Abstract
Mucopolysaccharidoses (MPS) are a family of lysosomal storage disorders which can lead to degenerative and irreversible skeletal, cardiovascular, pulmonary, and neurological damage. Current treatments, including hematopoietic stem cell transplantation and enzyme replacement therapy, have been found most effective if administered before clinical symptoms are present, highlighting the urgent need for the development of newborn screening.
This study analyzed 18,222 dried blood spot samples from newborns for both enzyme activity and glycosaminoglycan (GAG) concentration levels. GAG levels were measured using liquid chromatography tandem mass spectrometry. Results were compared to our previously established cutoff values for three subtypes of GAGs: dermatan sulfate (DS) and heparan sulfate (HS0S and HSNS). Samples that were high for two of the three GAGs were identified and screened a second time. Samples were also measured for iduronate-2-sulfatase and alfa-L-iduronidase activity.
A total of 300 samples were above the established cutoff values for at least two of the three GAGs after the first screening. One sample was determined through clinical and genetic testing to be a true positive for MPS II. The false positive rate after the first GAG screening was 1.64%. A Cochran's formula test showed that the samples available for the second screening were representative samples (p = .0000601). False positive rate after second GAG screening, extrapolated from the representative sample was 0.4%. False positive rate after enzyme activity assay by fluorimetry for IDUA and IDS enzymes was 0.21% and 0.18%. A combination of GAG and enzyme assays provided no false positive and false negative samples.
Two-tier screening involving a combination of enzyme activity and multiple GAGs should be considered the gold standard for the diagnosis of MPS patients.
Keywords: Newborn screening, Mucopolysaccharidosis, Fetal GAG contamination, Tandem mass spectrometry, Glycosaminoglycans
1. Introduction
Mucopolysaccharidoses (MPS) are a family of disorders that, when taken collectively, are one of the most common lysosomal storage disorders (LSD) [[1], [2], [3]]. MPS are caused by an inherited deficiency of specific lysosomal enzymes, which break down the long unbranched polysaccharides known as glycosaminoglycans (GAGs). Failure of these catabolic enzymes to break down their specific GAGs [chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate (HS), keratan sulfate (KS), and hyaluronic acid] leads to progressive build-up of these GAGs [4,5] and deterioration of several systems, including visceral organ damage (liver, kidney, lung, heart, etc.) and disease-specific manifestations. These can include corneal clouding, course facial features, restrictive and obstructive lung disease, tracheal obstruction, skeletal dysplasia, cardiac, respiratory, and neurological abnormalities [[6], [7], [8], [9], [10]].
Although disease manifestations are progressive and serious, infants are typically born without any easily identifiable clinical symptoms. Depending on the specific type and severity of the disease, symptoms typically present within the first three years of life and rapidly progress afterward [[10], [11], [12], [13]]. While outward clinical symptoms do not present at birth, MPS can be detected in the fetus (MPS I, II, III, IVA) and placenta (MPS II, VI) by measuring the accumulation of GAGs [[14], [15], [16], [17]]. Deficiency of enzyme activity and GAG accumulation can be measured at birth in blood [14,[18], [19], [20], [21]]. It is critical to develop a newborn screening method that will detect disease presence before the appearance of clinical signs and symptoms [[22], [23], [24], [25]].
In our previous pilot study, we established cutoff values for MPS I, II, and III through median absolute deviation (MAD) to distinguish patients from general newborns with cutoffs defined as median +7x MAD from the general newborns [18].
In the present study, we analyzed 18,222 dB samples from the general screening of newborns and have evaluated both GAG and the enzyme assays for MPS I and II to validate a two-tier strategy.
