Abstract
Objective
Biologics have an important role in the treatment of juvenile idiopathic arthritis (JIA). Long‐term safety data are limited. Direct comparison of different agents regarding occurrence of adverse events (AEs), especially of rare events, requires large quantities of patient years. In this analysis, long‐term safety with regard to AE of special interest (AESI) was compared between different biologics.
Methods
Patients with nonsystemic JIA were selected from the German BIKER registry. Safety assessments were based on AE reports. Number of AEs, serious AEs, and 25 predefined AESIs, including medically important infection, uveitis, inflammatory bowel disease, cytopenia, hepatic events, anaphylaxis, depression, pregnancy, malignancy, and death, were analyzed. Event rates and relative risks were calculated using AEs reported after first dose through 70 days after last dose.
Results
A total of 3873 patients entered the analysis with 7467 years of exposure to biologics. The most common AESIs were uveitis (n = 231) and medically important infections (n = 101). Cytopenia and elevation of transaminases were more frequent with tocilizumab (risk ratio [RR] 8.0, 95% confidence interval [CI] 4.2‐15, and RR 4.7, 95% CI 1.8‐12.2, respectively). Anaphylactic events were associated with intravenous route of administration. In patients ever exposed to biologics, eight malignancies were reported. Six pregnancies have been documented in patients with tumor necrosis factor inhibitors. No death occurred in this patient cohort during observation.
Conclusion
Surveillance of pharmacotherapy as provided by the BIKER registry is an import approach, especially for long‐term treatment of children. Overall, tolerance was acceptable. Differences between biologics were noted and should be considered in daily patient care.
Significance & Innovations.
Long‐term surveillance of biologic therapies remains an important challenge.
Medically important infections and uveitis were the most commonly reported events in all biologic treatments.
Adverse events of special interest depend in part on specific biologics used, such as cytopenias, liver enzyme elevations, anaphylaxis and psoriasis.
Introduction
Juvenile idiopathic arthritis (JIA) is the most common chronic inflammatory rheumatic disease in childhood with an estimated incidence of 10 to 20 per 100 000 children under 16 years of age 1. It can lead to severe disability, thus successful and timely treatment is crucial 2. The most common first‐line disease‐modifying anti‐rheumatic drug (DMARD) in JIA therapy is methotrexate (MTX). According to national and international guidelines and recommendations, patients with JIA who are refractory or intolerant to MTX treatment are eligible for treatment with biologics 3, 4. Currently, three tumor necrosis factor (TNF) inhibitors (TNFi)—etanercept (ETA), adalimumab (ADA), and golimumab (GOL)—as well as the interleukin (IL)‐6 inhibitor tocilizumab (TOC) and abatacept (ABA), an inhibitor of T‐cell activation, are approved for treatment of polyarticular JIA. The first TNFi infliximab (INF) is still used for treatment of JIA, although it has not been approved. This presents the pediatric rheumatologist with challenging choices, which should be made on an individual basis. As treatments may be continued for years, data on the tolerability of the individual biologics, particularly in long‐term use, should be taken into consideration. In particular, the detection of rare events requires the accumulation of a large quantity of patient years (PY). These data are scarce. The German BIKER registry has documented biological therapies in JIA since 2001 5 and covers the majority of pediatric JIA patients treated with biologics in Germany. The aim of this analysis was to compare the safety data on the different approved biologics in nonsystemic JIA.
Methods
The German BIKER registry has documented treatment of JIA with biologics since 2001. A biologics‐naïve cohort of patients starting treatment with MTX was recruited between 2005 and 2011. BIKER has been described in previous reports 5, 6. It was approved by the local ethics committees. Written informed consent was obtained, and pseudonymized data were collected for each patient. The BIKER registry is registered in the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP; http://www.encepp.eu/encepp/viewResource.htm?xml:id=20591).
Patient assessment was performed at baseline, after 3 and 6 months, and every 6 months thereafter. After discontinuation of a biologic, patients were followed up with every 6 months, patients transitioning to adult care are followed up by the JUMBO registry 7. Adverse events (AEs) are documented at every visit for the whole period from the last visit. Patients in the German BIKER registry diagnosed with nonsystemic JIA and treated with ETA, ADA, GOL, INF, TOC, or ABA and biologics‐naïve patients with MTX were selected for this analysis, including data documented until September 2018.
Definitions
According to ICH E6 Section 1.2 8, an AE is any untoward medical occurrence in a subject temporarily associated with a pharmaceutical product, even without causality or relationship; a serious AE (SAE) results either in death, is life‐threatening, requires hospitalization/prolongation of hospitalization or medical or surgical intervention to prevent a serious outcome, results in persistent or significant disability/incapacity, or is a congenital anomaly or birth defect. For the pharmaco‐surveillance of the biologics used in JIA treatment, 25 AEs of special interest (AESIs) were predefined: anaphylaxis, autoimmune disease—including psoriasis, bleeding disorder, inflammatory bowel disease, cytopenia, demyelination, gastrointestinal perforation, hepatic event, infection (either serious or medically important), malignancy, macrophage activation syndrome, cardiovascular event, depression/suicidality, pregnancy, thrombotic event, vasculitis, uveitis, cerebral ischemia, systemic lupus erythematosus, opportunistic infection, inefficacy, hepatitis B‐reactivation, arterial hypertension, sarcoidosis, serum sickness, and death.
An AESI was assigned to a therapy if it occurred during treatment or up to 70 days after discontinuation. If during these 70 days of follow‐up other therapies were started, the AESI was also counted for the new therapies, resulting in multiple counting of some AESIs. Malignancies were additionally attributed to the treatment if the patient was ever exposed.
Observation time was calculated as time from therapy start to end of treatment or end of follow‐up.
Statistical analyses show frequencies, rates per 100 PY, and relative risk (RR), both with 95% confidence interval (CI). For each biologic, the RR was calculated against all other biologics in this analysis. For MTX, RR was calculated against all biologic therapies in the analysis combined. Additionally, RR was calculated for TNFi versus TOC and ABA as well as TOC versus ABA. In addition biologic monotherapy and combination therapy with MTX were analyzed separately. AE rates were then compared for each of those therapies against all other treatments, which included a biologic. To detect the influence of concomitant steroid therapy, we compared AE rates in patients treated with biologics, who never received concomitant steroids during biologic treatment, with rates in patients who had received systemic steroids during biologic therapy.
Significance level was set at 5%, analyses were performed by SAS 9.3 (SAS Institute Inc.).
Results
From a total of 4500 patients with JIA included in the German BIKER registry, 3873 patients with nonsystemic JIA were identified with 3622 biologic therapies. The total exposure time to biologics was 7467 PY. For comparison, a biologics‐naïve cohort of 1404 patients starting treatment with MTX, with an exposure time of 3174 PY, documented in BIKER was used.
ETA was the most frequently used biologic, followed by ADA, TOC, ABA, GOL, and INF.
Baseline patient characteristics
There were differences between the cohorts treated with different biologics (Table 1). In the TOC and ABA cohorts, the frequency of patients who were female and had positive rheumatoid factor was higher, whereas presence of human leukocyte antigen–B27 was less frequent.
Table 1.
