liang, Email: liangxj@nanoctr.cn.
References
- 1.Sharfstein S.T. Non-protein biologic therapeutics. Curr. Opin. Biotechnol. 2018;53:65–75. doi: 10.1016/j.copbio.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Vabret N., Bhardwaj N., Greenbaum B.D. Sequence-specific sensing of nucleic acids. Trends Immunol. 2017;38:53–65. doi: 10.1016/j.it.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephenson M.L., Zamecnik P.C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc. Natl. Acad. Sci. USA. 1978;75:285–288. doi: 10.1073/pnas.75.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W.W., Li L., Li D., Liu J., Li X., Li W., Xu X., Zhang M.J., Chandler L.A., Lin H. The first approved gene therapy product for cancer Ad-p53 (Gendicine): 12 years in the clinic. Hum. Gene Ther. 2018;29:160–179. doi: 10.1089/hum.2017.218. [DOI] [PubMed] [Google Scholar]
- 5.Hoy S.M. Patisiran: first global approval. Drugs. 2018;78:1625–1631. doi: 10.1007/s40265-018-0983-6. [DOI] [PubMed] [Google Scholar]
- 6.Nellimarla S., Mossman K.L. Extracellular dsRNA: its function and mechanism of cellular uptake. J. Interferon Cytokine Res. 2014;34:419–426. doi: 10.1089/jir.2014.0002. [DOI] [PubMed] [Google Scholar]
- 7.Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Q., Wei T., Jia Y., Farbiak L., Zhou K., Zhang S., Wei Y., Zhu H., Siegwart D.J. Dendrimer-based lipid nanoparticles deliver therapeutic FAH mRNA to normalize liver function and extend survival in a mouse model of hepatorenal tyrosinemia type I. Adv. Mater. 2018;30:e1805308. doi: 10.1002/adma.201805308. [DOI] [PubMed] [Google Scholar]
- 9.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gan L.M., Lagerström-Fermér M., Carlsson L.G., Arfvidsson C., Egnell A.C., Rudvik A., Kjaer M., Collén A., Thompson J.D., Joyal J. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat. Commun. 2019;10:871. doi: 10.1038/s41467-019-08852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang R., Men K., Zhang X., Huang R., Tian Y., Zhou B., Yu C., Wang Y., Ji X., Hu Q., Yang L. Delivery of a modified mRNA encoding IL-22 binding protein (IL-22BP) for colon cancer gene therapy. J. Biomed. Nanotechnol. 2018;14:1239–1251. doi: 10.1166/jbn.2018.2577. [DOI] [PubMed] [Google Scholar]
- 12.Bobbin M.L., Rossi J.J. RNA interference (RNAi)-based therapeutics: delivering on the promise? Annu. Rev. Pharmacol. Toxicol. 2016;56:103–122. doi: 10.1146/annurev-pharmtox-010715-103633. [DOI] [PubMed] [Google Scholar]
- 13.Cullen B.R. RNA interference in mammals: the virus strikes back. Immunity. 2017;46:970–972. doi: 10.1016/j.immuni.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Ritchie W. MicroRNA target prediction. Methods Mol. Biol. 2017;1513:193–200. doi: 10.1007/978-1-4939-6539-7_13. [DOI] [PubMed] [Google Scholar]
- 15.Yang J.X., Rastetter R.H., Wilhelm D. Non-coding RNAs: an introduction. Adv. Exp. Med. Biol. 2016;886:13–32. doi: 10.1007/978-94-017-7417-8_2. [DOI] [PubMed] [Google Scholar]
- 16.Gorski S.A., Vogel J., Doudna J.A. RNA-based recognition and targeting: sowing the seeds of specificity. Nat. Rev. Mol. Cell Biol. 2017;18:215–228. doi: 10.1038/nrm.2016.174. [DOI] [PubMed] [Google Scholar]
- 17.Androsavich J.R. Assessing anti-miR pharmacology with miRNA polysome shift assay. Methods Mol. Biol. 2017;1517:103–113. doi: 10.1007/978-1-4939-6563-2_7. [DOI] [PubMed] [Google Scholar]
- 18.Bennett C.F., Baker B.F., Pham N., Swayze E., Geary R.S. Pharmacology of antisense drugs. Annu. Rev. Pharmacol. Toxicol. 2017;57:81–105. doi: 10.1146/annurev-pharmtox-010716-104846. [DOI] [PubMed] [Google Scholar]
- 19.Castanotto D., Stein C.A. Antisense oligonucleotides in cancer. Curr. Opin. Oncol. 2014;26:584–589. doi: 10.1097/CCO.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 20.Geary R.S. Antisense oligonucleotide pharmacokinetics and metabolism. Expert Opin. Drug Metab. Toxicol. 2009;5:381–391. doi: 10.1517/17425250902877680. [DOI] [PubMed] [Google Scholar]
- 21.Kirchner M., Schneider S. CRISPR-Cas: from the bacterial adaptive immune system to a versatile tool for genome engineering. Angew. Chem. Int. Ed. Engl. 2015;54:13508–13514. doi: 10.1002/anie.201504741. [DOI] [PubMed] [Google Scholar]
- 22.Barrangou R. The roles of CRISPR-Cas systems in adaptive immunity and beyond. Curr. Opin. Immunol. 2015;32:36–41. doi: 10.1016/j.coi.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Klompe S.E., Vo P.L.H., Halpin-Healy T.S., Sternberg S.H. Transposon-encoded CRISPR-Cas systems direct RNA-guided DNA integration. Nature. 2019;571:219–225. doi: 10.1038/s41586-019-1323-z. [DOI] [PubMed] [Google Scholar]
- 24.Wang J., DeClercq J.J., Hayward S.B., Li P.W., Shivak D.A., Gregory P.D., Lee G., Holmes M.C. Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery. Nucleic Acids Res. 2016;44:e30. doi: 10.1093/nar/gkv1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller J.B., Zhang S., Kos P., Xiong H., Zhou K., Perelman S.S., Zhu H., Siegwart D.J. Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew. Chem. Int. Ed. Engl. 2017;56:1059–1063. doi: 10.1002/anie.201610209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J., Chang J., Jiang Y., Meng X., Sun T., Mao L., Xu Q., Wang M. Fast and efficient CRISPR/Cas9 genome editing in vivo enabled by bioreducible lipid and messenger RNA nanoparticles. Adv. Mater. 2019;31:e1902575. doi: 10.1002/adma.201902575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Usman W.M., Pham T.C., Kwok Y.Y., Vu L.T., Ma V., Peng B., Chan Y.S., Wei L., Chin S.M., Azad A. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018;9:2359. doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nimjee S.M., White R.R., Becker R.C., Sullenger B.A. Aptamers as therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017;57:61–79. doi: 10.1146/annurev-pharmtox-010716-104558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y.X., Kwon Y.J. Aptamers: the “evolution” of SELEX. Methods. 2016;106:21–28. doi: 10.1016/j.ymeth.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Rozenblum G.T., Lopez V.G., Vitullo A.D., Radrizzani M. Aptamers: current challenges and future prospects. Expert Opin. Drug Discov. 2016;11:127–135. doi: 10.1517/17460441.2016.1126244. [DOI] [PubMed] [Google Scholar]
- 31.Ren A., Micura R., Patel D.J. Structure-based mechanistic insights into catalysis by small self-cleaving ribozymes. Curr. Opin. Chem. Biol. 2017;41:71–83. doi: 10.1016/j.cbpa.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott W.G., Horan L.H., Martick M. The hammerhead ribozyme: structure, catalysis, and gene regulation. Prog. Mol. Biol. Transl. Sci. 2013;120:1–23. doi: 10.1016/B978-0-12-381286-5.00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jimenez R.M., Polanco J.A., Lupták A. Chemistry and biology of self-cleaving ribozymes. Trends Biochem. Sci. 2015;40:648–661. doi: 10.1016/j.tibs.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scarborough R.J., Gatignol A. HIV and ribozymes. Adv. Exp. Med. Biol. 2015;848:97–116. doi: 10.1007/978-1-4939-2432-5_5. [DOI] [PubMed] [Google Scholar]
- 35.Zhou W., Ding J., Liu J. Theranostic DNAzymes. Theranostics. 2017;7:1010–1025. doi: 10.7150/thno.17736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong L., Zhao Z., Lv Y.F., Huan S.Y., Fu T., Zhang X.B., Shen G.L., Yu R.Q. DNAzyme-based biosensors and nanodevices. Chem. Commun. (Camb.) 2015;51:979–995. doi: 10.1039/c4cc06855f. [DOI] [PubMed] [Google Scholar]
- 37.Tang W., Hu J.H., Liu D.R. Aptazyme-embedded guide RNAs enable ligand-responsive genome editing and transcriptional activation. Nat. Commun. 2017;8:15939. doi: 10.1038/ncomms15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geary R.S., Khatsenko O., Bunker K., Crooke R., Moore M., Burckin T., Truong L., Sasmor H., Levin A.A. Absolute bioavailability of 2′-O-(2-methoxyethyl)-modified antisense oligonucleotides following intraduodenal instillation in rats. J. Pharmacol. Exp. Ther. 2001;296:898–904. [PubMed] [Google Scholar]