2. Materials and methods
2.1. Materials
All unsaturated disaccharides were provided by the Seikagaku Co. in Tokyo, Japan. These included: heparan ΔDi-0S [2-acetamido-2-deoxy-4-O-(4- deoxy-α- L-threo-hex- 4-enopyranosyluronic acid)-d-glucose] (HS-0S), heparan ΔDi-NS [2-deoxy-2-sulfamino 4-O-(4-deoxy-α-L-threo-hex-4-enopyranosyluronic acid)-d-glucose] (HS-NS), chondro Δ Di-0S [2-acetamido-2-deoxy 3-O-(β-D-gluco-4-enepyranosyluronic acid)-4-O-D-sulfo-galactose] (Di-0S). Stock solutions of standards, HS-0S (100 μg/mL), HS-NS (100 μg/mL), Di-0S (250 μg/mL), and IS (5 μg/mL) were diluted with Millipore Milli-Q H2O. The standard curves for all GAG concentration levels were prepared with the following concentrations of 8 standards from serial dilutions (in ng/mL): 1000, 500, 250, 125, 62.5, 31.5, 15.75, and 7.81 [26]. Samples were digested with chondroitinase B, heparitinase, and keratanase II. Acetonitrile optima® (A996–4), ammonium hydroxide ammoniaque optima® (A470–500), bovine serum albumin (BP1600–100), Corning Costar® Assay plate 96 well ref. 3797 (07–200-105), Thermo Scientific Hypercarb™ Porous Graphitic Carbon LC Columns (35005–052130), Thermo Scientific™ Uniguard™ Direct-connection guard cartridge holder (85200), Hypercarb 5 μm 10 × 2.1 mm drop-in guards (35005-012101) and Tris Base (BP152-500) were purchased from Thermo Fisher Scientific (Ottawa, Ontario). Acroprep™ advance 96 filter plates 10 K Omega (PN 8034) were purchased from Pall Co (Ann Arbor, MI). Recombinant human α-L-iduronidase (Laronidase) was purchased from Sanofi Genzyme (Cambridge, MA), and 4-methylumbelliferyl-α-L-idopyranosiduronic acid sodium salt, 4-methylumbelliferyl-α-L-idopyranosiduronic acid 2-sulfate disodium salt from Tronto Research Chemicals (Tronto, Canada). 4-methylumbelliferone sodium salt (4-MU), Tween20, sodium carbonate, molecular biology grade bovine serum albumin (BSA), and all other reagents were from Sigma Aldrich Corp (St. Louis, MO).
2.2. Samples
Dried blood spot (DBS) samples of 18,222 newborns were collected during routine newborn screening by Osaka City University (Osaka, Japan) and Gifu University (Gifu, Japan). Informed consent was obtained from the guardians of all infants. Samples were collected within 7 days after birth via heel prick. No samples were known to have MPS disorder before analysis. Samples were collected between 2012 and 2015 and were stored for a minimum of three years prior to testing. All samples were shipped to Nemours/AIDHC in cold temperature and stored until GAG assay at −20 °C. This study was approved by local protocols and IRBs at all local institutions, including Nemours/AIDHC (Protocol # 281498). Patients gave informed consent regarding the potential for samples to be included in this study and expectation that any abnormal results would be analyzed and reported to both themselves and for research purposes.
2.3. Sample preparation
DBS samples were collected and dried at room temperature before storage at −20 °C for at least 4 h [27]. For the rehydration of samples, two disks (3.3 mm) were obtained from the DBS spots using a DBS puncher (PerkinElmer®, Waltham, MA). Samples were treated as previously described [18]. Briefly, samples were placed into a 96 well filter plate (Omega) with 100 μL of 0.1% BSA solution, incubated for 15 min, and centrifuged for 15 min at 2500 RPM. The filtrate solution was discarded, and a cocktail mixture was added to these samples. This mixture contained 90 μL of 50 mM Tris HCL (pH 7.0), 10 μL of 5 μg/mL IS, 10 μL of 0.5 mU chondroitinase B (in 1% BSA), 1 mU heparitinase (in 1% BSA), and 10 μL of 1 mU keratanase II (in 1% BSA). Samples were then incubated at 37 °C water bath for 16 h with a new receiver plate. Samples were then centrifuged for 15 min, again at 2500 RPM. The filter plate was discarded, and the filtrate collected in the receiver plate was stored at −20 °C until injection in the LC-MS/MS [18,28].
2.4. LC-MS/MS
The mass spectrometer was a 1290 Infinity liquid chromatography system with a 6460 triple quad mass spectrometer (Agilent Technologies, Palo Alto, CA). Separation of digested disaccharides occurred on a Hypercarb column (2.0 mm i.d. 50 mm length; 5 μm particles; Thermo Scientific, USA). The method utilized was first developed by Oguma et al. [26] and further modified as described by Kubaski et al. [18]. In brief, the mobile phase was a gradient elution of 5 mM ammonium acetate, pH 11.0 (solution A) to 100% acetonitrile (solution B). The flow rate was 0.7 mL/min, and the gradient was as follows: at 0 min. 100% solution A, 1 min. 70% solution A, 2 min. 70% solution A, 2.20 min. 0% solution A, 2.60 min. 0% solution A, 2.61 min. 100% solution A, 5 min. 100% solution A. The mass spectrometer (Agilent Jet Stream Technology) was operated with electrospray ionization in the negative ion mode with a drying gas temperature of 350 °C, drying gas flow of 11 L/min, nebulizer pressure of 58 PSI, sheath gas temperature 412 °C, sheath gas flow of 11 L/min, capillary voltage of 4000 V and nozzle voltage of 2000 V. Precursor and product ions (m/z) were used to quantify each disaccharide as follows IS: 354.3, 193.1; DS: 378.3, 175.1; HS-NS: 416, 138; HS-0S: 378.3, 175.1 [26]. Dermatan sulfate (DS) was measured of Di-0S following digestion of Di-4S by a 4S-Sulfatase, which was present in the chondroitinase B enzyme solution. 5 μL of each sample was injected with a running time of 5 min.
