Abstract
Introduction
Besides therapeutic hypothermia or targeted temperature management no novel therapies have been developed to improve outcomes of patients after cardiac arrest (CA). Recent studies suggest that nitrite reduces neurological damage after asphyxial CA. Nitrite is also implicated as a new mediator of remote post conditioning produced by tourniquet inflation-deflation, which is under active investigation in CA. However, little is known about brain penetration or pharmacokinetics (PK). Therefore, to define the optimal use of this agent, studies on the PK of nitrite in experimental ventricular fibrillation (VF) are needed. We tested the hypothesis that nitrite administered after resuscitation from VF is detectable in cerebrospinal fluid (CSF), brain and other organ tissues, produces no adverse hemodynamic effects, and improves neurologic outcome in rats.
Methods
After return of spontaneous circulation (ROSC) of 5 min untreated VF, adult male Sprague-Dawley rats were given intravenous nitrite (8 μM, 0.13 mg/kg) or placebo as a 5 min infusion beginning at 5 min after CA. Additionally, sham groups with and without nitrite treatment were also studied. Whole blood nitrite levels were serially measured.
After 15 minutes, CSF, brain, heart and liver tissue were collected. In a second series, using a randomized and blinded treatment protocol, rats were treated with nitrite or placebo after arrest. Neurological deficit scoring (NDS) was performed daily and eight days after resuscitation, fear conditioning testing (FCT) and brain histology were assessed.
Results
In an initial series of experiments, rats (n=21) were randomized to 4 groups: VF-CPR and nitrite therapy (n=6), VF-CPR and placebo therapy (n=5), sham (n=5), or sham plus nitrite therapy (n=5). Whole blood nitrite levels increased during drug infusion to 57.14 ± 10.82 μM at 11 min post-resuscitation time (1 min after dose completion) in the VF nitrite group vs. 0.94±0.58 μM in the VF placebo group (p<0.001). There was a significant difference between the treatment and placebo groups in nitrite levels in blood between 7.5 and 15 minutes after CPR start and between groups with respect to nitrite levels in CSF, brain, heart and liver. In a second series (n=25 including 5 shams), 19 out of 20 animals survived until day 8. However, NDS, FCT and brain histology did not show any statistically significant difference between groups.
Conclusions
Nitrite, administered early after ROSC from VF, was shown to cross the blood brain barrier after a 5 min VF cardiac arrest. We characterized the PK of intravenous nitrite administration after VF and were able to demonstrate nitrite safety in this feasibility study.
Keywords: heart arrest, cardiopulmonary resuscitation, animals, laboratory, ventricular fibrillation, asphyxia
1. Introduction
Cardiac arrest (CA) remains a significant public health burden worldwide with one-half million cases in the US every year.[1] Targeted temperature management confers benefit when applied for 12–24 h after cardiac etiology (primarily ventricular fibrillation [VF]) CA arrest in adults.[2; 3; 4] Yet brain injury remains one of the leading cause of death after cardiac etiology CA [5; 6] and results in cognitive dysfunction in half of these survivors.[7] Our recent findings[6] confirm earlier studies[8; 9] that cardiac etiology CA is phenotypically distinct from asphyxial etiology CA in terms of heart and brain injury which may have important implications in regards to response to drug therapy.[6; 9] Thus a persistent need exists for novel neuroprotective therapies to treat CA, and that both pre-clinical and clinical studies should evaluate neuroprotective therapies across CA etiology-based phenotypes.
Nitrite (NO2−) is a potential novel therapy for brain and heart ischemia that could improve outcome after CA and/or augment the benefits of hypothermia. [10; 11; 12; 13; 14; 15; 16; 17; 18] Mechanistically, nitrite targets a number of important secondary injury pathways that appear to contribute to brain reperfusion injury, such as oxidative stress, mitochondrial injury, bioenergetic failure and secondary hypoperfusion. [18; 19; 20; 21; 22] Nitrite is converted locally in ischemic regions to nitric oxide (NO) and participates in cysteine S-nitrosation[18] potentially modulating blood flow, mitochondrial function and free radical generation.[12; 14; 15; 17; 18; 23] Lower pH results in a significant increase in NO release with heme and molybdenum containing enzymes facilitating nitrite reduction to NO particularly under hypoxic conditions[24; 25; 26; 27].
Our prior work demonstrated that a single dose of intravenous nitrite provided during cardiopulmonary resuscitation (CPR) improved cardiac function, survival, and neurological outcomes compared to saline placebo in a mouse-model of potassium chloride-induced CA.[16] In rat models, nitrite dosed shortly after return of spontaneous circulation (ROSC) was shown to reduce neurological damage after asphyxial (pulseless electrical activity) CA.[17; 18] It remains to be determined whether nitrite is effective after VF CA which is distinct from asphyxia in having greater cardiogenic shock but less neurological injury[6].
The goal of this study was to answer important questions which remained unresolved after these earlier studies. We determined whether nitrite crosses the blood brain barrier (BBB) and enters the cerebrospinal fluid (CSF) within minutes of dosing after VF similar to our findings in asphyxial CA[18]. We performed detailed pharmacokinetic (PK) modeling never previously reported after CA, which impact dosing and duration (bolus vs. infusion) in ongoing clinical trials (). Our recent phase I study confirmed nitrite safety when dosed during resuscitation after human CA[28; 29] nitrite levels in patients were quite variable and only modestly estimated PK models derived in critically ill lung transplant patients.[29] A major objective of this study was to determine how well our human PK models, which were derived in a distinct disease process (lung transplant), recapitulated PK modeling in rat VF CA where ischemic nitrite depletion creates a potential for increased tissue uptake.[16] Finally, we sought to test the hypothesis that nitrite would be neuroprotective when administered immediately after ROSC from VF-induced CA akin to our prior work in asphyxial CA.[17]
2. Material and methods
2.1. Rat Model
The Institutional Animal Care and Use committee at the University of Pittsburgh, PA, USA, approved this prospective study that included two separate series of experiments. These comply with the ARRIVE guidelines and have been carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals. Adult male Sprague Dawley rats (400 – 450 g; Hilltop Lab Animals, Scottdale, PA) were housed with unrestricted access to food and water. Rats were fasted for 16h prior to CA. Rats were anesthetized with 4% isoflurane in oxygen, then intubated with a 14-gauge intravenous cannula (Becton Dickinson, Sandy, UT), and placed supine. Rats were mechanically ventilated (Harvard Small Animal Ventilator 683, Harvard Apparatus, Holliston, MA) with a ventilation rate of 45/min. Anesthesia was maintained with 2% isoflurane in FiO2 0.5 for surgical procedures. Electrocardiogram, respiratory rate, arterial and central venous pressure were continuously monitored and recorded (Powerlab, ADinstruments, CO, USA). Rectal and tympanic probes were used to monitor temperature. Rectal temperature was held at 37.0±0.5°C with a temperature controlled operating table using lights and a heating pad for heat and a fan for reducing temperature. A Swan-Ganz pacing catheter (size 6F, Edwards Lifesciences LLC, CA, USA) was inserted into the right jugular vein for induction of VF. After surgery, isoflurane was reduced to 1% for 9 min, followed by 0% for 1 min prior to CA to minimize excessive anesthetic effects during the CA period. We obtained two baseline-blood gases and adjusted ventilation as needed prior to the insult. Data on oxygenation, electrolytes and lactate were serially obtained.
