Abstract
Polyunsaturated fatty acids (PUFA) and their cytochrome P450 (CYP450) metabolites have been linked to angiogenesis and vessel homeostasis. However, the role of individual CYP isoforms and their endogenous metabolites in those processes are not clear. Here, we focused on the role of Cyp2c44 in postnatal retinal angiogenesis and report that Cyp2c44 is highly expressed in Müller glial cells in the retina. The constitutive as well as inducible postnatal genetic deletion of Cyp2c44 resulted in an increased vessel network density without affecting vessel radial expansion during the first postnatal week. This phenotype was associated with an increased endothelial cell proliferation and attenuated Notch signaling. LC-MS/MS analyses revealed that levels of hydroxydocosahexaenoic acids (HDHA), i.e., 10-, 17- and 20-HDHA were significantly elevated in retinas from 5 day old Cyp2c44−/− mice compared to their wild-type littermates. Enzymatic activity assays revealed that HDHAs were potential substrates for Cyp2c44 which could account for the increased levels of HDHAs in retinas from Cyp2c44−/− mice. These data indicate that Cyp2c44 is expressed in the murine retina and, like the soluble epoxide hydrolase, is expressed in Müller glia cells. The enhanced endothelial cell proliferation and Notch inhibition seen in retinas from Cyp2c44-deficient mice indicate a role for Cyp2c44-derived lipid mediators in physiological angiogenesis.
Keywords: Docosahexenoic acid, Lipid mediators, Müller glia cells, Retinal angiogenesis, Soluble epoxide hydrolase
1. Introduction
The role of cytochrome P450 (CYP) enzymes in the vasculature was initially linked to the acute, endothelium-dependent regulation of tone by mechanisms involving endothelial cell hyperpolarization or an endothelium-derived hyperpolarizing factor [1,2]. Since those first reports it has become clear that metabolites of polyunsaturated fatty acids (PUFAs) generated by CYP enzymes can affect numerous signal transduction cascades as well as angiogenesis (for review see [3]). However, despite the fact that epoxides of arachidonic acid (the epoxyeicosatrienoic acids or EETs) exert potent angiogenic effects in vitro [4-7], as well as when applied to control animals e.g. in Matrigel plugs [6-8], there is a lack of evidence for CYP-derived metabolites in the regulation of physiological angiogenesis in vivo. Moreover, the angiogenic effects of the ω-6 PUFA epoxides are reportedly reversed in the presence of ω-3 fatty acids [9, 10] or cyclo-oxygenase inhibitors [11].
To date, most information about the role of endogenously generated CYP metabolites in angiogenesis has been obtained using animals lacking the soluble epoxide hydrolase (sEH), the enzyme that metabolizes fatty acid epoxides to their corresponding diols. sEH−/− mice demonstrate elevated tissue and circulating levels of EETs [12, 13], but rather than being associated with increased angiogenesis, the development of the retinal vascular plexus was attenuated in sEH−/− mice [9]. Similarly, the vascularization of Matrigel plugs and the vascular repair and recovery of blood flow after ischemic injury were attenuated in sEH−/− mice, especially following mobilization of bone marrow-derived progenitor cells [14]. A role for CYP-derived lipid mediators has been demonstrated in vivo e.g. in a humanized mouse models e.g. in mice overexpressing CYP enzymes [15, 16] but less has been done to assess the role of endogenously expressed CYP enzymes in regulating endothelial cell proliferation and angiogenesis in CYP knockout mice, simply because knocking down one specific CYP enzyme frequently results in the upregulation of another that can functionally compensate for it [17, 18].
The aim of this study was to determine the role of endogenous CYP-derived mediators in physiological angiogenesis by targeting the expression of a CYP enzyme using a combination of constitutive and inducible knockout mouse models. Cyp2c44 was chosen as a target given that: it is closely related to the human CYP2C8 and CYP2C9 isoforms [19], the enzyme generates EETs [20], and previous reports linking this enzyme with tumor growth [21] and signaling induced by vascular endothelial cell growth factor (VEGF) [22].
2. Materials and Methods
2.1. Chemicals and Reagents
All of the fatty acids, i.e., EETs, EpOMEs and HDHAs were obtained from Cayman Chemicals Europe (Tallinn, Estonia). NADPH was from Applichem (Darmstadt, Germany) and tamoxifen was from Sigma Aldrich (Deisenhofen, Germany). Other chemicals were purchased from Merck (Darmstadt, Germany) or Sigma Aldrich. The anti-Cyp2c44 antibody was generated as described [23], and the antibody directed against aquaporin 4 (AQP4) was from Santa Cruz (TX, USA), the antibody against CD31 was from BD biosciences (Heidelberg, Germany), the phospho histone 3 antibody was from Merck Millipore (Darmstadt, Germany), and the anti-ETS-related gene (ERG) antibody from Abcam (Cambridge, UK).
2.2. Animals
C57BL/6 mice (6-8 weeks old) were purchased from Charles River (Sulzfeld, Germany). Floxed Cyp2c44 mice were generated by TaconicArtemis (Cologne, Germany) using C57BL/6 embryonic stem cells for gene targeting. The positive selection cassettes flanked by FRT/F3 sites were deleted by crossing with a ubiquitously expressing FLP1 recombinase strain (TaconicArtemis). The conditional knock out allele was confirmed by PCR with primers flanking the loxP site modified genomic locus using the following primers: 5’-TTATATCATTGCTAGTGGCAATCG-3’, and 5’-GGCAGTTCTCTTCTATGTATGTGC-3’ resulting in a 233 bp product lengths for the wild-type allele and a 384 bp product for the conditional allele (loxP and F3). The Cre dependent recombination (excision) was assessed with PCR using the primer pair 5’-TTATATCATTGCTAGTGGCAATCG-3’ and 5’-TGGAGCTCTGTTCAGTAGTGTTCC-3’ that results in a (305 bp) product for the recombined locus. Genotyping was performed on genomic DNA derived from tail biopsies with the following PCR parameters: 95°C for 5 minutes, followed by 35 cycles, at 95°C for 30 seconds, 60°C for 30 seconds, 72°C for 30 seconds and 72°C for 10 minutes. Genotyping PCRs were performed with the following conditions 95°C for 5 minutes, followed by 30 cycles, at 95°C for 30 seconds, 60°C for 30 seconds, 72°C for 30 seconds and 72°C for 10 minutes (Supplementary Fig. S1). Floxed Cyp2c44 mice were crossed with Gt(ROSA)26Sortm16(Cre)Arte mice expressing Cre under the control of the endogenous Gt(ROSA)26Sor promoter (TaconicArtemis) to generate mice globally lacking Cyp2c44 (Cyp2c44−/−) or with Gt(ROSA)26Sortm(CreERT2)Arte mice (TaconicArtemis) to generate inducible Cyp2c44 knockout (Cyp2c44iKO) mice.
Mice were housed in conditions that conform to the Guide for the Care and Use of Laboratory Animals published by the U. S. National Institutes of Health (NIH publication no. 85-23). All experiments were approved by the governmental authorities (Regierungspräsidium Darmstadt: F28_38). Age- and strain-matched animals of both sexes (usually littermates) were used throughout. For the isolation of organs, mice were sacrificed using 4% isoflurane in air and subsequent exsanguination.
2.3. Immunohistochemistry
Retina:
Retinas were isolated, fixed in PBS containing 4% PFA for 2 hours at room temperature, washed in PBS containing 0.5% Triton X-100, 1 mmol/L CaCl2, 1 mmol/L MgCl2, and 1 mmol/L MnCl2 (pH 6. 8), permeabilized using 0.5% Triton X-100 overnight at 4°C, and blocked with the “mouse on mouse” blocking reagent (Vector Laboratories) before addition of the anti-Cyp2c44 antibody [23] overnight at 4°C. Afterwards samples were incubated with FITC-labelled Isolectin B4 and a fluorophore-conjugated secondary antibody (Alexa Fluor, Invitrogen, Karlsruhe, Germany).