2.5. Statistical analysis
All statistical analyses were conducted using SPSS, including specificity, false positive and false negative rates. We previously established the median absolute deviation (MAD) for MPS GAG concentration cutoff values using R software (R Core team, 2014). Median absolute deviation (MAD) was calculated for the establishment of these cutoff values due to the non-normal distribution of raw calculated values in the blood due to elevation for reasons other than MPS. This rendered the typical methodology of mean ± standard deviation inappropriate for the establishment of cutoff values.
2.6. Enzyme assay
For IDUA enzyme assay, one 3.2 mm disc was punched from the DBS card and placed into a black 96-well microplate. 20 μL of the reaction mixture (two parts elution buffer and 1 part substrate) was added to each well. Elution buffer contains 50 mmol/L formate buffer (pH 2.8) and 3.57 μmol/L d-saccharic acid-1, 4-lactone. A reaction mixture contained the elute solution and the substrate (4-methylumbelliferyl-α-L-idopyranosiduronic acid sodium salt) in a 2:1 ratio (v/v). The plate was covered well with foil to avoid influences from light, air, humidity. The plate was incubated at 37 °C in an orbital shaker for 20 h. One hundred and fifty microliter of glycine carbonate buffer (stop solution) was added to each well, and the plate was kept at room temperature for 30 min to stop the reaction. The fluorescence of the enzyme product, 4-methylumbelliferone (4-MU), was measured by microplate Fluorometer microplates (Fluoroskan Ascent, ThermoFisher Scientific) with excitation 360 nm and emission 450 nm. The fluorescence readings were corrected for blanks, and the results were compared with the fluorescence from a 4-MU calibration curve [29].
For IDS enzyme assay, 3.2 mm punch from DBS card was extracted by gentle mixing in 100 μL of 0.1% Tween20 in 96 microplates for 30 min at room temperature. Ten microliter of the extracts were incubated with the 10 μL artificial substrate containing 1.125 mmol/L 4-methylumbelliferyl-α-L-idopyranosiduronic acid 2-sulfate disodium salt in the presence of 1 μg/mL recombinant human α-L-iduronidase, 100 mM sodium acetate, 10 mM lead acetate, at acidic pH (5.0) for 20 h at 37 °C. The reaction was stopped by addition of 50 μL of 200 mM sodium bicarbonate (pH 10.1) and 0.01% Tween 20. The fluorescence of the enzyme product, 4-methylumberlliferone (4-MU), was measured by using a microplate Fluorometer microplates (Fluoroskan Ascent, ThermoFisher Scientific) with excitation 360 nm and emission 450 nm. The fluorescence readings were corrected for blanks, and the results were compared with the fluorescence from a 4-MU calibration curve [30].
3. Results
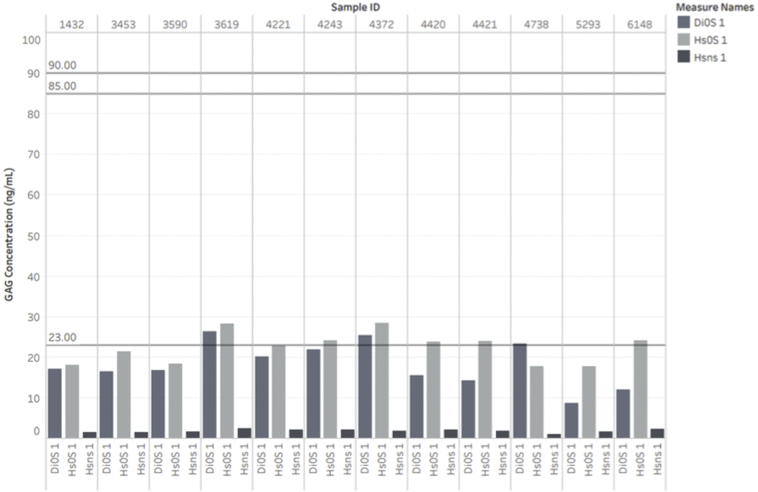
A total of 18,222 samples were analyzed from the general newborn population for GAG concentrations. No samples were known to have MPS disorder. The results of this assay showed that 300 total samples were above the acceptable cutoff values (>23 ng/mL for HS-NS, >88 ng/mL for DS, and/or >90 ng/mL for HS-0S) [18]. It was determined that one sample was a true positive who had been clinically and biochemically diagnosed with MPS II. Concentration levels of HS-0S, HS-NS, and Di-0S in this case with MPS II were 141.6 ng/mL, 26.04 ng/mL, and 30.9 ng/mL, respectively. Both HS0S and HSNS levels were above the established cutoff values of 90 ng/mL and 23 ng/mL, respectively. Therefore, the rate of true positive in this population was 1/18,222 or 0.005% (Table 1).
