Abstract
Objective
To report the 5‐year efficacy and safety of secukinumab in the treatment of patients with psoriatic arthritis (PsA) in the FUTURE 1 study (NCT01392326).
Methods
Following the 2‐year core trial, eligible patients receiving subcutaneous secukinumab entered a 3‐year extension phase. Results are presented for key efficacy endpoints for the secukinumab 150‐mg group (n = 236), including patients who escalated from 150 to 300 mg (approved doses) starting at week 156. Safety is reported for all patients (n = 587) who received 1 dose or more of study treatment.
Results
Overall, 81.8%% (193 of 236) of patients in the secukinumab 150‐mg group completed 5 years of treatment, of which 36.4% (86 of 236) had dose escalation from 150 to 300 mg. Sustained improvements were achieved with secukinumab across all key efficacy endpoints through 5 years. Overall, 71.0%/51.8%/36.3% of patients achieved American College of Rheumatology (ACR) 20/50/70 responses at 5 years. Efficacy improved in patients requiring dose escalation from 150 to 300 mg and was comparable with those who did not require dose escalation. Exposure‐adjusted incidence rates for selected adverse events per 100 patient‐years for any secukinumab dose were serious infections (1.8), Crohn's disease (0.2), Candida infection (0.9), and major adverse cardiac events (0.5).
Conclusion
Secukinumab provided sustained improvements in the signs and symptoms in the major clinical domains of PsA. Efficacy improved for patients requiring dose escalation from 150 to 300 mg during the study. Secukinumab was well tolerated with no new safety signals.
Introduction
Psoriatic arthritis (PsA) is a chronic, inflammatory disease characterized by peripheral arthritis, axial disease, dactylitis, enthesitis, and skin and nail psoriasis 1, 2. PsA can negatively affect patients’ daily functioning and quality of life as a result of permanent joint damage and disability 3. The reported prevalence of PsA in the general population is up to 1%, and it affects around 30% of patients with psoriasis 2, 4, 5.
Biologic therapies, such as anti–tumor necrosis factor (TNF) and anti–interleukin (IL)‐17A antibodies, are recommended for the treatment of PsA in patients who experience an inadequate response to first‐line treatment with nonsteroidal anti‐inflammatory drugs (NSAIDs) and/or disease‐modifying antirheumatic drugs (DMARDs) 6, 7, 8. The proinflammatory cytokine IL‐17A mediates multiple biological functions that result in joint and entheseal inflammation and structural damage, which are characteristic of PsA 9, 10. Recommendations from the European League Against Rheumatism (EULAR) 8 and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) 11 recognize targeting IL‐17A as a therapeutic strategy to manage all the main clinical manifestations of PsA.
Secukinumab, a human monoclonal antibody that directly inhibits IL‐17A, provided rapid and significant improvements in all key clinical manifestations of PsA in the FUTURE 1 study (NCT01392326) with improvements sustained through 3 years 12, 13, 14. These clinical benefits have been observed in patients naïve to biologic therapy and in those with an intolerance or inadequate response to agents targeting TNF 3, 8, 9, 11, 13, 14, 15, 16.
Data from FUTURE 1 have also shown that secukinumab significantly inhibits joint structural damage through 24 weeks 14, with benefits sustained out to 3 years 3; it is worth noting that radiographic data were only collected up to 3 years in this study 3, 17. Here, we present the final 5‐year efficacy and safety results, including efficacy results in patients who had a dose escalation from 150 to 300 mg during the study.
Methods
Study population
The study design of FUTURE 1 has been described in detail elsewhere 3. In brief, the population of the core study consisted of adults diagnosed with PsA, as classified by Classification Criteria for Psoriatic Arthritis (CASPAR) criteria 18, and with moderate to severe symptoms for 6 months or more, who must have 3 or more tender joints of 78 and 3 or more swollen joints of 76 at baseline. Patients were classed as anti‐TNF inadequate responders (anti‐TNF‐IR) if they had been taking an anti‐TNF agent at an adequate dose for 3 months or longer, had experienced an inadequate response, or had stopped treatment because of tolerability issues.
Clinical judgement of the investigator was used to assess the suitability of a patient to enter the extension study based upon overall improvement and response to therapy during the core study. Only participants who had completed the 2‐year core study and had signed the new informed consent form were included in the extension phase. Key exclusion criteria for the extension included patients who were deemed not to be benefiting from the study treatment based upon lack of improvement or worsening of their symptoms, patients receiving therapy with biologic immunomodulation agents other than secukinumab, and those with inflammatory disorders other than PsA, including active ongoing inflammatory bowel disease (IBD), which might confound the evaluation of therapy.