Müller cells:
Retinas were isolated and digested immediately with collagenase I (100 U/ml, Biochrom, Berlin, Germany) in DMEM/F12 medium for 30 minutes at 37°C. The reaction was stopped by the addition of DMEM with 10% FCS and the tissue was further dissociated by gentle trituration using a sterile Pasteur pipette [24]. Cells were fixed and permeabilized by using FIX & PERM cell fixation and cell permeabilization kit (Invitrogen) according to the standard protocol. Antibodies directed against Cyp2c44 and AQP-4 were used at 1:200 and 1:500 dilution, respectively.
2.4. Retinal angiogenesis
Freshly isolated eyes from 2, 5 and 7 day old wild-type and Cyp2c44−/− littermates were fixed in PBS containing 4% formalin for 2 hours at room temperature. Thereafter, the retinas were isolated, washed in PBS supplemented with 0.5% Triton X-100, 1 mmol/L CaCl2, 1 mmol/L MgCl2, and 1 mmol/L MnCl2 (pH 6. 8) and blocked as well as permeabilized in 1% BSA and 0.5% Triton X-100 overnight at 4°C. Endothelial cells were visualized using FITC-labelled Isolectin B4 (1:100, Sigma-Aldrich). Samples were visualized using a confocal microscope (LSM 780 scanning confocal microscope; Zeiss, Jena, Germany) and all quantification was performed using high resolution images and Axiovision software (Zeiss) as described [9].
2.5. Endothelial cell culture
Human umbilical vein endothelial cells were isolated and cultured as described [25] and used exclusively after only 1 passage. The use of human material in this study conforms to the principles outlined in the Declaration of Helsinki [26] and the isolation of endothelial cells was approved in written form by the ethics committee of the Goethe-University.
2.6. Cell proliferation
Cells were transfected with a control plasmid (pcDNA) or a plasmid encoding Cyp2c44 and after 48 hours cells were re-seeded onto 48 well plates (18000 cells) coated with fibronectin (25 μg/mL). Proliferation was assessed with Casy TT cell counter and analyzer system (Roche, Basel, Switzerland) in the absence and presence of VEGF (20 ng/ml) and 20-HDHA (1 μmol/L) in medium supplemented with 5% fetal calf serum (FCS).
2.7. RNA isolation and quantitative real time PCR (RT-qPCR)
Total RNA was extracted using TriReagent (Sigma-Aldrich). For the generation of cDNA, total RNA (1 μg) was reverse transcribed using the Superscript III (Life Technologies GmbH, Darmstadt, Germany) and random hexamer primers according to the manufacturer’s protocol. The amount of mRNA was quantified using the threshold cycle (c⊺) value using a SYBR green master mix (ABgene, Dreieich, Germany) with intron spanning primers in a Mx4000 multiplex qPCR system (Stratagene, Heidelberg, Germany). RNA amount was calculated relative to a housekeeping gene, i. e. 18S rRNA, using the ΔΔc⊺ method.
Primer sequences used in the study.
| Name | Forward primer | Reverse primer |
|---|---|---|
| 18S | CTTTGGTCGCTCGCTCCTC | CTGACCGGGTTGGTTTTGAT |
| Dll1 | CATCCGATACCCAGGTTGTC | ACGGCTTATGGTGAGTACAG |
| Jagged1 | TCTCTGACCCCTGCCATAAC | TTGAATCCATTCACCAGATCC |
| Notch 1 | CCTCAGATGGTGCTCTGATG | CTCAGGTCAGGGAGAACTAC |
| Hes1 | GAGGCGAAGGGCAAGAATAAA | GTGGACAGGAAGCGGGTCA |
| Hey1 | TGAGCTGAGAAGGCTGGTAC | ACCCCAAACTCCGATAGTC |
| VEGF | GCACTGGACCCTGGCTTTACTGCTGTA | GAACTTGATCACTTCATGGGACTTCTGCTC |
| Ang1 | GGCCACAGGCATCGAACCACC | CGGTGCCGATTTCAGCACGAAG |
| Pdgfa | CCTGTGCCCATTCGCAGGAAGAGA | TTGGCCACCTTGACACTGCGGTG |
2.8. Insect cell (Sf-9) culture and overexpression of Cyp2c44
Cyp2c44 was subcloned with Sall and NotI (NEB, Frankfurt, Germany) from a I.M.A.G.E. full length cDNA clone (IRAVp968E0871D; BioScience ImaGenes, Berlin, Germany) into the pFastBac vector (Invitrogen). The bacmid was generated using the Bac-to-Bac Baculovirus expression system (Invitrogen) according to the manufacturer’s instructions. For transposition the pFastbac-CYP vector was transformed by heat shock in DH10Bac E. coli cells (Invitrogen) and plated on agar supplemented with 50 μg/mL kanamycin, 7 μg/mL gentamycin, 10 μg/mL tetracycline (Applichem, Darmstadt, Germany), 100 μg/mL 5-brom-4-chlor-3-indolyl-β-D-galactopyranosid (Applichem) and 40 μg/mL isopropyl β-D-thiogalactopyranoside. The successful recombination was checked by PCR using the following primers: M13 Forward: 5’-GTTTTCCCAGTCACGAC-3’; M13 Reverse: 5’-CAGGAAACAGCTATGAC-3’ (Biospring, Frankfurt, Germany). After the cellfectine-mediated transfection of Cyp2c44 and human CYP oxidoreductase bacmids in Spodoptera frugiperda (Sf-9) cells (Invitrogen), virus generation and protein expression were achieved as described previously [27]. Sf-9 cells were grown in Ex-Cell 405 medium (with 10% heat-inactivated FCS, 100 U/mL of both penicillin and streptomycin) and infected at a density of 2×106 cells/mL with the recombinant Cyp2c44-baculoviruses (multiplicity of infection of 3; titrated against a human CYP oxidoreductase-baculovirus). After 24 hours hemin (5 μmol/L) and metyrapone (30 μmol/L) were added. The successful overexpression was verified by Western blotting for Cyp2c44 as well as FACS analysis for the viral envelope protein GP-64 (BD Biosciences, Heidelberg, Germany). Cells were harvested after additional 72 hours, resuspended in 0.1 mol/L potassium phosphate buffer (pH 7.4, containing 20% glycerol and 1 mmol/L EDTA, 1 mmol/L DTT, 0.1 mmol/L PMSF) and lysed by sonication. The cytosolic fraction was prepared by differential centrifugation (1000g for 10 minutes, 10.000g for 20 minutes and 100000g for 90 minutes; all at 4°C), the resulting pellet was homogenized in 50 μL potassium phosphate buffer and stored at −80°C until used. Microsomes were incubated with the PUFAs described in the results section and reactions started by the addition of NADPH (1 mmol/L). After 20 minutes, reactions were stopped by the addition of 40 μL of 0.4 mmol/L citric acid and placed on ice.
2.9. LC-MS/MS analyses
Samples (murine retina lysates or Sf-9 microsomes) were mixed with 500 μL methanol and 300 μL 10 mol/L sodium hydroxide and deuterated internal standards. The samples were hydrolyzed for 30 minutes at 60°C and then neutralized with acetic acid and adjusted to pH6.2. A solid phase extraction procedure using Agilent Bond-Elut-Certify II (Santa Clara, CA, USA) was performed as described [28]. The measurements were performed by LIPIDOMIX GmbH (Berlin, Germany) with a Triplequad LC-MS-MS instrument Agilent 6460/1200SL (Agilent Technologies, Waldbronn, Germany) equipped with a Phenomenex Kinetex Column (150 mm × 2.1 mm, 2.6 μm, Phenomenex, Aschaffenburg, Germany). Chromatography was achieved under gradient conditions with acetonitrile/0.1% formic acid in water as the mobile phase, a flow rate of 0.3 mL/min and a run time of 16 minutes. The injection volume was 7.5 μL. After optimization the following MS-MS conditions were used: electrospray ionization (ESI) in negative mode, capillary voltage 3500 V, nozzle voltage 1500 V, drying gas 210 °C/7 L/min, sheath gas 350 °C/11 L/min and nebulizer pressure 30 psi.