Table 1.
False positive rate as determined by GAG concentration assay using LC-MS/MS. 300 samples were found to be above the cutoff levels as previously established and described in Kubaski et al. [18].
| First screening | |
|---|---|
| Total samples | 18,222 |
|
300 |
|
1.64% |
|
0.00655% |
|
98.36% |
| Second screening | |
| Total samples | 212 |
|
55 |
|
0.41% |
Refers to anticipated false positives as opposed to acutal mesasured false positives, as described in the paper.
The rate of total false positives was, therefore, determined to be 299/18,222 or 1.64% (Table 1), and the rate of true negatives after the first assay was 98.4%.
Taken from the samples that were above the cutoff values of two or more of the three GAGs screened, 212 from a total of 300 samples were available to be rescreened for a second time, utilizing a second LC-MS/MS assay done in replicate. We conducted a Cochran Q test as described below to determine if this sample number could be considered replicative of the total population.
Case: The result of an assay belonging to a specific individual.
Sample: Not to be confused with a DBS; this will refer to the common statistical definition of “sample.”
300 is being treated as the proportion of the tested population of 18,222 with elevated GAGs. Of this 300, we had a representative simple random sample (henceforth “SRS”) of 212 cases with elevated GAGs. One out of 212 cases has been identified as a True Positive test for MPS II. We investigated that with 95% confidence these 212 cases represented the population of 300 total cases with elevated GAGs by using Cochran's Formula. Given 95% confidence, our results fell within a Z-score of Z = ∣ − 1.96, 1.96∣.
The results for an ideal sample size given a population of 300 high-GAG cases if a margin of error of 0.00601% or P = .0000601 or less was set up:
With 95% confidence, a sample size of 212 out of a population of 300 high-GAG cases represented the 300 and accurately predicted the true incidence of MPS to within +/− 0.00601% or p = .0000601.
Given that this is representative of the incidence, the apparent false positive rate through two separate screenings of GAG concentration levels was 0.47%.
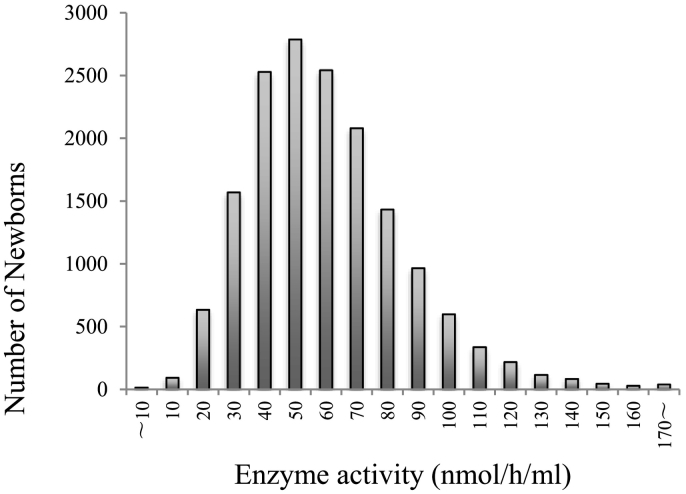
A total of 16,093 deidentified newborn samples were measured from the activity of α-L-iduronidase (IDUA). There was an average enzyme activity of 64.92 ± 6.835 μmol/L/h (Fig. 1). The 34 samples were less than mean - 2SD (13.67 μmol/L/h) after the first screening. This represented 0.21% percent of the population screened. Enzyme activity levels of samples below the cutoff level are shown in Fig. 2.
Fig. 1.
Distribution of samples measured for IDUA activity. There were 16,093 samples screened. The cutoff values were calculated as the activity level of mean - 2SD or 13.67 nmol/h/mL.
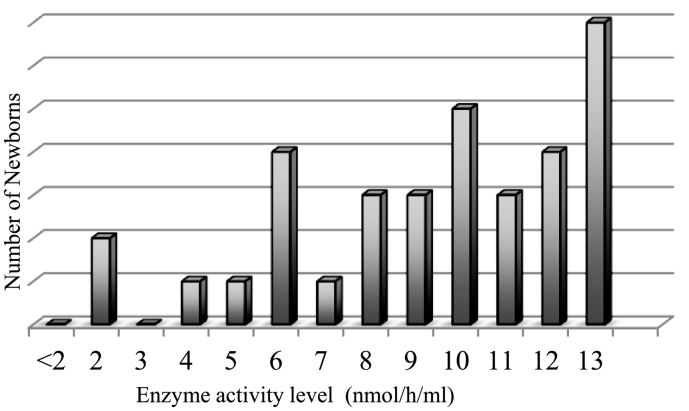
Fig. 2.