Study oversight and design
This study was approved by institutional review boards or ethics committees for each study center and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent prior to participation and again at the start of the extension study.
This extension study employed a parallel group, double‐blind design for the first year (up to and excluding week 156 [the 2‐year core study plus the first year of the extension study]), followed by an open‐label design for the next two years (week 156 onward). Blinding during the first year of the extension study was required to ensure that all patients had completed the core study and the data was locked before unblinding occurred. Investigators used their clinical judgment to decide if it was beneficial for patients to enter the extension study based upon overall improvement and response to therapy during the 2‐year period of the core study.
At 2 years in the core study, eligible patients completed assessments and continued on the same dose as that received during the core study (secukinumab at a dosage of 150 mg or 75 mg every 4 weeks). Study treatment dose adjustments were not permitted until week 156. During the open label study period starting at week 156, the secukinumab dose could be escalated from 75 to 150 or 300 mg or from 150 to 300 mg for patients whose signs and symptoms were not fully controlled with the current dose as judged by the investigator. Here, we report long‐term results for the secukinumab 150‐mg group, including patients who switched from placebo to secukinumab 150 mg at weeks 16 or 24 following the end of the placebo‐controlled period. We also report efficacy results for patients who escalated from secukinumab 150 to 300 mg (approved doses).
For all patients who discontinued or withdrew from the study, the investigator was to ensure that the patient completed an end‐of‐treatment visit (corresponding to the last visit for the patient's current period of treatment) 4 weeks after last study treatment, and also returned after an additional 8 weeks for a final follow‐up visit (12 weeks after last study treatment).
Endpoints and assessments
The primary endpoint of this extension study was to evaluate the long‐term efficacy of secukinumab with respect to the proportion of patients achieving 20% or greater, 50% or greater, or 70% or greater improvement in the American College of Rheumatology (ACR) criteria for improvement (ACR20, ACR50, and ACR70, respectively) over time, up to 5 years in patients with active PsA who completed the core study. ACR20/50/70 responses are reported for those patients who were originally randomized to secukinumab 150 mg during the core study to show the full 5‐year efficacy and separately for those who entered the extension study in the secukinumab 150 mg group (including patients both originally randomized to secukinumab 150 mg and those switched from placebo to secukinumab 150 mg at week 16 or 24).
Other assessments that continued for up to 5 years included resolution of dactylitis and enthesitis, which were measured using the Leeds Dactylitis and Leeds Enthesitis Indices, respectively; change from baseline in Health Assessment Questionnaire‐Disability Index (HAQ‐DI); Short Form 36‐item (SF‐36) health survey physical component summary (PCS); improvement (75% or greater and 90%) in Psoriasis Area and Severity Index responses (PASI 75/90); change from baseline in Disease Activity Score‐28 (DAS28; utilizing high‐sensitivity C‐reactive protein); the proportion of patients achieving low disease activity (LDA) and disease remission based on DAS28 scores of 3.2 or less and 2.6 or less, respectively, and the proportion of patients achieving minimal disease activity (MDA; defined as having 5 out of 7 of the following: 1 or fewer tender joint count, 1 or fewer swollen joint count, PASI ≤ 1 or Investigator's Global Assessment [IGA] score ≤ 1, patient pain visual analog scale [VAS] ≤ 15, patient global VAS ≤ 20, HAQ‐DI ≤ 0.5, tender entheseal points ≤ 1).
Long‐term safety and tolerability of secukinumab were assessed by monitoring vital signs, clinical laboratory variables, and treatment‐emergent adverse events (AEs) over time, up to 84 days after the last administration of treatment.
Statistical analysis
Efficacy data are presented using all observed data at the given time point of analysis based on the Full Analysis Set (FAS), which included all subjects with at least one efficacy assessment during the extension. For patients who discontinued during a specific period, the end‐of‐treatment visit (ie, final assessment 4 weeks after last study treatment) was considered the last week of the corresponding period. PASI assessments were conducted for subjects in whom at least 3% of the body surface area was affected by psoriatic skin involvement at baseline. Dactylitis/enthesitis assessments are presented among patients with dactylitis/enthesitis at baseline. Analysis is presented in a descriptive manner through week 260.