- 39.Yu R.Z., Kim T.W., Hong A., Watanabe T.A., Gaus H.J., Geary R.S. Cross-species pharmacokinetic comparison from mouse to man of a second-generation antisense oligonucleotide, ISIS 301012, targeting human apolipoprotein B-100. Drug Metab. Dispos. 2007;35:460–468. doi: 10.1124/dmd.106.012401. [DOI] [PubMed] [Google Scholar]
- 40.Geary R.S., Yu R.Z., Watanabe T., Henry S.P., Hardee G.E., Chappell A., Matson J., Sasmor H., Cummins L., Levin A.A. Pharmacokinetics of a tumor necrosis factor-α phosphorothioate 2′-O-(2-methoxyethyl) modified antisense oligonucleotide: comparison across species. Drug Metab. Dispos. 2003;31:1419–1428. doi: 10.1124/dmd.31.11.1419. [DOI] [PubMed] [Google Scholar]
- 41.Elmén J., Lindow M., Schütz S., Lawrence M., Petri A., Obad S., Lindholm M., Hedtjärn M., Hansen H.F., Berger U. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 42.Elmén J., Lindow M., Silahtaroglu A., Bak M., Christensen M., Lind-Thomsen A., Hedtjärn M., Hansen J.B., Hansen H.F., Straarup E.M. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008;36:1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakraborty C., Sharma A.R., Sharma G., Doss C.G.P., Lee S.S. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol. Ther. Nucleic Acids. 2017;8:132–143. doi: 10.1016/j.omtn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaus H.J., Owens S.R., Winniman M., Cooper S., Cummins L.L. On-line HPLC electrospray mass spectrometry of phosphorothioate oligonucleotide metabolites. Anal. Chem. 1997;69:313–319. doi: 10.1021/ac960557q. [DOI] [PubMed] [Google Scholar]
- 45.Griffey R.H., Greig M.J., Gaus H.J., Liu K., Monteith D., Winniman M., Cummins L.L. Characterization of oligonucleotide metabolism in vivo via liquid chromatography/electrospray tandem mass spectrometry with a quadrupole ion trap mass spectrometer. J. Mass Spectrom. 1997;32:305–313. doi: 10.1002/(SICI)1096-9888(199703)32:3<305::AID-JMS482>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Yu R.Z., Henry S., Geary R.S. Pharmacokinetics and clinical pharmacology considerations of GalNAc3-conjugated antisense oligonucleotides. Expert Opin. Drug Metab. Toxicol. 2019;15:475–485. doi: 10.1080/17425255.2019.1621838. [DOI] [PubMed] [Google Scholar]
- 47.Dirin M., Winkler J. Influence of diverse chemical modifications on the ADME characteristics and toxicology of antisense oligonucleotides. Expert Opin. Biol. Ther. 2013;13:875–888. doi: 10.1517/14712598.2013.774366. [DOI] [PubMed] [Google Scholar]
- 48.Darrow J.J. Luxturna: FDA documents reveal the value of a costly gene therapy. Drug Discov. Today. 2019;24:949–954. doi: 10.1016/j.drudis.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Adams D., Gonzalez-Duarte A., O’Riordan W.D., Yang C.C., Ueda M., Kristen A.V., Tournev I., Schmidt H.H., Coelho T., Berk J.L. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 50.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 51.Kanasty R., Dorkin J.R., Vegas A., Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 52.Dowdy S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017;35:222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]
- 53.Pastor F., Berraondo P., Etxeberria I., Frederick J., Sahin U., Gilboa E., Melero I. An RNA toolbox for cancer immunotherapy. Nat. Rev. Drug Discov. 2018;17:751–767. doi: 10.1038/nrd.2018.132. [DOI] [PubMed] [Google Scholar]
- 54.Xiao Y., Shi K., Qu Y., Chu B., Qian Z. Engineering nanoparticles for targeted delivery of nucleic acid therapeutics in tumor. Mol. Ther. Methods Clin. Dev. 2018;12:1–18. doi: 10.1016/j.omtm.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosi N.L., Giljohann D.A., Thaxton C.S., Lytton-Jean A.K., Han M.S., Mirkin C.A. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312:1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 56.Yang H., Li Y., Li T., Xu M., Chen Y., Wu C., Dang X., Liu Y. Multifunctional core/shell nanoparticles cross-linked polyetherimide-folic acid as efficient Notch-1 siRNA carrier for targeted killing of breast cancer. Sci. Rep. 2014;4:7072. doi: 10.1038/srep07072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huo S., Jin S., Ma X., Xue X., Yang K., Kumar A., Wang P.C., Zhang J., Hu Z., Liang X.J. Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano. 2014;8:5852–5862. doi: 10.1021/nn5008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Lépinay L.M., Pigeau B., Besga B., Vincent P., Poncharal P., Arcizet O. A universal and ultrasensitive vectorial nanomechanical sensor for imaging 2D force fields. Nat. Nanotechnol. 2017;12:156–162. doi: 10.1038/nnano.2016.193. [DOI] [PubMed] [Google Scholar]
- 59.Ginn S.L., Amaya A.K., Alexander I.E., Edelstein M., Abedi M.R. Gene therapy clinical trials worldwide to 2017: an update. J. Gene Med. 2018;20:e3015. doi: 10.1002/jgm.3015. [DOI] [PubMed] [Google Scholar]
- 60.Senapati S., Mahanta A.K., Kumar S., Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018;3:7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 62.Rosenblum D., Joshi N., Tao W., Karp J.M., Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018;9:1410. doi: 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 64.Essex S., Navarro G., Sabhachandani P., Chordia A., Trivedi M., Movassaghian S., Torchilin V.P. Phospholipid-modified PEI-based nanocarriers for in vivo siRNA therapeutics against multidrug-resistant tumors. Gene Ther. 2015;22:257–266. doi: 10.1038/gt.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zuckerman J.E., Davis M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015;14:843–856. doi: 10.1038/nrd4685. [DOI] [PubMed] [Google Scholar]
- 66.Rietwyk S., Peer D. Next-generation lipids in RNA interference therapeutics. ACS Nano. 2017;11:7572–7586. doi: 10.1021/acsnano.7b04734. [DOI] [PubMed] [Google Scholar]
- 67.Jensen S.A., Day E.S., Ko C.H., Hurley L.A., Luciano J.P., Kouri F.M., Merkel T.J., Luthi A.J., Patel P.C., Cutler J.I. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl. Med. 2013;5:209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lincoff A.M., Mehran R., Povsic T.J., Zelenkofske S.L., Huang Z., Armstrong P.W., Steg P.G., Bode C., Cohen M.G., Buller C., REGULATE-PCI Investigators Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention (REGULATE-PCI): a randomised clinical trial. Lancet. 2016;387:349–356. doi: 10.1016/S0140-6736(15)00515-2. [DOI] [PubMed] [Google Scholar]
- 69.Teng L., Xie J., Teng L., Lee R.J. Clinical translation of folate receptor-targeted therapeutics. Expert Opin. Drug Deliv. 2012;9:901–908. doi: 10.1517/17425247.2012.694863. [DOI] [PubMed] [Google Scholar]
- 70.Cui D., Zhang C., Liu B., Shu Y., Du T., Shu D., Wang K., Dai F., Liu Y., Li C. Regression of gastric cancer by systemic injection of RNA nanoparticles carrying both ligand and siRNA. Sci. Rep. 2015;5:10726. doi: 10.1038/srep10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Erel-Akbaba G., Carvalho L.A., Tian T., Zinter M., Akbaba H., Obeid P.J., Chiocca E.A., Weissleder R., Kantarci A.G., Tannous B.A. Radiation-induced targeted nanoparticle-based gene delivery for brain tumor therapy. ACS Nano. 2019;13:4028–4040. doi: 10.1021/acsnano.8b08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou J., Patel T.R., Fu M., Bertram J.P., Saltzman W.M. Octa-functional PLGA nanoparticles for targeted and efficient siRNA delivery to tumors. Biomaterials. 2012;33:583–591. doi: 10.1016/j.biomaterials.2011.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McNamara M.A., Nair S.K., Holl E.K. RNA-based vaccines in cancer immunotherapy. J. Immunol. Res. 2015;2015:794528. doi: 10.1155/2015/794528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fahrenkrog B., Aebi U. The nuclear pore complex: nucleocytoplasmic transport and beyond. Nat. Rev. Mol. Cell Biol. 2003;4:757–766. doi: 10.1038/nrm1230. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Y., Wang W., Guo S., Wang Y., Miao L., Xiong Y., Huang L. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat. Commun. 2016;7:11822. doi: 10.1038/ncomms11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maas S.L.N., Breakefield X.O., Weaver A.M. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27:172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lopez-Bertoni H., Kozielski K.L., Rui Y., Lal B., Vaughan H., Wilson D.R., Mihelson N., Eberhart C.G., Laterra J., Green J.J. Bioreducible polymeric nanoparticles containing multiplexed cancer stem cell regulating miRNAs inhibit glioblastoma growth and prolong survival. Nano Lett. 2018;18:4086–4094. doi: 10.1021/acs.nanolett.8b00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang M., Liu H., Li L., Cheng Y. A fluorinated dendrimer achieves excellent gene transfection efficacy at extremely low nitrogen to phosphorus ratios. Nat. Commun. 2014;5:3053. doi: 10.1038/ncomms4053. [DOI] [PubMed] [Google Scholar]
