Abstract
Background and Aims
Tumor necrosis factor (TNF) is a major pathogenic effector and a therapeutic target in inflammatory bowel disease (IBD), yet the basis for TNF-induced intestinal epithelial cell (IEC) death is unknown, because TNF does not kill normal IECs. Here, we investigated how chronic nuclear factor (NF)- κB activation, which occurs in human IBD, promotes TNF-dependent IEC death in mice.
Methods
Human IBD specimens were stained for p65 and cleaved caspase-3. C57BL/6 mice with constitutively active IKKβ in IEC (Ikkβ(EE)IEC), Ripk1D138N/D138N knockin mice, and Ripk3-/- mice were injected with TNF or lipopolysaccharide. Enteroids were also isolated from these mice and challenged with TNF with or without RIPK1 and RIPK3 inhibitors or butylated hydroxyanisole. Ripoptosome-mediated caspase-8 activation was assessed by immunoprecipitation.
Results
NF-κB activation in human IBD correlated with appearance of cleaved caspase-3. Congruently, unlike normal mouse IECs that are TNF-resistant, IECs in Ikkβ(EE)IEC mice and enteroids were susceptible to TNF-dependent apoptosis, which depended on the protein kinase function of RIPK1. Constitutively active IKKβ facilitated ripoptosome formation, a RIPK1 signaling complex that mediates caspase-8 activation by TNF. Butylated hydroxyanisole treatment and RIPK1 inhibitors attenuated TNF-induced and ripoptosome-mediated caspase-8 activation and IEC death in vitro and in vivo.
Conclusions
Contrary to common expectations, chronic NF-κB activation induced intestinal crypt apoptosis after TNF stimulation, resulting in severe mucosal erosion. RIPK1 kinase inhibitors selectively inhibited TNF destructive properties while preserving its survival and proliferative properties, which do not require RIPK1 kinase activity. RIPK1 kinase inhibition could be a potential treatment for IBD.
Keywords: IBD, RIPK1, Intestinal Epithelial Cell, Ripoptosome, Cell Death
Abbreviations used in this paper: BHA, butylated hydroxyanisole; cC-3, cleaved caspase-3; cC-8, cleaved caspase-8; CD, Crohn’s disease; CHX, cycloheximide; DPI, phenyleneiodonium; IB, immunoblotting; IBD, inflammatory bowel disease; IEC, intestinal epithelial cell; IHC, immunohistochemistry; i.p., intraperitoneally; LPS, lipopolysaccharide; Nec-1, necrostatin-1; NF, nuclear factor; PBS, phosphate-buffered saline; qPCR, quantitative polymerase chain reaction; TNF, tumor necrosis factor; UC, ulcerative colitis; WT, wild type
Graphical abstract
Summary.
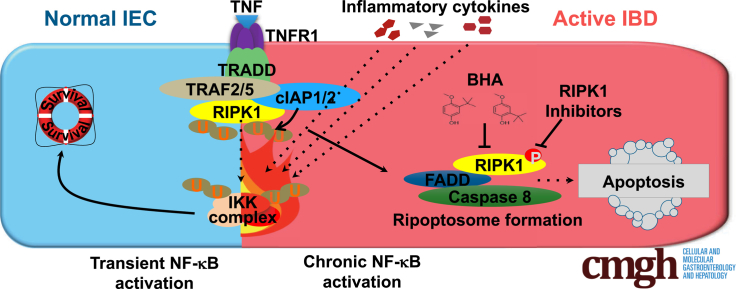
Here we describe how chronic nuclear factor–κB activation facilitates the formation of the ripoptosome, which enhances tumor necrosis factor–induced apoptosis in intestinal epithelial cells. Blockade of the kinase activity of RIPK1 prevents tumor necrosis factor's destructive properties while preserving its survival and proliferative functions.
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), represent a major cause of morbidity and mortality, affecting approximately 1.4 million Americans.1 IBDs are characterized by colon or small intestine inflammation and a relapsing course with clinically quiescent periods followed by bouts of severe and damaging inflammation associated with tissue destruction and mucosal erosion.1 Apoptotic and necrotic features are often found in the crypt compartment of the affected intestinal tissue.2 IBD etiology depends on genetic susceptibility and environmental triggers.3 Although most research efforts have focused on immune cells, it is becoming increasingly clear that intestinal epithelial cells (IEC) are also important players in IBD pathogenesis4 and their ability to regenerate and heal the injured mucosa is critical for long-term remission.5 Interestingly, single nucleotide polymorphisms in ubiquitously expressed genes encoding nuclear factor (NF)-κB-regulated molecules show strong association with IBD3,6 and activated/nuclear NF-κB is present in both IEC and lamina propria macrophages of active disease areas.7 NF-κB stimulates transcription of numerous genes implicated in IBD pathogenesis, including TNF, which codes for the prototypical inflammatory cytokine tumor necrosis factor (TNF). TNF inhibition is one of the main therapeutic options in IBD7 leading to reduced IEC apoptosis and enhanced mucosal repair.8 Unlike other TNF-dependent inflammatory diseases, such as psoriasis, extensive epithelial cell death is a cardinal feature of IBD, yet the basis for TNF-induced IEC death is unknown, because TNF is not cytotoxic to normal IEC unless treated with protein synthesis inhibitors.
In fact, in most cell types, IEC included, transient TNF signaling inhibits apoptosis because of IKKβ-dependent NF-κB activation.9 Correspondingly, ablation of IKKβ10,11 or its regulatory subunit IKKγ/NEMO12 renders IEC susceptible to TNF-induced death. Elevated expression of A20, a protein thought to be a negative regulator of NF-κB, also sensitizes IEC to TNF-mediated killing but this effect is not due to NF-κB inhibition.13 Indeed, IKK or NF-κB deficiencies have not been described in IBD.
To determine whether persistent NF-κB activation in IBD7 has a pathogenic function, we generated Ikkβ(EE)IEC mice in which a constitutively active IKKβ(EE) variant is expressed in IEC from the villin promoter.14 Surprisingly, instead of being resistant to TNF-induced mucosal erosion, Ikkβ(EE)IEC mice display severe TNF-dependent epithelial layer destruction when challenged with TNF or various stimuli that induce TNF production.14 The mechanism by which constitutive IKKβ/NF-κB activation renders mouse IEC susceptible to TNF-induced killing, rather than prevent it, is unknown, but is likely to be relevant to the effect of chronic NF-κB activation in IEC of active IBD lesions. We have therefore investigated the mechanisms by which TNF induces IEC death in Ikkβ(EE)IEC mice.
We focused our studies on the function of RIPK1, a protein kinase that serves as a key regulator of life and death in TNF-exposed cells. Under conditions in which RIPK1 is subject to K63-linked and linear ubiquitination, TNFR1 engagement induces cell survival, but when the RIPK1 ubiquitination pattern is altered, TNF induces 1 of 2 forms of programmed cell death: necroptosis15,16 or noncanonical apoptosis that is not inhibited by NF-κB.17 The latter depends on formation of a RIPK1-dependent signaling complex that also contains FADD and caspase-8, known as complex IIb or the ripoptosome.17 However, in cells that are completely deficient of RIPK1, which is needed for NF-κB activation,18 TNF leads to a classical apoptotic response that is NF-κB preventable.19, 20, 21 Adding to the complexities of TNF-mediated cell death and its dependence on NF-κB inhibition or RIPK1 kinase activation, we found that elevated A20 expression facilitates ripoptosome formation and RIPK1 activation.13 Here we describe the role of RIPK1 in TNF-mediated IEC killing and mucosal erosion in Ikkβ(EE)IEC mice.