Graphical representation (line plots) of ACR20, ACR50, and ACR70 response over time are provided for patients initially randomized to the 150‐mg dose. Sankey‐style bar charts for the ACR and PASI responses for all patients (including patients who were initially randomized to placebo followed by secukinumab 150 mg) that escalated from 150 to 300 mg and had efficacy assessments at both up to 32 and up to 56 weeks after escalation were also drawn from the observed data. The Sankey‐style overlay presents ACR and PASI responses using mutually exclusive definitions.
The safety set included all patients who took at least one dose of study treatment during the treatment period in the core or extension study. Evaluation of AEs is based on the secukinumab dose taken prior to the AE and presented as exposure‐adjusted incidence rates (EAIRs) per 100 patient‐years over the entire treatment period for each treatment dose and overall. If a patient experienced an AE after dose escalation, the patient was counted at the escalated dose.
Results
Baseline characteristics and subject disposition
Baseline demographics and disease characteristics for patients in the secukinumab 150‐mg FAS group (including patients who switched from placebo to secukinumab 150 mg) are shown in Table 1. The patient retention rates in this 5‐year study were high, with over 80% of patients who entered the extension study completing the full treatment period (Figure 1). Of the 236 FAS patients in the secukinumab 150‐mg group, 193 (81.8%) completed 5 years of treatment, with 86 of 236 (36.4%) patients having escalated to 300 mg; of these 193 patients, 149 (77.2%) were TNF‐naïve.
Table 1.
Demographics and baseline characteristics of patients who entered the extension phase receiving secukinumab 150 mga
| Characteristicb | Secukinumab 150 mg (N = 236) |
|---|---|
| Age (years) | 49.3 (11.57) |
| Female, n (%) | 121 (51.3) |
| Weight (kg) | 82.7 (19.70) |
| BMI (kg/m2) | 28.5 (6.47) |
| Time since first diagnosis of PsA (years) | 7.63 (7.927) |
| Disease history and baseline characteristics | |
| Anti–TNF‐naïve, n (%) | 178 (75.4) |
| Methotrexate use at randomization, n (%) | 148 (62.7) |
| Systemic glucocorticoid use at randomization, n (%) | 37 (15.7) |
| Tender joint count (78 joints) | 23.3 (16.90) |
| Swollen joint count (76 joints) | 13.1 (10.25) |
| HAQ‐DI | 1.142 (0.667) |
| DAS28‐CRP | 4.72 (1.079) |
| Psoriasis (≥3% body surface area), n (%) | 130 (55.1) |
| Presence of dactylitis, n (%) | 123 (52.1) |
| Presence of enthesitis, n (%) | 141 (59.7) |
Abbreviation: BMI, body mass index; DAS28‐CRP, Disease Activity Score‐28‐C‐reactive protein; HAQ‐DI, Health Assessment Questionnaire‐Disability Index; PsA, psoriatic arthritis; TNF, tumor necrosis factor.
Baseline characteristics were recorded at baseline of the core study.
Results are mean (standard deviation) unless otherwise stated.
Figure 1.
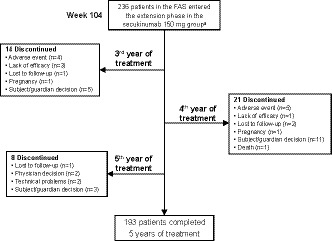
Patient disposition during the extension phase.
Disease activity assessed by ACR responses
The ACR20/50/70 responses reported during the core study for the group of patients who were originally randomized to secukinumab 150 mg were sustained through 5 years (Figure 2) with responses of 67.9%/52.7%/37.4% at 5 years. ACR responses were similar in the secukinumab 150 mg group that included patients who switched from placebo at week 16 or 24 (Table 2).
Figure 2.
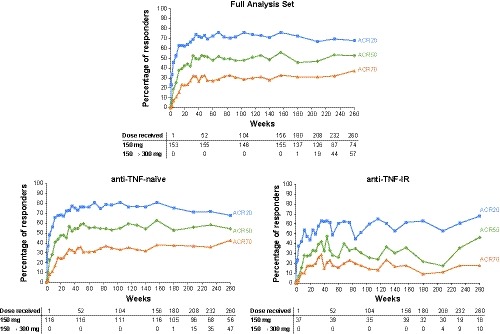
ACR20/50/70 responses up to 5 years in the secukinumab 150‐mg group. To show the full 5‐year efficacy, results are shown for the group of patients who were originally randomized to secukinumab 150 mg during the core study and entered the extension study in the secukinumab 150 mg group; patients who switched from placebo to secukinumab 150 mg at weeks 16 and 24 are not included in these figures. ACR 20/50/70, American College of Rheumatology criteria for 20%/50%/70% improvement in disease activity.