Baseline characteristics of patients contributing to the biologics cohorts. Biologics‐naïve patients served as controls (MTX cohort). P values with χ2 test or Fisher's exact test and Mann‐Whitney U, where appropriate
| Treatment Cohort | ETA | ADA | GOL | INF | TOC | ABA | MTX |
|---|---|---|---|---|---|---|---|
| n | 2338 | 828 | 86 | 63 | 215 | 91 | 1404 |
| Female, n (%) | 1600 (68.4) | 578 (69. 9) | 60 (69.8) | 39 (61.9) | 173 (80.5) | 75 (82.4) | 967 (68.9) |
| RF positive, n (%) | 205 (8.8) | 52 (6.3) | 8 (9.3) | 1 (1.6) | 23 (10.7) | 9 (9.9) | 53 (3.8) |
| ANA positive, n (%) | 1144 (48.9) | 480 (58.0) | 40 (46.5) | 41 (65.1) | 119 (55.4) | 46 (50.6) | 711 (50.6) |
| HLA B27 positive, n (%) | 581 (24.9) | 161 (19.5) | 18 (20.9) | 17 (27.0) | 21 (9.8) | 13 (14.3) | 246 (17.5) |
| Age at diagnosis of JIA, mean (SD) | 8.1 (4.6) | 7.0 (4.7) | 6.7 (4.7) | 5.3 (3.8) | 6.8 (4.4) | 6.9 (4.2) | 7.7 (4.6) |
| Disease duration, mean (SD) | 4.07 (3.5) | 5.35 (3.9) | 6.4 (4.7) | 7.2 (3.6) | 5.83 (4.1) | 6.82 (3.75) | 2.05 (2.7) |
| Uveitis, n (%) | 153 (15.4) | 225 (27.2) | 17 (19.8) | 18 (28.6) | 35 (16.3) | 14 (15.4) | 104 (7.4) |
| Pretreatment with biologic, n (%) | 42 (1.8) | 387 (46.8) | 57 (66.3) | 57 (90.5) | 144 (67.0) | 78 (85.7) | 0 |
| MTX, n (%)a | 1501 (64.2) | 491 (59.4) | 58 (67.4) | 38 (60.3) | 108 (50.2) | 55 (60.4) | 1404 (100) |
| Systemic steroids, n (%)a | 763 (32.6) | 235 (28.4) | 19 (22.1) | 23 (36.5) | 79 (36.7) | 49 (53.9) | 334 (23.8) |
| Number of active joints, mean (SD) | 6.7 (8.0) | 3.7 (5.3) | 5.5 (8.0) | 3.9 (6.9) | 6.1 (6.6) | 4.7 (4.9) | 6.0 (7.6) |
| Number of joints with LOM, mean (SD) | 7.3 (8.8) | 4.4 (5.9) | 5.9 (8.1) | 4.0 (6.6) | 5.8 (6.3) | 5.7 (6.5) | 5.9 (7.7) |
| Patient Global; VAS mean (SD) | 43.5 (27.1) | 31.0 (25.3) | 34.6 (25.8) | 33.9 (27.7) | 39.3 (25.0) | 41.0 (24.0) | 39.1 (26.0) |
| Physician Global VAS mean (SD) | 51.2 (26.5) | 40.2 (27.6) | 36.8 (26.0) | 40.8 (34.0) | 52.4 (28.0) | 48.9 (27.0) | 46.6 (25.5) |
| ESR (mm/h) mean (SD) | 22.4 (22.0) | 15.8 (15.8) | 16.9 (23.4) | 19.1 (20.3) | 18.8 (20.6) | 17.6 (17.5) | 23.2 (21.6) |
| JADAS‐10, mean (SD) | 15.2 (7.3) | 10.6 (7.0) | 11.9 (7.5) | 10.7 (8.8) | 14.2 (6.9) | 13.1 (6.6) | 13.8 (7.0) |
Abbreviation: ABA, abatacept; ADA, adalimumab; ANA, antinuclear antibodies; ESR, erythrocyte sedimentation rate; ETA, etanercept; GOL, golimumab; HLA, human leukocyte antigen, INF, infliximab; JADAS, Juvenile Arthritis Disease Activity Score; LOM, Limitation of motion; MTX, methotrexate; RF, rheumatoid factor; TOC, tocilizumab; VAS, visual analogue scale.
Concomitant treatment at baseline visit.
Disease duration before start of therapy also differed between the cohorts. Patients with ETA had the shortest disease duration (mean 4.1 years, SD ±3.5), followed by patients starting with ADA and TOC (5.4 ± 3.9 years and 5.8 ± 4.1 years), ABA (6.2 ± 3.7 years), GOL (6.4 ± 4.7 years), and INF (7.2 ± 3.6 years). Pretreatment with other biologics was documented only in 1.8% of patients treated with ETA; for ADA, GOL, INF, TOC, and ABA, the frequencies were 46.8%, 66.3%, 90.5%, 67.0%, and 85.7%, respectively. Patients with known uveitis were more likely to receive TNF antibodies. Concomitant treatment at baseline with MTX was slightly lower in patients treated with TOC. The percentage of patients receiving concomitant systemic steroids at baseline was highest in patients starting ABA (54%). Baseline Juvenile Arthritis Disease Activity Score (JADAS)‐10 was highest in patients starting ETA (15.2 ± 7.3) and TOC (14.2 ± 6.9), followed by ABA (13.1 ± 6.6), GOL (11.9 ± 7.5), INF (10.7 ± 8.8), and ADA (10.6 ± 7.0). Active joint counts at baseline had a similar distribution.
Safety
Altogether, 733 AESIs were reported. In the biologics cohort, 163 were SAEs with higher rates in the GOL (RR 2.5; 95% CI, 1.0‐6.0) and INF (RR 3.5; 95% CI, 1.7‐7.1) cohorts, whereas biologics‐naïve patients with MTX had a lower SAE rate (RR 0.3; 95% CI, 0.2‐0.5), (Table 2). No differences were found between patients receiving TNFi, TOC, or ABA (Table 3). However, patients treated with any biologic, who had never been treated with steroids concomitantly to the biologic, had a lower risk for SAE (RR 0.65; 95% CI, 0.48‐0.87) and there was no difference in the rate of AEs (RR 1.18; 95% CI, 0.99‐1.4). Selected AESI categories are shown in Figures 1 and 2.
Table 2.