Results
NF-κB and Caspase-3 Activation in Human IBD
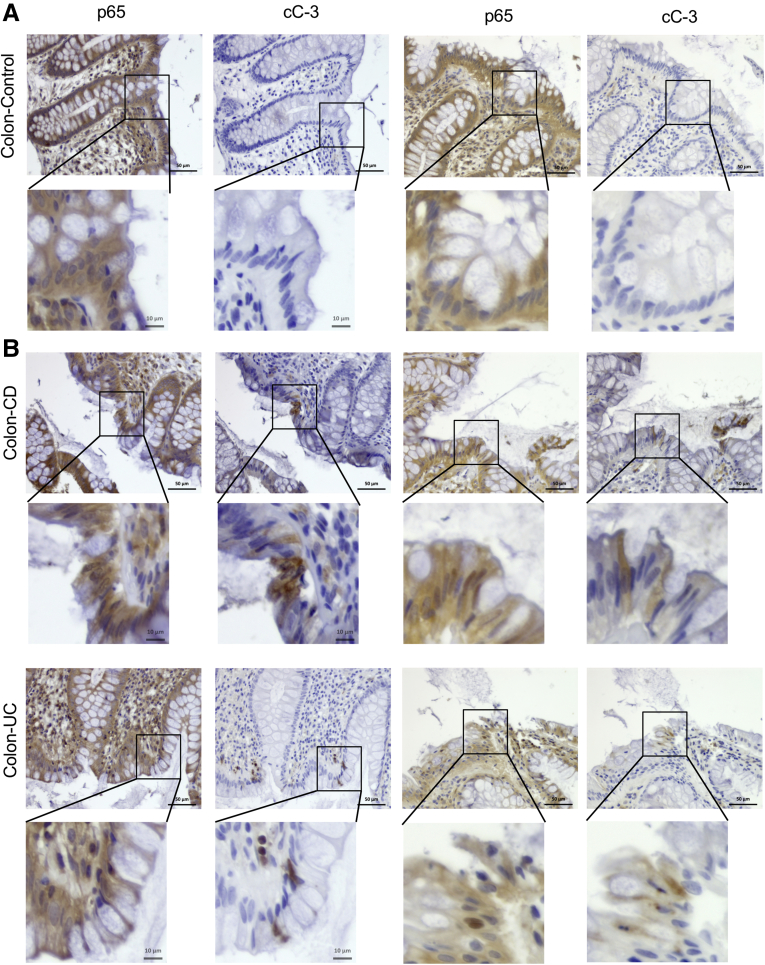
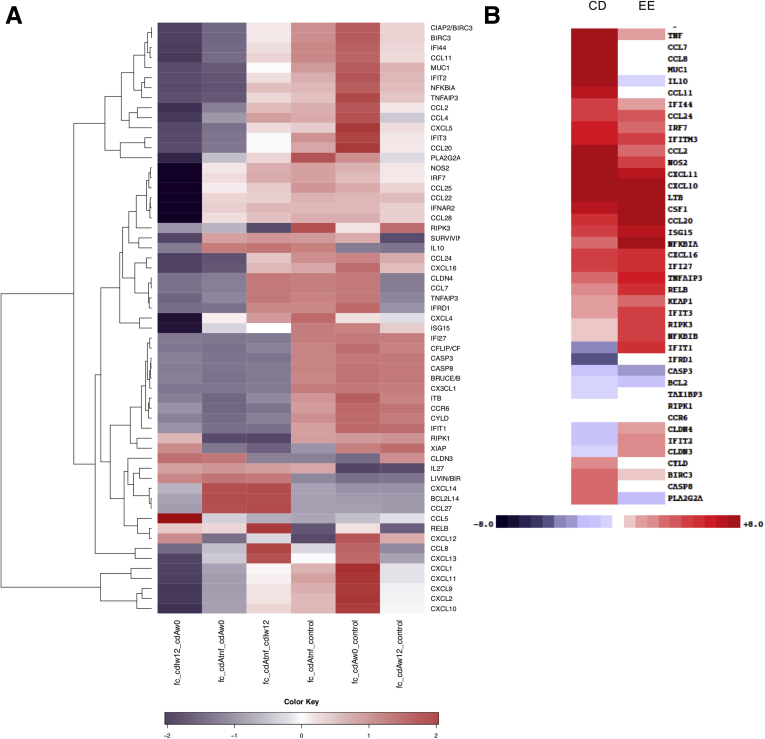
We conducted immunohistochemistry (IHC) analysis of human tissue specimens from healthy individuals and patients suffering with either ileal or colonic CD or UC to determine the correlation between NF-κB activation and cell death. As previously described,13 we examined 10 normal colon specimens, 10 samples with active UC, and 10 samples with colonic CD, as well as 4 active ileitis samples and 5 inactive ileal CD samples, all of which were stained for p65/RelA and cleaved caspase-3 (cC-3). In general, normal colonic or ileal specimens contained hardly any IEC that were positive for cC-3 or nuclear p65 (Figure 1A). As described,13 active IBD specimens contained more cC-3-positive cells than control samples. Interestingly, the areas enriched for cC-3-positive cells correlated with areas harboring cells with nuclear p65 (Figure 1A and B). Active UC specimens contained more cC-3- and p65-positive cells than CD specimens and hardly any differences were observed between colonic and ileal CD (Table 1). Our finding of activated/nuclear NF-κB in IEC is consistent with other reports.7,22 In addition, mining of publicly available data sets also showed upregulation of numerous NF-κB target genes including TNFAIP3, CXCL10, CXCL11, CCL2, CCL8, CSF1, CCL11, and CCL20 in active IBD areas that decreased after anti-TNF therapy (Figure 2A).23
Figure 1.
Active IBD tissue displays spatially adjacent NF-κB and caspase-3 activation in intestinal epithelial cells. Human control (A), CD and UC colon sections (B) were stained with antibodies to cC-3 and p65(RelA). Magnification bars: 50 μm. Arrows show positive cells. Results are representative for 15 healthy, 14 CD, and 10 UC specimens.
Table 1.
Number of Samples and the Corresponding Percentages of Nuclear p65 and Cleaved Caspase 3 Expression Level in IEC of Control Tissue and Active IBD Specimens
| Expression level | C ileum | C colon | CD ileum | CD colon | UC colon |
|---|---|---|---|---|---|
| Nuclear p65 expression level | |||||
| 0 | 4 (80) | 7 (70) | 1 (25) | 0 | 0 |
| 1 | 1 (20) | 3 (30) | 1 (25) | 2 (20) | 1 (10) |
| 2 | 0 | 0 | 2 (50) | 5 (50) | 4 (40) |
| 3 | 0 | 0 | 0 | 3 (30) | 5 (50) |
| Total | 5 | 10 | 4 | 10 | 10 |
| Caspase-3 expression level | |||||
| 0 | 2 (40) | 5 (50) | 1 (25) | 0 | 0 |
| 1 | 3 (60) | 5 (50) | 1 (25) | 3 (30) | 2 (20) |
| 2 | 0 | 0 | 2 (50) | 5 (50) | 4 (40) |
| 3 | 0 | 0 | 0 | 2 (20) | 4 (40) |
| Total | 5 | 10 | 4 | 10 | 10 |
C, control; CD, Crohn’s disease; IBD, inflammatory bowel disease; IEC, intestinal epithelial cells; UC, ulcerative colitis.
Figure 2.
Anti-TNF treatment decreases expression of NF-κB-related genes in human IBD tissue. (A) Heat map showing differentially expressed NF-κB-related genes between healthy control subjects and CD subjects, before and after anti-TNF therapy, in tissue biopsies described in array GSE52746.23 Fc_cdIw12_cdAw0 means fold-change between CD samples from inactive areas after 12 weeks of anti-TNF therapy and CD samples from active areas at time 0; fc_cdAtnf_cdAw0 means fold-change between CD samples from active areas after 12 weeks of anti-TNF therapy and CD samples from active areas at time 0; fc_cdAtnf_cdIw12 means fold change between CD samples from active areas after 12 weeks of anti-TNF therapy and CD samples from inactive areas after 12 weeks of anti-TNF therapy; fc_cdAtnf_control means fold change between CD samples from active areas after 12 weeks of anti-TNF therapy and healthy control samples; fc_cdAw0_control means fold change between CD samples from active areas at time 0 and healthy control samples; fc_cdAw12_control means fold change between CD samples from active areas after 12 weeks of anti-TNF therapy and healthy control samples. (B) Comparison between genes that are differentially expressed between WT and Ikkβ(EE)IEC enterocytes14 and those that are differentially expressed between CD and normal human ileum (left).13
TNF-Induced Apoptosis in Ikkβ(EE)IEC Mice
To determine the pathogenic function of persistent NF-κB activation we used Ikkβ(EE)IEC mice, which instead of being resistant to TNF-induced mucosal erosion are highly sensitive to TNF.14 Of note, many of the genes found to be up-regulated in human IBD and described in our previous work13 were also up-regulated in Ikkβ(EE)IEC mice relative to the wild-type (WT) mouse epithelium (Figure 2B).14
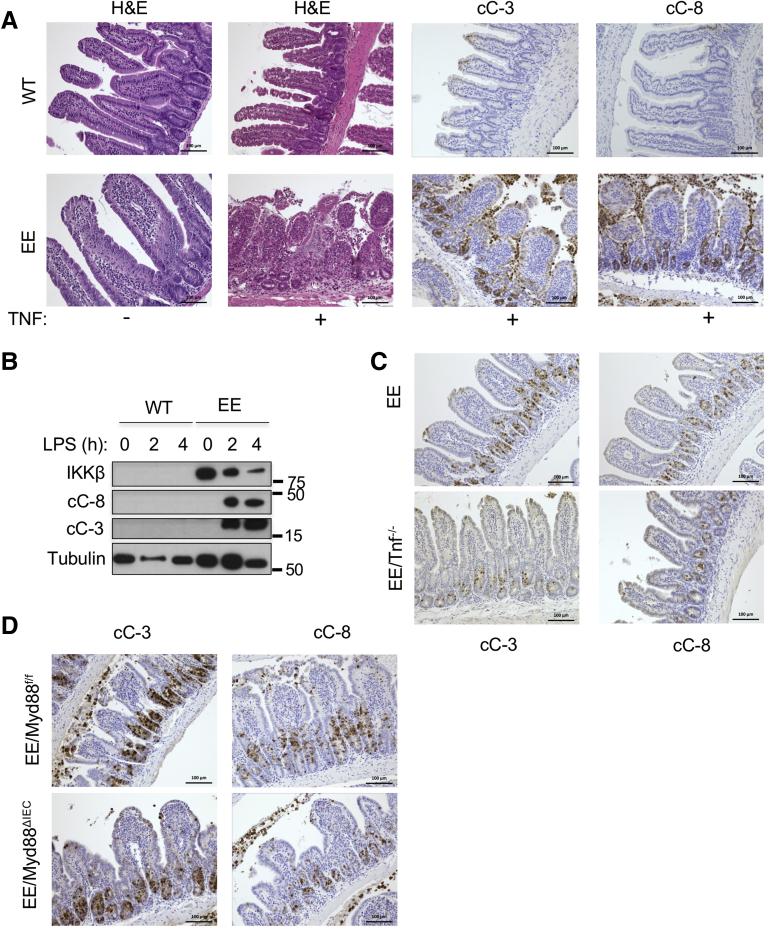
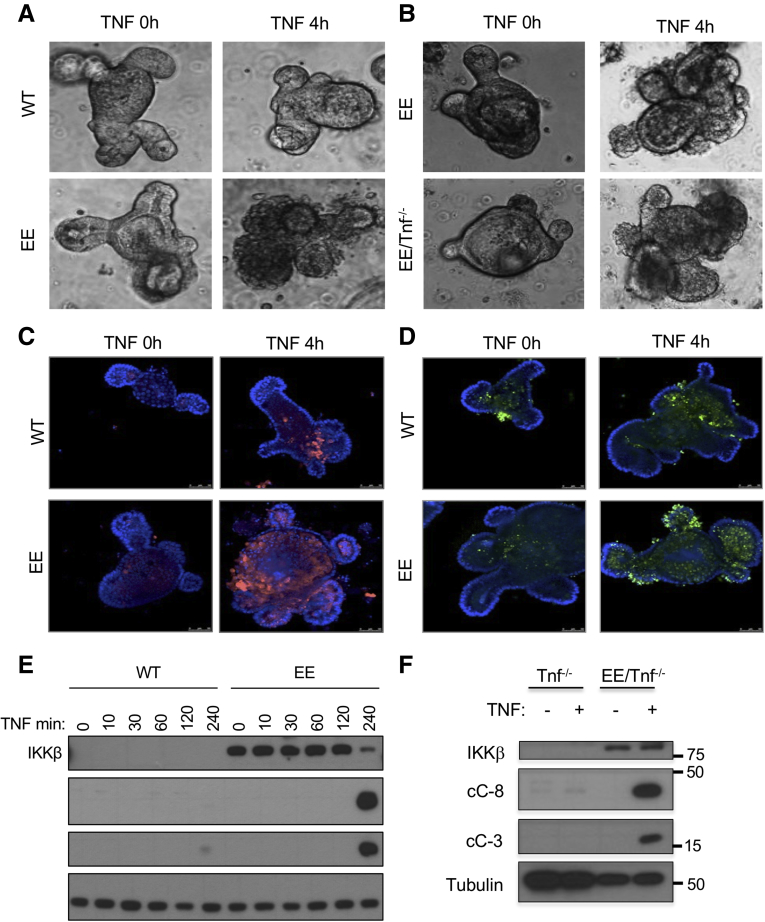
Because TNF can trigger either apoptosis or necroptosis, we used IHC with antibodies specific for activated/cleaved caspase-8 (cC-8) or cC-3 to determine the type of cell death affecting the Ikkβ(EE)IEC small bowel epithelium after administration of TNF or lipopolysaccharide (LPS). Treatment of Ikkβ(EE)IEC mice with either agent activated both caspases (Figure 3A and B). TNF-treated WT mice showed only a mild and transient caspase-3 activation near the villi tips, but not within the crypt compartment, and hardly any caspase-8 activation, correlating with little or no mucosal damage. TNF-treated Ikkβ(EE)IEC mice, however, displayed activation of both caspases in villi and especially within crypt compartments, leading to cell shedding and tissue damage (0.02 ± 0.03 cC-3+ and 0.01 ± 0.02 cC-8+ cells per crypt in WT vs 7.01 ± 1.15 cC-3+ and 4.35 ± 2.19 cC-8+ cells per crypt in Ikkβ(EE)IEC mice; P < .001 and P < .001). Immunoblotting (IB) analysis of the intestinal crypt fraction of Ikkβ(EE)IEC mice intraperitoneally (i.p.) injected with LPS showed strong and sustained cleavage of caspases-3 and -8 and delayed degradation of IKKβ(EE) protein, likely because of caspase activation (Figure 3B). As described,14 Ikkβ(EE)IEC/Tnf–/– mice remained hypersensitive to exogenous TNF and showed caspase-3 and -8 activation after its administration (6.99 ± 1.50 cC-3+ and 3.38 ± 0.59 cC-8+ cells per crypt in Ikkβ(EE)IEC vs 5.07 ± 0.78 cC-3+ and 1.93 ± 0.61 cC-8+ cells per crypt in Ikkβ(EE)IEC/Tnf–/– mice; P = .015 and P < .001) (Figure 3C). Similar results were obtained in TNF-treated Ikkβ(EE)IEC/Myd88ΔIEC mice (5.72 ± 2.22 cC-3+ and 4.35 ± 1.12 cC-8+ cells per crypt in Ikkβ(EE)IEC/Myd88F/F mice vs 8.18 ± 1.17 cC-3+ and 5.61 ± 0.76 cC-8+ cells per crypt in Ikkβ(EE)IEC/Myd88ΔIEC mice; P = .015 and P = .02) (Figure 3D), suggesting an inherent ability of persistent IKKβ activation to sensitize IEC to TNF-induced apoptosis independently of IEC-autonomous toll-like receptor signaling.