Table 2.
Summary of efficacy results during the extension phasea
| Efficacy Endpoints | Secukinumab 150 mg (N = 236) | ||||
|---|---|---|---|---|---|
| Year 2b | Year 3b | Year 4b | Year 5c | ||
| ACR20 | 158/213 (74.2) | 168/229 (73.4) | 142/211 (67.3) | 137/193 (71.0) | |
| ACR50 | 99/213 (46.5) | 122/229 (53.3) | 103/211 (48.8) | 100/193 (51.8) | |
| ACR70 | 58/213 (27.2) | 71/229 (31.0) | 64/211 (30.3) | 70/193 (36.3) | |
| PASI 75d | 93/117 (79.5) | 95/127 (74.8) | 86/120 (71.7) | 83/103 (80.6) | |
| PASI 90d | 73/117 (62.4) | 72/127 (56.7) | 68/120 (56.7) | 69/103 (67.0) | |
| Resolution of dactylitise | 92/107 (86.0) | 106/121 (87.6) | 102/113 (90.3) | 93/99 (93.9) | |
| Resolution of enthesitisf | 96/130 (73.8) | 106/137 (77.4) | 94/123 (76.4) | 89/113 (78.8) | |
| DAS28‐CRP remission | 101/213 (47.4) | 116/229 (50.7) | 111/212 (52.4) | 118/194 (60.8) | |
| DAS28‐CRP LDA | 128/213 (60.1) | 149/229 (65.1) | 143/212 (67.5) | 147/194 (75.3) | |
| Minimal Disease Activity | 76/215 (35.3) | 79/232 (34.1) | 82/216 (38.0) | 77/195 (39.5) | |
| HAQ‐DI | BL (SD) |
1.12 (0.661) [n = 213] |
1.14 (0.671) [n = 229] |
1.13 (0.662) [n = 210] |
1.09 (0.650) [n = 193] |
| mean change from BL (SD) |
−0.40 (0.587) [n = 213] |
−0.39 (0.632) [n = 229] |
−0.37 (0.644) [n = 210] |
−0.39 (0.626) [n = 193] |
|
| SF‐36 PCS | BL (SD) |
38.39 (7.929) [n = 207] |
38.15 (7.80) [n = 230] |
38.19 (7.734) [n = 213] |
38.39 (7.811) [n = 192] |
| mean change from BL (SD) |
5.72 (7.893) [n = 207] |
5.43 (8.417) [n = 230] |
5.52 (8.350) [n = 213] |
6.04 (8.341) [n = 192] |
|
Abbreviation: ACR, American College of Rheumatology; BL, baseline; DAS28‐CRP, Disease Activity Score 28‐joint count C‐reactive protein; HAQ‐DI, Health Assessment Questionnaire Disability Index; LDA, low disease activity; M, The total number of patients evaluated in the treatment group; PASI90, 90% improvement in Psoriasis Area and Severity Index; SF‐36 PCS, Short Form‐36 Physical Component Summary.
Results are n/M (%) unless otherwise stated. Results are shown for patients who entered the extension phase in the secukinumab 150‐mg group, including patients who switched from placebo to secukinumab 150 mg at weeks 16/24.
All patients received secukinumab 150 mg.
Includes 83 patients who had dose escalation from 150 to 300 mg based on physician's judgement from week 156 onward.
Patients with psoriasis affecting ≥3% body surface area at baseline (n = 89 [150 mg] and 82 [75 mg]).
Patients (n = 83 [150 mg] and 77 [75 mg]) with dactylitis at baseline, as measured using the Leeds Dactylitis Index.
Patients (n = 99 [150 mg] and 91 [75 mg]) with enthesitis at baseline, as measured using the Leeds Enthesitis Index.
ACR response rates achieved in the core study were sustained through 5 years in both anti–TNF‐naïve and anti–TNF‐IR groups, with generally higher responses observed in anti–TNF‐naïve patients across the entire treatment period (Figure 2). ACR responses were generally similar regardless of whether patients were receiving concomitant methotrexate or not (Supplementary Table 1).