AESI numbers reported, rates per 100 patient years and RRs. For ETA, ADA, GOL, INF, TOC, ABA (both monotherapy and combination with MTX), the relative risk was calculated against all other biologics of this group. For TNF, the RR was calculated versus ABA+TOC. For MTX, the RR was calculated against all biologics. Patients may have multiple therapies, and AESIs may be counted under several therapies. AESI categories with at least three events are reported in this table
| Total n | ETA | ADA | GOL | INF | TNFi | TOC | ABA | MTX | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | 3873 | 2339 | 828 | 86 | 63 | 215 | 91 | 1404 | ||
| Numbers of all AESI categories | ||||||||||
| Total SAE (n) |
N Rate RR |
184 |
109 2.02 (1.68‐2.44) 0.78 (0.56‐1.08) |
27 1.86 (1.27‐2.71) 0.82 (0.54‐1.24) |
5 5.32 (2.21‐12.77) 2.48 (1.02‐6.04) |
8 7.39 (3.70‐14.78) 3.51 (1.72‐7.14) |
149 2.12 (1.80‐2.48) 0.64 (0.37‐1.11) |
10 3.27 (1.76‐6.08) 1.53 (0.81‐2.90) |
4 3.39 (1.27‐9.04) 1.57 (0.58‐4.23) |
21 0.66 (0.43‐1.01) 0.30 (0.19‐0.48) |
| Multiple SAEs | 6 | |||||||||
| Total AESI (n) |
N Rate RR |
758 |
362 6.72 (6.06‐7.45) 0.49 (0.42‐0.57) |
175 12.04 (10.38‐13.96) 1.53 (1.28‐1.82) |
23 24.45 (16.25‐36.80) 2.88 (1.90‐4.37) |
22 20.33 (13.39‐30.87) 2.39 (1.56‐3.65) |
576 8.18 (7.54‐8.87) 0.47 (0.37‐0.61) |
54 17.66 (13.52‐23.05) 2.12 (1.61‐2.81) |
13 11.02 (6.40‐18.98) 1.27 (0.74‐2.21) |
109 3.43 (2.85‐4.14) 0.40 (0.32‐0.48) |
| AESI categories with more than 2 reports | ||||||||||
| AESI (n) | 755 | 359 | 175 | 23 | 22 | 573 | 54 | 13 | 109 | |
| Multiple AESIs | 25 | 9 | 10 | 2 | 16 | 3 | 1 | |||
| Exposure years | 5387 | 1454 | 94 | 108 | 7043 | 306 | 118 | 3174 | ||
| Anaphylaxis |
N Rate RR |
21 |
4 0.07 (0.03‐0.20) 0.08 (0.03‐0.23) |
5 0.34 (0.14‐0.83) 1.09 (0.41‐2.92) |
0 |
5 4.62 (1.92‐11.10) 17.89 (6.68‐47.92) |
14 0.20 (0.12‐0.34) 0.08 (0.04‐0.19) |
7 2.29 (1.09‐4.80) 9.64 (4.00‐23.25) |
3 2.54 (0.82‐7.89) 8.90 (2.66‐29.84) |
2 0.06 (0.02‐0.25) 0.20 (0.05‐0.83) |
| Bleeding event |
N Rate RR |
9 |
3 0.06 (0.02‐0.17) 0.29 (0.06‐1.29) |
3 0.21 (0.07‐0.64) 3.10 (0.69‐13.86) |
0 | 0 |
6 0.09 (0.04‐0.19) 0.36 (0.04‐3.00) |
0 |
1 0.85 (0.12‐6.02) 10.39 (1.25‐86.27) |
2 0.06 (0.02‐0.25) 0.67 (0.14‐3.24) |
| Inflammatory bowel disease |
N Rate RR |
20 |
17 0.32 (0.20‐0.51) 2.19 (0.64‐7.46) |
2 0.14 (0.03‐0.55) 0.46 (0.11‐1.98) |
0 |
1 0.92 (0.13‐6.56) 3.58 (0.48‐26.73) |
20 0.28 (0.18‐0.44) |
0 | 0 | 0 |
| Cytopenia |
N Rate RR |
59 |
30 0.56 (0.39‐0.80) 0.68 (0.38‐1.24) |
4 0.28 (0.10‐0.73) 0.38 (0.14‐1.07) |
0 |
1 0.92 (0.13‐6.56) 1.48 (0.20‐10.72) |
35 0.50 (0.36‐0.69) 0.18 (0.09‐0.34) |
12 3.92 (2.23‐6.91) 8.03 (4.17‐15.46) |
0 |
12 0.38 (0.21‐0.67) 0.60 (0.32‐1.13) |
| Hepatic event |
N Rate RR |
41 |
15 0.28 (0.17‐0.46) 0.39 (0.19‐0.79) |
8 0.55 (0.28‐1.10) 1.50 (0.67‐3.38) |
0 |
1 0.92 (0.13‐6.56) 2.34 (0.32‐17.21) |
24 0.34 (0.23‐0.51) 0.24 (0.10‐0.59) |
5 1.63 (0.68‐3.93) 4.68 (1.79‐12.23) |
1 0.85 (0.12‐6.02) 2.15 (0.29‐15.77) |
11 0.35 (0.19‐0.63) 0.86 (0.43‐1.72) |
| Medically important infection |
N Rate RR |
101 |
58 1.08 (0.83‐1.39) 0.86 (0.54‐1.37) |
17 1.17 (0.73‐1.88) 1.05 (0.62‐1.79) |
5 5.32 (2.21‐12.77) 4.96 (2.01‐12.25) |
1 0.92 (0.13‐6.56) 0.82 (0.11‐5.89) |
81 1.15 (0.92‐1.43) 1.62 (0.51‐5.14) |
3 0.98 (0.32‐3.04) 0.87 (0.27‐2.75) |
0 |
20 0.63 (0.41‐0.98) 0.56 (0.34‐0.91) |
| Suspected malignancy |
N Rate RR |
7 |
3 0.06 (0.02‐0.17) 0.58 (0.10‐3.47) |
0 | 0 |
2 1.85 (0.46‐7.39) 45.33 (7.57‐271.3) |
5 0.07 (0.03‐0.17) |
0 | 0 |
3 0.09 (0.03‐0.29) 1.41 (0.34‐5.91) |
| Depression/ Suicidality |
N Rate RR |
21 |
11 0.20 (0.11‐0.37) 0.71 (0.26‐1.91) |
3 0.21 (0.07‐0.64) 0.89 (0.25‐3.09) |
0 | 0 |
14 0.20 (0.12‐0.34) 0.28 (0.08‐0.98) |
2 0.65 (0.16‐2.61) 3.12 (0.71‐13.65) |
1 0.85 (0.12‐6.02) 3.89 (0.52‐29.37) |
5 0.16 (0.07‐0.38) 0.69 (0.26‐1.88) |
| Pregnancy |
N Rate RR |
6 |
4 0.07 (0.03‐0.20) 0.77 (0.14‐4.22) |
2 0.14 (0.03‐0.55) 2.07 (0.38‐11.29) |
0 | 0 |
6 0.09 (0.04‐0.19) |
0 | 0 |
1 0.03 (0.00‐0.22) 0.39 (0.05‐3.26) |
| Thrombosis |
N Rate RR |
3 | 0 |
3 0.21 (0.07‐0.64) |
0 | 0 |
3 0.04 (0.01‐0.13) |
0 | 0 | 0 |
| Inefficacy |
N Rate RR |
112 |
57 1.06 (0.82‐1.37) 0.40 (0.28‐0.58) |
25 1.72 (1.16‐2.55) 1.19 (0.76‐1.85) |
8 8.51 (4.25‐17.01) 6.03 (2.94‐12.38) |
1 0.92 (0.13‐6.56) 0.61 (0.09‐4.39) |
89 1.26 (1.03‐1.56) 0.23 (0.15‐0.37) |
19 6.21 (3.96‐9.74) 4.78 (2.92‐7.84) |
2 1.70 (0.42‐6.78) 1.13 (0.28‐4.59) |
2 0.06 (0.02‐0.25) 0.04 (0.01‐0.17) |
| Hypertension |
N Rate RR |
8 |
4 0.07 (0.03‐0.20) 0.51 (0.12‐2.30) |
3 0.21 (0.07‐0.64) 3.10 (0.69‐13.86) |
0 | 0 |
7 0.10 (0.05‐0.21) |
0 | 0 |
1 0.03 (0.00‐0.22) 0.34 (0.04‐2.73) |
| Vasculitis |
N Rate RR |
3 |
3 0.06 (0.02‐0.17) |
0 | 0 | 0 |
3 0.04 (0.01‐0.13) |
0 | 0 |
1 0.03 (0.00‐0.22) 0.78 (0.08‐7.54) |