IDUA levels of samples under the cutoff level of 13.67 nmol/h/mL. From the samples tested, 34 (0.21%) samples were under the cutoff level.
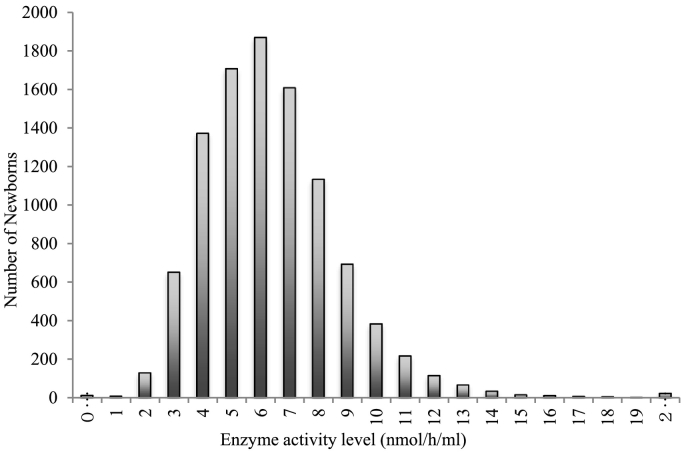
Since MPS II is an X-linked recessive genetic disorder, exclusively male samples were screened. 10,055 deidentified male samples were screened for iduronate-2-sulfatase (IDS) levels. The average enzyme activity was 6.64 nmol/h/mL with a standard deviation of 0.895 mol/L/h (Fig. 3).
Fig. 3.
Distribution of IDS activity levels in 10,055 samples screened for deficiency. The cutoff value was calculated as the activity level of mean - 2SD or 1.79 nmol/h/mL.
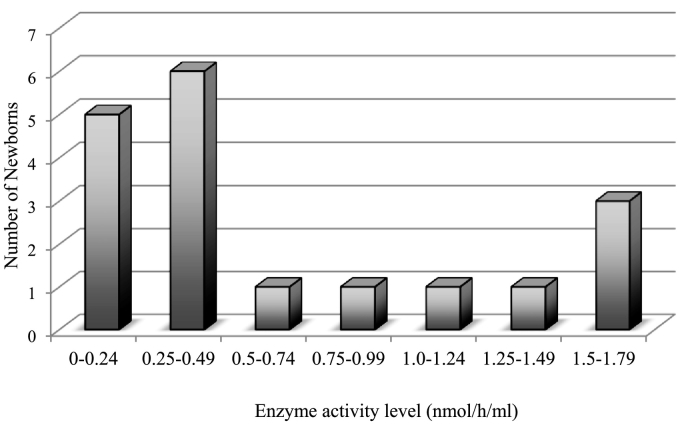
When 1.79 nmol/h/mL was set to the cutoff value of 1st screening (below mean – 2SD), 18 samples out of the 10,055 male samples screened were positive (0.18%) (Fig. 4).
Fig. 4.
IDS levels of samples under the cutoff level of 1.79 nmol/h/mL. From the samples tested, 18 (0.18%) samples were under the cutoff level.
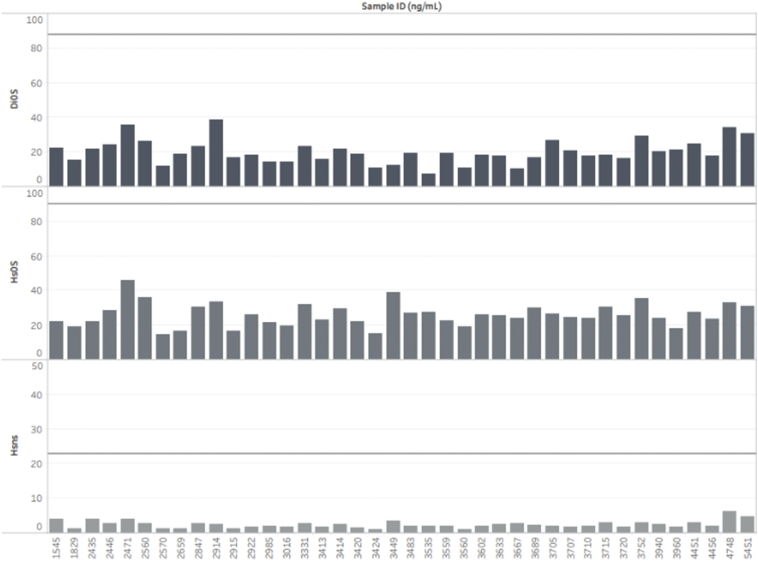
DBS samples whose enzyme activity was below mean – 2SD for either IDUA or IDS activity were also screened for GAG concentrations of HS-0S, DS, and HSNS (Fig. 5, Fig. 6).
Fig. 5.