Disease remission/LDA based on DAS28‐CRP
The proportions of patients who achieved disease remission and LDA states according to DAS28‐CRP in the secukinumab 150‐mg group (including those who switched from placebo to secukinumab 150 mg) at 2 years during the core study improved yearly through 5 years (Table 2). At 5 years, 60.8% and 75.3% of patients in the secukinumab 150‐mg group achieved DAS28‐CRP disease remission and LDA states, respectively.
Resolution of enthesitis and dactylitis
Resolution of dactylitis and enthesitis achieved with secukinumab 150 mg up to 2 years were sustained through 5 years, with 93.9% and 78.8% of patients achieving complete resolution of dactylitis and enthesitis, respectively, at 5 years (Table 2).
Efficacy across other endpoints
Efficacy responses reported during the core study with secukinumab 150 mg were also sustained throughout the extension study for the following parameters: PASI90 and PASI75, HAQ‐DI response, SF‐36 PCS, and MDA (Table 2). At 5 years, high PASI75 (80.6%) and PASI90 (67.0%) responses were observed, and MDA response was 39.5% (Table 2).
Efficacy following dose escalation
The majority of patients showed improvements in their ACR and PASI responses after dose escalation from 150 to 300 mg (Figure 3). Lower proportions of patients were ACR nonresponders (fewer than 20) after escalation from 150 to 300 mg at 12 to 32 weeks (40 of 126 patients, 31.7%) and 36 to 56 weeks (35 of 126 patients, 27.8%) compared with before escalation (54 of 126, 42.9%). Responses for higher hurdle ACR endpoints (ACR50 and ACR70) were achieved by greater proportions of patients at 12 to 32 weeks (55 of 126 patients, 43.7%) and 36 to 56 weeks (64 of 126 patients, 50.8%) after escalation from 150 to 300 mg compared with before dose escalation (40 of 126 patients, 31.7%) (Figure 3). At 5 years in the secukinumab group (including patients who switched from placebo to secukinumab 150 mg), ACR20/50/70 responses were 71.8%/51.8%/39.1%, respectively, in those receiving 150 mg (n = 110) and 69.9%/51.8%/32.5%, respectively, in those who had escalated to 300 mg (n = 83).
Figure 3.
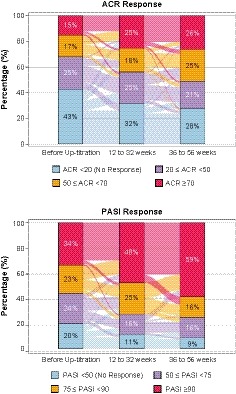
American College of Rheumatology (ACR) and Psoriasis Area and Severity Index (PASI) responses up to 56 weeks after dose escalation from 150 to 300 mg. Before escalation is defined as the last assessment done on or before patient took the escalated dose. Patients who entered the extension phase in the secukinumab 150‐mg group (including patients who switched from placebo to secukinumab 150 mg at weeks 16 and 24) and who had both pre‐ and all postdose escalation assessments data available are included in the analysis for ACR (n = 126) and PASI (n = 80) responses.
Similarly, lower proportions of patients were PASI nonresponders (less than 50) after escalation from 150 to 300 mg at 12 to 32 weeks (9 of 80 patients, 11.3%) and 36 to 56 weeks (7 of 80 patients, 8.8%) compared with those before escalation (16 of 80, 20.0%). Responses for the high hurdle endpoint PASI90 were achieved by greater proportions of patients at 12 to 32 weeks (38 of 80 patients, 47.5%) and 36 to 56 weeks (47 of 80 patients, 58.8%) after dose escalation compared with before escalation (27 of 80 patients, 33.8%). At 5 years in the secukinumab group (including patients who switched from placebo to secukinumab 150 mg), PASI75/PASI90 responses were 86.3% and 78.4%, respectively, in those receiving 150 mg (n = 51) and 75.0% and 55.8%, respectively, in those who had escalated to 300 mg (n = 52).
Safety
Safety is reported as EAIR/100 patient‐years for all patients (N = 587) who were administered at least one dose of study treatment during the core or the extension study. The type, incidence, and severity of AEs over the 260‐week treatment period were consistent with those reported at year 1 and year 2. The EAIR of AEs and serious AEs observed with secukinumab are shown in Table 3. The majority of AEs with the highest EAIRs were infections usually involving the upper respiratory tract (EAIR = 8.0 for any dose of secukinumab), whereas the most common AE was nasopharyngitis (EAIR = 8.6 for any dose of secukinumab).