Metabolite analysis was performed using multiple reaction monitoring (MRM), as established previously [29,30]. The internal standards added to the samples before extraction included 10 ng each of 20-HETE-d 6, 14,15-EET-d 8, 14,15-DHET-d 11, prostaglandin E2 (PGE2)-d4, leukotriene B4 (LTB4)-d5, and 15-HETE-d 8 (Cayman Chemical, Ann Arbor, MI) and served for the quantification of groups of similar metabolites. Calibration curves for the quantification of individual metabolites were established using authentic unlabeled standard compounds as purchased from Cayman Chemical and measuring the changes in the relative peak area in response to different target compound/internal standard-concentration ratios. Linearity was r2 > 0.99 over a range from 1 to 20 ng absolute for all metabolites determined. Metabolite identities rely on their characteristic retention times in combination with two characteristic transitions (quantifier and qualifier) resulting from metabolite specific fragmentation.
The eicosanoid profile in murine plasma and the metabolism of 17- or 20-HDHA by Sf-9 microsomes were performed after liquid–liquid extraction of 100 ul sample. Briefly, samples were extracted twice into ethyl acetate, evaporated under nitrogen and resuspended in methanol/water (vol. 1:2). The metabolites were analyzed using a C18 reversed-phase chromatographic column (Gemini NX C18; Phenomenex) that was coupled to a tandem MS system (API 4000; AB Sciex). Quantification was performed using internal standards (with deuterated standards) and one precursor to product ion transition per analyte as described [14,31]. In all cases, a calibration curve from 0.5 to 2500 ng/ml was generated. All of fatty acid epoxides, diols and deuterated analogs were from Cayman Europe (Tallinn, Estonia).
2.10. Statistical analysis
Data are expressed as mean ± SEM. Statistical evaluation was performed using Student’s t test for unpaired data, one-way ANOVA followed by a Bonferroni t test or ANOVA for repeated measures where appropriate. Values of P<0.05 were considered statistically significant.
3. Results
3.1. Expression of Cyp2c44 in the murine retina
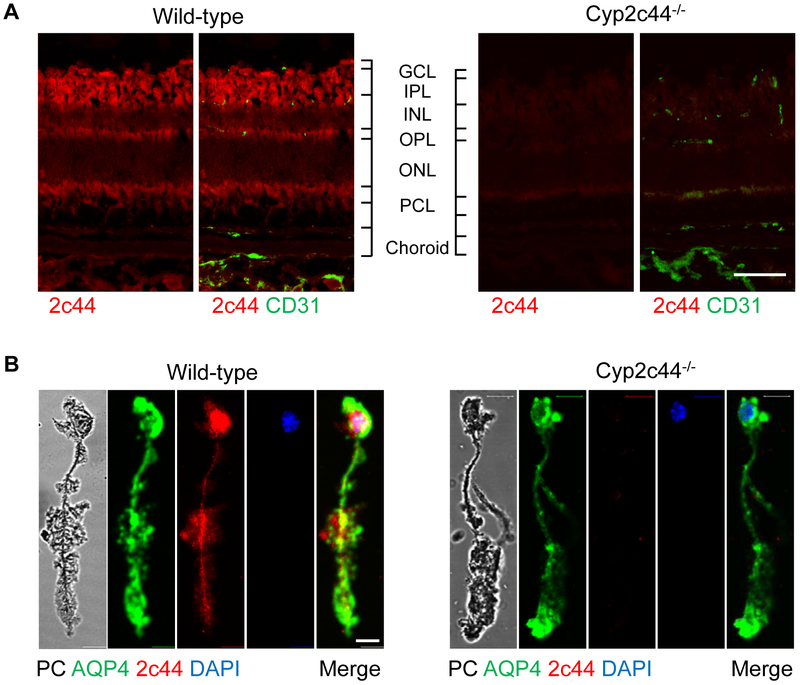
A specific antibody was used to determine the presence and localization of Cyp2c44 in cross-sections of the adult murine retina. The Cyp2c44 signal failed to colocalize with that of Isolectin B4 indicating that the enzyme was not expressed in any of the endothelial cell layers (Fig. 1A). Rather, Cyp2c44 was detected in the ganglion cell, inner plexiform, and outer plexiform layers which indicated that it may expressed in retinal Müller glia cells. Indeed, Cyp2c44 was highly expressed in Müller cells freshly dissociated from adult retinas, identified by their typical morphology and the expression of aquaporin 4 (Fig. 1B).
Figure 1. Cyp2c44 is expressed in the murine retina.
(A) Cryosections of the retina from adult wild-type and Cyp2c44−/− mice; Cyp2c44 = red, CD31 = green; bar = 100 μm. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PCL, retinal pigment cell layer; bar = 100 μm. (B) Cyp2c44 (red) and aquaporin 4 (blue) expression in Müller glia cells freshly isolated from wild-type and Cyp2c44−/− mice. Cell nuclear were labelled with DAPI (blue). PC: phase contrast image; bar = 10 μm. The images are representative of data from at least 3 additional experiments.
Cyp2c44−/− mice were generated by crossing floxed Cyp2c44 mice with animals expressing Cre under the control of the CMV promoter (Supplementary Fig. S1). Deletion of Cyp2c44 resulted in altered plasma levels of 14,15-dihydroxyeicosatrienoic acid (DHET) without affecting levels of other arachidonic acid-derived epoxides and diols (Supplementary Fig. S2A). Interestingly, metabolites of linoleic acid and docosahexenoic acid (DHA) and were more affected than those of arachidonic acid with significantly less 9,10-epoxyoctadecenoic acid (9,10-EpOME) and 9,10- dihydroxyoctadecenoic acid (9,10-DiHOME) as well as 19,20-epoxydocosapentaenoic acid (19,20-EDP) and 19,20-dihydroxydocosapentaenoic acid (DHDP) detected in samples from Cyp2c44−/− mice (Supplementary Fig. S2B-C). No Cyp2c44 signal was detected in retinal cross sections or Müller cells isolated from these mice (Fig. 1A-B). Thus, the expression of Cyp2c44 in the murine retina is similar to that of the sEH as both are expressed mainly in Müller glia cells [9].