Figure 3.
IKKβ(EE)-expressing intestinal crypts are highly susceptible to TNF-induced apoptosis independently of toll-like receptor signaling. (A) WT and Ikkβ(EE)IEC mice were analyzed 4 hours after TNF injection (2 μg intravenously). Jejunal sections were stained with either H&E or antibodies to cC-3 or cC-8. Magnification bars: 100 μm. (B) Lysates of WT and Ikkβ(EE)IEC intestinal mucosa were analyzed at different times after LPS injection (5 mg/kg) by IB. (C) Ikkβ(EE)IEC and Ikkβ(EE)IEC/Tnf–/– mice were injected with TNF (2 μg intravenously). Jejunal sections were prepared 4 hours later and stained with antibodies to either cC-3 or cC-8. Magnification bars: 100 μm. (D) Ikkβ(EE)IEC/Myd88ΔIEC and Ikkβ(EE)IEC/Myd88F/F mice were analyzed 4 hours after TNF injection as above.
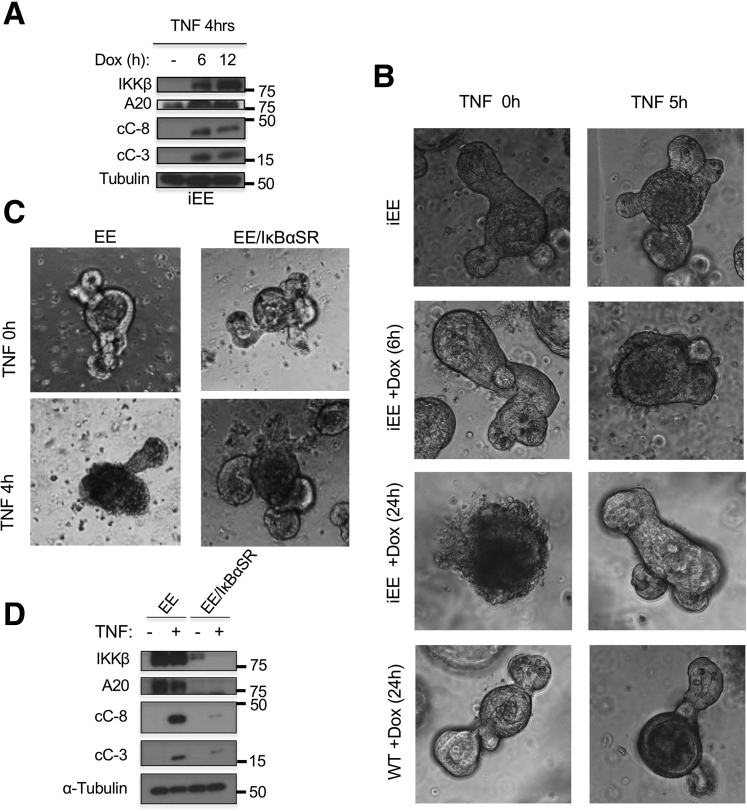
To further investigate this phenomenon and determine whether or not it depends on microbiota and immune cells, we established enteroid cultures.24 Whereas WT enteroids exhibited minimal TNF-induced death, Ikkβ(EE)IEC and Ikkβ(EE)IEC/Tnf–/– enteroids underwent extensive cell death, especially within crypt domains, after incubation with TNF (1.9% ± 3.8% WT dead organoids vs 95.5% ± 5.4% dead in Ikkβ(EE)IEC organoids; P < .001) (Figure 4A and B), leading to their rapid disintegration. cC-3 and TUNEL immunofluorescent staining (Figure 4C and D) and IB analysis of caspases-3 and -8 (Figure 4E and F) correlated and confirmed that Ikkβ(EE)IEC or Ikkβ(EE)IEC/Tnf-/- enteroids underwent TNF-induced apoptosis mainly within crypt domains. Inducible expression of IKKβ(EE) in WT enteroids also conferred susceptibility to TNF-induced apoptosis (Figure 5A). Doxycycline, which was used to induce IKKβ(EE) expression, did not cause cell death, neither did it sensitize WT enteroids to TNF-induced apoptosis (Figure 5B).
Figure 4.
TNF induces apoptosis in IKKβ(EE)-expressing intestinal enteroids. (A) WT and Ikkβ(EE)IEC enteroids were photographed under brightfield 4 hours after TNF stimulation (40 ng/mL). Original magnification ×200. (B) Ikkβ(EE)IEC and Ikkβ(EE)IEC/Tnf–/– enteroids were photographed under brightfield before and after incubation with TNF (40 ng/mL). Original magnification ×200. (C) WT and Ikkβ(EE)IEC enteroids were stained with cC-8 antibody and visualized by confocal microscopy at the indicated times after TNF (40 ng/mL) addition. Magnification bars: 100 μm. (D) WT and Ikkβ(EE)IEC enteroids were TUNEL stained and analyzed by confocal microscopy before and after incubation with TNF. Apoptotic cells are bright green. Magnification bars: 100 μm. (E) Lysates of WT and Ikkβ(EE)IEC enteroids were IB analyzed at different times after TNF addition. (F) Lysates of Tnf-/- and Ikkβ(EE)IEC/Tnf–/– enteroids incubated with or without TNF were analyzed for caspase activation by IB.
Figure 5.
Susceptibility of Ikkβ(EE)IECenteroids to TNF-induced death is NF-κB-dependent. (A) WT enteroids transduced with an inducible IKKβ(EE) construct were preincubated with or without doxycycline as indicated, lysed, and IB analyzed 4 hours after TNF addition. (B) Control and inducible-IKKβ(EE) transduced WT enteroids were incubated for 24 hours with doxycycline before TNF stimulation as indicated. Original magnification Χ200. (C) Control and IκBαSR-transduced Ikkβ(EE)IEC enteroids were incubated with TNF as indicated and photographed under bright field. Original magnification ×200. (D) Control and IκBαSR-transduced Ikkβ(EE)IEC enteroids incubated with or without TNF were lysed and IB analyzed.
To determine whether the elevated susceptibility of Ikkβ(EE)IEC enteroids to TNF-induced death was NF-κB-dependent, we transduced them with a lentivirus expressing IκBα super-repressor (IκBαSR). Enteroids containing IκBαSR no longer exhibited TNF-induced crypt apoptosis (Figure 5C), but unexpectedly IkBαSR expression led to reduced IKKβ(EE) expression (Figure 5D).