GAG levels in samples showing below the cutoff level of the IDUA enzyme. The solid line represents the cutoffs for MPS I and II in newborn MPS patients as established by Kubaski et al. [18].
Fig. 6.
GAG levels in samples showing below the cutoff level of the IDS enzyme. The solid line represents the cutoffs for MPS I and II in newborn MPS patients, as previously described.
No sample provided the elevation of any specific GAG, suggesting that these are false positive samples by enzyme assay (IDUA or IDS). Overall, the combination of both GAG and enzyme assays yielded neither false positive nor false negative results in MPS I and II.
4. Discussion
The major clinical symptoms for MPS disorders do not become apparent at birth. Delay of diagnosis severely limits the treatment window for patients. GAG levels or enzyme levels, on the other hand, will be elevated or reduced from birth in MPS patients, respectively [16,19]. Although GAGs are elevated due to other disorders, the number of false positives is reduced by requiring elevation of at least two GAGs. We have previously shown that MPS newborns have high levels or more than one GAG subtype [18,28,31].
In this study, we screened 18,222 general newborn DBS samples. Samples were flagged as suspicious if they were above the previously established cutoff values in at least two of the three GAGs screened for. There were 300 samples above the cutoff levels after the first screening. Since MPS has an incidence rate of 1:25,000, it is not possible that all samples were true positives. We conducted a second screen using the same method as the first, for all available samples. There were 212 samples available to be rescreened. We conducted a Cochran Q test to determine if this sample size was representative of the entire sample population size. This sample size was found to be representative of the population with a margin of error of 0.00601%.
After the second screening, 55 out of 212 samples remained high for at least two of the three GAGs. We found that the false positive rate after the first GAG screening was 1.64%. There were 212 samples able to be rescreened, and we found that 55 of these samples remained high in at least two GAGs. One of these samples was true positive so that the remaining samples were known to be false positives. Since this sample was found to be representative of the total, we applied this false positive rate after the second screening to the entire population giving an anticipated false positive rate of 0.41%.
Separately, IDUA and IDS enzyme assays were conducted on deidentified newborn DBS samples. Fifty-two samples were found to have enzyme activity levels below the cutoff values from combined results of the IDUA and IDS enzyme assays. These samples were then analyzed for GAG concentration by LC-MS/MS. In this way, the one true positive sample, a male with MPS II was identified. He had an IDS level that was below the cutoff and was higher than the cut off for all three GAG's screened.
When conducting enzyme activity tests by fluorimetry, there was a false positive rate of 0.21% and 0.18% for IDUA and IDS enzyme activity in samples screened, respectively. When DBS samples with low enzyme activity levels were screened for measurement of GAGs, there were no samples with the GAG elevated above cutoff levels. This finding indicates a false positive rate of 0% when both enzyme activity and GAG levels are measured. Since samples were collected a minimum of three years prior to testing, we know that there was only one affected sample in all samples tested, which was detected as a true positive. The false negative rate was, therefore, 0%. It is notable that this study is limited by the quantification of enzymes by fluorimetry instead of tandem mass spectrometry, consequently larger false-positive rates are expected.
After both biochemical and GAG assays were conducted, we compared the samples with known clinical data. One out of the 18,222 samples screened for GAG concentrations was later found to be affected with MPS II. Both HS0S and HSNS levels were above the previously established cutoff values of 90 ng/mL and 23 ng/mL, respectively. DS levels were not above the previously established cutoff level.
Since we have identified only one patient with MPS in the limited number of samples, it may not be possible to calculate an accurate false negative or positive rate. It is critical to screen any affected patients at the asymptomatic stage. We have determined the cutoff values of threes GAGs (DS and HS) by using newborn samples with MPS I, II, or III. In a future study, it is required to determine the cutoff values of keratan sulfate for newborn patients with MPS IV.
We have identified one MPS II patient with the established cutoff values for HS-0S, HS-NS, and DS, indicating the utility of GAG screening as part of NBS for MPS I, II, and III. In two-tier approaches with GAG quantification and enzyme assay, false positives rates have been dramatically reduced. Our results indicate that screening for MPS should involve a two-tier approach by using both enzyme activity and GAG assays as the gold standard for screening of MPS at newborns.
5. Conclusion
Two-tier screening involving a combination of enzyme activity and multiple GAG concentration by MS/MS should be considered the gold standard for the diagnosis of MPS patients in newborns. A combination assay for GAG measurement as well as enzyme activity, taken from the general newborn population, allows for effective discrimination of non-affected from affected samples. This strategy enhances the value of both methods as potential newborn screening for MPS.
Acknowledgments
This work was supported by grants from the Japanese MPS Society, the Austrian MPS Society, Bennett Foundation, and International Morquio Organization (Carol Ann Foundation). S.T. was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of NIH under grant numbers P20GM103464 and P30GM114736. M.S. was supported by the University of Delaware and the A.I duPont/Nemours Hospital for Children. F.K. was supported by Coordenação de aperfeiçoamento de pessoal de Nível Superior (CAPES), Brazil. The content of the article has not been influenced by the sponsors. We also appreciate individual patients who participated in this project.