3.2. Consequences of Cyp2c44 deletion on the retinal vascular network
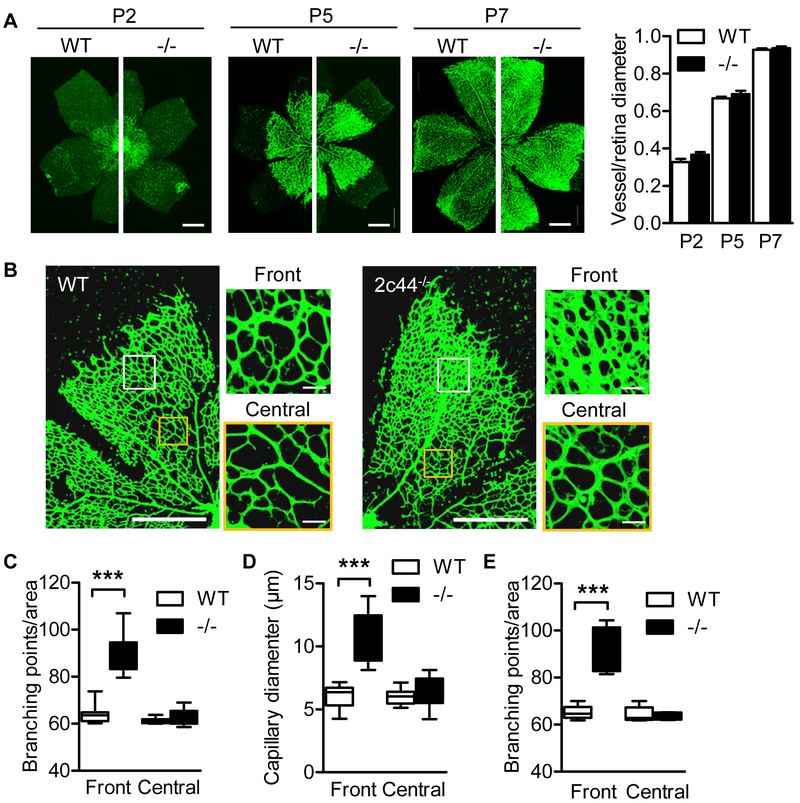
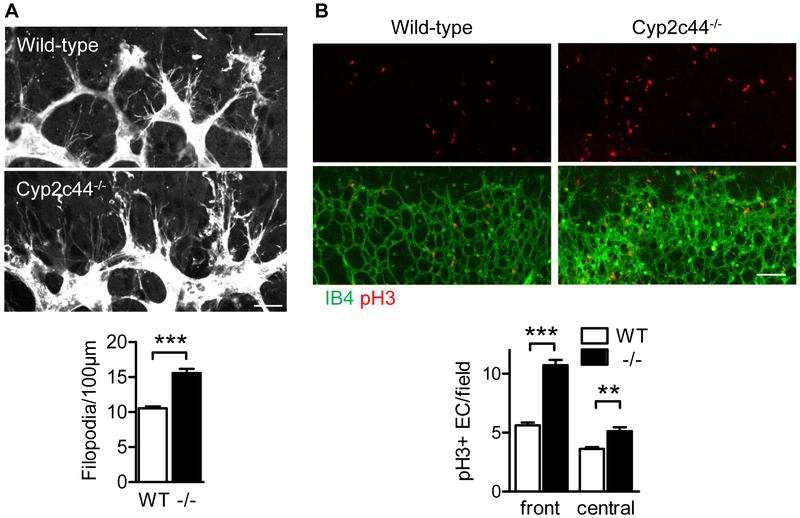
The importance of Cyp2c44 in sprouting angiogenesis was studied during retinal development in the first postnatal week. Wild-type and Cyp2c44−/− littermates displayed normal vessel radial expansion from the optic nerve to the periphery on postnatal days (P) 2, 5 and 7 (Fig. 2A). However, a significant increase in vessel network density as well as branching and vessel diameter was observed at the angiogenic front in retinas from Cyp2c44−/− mice versus their wild-type littermates at P5 (Fig. 2B-D) and remained significantly elevated at P7 (Fig. 2E). The increase in retinal vessel density at the angiogenic front in Cyp2c44−/− mice on P5 was associated with a significant increase in tip cell filopodia numbers (Fig 3A) as well as elevated numbers of phosphorylated histone 3 positive endothelial cells (Fig. 3B), implying that the loss of Cyp2c44 promoted endothelial cell proliferation.
Figure 2. Consequences of Cyp2c44 deletion on retinal angiogenesis.
(A) Isolectin B4 staining was assessed in whole-mounts of the retinal vasculature from wild-type (WT) and Cyp2c44−/− (−/−) mice on postnatal days (P) 2, 5 and 7; bar = 500 μm. (B) Higher magnification images of P5 retinas from wild-type and Cyp2c44−/− mice. The front and central areas indicated by the white and yellow boxes show the position of the right hand images; bars = 500 and 20 μm. (C&D) Quantification of branching points (C) and vessel diameter (D) in P5 retinas from wild-type and Cyp2c44−/− mice. (E) Quantification of branching points in retinas from wild-type and Cyp2c44−/− mice at P7. The graphs summarise data from 6-14 mice in each group; ***P<0.001.
Figure 3. Consequences of Cyp2c44 deletion on tip cell filopodia and proliferation at the angiogenic front.
(A) High magnification images and quantification of Isolectin B4–stained tip cells and filopodia in P5 retinas from wild-type (WT) and Cyp2c44−/− (−/−) littermates. Bar = 20 μm. (B) Proliferation assessed by phosphorylated histone 3 (pH3) staining (red) at the angiogenic front of P5 retinas. The vasculature was visualised using Isolectin B4; bar = 100 μm. The graphs summarise data from 6 mice in each group; **P<0.01; ***P<0.001.
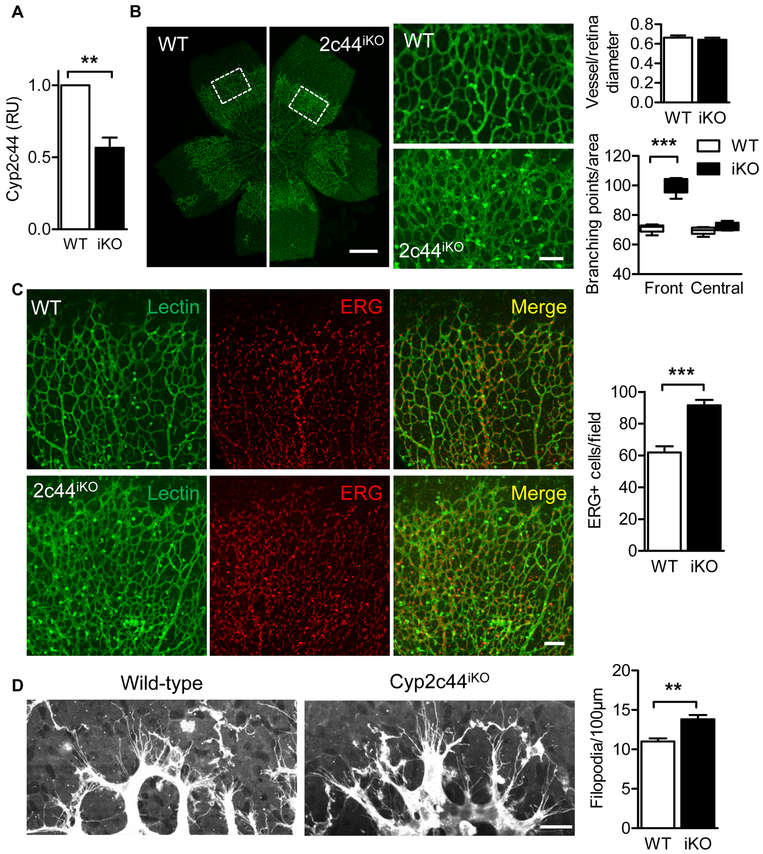
In order to confirm the defects observed in the constitutive Cyp2c44−/− mice, an inducible Cyp2c44 knockout strain (Cyp2c44iKO) was generated by breeding floxed 2C44 mice with CreERT2 mice. The postnatal deletion of genes is usually associated with a milder phenotype and treating the Cyp2c44iKO mice with tamoxifen (from P1 to P4) resulted in ~45% reduction in Cyp2c44 expression on P5 (Fig. 4A). Despite the incomplete downregulation of Cyp2c44 in the latter model the acute postnatal down-regulation of the enzyme increased vessel density without affecting vessel radial expansion (Fig. 4B), thus phenocopying the Cyp2c44−/− mice. Consistent with the increased endothelial cell proliferation observed in retinas from Cyp2c44−/− mice, ERG staining to mark endothelial cell nuclei was increased in Cyp2c44iKO retinas (Fig. 4C). This indicates that the increased vessel density could be mainly attributed to cell proliferation rather than a change in cell morphology. Also at the angiogenic front filopodia numbers were elevated in retinas from Cyp2c44iKO mice (Fig. 4D).
Figure 4. Retinal angiogenesis following the acute knockout of Cyp2c44.
Wild-type (WT) or Cyp2c44iKO (iKO) mice were treated with tamoxifen from P1 to P4. (A) Cyp2c44 expression on P5 determined by RT-qPCR. (B) Isolectin B4 staining in retinal whole mounts from the same animals; bars = 500μm. White boxes indicated the cropped areas shown on the right panels; bar = 100 μm. (C) Quantification of vascularization. (D) Quantification of vessel of branching points in P5 retinas from wild-type and Cyp2c44iKO mice. (E) ERG (endothelial nuclear, red) and Isolectin B4 (green) staining in P5 retinas from wild-type and Cyp2c44iKO mice; bar = 100 μm. (F) High magnification images and quantification of Isolectin B4–stained tip cells and filopodia on P5 retinas. Bar = 20 μm. The graph summarises data from 5-9 mice in each group; **P<0.01, *** P<0.001.