RIPK1 Kinase Activity Is Required for TNF-Induced Tissue Damage
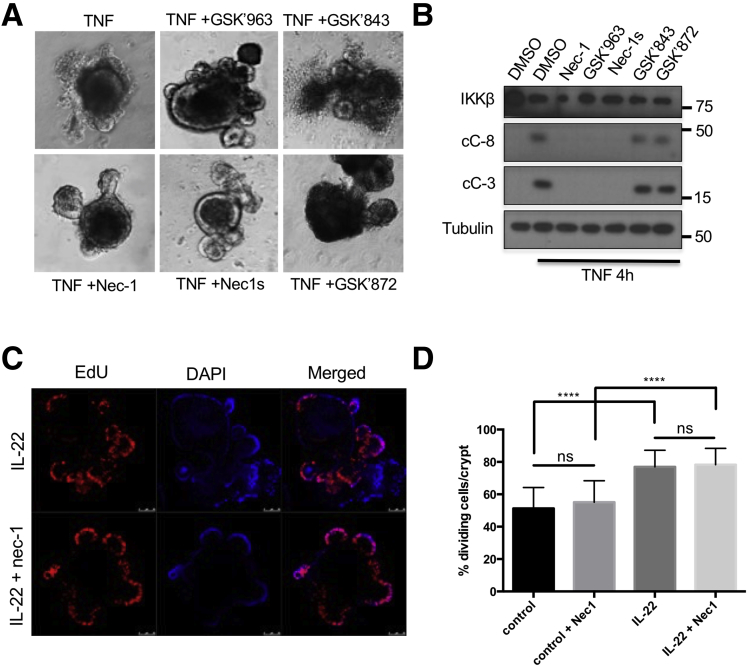
TNFR1 engagement triggers assembly of several distinct signaling complexes, the first of which is complex I, which includes TRADD, RIPK1, TRAF2, and cIAP1/2, and is responsible for IKK and NF-κB activation and inhibition of apoptosis. The latter is mediated by induction of antiapoptotic genes, including those coding for c-FLIP, a caspase-8 inhibitor,25 and survivin, a caspase-3 inhibitor.26 Complex I formation and NF-κB activation depend on the scaffold functions of RIPK1 but not on its kinase activity.16 Notably, and despite its susceptibility to TNF-induced apoptosis, the intestinal epithelium of Ikkβ(EE)IEC mice exhibited elevated expression of antiapoptotic molecules, including BIRC3, BCL2, and BCL2L1.14 Persistent TNFR1 engagement, however, results in receptor internalization, which can promote cell death through either RIPK1 kinase-dependent apoptosis or necroptosis.16,27 To query the involvement of RIPK1 kinase activity in the observed response of Ikkβ(EE)IEC enteroids, we examined the ability of necrostatin-1 (Nec-1), a RIPK1 inhibitor,28 to block TNF-induced killing. Indeed, Nec-1 effectively blocked TNF-induced Ikkβ(EE)IEC enteroid apoptosis (Figure 6A and B), without any effect on crypt proliferation (Figure 6C and D). Nec-1, however, can also inhibit other kinases, such as PAK1.29 We therefore examined the effect of more specific RIPK1 kinase inhibitors and inhibitors of RIPK3, a key inducer of necroptosis.30,31 The RIPK1 inhibitors (GSK’963 and GSK’728 or Nec-1s, which is the active enantiomer of the optimized necrostatin 7-Cl-O-Nec-1), but not the RIPK3 selective inhibitors (GSK’843 and GSK’872), prevented the TNF-induced death of Ikkβ(EE)IEC intestinal enteroids and blocked caspase-3 and -8 cleavage (95.5% ± 5.4% dead organoids after control treatment vs 2.8% ± 3.3% after Nec-1, 3.0% ± 3.5% after GSK’963, 4.9% ± 0.5% after Nec-1s, 95.7% ±5.8% after GSK’843, and 97.7% ± 4.5% after GSK’872 treatments) (Figure 6A and B).
Figure 6.
RIPK1 kinase activity is required for TNF-induced enteroid death. (A) Ikkβ(EE)IEC enteroids were incubated with TNF (40 ng/mL) in the absence or presence of RIPK1 or RIPK3 inhibitors and photographed 4 hours later. Original magnification ×200. (B) Lysates of Ikkβ(EE)IEC enteroids treated as above were subjected to IB analysis after 4 hours. (C) WT enteroids were treated for 24 hours with interleukin-22 (10 ng/mL) in the absence or presence of Nec-1. Two hours before fixation, EdU was added to the culture medium. ****P ≤ .0001. (D) Percentages of EdU positive cells per crypt are shown. IL, interleukin; ns, not significant.
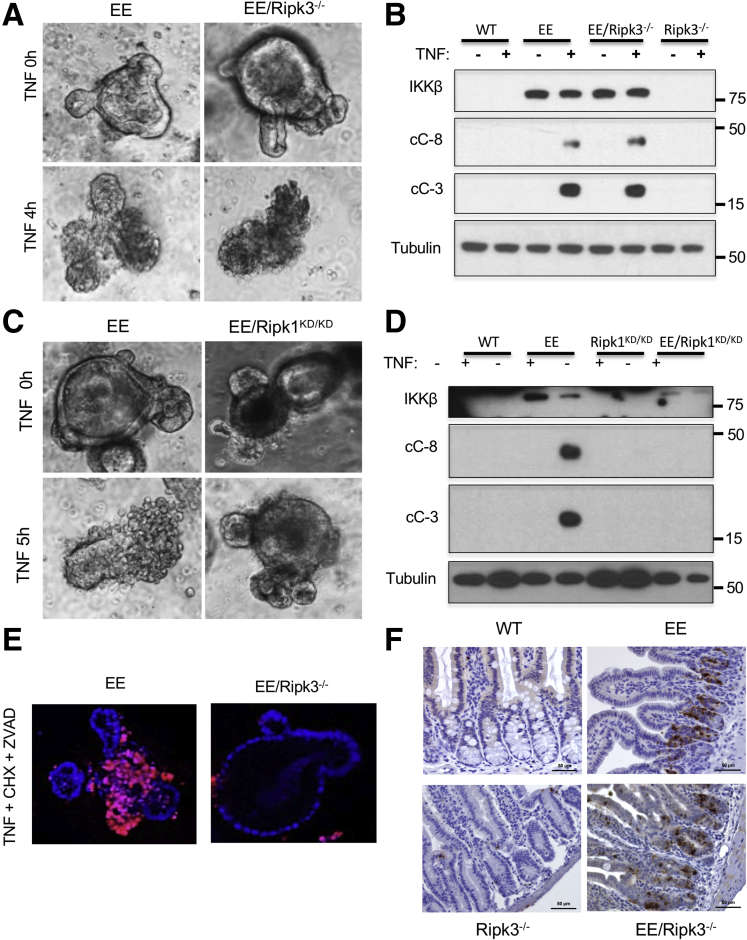
To further validate the role of RIPK1 in TNF-induced apoptosis and tissue damage in Ikkβ(EE)IEC mice, we crossed the latter to Ripk1D138N/D138N homozygous knockin mice, which express a catalytically inactive version of RIPK1 that retains its scaffold function.32 We also crossed Ikkβ(EE)IEC mice with Rip3-/- mice33 to genetically rule out a role for RIPK3-dependent necroptosis in TNF-induced mucosal erosion in our model. Notably, Ripk3 ablation did not prevent TNF-induced apoptosis (Figure 7A and B) but RIPK1 kinase inactivation completely blocked TNF-induced crypt cell death and caspase-3 activation in Ikkβ(EE)IEC enteroids (95.5% ± 5.4% dead Ikkβ(EE)IEC organoids vs 2.0% ± 4.0% dead Ikkβ(EE)IEC/Ripk1D138N/D138N organoids) (Figure 7C and D). As expected,34,35 Ripk3 gene ablation prevented necroptosis in enteroids incubated with TNF + cycloheximide (CHX) + zVAD (5.59 ± 3.12 cC-3+ cells per crypt in Ikkβ(EE)IEC mice vs 6.79 ± 1.02 cC-3+ cells per crypt in Ikkβ(EE)IEC/Rip3-/- mice; P = .91) (Figure 7E and F).
Figure 7.
TNF-induced mucosal damage and IEC death is RIPK3-independent. (A) Ikkβ(EE)IEC and Ikkβ(EE)IEC/Rip3-/- enteroids were photographed under brightfield before and 4 hours after incubation with TNF (40 ng/mL). Original magnification ×200. (B) WT, Ikkβ(EE)IEC, Rip3-/- and Ikkβ(EE)IEC/Rip3-/- enteroids were incubated with or without TNF, lysed, and IB analyzed 5 hours later. (C) Ikkβ(EE)IEC and Ikkβ(EE)IEC/Ripk1D138N/D138N enteroids were photographed under brightfield before and 5 hours after TNF addition. Original magnification ×200. (D) WT, Ikkβ(EE)IEC, Ripk1D138N/D138N, and Ikkβ(EE)IEC/Ripk1D138N/D138N enteroids were incubated with or without TNF, lysed, and IB analyzed 5 hours later. (E) Ikkβ(EE)IEC and Ikkβ(EE)IEC/Rip3-/- enteroids were incubated with TNF (40 ng/mL), CHX (25 μg/mL), and zVAD (10 μg/mL) for 4 hours, stained with Hoechst 33342 and propidium iodide and examined by confocal microscopy. Original magnification ×200. (F) WT, Ikkβ(EE)IEC, Rip3-/-, and Ikkβ(EE)IEC/Rip3-/- mice were analyzed 4 hours after LPS (0.5 μg/g i.p.) injection. Jejunal sections were stained with antibody to cC-3 and photographed. Magnification bars: 100 μm.