| Evolving autoimmune disease (including psoriasis) |
N Rate RR |
27 |
6 0.11 (0.05‐0.25) 0.11 (0.04‐0.26) |
14 0.96 (0.57‐1.63) 4.14 (1.97‐8.68) |
2 2.13 (0.53‐8.50) 6.03 (1.43‐25.40) |
1 0.92 (0.13‐6.56) 2.52 (0.34‐18.53) |
23 0.33 (0.22‐0.49) 0.28 (0.11‐0.73) |
2 0.65 (0.16‐2.61) 1.80 (0.43‐7.59) |
3 2.54 (0.82‐7.89) 7.48 (2.26‐24.77) |
1 0.03 (0.00‐0.22) 0.08 (0.01‐0.62) |
| Psoriasis |
N Rate RR |
17 |
2 0.04 (0.01‐0.15) 0.05 (0.01‐0.21) |
10 0.69 (0.37‐1.28) 5.17 (2.04‐13.10) |
1 1.06 (0.15‐7.55) 4.61 (0.61‐34.65) |
1 0.92 (0.13‐6.56) 4.00 (0.53‐30.06) |
14 0.20 (0.12‐0.34) 0.21 (0.07‐0.64) |
1 0.33 (0.05‐2.32) 1.38 (0.18‐10.35) |
3 2.54 (0.82‐7.89) 12.46 (3.61‐43.05) |
0 |
|
Uveitis, Iridozyclitis manifestation |
N Rate RR |
78 |
53 0.98 (0.75‐1.29) 2.27 (1.12‐4.61) |
8 0.55 (0.28‐1.10) 0.61 (0.29‐1.29) |
0 | 0 |
58 0.82 (0.64‐1.07) 0.87 (0.32‐2.40) |
0 |
1 0.85 (0.12‐6.02) 1.02 (0.14‐7.37) |
21 0.66 (0.43‐1.01) 0.80 (0.49‐1.31) |
| Uveitis flare |
N Rate RR |
153 |
58 1.08 (0.83‐1.39) 0.27 (0.19‐0.38) |
64 4.40 (3.45‐5.63) 3.44 (2.47‐4.79) |
7 7.44 (3.55‐15.61) 4.09 (1.92‐8.75) |
8 7.39 (3.70‐14.78) 4.09 (2.00‐8.35) |
136 1.93 (1.63‐2.28) 1.64 (0.67‐4.00) |
3 0.98 (0.32‐3.04) 0.51 (0.16‐1.60) |
1 0.85 (0.12‐6.02) 0.45 (0.06‐3.18) |
14 0.44 (0.26‐0.74) 0.23 (0.13‐0.40) |
| Uveitis all |
N Rate RR |
231 |
111 2.06 (1.71‐2.48) 0.47 (0.35‐0.61) |
72 4.95 (3.93‐6.24) 2.27 (1.71‐3.03) |
7 7.44 (3.55‐15.61) 2.80 (1.32‐5.95) |
8 7.39 (3.70‐14.78) 2.79 (1.38‐5.66) |
194 2.75 (2.39‐3.17) 1.30 (0.66‐2.53) |
3 0.98 (0.32‐3.04) 0.35 (0.11‐1.10) |
2 1.70 (0.42‐6.78) 0.62 (0.15‐2.50) |
35 1.10 (0.79‐1.54) 0.41 (0.28‐0.58) |
| Herpes Zoster |
N Rate RR |
35 |
22 0.41 (0.27‐0.62) 0.94 (0.43‐2.05) |
6 0.41 (0.19‐0.92) 0.99 (0.41‐2.42) |
1 1.06 (0.15‐7.55) 2.61 (0.36‐19.16) |
1 0.92 (0.13‐6.56) 2.27 (0.31‐16.62) |
30 0.43 (0.30‐0.61) 1.81 (0.25‐13.24) |
1 0.33 (0.05‐2.32) 0.78 (0.11‐5.72) |
0 |
5 0.16 (0.07‐0.38) 0.38 (0.15‐0.98) |
| Varicella |
N Rate RR |
26 |
11 0.20 (0.11‐0.37) 0.53 (0.21‐1.32) |
8 0.55 (0.28‐1.10) 3.01 (1.21‐7.48) |
0 | 0 |
19 0.27 (0.17‐0.42) |
0 | 0 |
8 0.25 (0.13‐0.50) 0.99 (0.43‐2.26) |
Abbreviation: ABA, abatacept; ADA, adalimumab; AE, adverse event; AESI, AE of special interest; ETA, etanercept; GOL, golimumab; INF, infliximab; MTX, methotrexate; RR, risk ratio; SAE, serious adverse event; TNFi, tumor necrosis factor α inhibitor; TOC, tocilizumab.
Table 3.
AESI rates/100 patient years and RRs comparing TNF versus TOC or ABA, respectively, and TOC versus ABA
| Statistics | TNFi | TOC | ABA | |
|---|---|---|---|---|
| Exposure years | 7043 | 306 | 118 | |
| Total SAEs |
N Rate RR vs. TNF RR TOC vs. ABA |
149 2.12 (1.80‐2.48) |
10 3.27 (1.76‐6.08) 1.55 (0.81‐2.93) 0.96 (0.30‐3.07) |
4 3.39 (1.27‐9.04) 1.60 (0.59‐4.33) |
| Total AESI |
N Rate RR vs. TNF RR TOC vs. ABA |
576 8.18 (7.54‐8.87) |
54 17.66 (13.52‐3.05) 2.16 (1.63‐2.85) 1.60 (0.87‐2.93) |
13 11.02 (6.40‐18.98) 1.35 (0.78‐2.34) |
|
Anaphylaxis |
N Rate RR vs. TNF RR TOC vs. ABA |
14 0.20 (0.12‐0.34) |
7 2.29 (1.09‐4.80) 11.51 (4.65‐28.53) 0.90 (0.23‐3.48) |
3 2.54 (0.82‐7.89) 12.80 (3.68‐44.53) |
| Bleeding |
N Rate RR vs. TNF |
6 0.08 (0.04‐0.19) |
0 |
1 0.85 (0.12‐6.02) 9.95 (1.20‐82.68) |
| Cytopenia |
N Rate RR vs. TNF |
35 0.50 (0.36‐0.69) |
12 3.92 (2.23‐6.91) 7.90 (4.10‐15.21) |
0 |
| Hepatic events |
N Rate RR vs. TNF RR TOC vs. ABA |
24 0.34 (0.23‐0.51) |
5 1.63 (0.68‐3.93) 4.80 (1.83‐12.57) 1.93 (0.23‐16.50) |
1 0.85 (0.12‐6.02) 2.49 (0.34‐18.39) |
| Medically important infection |
N Rate RR vs. TNF |
81 1.15 (0.92‐1.43) |
3 0.98 (0.32‐3.04) 0.85 (0.27‐2.70) |
0 |
| Depression/Suicidality |
N Rate RR vs. TNF RR TOC vs. ABA |
14 0.20 (0.12‐0.34) |
2 0.65 (0.16‐2.61) 3.29 (0.75‐14.48) 0.77 (0.07‐8.51) |
1 0.85 (0.12‐6.02) 4.27 (0.56‐32.44) |
| Inefficacy |
N Rate RR vs. TNF RR TOC vs. ABA |
89 1.26 (1.03‐1.56) |
19 6.21 (3.96‐9.74) 4.92 (3.00‐8.07) 3.66 (0.85‐15.73) |
2 1.70 (0.42‐6.78) 1.34 (0.33‐5.45) |
| Evolving autoimmune diseases |
N Rate RR vs. TNF RR TOC vs. ABA |
23 0.33 (0.22‐0.49) |
2 0.65 (0.16‐2.61) 2.00 (0.47‐8.49) 0.26 (0.04‐1.54) |
3 2.54 (0.82‐7.89) 7.79 (2.34‐25.94) |
| Herpes Zoster |
N Rate RR vs. TNF |
30 0.43 (0.30‐0.61) |
1 0.33 (0.05‐2.32) 0.77 (0.10‐5.63) |
0 |
| Psoriasis |
N Rate RR vs. TNF RR TOC vs. ABA |
14 0.20 (0.12‐0.34) |
1 0.33 (0.05‐2.32) 1.64 (0.22‐2.51) 0.13 (0.01‐1.24) |
3 2.54 (0.82‐7.89) 12.80 (3.68‐44.53) |
| Uveitis all events |
N Rate RR vs. TNF RR TOC vs. ABA |
194 2.75 (2.39‐3.17) |
3 0.98 (0.32‐3.04) 0.36 (0.11‐1.11) 0.58 (0.10‐3.46) |
2 1.70 (0.42‐6.78) 0.62 (0.15‐2.48) |
Abbreviation: ABA, abatacept; AESI, adverse event of special interest; RR, risk ratio; SAE, serious adverse event; TNFi, tumor necrosis factor α inhibitor; TOC, tocilizumab.