- 79.Wang H., Wang Y., Wang Y., Hu J., Li T., Liu H., Zhang Q., Cheng Y. Self-assembled fluorodendrimers combine the features of lipid and polymeric vectors in gene delivery. Angew. Chem. Int. Ed. Engl. 2015;54:11647–11651. doi: 10.1002/anie.201501461. [DOI] [PubMed] [Google Scholar]
- 80.Zhang T., Huang Y., Ma X., Gong N., Liu X., Liu L., Ye X., Hu B., Li C., Tian J.H. Fluorinated oligoethylenimine nanoassemblies for efficient siRNA-mediated gene silencing in serum-containing media by effective endosomal escape. Nano Lett. 2018;18:6301–6311. doi: 10.1021/acs.nanolett.8b02553. [DOI] [PubMed] [Google Scholar]
- 81.Yu M., Xue Y., Ma P.X., Mao C., Lei B. Intrinsic ultrahigh drug/miRNA loading capacity of biodegradable bioactive glass nanoparticles toward highly efficient pharmaceutical delivery. ACS Appl. Mater. Interfaces. 2017;9:8460–8470. doi: 10.1021/acsami.6b13874. [DOI] [PubMed] [Google Scholar]
- 82.Wang L.H., Wu D.C., Xu H.X., You Y.Z. High DNA-binding affinity and gene-transfection efficacy of bioreducible cationic nanomicelles with a fluorinated core. Angew. Chem. Int. Ed. Engl. 2016;55:755–759. doi: 10.1002/anie.201508695. [DOI] [PubMed] [Google Scholar]
- 83.Jia Y., Niu D., Li Q., Huang H., Li X., Li K., Li L., Zhang C., Zheng H., Zhu Z. Effective gene delivery of shBMP-9 using polyethyleneimine-based core-shell nanoparticles in an animal model of insulin resistance. Nanoscale. 2019;11:2008–2016. doi: 10.1039/c8nr08193j. [DOI] [PubMed] [Google Scholar]
- 84.Tian M., Zhou Z., Tan S., Fan X., Li L., Ullah N. Formulation in DDA-MPLA-TDB liposome enhances the immunogenicity and protective efficacy of a DNA vaccine against Mycobacterium tuberculosis infection. Front. Immunol. 2018;9:310. doi: 10.3389/fimmu.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arnon S., Dahan N., Koren A., Radiano O., Ronen M., Yannay T., Giron J., Ben-Ami L., Amir Y., Hel-Or Y. Thought-controlled nanoscale robots in a living host. PLoS ONE. 2016;11:e0161227. doi: 10.1371/journal.pone.0161227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deng W., Chen W., Clement S., Guller A., Zhao Z., Engel A., Goldys E.M. Controlled gene and drug release from a liposomal delivery platform triggered by X-ray radiation. Nat. Commun. 2018;9:2713. doi: 10.1038/s41467-018-05118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fraley R., Subramani S., Berg P., Papahadjopoulos D. Introduction of liposome-encapsulated SV40 DNA into cells. J. Biol. Chem. 1980;255:10431–10435. [PubMed] [Google Scholar]
- 88.Dong Y., Love K.T., Dorkin J.R., Sirirungruang S., Zhang Y., Chen D., Bogorad R.L., Yin H., Chen Y., Vegas A.J. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc. Natl. Acad. Sci. USA. 2014;111:3955–3960. doi: 10.1073/pnas.1322937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., Cho C.K., Sah D.W., Stebbing D., Crosley E.J., Yaworski E. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 90.Maier M.A., Jayaraman M., Matsuda S., Liu J., Barros S., Querbes W., Tam Y.K., Ansell S.M., Kumar V., Qin J. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013;21:1570–1578. doi: 10.1038/mt.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Love K.T., Mahon K.P., Levins C.G., Whitehead K.A., Querbes W., Dorkin J.R., Qin J., Cantley W., Qin L.L., Racie T. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl. Acad. Sci. USA. 2010;107:1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Brien P.J., Elahipanah S., Rogozhnikov D., Yousaf M.N. Bio-orthogonal mediated nucleic acid transfection of cells via cell surface engineering. ACS Cent. Sci. 2017;3:489–500. doi: 10.1021/acscentsci.7b00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li L., Zahner D., Su Y., Gruen C., Davidson G., Levkin P.A. A biomimetic lipid library for gene delivery through thiol-yne click chemistry. Biomaterials. 2012;33:8160–8166. doi: 10.1016/j.biomaterials.2012.07.044. [DOI] [PubMed] [Google Scholar]
- 94.Li Y., Liu R., Shi Y., Zhang Z., Zhang X. Zwitterionic poly(carboxybetaine)-based cationic liposomes for effective delivery of small interfering RNA therapeutics without accelerated blood clearance phenomenon. Theranostics. 2015;5:583–596. doi: 10.7150/thno.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ortiz Mellet C., García Fernández J.M., Benito J.M. Cyclodextrin-based gene delivery systems. Chem. Soc. Rev. 2011;40:1586–1608. doi: 10.1039/c0cs00019a. [DOI] [PubMed] [Google Scholar]
- 96.Davis M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 97.Barata P., Sood A.K., Hong D.S. RNA-targeted therapeutics in cancer clinical trials: current status and future directions. Cancer Treat. Rev. 2016;50:35–47. doi: 10.1016/j.ctrv.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 98.Fang J.K., Chen L., Lu X.G., Cao D., Guo L.L., Zhang Y.S., Li L.B., Zhang L.F., Kuang Y.T., Wang S.L. Optimization of transforming growth factor-β1 siRNA loaded chitosan-tripolyphosphate nanoparticles for the treatment of colorectal cancer hepatic metastasis in a mouse model. J. Biomed. Nanotechnol. 2016;12:1489–1500. doi: 10.1166/jbn.2016.2265. [DOI] [PubMed] [Google Scholar]
- 99.Sun P., Huang W., Jin M., Wang Q., Fan B., Kang L., Gao Z. Chitosan-based nanoparticles for survivin targeted siRNA delivery in breast tumor therapy and preventing its metastasis. Int. J. Nanomedicine. 2016;11:4931–4945. doi: 10.2147/IJN.S105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siahmansouri H., Somi M.H., Babaloo Z., Baradaran B., Jadidi-Niaragh F., Atyabi F., Mohammadi H., Ahmadi M., Yousefi M. Effects of HMGA2 siRNA and doxorubicin dual delivery by chitosan nanoparticles on cytotoxicity and gene expression of HT-29 colorectal cancer cell line. J. Pharm. Pharmacol. 2016;68:1119–1130. doi: 10.1111/jphp.12593. [DOI] [PubMed] [Google Scholar]
- 101.Park K., Yang J.A., Lee M.Y., Lee H., Hahn S.K. Reducible hyaluronic acid-siRNA conjugate for target specific gene silencing. Bioconjug. Chem. 2013;24:1201–1209. doi: 10.1021/bc4001257. [DOI] [PubMed] [Google Scholar]
- 102.Kim J.S., Oh M.H., Park J.Y., Park T.G., Nam Y.S. Protein-resistant, reductively dissociable polyplexes for in vivo systemic delivery and tumor-targeting of siRNA. Biomaterials. 2013;34:2370–2379. doi: 10.1016/j.biomaterials.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 103.Patel A.K., Kaczmarek J.C., Bose S., Kauffman K.J., Mir F., Heartlein M.W., DeRosa F., Langer R., Anderson D.G. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater. 2019;31:e1805116. doi: 10.1002/adma.201805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li J., He Y., Wang W., Wu C., Hong C., Hammond P.T. Polyamine-mediated stoichiometric assembly of ribonucleoproteins for enhanced mRNA delivery. Angew. Chem. Int. Ed. Engl. 2017;56:13709–13712. doi: 10.1002/anie.201707466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jarzębińska A., Pasewald T., Lambrecht J., Mykhaylyk O., Kümmerling L., Beck P., Hasenpusch G., Rudolph C., Plank C., Dohmen C. A single methylene group in oligoalkylamine-based cationic polymers and lipids promotes enhanced mRNA delivery. Angew. Chem. Int. Ed. Engl. 2016;55:9591–9595. doi: 10.1002/anie.201603648. [DOI] [PubMed] [Google Scholar]