Table 3.
Safety summary across the entire 260‐week treatment perioda
| Category | Any Secukinumab Dose (N = 587) |
|---|---|
| Exposure to treatment – d, mean (SD) | 1443.6 (616.7) |
| Death, n (%) | 6 (1.0) |
| Discontinued due to AEs, n (%) | 49 (8.3) |
| Any AE | 126.7 |
| Serious AEs | 7.5 |
| Most Common AEs | |
| Nasopharyngitis | 8.6 |
| Upper respiratory tract infection | 8.0 |
| AEs of special interest | |
| Serious infections | 1.8 |
| Candida infections | 0.9 |
| Crohn's disease | 0.2 |
| MACE | 0.5 |
| Malignancies | 0.8 |
Abbreviation: AE, adverse event; EAIR, exposure‐adjusted incidence rate; MACE, major adverse cardiac event.
Data are EAIR, unless otherwise stated.
Six deaths were reported during this 5‐year study. Two of these deaths occurred during the core study: one died of stroke/cerebrovascular accident (a patient receiving secukinumab 75 mg), and one died of myocardial infarction (a patient receiving secukinumab 150 mg); but the other four deaths occurred during the extension phase: one patient receiving secukinumab 75 mg died from squamous cell carcinoma of the pharynx, the other three patients, in the secukinumab 150‐mg group, died from acute myocardial infarction, cardiac failure, and septic shock, respectively.
The EAIR for Crohn's disease was 0.2 for the entire 5‐year period. Confirmed cases of Crohn's disease were reported during the core study for one patient in the secukinumab 75‐mg group (23‐year‐old male with no history of IBD; the event was graded as severe and the patient discontinued; the investigator did not suspect a relationship to study treatment) and one patient in the placebo group (exacerbation). One additional confirmed case of Crohn's disease was reported for one patient in the secukinumab 75‐mg group during the extension study (68‐year‐old male with no history of IBD; the event was graded as moderate in severity and the patient remained in the study; the investigator did not suspect a relationship to study treatment).
The EAIR for Candida infections was 0.9 for patients treated with any dose of secukinumab during the entire treatment period. The infections were located in the skin and mucus membranes, none were systemic or invasive, and all except one were nonserious, mild, or moderate in severity, responded to standard therapy, and did not lead to study treatment discontinuation. The EAIR for malignancies was 0.8 for patientss treated with any dose of secukinumab during the entire treatment period.
Overall, the incidence of treatment‐emergent antidrug antibodies (ADAs) was low (detected in eight patients) throughout the 5‐year period; three patients were positive at baseline and continued to show ADAs, and two patients showed neutralizing antibodies at week 24. A clear relationship between ADA formation and deviating pharmacokinetics or immunogenicity‐related AEs was not observed.
Discussion
Secukinumab demonstrated sustained improvements in the signs and symptoms, function, and health‐related quality of life in patients with active PsA. The patient retention rates in this study were high, with over 80% of patients who entered the extension study completing the full 5‐year treatment period. These are the first 5‐year findings reported for secukinumab in PsA, and the data add to the growing body of evidence supporting the use of IL‐17 inhibitors for the treatment of PsA as recognized in the guidelines from the EULAR 8, GRAPPA 11, and recently the ACR/National Psoriasis Foundation (NPF) 19.
This robust study also provides data on the benefits of dose escalation in patients whose symptoms are inadequately controlled on 150 mg. Improvement in ACR and PASI responses was achieved in patients who escalated from secukinumab 150 to 300 mg, indicating a benefit of dose escalation in patients who require additional control of symptoms. In agreement with previous studies 3, 8, 9, 11, 13, 14, 15, 16, secukinumab treatment in FUTURE 1 was shown to be efficacious in both anti–TNF‐naïve and anti–TNF‐IR patients, with clinical responses generally higher in anti–TNF‐naïve patients than in anti–TNF‐IR patients. These results support the effectiveness of long‐term treatment with secukinumab for biologic‐naïve patients as well as for patients who have previously failed anti‐TNF therapy.