Figure 1.
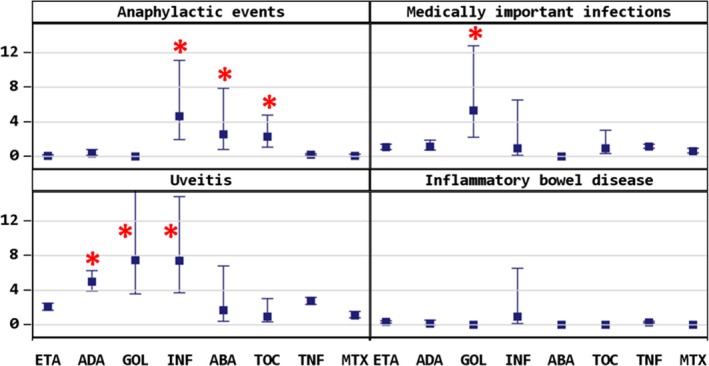
Anaphylactic events, medically important infections, uveitis, and inflammatory bowel disease. Asterisks indicate significance of P < 0.05.
Figure 2.
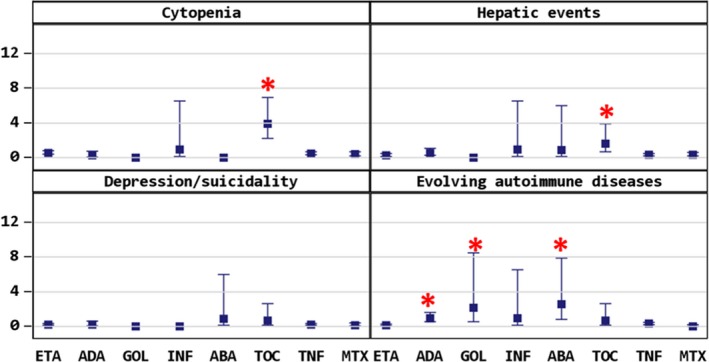
Cytopenia, hepatic events, depression/suicidality, and evolving autoimmune disease (including psoriasis). Asterisks indicate significance of P < 0.05.
Anaphylactic events occurred significantly more often with intravenously administered biologics: INF (4.62/100 PY; 95% CI, 1.9‐11.1), TOC (2.29/100 PY; 95% CI, 1.1‐4.8), and ABA (2.54/100 PY; 95% CI, 0.8‐7.9). When comparing any therapy with TNFi, TOC and ABA also had a higher incidence of anaphylactic events (Table 3). MTX did not seem to reduce the risk, and with TOC and INF, the vast majority of anaphylactic events occurred in patients with combination treatment; only with ABA did the reported events occur in patients with ABA monotherapy (Supplementary Table 1).
Cytopenias were reported with a nearly 8‐fold risk (95% CI, 4.2‐15.5) in patients receiving TOC compared with other biologics or with TNFi (Table 3). The risk was significantly increased in both patients with TOC monotherapy and patients with TOC and MTX (Supplementary Table 1).
Medically important infections had a higher incidence in patients treated with GOL (5.32/100 PY; 95% CI, 2.2‐12.8), all in patients with GOL and MTX combination treatment. Rates in patients undergoing all other treatments were comparable. There were no significant differences between patients receiving any TNFi and patients receiving TOC. Biologics‐naïve patients with MTX had a lower risk (0.63/100 PY; RR 0.56; 95% CI, 0.34‐0.91).
Opportunistic infections were mainly herpes zoster reactivation and had comparable rates in all treatment groups; again, patients in the MTX cohort had a lower risk. One case of latent tuberculosis was reported to the registry.
Uveitis (manifestation and flare) occurred significantly more frequently in patients receiving TNF antibodies ADA (4.95/100 PY; RR 2.3; 95% CI, 1.7‐3.0), GOL (7.4/100 PY; RR 2.8; 95% CI, 1.3‐6.0), and INF (7.4/100 PY; RR 2.8; 95% CI, 1.4‐5.7). Although fewer patients with ABA or TOC had uveitis events, there was no significant difference when compared with each other or with TNFi therapy. Although patients treated with ETA had a lower risk for uveitis flare, mainly when treated in combination with MTX (Supplementary Table 1), they had about a 2‐fold increased risk (95% CI, 1.1‐3.2) for first manifestation of uveitis if they received ETA monotherapy. Patients receiving TNF antibodies (ADA, GOL, INF) all had a higher incidence of uveitis flares (RR 3.4 [2.5‐4.8]; RR 4.1 [1.9‐8.8]; RR 4.1 [2.0‐8.4]). The risk for new uveitis manifestation was lowest in ADA in combination with MTX but did not reach significance (RR 0.45; 0.2‐1.2). Patients with MTX monotherapy also had a low risk for uveitis flare (RR 0.23 [0.1‐0.4]). Rates of uveitis flare were significantly higher in patients who had received systemic steroids concomitantly with a biologic (RR 5.14; 95% CI, 1.64‐16.15).
Most evolving autoimmune events were reports on manifestation of psoriasis. Psoriasis was seen in patients treated with ADA (n = 10; 0.69/100 PY; RR 5.2; 95% CI, 2.0‐13.1) and ABA (n = 3; 2.54/100 PY; RR 12.5; 95% CI, 3.6‐43.0) significantly more often. ETA‐treated patients had a lower risk for psoriasis (n = 2; 0.04/100 PY; RR 0.05; 95% CI, 0.01‐0.2). Other autoimmune events were type 1 diabetes mellitus in two patients, celiac disease in two patients, and alopecia areata and autoimmune hemolytic anemia in one patient each.
Inflammatory bowel disease (IBD) was reported only in patients treated with TNFi, mainly in patients treated with ETA (n = 17; 0.32/100 PY; 95% CI, 0.2‐0.5) and less frequently in patients with ADA (n = 2; 0.14/100 PY; 95% CI, 0.03‐0.6) or INF (n = 1; 0.92/100 PY; 95% CI, 0.1‐6.6); only for ETA monotherapy was there a significantly increased risk (RR 3.0; 95% CI, 1.25‐7.2).
Vasculitic events were reported in three ETA‐treated patients: Henoch‐Schoenlein purpura, leukocytoclastic vasculitis with skin involvement, and cutaneous vasculitis.
Hepatic events are described in detail as follows: relevant elevation of transaminase leading to treatment discontinuation or accompanied by hyperbilirubinemia (n = 35 reports in 33 patients), cholecystitis in two patients with ETA, hepatic steatosis in two patients receiving ETA, one case of centrolobular cell necrosis of the liver in an ETA‐treated patient, and hepatitis in one patient receiving TOC and leflunomide. Altogether, patients treated with ETA were less likely to experience hepatic events (0.28/100 PY; 95% CI, 0.2‐0.5), whereas in patients with TOC, this applied in particular to patients with concomitant treatment with MTX (Supplementary Table 1) had an increased risk (1.63/100 PY; RR 4.68; 95% CI, 1.8‐12.2) compared with all other biologics and compared with TNFi, but not compared to ABA (Table 3).