2.2. Induction of VF
Immediately before VF induction, mechanical ventilation and temperature control were discontinued. VF was induced over a period of 90 sec with 12V/50 Hz alternating current and was confirmed by a precipitous reduction in the arterial blood pressure and ECG recordings. After 90 sec, the voltage was reduced 50% for the remaining 30 sec to minimize any electrical injury to the heart. After 2 min of VF induction, the current was turned off, the pacing catheter removed and the vein and skin incision sutured. VF was left untreated for 3 additional min resulting in a 5 min total no flow CA time.
2.3. CPR
Epinephrine 20 μg/kg and sodium bicarbonate 1 mmol/kg were given intravenously 15 sec before start of CPR and flushed by 1ml of normal saline. CPR was initiated 5 min after VF induction. Mechanical ventilation during CPR was performed at a rate equivalent to that used in the final baseline period (generally 40 breaths/min). Chest compressions were delivered manually with three fingers in the middle of the sternum with equal compression-relaxation periods at a depth adjusted to achieve a systolic blood pressure >40 mmHg and a rate of 200/min guided by the sound of a metronome (Steinway Metronome, Steinway Musical Instruments, iTunes App). After 60 sec of CPR, a single monophasic defibrillation attempt with 20 joules was delivered (Physio-Control Lifepak 8 defibrillator, Physio-Control, WA, USA) with immediate resumption of CPR. After 90 sec, CPR was briefly paused to assess the blood pressure and ECG. If ROSC had not occurred at that point, CPR was continued and a second dose of epinephrine (20 μg/kg) was given intravenously before delivering a second shock. Defibrillation was repeated every 30 sec at 20 J if necessary up to 3 min after initiation of CPR. If ROSC was not achieved, CPR attempts were terminated and the animal excluded from further study.
2.4. Whole blood, CSF and tissue nitrite sampling for PK
Immediately after ROSC, rats were randomized to nitrite or placebo. FiO2 and ventilation rate were adapted according to arterial blood gas at 5 min post-resuscitation time (RT) based on a standardized protocol. Temperature control was reactivated and rectal temperature was maintained at 37±0.5°C. Hemodynamics and temperature were measured over 15 min after CPR. Rats received either 1ml of 8μM (0.13 mg/kg) sodium nitrite (NaNO2 in plasmalyte) or vehicle placebo (1ml plasmalyte) intravenously over a 5-min period from 5 to 10 min RT in a randomized blinded fashion. Whole blood (50 μl) was obtained for nitrite measurement at baseline (prior to VF) and after CPR at 5, 7.5, 10, 11, 12, 13, 14 and 15 min RT. After each blood sampling we returned an initial 100 μl blood waste and flushed our lines with 50–100 μl plasmalyte. This represents data from the time period during and immediately after the therapeutic infusion. After 15 min, rats were placed into the prone position and CSF was obtained from the cisterna magna using a 25G 5/8 needle.14 CSF was centrifuged and the supernatant frozen. Rats were transcardially perfused with nitrite-free water and brain-, liver- and heart-tissue were extracted and homogenized in a cyanide-based nitrite preservation solution.[16; 17] Whole blood samples for the assessment of nitrite were preserved using a ferricyanide-based solution.[16] CSF, tissue and blood samples were analyzed by a tri-iodide based reductive chemiluminescent method using an ozone chemiluminescence detector (Model 280i nitric oxide analyzer [NOA], Sievers Instruments, CO, USA) to measure nitrite levels.[16; 17]
2.5. Rat VF survival study
The same CA and resuscitation protocol described above was used for the 8-day survival experiments which assessed PK and outcomes. This was a randomized, investigator-blinded study. After ROSC, rats were randomized to NaNO2 (8 μM) vs. placebo therapy delivered in blinded fashion over a time period of 5 min from 5 to 10 min RT. Whole blood nitrite levels were measured at baseline, 4, 11, 18, 25, 30, 45 and 60 min. These data were combined with early nitrite samples obtained in the experiments above to a dose-concentration nitrite PK model. Weaning from the ventilator was performed at 30 min RT, extubation and removal of central catheters were carried out at the end of ICU phase (60 min RT). Using a midline laparotomy incision, a Mini-mitter probe (Mini-Mitter Co., Sunriver, OR) was placed into the peritoneal cavity to allow postoperative temperature control (37°C for ≥16h after ROSC) and continuous monitoring of movement. Rats received a subcutaneous bolus of 10ml dextrose 5% in 0.9% sodium chloride (D5NS) before being returned to their cage. Rats were allowed unrestricted access to food and water and were monitored for weight and activity every 24h by a technician.
2.6. Sham control group
Sham rats underwent the same surgical procedures, blood withdrawals and testing as the CA rats, but no CA was induced. Five ml of plasmalyte were administered over 5 min at RT 5 of 60 min. An ICU phase of 60 min was performed during which sham animals remained intubated and sedated.