The safety profile of secukinumab was consistent with that previously reported for PsA 3, 9, 12, 13, 14, 15, 16, 17 and psoriasis 6. No new or unexpected safety signals were observed in patients with PsA over a treatment period of up to 5 years. The rates of Candida infections, major adverse cardiac events, and malignancies reported were low throughout the 5‐year treatment period and consistent with results of an analysis of long‐term safety of secukinumab in patients with psoriasis, PsA, and ankylosing spondylitis 20. The low rate of Crohn's disease reported in the study is in line with findings of a recent retrospective analysis of 21 clinical trials of psoriasis, PsA, and ankylosing spondylitis, which found that IBD events were uncommon with secukinumab treatment 21. It should be noted that patients with active ongoing inflammatory disease were excluded from this study, so patients with a history of IBD were likely not enrolled.
Limitations of this study included a lack of a long‐term comparator due to the fact that long‐term treatment with placebo is considered unethical; the placebo‐controlled period of the core trial was, therefore, only up to week 16. There was no active comparator included, and results could be potentially biased as patients remaining in the study are those patients benefiting from secukinumab. Although efficacy responses in the current study were sustained irrespective of previous anti‐TNF exposure, patients eligible for inclusion in this study could have been treated with no more than three anti‐TNF agents; this may be viewed as a limitation of the study. The inclusion of patients in this extension study based on the investigator's judgement of their overall response to therapy likely resulted in a population of preselected responders.
Results from this long‐term extension study confirm the benefit of IL‐17 inhibition with secukinumab for sustained improvements in the signs and symptoms of major clinical domains of PsA. With no new safety concerns over a treatment period of up to 5 years, results from the 5‐year phase III FUTURE 1 study support the long‐term efficacy, safety, and tolerability of secukinumab in the treatment of patients with PsA.
Author Contributions
Study conception and design
Pricop, Mpofu.
Acquisition of data
Mease, Kavanaugh, Reimold, Tahir, Rech, Hall, Geusens, Pellet, Delicha, Pricop, Mpofu.
Analysis and interpretation of data
Mease, Kavanaugh, Reimold, Tahir, Rech, Hall, Geusens, Pellet, Delicha, Pricop, Mpofu.
Supporting information
Supplementary Figure 1. ACR20/50 responses by concomitant methotrexate use up to 5 years in the secukinumab 150‐mg group. To show the full 5‐year efficacy, results are shown for the group of patients who were originally randomized to secukinumab 150 mg during the core study and entered the extension study in the secukinumab 150‐mg group. ACR 20/50, American College of Rheumatology criteria for 20%/50% improvement in disease activity.
Acknowledgments
Medical writing support was provided by Martin Wallace, PhD, of Novartis Ireland, Ltd., in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). The funding for this writing support was provided by Novartis.
Additionally, Novartis is committed to sharing with qualified external researchers access to patient‐level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on http://www.clinicalstudydatarequest.com
Dr. Mease was recipient of grant/research support from AbbVie, Amgen, BMS, Celgene, Crescendo Bioscience, Genentech, Janssen, Lilly, Merck, Novartis, Pfizer, and UCB; has consulted for AbbVie, Amgen, Bristol‐Myers Squibb, Celgene, Crescendo Bioscience, Genentech, Janssen, Lilly, Merck, Novartis, Pfizer, and UCB; participated in speakers bureau for AbbVie, Amgen, Bristol‐Myers Squibb, Celgene, Crescendo Bioscience, Genentech, Janssen, Lilly, Merck, Novartis, Pfizer, and UCB. Dr. Kavanaugh has consulted for Novartis. Dr. Reimold has received grant/research support from AbbVie. Dr. Tahir participated in speakers bureau for Novartis, Eli Lilly, and AbbVie. Dr. Rech has participated in speakers bureau for AbbVie, Bristol‐Myers Squibb, Celgene, Fresenius, Medicap, MSD, Novartis, Pfizer, and Roche. Dr. Geusens has received grant/research support from Pfizer, Abbott, Lilly, Amgen, MSD, Will, Bio Minerals, and Roche; participated in speakers bureau for Pfizer, Abbott, Lilly, Amgen, MSD, Will, Bio Minerals, and Roche. Drs. Pellet, Delicha, Pricop, and Mpofu are employees of Novartis. No other disclosures relevant to this article were reported.