Depression or suicidality was documented in 20 reports of 17 patients receiving biologics, 11 patients with ETA treatment, 3 with ADA, 2 with TOC, and 1 treated in the ABA cohort. The slight differences in the rates were not significant.
Bleeding events occurred in seven patients with biologics, hematochezia in four patients (three with ADA, one with ETA), and petechiae in three patients (two with ETA and one with ADA).
In all, eight cases of suspected malignancies in patients ever exposed to biologics and three cases in biologics‐naïve patients treated with MTX only have been reported to BIKER. Of these, five patients received biologics at the time of diagnosis of malignancy (Table 2, all TNFi). Two cases of acute lymphatic leukaemia (both in patients treated with MTX only); two cases of lymphoproliferative disorder (one in a patient treated with MTX only); one case of Hodgkin's lymphoma; one case of non‐Hodgkin's lymphoma; one case each of anaplastic ependymoma, thyroid carcinoma, yolk sac carcinoma, and cervix dysplasia reported to the registry have been described earlier 9, 10. Since then, only one new case has been reported in a 17‐year‐old male. He was diagnosed with seronegative polyarthritis and uveitis at the age of 2.5 years and had been treated with ETA cumulatively for 4.5 years before diagnosis of a malignant lymphoproliferative disorder. Rates for malignant diseases for patients ever exposed to ETA were 0.08/100 PY (n = 7), for ADA 0.04/100 PY (n = 1), for INF 0.91/100 PY (n = 2), for ABA 0.4/100 PY (n = 1), and for MTX 0.05/100 PY (n = 3). If a patient had received multiple therapies before diagnosis of malignancy, the event was counted for all biologics ever received. Comparing the rates of patients treated with ETA, ADA, or ABA to rates in biologics‐naïve patients with MTX, no significant difference could be observed (ETA: RR 1.75; 95% CI, 0.45‐6.8; ADA: RR 0.95; 95% CI, 0.9‐9.1; ABA: RR 8.9; 95% CI, 0.9‐86.0); also, if only hematologic malignancies were compared (ETA: n = 4, MTX: n = 3; RR 1.0; 95% CI, 0.2‐4.5). For INF, an increased RR was calculated: RR 19.4 (95% CI, 3.2‐116.1).
One case of suspected demyelination was documented in a female patient receiving ETA. She had no clinical symptoms, and an magnetic resonance imaging (MRI) scan of the cranium was performed for other reasons that showed minor changes in periventricular white matter. ETA was stopped but resumed later when further diagnostic tests were not suggestive of multiple sclerosis.
A case of suspected transient ischemic attack was reported in a patient with known thrombophilia upon ongoing anticoagulation. The patient with multiple comorbidities reported a transient hemiparalysis. Cranial MRI showed an old lesion but no other abnormalities. She had been treated with ETA for 2.5 years and MTX for 2 years at the time of the event.
Arterial hypertension was reported in patients receiving ETA (n = 4; 0.07/100 PY; 95% CI, 0.03‐0.2) or ADA (n = 3; 0.19/100 PY; 95% CI, 0.06‐0.6).
Thrombotic events (two cases of deep vein thrombosis, one case of thrombophlebitis) were reported in three patients receiving ADA.
There were 112 reports of inefficacy, with a higher frequency in patients with GOL (RR 6.03; 95% CI, 2.94‐12.38) and TOC (RR 4.78; 95% CI, 2.92‐7.84).
In all, six pregnancies in patients receiving TNFi at the time of conception were documented. Pregnancy outcomes were a miscarriage, two induced abortions, and three deliveries of healthy children.
There were no cases of gastrointestinal perforation, cardiovascular events, hepatitis B reactivation, sarcoidosis, systemic lupus erythematosus, or serum sickness reported in the analyzed population. No death occurred in this patient cohort during observation.
Discussion
The German BIKER‐registry is one of the largest national registries on the use of biologics in JIA. It has accumulated a large quantity of data and observation years. Thus it is possible to draw some conclusions about the occurrence of rare events in JIA patients treated with biologics. The data confirm known safety profiles of some drugs. Cytopenias and elevation of transaminases are known side effects of TOC 11. These events were seen significantly more often in TOC‐treated patients in this analysis. In case of hepatic events, concomitant MTX treatment may have had an influence as well. Anaphylactic events in our patients were clearly associated with intravenous route of administration (INF, TOC, ABA).
Infectious events are of particular interest as they occur frequently in the pediatric population and with biologics in particular as they are proteins targeting molecules of the immune system. The rates for medically important infections in this analysis were comparable between the different therapies with the exception of GOL with a 5‐fold increased risk. There may be a bias due to the low number of patients and exposure years. The obligatory comedication with MTX might be a contributing factor as these events were only seen in patients with concomitant MTX. Furthermore, patients in the GOL‐cohort had experienced a higher number of treatment failures with other biologics and thus constitute a negative selection. The total number of medically important infections was five, with no infection occurring more than once. Interestingly biologics‐naïve patients treated with MTX had a lower risk for medically important infections compared with all biologic therapies combined, underlining the high tolerability of MTX, which is still recommended as the first DMARD of choice. When interpreting these data, it has to be kept in mind that these patients had a shorter duration of their JIA and possibly a less severe disease course if MTX monotherapy was sufficient for control of the disease. A study comparing Medicaid data of 8479 JIA patients and 360 489 children diagnosed with attention‐deficit hyperactivity disorder 12 calculated a rate of hospitalized bacterial infection of 1.0 (0.9‐1.0) for the non‐JIA cohort and a 2‐fold risk for JIA patients not currently treated with MTX or TNFi. However, the risk did not increase with MTX or TNFi treatment, but a significant increase was seen with use of glucocorticosteroids. The infection rates in the ETA, ADA, INF, TOC, and ABA cohorts from the BIKER registry were nearer the non‐JIA group in the analysis of Medicaid data. As definitions for medically important infections vary, a direct comparison of the rates should be interpreted carefully. The Medicaid analysis was done in the United States, so the overall infection rate might also differ from rates in Europe.
Opportunistic infections were very rare events in BIKER. Most reports were herpes zoster reactivation. A case of Pneumocystis jiroveci pneumonia was reported after data closure in a 16‐year‐old male patient with seronegative polyarthritis and concomitant X‐linked hypophosphatemia presenting with exertion dyspnea, fatigue, and abnormal pulmonary function test. He was treated with ADA and MTX for JIA and burosumab, an IgG‐1 antibody inhibiting fibroblast growth factor 23 for hypophosphatemia. Antibiotic treatment led to recovery, and ADA and MTX treatment were resumed. A case of latent tuberculosis was reported to the BIKER registry with a positive Quantiferon Gold test in a patient with ongoing MTX and previous ETA treatment. Clinical symptoms or changes in chest radiograph were never present. The patient resumed TNFi treatment while receiving INH‐prophylaxis. No reactivation of tuberculosis was reported.
Recently, a comparison of data from the BIKER registry with data of Pharmachild, a large multinational retrospective registry, was published 13. The percentages of infections and serious infections were comparable, but the Pharmachild registry reported 27 cases of tuberculosis. They did not stratify their AESI according to therapy. This difference is probably due to different incidence rates in different countries and a very low risk of tuberculosis infection in Germany. A validated test for tuberculosis before the start of biologic treatment is recommended and performed in Germany and may also contribute to the low incidence of tuberculosis in BIKER.
Interestingly, the risk for first uveitis manifestation was increased in patients with ETA monotherapy. It has been shown before that ETA monotherapy is associated with a higher incidence of uveitis onset 14. This might be due to insufficient effectiveness of ETA for uveitis treatment. With ADA, the risk for uveitis was lower but did not reach statistical significance. ADA is recommended for treatment of JIA‐related uveitis after its efficacy was demonstrated in a randomized controlled trial 15, 16. The risk for uveitis flare was higher in patients receiving TNFi antibodies (ADA, GOL, INF). This is probably due to a treatment bias, as patients with known uveitis are more likely to be treated with these biologics.