2.7. Neurologic Outcomes
Neurologic Deficit Score (NDS)[30] was assessed at post-arrest days 1, 2 and 8. On day 7, a 2-day-fear conditioning protocol[6; 31] was initiated. Rats were placed in a Plexiglas conditioning box (Habitest operant cage, Coulbourn Instruments, Holliston, MA) with steel grid bottom. Rats were conditioned to associate a tone (85 dB; 3 kHz) for 10 sec with brief pain delivered by an electrical foot shock (1.0 mA over 1 sec). Conditioning consisted of 5 cue-shock cycles each consisting of 60 s of silence and 10 s of cue with a shock in the final sec (total time 420 sec). On day 8, rats were again placed in the Plexiglas box with the floor and walls disguised and a distracting odor and soft white noise (60 dB; 500 Hz) intended to conceal the contextual clues that this is the same chamber where shocks were performed the day prior. Rats were allowed to explore 120 sec at which point the conditioned tone was applied for 60 sec without shock followed by 60 sec silence (total 240 sec). During this entire period, freezing (remaining motionless) is recorded via video and quantified using FreezeFrame (Coulbourn Instruments, Holliston, MA). Cue mediated freezing was calculated as the percent time spent frozen during the 2 min after the start of the conditioned cue on day 2, indicative of memory and extinction, minus the percent time frozen in the initial 2 min which is indicative of non-specific sensitization.[32]
On day 8, after completion of the fear conditioning testing, rats were anesthetized with 2–4% isoflurane and trans-cardiac perfusion with paraformaldehyde was performed followed by necropsy. Brains were removed, further fixed and sectioned. Formalin-fixed brains were divided into 8 coronal slices 3 mm apart and embedded in paraffin blocks. Five μM sections were stained with hematoxylin and eosin (Millipore, Temecula, CA). Surviving hippocampal CA1 neurons were then quantified using haematoxylin and eosin stain (H&E) and counted.
2.8. PK analysis
Whole blood nitrite concentration vs. time profiles for each rat were analyzed individually by nonlinear regression using WinNonlin Phoenix (Pharsight, Mountain View, CA). Profiles were simultaneously fit to an intravenous infusion, no lag time, 2 compartmental, first order elimination model based on inspections of goodness of fit. Calculated parameter estimates included predicted maximal concentration (CMax), apparent volume of distribution at steady state (VSS), systemic clearance (CLS), and terminal elimination half-life (beta half-life, T1/2,β) and were expressed as mean ± standard error.
2.9. Statistics
Data are reported as mean and standard deviation or median and interquartile range, if not normally distributed. Statistics were performed with STATA SE 13.0 Statistics (College Station, TX). Group comparisons were made with a one-way analysis of variance and a post-hoc Bonferroni adjustment. Comparisons of 2 groups were performed with t-test (parametric) or Mann Whitney U test (non-parametric). Figures were designed with GraphPad Prism 7.0 (La Jolla, CA). A two-tailed p-value less than 0.05 was considered as statistically significant.
All authors had full access to the data and take full responsibility to the integrity of the data. All authors have read and agreed to the content of the manuscript as written.
3. Results
3.1. Nitrite tissue distribution and pharmacokinetics
In the first series of experiments, rats (n=21) weighing 427 ±12 g and were randomized to 4 groups after ROSC: VF and nitrite therapy (n=6), VF and placebo therapy (n=5), sham (n=5), or sham and nitrite therapy (n=5). Time to ROSC was not different between VF groups (VF and nitrite 111±60 sec, VF and placebo 152±58 sec; p=0.28). CSF was withdrawn at 17 ±1 min after start of CPR with no difference between groups. CSF sampling was successful in 17/21 rats due to the technically challenging nature of the procedure.
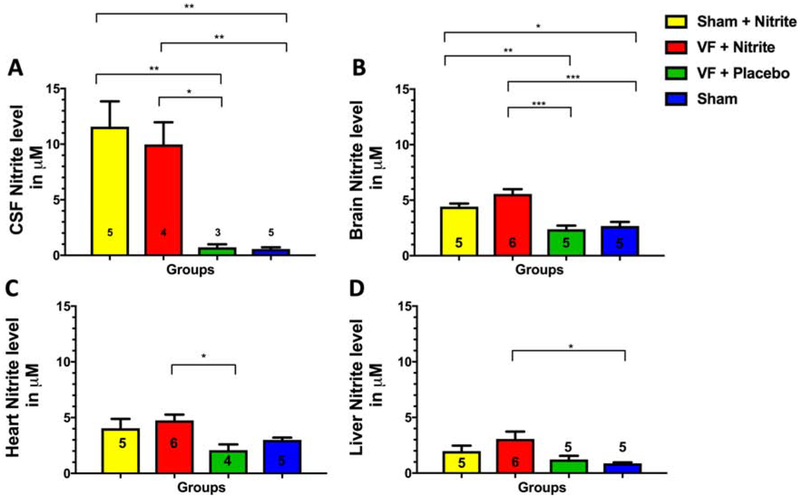
Nitrite levels in CSF at 15 min increased significantly compared to placebo whether dosed after VF or sham surgery (Figure 1A) with the increase exceeding 10-fold greater than placebo. CSF nitrite levels were significantly higher than all tissue levels (p=0.02 to <0.0001). Brain (Figure 1B), heart (Figure 1C) and liver (Figure 1D) tissue all had more modest 2–3-fold increases in nitrite level compared to placebo which were similar between VF and sham. Liver levels of nitrite were significantly lower in nitrite-treated sham (p=0.024) and VF (p=0.01) rats compared to brain with a similar trend (p=0.056–0.09) noted between heart and liver (Supplemental Figure 1). We did not observe significant nitrite depletion after CA comparing the placebo treated VF group to sham. Due to technical reasons, 3 samples of included animals could not be analyzed. Simultaneous levels of nitrite (μM) in whole blood, CSF and brain tissue at approximately 15 min RT after VF/treatment were 23.4 ± 2.5, 10.0 ± 4.0 and 5.6 ± 1.0.
Figure 1. Results of the tissue 15-minute model.
Nitrite levels in (A) CSF, (B) brain, (C) heart and (D) liver tissue homogenates. Data are shown as mean (± standard error). Numbers of rats per group are shown within each bar.