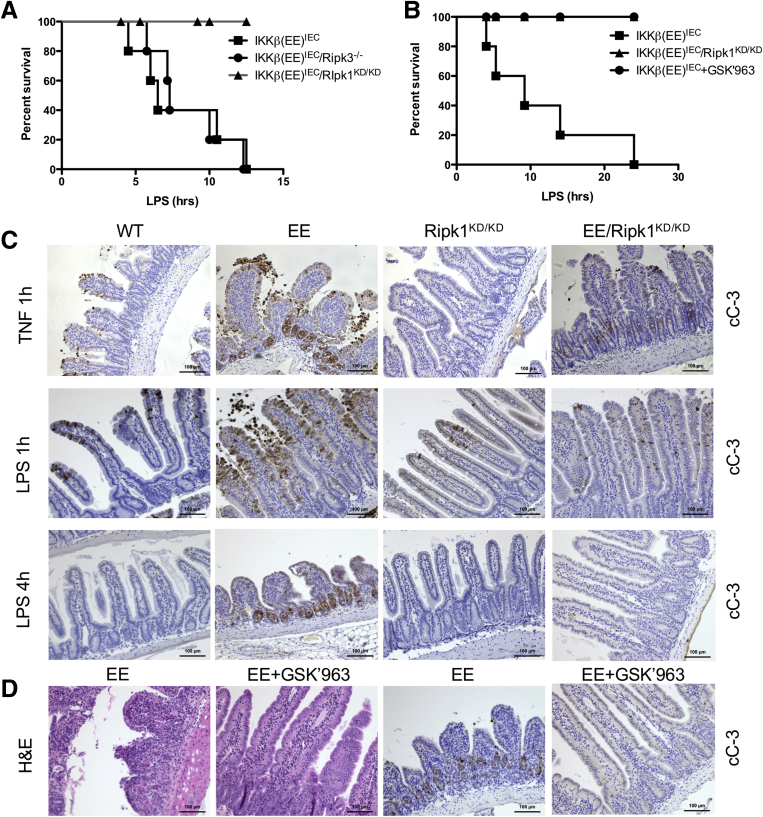
Ikkβ(EE)IEC mice are highly sensitive to TNF-induced mucosal damage and even a low dose of LPS, which induces TNF expression, causes their rapid death.14 Whereas Ripk3 ablation did not decrease LPS-induced mortality, or LPS-induced caspase-3 or -8 activation (not shown) in Ikkβ(EE)IEC mice (Figures 4A and 5D), RIPK1 inactivation was fully protective (Figure 8A and B). RIPK1 inactivation also inhibited TNF- and LPS-induced caspase-3 activation in Ikkβ(EE)IEC intestinal villi and crypts (5.25 ± 1 cC-3+ cells per crypt in Ikkβ(EE)IEC vs 1.1 ± 0.35 cC-3+ cells in Ikkβ(EE)IEC/Ripk1D138N/D138N mice, 4 hours after LPS administration; P < .001), as assessed by IHC (Figure 8C). Furthermore, the specific RIPK1 inhibitor GSK’963 prevented LPS-induced tissue damage, apoptosis (5.5 ± 0.3 cC-3+ cells per crypt in Ikkβ(EE)IEC mice vs 3.2 ± 0.6 cC-3+ cells in GSK’963-treated Ikkβ(EE)IEC mice; P = .002) and death in Ikkβ(EE)IEC mice challenged with LPS (Figure 8B and D). Curiously, within 1 hour after TNF or LPS administration, WT mice contained a few cC-3+ IEC in their villi, whose number was also reduced on RIPK1 inactivation (4.5 ± 0.8 cC-3+ cells per villus in WT mice vs 1.5 ± 0.5 cC-3+ cells per villus in Ripk1D138N/D138N mice; P = .01) (Figure 8C).
Figure 8.
RIPK1 kinase activity is required for TNF-induced death in intestinal crypts. (A) Ikkβ(EE)IEC, Ikkβ(EE)IEC/Ripk1D138N/D138N, and Ikkβ(EE)IEC/Ripk3-/- mice were injected with LPS (0.5 mg/kg, Escherichia coli O111:B4) and their survival was monitored over a 36-hour period (n = 5 per group). (B) Ikkβ(EE)IEC mice were injected with LPS in the absence or presence of GSK’963 (50 mg/kg) and their survival analyzed over a 36-hour period and compared with that of LPS-injected Ikkβ(EE)IEC/Ripk1D138N/D138N mice (n = 5 per group). (C) The strains listed above along with WT mice were injected with TNF (2 μg intravenously) or LPS (0.5 mg/kg i.p.). Jejunal sections were collected at indicated times and stained for cC-3. Magnification bars: 100 μm. (D) Ikkβ(EE)IEC were injected with LPS (0.5 mg/kg i.p) in the absence or presence of GSK’963 (50 mg/kg). Jejunal sections were collected after 4 hours of LPS injection and stained for H&E or cC-3. Magnification bars: 100 μm.
Ripoptosome Formation and Protection by Antioxidants
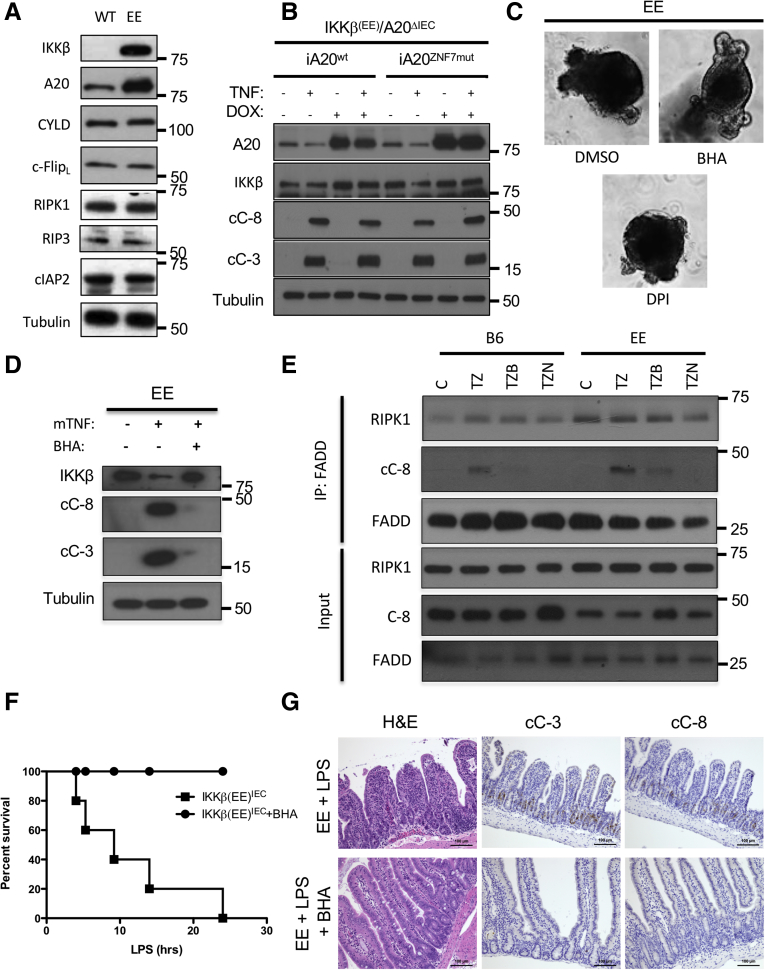
RIPK1 activation as a kinase and RIPK1-dependent apoptosis depend on formation of the ripoptosome, or complex IIb, which also contains FADD, A20, and caspase-8.13,17,36 We have recently shown that A20 overexpression in IECs induces ripoptosome formation and activation in response to TNF.13 Because A20 expression is elevated in IKKβ(EE)-expressing IEC (Figures 9A and 2B) we examined its role in TNF-induced Ikkβ(EE)IEC enteroid death by crossing Ikkβ(EE)IEC mice with A20ΔIEC mice, which specifically lack A20 in IEC.13 We then infected IKKβ(EE)/A20ΔIEC enteroids with a doxycycline inducible vector coding for either WT A20 or an A20 variant with a C764A/C767A double substitution that impairs the linear ubiquitin binding ability of zinc finger 7 (A20ZNF7mut), which is needed for RIPK1 activation.13 As expected, TNF induced caspase activation in IKKβ(EE)/A20ΔIEC (29930103). Surprisingly, expression of the protective A20ZNF7mut did not prevent caspase-3 and -8 activation as effectively as WT A20 (Figure 9B), demonstrating that A20 upregulation is not responsible for TNF-induced apoptosis in Ikkβ(EE)IEC IEC.
Figure 9.
TNF-induced apoptosis of IKKβ(EE)-expressing IEC involves ripoptosome formation and can be inhibited by BHA. (A) WT and Ikkβ(EE)IEC enteroids were IB analyzed with antibodies to the indicated proteins. (B) Ikkβ(EE)IEC/A20ΔIEC enteroids infected with the indicated A20 constructs and preincubated with doxycycline were stimulated without or with TNF (40 μg/mL) for 4 hours and IB analyzed with the indicated antibodies. (C) Ikkβ(EE)IEC enteroids were incubated with dimethyl sulfoxide, BHA (10 μM), or diphenylene iodonium (5 μM) together with TNF (40 ng/mL) and photographed under brightfield 4 hours later. Original magnification ×200. (D) Lysates of Ikkβ(EE)IEC enteroids treated with BHA and/or TNF as indicated were IB analyzed after 4 hours. (E) WT and Ikkβ(EE)IEC enteroids were stimulated without (C) or with TNF together with zVAD (10 μg/mL) (TZ), BHA (TZB), or Nec-1 (50 nM) (TZN) for 2 hours. Complex IIb was isolated by FADD immunoprecipitation and IB analyzed with the indicated antibodies. (F) Ikkβ(EE)IEC mice were treated with or without BHA (20 mg/kg), challenged with LPS (0.5 mg/kg Escherichia coli O111:B4), and their survival was monitored over a 36-hour period (n = 5 mice per group). (G) Ikkβ(EE)IEC mice treated as above were analyzed 4 hours after LPS injection. Jejunal sections were stained with either H&E or antibodies to cC-8 or cC-3. Magnification bars: 100 μm. DMSO, dimethyl sulfoxide; DPI, diphenylene iodonium
Previous studies have shown that RIPK1 activation increases reactive oxygen species production after TNF stimulation37 to potentiate RIPK1-dependent cell death.38,39 We therefore examined the effect of the antioxidant butylated hydroxyanisole (BHA), which was shown to inhibit ripoptosome formation,38 on TNF-mediated apoptosis and caspase activation in Ikkβ(EE)IEC enteroids. Consistent with previous reports, BHA, but not diphenyleneiodonium (DPI), a NOX inhibitor, fully prevented TNF-induced apoptosis and caspase activation (95.5% ± 5.4% dead organoids after control treatment vs 23.7% ± 20.0% dead organoids after BHA or 97.9% ± 4.1% after DPI treatment) (Figure 9C and D). BHA treatment also inhibited ripoptosome-mediated caspase-8 activation, assessed by caspase-8 coprecipitation with FADD, almost as effectively as Nec-1 (Figure 9E). Accordingly, BHA administration to Ikkβ(EE)IEC mice abrogated LPS-induced mortality and IEC apoptosis (5.85 ± 1.04 cC-3+ and 3.01 ± 0.83 cC-8+ cells per crypt after control treatment vs 0.00 ± 0.00 cC-3+ and 0.00 ± 0.00 cC-8+ cells per crypt in BHA-treated mice; P < .001 and P < .001) (Figure 9F and G).