3.3. Consequences of Cyp2c44 deletion on gene expression
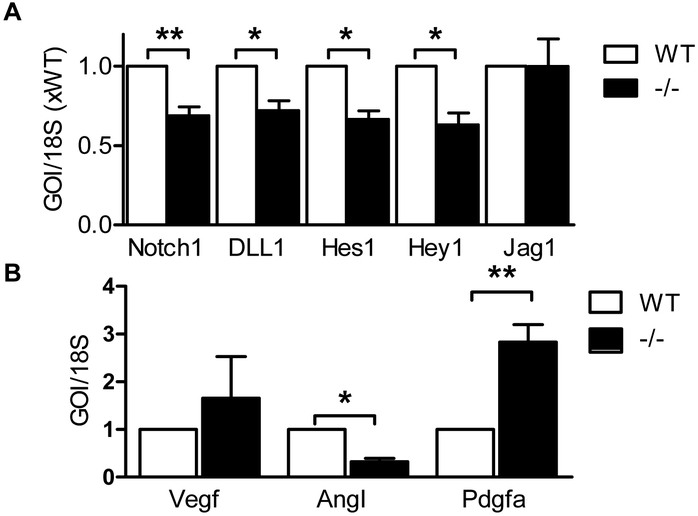
The increase in vascular diameter at the angiogenic front was reminiscent of the effects elicited by Notch inhibition which triggers the excessive formation of tip cells, increased sprouting and branching, and endothelial hyperproliferation [32]. Indeed, the expression of Notch 1, Dll1 Hes1 and Hey1 were all attenuated in retinas from 5 day old Cyp2c44−/− mice versus their wild-type littermates (Fig. 5A). In the same samples there was a tendency towards increased VEGF expression that failed to reach significance, a decrease in angiopoietin I and an increase in platelet-derived growth factor A expression (Fig. 5B).
Figure 5. Attenuated of Notch signaling in Cyp2c44−/− mice.
(A) Expression of genes of interest (GOI) involved in Notch signaling was assessed in P5 retinas from wild-type (WT) and Cyp2c44−/− (−/−) mice. (B) Expression of VEGF, angiopoietin I (AngI) and platelet-derived growth factor A (pdgfa) expression in P5 retinas from wild-type and Cyp2c44−/− mice. The graphs summarize data from 5-9 mice in each group; *P<0.05, *P<0.01.
3.4. Enhanced retina HDHA levels in Cyp2c44−/− mice
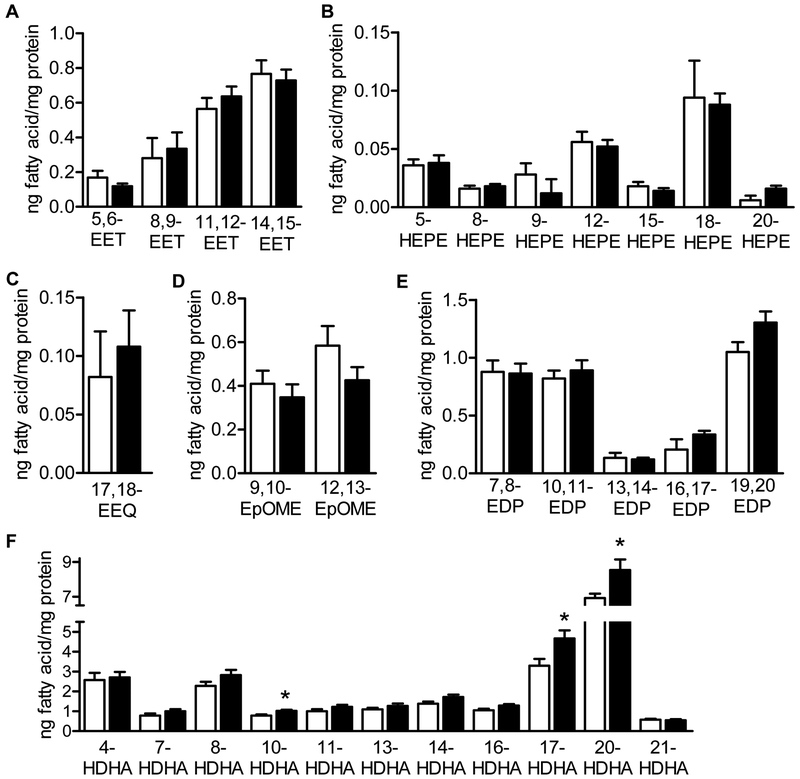
The altered lipid profile in the plasma from Cyp2c44−/− animals does not necessarily reflect the lipid profile of the retina which contains much more DHA and DHA-derived metabolites than arachidonic acid and arachidonic acid-derived metabolites [9,33]. To determine which of the Cyp2c44 substrates or products could account for the increase in retinal vessel density, LC-MS/MS was used to assess the levels of epoxides and diols in retinas from 5 day old wild-type and Cyp2c44−/− littermates. Surprisingly, none of epoxides of arachidonic acid, linoleic acid or DHA were altered in retinas from Cyp2c44−/− mice (Fig. 6A-E). However, in the same samples, several hydroxydocosahexaenoic acids (HDHA), i.e., 10-, 17- and 20-HDHA were more abundant in P5 retinas from Cyp2c44−/− mice compared to their wild-type littermates (Fig. 6F,). This is of interest as at least 17-HDHA is a marker for the formation of anti-inflammatory D-resolvins [34].
Figure 6. PUFA product profile in retinas isolated from 5 day old wild-type (WT) or Cyp2c44−/− littermates.
Retinas were isolated from 5 day old wild-type (WT) and Cyp2c44−/− (−/−) littermates and lipid metabolite levels were determined by LC-MS/MS. (A) Epoxyeicosatrienoic acids (EET). (B) Hydroxyeicosapentaenoic acids (HEPE). (C) Epoxyeicosatetraenoic acid (EEQ). (D) Epoxyoctadecenoic acid (EpOME). (E) Epoxydocosapentaenoic acid (EDP). (F) Hydroxy docosahexaenoic acids (HDHA). The graphs summarize data from 5 different samples each representing a pool from 4 different retinas; *P<0.05.
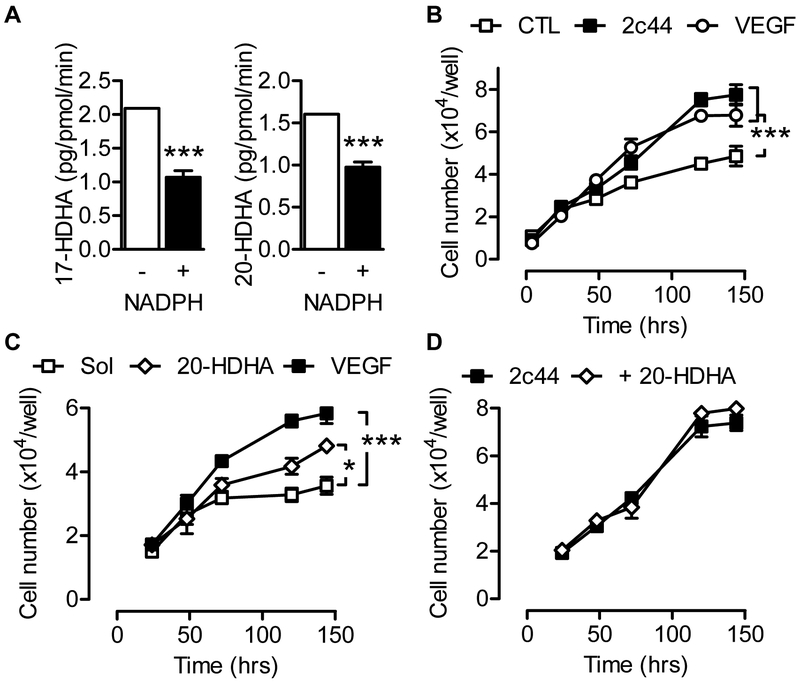
HDHAs are generated by auto-oxidation from DHA in vitro and can also be generated by enzymatic transformation e.g., via lipoxygenases in vivo [34] (see Supplementary Fig. S3 for structures), but there is no known link to CYP2C enzymes. The observed increase in HDHA levels in Cyp2c44−/− retinas suggested that they may be metabolized by Cyp2c44. Therefore, Cyp2c44 metabolism was assessed in microsomes prepared from Sf-9 overexpressing Cyp2c44 and the cytochrome reductase. The enzyme was able to metabolize arachidonic acid to EETs as well as hydroxyeicosatetraenoic acids (HETEs), eicosapentaenoic acid (EPA) to epoxyeicosatetraenoic acids (EEQs) and hydroxyeicosapentaenoic acids (HEPEs), and linoleic acid to EpOME as well as DHA to EDPs and HDHAs. However, only low amounts of the 10-, 17- and 20-HDHA detected in the retina were generated by the microsomal preparations indicating that Cyp2c44 may be involved in the metabolism of HDHA rather than its generation (Supplementary Fig. S4). Indeed, the incubation of Cyp2c44-expressing microsomes with 17- or 20-HDHA results in their rapid metabolism in the presence of NADPH (Fig. 7A) to as yet unidentified metabolites.