Whole blood nitrite levels increased during drug infusion to 57.14 ± 10.82 μM at 11 min RT (1 min after dose completion) in the VF nitrite group vs. 0.94±0.58 μM in the VF placebo group (p<0.001) (Figure 2A). Whole blood nitrite concentrations over time were best fit by a two-compartment model for all rats (Figure 2B). PK profiles exhibited a rapid distribution phase (declining 65% from peak values in the first 7 min following the completion of the infusion) with a more gradual terminal elimination (Figure 2B). In VF rats, the model predicted maximal concentration was 53.43±3.81 μmol/L, the apparent volume of distribution at steady state (VSS) was 0.69±0.13 L/kg, the systemic clearance (CLS) was 22.52±2.06 mL/min/kg, and terminal elimination half-life (beta half-life, T1/2,β) was 29.66±8.73 min. VF (compared to sham surgery) had little or no impact on both nitrite PK and endogenous levels as evidenced by these nearly superimposable concentration-time profiles.
Figure 2. Pharmacokinetics (PK) of nitrite in blood.
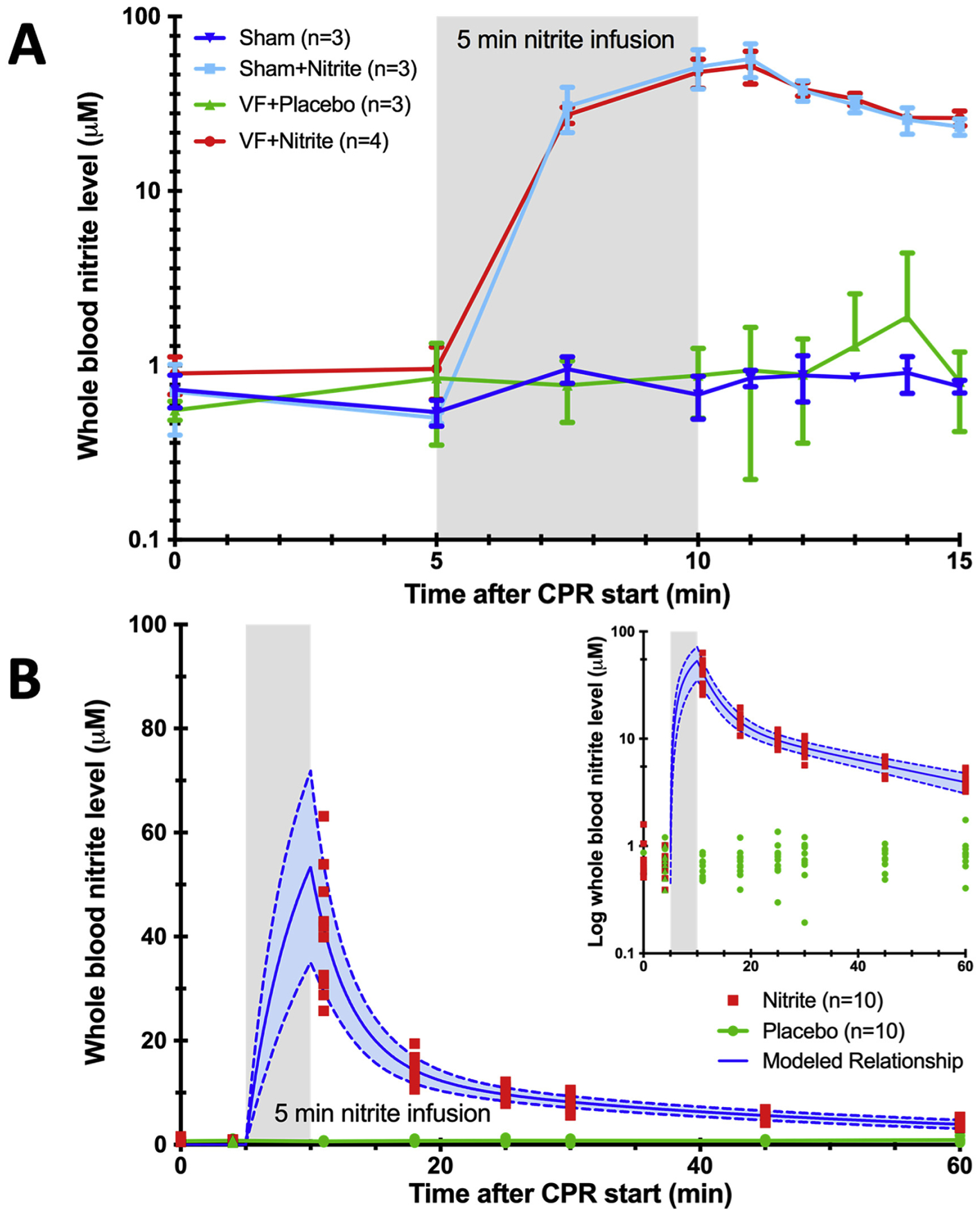
(A) Whole blood nitrite levels 15 minutes after start of resuscitation from short term experiments (CSF and tissue harvest after 15 min). (B) Whole blood nitrite levels up to 60 minutes after start of resuscitation from 8-days survival experiments including best fit PK modeling (solid line is the mean and dashed line is the standard deviation).