Discussion
TNF is a major pathogenic factor in IBD and its blockade represents one of the most effective therapeutic options for these diseases. However, how TNF triggers IEC killing and mucosal erosion has remained a mystery. Although certain genetic mutations associated with IBD, such as ATGL1T300A, increase IEC susceptibility to TNF3,40, 41, 42 and several studies have identified, in different ethnic cohorts, 235 genetic markers in 200 susceptibility loci, some of them involving the NF-κB pathway, such as TNFAIP3 and NFKB1,3,43, 44, 45 TNF is a poor killer of normal IEC and can promote antiapoptotic NF-κB activation.11 Not surprising, NF-κB-deficient IEC are highly susceptible to TNF-induced killing, which depends on conventional, caspase-8-dependent, apoptosis.10, 11, 12,46 However, rather unexpectedly, we found that excessive IKKβ-mediated NF-κB activation reduces the threshold needed for induction of TNF-induced IEC death.14 Here we show that the TNF induced death of IKKβ(EE)-expressing IEC depends on RIPK1 kinase activation and presumably ripoptosome formation.
TNF-induced ripoptosome formation in IEC and RIPK1-dependent apoptosis are also facilitated by elevated expression of the deubiquitinase A20.13 Since expression of TNFAIP3, the gene coding for A20, is stimulated by NF-κB and is elevated in Ikkβ(EE)IEC mice, a likely explanation to the reduced threshold for TNF-induced mucosal erosion and IEC death driven by IKKβ(EE) is A20. Although A20 ablation abrogated the TNF-induced death of IKKβ(EE)-expressing IEC, reconstitution of A20 expression with a variant, A20ZNF7mut, that no longer enhances TNF-induced ripoptosome formation13 led to full restoration of TNF-induced apoptosis. These results suggest that IKKβ(EE) expression overcomes the requirement for linear ubiquitin binding by the seventh ZnF of A20.13 Exactly how IKKβ(EE) expression does that is not entirely clear, but our results show that its ability to reduce the threshold for TNF-induced death is NF-κB mediated. Because TNF-induced ripoptosome formation and RIPK1-mediated apoptosis in IKKβ (EE)-expressing IEC is inhibited by BHA, it is plausible that NF-κB activation may promote the accumulation of reactive oxygen species or other pro-oxidants. Curiously, oxidative stress is also thought to be involved in IBD pathogenesis,47 and our results show that NF-κB is activated in active human IBD. Indeed, in previous studies we found that Ikkβ(EE)IEC mice exhibit higher levels of oxidative DNA damage, which facilitates loss of APC heterozygocity to cause that formation of colonic adenomas and premalignant aberrant crypt foci,48 a pathologic process that also occurs in patients with IBD.49,50 It is also plausible that chronic NF-κB signaling affects Paneth cell differentiation or alters autophagy signaling, thereby increasing the susceptibility to TNF-induced cell death, in a similar manner to the effect of the ATG16L1 or NOD2 gene mutations.51 Overall, our results suggest that RIPK1 inhibition and treatment with antioxidants may attenuate these pathologies, especially in conjunction with anti-TNF drugs.
Materials and Methods
Mice
Ikkβ(EE)IEC and Ripk1D138N mice were described.14,32 Ripk3-/- mice were obtained from Dr. Vishva Dixit (Genentech).52 A20F/F mice were obtained from Dr. Avril Ma.53 Ikkβ(EE)IEC mice were crossed to Tnf-/-, Ripk3-/-, and Ripk1D138N/D138N mice, and maintained in the C57BL/6J background originally acquired from Jackson Laboratories. Mice used in this work were 8–12 weeks old and were maintained under specific pathogen free (SPF) conditions at a University of California San Diego facility, accredited by the American Association for Accreditation of Laboratory Animal Care. All animal protocols were approved by the institutional review board, following National Institutes of Health guidelines. Mice were fed autoclaved standard chow and all of the different strains were cohoused to minimize microbiome fluctuations.
Reagents
LPS (Escherichia coli O111:B4) was purchased from Sigma (St. Louis, MO) and was i.p. injected at 0.5 mg/kg, unless otherwise indicated. TNF was purchased from R&D systems (Minneapolis, MN) and injected at 2 μg per mouse unless otherwise indicated. In vitro, TNF was used at 40 ng/mL. CHX (25 μg/mL), zVAD-FMK (50 μM), BHA (10 μM), Nec-1 (100 μM), and DPI (5 μM) were purchased from Sigma. RIPK1 (Nec-1s: 50 nM and 963: 50 nM) and RIPK3 (843: 5 μM, 872: 5 μM) inhibitors were synthesized at GSK and purified before use at the previously determined doses.30,31 For in vivo studies, BHA (20 mg/kg) and the RIPK1 inhibitor GSK’963 (50 mg/kg) were dissolved in saline with 5% dimethyl sulfoxide and 6% Cavitron and were i.p. injected.
Human Samples
Archived specimens of ileum and colon for IHC analyses were obtained from patients who had undergone routine colonoscopy for clinical indications. Prospective ileum and colon specimens used for RNA analysis were obtained from patients with CD or UC and healthy control subjects undergoing colonoscopy as part of routine medical care at UCSD. The recruited participants had a diagnosis of CD or UC and were older than 18 years. Healthy control subjects were undergoing colonoscopy as part of routine colon cancer screening. Healthy control subjects were not included if they reported gastrointestinal symptoms. The clinical status of the patients was verified by chart review of all medical records, laboratory and pathologic data, and all specimens were deidentified before use. All participants provided written informed consent. The institutional review boards at UCSD and at VA San Diego Healthcare System approved the study.
RNAseq and Bioinformatics
Total RNA was isolated from human intestinal tissues and its quality was assessed on an Agilent Bioanalyzer (Santa Clara, CA). Samples that were determined to have an RNA Integrity Number of 7 or greater were used to generate RNA libraries using Illumina TruSeq Stranded Total RNA Sample Prep Kit starting with 100 ng of RNA. Libraries were then sequenced using the Illumina Hiseq2500 sequencer (San Diego, CA). Ikkβ(EE)IEC expression array profiling was published14 and submitted to GEO (GSE29701). The fastq files were aligned to human and mouse transcriptomes of the Genome Reference Consortium GRCh.37/hg19 and GRCm.38/mm10, respectively, using the STAR aligner.54 Transcript-level counts were calculated with an expectation maximization algorithm RSEM.55 Transcript-level summaries were processed into gene-level summaries by combining all transcript counts from the same gene. Gene counts from different samples were normalized and analyzed for differential expression using DESeq.56 Because at least 1 of the samples does not have biologic replicates, we used an ansatz in which dispersion of gene counts is estimated from all available samples as if they were replicates. All presented measures of statistical significance should be viewed in this light. Where comparisons are made between expression patterns of human and the mouse model, we used the Homologene database to translate mouse genes into their human homologues, if such a homologue exists, and compared mouse expression data with their human counterparts.
Real-Time Quantitative Polymerase Chain Reaction
Intestinal human tissue from healthy control subjects and patients with CD was collected by colonoscopy and total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA) and reverse-transcribed with random hexamers and Superscript II Kit (Invitrogen). Quantitative polymerase chain reaction (qPCR) was performed with SYBR Green PCR Master Mix Kit (Applied Biosystems, Foster City, CA). The relative amounts of each transcript were compared with those of GAPDH and normalized to untreated samples by the ΔΔCt method. Primers are available on request.
Histologic Analysis and IHC
Intestines were removed, opened longitudinally, cleaned, processed as "Swiss rolls," and fixed in 10% phosphate-buffered formalin for 24 hours. Fixed tissues were embedded in paraffin, and 5 μm sections were prepared and stained with H&E. For IHC, sections from murine and human samples were incubated overnight at 4°C with anti-p65/RelA, anti-cC-3, or anti-cC-8 antibodies (1697, 9661, and 8592, respectively; Cell Signaling, Danvers, MA) at a 1:200 dilution. Antigen retrieval was with citrate buffer pH 6.0 at 96°C for 20 minutes. All sections were counterstained with hematoxylin and photographed using an Axioplan 200 microscope with AxioVision Release 4.5 software (Zeiss, Oberkochen, Germany).
Enteroid Isolation and Culture
Small intestinal organoids (enteroids) were cultured as described.24 Briefly, crypts were collected from small intestines of mice after 30-minute incubation in phosphate-buffered saline (PBS; pH 7.4) containing 2-mM EDTA in 6°C. Enteroids were plated in Matrigel (BD Bioscience, San Jose, CA) and maintained in DMEM/F12 (Life Technologies, Carlsbad, CA) containing B27 and N2 supplements (Life Technologies), 1.25 mM N-acetyl L-cysteine (Sigma), 100 ng/mL noggin (GoldBio, St. Louis, MO), 50 ng/mL mEGF (Biosource, San Diego, CA), and 10% Rspo1-Fc-conditioned medium (the Rspo1-Fc-expressing cell line was a generous gift from Dr. Calvin Kuo, Stanford).
Immunoblotting and Immunoprecipitation
Whole cell extracts were obtained by lysing enteroids in ice-cold RIPA lysis buffer (Cell Signaling) containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM EDTA, 1% Triton X-100, and 1% deoxycholate, supplemented with a protease inhibitor cocktail (Roche, Basel, Switzerland). Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes that were incubated with antibodies against IKKβ (Millipore, Billerica, MA), A20 (Thermofisher, Waltham, MA), IκBα, p65, (Santa Cruz Biotechnology Inc, Santa Cruz, CA), RIPK1, IKKγ, and TNF (R&D Systems, Minneapolis, MN), RIPK3 (eBioscience, Waltham, MA), cC-3, cC-8, A20, and ERK1/2 (Cell Signaling), actin, and tubulin (Sigma). For immunoprecipitation, whole cell extracts were incubated with the indicated antibodies and Protein G Dynabeads (Life Technologies) overnight at 4°C. Immunocomplexes were washed with lysis buffer and analyzed by IB. To isolate protein complexes by coimmunoprecipitation, the indicated antibodies and cell lysates were incubated in 10 mM Tris, pH 7.4, 150 mM NaCl, and 0.2% Nonident P-40.
Proliferation Assay
Enteroids in Matrigel were stimulated with interleukin-22 (10 ng/mL) for 24 hours. EdU (Click-iT EdU Alexa Fluor 594 Imaging Kit, Thermo Fisher C10339) was added at 10 μM 2 hours before fixation. EdU staining was performed as indicated by the manufacturer.
Cell Survival Assay
3-.4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was dissolved in PBS at 5 mg/mL. Organoids were stimulated as indicated and 4 hours post-treatment MTT was added to the media to a final concentration of 0.5 mg/mL. Thirty minutes post-MTT, cell viability was assessed through microscopic visualization of organoids and quantified.