References
- 1.Muenzer J. The mucopolysaccharidoses: a heterogeneous group of disorders with variable pediatric presentations. J. Pediatr. 2004 May;144(5 Suppl):S27–S34. doi: 10.1016/j.jpeds.2004.01.052. [DOI] [PubMed] [Google Scholar]
- 2.Galimberti C., Madeo A., Di Rocco M., Fiumara A. Mucopolysaccharidoses: early diagnostic signs in infants and children. Ital. J. Pediatr. 2018 Nov;44(Suppl. 2) doi: 10.1186/s13052-018-0550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan S.A., Peracha H., Ballhausen D., Wiesbauer A., Rohrbach M., Gautschi M. Epidemiology of mucopolysaccharidoses. Mol. Genet. Metab. 2017;121(3):227–240. doi: 10.1016/j.ymgme.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byers S., Rozaklis T., Brumfield L.K., Ranieri E., Hopwood J.J. Glycosaminoglycan accumulation and excretion in the mucopolysaccharidoses: characterization and basis of a diagnostic test for MPS. Mol. Genet. Metab. 1998 Dec;65(4):282–290. doi: 10.1006/mgme.1998.2761. [DOI] [PubMed] [Google Scholar]
- 5.Gallegos-Arreola M.P., Machorro-Lazo M.V., Flores-Martínez S.E., Zúñiga-González G.M., Figuera L.E., González-Noriega A. Urinary glycosaminoglycan excretion in healthy subjects and in patients with mucopolysaccharidoses. Arch. Med. Res. 2000;31(5):505–510. doi: 10.1016/s0188-4409(00)00104-1. 2000 Sep-Oct. [DOI] [PubMed] [Google Scholar]
- 6.Stapleton M., Arunkumar N., Kubaski F., Mason R.W., Tadao O., Tomatsu S. Clinical presentation and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2018;125(1–2):4–17. doi: 10.1016/j.ymgme.2018.01.003. 09. [DOI] [PubMed] [Google Scholar]
- 7.Montaño A.M., Tomatsu S., Gottesman G.S., Smith M., Orii T. International Morquio A registry: clinical manifestation and natural course of Morquio A disease. J. Inherit. Metab. Dis. 2007 Apr;30(2):165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 8.Lin H.Y., Chuang C.K., Chen M.R., Lin S.J., Chiu P.C., Niu D.M. Clinical characteristics and surgical history of Taiwanese patients with mucopolysaccharidosis type II: data from the hunter outcome survey (HOS) Orphanet J. Rare Dis. 2018;13(1):89. doi: 10.1186/s13023-018-0827-1. 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruijter G.J., Valstar M.J., van de Kamp J.M., van der Helm R.M., Durand S., van Diggelen O.P. Clinical and genetic spectrum of Sanfilippo type C (MPS IIIC) disease in the Netherlands. Mol. Genet. Metab. 2008 Feb;93(2):104–111. doi: 10.1016/j.ymgme.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Wraith J.E., Jones S. Mucopolysaccharidosis type I. Pediatr. Endocrinol. Rev. 2014 Sep;12(Suppl. 1):102–106. [PubMed] [Google Scholar]
- 11.Melbouci M., Mason R.W., Suzuki Y., Fukao T., Orii T., Tomatsu S. Growth impairment in mucopolysaccharidoses. Mol. Genet. Metab. 2018;124(1):1–10. doi: 10.1016/j.ymgme.2018.03.004. 05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guffon N., Journeau P., Brassier A., Leger J., Chevallier B. Growth impairment and limited range of joint motion in children should raise suspicion of an attenuated form of mucopolysaccharidosis: expert opinion. Eur. J. Pediatr. 2019 Apr;178(4):593–603. doi: 10.1007/s00431-019-03330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parini R., Jones S.A., Harmatz P.R., Giugliani R., Mendelsohn N.J. The natural history of growth in patients with hunter syndrome: data from the hunter outcome survey (HOS) Mol. Genet. Metab. 2016 Apr;117(4):438–446. doi: 10.1016/j.ymgme.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Aboul Nasr A., Fateen E. Prenatal diagnosis of mucopolysaccharidoses (MPS): the first Egyptian experience. Bratisl. Lek. Listy. 2004;105(9):310–314. [PubMed] [Google Scholar]
- 15.Natowicz M.R., Isman F., Prence E.M., Cedrone P., Allen J.J. Rapid prenatal testing for human beta-glucuronidase deficiency (MPS VII) Genet. Test. 2003;7(3):241–243. doi: 10.1089/109065703322537269. [DOI] [PubMed] [Google Scholar]