3.2. Outcomes from VF in rats treated with nitrite vs. placebo
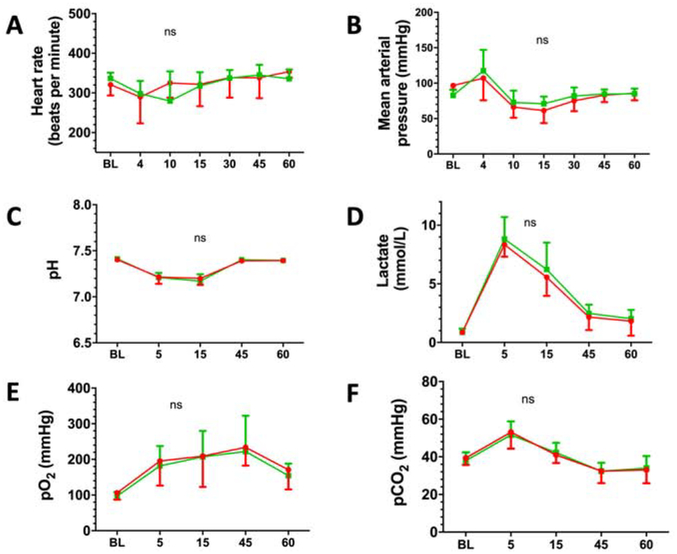
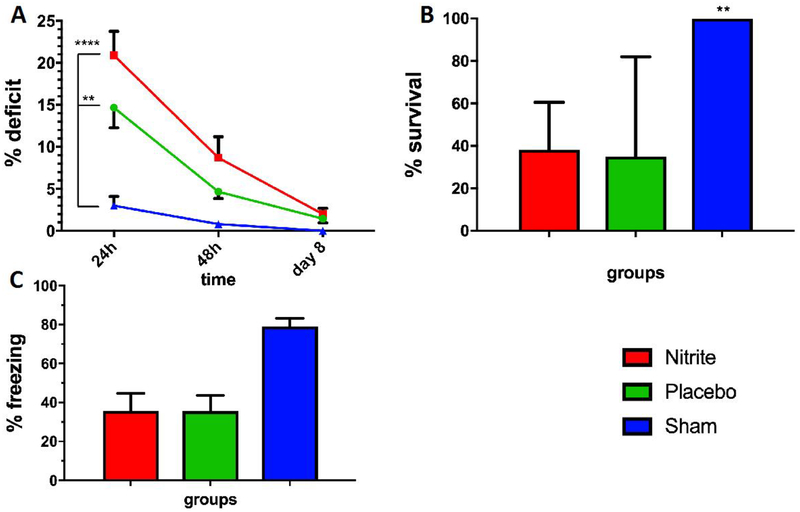
We randomized 20 rats to nitrite vs. placebo-treatment delivered 5 min after ROSC. Median weight was 431 ± 14g. Nineteen of 20 (95%) rats randomized survived until day 8. One rat in the placebo group died at 2 days after VF. CPR-duration in the nitrite group was 99 ± 39s vs. 118 ± 31s in the placebo group (p=0.86). Between groups, no significant differences were found in baseline labs or physiologic variables. (Table 1/Figure 3). No differences were noted in arterial blood gases at 5 (post-VF but pre-drug therapy) or 15 min (post-drug therapy) after VF (Table 2/Figure 3). No significant differences were noted in NDS at days 1, 2 or 8 (Figure 4A), CA1 neuronal survival (Figure 4B) or freezing after conditioned fear (Figure 4C) between nitrite and placebo treated groups. Due to technical reasons, histology from one rat in the placebo group (Figure 4B) was excluded.
Table 1.
Baseline characteristics prior to cardiac arrest, mean (±standard deviation)
| Parameter | Placebo (n=9) | Nitrite (n=10) | P-value |
|---|---|---|---|
| pH | 7.40 (0.02) | 7.41 (0.03) | 0.49 |
| BE (mmol/l) | −0.5 (2.2) | −0.1 (2.2) | 0.67 |
| Lactate (mmol/l) | 0.9 (0.3) | 0.9 (0.3) | 0.96 |
| pO2 (mmHg) | 97 (11.4) | 106 (18.7) | 0.24 |
| pCO2 (mmHg) | 37.9 (4.4) | 39.4 (3.6) | 0.46 |
| Hb (g/dl) | 14.8 (1.5) | 14.9 (0.5) | 0.96 |
| Na+ (mmol/l) | 142 (3) | 144 (3) | 0.37 |
| K+ (mmol/l) | 3.7 (0.6) | 3.8 (0.4) | 0.63 |
| MAP (mmHg) | 83 (8) | 82 (9) | 0.81 |
| Heart rate (bpm) | 339 (12) | 321 (27) | 0.08 |
| Body Temp. (°C) | 37.0 (0.4) | 37.1 (0.3) | 0.50 |
| Weight (g) | 435 (13) | 427 (15) | 0.24 |
Abbreviations: BE: base excess; Hb: Haemoglobin; Na+: Sodium; K+: Potassium; MAP: mean arterial pressure; bpm: beats per minute;
Figure 3. Physiological and biochemical results of the 8-days survival model.
Sequential measurements of physiologic and biochemical variables where made at baseline (BL) and after defined times (in minutes) after return of spontaneous circulation (e.g. 5 = 5 min after ROSC). Over a total time period of 60 minutes during ICU phase, Nitrite (red circles, n=10) and placebo (green circles, n=9) treated animals showed no significant difference in (A) heart rate, (B) blood pressure, (C) arterial pH, (D) lactic acid, (E) paO2 and (F) paCO2. Data are shown as mean (± standard error). For better visualization, error bars are directed only in one direction, above for placebo (green) and below for Nitrite (red).
Table 2.
Characteristics 5 minutes and 15 minutes post CPR start, mean (±standard deviation)
| Timepoint post CPR start | Parameter | Placebo (n=9) | Nitrite (n=10) | p-value |
|---|---|---|---|---|
| 5 min | pH | 7.21 (0.05) | 7.21 (0.07) | 0.88 |
| Lactate (mmol/l) | 8.8 (1.9) | 8.3 (1.0) | 0.50 | |
| pO2 (mmHg) | 182 (56) | 196 (69) | 0.64 | |
| pCO2 (mmHg) | 52 (7) | 53 (9) | 0.70 | |
| MAP (mmHg) | 117 (30) | 107 (31) | 0.47 | |
| Heart rate (bpm) | 298 (32) | 290 (66) | 0.73 | |
| 15 min | pH | 7.17 (0.07) | 7.20 (0.07) | 0.39 |
| Lactate (mmol/l) | 6.2 (2.3) | 5.6 (1.6) | 0.47 | |
| pO2 (mmHg) | 207 (73) | 209 (86) | 0.96 | |
| pCO2 (mmHg) | 42 (5) | 41 (4) | 0.56 |
Figure 4. Outcomes from 8-days survival model after VF CA.
(A) Neurological deficit score at 24, 48 hours and 8 days after CPR, (B) CA1 neuronal survival in % 8 days after CPR and (C) fear conditioning freezing on day 2 showed no significant differences between nitrite (green bar, n=10) compared to placebo (red bar, n=9). Sham shown as blue bar, n=5. Data are shown as mean (± standard error).