- 106.Boussif O., Lezoualc’h F., Zanta M.A., Mergny M.D., Scherman D., Demeneix B., Behr J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang D., Wang M., Wang T., Zhang B., Liu C., Zhang N. Multifunctionalized polyethyleneimine-based nanocarriers for gene and chemotherapeutic drug combination therapy through one-step assembly strategy. Int. J. Nanomedicine. 2017;12:8681–8698. doi: 10.2147/IJN.S142966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu H.-H., Huang C.-H., Shiue T.-Y., Wang F.-S., Chang K.-K., Chen Y., Peng C.H. Highly efficient gene release in spatiotemporal precision approached by light and pH dual responsive copolymers. Chem. Sci. (Camb.) 2018;10:284–292. doi: 10.1039/c8sc01494a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng H., Wu Z., Wu C., Wang X., Liow S.S., Li Z., Wu Y.L. Overcoming STC2 mediated drug resistance through drug and gene co-delivery by PHB-PDMAEMA cationic polyester in liver cancer cells. Mater. Sci. Eng. C. 2018;83:210–217. doi: 10.1016/j.msec.2017.08.075. [DOI] [PubMed] [Google Scholar]
- 110.Cheng Y., Wei H., Tan J.K., Peeler D.J., Maris D.O., Sellers D.L., Horner P.J., Pun S.H. Nano-sized sunflower polycations as effective gene transfer vehicles. Small. 2016;12:2750–2758. doi: 10.1002/smll.201502930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Olden B.R., Cheng Y., Yu J.L., Pun S.H. Cationic polymers for non-viral gene delivery to human T cells. J. Control. Release. 2018;282:140–147. doi: 10.1016/j.jconrel.2018.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cheng C., Convertine A.J., Stayton P.S., Bryers J.D. Multifunctional triblock copolymers for intracellular messenger RNA delivery. Biomaterials. 2012;33:6868–6876. doi: 10.1016/j.biomaterials.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lyu Y., Cui D., Sun H., Miao Y., Duan H., Pu K. Dendronized semiconducting polymer as photothermal nanocarrier for remote activation of gene expression. Angew. Chem. Int. Ed. Engl. 2017;56:9155–9159. doi: 10.1002/anie.201705543. [DOI] [PubMed] [Google Scholar]
- 114.Singh Y., Meher J.G., Raval K., Khan F.A., Chaurasia M., Jain N.K., Chourasia M.K. Nanoemulsion: concepts, development and applications in drug delivery. J. Control. Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 115.Kihara Y., Ichikawa T., Abe S., Nemoto N., Ishihara T., Hirano N., Haruki M. Synthesis of alkyne-functionalized amphiphilic polysiloxane polymers and formation of nanoemulsions conjugated with bioactive molecules by click reactions. Polym. J. 2014;46:175–183. [Google Scholar]
- 116.Rai V.K., Mishra N., Yadav K.S., Yadav N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J. Control. Release. 2018;270:203–225. doi: 10.1016/j.jconrel.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 117.Chen G., Wang K., Wu P., Wang Y., Zhou Z., Yin L. Development of fluorinated polyplex nanoemulsions for improved small interfering RNA delivery and cancer therapy. Nano Res. 2018;11:3746–3761. [Google Scholar]
- 118.Mahmood A., Prüfert F., Efiana N.A., Ashraf M.I., Hermann M., Hussain S., Bernkop-Schnürch A. Cell-penetrating self-nanoemulsifying drug delivery systems (SNEDDS) for oral gene delivery. Expert Opin. Drug Deliv. 2016;13:1503–1512. doi: 10.1080/17425247.2016.1213236. [DOI] [PubMed] [Google Scholar]
- 119.Kim I.D., Shin J.H., Kim S.W., Choi S., Ahn J., Han P.L., Park J.S., Lee J.K. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol. Ther. 2012;20:829–839. doi: 10.1038/mt.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu C., Wan T., Wang H., Zhang S., Ping Y., Cheng Y. A boronic acid-rich dendrimer with robust and unprecedented efficiency for cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci. Adv. 2019;5:eaaw8922. doi: 10.1126/sciadv.aaw8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li X., Sun A., Liu Y., Zhang W., Pang N., Cheng S., Qi X. Amphiphilic dendrimer engineered nanocarrier systems for co-delivery of siRNA and paclitaxel to matrix metalloproteinase-rich tumors for synergistic therapy. NPG Asia Mater. 2018;10:238–254. [Google Scholar]
- 122.Wang H., Jiang Y., Peng H., Chen Y., Zhu P., Huang Y. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv. Drug Deliv. Rev. 2015;81:142–160. doi: 10.1016/j.addr.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 123.Navarro G., Pan J., Torchilin V.P. Micelle-like nanoparticles as carriers for DNA and siRNA. Mol. Pharm. 2015;12:301–313. doi: 10.1021/mp5007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lao Y.H., Li M., Gao M.A., Shao D., Chi C.W., Huang D., Chakraborty S., Ho T.C., Jiang W., Wang H.X. HPV oncogene manipulation using nonvirally delivered CRISPR/Cas9 or Natronobacterium gregoryi Argonaute. Adv. Sci. (Weinh.) 2018;5:1700540. doi: 10.1002/advs.201700540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Biju V., Anas A., Akita H., Shibu E.S., Itoh T., Harashima H., Ishikawa M. FRET from quantum dots to photodecompose undesired acceptors and report the condensation and decondensation of plasmid DNA. ACS Nano. 2012;6:3776–3788. doi: 10.1021/nn2048608. [DOI] [PubMed] [Google Scholar]
- 126.Tang J., Howard C.B., Mahler S.M., Thurecht K.J., Huang L., Xu Z.P.J.N. Enhanced delivery of siRNA to triple negative breast cancer cells in vitro and in vivo through functionalizing lipid-coated calcium phosphate nanoparticles with dual target ligands. Nanoscale. 2018;10:4258–4266. doi: 10.1039/c7nr08644j. [DOI] [PubMed] [Google Scholar]
- 127.Wu Z., Chen J., Sun Y., Shen M., Huang L., Xu Q., Duan Y., Li Y. Tumor microenvironment-response calcium phosphate hybrid nanoparticles enhanced siRNAs targeting tumors In Vivo. J. Biomed. Nanotechnol. 2018;14:1816–1825. doi: 10.1166/jbn.2018.2606. [DOI] [PubMed] [Google Scholar]
- 128.Paine P.L., Moore L.C., Horowitz S.B. Nuclear envelope permeability. Nature. 1975;254:109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- 129.Lei Y., Tang L., Xie Y., Xianyu Y., Zhang L., Wang P., Hamada Y., Jiang K., Zheng W., Jiang X. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017;8:15130. doi: 10.1038/ncomms15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yi Y., Kim H.J., Mi P., Zheng M., Takemoto H., Toh K., Kim B.S., Hayashi K., Naito M., Matsumoto Y. Targeted systemic delivery of siRNA to cervical cancer model using cyclic RGD-installed unimer polyion complex-assembled gold nanoparticles. J. Control. Release. 2016;244(Pt B):247–256. doi: 10.1016/j.jconrel.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 131.Randeria P.S., Seeger M.A., Wang X.-Q., Wilson H., Shipp D., Mirkin C.A., Paller A.S. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc. Natl. Acad. Sci. USA. 2015;112:5573–5578. doi: 10.1073/pnas.1505951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qiu C., Wei W., Sun J., Zhang H.-T., Ding J.-S., Wang J.-C., Zhang Q. Systemic delivery of siRNA by hyaluronan-functionalized calcium phosphate nanoparticles for tumor-targeted therapy. Nanoscale. 2016;8:13033–13044. doi: 10.1039/c6nr04034a. [DOI] [PubMed] [Google Scholar]
- 133.Dinari A., Moghadam T.T., Abdollahi M., Sadeghizadeh M. Synthesis and characterization of a nano-polyplex system of GNRs-PDMAEA-pDNA: an inert self-catalyzed degradable carrier for facile gene delivery. Sci. Rep. 2018;8:8112. doi: 10.1038/s41598-018-26260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Conde J., Oliva N., Zhang Y., Artzi N. Local triple-combination therapy results in tumour regression and prevents recurrence in a colon cancer model. Nat. Mater. 2016;15:1128–1138. doi: 10.1038/nmat4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vindigni G., Raniolo S., Ottaviani A., Falconi M., Franch O., Knudsen B.R., Desideri A., Biocca S. Receptor-mediated entry of pristine octahedral DNA nanocages in mammalian cells. ACS Nano. 2016;10:5971–5979. doi: 10.1021/acsnano.6b01402. [DOI] [PubMed] [Google Scholar]