Immunofluorescence
Enteroids in Matrigel were fixed with 4% paraformaldehyde overnight at 4°C. Fixed enteroids were blocked with PBS containing 0.2% Triton X-100 and 1% bovine serum albumin for 30 minutes, immunostained with primary antibodies overnight at 4°C and with Alexa Fluor 594-conjugated goat antirabbit IgG secondary antibody for 1 hour at room temperature. Immunostained enteroids were gently mounted on the slide glass and imaged under the Zeiss confocal microscope.
Constructs and Lentivirus Transduction of Enteroids
The inducible-IKKβ(EE) lentiviral expression constructs were made by subcloning PCR amplified attB-flanked IKKβ(EE),57 or A2058 and the different A20 mutants59 cDNAs were PCR amplified and cloned into pINDUCER2060 using the Gateway Clonase II system (Life Technologies). All constructs were verified by sequencing.
The lentiviral constructs were transfected along with pMD2.G (Addgene, Plasmid 12259) and psPAX2 (Addgene, Plasmid 12260) into 293T cells to produce viral particles used to infect enteroids as described.61 Briefly, 2 days before virus infection, enteroids were supplemented with 50% Wnt3a conditioned medium (the Wnt3a-expressing cell line was a generous gift from Dr. Karl Willert, UCSD) and 10 mM nicotinamide. Single cells were obtained by digesting the enteroids with TrypLE (Life Technologies) for 5 minutes at 37°C. Cells were mixed with high-titer lentivirus plus polybrene (8 μg/mL, Santa Cruz) and Y-27632 (10 μM, Sigma) in a 48-well culture plate and centrifuged at 600 × g at 32°C for 60 minutes, followed by 5 hours incubation at 37°C. Cells were then plated in Matrigel and cultured in media with 50% Wnt3a, nicotinamide, and Y-27632 for 2 days, followed by selection with puromycin (1 μg/mL) or G418 (800 ng/mL) in regular media for 1 week.
Statistical Analysis
Data are expressed as means ± standard error. For comparison between 2 groups, a 2-tailed Student t test or a Mann-Whitney test was applied depending on the normality of the distribution of the variables. All statistical analyses were performed using GraphPad Prism statistical package. Results were considered significant at P < .05.
Acknowledgements
The authors acknowledge Genentech and Dr. Vishva Dixit for the provision of Rip3-/- mice. All authors had access to the study data and had reviewed and approved the final manuscript.
Footnotes
Author contributions Jerry Wong, Monica Guma, and Michael Karin designed the study. Jerry Wong, Monica Guma, Ricard Garcia-Carbonell, Matija Zelic, Shih-Jing Yao, and Shalin A. Desai performed research. Philip A. Harris, Brigid S Boland, Joan Font-Burgada, Koji Taniguchi, Soumita Das, Núria Planell, Azucena Salas, John Bertin, Manolis Pasparakis, Pete J. Gough, and Michelle Kelliher contributed new constructs, chemical inhibitors, enteroid isolation, mice and human inflammatory bowel disease samples, and transcriptome data. Jerry Wong, Monica Guma, Ricard Garcia-Carbonell, and Michael Karin analyzed data. Jerry Wong, Ricard Garcia-Carbonell, Monica Guma, and Michael Karin wrote the paper.
Conflicts of interest These authors disclose the following: Philip A. Harris, John Bertin, and Pete J. Gough are employees and shareholders of GlaxoSmithKline. The remaining authors disclose no conflicts.
Funding Supported by fellowships from the Canadian Institutes of Health Research (J.W.) and Boehringer-Ingelheim Fonds (R.G.-C.); the National Institutes of Health1K08AR064834 (M.G.), R01AI075118 (M. Kelliher), and T32DK007202 (B.S.B.); M.P. was funded by the ERC (2012-ADG_20120314), DFG (SFB670, SFB829, SPP1656), European CommissionFP7 grants 223404 (Masterswitch) and 223151 (InflaCare), the Deutsche Krebshilfe (Grant 110302), and the Helmholtz Alliance Preclinical Comprehensive Cancer Center; M.K. was supported by NIHR01AI043477, is an American Cancer Society Research Professor, and holds the Ben and Wanda Hildyard Chair for Mitochondrial and Metabolic Diseases. M.G. and M. Karin were also supported by GlaxoSmithKline.
Contributor Information
Michael Karin, Email: karinoffice@ucsd.edu.
Monica Guma, Email: mguma@ucsd.edu.
References
- 1.Abraham C., Cho J.H. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunther C., Neumann H., Neurath M.F., Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062–1071. doi: 10.1136/gutjnl-2011-301364. [DOI] [PubMed] [Google Scholar]
- 3.Khor B., Gardet A., Xavier R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto R., Watanabe M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J Gastroenterol. 2016;51:11–21. doi: 10.1007/s00535-015-1098-4. [DOI] [PubMed] [Google Scholar]
- 5.Neurath M.F., Travis S.P. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–1635. doi: 10.1136/gutjnl-2012-302830. [DOI] [PubMed] [Google Scholar]
- 6.Vereecke L., Beyaert R., van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009;30:383–391. doi: 10.1016/j.it.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Kaser A., Zeissig S., Blumberg R.S. Inflammatory bowel disease. Ann Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeissig S., Bojarski C., Buergel N., Mankertz J., Zeitz M., Fromm M., Schulzke J.D. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin A., Karin M. NF-kappaB in cancer: a marked target. Semin Cancer Biol. 2003;13:107–114. doi: 10.1016/s1044-579x(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 10.Egan L.J., Eckmann L., Greten F.R., Chae S., Li Z.W., Myhre G.M., Robine S., Karin M., Kagnoff M.F. IkappaB-kinasebeta-dependent NF-kappaB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci U S A. 2004;101:2452–2457. doi: 10.1073/pnas.0306734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L.W., Egan L., Li Z.W., Greten F.R., Kagnoff M.F., Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- 12.Nenci A., Becker C., Wullaert A., Gareus R., van Loo G., Danese S., Huth M., Nikolaev A., Neufert C., Madison B., Gumucio D., Neurath M.F., Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Carbonell R., Wong J., Kim J.Y., Close L.A., Boland B.S., Wong T.L., Harris P.A., Ho S.B., Das S., Ernst P.B., Sasik R., Sandborn W.J., Bertin J., Gough P.J., Chang J.T., Kelliher M., Boone D., Guma M., Karin M. Elevated A20 promotes TNF-induced and RIPK1-dependent intestinal epithelial cell death. Proc Natl Acad Sci U S A. 2018;115:E9192–E9200. doi: 10.1073/pnas.1810584115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guma M., Stepniak D., Shaked H., Spehlmann M.E., Shenouda S., Cheroutre H., Vicente-Suarez I., Eckmann L., Kagnoff M.F., Karin M. Constitutive intestinal NF-kappaB does not trigger destructive inflammation unless accompanied by MAPK activation. J Exp Med. 2011;208:1889–1900. doi: 10.1084/jem.20110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasparakis M., Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 16.Weinlich R., Green D.R. The two faces of receptor interacting protein kinase-1. Mol Cell. 2014;56:469–480. doi: 10.1016/j.molcel.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L., Du F., Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Kanayama A., Seth R.B., Sun L., Ea C.K., Hong M., Shaito A., Chiu Y.H., Deng L., Chen Z.J. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Micheau O., Thome M., Schneider P., Holler N., Tschopp J., Nicholson D.W., Briand C., Grutter M.G. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 20.Feng P., Liang C., Shin Y.C., Xiaofei E., Zhang W., Gravel R., Wu T.T., Sun R., Usherwood E., Jung J.U. A novel inhibitory mechanism of mitochondrion-dependent apoptosis by a herpesviral protein. PLoS Pathog. 2007;3:e174. doi: 10.1371/journal.ppat.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Donnell M.A., Perez-Jimenez E., Oberst A., Ng A., Massoumi R., Xavier R., Green D.R., Ting A.T. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogler G., Brand K., Vogl D., Page S., Hofmeister R., Andus T., Knuechel R., Baeuerle P.A., Scholmerich J., Gross V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 23.Leal R.F., Planell N., Kajekar R., Lozano J.J., Ordas I., Dotti I., Esteller M., Masamunt M.C., Parmar H., Ricart E., Panes J., Salas A. Identification of inflammatory mediators in patients with Crohn's disease unresponsive to anti-TNFalpha therapy. Gut. 2015;64:233–242. doi: 10.1136/gutjnl-2013-306518. [DOI] [PubMed] [Google Scholar]
- 24.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 25.Kreuz S., Siegmund D., Scheurich P., Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawakami H., Tomita M., Matsuda T., Ohta T., Tanaka Y., Fujii M., Hatano M., Tokuhisa T., Mori N. Transcriptional activation of survivin through the NF-kappaB pathway by human T-cell leukemia virus type I tax. Int J Cancer. 2005;115:967–974. doi: 10.1002/ijc.20954. [DOI] [PubMed] [Google Scholar]