4. Discussion
Using a rat model of VF CA,[6] we demonstrate that nitrite, administered early after ROSC, rapidly crosses the BBB and achieves levels in CSF that are approximately half of that seen simultaneously in whole blood. Nitrite therapy increases brain nitrite levels to a similar extent as other tissues. We characterized the PK of intravenous nitrite administration after VF and report on laboratory and physiologic data which speak to nitrite safety as recently noted in phase I clinical trials.[28] In contrast to our prior reports in asphyxial and KCl induced CA [26, 27], we failed to see a benefit for nitrite therapy in terms of post-VF brain injury, although there was no signal for harm either.
This is the first study to measure and characterize nitrite’s PK not only after CA but in any form of critical illness or brain injury. Our study clearly shows that regardless of the presence or absence (i.e. sham data) of brain injury, nitrite is able to rapidly cross the BBB to enter brain tissue and CSF after a single IV dose. CSF levels are approximately half that of whole blood which is what one would expect plasma levels to be at this time[33] implying diffusion into CSF with minimal impediment or metabolism. This is surprising given that nitrite is an anion and is not lipophilic as are most drugs which cross the BBB. The implication is there is some form of facilitated transport assisting nitrite entry.[34] Prior studies have suggested anion exchanger 1 (AE1) as a candidate for facilitated nitrite entry into erythrocytes.[35] The lower levels of nitrite in brain tissue compared to blood/CSF imply metabolism to nitrate, S-nitrosothiols or iron- nitrosyls [10; 18] though we did not assay these quantities specifically. Furthermore, the degree to which tissue nitrite increases in brain, which is comparable to heart, demonstrates that the BBB does not impede nitrite from reaching its target. Nitrite levels were lowest in liver which could be explained by extensive metabolism by mitochondria[10; 14], xanthine oxidase[36], mARC-1/2[26] and cytochrome P450[37] systems which are present in higher quantities within this tissue type. These findings support the use of nitrite to target brain injury even in the absence of BBB breakdown as we have previously demonstrated is the case in animal models of asphyxial and potassium-chloride-induced CA.[38; 39] It is also possible that in human CA, longer ischemic times and perhaps other comorbidities, that are commonly observed, may result in BBB breakdown.
The PK and safety observed for nitrite therapy after CA are interesting and corroborate our human modeling. We found that whole blood nitrite was reduced considerably in the first five minutes after dose completion. This finding explains why a two compartment PK model best fits our data and is highly suggestive of early redistribution. Prior studies have demonstrated that ischemia depletes nitrite in the blood and tissue.[13; 16; 40] In the setting of global ischemia (CA) one would expect systemic depletion as the tissues and blood metabolize nitrite at a steadily increasing rate as oxygen tension and pH decline.[25; 40] The result is a global nitrite deficiency unique to CA which explains why nitrite dosed early after reperfusion results in high rates of redistribution to restore the global tissue deficiency present after CA. One would expect this initial rapid elimination is greater after CA than focal ischemic disease (e.g. myocardial infarction or stroke) though direct comparisons have not been made. Recent human data (n=120) from nitrite dosing in CA support this concept of global ischemic nitrite consumption with reduced plasma levels from those anticipated based on PK models from healthy control models.[29] Since it is not feasible to perform serial blood sampling in the early post-resuscitation period after human CA, our present PK characterization provides the only data on this topic. The terminal half time for nitrite elimination which we observed (~30 min) is close to that noted in healthy humans (42 min).[33] Of note the human studies were performed in healthy volunteers so the small increase in elimination rate may represent the persistent effects of global ischemia-reperfusion injury within our model which may be of relevance to ongoing human studies of nitrite after CA (). Since longer low and no flow ischemic times are commonly encountered in human CA compared to our animal models, the impact of both redistribution due to global nitrite depletion and terminal elimination due to persistent ischemia are likely to be even greater within human disease.
Our VF model results in significant myocardial dysfunction and shock as demonstrated by the observed lactate elevations. We recently reported this greater hemodynamic insult to be an important feature of VF compared to asphyxial CA in both rodent models and human CA.[41] Despite this we did not note any evidence of hypotension or hemodynamic instability after nitrite administration, even with peak whole blood levels of over 50 μM. This is consistent with human safety data in healthy volunteers[33], patients with heart failure[42], and a large phase 1 study of nitrite dosed during CPR after out of hospital cardiac arrest, and further supports is safety with regard to hemodynamics at the doses used [29].
We observed significant brain injury within our VF CA model but did not see a neurologic improvement resulting from nitrite therapy. This contrasts to prior results in a rat model of asphyxial CA[17] and a mouse model of potassium chloride-induced CA[16] where large effects (>25%) were noted. It is possible that nitrite is less effective in VF CA, which is to say cardiac etiology CA, due to differences in the pathophysiology of injury[6] and hence response to therapy. There is precedent for this form of heterogeneous response to therapies within different models of CA.[9] Greater brain oxidative stress and mitochondrial injury[8; 9] produced by asphyxia vs. VF may make the former more nitrite responsive.[18] Cerebral oxidative stress increases substantially after prolonged (>10 min) VF[22] such that our model, with the brief CA duration used in this study, may not be sufficiently severe to capture potential benefits of nitrite therapy. It should be noted that we were not powered to detect small differences in outcome as a 5 or 10% reduction in CA1 loss would require 1470 or 367 rats per group (α=0.05; 1-β=0.80), respectively. By comparison, our prior data indicated a need for n=6 per group to see similar effects as previously observed. Given that in humans, CA duration is often greater than 5 min, we speculate that it is possible that benefit could be observed even in VF CA where post-arrest brain injury will exceed what we model in the lab. The ongoing nitrite clinical trial () is enrolling 1500 subjects to answer this question. Since the extent of brain injury produced by VF is much less than similar durations of asphyxia[6; 9] it is also possible that any benefits from nitrite against ischemia-reperfusion[10; 12; 13; 14; 15; 16; 17; 18] may be offset by potential adverse effects such as increased free radical (e.g. peroxynitrite) production.[43; 44]
Our study has limitations. Our small sample size with a high survival rate and modest neuronal cell loss may have limited statistical power to detect a protective effect. Also, our 5-minute model of VF CA might not be fully comparable to an out-of hospital cardiac arrest with long lasting CPR. Our PK characterization in VF may not be generalizable to other forms of CA or illness although our findings are close to estimates derived in healthy subjects and lung transplant recipients[29].