- 136.Jiang D., Ge Z., Im H.-J., England C.G., Ni D., Hou J., Zhang L., Kutyreff C.J., Yan Y., Liu Y. DNA origami nanostructures can exhibit preferential renal uptake and alleviate acute kidney injury. Nat. Biomed. Eng. 2018;2:865–877. doi: 10.1038/s41551-018-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang H., Demirer G.S., Zhang H., Ye T., Goh N.S., Aditham A.J., Cunningham F.J., Fan C., Landry M.P. DNA nanostructures coordinate gene silencing in mature plants. Proc. Natl. Acad. Sci. USA. 2019;116:7543–7548. doi: 10.1073/pnas.1818290116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ezzat K., Aoki Y., Koo T., McClorey G., Benner L., Coenen-Stass A., O’Donovan L., Lehto T., Garcia-Guerra A., Nordin J. Self-assembly into nanoparticles is essential for receptor mediated uptake of therapeutic antisense oligonucleotides. Nano Lett. 2015;15:4364–4373. doi: 10.1021/acs.nanolett.5b00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun W., Ji W., Hall J.M., Hu Q., Wang C., Beisel C.L., Gu Z. Self-assembled DNA nanoclews for the efficient delivery of CRISPR-Cas9 for genome editing. Angew. Chem. Int. Ed. Engl. 2015;54:12029–12033. doi: 10.1002/anie.201506030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zakeri B., Lu T.K. DNA nanotechnology: new adventures for an old warhorse. Curr. Opin. Chem. Biol. 2015;28:9–14. doi: 10.1016/j.cbpa.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li S., Jiang Q., Liu S., Zhang Y., Tian Y., Song C., Wang J., Zou Y., Anderson G.J., Han J.Y. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018;36:258–264. doi: 10.1038/nbt.4071. [DOI] [PubMed] [Google Scholar]
- 142.Afonin K.A., Desai R., Viard M., Kireeva M.L., Bindewald E., Case C.L. Co-transcriptional production of RNA-DNA hybrids for simultaneous release of multiple split functionalities. Nucleic Acids Res. 2014;42:2085–2097. doi: 10.1093/nar/gkt1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guo P.X., Erickson S., Anderson D. A small viral RNA is required for in vitro packaging of bacteriophage phi 29 DNA. Science. 1987;236:690–694. doi: 10.1126/science.3107124. [DOI] [PubMed] [Google Scholar]
- 144.Khaled A., Guo S., Li F., Guo P. Controllable self-assembly of nanoparticles for specific delivery of multiple therapeutic molecules to cancer cells using RNA nanotechnology. Nano Lett. 2005;5:1797–1808. doi: 10.1021/nl051264s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pi F., Binzel D.W., Lee T.J., Li Z., Sun M., Rychahou P., Li H., Haque F., Wang S., Croce C.M. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat. Nanotechnol. 2018;13:82–89. doi: 10.1038/s41565-017-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shu D., Shu Y., Haque F., Abdelmawla S., Guo P. Thermodynamically stable RNA three-way junction for constructing multifunctional nanoparticles for delivery of therapeutics. Nat. Nanotechnol. 2011;6:658–667. doi: 10.1038/nnano.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dabkowska A.P., Michanek A., Jaeger L., Chworos A., Nylander T., Sparr E. Supported fluid lipid bilayer as a scaffold to direct assembly of RNA nanostructures. Methods Mol. Biol. 2017;1632:107–122. doi: 10.1007/978-1-4939-7138-1_7. [DOI] [PubMed] [Google Scholar]
- 148.Boerneke M.A., Dibrov S.M., Hermann T. Crystal-structure-guided design of self-assembling RNA nanotriangles. Angew. Chem. Int. Ed. Engl. 2016;55:4097–4100. doi: 10.1002/anie.201600233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grabow W.W., Jaeger L. RNA self-assembly and RNA nanotechnology. Acc. Chem. Res. 2014;47:1871–1880. doi: 10.1021/ar500076k. [DOI] [PubMed] [Google Scholar]
- 150.Afonin K.A., Viard M., Koyfman A.Y., Martins A.N., Kasprzak W.K., Panigaj M., Desai R., Santhanam A., Grabow W.W., Jaeger L. Multifunctional RNA nanoparticles. Nano Lett. 2014;14:5662–5671. doi: 10.1021/nl502385k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Afonin K.A., Bindewald E., Yaghoubian A.J., Voss N., Jacovetty E., Shapiro B.A., Jaeger L. In vitro assembly of cubic RNA-based scaffolds designed in silico. Nat. Nanotechnol. 2010;5:676–682. doi: 10.1038/nnano.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Halman J.R., Satterwhite E., Roark B., Chandler M., Viard M., Ivanina A., Bindewald E., Kasprzak W.K., Panigaj M., Bui M.N. Functionally-interdependent shape-switching nanoparticles with controllable properties. Nucleic Acids Res. 2017;45:2210–2220. doi: 10.1093/nar/gkx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Y., Schulman R. DNA nanostructures that self-heal in serum. Nano Lett. 2019;19:3751–3760. doi: 10.1021/acs.nanolett.9b00888. [DOI] [PubMed] [Google Scholar]
- 154.Wolfe B.R., Porubsky N.J., Zadeh J.N., Dirks R.M., Pierce N.A. Constrained multistate sequence design for nucleic acid reaction pathway engineering. J. Am. Chem. Soc. 2017;139:3134–3144. doi: 10.1021/jacs.6b12693. [DOI] [PubMed] [Google Scholar]
- 155.Bindewald E., Afonin K.A., Viard M., Zakrevsky P., Kim T., Shapiro B.A. Multistrand structure prediction of nucleic acid assemblies and design of RNA switches. Nano Lett. 2016;16:1726–1735. doi: 10.1021/acs.nanolett.5b04651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zadeh J.N., Steenberg C.D., Bois J.S., Wolfe B.R., Pierce M.B., Khan A.R., Dirks R.M., Pierce N.A. NUPACK: analysis and design of nucleic acid systems. J. Comput. Chem. 2011;32:170–173. doi: 10.1002/jcc.21596. [DOI] [PubMed] [Google Scholar]
- 157.Tsang H.H., Wiese K.C. SARNA-Predict: accuracy improvement of RNA secondary structure prediction using permutation-based simulated annealing. IEEE/ACM Trans. Comput. Biol. Bioinformatics. 2010;7:727–740. doi: 10.1109/TCBB.2008.97. [DOI] [PubMed] [Google Scholar]
- 158.Martinez H.M., Maizel J.V., Jr., Shapiro B.A. RNA2D3D: a program for generating, viewing, and comparing 3-dimensional models of RNA. J. Biomol. Struct. Dyn. 2008;25:669–683. doi: 10.1080/07391102.2008.10531240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Adleman L.M. Molecular computation of solutions to combinatorial problems. Science. 1994;266:1021–1024. doi: 10.1126/science.7973651. [DOI] [PubMed] [Google Scholar]
- 160.Shibata T., Fujita Y., Ohno H., Suzuki Y., Hayashi K., Komatsu K.R., Kawasaki S., Hidaka K., Yonehara S., Sugiyama H. Protein-driven RNA nanostructured devices that function in vitro and control mammalian cell fate. Nat. Commun. 2017;8:540. doi: 10.1038/s41467-017-00459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zadeh J.N., Wolfe B.R., Pierce N.A. Nucleic acid sequence design via efficient ensemble defect optimization. J. Comput. Chem. 2011;32:439–452. doi: 10.1002/jcc.21633. [DOI] [PubMed] [Google Scholar]
- 162.Siuti P., Yazbek J., Lu T.K. Synthetic circuits integrating logic and memory in living cells. Nat. Biotechnol. 2013;31:448–452. doi: 10.1038/nbt.2510. [DOI] [PubMed] [Google Scholar]
- 163.Douglas S.M., Bachelet I., Church G.M. A logic-gated nanorobot for targeted transport of molecular payloads. Science. 2012;335:831–834. doi: 10.1126/science.1214081. [DOI] [PubMed] [Google Scholar]
- 164.Krishnan Y., Simmel F.C. Nucleic acid based molecular devices. Angew. Chem. Int. Ed. Engl. 2011;50:3124–3156. doi: 10.1002/anie.200907223. [DOI] [PubMed] [Google Scholar]
- 165.Yin P., Choi H.M., Calvert C.R., Pierce N.A. Programming biomolecular self-assembly pathways. Nature. 2008;451:318–322. doi: 10.1038/nature06451. [DOI] [PubMed] [Google Scholar]
- 166.Dirks R.M., Pierce N.A. Triggered amplification by hybridization chain reaction. Proc. Natl. Acad. Sci. USA. 2004;101:15275–15278. doi: 10.1073/pnas.0407024101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qian L., Winfree E. Scaling up digital circuit computation with DNA strand displacement cascades. Science. 2011;332:1196–1201. doi: 10.1126/science.1200520. [DOI] [PubMed] [Google Scholar]