- 16.Kubaski F., Brusius-Facchin A.C., Mason R.W., Patel P., Burin M.G., Michelin-Tirelli K. Elevation of glycosaminoglycans in the amniotic fluid of a fetus with mucopolysaccharidosis VII. Prenat. Diagn. 2017 My;37(5):435–439. doi: 10.1002/pd.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D., Lin Y., Huang Y., Zhang W., Jiang M., Li X. Early prenatal diagnosis of lysosomal storage disorders by enzymatic and molecular analysis. Prenat. Diagn. 2018;38(10):779–787. doi: 10.1002/pd.5329. 09. [DOI] [PubMed] [Google Scholar]
- 18.Kubaski F., Mason R.W., Nakatomi A., Shintaku H., Xie L., van Vlies N.N. Newborn screening for mucopolysaccharidoses: a pilot study of measurement of glycosaminoglycans by tandem mass spectrometry. J. Inherit. Metab. Dis. 2017;40(1):151–158. doi: 10.1007/s10545-016-9981-6. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akella R.R., Kadali S. Amniotic fluid glycosaminoglycans in the prenatal diagnosis of mucopolysaccharidoses - a useful biomarker. Clin. Chim. Acta. 2016 Sep;460:63–66. doi: 10.1016/j.cca.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Whiteman P., Henderson H. A method for the determination of amniotic-fluid glycosaminoglycans and its application to the prenatal diagnosis of hurler and Sanfilippo diseases. Clin. Chim. Acta. 1977 Aug;79(1):99–105. doi: 10.1016/0009-8981(77)90466-1. [DOI] [PubMed] [Google Scholar]
- 21.Mossman J., Patrick A.D. Prenatal diagnosis of mucopolysaccharidosis by two-dimensional electrophoresis of amniotic fluid glycosaminoglycans. Prenat. Diagn. 1982 Jul;2(3):169–176. doi: 10.1002/pd.1970020305. [DOI] [PubMed] [Google Scholar]
- 22.Khan S.A., Mason R.W., Giugliani R., Orii K., Fukao T., Suzuki Y. Glycosaminoglycans analysis in blood and urine of patients with mucopolysaccharidosis. Mol. Genet. Metab. 2018;125(1–2):44–52. doi: 10.1016/j.ymgme.2018.04.011. 09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin R., Beck M., Eng C., Giugliani R., Harmatz P., Muñoz V. Recognition and diagnosis of mucopolysaccharidosis II (Hunter syndrome) Pediatrics. 2008 Feb;121(2):e377–e386. doi: 10.1542/peds.2007-1350. [DOI] [PubMed] [Google Scholar]
- 24.Hendriksz C.J., Harmatz P., Beck M., Jones S., Wood T., Lachman R. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol. Genet. Metab. 2013;110(1–2):54–64. doi: 10.1016/j.ymgme.2013.04.002. 2013 Sep-Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendriksz C.J., Berger K.I., Giugliani R., Harmatz P., Kampmann C., Mackenzie W.G. International guidelines for the management and treatment of Morquio A syndrome. Am. J. Med. Genet. A. 2015 Jan;167A(1):11–25. doi: 10.1002/ajmg.a.36833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oguma T., Tomatsu S., Montano A.M., Okazaki O. Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal. Biochem. 2007 Sep;368(1):79–86. doi: 10.1016/j.ab.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Grüner N., Stambouli O., Ross R.S. Dried blood spots—preparing and processing for use in immunoassays and in molecular techniques. J. Vis. Exp. 2015 Mar;97 doi: 10.3791/52619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomatsu S., Shimada T., Mason R.W., Kelly J., LaMarr W.A., Yasuda E. Assay for Glycosaminoglycans by tandem mass spectrometry and its applications. J. Anal. Bioanal. Tech. 2014 Mar;2014(Suppl. 2):6. doi: 10.4172/2155-9872.S2-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin S.P., Lin H.Y., Wang T.J., Chang C.Y., Lin C.H., Huang S.F. A pilot newborn screening program for Mucopolysaccharidosis type I in Taiwan. Orphanet J. Rare Dis. 2013 Sep;8:147. doi: 10.1186/1750-1172-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tolun A., Graham C., Wang T., AE E., Pamula V., Millington D. A novel fluorometric enzyme analysis method for hunter syndrome using dried blood spots. Mol. Genet. Metab. 2013;105(3):519–521. doi: 10.1016/j.ymgme.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Kemper A.R., Brosco J., Green N.S., Ojodu J., Jones E., Comeau A. Health resources and services administration: Maternal and Child Health Bureau; 2015. Newborn Screening for Mucopolysaccharidosis Type 1 (MPS I): A Systematic Review of Evidence Report of Final Findings. [Google Scholar]