Figure 7. Effect of Cyp2c44 overexpression and HDHA on endothelial cell proliferation.
(A) Metabolism of 17- and 20-HDHA (each 1 μmol/L) by Cyp2c44 containing microsomes (50 pmol) isolated from Cyp2c44 overexpressing Sf9 cells. (B) Human endothelial cells were transiently transfected with either a control plasmid or a Cyp2c44 overexpression plasmid and proliferation followed over 120 hours. (C) Effect of solvent (Sol; DMSO), 20-HDHA (1 μmol/L) versus VEGF (20 ng/mL) on the proliferation of human endothelial cells. (D) Effect of solvent (Sol; DMSO) and 20-HDHA (1 μmol/L) on the proliferation of human endothelial cells overexpressing Cyp2c44. The graph summarises data from 4 independent experiments, each using a different batch of endothelial cells; *P<0.05, *** P<0.001.
3.5. Effect of 20-HDHA on endothelial cell proliferation in vitro
To determine whether or not Cyp2c44 and HDHAs could affect endothelial cell function, human endothelial cells that expressed little or no endogenous CYP enzymes [35] were transfected with a control plasmid or a plasmid encoding Cyp2c44. The overexpression of Cyp2c44 resulted in a marked increase in endothelial cell proliferation (Fig. 7B) without any clear effect on cell migration (Supplementary Fig. S5). The effect elicited by the overexpression of Cyp2c44 was comparable with that of VEGF stimulation and is consistent with a previous report [22]. The application of 20-HDHA (1 μmol/L) also increased endothelial cell proliferation (Fig. 7C), but only in endothelial cells that lacked CYP expression as no 20-HDHA-induced increase in proliferation was detected in cells expressing Cyp2c44 (Fig. 7D).
4. Discussion
The results of the present investigation revealed that Cyp2c44 is expressed in Müller glia cells in the murine retina. The constitutive as well as the acute deletion of the enzyme modulated sprouting angiogenesis by promoting endothelial cell proliferation and vessel diameter expansion as well as tip cell filopodia numbers. Mechanistically, the effect of Cyp2c44 deletion could be attributed to the accumulation of HDHAs in the retina, and at least 20-HDHA was able to increase endothelial cell proliferation in vitro – but only in cells lacking Cyp2c44. Thus it appears that in vivo Cyp2c44 limits retinal angiogenesis by ensuring the continuous metabolism of HDHAs.
Cyp2c44 is closely related to the human CYP2C8 and CYP2C9 isoforms and is reported to be the predominant epoxygenase in the mouse kidney [19]. It is expressed in the cortical collecting duct and plays a role, in the regulation of blood pressure by inhibiting the epithelial sodium channel (ENaC) [20]. The genetic deletion of Cyp2c44 blunts the inhibition of ENaC to cause salt-sensitive hypertension and 11,12-EET was reported to inhibit ENaC in wild-type and Cyp2c44−/− mice [20]. Cyp2c44 is particularly interesting with respect to angiogenesis because of the fact that its expression can be induced by VEGF [22], and its 11,12- and 14,15-EET metabolites increase cell proliferation, tubulogenic activity, and the phosphorylation states of the ERK1/2 and Akt kinases in cultured cells [36] as well as in vivo models (for review see [3]). The finding that the deletion of Cyp2c44 resulted in an increase rather than a decrease in endothelial cell proliferation as well as an increase in flipodia formation was therefore initially unexpected. Indeed, the lack of Cyp2c44 was expected to prevent retinal epoxide generation and thus attenuate postnatal sprouting angiogenesis.
However, the genetic deletion of CYP enzymes has been reported to result in the compensatory upregulation of alternative isoforms to at least partially compensate for the loss of CYP activity. For example, the increased expression of Cyp4a12 was found to underlie the hypertensive phenotype of Cyp4a14−/− mice [17]. To avoid such an effect studies were repeated using Cyp2c44iKO mice in which tamoxifen was used to acutely downregulate Cyp2c44 expression postnatally. Although the knockdown of the enzyme was incomplete, the inducible model phenocopied that of the constitutive knockout mouse indicating that the effects observed could be attributed to the loss of Cyp2c44. However, it is important to note that such experiments cannot rule out that the expression of other CYP isoforms was altered under the experimental conditions studied.
Ours is not the first report of Cyp2c protein expression in the murine retina as an antibody recognizing a cluster of different Cyp2c isoforms was previously used to implicate Cyp2c in monocytes/macrophages throughout the retina in pathologic neovascularization [16]. However, although not a focus of the latter study a clear Cyp2c signal was detected in the ganglion cell layer, inner nuclear layers, and outer nuclear layer of the retina, consistent with expression on Müller cells [16]. A selective Cyp2c44 antibody was used in the present study to demonstrate the enrichment of Cyp2c44 in Müller glia cells in the retina. The coexpression of Cyp2c44 and the sEH in Müller glia cells strongly supported the hypothesis that the Müller glia cells act as a primary source of lipid mediators in the retina and the processing of lipid mediators in these cells consequently results in the modulation of vascular function development and possibly also vascular function. More recently, fenofibrate was reported to bind to and inhibit CYP2C with higher affinity than to peroxisome proliferator-activated receptor (PPAR)α and to inhibit oxygen-induced retinopathy in mice by reducing the generation of Cyp2c ω-3 and ω-6 PUFA metabolites [37]. Although Cyp2c44 expression is upregulated by hypoxia and the enzyme would be expected to be implicated in retinopathy, it remains to be determined whether or not fenofibrate affects Cyp2c44 activity or whether or not the phenotype observed reflected alterations in the expression or activity of the sEH. Certainly, PPARα agonist such as fenofibrate have been reported to induce the expression of the sEH [38], however, this does not appear to be a universal observation as the compound failed to affect sEH expression in a mouse model of hypertension [39].
The vascular defects in the retinas from sEH−/− mice were attributed to activation of the Notch signaling cascade [9] and it was expected that the phenotype observed in retinas from Cyp2c44−/− mice may be associated with opposite effects i.e. Notch inhibition. This was indeed the case, with Notch 1, Dll1 Hes1 and Hey1 levels all suppressed in retinas from Cyp2c44−/− mice. Also, the increase in vascular diameter at the angiogenic front was reminiscent of the effects elicited by Notch inhibition which triggers the excessive formation of tip cells, increased sprouting and branching, and endothelial hyperproliferation [32,40-43]. Although altered Notch signaling was previously linked to 19,20-DHDP which acts as a γ-secretase inhibitor [9], lipid profiles from 5 day old Cyp2c44−/− retinas revealed a completely unaltered ω-3 and ω-6 PUFA metabolite profile - despite the fact that in vitro analyses demonstrated the ability of the enzyme to metabolize arachidonic acid, linoleic acid, EPA and DHA. However both PDGFA and VEGF were increased in retinas from Cyp2c44−/− mice indicating that more than one mechanism may underlie the effects observed. Our study also highlights the differential consequences of Cyp2c44 expression in different models i.e. while Cyp2c44 deletion increased endothelial cell proliferation in the retina in vivo, the overexpression of Cyp2c44 increased the proliferation of human endothelial cells in vitro. These differential effects are most likely attributable to the markedly different lipid composition of the cells studied and in particular the high concentrations of DHA and HDHA in the retina [9,33].