References
- 1. Smolen JS, Schöls M, Braun J, Dougados M, FitzGerald O, Gladman DD, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2018;77:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gladman D, Antoni C, Mease P, Clegg D, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64 Suppl 2:ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kavanaugh A, Mease PJ, Reimold AM, Tahir H, Rech J, Hall S, et al. Secukinumab for long‐term treatment of psoriatic arthritis: a two‐year followup from a phase iii, randomized, double‐blind placebo‐controlled study. Arthritis Care Res (Hoboken) 2017;69:347–55. [DOI] [PubMed] [Google Scholar]
- 4. Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol 2003;4:441–7. [DOI] [PubMed] [Google Scholar]
- 5. Henes JC, Ziupa E, Eisfelder M, Adamczyk A, Knaudt B, Jacobs F, et al. High prevalence of psoriatic arthritis in dermatological patients with psoriasis: a cross‐sectional study. Rheumatol Int 2014;34:227–34. [DOI] [PubMed] [Google Scholar]
- 6. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014;371:326–38. [DOI] [PubMed] [Google Scholar]
- 7. Ash Z, Gaujoux‐Viala C, Gossec L, Hensor EM, FitzGerald O, Winthrop K, et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta‐analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2012;71:319–26. [DOI] [PubMed] [Google Scholar]
- 8. Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 9. Mease PJ, Kavanaugh A, Reimold AM, Tahir H, Rech J, Hall S, et al. Sustained improvements in the signs and syptoms of active psoriatic arthritis through 3 years: efficacy and safety results from a phase 3 trial [abstract]. Arthritis Rheumatol 2016; 68 Suppl 10 URL: https://acrabstracts.org/abstract/secukinumab-provides-sustained-improvements-in-the-signs-and-symptoms-of-active-psoriatic-arthritis-through-3-years-efficacy-and-safety-results-from-aphase-3-trial/. [Google Scholar]
- 10. Lubrano E, Perrotta FM. Beyond TNF inhibitors: new pathways and emerging treatments for psoriatic arthritis. Drugs 2016;76:663–73. [DOI] [PubMed] [Google Scholar]
- 11. Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta‐Felquer M, Armstrong AW, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 12. McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, et al. Efficacy and safety of secukinumab, a fully human anti‐interleukin‐17A monoclonal antibody, in patients with moderate‐to‐severe psoriatic arthritis: a 24‐week, randomised, double‐blind, placebo‐controlled, phase II proof‐of‐concept trial. Ann Rheum Dis 2014;73:349–56. [DOI] [PubMed] [Google Scholar]
- 13. McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti‐interleukin‐17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2015;386:1137–46. [DOI] [PubMed] [Google Scholar]
- 14. Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin‐17a in patients with psoriatic arthritis. N Engl J Med 2015;373:1329–39. [DOI] [PubMed] [Google Scholar]
- 15. Kavanaugh A, McInnes IB, Mease PJ, Hall S, Chinoy H, Kivitz AJ, et al. Efficacy of subcutaneous secukinumab in patients with active psoriatic arthritis stratified by prior tumor necrosis factor inhibitor use: results from the randomized placebo‐controlled FUTURE 2 study. J Rheumatol 2016;43:1713–7. [DOI] [PubMed] [Google Scholar]
- 16. McInnes IB, Mease PJ, Ritchlin CT, Rahman P, Gottlieb AB, Kirkham B, et al. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology (Oxford) 2017;56:1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mease PJ, Kavanaugh A, Reimold A, Tahir H, Rech J, Hall S, et al. Secukinumab in the treatment of psoriatic arthritis: efficacy and safety results through 3 years from the year 1 extension of the randomised phase III FUTURE 1 trial. RMD Open 2018;4:e000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 19. Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the treatment of psoriatic arthritis. Arthritis Care Res (Hoboken) 2019;71:2–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deodhar A, Mease PJ, McInnes IB, Baraliakos X, Reich K, Blauvelt A, et al. Long‐term safety of secukinumab in patients with moderate‐to‐severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post‐marketing surveillance data. Arthritis Res Ther 2019;21:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schreiber S, Colombel JF, Feagan BG, Reich K, Deodhar AA, McInnes IB, et al. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis 2019;78:473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. ACR20/50 responses by concomitant methotrexate use up to 5 years in the secukinumab 150‐mg group. To show the full 5‐year efficacy, results are shown for the group of patients who were originally randomized to secukinumab 150 mg during the core study and entered the extension study in the secukinumab 150‐mg group. ACR 20/50, American College of Rheumatology criteria for 20%/50% improvement in disease activity.