IBD was reported only in TNFi‐treated patients in BIKER, in the vast majority treated with ETA, and significantly more frequently in those treated with ETA monotherapy, which was ineffective in a randomized placebo‐controlled trial in Crohn's disease 17. Thus, patients with spondylarthritis associated with IBD are more likely to show gastrointestinal manifestation with ETA. The proportion of IBD was similar in the Pharmachild registry 13.
About 60% of cases with evolving autoimmune disease were reports about manifestation of psoriasis. Roughly half of patients with psoriatic arthritis develop arthritis before skin manifestation. Surprising is an increased risk of psoriasis manifestation in ADA‐treated patients compared with other biologics, as ADA is used for treatment of psoriasis. This paradox phenomenon has been described before. In a Finnish multicenter observational study with 348 patients with JIA treated with biologics, psoriasis events were only reported in patients receiving TNFi antibodies (ADA and INF) 18.
Other autoimmune events, such as Celiac disease or type 1 diabetes, were rare and are associated with JIA.
Several suspected malignancies have been reported to the BIKER registry. As demonstrated before, incidence rates of the malignancies reported to BIKER are higher than in the general population 10. The background risk for malignancies in JIA patients might be higher than that of the general population. In a nationwide cohort study in the United States, Beukelman et al 19 found a threefold increased risk for malignancies in patients with JIA compared with children with ADHD. In a more recent study, they compared patients with JIA who were using TNFi with those who were not using TNFi and found that treatment with TNFi did not appear significantly associated with the development of malignancy 20. In our analysis, the rates for malignancies were higher in patients ever treated with INF or ABA, although the numbers are too low to draw conclusions. When comparing the rates of suspected malignancies and hematologic malignancies in patients treated with ETA with the rates in biologics‐naïve patients treated with MTX therapy, there was no significant difference. For INF, an increased risk was calculated against biologics‐naïve MTX‐treated patients. The two malignancies in patients who had been treated with INF were suspected but not confirmed malignancies (one case of cervical dysplasia and one case showing polymorphic lymphoproliferative alterations without clonality in molecular pathologic analysis). Additionally, the observation years for INF treatment were few, thus no reliable RR can be deduced. The Pharmachild registry reported a similar rate of malignancies, although with fewer hematopoietic malignancies 13.
Depression or suicidality was reported in 20 patients. Chronic diseases tend to increase the risk for depressive symptoms, this was also shown in JIA, when actively screening for depressive symptoms 21. It can be speculated that a high disease activity poses a risk factor for depression and that successful treatment consecutively decreases that risk. The rates for depression and suicidality in the BIKER registry were relatively low, with no significant differences between treatment cohorts. The rates in patients with ABA and TOC were slightly higher, this might be due to a selection of patients with more severe and treatment refractory disease, as these biologics are more often prescribed as second‐line biologics. Underreporting of depressive symptoms could be a factor in this cohort. In routine clinical care, screening for depressive symptoms could be an approach to detect depressive symptoms and provide adequate care.
Only one case of suspected demyelination was reported, being a very rare event that is even more rare in the pediatric population. In the Pharmachild registry, demyelination was also a rare event 13.
This analysis has numerous limitations. A higher rate of an event observed is not attributed to a higher risk of the drug itself as a cause because the different biologic cohorts differ in a number of aspects. ETA has been approved for polyarticular JIA for 18 years, ADA for 10, TOC for 7, ABA for 8 (as a second‐line biologic), and GOL for nearly 3 years. This is mirrored in an overwhelming proportion of patient numbers and observation years in the ETA cohort. As one limitation of this analysis, this renders some comparisons and interpretation of AESI rates and RRs, especially with rare events, difficult. In the Dutch ABC registry, ETA was also the most frequently prescribed biologic for nonsystemic JIA 22, the most important factor in decision making being greater experience with this drug. ADA was prescribed by Dutch pediatric rheumatologists more frequently in patients with concomitant or preceding uveitis. This could also be demonstrated in this analysis. ETA was most commonly used as a first‐line biologic, whereas the other biologics, especially INF, ABA, and TOC were used as second‐ or third‐line biologics. This seemed to have an influence on efficacy. Inefficacy was reported more often with biologics that were mostly used as second‐line biologics and less frequently in biologics‐naïve patients treated with MTX and patients with ETA posing as a possible selection bias. Other indicators are a higher proportion of rheumatoid factor–positive and female patients in these cohorts. It could be speculated that patients with ongoing disease activity who had been exposed to different DMARDs experience more adverse events.
The analysis also considered concomitant medications. The AESI rates in patients receiving biologic monotherapy and combination treatment with MTX were compared separately with all other biologic treatments. As in some AESI categories, this resulted in low numbers of events making the interpretation of the results more difficult. Interestingly, concomitant MTX in ETA‐treated patients seemed to have a preventive effect on the development of inflammatory bowel disease and uveitis manifestation but not on manifestation of psoriasis in ADA‐treated patients. Additionally, AESI rates were compared in patients having received a biologic (for this analysis all biologics were combined) without concomitant systemic steroid treatment with patients who had received systemic steroids at any time during their course of biologic treatment. Interestingly patients who had had concomitant systemic steroid had a higher rate of SAE and of uveitis flares. This might be due to bias, as patients with known uveitis also often receive systemic steroids to resolve active uveitis quickly.
An explanation for the less frequent use of TOC and ABA may be the intravenous route of administration. For TOC and, recently, ABA, subcutaneous application is now approved for JIA. It can be speculated that this will lead to more frequent use of these drugs.
Further limitations are the nonrandomized approach arising from a registry setting. Physicians’ decisions may include multiple factors. Additionally, patients are switching between treatments. In some cases, AESI could be attributed to two biologic therapies because of the 70‐day follow‐up period after discontinuation. In this case, AESIs were counted under both treatments.
On the other hand, data from routine clinical care with an unselected patient population are very valuable for collecting huge amounts of experience and observation time, especially in a disease as rare as JIA. The experience gained in this setting can support decision making in the real world and delivers information for patient/parent shared decision making. Long‐term surveillance of JIA therapy with biologics is an important task. In summary, a number of AESIs have been observed in JIA patients. No new safety signals were identified. Taken together, the safety of these biologic therapies seems acceptable for long‐term use in JIA.
Conclusion
Long‐term surveillance of biologic therapies in JIA is an important task. Through registries, it is possible to establish risks of rare events. The safety profiles of the biologics in this analysis were acceptable with no new safety signals in patients with JIA.
Author Contributions
Klein, Becker, Minden, Hospach, Schwarz, Foeldvari, Huegle, Borte, Weller‐Heinemann, Dressler, Kuemmerle‐Deschner, Oommen, Foell, Trauzeddel, Rietschel, Horneff were responsible for critically revising the manuscript. Klein, Becker, Minden, Hospach, Schwarz, Foeldvari, Huegle, Borte, Weller‐Heinemann, Dressler, Kuemmerle‐Deschner, Oommen, Foell, Trauzeddel, Rietschel, Horneff approved the final version of the manuscript.
Study conception and design
Klein, Horneff.
Acquisition of data
Klein, Minden, Hospack, Schwarz, Foeldvari, Huegle, Borte, Weller‐Heinemann, Dressler, Kuemmerle‐Deschner, Oommen, Foell, Trauzeddel, Rietschel, Horneff.
Analysis and interpretation of data
Klein, Becker, Horneff.