HDHAs are currently of interest given that they can be further metabolized to produce a series of specialized “pro-resolving” lipid mediators termed the protectins, resolvins and maresins [44]. Several HDHAs were increased in retinas from Cyp2c44−/− mice retinas and microsomes from Cyp2c44-expressing Sf-9 cells rapidly metabolized 17- and 20-HDHA. Although the detailed chemical catalysis and potential metabolites generated remain unidentified, this observation strongly indicates that HDHAs may act as direct substrates of Cyp2c44. Little is known about the biological actions of HDHA metabolites but 17-oxo-DHA has been attributed anti-inflammatory properties at least partly attributable to the nuclear accumulation of the transcription factor Nrf2 (nuclear factor, erythroid derived 2, like 2) [45,46], which has in turn been linked with Notch activation (for review see [47]). 17-Oxo-DHA is known to be generated in activated macrophages by cyclooxygenase-2 but to-date nothing is known about the role of CYP enzymes. Moreover, the lack of available standards has meant that it has not yet been possible to determine the role of Cyp2c44 in its generation. Whether or not altered Cyp2c44 expression also has consequences on the retinal levels of pro-resolving mediators remains to be determined but 17S-hydroxy-containing docosanoids and 17S series resolvins were reportedly biosynthesized via epoxide-containing intermediates in murine brain, human blood, and glial cells [48].
5. Conclusions
On the basis of the data presented we conclude that Cyp2c44 is expressed in the normal murine retina and that its constitutive or acute deletion modulates postnatal angiogenesis in a manner reminiscent of Notch inhibition. Mechanistically, Cyp2c44 metabolized a series of ω-3 and ω-6 PUFAs in vitro but the most pronounced difference between retinas from wild-type and Cyp2c44−/− littermates was in the levels of three HDHA regioisomers (i.e. 10-, 17- and 20-HDHA), at least two of which could be further metabolized by Cyp2c44. At this stage there are of course a number of unknowns, including the identity of the source of the HDHA and whether or not the phenotype observed can be attributed to the Cyp2c44-dependent elimination of a bioactive metabolite, or its generation. It will be interesting to determine whether or not this mechanism is preserved in humans and whether the CYP2C-dependent metabolism of HDHAs has consequences on the retinal vasculature. It will also be interesting to determine whether or not other mouse CYPs can also metabolize HDHA and what the relative role of Cyp2c44 is compared to other enzymes.
Supplementary Material
Highlights.
Cyp2c44 is expressed in Müller glia cells in the murine retina.
Cyp2c44 deletion increases retinal endothelial cell proliferation.
Hydroxydocosahexaenoic acids are potential substrates for Cyp2c44.
Acknowledgements
The authors are indebted to Isabel Winter, Mechtild Piepenbrock-Gyamfi and Katharina Herbig for expert technical assistance.
Funding
This work was supported by the Dr. Hans Kröner-Graduiertenkolleg funded by the Else Kröner-Fresenius-Stiftung and the Deutsche Forschungsgemeinschaft (TR-SFB 23/A6, SFB 1039/A6 and Exzellenzcluster 147 "Cardio-Pulmonary Systems").
Abbreviations:
- CYP
cytochrome P450
- DHDP
dihydroxydocosapentaenoic acid
- DHET
dihydroxyeicosatrienoic acid
- DiHOME
dihydroxyoctadecenoic acid
- EDP
epoxydocosapentaenoic acid
- EEQ
epoxyeicosatetraenoic acid
- EET
epoxyeicosatrienoic acid
- ENaC
epithelial sodium channel
- EPA
eicosapentaenoic acid
- EpOME
epoxyoctadecenoic acid
- ERG
ETS-related gene
- GCL
ganglion cell layer
- HDHA
hydroxydocosahexaenoic acid
- HEPE
hydroxyeicosapentaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- INL
inner nuclear layer
- IPL
inner plexiform layer
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- P
postnatal day
- PCL
retinal pigment cell layer
- pH3
phosphorylated histone 3
- PUFA
polyunsaturated fatty acids
- sEH
soluble epoxide hydrolase
- VEGF
vascular endothelial cell growth factor
Footnotes
Conflict of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campbell WB, Gebremedhin D, Pratt PF, Harder DR, Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors, Circ. Res 78 (1996) 415–423. [DOI] [PubMed] [Google Scholar]
- 2.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R, Cytochrome P450 2C is an EDHF synthase in coronary arteries, Nature 401 (1999) 493–497. [DOI] [PubMed] [Google Scholar]
- 3.Fleming I, The pharmacology of the cytochrome P450/soluble epoxide axis in the vasculature, Pharmacol. Rev 66 (2014) 1–36. [DOI] [PubMed] [Google Scholar]
- 4.Munzenmaier DH, Harder DR, Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release, Am J Physiol Heart Circ Physiol 278 (2000) H1163–H1167. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Harder DR, Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic acid, Stroke 33 (2002) 2957–2964. [DOI] [PubMed] [Google Scholar]
- 6.Medhora M, Daniels J, Mundey K, Fisslthaler B, Busse R, Jacobs ER, Harder DR, Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells, Am J Physiol Heart Circ Physiol 284 (2003) H215–H224. [DOI] [PubMed] [Google Scholar]
- 7.Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R, Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR), FASEB J. 17 (2003) 770–772. [DOI] [PubMed] [Google Scholar]
- 8.Webler AC, Popp R, Korff T, Michaelis UR, Urbich C, Busse R, Fleming I, Cytochrome P450 2C9-induced angiogenesis is dependent on EphB4, Arterioscler. Thromb. Vasc. Biol 28 (2008) 1123–1129. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Popp R, Frömel T, Ehling M, Awwad K, Adams RH, Hammes HP, Fleming I, Müller glia cells regulate Notch signaling and retinal angiogenesis via the generation of 19,20-dihydroxydocosapentaenoic acid, J. Exp. Med 211 (2014) 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanai R, Mulki L, Hasegawa E, Takeuchi K, Sweigard H, Suzuki J, Gaissert P, Vawas DG, Sonoda KH, Rothe M, Schunck WH, Miller JW, Connor KM, Cytochrome P450-generated metabolites derived from ω-3 fatty acids attenuate neovascularization, PNAS 111 (2014) 9603–9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michaelis UR, Falck JR, Schmidt R, Busse R, Fleming I, Cytochrome P4502C9-derived epoxyeicosatrienoic acids induce the expression of cyclooxygenase-2 in endothelial cells, Arterioscler. Thromb. Vase. Biol 25 (2005) 321–326. [DOI] [PubMed] [Google Scholar]
- 12.Sinai CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ, Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation, J. Biol. Chem 275 (2000) 40504–40510. [DOI] [PubMed] [Google Scholar]