- 168.Seelig G., Soloveichik D., Zhang D.Y., Winfree E. Enzyme-free nucleic acid logic circuits. Science. 2006;314:1585–1588. doi: 10.1126/science.1132493. [DOI] [PubMed] [Google Scholar]
- 169.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 170.Yatvin M.B., Weinstein J.N., Dennis W.H., Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 171.Chiper M., Tounsi N., Kole R., Kichler A., Zuber G. Self-aggregating 1.8kDa polyethylenimines with dissolution switch at endosomal acidic pH are delivery carriers for plasmid DNA, mRNA, siRNA and exon-skipping oligonucleotides. J. Control. Release. 2017;246:60–70. doi: 10.1016/j.jconrel.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 172.Xu X., Wu J., Liu Y., Yu M., Zhao L., Zhu X., Bhasin S., Li Q., Ha E., Shi J., Farokhzad O.C. Ultra‐pH‐responsive and tumor‐penetrating nanoplatform for targeted siRNA delivery with robust anti‐cancer efficacy. Angew. Chem. Int. Ed. Engl. 2016;55:7091–7094. doi: 10.1002/anie.201601273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu X., Wu J., Liu Y., Saw P.E., Tao W., Yu M., Zope H., Si M., Victorious A., Rasmussen J. Multifunctional envelope-type siRNA delivery nanoparticle platform for prostate cancer therapy. ACS Nano. 2017;11:2618–2627. doi: 10.1021/acsnano.6b07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Adamo G., Grimaldi N., Campora S., Bulone D., Bondì M.L., Al-Sheikhly M., Sabatino M.A., Dispenza C., Ghersi G. Multi-functional nanogels for tumor targeting and redox-sensitive drug and siRNA delivery. Molecules. 2016;21:1594. doi: 10.3390/molecules21111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Karimi M., Ghasemi A., Sahandi Zangabad P., Rahighi R., Moosavi Basri S.M., Mirshekari H., Amiri M., Shafaei Pishabad Z., Aslani A., Bozorgomid M. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016;45:1457–1501. doi: 10.1039/c5cs00798d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tran K.N., Tran P.H., Vo T.V., Tran T.T. Design of fucoidan functionalized - iron oxide nanoparticles for biomedical applications. Curr. Drug Deliv. 2016;13:774–783. doi: 10.2174/1567201812666151020100921. [DOI] [PubMed] [Google Scholar]
- 177.Ding Y., Su S., Zhang R., Shao L., Zhang Y., Wang B., Li Y., Chen L., Yu Q., Wu Y., Nie G. Precision combination therapy for triple negative breast cancer via biomimetic polydopamine polymer core-shell nanostructures. Biomaterials. 2017;113:243–252. doi: 10.1016/j.biomaterials.2016.10.053. [DOI] [PubMed] [Google Scholar]
- 178.Xin H., Namgung B., Lee L.P. Nanoplasmonic optical antennas for life sciences and medicine. Nat. Rev. Mater. 2018;3:228–243. [Google Scholar]
- 179.Wang Z., Li S., Zhang M., Ma Y., Liu Y., Gao W., Zhang J., Gu Y. Laser-triggered small interfering RNA releasing gold nanoshells against heat shock protein for sensitized photothermal therapy. Adv. Sci. (Weinh.) 2016;4:1600327. doi: 10.1002/advs.201600327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang Y., Xie X., Yang Y., Li Z., Yu F., Gong W., Li Y., Zhang H., Wang Z., Mei X. Polymer nanoparticles modified with photo-and pH-dual-responsive polypeptides for enhanced and targeted cancer therapy. Mol. Pharm. 2016;13:1508–1519. doi: 10.1021/acs.molpharmaceut.5b00977. [DOI] [PubMed] [Google Scholar]
- 181.Kowal J., Tkach M., Théry C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 182.Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016;106(Pt A):148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 183.Batrakova E.V., Kim M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., Lee J.J., Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lin Y., Wu J., Gu W., Huang Y., Tong Z., Huang L., Tan J. Exosome-Liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv. Sci. (Weinh.) 2018;5:1700611. doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen R., Huang H., Liu H., Xi J., Ning J., Zeng W., Shen C., Zhang T., Yu G., Xu Q. Friend or foe? Evidence indicates endogenous exosomes can deliver functional gRNA and Cas9 protein. Small. 2019;15:e1902686. doi: 10.1002/smll.201902686. [DOI] [PubMed] [Google Scholar]
- 187.van Zandwijk N., Pavlakis N., Kao S.C., Linton A., Boyer M.J., Clarke S., Huynh Y., Chrzanowska A., Fulham M.J., Bailey D.L. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017;18:1386–1396. doi: 10.1016/S1470-2045(17)30621-6. [DOI] [PubMed] [Google Scholar]
- 188.MacDiarmid J.A., Brahmbhatt H. Minicells: versatile vectors for targeted drug or si/shRNA cancer therapy. Curr. Opin. Biotechnol. 2011;22:909–916. doi: 10.1016/j.copbio.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 189.Santamaria A. Historical overview of nanotechnology and nanotoxicology. Methods Mol. Biol. 2012;926:1–12. doi: 10.1007/978-1-62703-002-1_1. [DOI] [PubMed] [Google Scholar]
- 190.Ballarín-González B., Howard K.A. Polycation-based nanoparticle delivery of RNAi therapeutics: adverse effects and solutions. Adv. Drug Deliv. Rev. 2012;64:1717–1729. doi: 10.1016/j.addr.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 191.Borgheti-Cardoso L.N., Kooijmans S.A.A., Fens M.H.A.M., van der Meel R., Vicentini F.T.M.C., Fantini M.C.A., Bentley M.V.L.B., Schiffelers R.M. In situ gelling liquid crystalline system as local siRNA delivery system. Mol. Pharm. 2017;14:1681–1690. doi: 10.1021/acs.molpharmaceut.6b01141. [DOI] [PubMed] [Google Scholar]
- 192.Adams D., Suhr O.B., Dyck P.J., Litchy W.J., Leahy R.G., Chen J., Gollob J., Coelho T. Trial design and rationale for APOLLO, a phase 3, placebo-controlled study of patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy. BMC Neurol. 2017;17:181. doi: 10.1186/s12883-017-0948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hong E., Halman J.R., Shah A.B., Khisamutdinov E.F., Dobrovolskaia M.A., Afonin K.A. Structure and composition define immunorecognition of nucleic acid nanoparticles. Nano Lett. 2018;18:4309–4321. doi: 10.1021/acs.nanolett.8b01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Johnson M.B., Halman J.R., Satterwhite E., Zakharov A.V., Bui M.N., Benkato K., Goldsworthy V., Kim T., Hong E., Dobrovolskaia M.A. Programmable nucleic acid based polygons with controlled neuroimmunomodulatory properties for predictive QSAR modeling. Small. 2017;13:13. doi: 10.1002/smll.201701255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sugiyama T., Gursel M., Takeshita F., Coban C., Conover J., Kaisho T., Akira S., Klinman D.M., Ishii K.J. CpG RNA: identification of novel single-stranded RNA that stimulates human CD14+CD11c+ monocytes. J. Immunol. 2005;174:2273–2279. doi: 10.4049/jimmunol.174.4.2273. [DOI] [PubMed] [Google Scholar]
- 196.Kim D., Kim H., Han S., Scatena M., Kim D.H., Lee J.B. Immunostimulatory effects triggered by self-assembled microspheres with tandem repeats of polymerized RNA strands. Adv. Healthc. Mater. 2019;8:e1801395. doi: 10.1002/adhm.201801395. [DOI] [PubMed] [Google Scholar]