- 27.Ofengeim D., Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Cell Biol. 2013;14:727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- 28.Degterev A., Hitomi J., Germscheid M., Ch'en I.L., Korkina O., Teng X., Abbott D., Cuny G.D., Yuan C., Wagner G., Hedrick S.M., Gerber S.A., Lugovskoy A., Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi N., Duprez L., Grootjans S., Cauwels A., Nerinckx W., DuHadaway J.B., Goossens V., Roelandt R., Van Hauwermeiren F., Libert C., Declercq W., Callewaert N., Prendergast G.C., Degterev A., Yuan J., Vandenabeele P. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandal P., Berger S.B., Pillay S., Moriwaki K., Huang C., Guo H., Lich J.D., Finger J., Kasparcova V., Votta B., Ouellette M., King B.W., Wisnoski D., Lakdawala A.S., DeMartino M.P., Casillas L.N., Haile P.A., Sehon C.A., Marquis R.W., Upton J., Daley-Bauer L.P., Roback L., Ramia N., Dovey C.M., Carette J.E., Chan F.K., Bertin J., Gough P.J., Mocarski E.S., Kaiser W.J. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger S.B., Harris P., Nagilla R., Kasparcova V., Hoffman S., Swift B., Dare L., Schaeffer M., Capriotti C., Ouellette M., King B.W., Wisnoski D., Reilly M., Marquis R.W., Bertin J., Gough P.J. Characterization of GSK’963: a structurally distinct, potent and selective inhibitor of RIP1 kinase. Cell Death Discov. 2015;1 doi: 10.1038/cddiscovery.2015.9. 15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polykratis A., Hermance N., Zelic M., Roderick J., Kim C., Van T.M., Lee T.H., Chan F.K., Pasparakis M., Kelliher M.A. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J Immunol. 2014;193:1539–1543. doi: 10.4049/jimmunol.1400590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton K., Sun X., Dixit V.M. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang D.W., Shao J., Lin J., Zhang N., Lu B.J., Lin S.C., Dong M.Q., Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 35.Vandenabeele P., Declercq W., Van Herreweghe F., Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3:re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 36.Vanden Berghe T., Linkermann A., Jouan-Lanhouet S., Walczak H., Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y.S., Morgan M.J., Choksi S., Liu Z.G. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Shindo R., Kakehashi H., Okumura K., Kumagai Y., Nakano H. Critical contribution of oxidative stress to TNFalpha-induced necroptosis downstream of RIPK1 activation. Biochem Biophys Res Commun. 2013;436:212–216. doi: 10.1016/j.bbrc.2013.05.075. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y., Su S.S., Zhao S., Yang Z., Zhong C.Q., Chen X., Cai Q., Yang Z.H., Huang D., Wu R., Han J. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat Commun. 2017;8:14329. doi: 10.1038/ncomms14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuzawa-Ishimoto Y., Shono Y., Gomez L.E., Hubbard-Lucey V.M., Cammer M., Neil J., Dewan M.Z., Lieberman S.R., Lazrak A., Marinis J.M., Beal A., Harris P.A., Bertin J., Liu C., Ding Y., van den Brink M.R.M., Cadwell K. Autophagy protein ATG16L1 prevents necroptosis in the intestinal epithelium. J Exp Med. 2017;214:3687–3705. doi: 10.1084/jem.20170558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pott J., Kabat A.M., Maloy K.J. Intestinal epithelial cell autophagy is required to protect against TNF-induced apoptosis during chronic colitis in mice. Cell Host Microbe. 2018;23:191–202. doi: 10.1016/j.chom.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 42.Aden K., Tran F., Ito G., Sheibani-Tezerji R., Lipinski S., Kuiper J.W., Tschurtschenthaler M., Saveljeva S., Bhattacharyya J., Hasler R., Bartsch K., Luzius A., Jentzsch M., Falk-Paulsen M., Stengel S.T., Welz L., Schwarzer R., Rabe B., Barchet W., Krautwald S., Hartmann G., Pasparakis M., Blumberg R.S., Schreiber S., Kaser A., Rosenstiel P. ATG16L1 orchestrates interleukin-22 signaling in the intestinal epithelium via cGAS-STING. J Exp Med. 2018;215:2868–2886. doi: 10.1084/jem.20171029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson C.A., Boucher G., Lees C.W., Franke A., D'Amato M., Taylor K.D., Lee J.C., Goyette P., Imielinski M., Latiano A., Lagace C., Scott R., Amininejad L., Bumpstead S., Baidoo L., Baldassano R.N., Barclay M., Bayless T.M., Brand S., Buning C., Colombel J.F., Denson L.A., De Vos M., Dubinsky M., Edwards C., Ellinghaus D., Fehrmann R.S., Floyd J.A., Florin T., Franchimont D., Franke L., Georges M., Glas J., Glazer N.L., Guttery S.L., Hamiltonians T., Hayward N.K., Hugo J.P., Jobin G., Lukens D., Lawrence I., Lemann M., Levine A., Lobule C., Louis E., McGovern D.P., Milla M., Montgomery G.W., Morley K.I., Mowat C., Ng A., Newman W., Popoff R.A., Papa L., Palmieri O., Peyrin-Biroulet L., Panes J., Phillips A., Prescott N.J., Proctor D.D., Roberts R., Russell R., Rutgeerts P., Sanderson J., Sans M., Schumm P., Seibold F., Sharma Y., Simms L.A., Seielstad M., Steinhart A.H., Targan S.R., van den Berg L.H., Vatn M., Verspaget H., Walters T., Wijmenga C., Wilson D.C., Westra H.J., Xavier R.J., Zhao Z.Z., Ponsioen C.Y., Andersen V., Torkvist L., Gazouli M., Anagnou N.P., Karlsen T.H., Kupcinskas L., Sventoraityte J., Mansfield J.C., Kugathasan S., Silverberg M.S., Halfvarson J., Rotter J.I., Mathew C.G., Griffiths A.M., Gearry R., Ahmad T., Brant S.R., Chamaillard M., Satsangi J., Cho J.H., Schreiber S., Daly M.J., Barrett J.C., Parkes M., Annese V., Hakonarson H., Radford-Smith G., Duerr R.H., Vermeire S., Weersma R.K., Rioux J.D. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franke A., McGovern D.P., Barrett J.C., Wang K., Radford-Smith G.L., Ahmad T., Lees C.W., Balschun T., Lee J., Roberts R., Anderson C.A., Bis J.C., Bumpstead S., Ellinghaus D., Festen E.M., Georges M., Green T., Haritunians T., Jostins L., Latiano A., Mathew C.G., Montgomery G.W., Prescott N.J., Raychaudhuri S., Rotter J.I., Schumm P., Sharma Y., Simms L.A., Taylor K.D., Whiteman D., Wijmenga C., Baldassano R.N., Barclay M., Bayless T.M., Brand S., Buning C., Cohen A., Colombel J.F., Cottone M., Stronati L., Denson T., De Vos M., D'Inca R., Dubinsky M., Edwards C., Florin T., Franchimont D., Gearry R., Glas J., Van Gossum A., Guthery S.L., Halfvarson J., Verspaget H.W., Hugot J.P., Karban A., Laukens D., Lawrance I., Lemann M., Levine A., Libioulle C., Louis E., Mowat C., Newman W., Panes J., Phillips A., Proctor D.D., Regueiro M., Russell R., Rutgeerts P., Sanderson J., Sans M., Seibold F., Steinhart A.H., Stokkers P.C., Torkvist L., Kullak-Ublick G., Wilson D., Walters T., Targan S.R., Brant S.R., Rioux J.D., D'Amato M., Weersma R.K., Kugathasan S., Griffiths A.M., Mansfield J.C., Vermeire S., Duerr R.H., Silverberg M.S., Satsangi J., Schreiber S., Cho J.H., Annese V., Hakonarson H., Daly M.J., Parkes M. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J.Z., van Sommeren S., Huang H., Ng S.C., Alberts R., Takahashi A., Ripke S., Lee J.C., Jostins L., Shah T., Abedian S., Cheon J.H., Cho J., Dayani N.E., Franke L., Fuyuno Y., Hart A., Juyal R.C., Juyal G., Kim W.H., Morris A.P., Poustchi H., Newman W.G., Midha V., Orchard T.R., Vahedi H., Sood A., Sung J.Y., Malekzadeh R., Westra H.J., Yamazaki K., Yang S.K., International Multiple Sclerosis Genetics C. International IBDGC. Barrett J.C., Alizadeh B.Z., Parkes M., Bk T., Daly M.J., Kubo M., Anderson C.A., Weersma R.K. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–986. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greten F.R., Eckmann L., Greten T.F., Park J.M., Li Z.W., Egan L.J., Kagnoff M.F., Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Pereira C., Gracio D., Teixeira J.P., Magro F. Oxidative stress and DNA damage: implications in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:2403–2417. doi: 10.1097/MIB.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 48.Shaked H., Hofseth L.J., Chumanevich A., Chumanevich A.A., Wang J., Wang Y., Taniguchi K., Guma M., Shenouda S., Clevers H., Harris C.C., Karin M. Chronic epithelial NF-kappaB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc Natl Acad Sci U S A. 2012;109:14007–14012. doi: 10.1073/pnas.1211509109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lakatos P.L., Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008;14:3937–3947. doi: 10.3748/wjg.14.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abraham B.P. Cancer surveillance in ulcerative colitis and Crohn's disease: new strategies. Curr Opin Gastroenterol. 2016;32:32–37. doi: 10.1097/MOG.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 51.Brain O., Allan P., Simmons A. NOD2-mediated autophagy and Crohn disease. Autophagy. 2010;6:412–414. doi: 10.4161/auto.6.3.11389. [DOI] [PubMed] [Google Scholar]
- 52.Newton K., Dugger D.L., Wickliffe K.E., Kapoor N., de Almagro M.C., Vucic D., Komuves L., Ferrando R.E., French D.M., Webster J., Roose-Girma M., Warming S., Dixit V.M. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 53.Rott H.D., Fahsold R. Tuberous sclerosis in two sibs of normal parents. Clin Genet. 1991;39:306–308. doi: 10.1111/j.1399-0004.1991.tb03031.x. [DOI] [PubMed] [Google Scholar]
- 54.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anders S., Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delhase M., Hayakawa M., Chen Y., Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 58.Wertz I.E., O'Rourke K.M., Zhou H., Eby M., Aravind L., Seshagiri S., Wu P., Wiesmann C., Baker R., Boone D.L., Ma A., Koonin E.V., Dixit V.M. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 59.Lu T.T., Onizawa M., Hammer G.E., Turer E.E., Yin Q., Damko E., Agelidis A., Shifrin N., Advincula R., Barrera J., Malynn B.A., Wu H., Ma A. Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme. Immunity. 2013;38:896–905. doi: 10.1016/j.immuni.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meerbrey K.L., Hu G., Kessler J.D., Roarty K., Li M.Z., Fang J.E., Herschkowitz J.I., Burrows A.E., Ciccia A., Sun T., Schmitt E.M., Bernardi R.J., Fu X., Bland C.S., Cooper T.A., Schiff R., Rosen J.M., Westbrook T.F., Elledge S.J. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc Natl Acad Sci U S A. 2011;108:3665–3670. doi: 10.1073/pnas.1019736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koo B.K., Stange D.E., Sato T., Karthaus W., Farin H.F., Huch M., van Es J.H., Clevers H. Controlled gene expression in primary Lgr5 organoid cultures. Nat Methods. 2012;9:81–83. doi: 10.1038/nmeth.1802. [DOI] [PubMed] [Google Scholar]