- 13.Jung O, Jansen F, Mieth A, Barbosa-Sicard E, Pliquett RU, Babelova A, Morisseau C, Hwang SH, Tsai C, Hammock BD, Schaefer L, Geisslinger G, Amann K, Brandes RP, Inhibition of the soluble epoxide hydrolase promotes albuminuria in mice with progressive renal disease, PLoS ONE 5 (2010) e11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frömel T, Jungblut B, Hu J, Trouvain C, Barbosa-Sicard E, Popp R, Liebner S, Dimmeler S, Hammock BD, Fleming I, Soluble epoxide hydrolase regulates hematopoietic progenitor cell function via generation of fatty acid diols, Proc. Natl. Acad. Sci. USA 109 (2012) 9995–10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, Barnés CM, Mammoto A, Mammoto T, Luria A, Benny O, Chaponis DM, Dudley AC, Greene ER, Vergilio JA, Pietramaggiori G, Scherer-Pietramaggiori SS, Short SM, Seth M, Lih FB, Tomer KB, Yang J, Schwendener RA, Hammock BD, Falck JR, Manthati VL, Ingber DE, Kaipainen A, D'Amore PA, Kieran MW, Zeldin DC, Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice, J. Clin. Invest 122 (2012) 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao Z, Fu Z, Stahl A, Joyal J. S. b., Hatton C, Juan A, Hurst C, Evans L, Cui Z, Pei D, Gong Y, Xu D, Tian K, Bogardus H, Edin ML, Lih F, Sapieha P, Chen J, Panigrahy D, Hellstrom A, Zeldin DC, Smith LEH, Cytochrome P450 2C8 ω3-long-chain polyunsaturated fatty acid metabolites increase mouse retinal pathologic neovascularization, Arterioscler. Thromb. Vasc. Biol 34 (2014) 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holla VR, Adas F, Imig JD, Zhao X, Price E Jr., Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH, Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension, Proc. Natl. Acad. Sci. USA 98 (2001) 5211–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price E, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH, Salt-sensitive hypertension is associated with dysfunctionalCyp4a10 gene and kidney epithelial sodium channel, J Clin. Invest 116 (2006) 1696–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capdevila J, Wang W, Role of cytochrome P450 epoxygenase in regulating renal membrane transport and hypertension, Curr. Opin. Nephrol Hypertens 22 (2013) 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun P, Antoun J, Lin DH, Yue P, Gotlinger KH, Capdevila J, Wang WH, Cyp2c44 epoxygenase is essential for preventing the renal sodium absorption during increasing dietary potassium intake, Hypertension 59 (2012) 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pozzi A, Popescu V, Yang S, Mei S, Shi M, Puolitaival SM, Caprioli RM, Capdevila JH, The anti-tumorigenic properties of peroxisomal proliferator-activated receptor α are arachidonic acid epoxygenase-mediated, J. Biol. Chem 285 (2010) 12840–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang S, Wei S, Pozzi A, Capdevila JH, The arachidonic acid epoxygenase is a component of the signaling mechanisms responsible for VEGF-stimulated angiogenesis, Arch. Biochem. Biophys 489 (2009) 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLozier TC, Tsao CC, Coulter SJ, Foley J, Bradbury JA, Zeldin DC, Goldstein JA, CYP2C44, a new murine CYP2C that metabolizes arachidonic acid to unique stereospecific products, J. Pharmacol. Exp. Ther 310 (2004) 845–854. [DOI] [PubMed] [Google Scholar]
- 24.Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL, The transcriptome of retinal Muller glial cells, J Comp Neurol. 509 (2008) 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busse R, Lamontagne D, Endothelium-derived bradykinin is responsible for the increase in calcium produced by angiotensin-converting enzyme inhibitors in human endothelial cells, Naunyn Schmiedebergs Arch. Pharmacol 344 (1991) 126–129. [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association, Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects, Cardiovasc. Res 35 (1997) 2–3. [PubMed] [Google Scholar]
- 27.Schwarz D, Kisselev P, Honeck H, Cascorbi I, Schunck WH, Roots I, Coexpression of human cytochrome P4501A1 (CYP1A1) variants and human NADPH-cytochrome P450 reductase in the baculovirus/ insect cell system, Xenobiotica 31 (2001) 345–356. [DOI] [PubMed] [Google Scholar]
- 28.Rivera J, Ward N, Hodgson J, Puddey IB, Falck JR, Croft KD, Measurement of 20-hydroxyeicosatetraenoic acid in human urine by gas chromatography-mass spectrometry, Clin Chem. 50 (2004) 224–226. [DOI] [PubMed] [Google Scholar]
- 29.Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von SC, Luft FC, Muller DN, Rothe M, Schunck WH, Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of ω-3 fatty acids, J Biol Chem 285 (2010) 32723–32733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer R, Konkel A, Mehling H, Blossey K, Gapelyuk A, Wessel N, von Schacky C, Dechend R, Muller DN, Rothe M, Luft FC, Weylandt K, Schunck WH, Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway, J. Lipid Res 55 (2014) 1150–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R, Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis, J. Cell Sci 118 (2005) 5489–5498. [DOI] [PubMed] [Google Scholar]
- 32.Ehling M, Adams S, Benedito R, Adams RH, Notch controls retinal blood vessel maturation and quiescence, Development 140 (2013) 3051–3061. [DOI] [PubMed] [Google Scholar]
- 33.Arterburn LM, Hall EB, Oken H, Distribution, interconversion, and dose response of n-3 fatty acids in humans, Am J Clin Nutr. 83 (2006) S1467–1476S. [DOI] [PubMed] [Google Scholar]
- 34.Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B, Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation, Prostagland. Other Lipid Mediat 97 (2012) 73–82. [DOI] [PubMed] [Google Scholar]
- 35.Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R, Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells, Arterioscler. Thromb. Vase. Biol 27 (2007) 2612–2618. [DOI] [PubMed] [Google Scholar]
- 36.Michaelis UR, Fleming I, From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: epoxyeicosatrienoic acids (EETs) and cell signaling, Pharmacol. Ther 111 (2006) 584–595. [DOI] [PubMed] [Google Scholar]
- 37.Gong Y, Shao Z, Fu Z, Edin ML, Sun Y, Liegl RG, Wang Z, Liu CH, Burnim SB, Meng SS, Lih FB, SanGiovanni JP, Zeldin DC, Hellström A, Smith LEH, Fenofibrate inhibits cytochrome P450 epoxygenase 2C activity to suppress pathological ocular angiogenesis, EBioMedicine 13 (2016) 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL, Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor α, Drug Metab Dispos 35 (2007) 1126. [DOI] [PubMed] [Google Scholar]
- 39.Lee DL, Wilson JL, Duan R, Hudson T, El-Marakby A, Peroxisome proliferator-activated receptor-α activation decreases mean arterial pressure, plasma interleukin-6, and COX-2 while increasing renal CYP4A expression in an acute model of DOCA-salt hypertension, PPAR. Res 2011 (2011) 502631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C, Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis, Nature 445 (2007) 776–780. [DOI] [PubMed] [Google Scholar]
- 41.Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J, Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis, Development 134 (2007) 839–844. [DOI] [PubMed] [Google Scholar]
- 42.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ, Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting, Proc. Natl. Acad. Sci. USA 104 (2007) 3219–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siekmann AF, Lawson ND, Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries, Nature 445 (2007) 781–784. [DOI] [PubMed] [Google Scholar]
- 44.Serhan CN, Pro-resolving lipid mediators are leads for resolution physiology, Nature 510 (2014) 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cipollina C, Di Vincenzo S, Gerbino S, Siena L, Gjomarkaj M, Pace E, Dual antioxidant and anti-inflammatory actions of the electrophilic cyclooxygenase-2-derived 17-oxo-DHA in lipopolysaccharide- and cigarette smoke-induced inflammation, Biochimica et Biophysica Acta (BBA) - General Subjects 1840 (2014) 2299–2309. [DOI] [PubMed] [Google Scholar]
- 46.Gruber F, Ornelas CM, Karner S, Narzt MS, Nagelreiter IM, Gschwandtner M, Bochkov V, Tschachler E, Nrf2 deficiency causes lipid oxidation, inflammation, and matrix-protease expression in DHA-supplemented and UVA-irradiated skin fibroblasts, Free Radic. Biol. Med 88, Part B (2015) 439–451. [DOI] [PubMed] [Google Scholar]
- 47.Wakabayashi N, Chartoumpekis DV, Kensler TW, Crosstalk between Nrf2 and Notch signaling, Free Radic. Biol. Med 88, Part B (2015) 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN, Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells: autacoids in anti-inflammation, J. Biol. Chem 278 (2003) 14677–14687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.