- 197.Durbin J.K., Miller D.K., Niekamp J., Khisamutdinov E.F. Modulating immune response with nucleic acid nanoparticles. Molecules. 2019;24:E3740. doi: 10.3390/molecules24203740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Guo S., Li H., Ma M., Fu J., Dong Y., Guo P. Size, shape, and sequence-dependent immunogenicity of RNA nanoparticles. Mol. Ther. Nucleic Acids. 2017;9:399–408. doi: 10.1016/j.omtn.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Guo S., Xu C., Yin H., Hill J., Pi F., Guo P. Tuning the size, shape and structure of RNA nanoparticles for favorable cancer targeting and immunostimulation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020;12:e1582. doi: 10.1002/wnan.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hong E., Halman J.R., Shah A., Cedrone E., Truong N., Afonin K.A., Dobrovolskaia M.A. Toll-like receptor-mediated recognition of nucleic acid nanoparticles (NANPs) in human primary blood cells. Molecules. 2019;24:E1094. doi: 10.3390/molecules24061094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Watanabe N., Yano K., Tsuyuki K., Okano T., Yamato M. Re-examination of regulatory opinions in Europe: possible contribution for the approval of the first gene therapy product Glybera. Mol. Ther. Methods Clin. Dev. 2015;2:14066. doi: 10.1038/mtm.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Harrington K.J., Puzanov I., Hecht J.R., Hodi F.S., Szabo Z., Murugappan S., Kaufman H.L. Clinical development of talimogene laherparepvec (T-VEC): a modified herpes simplex virus type-1-derived oncolytic immunotherapy. Expert Rev. Anticancer Ther. 2015;15:1389–1403. doi: 10.1586/14737140.2015.1115725. [DOI] [PubMed] [Google Scholar]
- 203.Al-Zaidy S., Pickard A.S., Kotha K., Alfano L.N., Lowes L., Paul G., Church K., Lehman K., Sproule D.M., Dabbous O. Health outcomes in spinal muscular atrophy type 1 following AVXS-101 gene replacement therapy. Pediatr. Pulmonol. 2019;54:179–185. doi: 10.1002/ppul.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rao V.K., Kapp D., Schroth M. Gene therapy for spinal muscular atrophy: an emerging treatment option for a devastating disease. J. Manag. Care Spec. Pharm. 2018;24(12-a, Suppl):S3–S16. doi: 10.18553/jmcp.2018.24.12-a.s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liebow A., Li X., Racie T., Hettinger J., Bettencourt B.R., Najafian N., Haslett P., Fitzgerald K., Holmes R.P., Erbe D. An investigational RNAi therapeutic targeting glycolate oxidase reduces oxalate production in models of primary hyperoxaluria. J. Am. Soc. Nephrol. 2017;28:494–503. doi: 10.1681/ASN.2016030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Janas M.M., Zlatev I., Liu J., Jiang Y., Barros S.A., Sutherland J.E., Davis W.P., Liu J., Brown C.R., Liu X. Safety evaluation of 2′-deoxy-2′-fluoro nucleotides in GalNAc-siRNA conjugates. Nucleic Acids Res. 2019;47:3306–3320. doi: 10.1093/nar/gkz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sardh E., Harper P., Balwani M., Stein P., Rees D., Bissell D.M., Desnick R., Parker C., Phillips J., Bonkovsky H.L. Phase 1 trial of an RNA interference therapy for acute intermittent porphyria. N. Engl. J. Med. 2019;380:549–558. doi: 10.1056/NEJMoa1807838. [DOI] [PubMed] [Google Scholar]
- 208.Stoekenbroek R.M., Kallend D., Wijngaard P.L., Kastelein J.J. Inclisiran for the treatment of cardiovascular disease: the ORION clinical development program. Future Cardiol. 2018;14:433–442. doi: 10.2217/fca-2018-0067. [DOI] [PubMed] [Google Scholar]
- 209.Kosmas C.E., Muñoz Estrella A., Sourlas A., Silverio D., Hilario E., Montan P.D., Guzman E. Inclisiran: a new promising agent in the management of hypercholesterolemia. Diseases. 2018;6:E63. doi: 10.3390/diseases6030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sehgal A., Blomenkamp K.S., Qian K., Simon A., Haslett P., Barros S., Teckman J. 279 Pre-clinical evaluation of ALN-AAT to ameliorate liver disease associated with alpha-1-antitrypsin deficiency. Gastroenterology. 2015;148(Suppl 1):S-975. [Google Scholar]
- 211.Borrelli M.J., Youssef A., Boffa M.B., Koschinsky M.L. New frontiers in Lp(a)-targeted therapies. Trends Pharmacol. Sci. 2019;40:212–225. doi: 10.1016/j.tips.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 212.Dindo M., Conter C., Oppici E., Ceccarelli V., Marinucci L., Cellini B. Molecular basis of primary hyperoxaluria: clues to innovative treatments. Urolithiasis. 2019;47:67–78. doi: 10.1007/s00240-018-1089-z. [DOI] [PubMed] [Google Scholar]
- 213.Solano E.C., Kornbrust D.J., Beaudry A., Foy J.W., Schneider D.J., Thompson J.D. Toxicological and pharmacokinetic properties of QPI-1007, a chemically modified synthetic siRNA targeting caspase 2 mRNA, following intravitreal injection. Nucleic Acid Ther. 2014;24:258–266. doi: 10.1089/nat.2014.0489. [DOI] [PubMed] [Google Scholar]
- 214.Moreno-Montañés J., Bleau A.M., Jimenez A.I. Tivanisiran, a novel siRNA for the treatment of dry eye disease. Expert Opin. Investig. Drugs. 2018;27:421–426. doi: 10.1080/13543784.2018.1457647. [DOI] [PubMed] [Google Scholar]
- 215.Denise H., Moschos S.A., Sidders B., Burden F., Perkins H., Carter N., Stroud T., Kennedy M., Fancy S.A., Lapthorn C. Deep sequencing insights in therapeutic shRNA processing and siRNA target cleavage precision. Mol. Ther. Nucleic Acids. 2014;3:e145. doi: 10.1038/mtna.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Phadke A.P., Jay C.M., Wang Z., Chen S., Liu S., Haddock C., Kumar P., Pappen B.O., Rao D.D., Templeton N.S. In vivo safety and antitumor efficacy of bifunctional small hairpin RNAs specific for the human stathmin 1 oncoprotein. DNA Cell Biol. 2011;30:715–726. doi: 10.1089/dna.2011.1240. [DOI] [PubMed] [Google Scholar]
- 217.Barve M., Wang Z., Kumar P., Jay C.M., Luo X., Bedell C., Mennel R.G., Wallraven G., Brunicardi F.C., Senzer N. Phase 1 trial of Bi-shRNA STMN1 BIV in refractory cancer. Mol. Ther. 2015;23:1123–1130. doi: 10.1038/mt.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rao D.D., Jay C., Wang Z., Luo X., Kumar P., Eysenbach H., Ghisoli M., Senzer N., Nemunaitis J. Preclinical justification of pbi-shRNA EWS/FLI1 lipoplex (LPX) treatment for Ewing’s sarcoma. Mol. Ther. 2016;24:1412–1422. doi: 10.1038/mt.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Keam S.J. Inotersen: first global approval. Drugs. 2018;78:1371–1376. doi: 10.1007/s40265-018-0968-5. [DOI] [PubMed] [Google Scholar]
- 220.Benson M.D., Waddington-Cruz M., Berk J.L., Polydefkis M., Dyck P.J., Wang A.K., Planté-Bordeneuve V., Barroso F.A., Merlini G., Obici L. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018;379:22–31. doi: 10.1056/NEJMoa1716793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Geary R.S., Henry S.P., Grillone L.R. Fomivirsen: clinical pharmacology and potential drug interactions. Clin. Pharmacokinet. 2002;41:255–260. doi: 10.2165/00003088-200241040-00002. [DOI] [PubMed] [Google Scholar]
- 222.Geary R.S., Baker B.F., Crooke S.T. Clinical and preclinical pharmacokinetics and pharmacodynamics of mipomersen (Kynamro®): a second-generation antisense oligonucleotide inhibitor of apolipoprotein B. Clin. Pharmacokinet. 2015;54:133–146. doi: 10.1007/s40262-014-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nandakumar R., Matveyenko A., Thomas T., Pavlyha M., Ngai C., Holleran S., Ramakrishnan R., Ginsberg H.N., Karmally W., Marcovina S.M., Reyes-Soffer G. Effects of mipomersen, an apolipoprotein B100 antisense, on lipoprotein (a) metabolism in healthy subjects. J. Lipid Res. 2018;59:2397–2402. doi: 10.1194/jlr.P082834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hoy S.M. Nusinersen: first global approval. Drugs. 2017;77:473–479. doi: 10.1007/s40265-017-0711-7. [DOI] [PubMed] [Google Scholar]
- 225.Lim K.R., Maruyama R., Yokota T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des. Devel. Ther. 2017;11:533–545. doi: 10.2147/DDDT.S97635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hosten T.A., Zhao K., Han H.Q., Liu G., He X.H. Alicaforsen: an emerging therapeutic agent for ulcerative colitis and refractory pouchitis. Gastroenterol. Res. 2014;7:51–55. doi: 10.14740/gr599w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.van der Ree M.H., de Vree J.M., Stelma F., Willemse S., van der Valk M., Rietdijk S., Molenkamp R., Schinkel J., van Nuenen A.C., Beuers U. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: a phase 1B, double-blind, randomised controlled trial. Lancet. 2017;389:709–717. doi: 10.1016/S0140-6736(16)31715-9. [DOI] [PubMed] [Google Scholar]