5. Conclusions
Using a rat model of VF CA, we characterized the PK of intravenous nitrite administration after rat VF and report safety and rapid brain penetration on par with other organs. However, contrasting studies in models of different CA phenotypes, despite showing safety, we failed to detect a benefit for nitrite therapy in reducing the modest brain injury from VF CA.
Supplementary Material
Highlights.
Nitrite rapidly crosses the blood brain barrier in a VF cardiac arrest rat model with cerebrospinal fluid levels approximately half that of whole blood
The pharmacokinetics of intravenous nitrite best fit a two compartmental model with early rapid redistribution followed by a more gradual elimination
Nitrite was well tolerated hemodynamically and did not worsen post-resuscitation shock which is common in this model and human VF cardiac arrest
Sources of Funding:
This work was supported by the Laerdal Foundation for Acute Medicine and the Max Kade Foundation (TU); and by the National Institutes of Health [grant numbers K08NS069817 (CD), R01HL129722 (CD, PE, FK)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: Dr. Dezfulian is the principal investigator for an investigator initiated phase 2 randomized clinical trial of inhaled nitric oxide after out-of-hospital cardiac arrest () funded by Mallinckrodt Pharmaceuticals, Inc. Drs. Dezfulian, Empey and Kim are investigators in a phase 2 randomized clinical trial of nitrite after out-of-hospital cardiac arrest () funded by NHLBI.
References
- [1].Members WG, Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, Heart Disease and Stroke Statistics—2012 Update: A Report From the American Heart Association, Circulation 125 (2012) e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K, Treatment of Comatose Survivors of Out-of-Hospital Cardiac Arrest with Induced Hypothermia, New Engl J Med 2002, pp. 557–563. [DOI] [PubMed] [Google Scholar]
- [3].T. Hypothermia after Cardiac Arrest Study Group, Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest, New Engl J Med 2002, pp. 549–556. [DOI] [PubMed] [Google Scholar]
- [4].Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Åneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Køber L, Langørgen J, Lilja G, Møller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H, Targeted Temperature Management at 33°C versus 36°C after Cardiac Arrest, New England Journal of Medicine 369 (2013) 2197–2206. [DOI] [PubMed] [Google Scholar]
- [5].Winther-Jensen M, Pellis T, Kuiper M, Koopmans M, Hassager C, Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Friberg H, Gasche Y, Horn J, Hovdenes J, Stammet P, Wanscher M, Wise MP, Åneman A, Kjaergaard J, Mortality and neurological outcome in the elderly after target temperature management for out-of-hospital cardiac arrest, Resuscitation 91 (2015) 92–98. [DOI] [PubMed] [Google Scholar]
- [6].Uray T, Lamade A, Elmer J, Drabek T, Stezoski JP, Misse A, Janesko-Feldman K, Garman RH, Chen N, Kochanek PM, Dezfulian C, Callaway CW, Doshi AA, Frisch A, Guyette FX, Reynolds JC, Rittenberger JC, Phenotyping Cardiac Arrest: Bench and Bedside Characterization of Brain and Heart Injury Based on Etiology, Crit Care Med 46 (2018) e508–e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lilja G, Nielsen N, Friberg H, Horn J, Kjaergaard J, Nilsson F, Pellis T, Wetterslev J, Wise MP, Bosch F, Bro-Jeppesen J, Brunetti I, Buratti AF, Hassager C, Hofgren C, Insorsi A, Kuiper M, Martini A, Palmer N, Rundgren M, Rylander C, van der Veen A, Wanscher M, Watkins H, Cronberg T, Cognitive Function in Survivors of Out-of-Hospital Cardiac Arrest After Target Temperature Management at 33°C Versus 36°C, Circulation 131 (2015) 1340–1349. [DOI] [PubMed] [Google Scholar]
- [8].Kamohara T, Weil MH, Tang W, Sun S, Yamaguchi H, Klouche K, Bisera J, A Comparison of Myocardial Function after Primary Cardiac and Primary Asphyxial Cardiac Arrest, American Journal of Respiratory and Critical Care Medicine 164 (2001) 1221–1224. [DOI] [PubMed] [Google Scholar]
- [9].Vaagenes P, Safar P, Moossy J, Rao G, Diven W, Ravi C, Arfors K, Asphyxiation versus ventricular fibrillation cardiac arrest in dogs.: Differences in cerebral resuscitation effects—a preliminary study, Resuscitation 35 (1997) 41–52. [DOI] [PubMed] [Google Scholar]
- [10].Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ, Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver, J Clin Invest 115 (2005) 1232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pluta RM, Dejam A, Grimes G, Gladwin MT, Oldfield EH, Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage, Jama 293 (2005) 1477–84. [DOI] [PubMed] [Google Scholar]
- [12].Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, Roh JK, Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury, Stroke 37 (2006) 2744–50. [DOI] [PubMed] [Google Scholar]
- [13].Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ, Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury, Proc Natl Acad Sci U S A 104 (2007) 19144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT, Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer, J Exp Med 204 (2007) 2089–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, Aletras AH, Cannon RO 3rd, Gladwin MT, Arai AE, Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction, Circulation 117 (2008) 2986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT, Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I, Circulation 120 (2009) 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dezfulian C, Alekseyenko A, Dave KR, Raval AP, Do R, Kim F, Perez-Pinzon MA, Nitrite Therapy is Neuroprotective and Safe in Cardiac Arrest Survivors, Nitric Oxide 26 (2012) 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dezfulian C, Kenny E, Lamade A, Misse A, Krehel N, St Croix C, Kelley EE, Jackson TC, Uray T, Rackley J, Kochanek PM, Clark RS, Bayir H, Mechanistic characterization of nitrite-mediated neuroprotection after experimental cardiac arrest, J Neurochem 139 (2016) 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH, Oxidative Stress in Ischemic Brain Damage: Mechanisms of Cell Death and Potential Molecular Targets for Neuroprotection, Antioxidants & Redox Signaling 14 (2010) 1505–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu Y, Fiskum G, Schubert D, Generation of reactive oxygen species by the mitochondrial electron transport chain, J Neurochem 80 (2002) 780–7. [DOI] [PubMed] [Google Scholar]