Supporting information
Acknowledgments
This study would not have been possible without the collaboration of numerous German and Austrian pediatric rheumatologists, patients and their parents. The German BIKER registry was approved by the ethics committee of the physician board Aerztekammer Nordrhein, Duesseldorf. Written consent was obtained from patients and parents and repeated if patient became adult. Only pseudonymized data were collected.
The German Registry is supported by an unrestricted grant from Pfizer, Germany; AbbVie, Germany Merck Sharp & Dohme (MSD), Germany; and Roche, Germany. AbbVie, Pfizer, MSD, and Roche had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by the study sponsors.
The BIKER registry is registered in the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP; http://www.encepp.eu/encepp/viewResource.htm?xml:id=20591).
Dr. Klein has received congress travel fees from Sobi and Sandoz as well as advisory board honoraria from Celgene. Dr. Minden has received honoraria from AbbVie, Biermann, Medac, and Sanofi. Dr. Hospach has received advisory board honoraria from Novartis, Chugai‐Roche, and Sobi. Dr. Foeldvari discloses advisory board participation for Novartis, Genzyme, Bayer, Lilly, Pfizer, Abbvie, and Sanofi. Dr. Borte has received research support from Pfizer and Shire. Dr. Weller‐Heinemann has received speaker honoraria from Pfizer, Abbvie, Novartis, Sobi, and Roche. Dr. Dressler has received honoraria for lectures from Pfizer, Abbvie, and Novartis. Dr. Kuemmerle‐Deschner has received consultants/speakers fees from Novartis and Sobi, as well as pharmaceuticals and grant support from Sobi and Novartis. Dr. Oommen has received travel fees from Shire, Novartis, and CSL‐Behring as well as advisory board honoraria from Novartis. Dr. Foell has received speaking fees from Sobi and Novartis, and research support from Novartis and Pfizer. Dr. Rietschel has received speaking fees or advisory board honoraria from Pfizer, Abbvie, Novartis, Chugai, and Sobi. Dr. Horneff has received grants and honorary fees from Abbvie, Pfizer, Novartis, and Roche/Chugai. No other disclosures relevant to this article were reported.
References
- 1. Danner S, Sordet C, Terzic J, Donato L, Velten M, Fischbach M, et al. Epidemiology of juvenile idiopathic arthritis in Alsace, France. J Rheumatol 2006;33:1377–81. [PubMed] [Google Scholar]
- 2. Minden K, Niewerth M, Listing J, Zink A, and German Study Group of Pediatric Rheumatologists . Health care provision in pediatric rheumatology in Germany–national rheumatologic database. J Rheumatol 2002;29:622–8. [PubMed] [Google Scholar]
- 3. Dueckers G, Guellac N, Arbogast M, Dannecker G, Foeldvari I, Frosch M, et al. Interdisziplinäre S2‐therapieleitlinie der juvenilen idiopathischen arthritis. Klin Padiatr 2011;223:386–94. [DOI] [PubMed] [Google Scholar]
- 4. Beukelman T, Patkar NM, Saag KG, Tolleson‐Rinehart S, Cron RQ, DeWitt EM, et al. American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res 2011;63:465–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horneff G, Schmeling H, Biedermann T, Foeldvari I, Ganser G, Girschick H, et al. The German etanercept registry for treatment of juvenile idiopathic arthritis. Ann Rheum Dis 2004;63:1638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horneff G, De Bock F, Foeldvari I, Girschick HJ, Michels H, Moebius D, et al. Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA Registry. Ann Rheum Dis 2009;68:519–25. [DOI] [PubMed] [Google Scholar]
- 7. Minden K, Niewerth M, Zink A, Seipelt E, Foeldvari I, Girschick H, et al. Long‐term outcome of patients with JIA treated with etanercept, results of the biologic register JuMBO. Rheumatology (Oxford) 2012;51:1407–15. [DOI] [PubMed] [Google Scholar]
- 8. ICH Harmonised Guideline . Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). 2016. URL: http://www.ich.org.
- 9. Horneff G, Foeldvari I, Minden K, Moebius D, Hospach T. Report on malignancies in the German juvenile idiopathic arthritis registry. Rheumatology (Oxford) 2011;50:230–6. [DOI] [PubMed] [Google Scholar]
- 10. Horneff G, Klein A, Oommen PT, Hospach A, Foeldvari I, Feddersen I, et al. Update on malignancies in children with juvenile idiopathic arthritis in the German BIKER Registry. Clin Exp Rheumatol 2016;34:1113–20. [PubMed] [Google Scholar]
- 11. Brunner HI, Ruperto N, Zuber Z, Keane C, Harari O, Kenwright A, et al. Efficacy and safety of tocilizumab in patients with polyarticular‐course juvenile idiopathic arthritis: results from a phase 3, randomised, double‐blind withdrawal trial. Ann Rheum Dis 2015;74:1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beukelman T, Xie F, Chen L, Baddley JW, Delzell E, Grijalva CG, et al. Rates of hospitalized bacterial infection associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum 2012;64:2773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swart J, Giancane G, Horneff G, Magnusson B, Hofer M, Alexeeva E, et al. Pharmacovigilance in juvenile idiopathic arthritis patients treated with biologic or synthetic drugs: combined data of more than 15,000 patients from Pharmachild and national registries. Arthritis Res Ther 2018;20:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klotsche J, Niewerth M, Haas JP, Huppertz HI, Zink A, Horneff G, et al. Long‐term safety of etanercept and adalimumab compared to methotrexate in patients with juvenile idiopathic arthritis (JIA). Ann Rheum Dis 2016;75:855–61. [DOI] [PubMed] [Google Scholar]
- 15. Heiligenhaus A, Minden K, Tappeiner C, Baus H, Bertram B, Deuter C, et al. Update of the evidence based, interdisciplinary guideline for anti‐inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Semin Arthritis Rheum 2019;49:43–55. [DOI] [PubMed] [Google Scholar]
- 16. Ramanan AV, Dick AD, Jones AP, McKay A, Williamson PR, Compeyrot‐Lacassagne S, et al. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N Engl J Med 2017;376:1637–46. [DOI] [PubMed] [Google Scholar]
- 17. Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, Baerg RD, et al. Etanercept for active Crohn's disease: a randomized, double‐blind, placebo‐controlled trial. Gastroenterology 2001;121:1088–94. [DOI] [PubMed] [Google Scholar]
- 18. Tarkiainen M, Tynjälä P, Vähäsalo P, Lahdenne P. Occurrence of adverse events in patients with JIA receiving biologic agents: long‐term follow‐up in a real‐life setting. Rheumatology (Oxford) 2015;54:1170–6. [DOI] [PubMed] [Google Scholar]
- 19. Beukelman T, Haynes K, Curtis JR, Xie F, Chen L, Bemrich‐Stolz CJ, et al. Rates of malignancy associated with juvenile idiopathic arthritis and its treatment. Arthritis Rheum 2012;64:1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beukelman T, Xie F, Chen L, Horton DB, Lewis JD, Mamtani E, et al. Risk of malignancy associated with paediatric use of tumour necrosis factor inhibitors. Ann Rheum Dis 2018;77:1012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanns L, Cordingley L, Galloway J, Norton S, Carvalho LA, Christie D, et al. Depressive symptoms, pain and disability for adolescent patients with juvenile idiopathic arthritis: results from the Childhood Arthritis Prospective Study. Rheumatology (Oxford) 2018;57:1381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anink J, Otten MH, Gorter AL, Prince FH, van Rossum MA, van den Berg JM, et al. Treatment choices of paediatric rheumatologists for juvenile idiopathic arthritis: etanercept or adalimumab? [research support]. Rheumatology (Oxford) 2013;52:1674–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