- 228.Gomez I.G., MacKenna D.A., Johnson B.G., Kaimal V., Roach A.M., Ren S., Nakagawa N., Xin C., Newitt R., Pandya S. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J. Clin. Invest. 2015;125:141–156. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gallant-Behm C.L., Piper J., Lynch J.M., Seto A.G., Hong S.J., Mustoe T.A., Maari C., Pestano L.A., Dalby C.M., Jackson A.L. A microRNA-29 mimic (remlarsen) represses extracellular matrix expression and fibroplasia in the skin. J. Invest. Dermatol. 2019;139:1073–1081. doi: 10.1016/j.jid.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 230.Figlin R.A. Personalized immunotherapy (AGS-003) when combined with sunitinib for the treatment of metastatic renal cell carcinoma. Expert Opin. Biol. Ther. 2015;15:1241–1248. doi: 10.1517/14712598.2015.1063610. [DOI] [PubMed] [Google Scholar]
- 231.Sun N., Ning B., Hansson K.M., Bruce A.C., Seaman S.A., Zhang C., Rikard M., DeRosa C.A., Fraser C.L., Wågberg M. Modified VEGF-A mRNA induces sustained multifaceted microvascular response and accelerates diabetic wound healing. Sci. Rep. 2018;8:17509. doi: 10.1038/s41598-018-35570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Katz B., Goldbaum M. Macugen (pegaptanib sodium), a novel ocular therapeutic that targets vascular endothelial growth factor (VEGF) Int. Ophthalmol. Clin. 2006;46:141–154. doi: 10.1097/01.iio.0000212130.91136.31. [DOI] [PubMed] [Google Scholar]
- 233.Stein C.A., Castanotto D. FDA-approved oligonucleotide therapies in 2017. Mol. Ther. 2017;25:1069–1075. doi: 10.1016/j.ymthe.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bates P.J., Reyes-Reyes E.M., Malik M.T., Murphy E.M., O’Toole M.G., Trent J.O. G-quadruplex oligonucleotide AS1411 as a cancer-targeting agent: uses and mechanisms. Biochim. Biophys. Acta, Gen. Subj. 2017;1861(5 Pt B):1414–1428. doi: 10.1016/j.bbagen.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 235.Leung E., Landa G. Update on current and future novel therapies for dry age-related macular degeneration. Expert Rev. Clin. Pharmacol. 2013;6:565–579. doi: 10.1586/17512433.2013.829645. [DOI] [PubMed] [Google Scholar]
- 236.Mitsuyasu R.T., Merigan T.C., Carr A., Zack J.A., Winters M.A., Workman C., Bloch M., Lalezari J., Becker S., Thornton L. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat. Med. 2009;15:285–292. doi: 10.1038/nm.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Morrow P.K., Murthy R.K., Ensor J.D., Gordon G.S., Margolin K.A., Elias A.D., Urba W.J., Weng D.E., Rugo H.S., Hortobagyi G.N. An open-label, phase 2 trial of RPI.4610 (angiozyme) in the treatment of metastatic breast cancer. Cancer. 2012;118:4098–4104. doi: 10.1002/cncr.26730. [DOI] [PubMed] [Google Scholar]
- 238.Liao W.H., Yang L.F., Liu X.Y., Zhou G.F., Jiang W.Z., Hou B.L., Sun L.Q., Cao Y., Wang X.Y. DCE-MRI assessment of the effect of Epstein-Barr virus-encoded latent membrane protein-1 targeted DNAzyme on tumor vasculature in patients with nasopharyngeal carcinomas. BMC Cancer. 2014;14:835. doi: 10.1186/1471-2407-14-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Scarozza P., Schmitt H., Monteleone G., Neurath M.F., Atreya R. Oligonucleotides—a novel promising therapeutic option for IBD. Front. Pharmacol. 2019;10:314. doi: 10.3389/fphar.2019.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wu Y., Zeng J., Roscoe B.P., Liu P., Yao Q., Lazzarotto C.R., Clement K., Cole M.A., Luk K., Baricordi C. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat. Med. 2019;25:776–783. doi: 10.1038/s41591-019-0401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hu W., Zi Z., Jin Y., Li G., Shao K., Cai Q., Ma X., Wei F. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol. Immunother. 2019;68:365–377. doi: 10.1007/s00262-018-2281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., Meng M., Fritz D., Vascotto F., Hefesha H. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 243.Oberli M.A., Reichmuth A.M., Dorkin J.R., Mitchell M.J., Fenton O.S., Jaklenec A., Anderson D.G., Langer R., Blankschtein D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tangsangasaksri M., Takemoto H., Naito M., Maeda Y., Sueyoshi D., Kim H.J., Miura Y., Ahn J., Azuma R., Nishiyama N. siRNA-loaded polyion complex micelle decorated with charge-conversional polymer tuned to undergo stepwise response to intra-tumoral and intra-endosomal pHs for exerting enhanced RNAi efficacy. Biomacromolecules. 2016;17:246–255. doi: 10.1021/acs.biomac.5b01334. [DOI] [PubMed] [Google Scholar]
- 245.Woodrow K.A., Cu Y., Booth C.J., Saucier-Sawyer J.K., Wood M.J., Saltzman W.M. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat. Mater. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Brown P.K., Qureshi A.T., Moll A.N., Hayes D.J., Monroe W.T. Silver nanoscale antisense drug delivery system for photoactivated gene silencing. ACS Nano. 2013;7:2948–2959. doi: 10.1021/nn304868y. [DOI] [PubMed] [Google Scholar]
- 247.Chahal J.S., Khan O.F., Cooper C.L., McPartlan J.S., Tsosie J.K., Tilley L.D., Sidik S.M., Lourido S., Langer R., Bavari S. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA. 2016;113:E4133–E4142. doi: 10.1073/pnas.1600299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Khan O.F., Zaia E.W., Jhunjhunwala S., Xue W., Cai W., Yun D.S., Barnes C.M., Dahlman J.E., Dong Y., Pelet J.M. Dendrimer-inspired nanomaterials for the in vivo delivery of siRNA to lung vasculature. Nano Lett. 2015;15:3008–3016. doi: 10.1021/nl5048972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Baba M., Itaka K., Kondo K., Yamasoba T., Kataoka K. Treatment of neurological disorders by introducing mRNA in vivo using polyplex nanomicelles. J. Control. Release. 2015;201:41–48. doi: 10.1016/j.jconrel.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 250.Zhao M., Li M., Zhang Z., Gong T., Sun X. Induction of HIV-1 gag specific immune responses by cationic micelles mediated delivery of gag mRNA. Drug Deliv. 2016;23:2596–2607. doi: 10.3109/10717544.2015.1038856. [DOI] [PubMed] [Google Scholar]
- 251.Fraga M., Bruxel F., Diel D., de Carvalho T.G., Perez C.A., Magalhães-Paniago R., Malachias Â., Oliveira M.C., Matte U., Teixeira H.F. PEGylated cationic nanoemulsions can efficiently bind and transfect pIDUA in a mucopolysaccharidosis type I murine model. J. Control. Release. 2015;209:37–46. doi: 10.1016/j.jconrel.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 252.Lee H., Lytton-Jean A.K., Chen Y., Love K.T., Park A.I., Karagiannis E.D., Sehgal A., Querbes W., Zurenko C.S., Jayaraman M. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat. Nanotechnol. 2012;7:389–393. doi: 10.1038/nnano.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jang M., Kim J.H., Nam H.Y., Kwon I.C., Ahn H.J. Design of a platform technology for systemic delivery of siRNA to tumours using rolling circle transcription. Nat. Commun. 2015;6:7930. doi: 10.1038/ncomms8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wu C., Li J., Pang P., Liu J., Zhu K., Li D., Cheng D., Chen J., Shuai X., Shan H. Polymeric vector-mediated gene transfection of MSCs for dual bioluminescent and MRI tracking in vivo. Biomaterials. 2014;35:8249–8260. doi: 10.1016/j.biomaterials.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 255.Shan Y., Luo T., Peng C., Sheng R., Cao A., Cao X., Shen M., Guo R., Tomás H., Shi X. Gene delivery using dendrimer-entrapped gold nanoparticles as nonviral vectors. Biomaterials. 2012;33:3025–3035. doi: 10.1016/j.biomaterials.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 256.Israel L.L., Lellouche E., Ostrovsky S., Yarmiayev V., Bechor M., Michaeli S., Lellouche J.P. Acute in vivo toxicity mitigation of PEI-coated maghemite nanoparticles using controlled oxidation and surface modifications toward siRNA delivery. ACS Appl. Mater. Interfaces. 2015;7:15240–15255. doi: 10.1021/acsami.5b02743. [DOI] [PubMed] [Google Scholar]
- 257.Lostalé-Seijo I., Montenegro J. Synthetic materials at the forefront of gene delivery. Nat. Rev. Chem. 2018;2:258–277. [Google Scholar]