- [21].Drabek T, Foley LM, Janata A, Stezoski J, Kevin Hitchens T, Manole MD, Kochanek PM, Global and regional differences in cerebral blood flow after asphyxial versus ventricular fibrillation cardiac arrest in rats using ASL-MRI, Resuscitation 85 (2014) 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Basu S, Liu X, Nozari A, Rubertsson S, Miclescu A, Wiklund L, Evidence for Time-dependent Maximum Increase of Free Radical Damage and Eicosanoid Formation in the Brain as Related to Duration of Cardiac Arrest and Cardio-pulmonary Resuscitation, Free Radical Research 37 (2003) 251–256. [DOI] [PubMed] [Google Scholar]
- [23].Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO 3rd, Gladwin MT, Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation, Nat Med 9 (2003) 1498–505. [DOI] [PubMed] [Google Scholar]
- [24].Fens MH, Larkin SK, Oronsky B, Scicinski J, Morris CR, Kuypers FA, The capacity of red blood cells to reduce nitrite determines nitric oxide generation under hypoxic conditions, PLoS One 9 (2014) e101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT, Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control, J Clin Invest 115 (2005) 2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sparacino-Watkins CE, Tejero J, Sun B, Gauthier MC, Thomas J, Ragireddy V, Merchant BA, Wang J, Azarov I, Basu P, Gladwin MT, Nitrite reductase and nitric-oxide synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2, J Biol Chem 289 (2014) 10345–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].DeMartino AW, Kim-Shapiro DB, Patel RP, Gladwin MT, Nitrite and nitrate chemical biology and signalling, Br J Pharmacol 176 (2019) 228–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dezfulian C, Olsufka M, Fly D, Scruggs S, Do R, Maynard C, Nichol G, Kim F, Hemodynamic effects of IV sodium nitrite in hospitalized comatose survivors of out of hospital cardiac arrest, Resuscitation 122 (2018) 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim F, Dezfulian C, Empey PE, Morrell M, Olsufka M, Scruggs S, Kudenchuk P, May S, Maynard C, Sayre MR, Nichol G, Usefulness of Intravenous Sodium Nitrite During Resuscitation for the Treatment of Out-of-Hospital Cardiac Arrest, Am J Cardiol 122 (2018) 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R, Outcome Model of Asphyxial Cardiac Arrest in Rats, J Cereb Blood Flow Metab 15 (1995) 1032–1039. [DOI] [PubMed] [Google Scholar]
- [31].Curzon P, Rustay NR, Browman KE, Cued and Contextual Fear Conditioning for Rodents, (2009). [PubMed] [Google Scholar]
- [32].Kamprath K, Wotjak CT, Nonassociative learning processes determine expression and extinction of conditioned fear in mice, Learn Mem 11 (2004) 770–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO 3rd, Schechter AN, Gladwin MT, Nitrite Infusion in Humans and Nonhuman Primates. Endocrine Effects, Pharmacokinetics, and Tolerance Formation, Circulation 116 (2007) 1821–1831. [DOI] [PubMed] [Google Scholar]
- [34].Shingles R, Roh MH, McCarty RE, Direct measurement of nitrite transport across erythrocyte membrane vesicles using the fluorescent probe, 6-methoxy-N-(3-sulfopropyl) quinolinium, J Bioenerg Biomembr 29 (1997) 611–6. [DOI] [PubMed] [Google Scholar]
- [35].Jensen FB, Nitrite transport into pig erythrocytes and its potential biological role, Acta Physiol Scand 184 (2005) 243–51. [DOI] [PubMed] [Google Scholar]
- [36].Li H, Samouilov A, Liu X, Zweier JL, Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues, J Biol Chem 276 (2001) 24482–9. [DOI] [PubMed] [Google Scholar]
- [37].Li H, Liu X, Cui H, Chen YR, Cardounel AJ, Zweier JL, Characterization of the mechanism of cytochrome P450 reductase-cytochrome P450-mediated nitric oxide and nitrosothiol generation from organic nitrates, J Biol Chem 281 (2006) 12546–54. [DOI] [PubMed] [Google Scholar]
- [38].Lahoud-Rahme MS, Stezoski J, Kochanek PM, Melick J, Tisherman SA, Drabek T, Blood-brain barrier integrity in a rat model of emergency preservation and resuscitation, Resuscitation 80 (2009) 484–8. [DOI] [PubMed] [Google Scholar]
- [39].Tress EE, Clark RS, Foley LM, Alexander H, Hickey RW, Drabek T, Kochanek PM, Manole MD, Blood brain barrier is impermeable to solutes and permeable to water after experimental pediatric cardiac arrest, Neurosci Lett 578 (2014) 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zweier JL, Wang P, Samouilov A, Kuppusamy P, Enzyme-independent formation of nitric oxide in biological tissues, Nat Med 1 (1995) 804–9. [DOI] [PubMed] [Google Scholar]
- [41].Uray T, Lamade A, Elmer J, Drabek T, Stezoski JP, Misse A, Janesko-Feldman K, Garman RH, Chen N, Kochanek PM, Dezfulian C, Phenotyping Cardiac Arrest: Bench and Bedside Characterization of Brain and Heart Injury Based on Etiology, Crit Care Med (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ormerod JOM, Arif S, Mukadam M, Evans JDW, Beadle R, Fernandez BO, Bonser RS, Feelisch M, Madhani M, Frenneaux MP, Short-Term Intravenous Sodium Nitrite Infusion Improves Cardiac and Pulmonary Hemodynamics in Heart Failure Patients, Circulation: Heart Failure 8 (2015) 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dohi K, Ohtaki H, Inn R, Ikeda Y, Shioda HS, Aruga T, Peroxynitrite and caspase-3 expression after ischemia/reperfusion in mouse cardiac arrest model, Acta Neurochir Suppl 86 (2003) 87–91. [DOI] [PubMed] [Google Scholar]
- [44].Forman LJ, Liu P, Nagele RG, Yin K, Wong PY, Augmentation of nitric oxide, superoxide, and peroxynitrite production during cerebral ischemia and reperfusion in the rat, Neurochem Res 23 (1998) 141–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.